Congenital Lobar Emphysema (CLE) is a rare condition presenting with respiratory distress soon after birth. It is often difficult to diagnose but its early recognition and surgical management can be life saving.
CASE REPORT
A 20 days old, full term, male baby weighing 3 kg delivered vaginally at a peripheral maternity hospital to a primigravida mother, presented to our hospital with respiratory distress since birth. The baby had cried immediately after birth, color was pink and peripheries were warm. The heart rate was 140/min and respiratory rate was 40/min. However, soon after birth were he started developing respiratory distress with suprasternal and intercostal recession. On auscultation, air entry on left side of the chest was decreased, heart sounds better heard on the right side. Pulse oximeter showed a saturation of 85% while using an oxygen hood.
A chest x ray (Figure 1) reported left pneumothorax with consolidation on the right side. Consequently, a chest tube was put in the left 4th intercostal space. However, the distress remained and the intercostal tube column was not moving. Repositioning it did not improve the status. Since respiratory distress persisted with antibiotics and other standard care the child was referred to our hospital. A CT scan (Figure 2) was performed which revealed gross mediastinal shift to the right side. The heart and other mediastinal contents appeared to be normal. There was evidence of collapse consolidation in right upper and left lower lung lobes with emphysema in left upper lobe and no pneumothorax. The chest tube was reported to be lying in emphysematous LUL. A diagnosis of congenital lobar emphysema was made and surgery was planned. The preoperative blood investigations revealed Hb of 11.9 g%, normal leukocyte, platelet counts and normal biochemistry. ABG analysis revealed a pH of 7.48, pO2 of 55 mmHg and pCO2 of 26 mmHg on FiO2 of 0.4. Echocardiography was normal. In the pre anesthesia check up, the baby had respiratory distress with RR of 56/min with suprasternal and intercostal recession. The saturation with an oxyhood was 85% and on room air was 75%. The air entry was decreased on left side and intercostal tube was in situ. The child was scheduled for left lobectomy on the next day, blood was ordered and no sedative premedication was prescribed.
Figure 1.
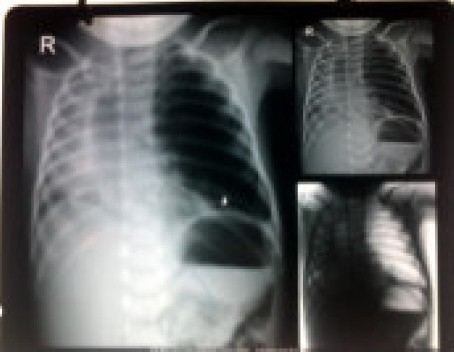
Chest X-ray film
Figure 2.
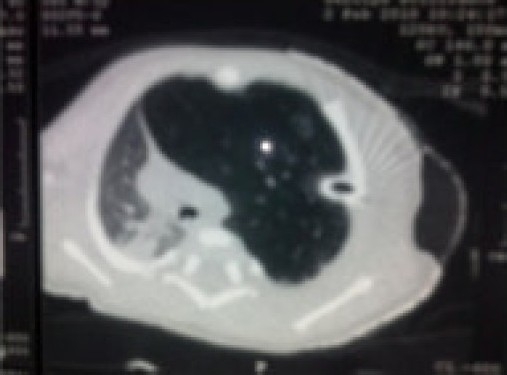
CT Scan film which confirmed the diagnosis of CLE
In the OT, ECG, saturation and temperature monitoring was started and a peripheral line secured. Standard precautions to avoid hypothermia were taken and premedication with iv atropine 0.03mg and fentanyl 0.006 mg was given. A surgeon was washed up and ready to perform an emergency thoracotomy if the need arose. Induction was done by a mask with 100% O2 and sevoflurane 2-4%. After achieving an adequate depth of anaesthesia, trachea was intubated with size 3.0mm ETT fixed at 9 cm. Gentle assisted ventilation was done with hand using a Jackson Rees circuit, neonate placed in the right lateral position and anaesthesia maintained with sevoflurane 2-3% in 100% O2. Local anaesthetic 0.25% (2 ml) bupivacaine was infiltrated in the line of incision before starting surgery. Intraoperative analgesia was provided with intravenous ketamine given to a total of 10 mg in divided doses. A left upper lobectomy was performed through the 4th intercostal space with stable hemodynamics throughout the two hour surgical procedure. After lobectomy, the left lower lobe was expanded using gentle manual recruitment maneuver. Blood and fluid loss monitored and replaced with 120 ml of warmed Ringer's Lactate and 20 ml of fresh whole blood. At the end of the surgery, an intercostal block was given by the surgeon under direct vision using 2 ml of 0.25% bupivacaine. The saturation rose up to 100% after the lobectomy and the trachea was extubated after discontinuation of sevoflurane when full airway reflexes had returned. The baby was shifted to the post anesthesia care unit with no evidence of respiratory distress. Post operative analgesia was provided by rectal paracetamol. The patient made a rapid uneventful recovery and was discharged on 7th post operative day.
DISCUSSION
CLE refers to an idiopathic postnatal abnormal overdistension of an otherwise anatomically normal lobe of the lung characterized by expiratory air trapping within the lobe producing compression and atelectasis of adjacent lobes.1–3 Other causes of bronchial obstruction2, either intrinsic (bronchial stenosis or cysts) or extrinsic (compression due to abnormal vessels), may also be responsible. Intrinsic obstruction is more common where the cartilage of the affected bronchus fails to develop, resulting in focal bronchial collapse and air trapping on expiration. This hyperinflation leads to medistinal shift, decreased venous return, hypotension and hypoxia.4 Usually only one lobe is involved, with about 50% of cases affecting left upper lobe, 30% affecting right middle lobe, and 20% affecting right upper lobe.4
CLE usually presents in full term infants between the newborn period and 6 months of age, with approximately 50% presenting within the first two days of life. Congenital heart disease or abnormalities of the great vessels occur in approximately 15% of infants. Depending on the degree of bronchial obstruction, the clinical presentation is variable with the majority presenting with tachycardia, tachypnea and retractions. If hypoxemia ensues, the infant may become increasingly agitated, anxious and cyanosed with grunting and coughing. Physical examination may reveal asymmetric chest expansion with focal hyperresonance and diminished breath sounds over the affected lobe. Chest X-ray shows typical findings including hyperinflation of the involved lobe, atelectasis of adjacent lung, mediastinal shift and flattening of the ipsilateral diaphragm.3 However this picture is often confused with pneumothorax or pneumonia. A CT scan is diagnostic and can identify the point of obstruction, if intraluminal or extrinsic. Ventilation-Perfusion scans show decreased perfusion of affected lobe (secondary to vessel compression) and increased perfusion of unaffected lobe (secondary to shunting).
Thoracotomy and complete lobectomy are usually required for CLE. The physiological considerations for anaesthetizing these patients include ventilation and perfusion impairment of the lung in an infant undergoing thoracic surgery in the lateral decubitus position. Unlike adults, infants with unilateral lung disease do not improve their oxygenation when the healthy lung is dependent and the diseased lung is nondependent.5 In infants, oxygenation is optimized when the healthy lung is nondependent.6 This difference is due to the more compliant chest wall of the infant, which cannot completely support the dependent lung. The FRC is consequently closer to the residual volume and airway closure becomes more likely in the dependent lung.7 The advantage of the abdominal hydrostatic pressure gradient to the dependent diaphragm is lesser in infants. Due to their small size; the favorable increase in perfusion to the dependent lung is also less. An infant's ability to tolerate one-lung ventilation (OLV) is affected by these considerations as the position for thoracic surgery is unfavorable for them when compared to adults.
Inhalation induction is preferred because spontaneous ventilation should be maintained until either the chest is opened or OLV of the contralateral lung is achieved.4 Positive pressure ventilation, due to a ball-valve effect, can lead to overdistension of the emphysematous lobe, worsening of mediastinal shift, reduction in cardiac output and hemodynamic collapse. However gentle assisted manual ventilation may be needed if there is hypoventilation during induction due to poor respiratory reserve. Nitrous oxide should be avoided.
The optimum ventilatory technique has been discussed by many authors. Tempe suggests that IPPV is best avoided as the critical airway pressure is not known.3 Gentle manual ventilation keeping the airway pressure at 20-25 cm of H2O before thoracotomy has been described.4 PRVC mode is an attractive option for mechanical ventilation if available. High frequency ventilation has been used successfully in the patients with CLE, as low airway pressures are especially suitable.8 The risk of overdistension leading to a catastrophic hemodynamic crisis should never be underestimated and the surgeon should be ready for immediate thoracotomy during induction.
OLV is not generally indicated for open thoracotomies in neonates and infants because the surgeon is usually able to manually retract the lung. In neonates and infants, the only options for lung isolation are either mainstem intubation or placement of bronchial blockers. Use of double lumen Marraro tubes has been reported as well.9
Maintenance of anaesthesia is usually done with inhalational agents. Opiates like morphine are avoided due to the risk of postoperative respiratory depression. Epidural analgesia with a caudal catheter inserted up to the thoracic level and using local anesthetics has been described for management of 3 cases.10 Intravenous ketamine in boluses of 1.5-2mgkg-1 has been recommended for intraoperative analgesia.2,4
In most of the cases the trachea can be extubated at the end of the surgery. Elective postoperative ventilation is usually not required especially if excision is limited to one lobe and analgesia is adequate which allows infant to breathe spontaneously.
REFERENCES
- 1.Husain A, Kumar V. The Lung. In: Kumar V, Abbas A, Fausto N, editors. Robbins and Cotran Pathologic Basis of Disease. 7th edition. Philadelphia, Pa: Saunders Elsevier; 2005. Chapter 15. [Google Scholar]
- 2.Lakshmi Vas. Congenital Lobar Emphysema. In: Litman RS, editor. Pediatric Anesthesia Practice. Cambridge: University Press; 2007. pp. 85–87. [Google Scholar]
- 3.Tempe DK, Virmani S, Javetkar S, et al. Congenital lobar emphysema: pitfalls and management. Ann Cardiac Anesth. 2010;13:1. doi: 10.4103/0971-9784.58836. [DOI] [PubMed] [Google Scholar]
- 4.Cote CJ. The anesthetic management of congenital lobar emphysema. Anesthesiology. 1978;49:296–8. doi: 10.1097/00000542-197810000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Remolina C, Khan AU, Santiago TV, et al. Positional hypoxemia in unilateral lung disease. N Eng J Med. 1981;304(9):523–525. doi: 10.1056/NEJM198102263040906. [DOI] [PubMed] [Google Scholar]
- 6.Second Edition. Elsevier Inc; 2007. Benumof's Airway Management: Chapter 24: Separation of two lungs. [Google Scholar]
- 7.Mansell A, Bryan C, Levison H. Airway closure in children. J Appl Physiol. 1972;33(6):711–714. doi: 10.1152/jappl.1972.33.6.711. [DOI] [PubMed] [Google Scholar]
- 8.Goto H, Boozalis ST, Benson KT, et al. High frequency jet ventilation for resection of congenital lobar emphysema. Anesth Analg. 1987;66:684–6. [PubMed] [Google Scholar]
- 9.Pawar DK, Marraro GA. One Lung ventilation in infants and children: experience with Marraro double lumen tube. Pediatr Anesth. 2005;15(3):204–8. doi: 10.1111/j.1460-9592.2005.01421.x. [DOI] [PubMed] [Google Scholar]
- 10.Raghavendran S, Diwan R, Shah T, et al. Continuous caudal epidural analgesia for congenital lobar emphysema: a report of three cases. Anesth Analg. 2001;93:348–50. doi: 10.1097/00000539-200108000-00022. [DOI] [PubMed] [Google Scholar]


