ParaVertebral Block (PVB) involve injection of local anaesthetic in a space immediately lateral to where the spinal nerves emerge from the intervertebral foramina. This technique is being used increasingly for not only intra-operative and post-operative analgesia but also as a sole anaesthetic technique for carrying out various procedures. This popularity is mainly due to the ease of the technique and fewer complications.
History
The concept of ParaVertebral Block was pioneered by Hugo Sellheim of Leipzig in 1905. It was further refined by Lawen (1911) and Kappis (1919).1 The technique however remained neglected till the late 1970s, when a renewed interest developed in the topic due to efforts from Eason and Wyatt who presented a reappraisal on Thoracic ParaVertebral Block (TPVB).2 They found it to be an accurate, simple and safe method which carried significant advantages over intercostal or epidural block. It was initially utilized as an alternative to spinal anaesthesia which would minimize the cardiovascular and respiratory effects of central neuraxial block. However, after its initial description PVB were used sparingly to provide anaesthesia and analgesia.
More recently, there has been renewed interest in this technique for the treatment of acute and chronic pain. Because of the ability to provide long-lasting unilateral anaesthesia, PVB have been successfully used to provide analgesia for multiple thoracic and abdominal procedures in both children and adults.3
Anatomy of the Paravertebral space
The paravertebral space (Figure 1) is a wedge-shaped anatomical compartment adjacent to the vertebral bodies. Klein et al (2004) described an endoscopic technique that permits imaging of the contents and boundaries of the thoracic paravertebral space in cadavers.4 In the dorsal region, the paravertebral space is defined anterolaterally by the parietal pleura, posteriorly by the superior costotransverse ligament, medially by the vertebrae and intervertebral foramina, superiorly and inferiorly by the heads of the ribs. Within this space, the spinal root emerges from the intervertebral foramen and divides into dorsal and ventral rami. The sympathetic chain lies in the same fascial plane, just anterior to the intercostal nerve and communicates with it via the rami communicantes. Hence, PVB produces unilateral sensory, motor and sympathetic blockade.
Figure 1.
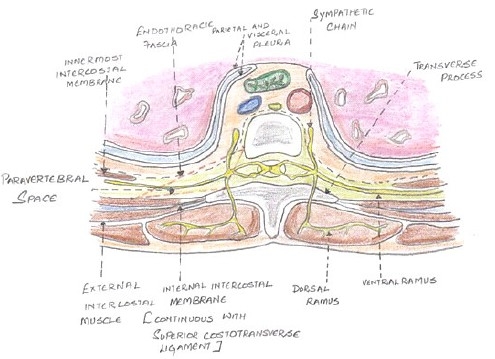
Anatomy of the Paravertebral space
Each space is not an isolated structure but can communicate superiorly and inferiorly across the heads and necks of the ribs with the spaces above and below. Interposed between the parietal pleura and the superior costotransverse ligament is the endothoracic fascia, which is the deep fascia of thorax. This fascia divides the space into 2 compartments, anterior “extrapleural paravertebral compartment” and the posterior “subendothoracic paravertebral compartment”. The nerves are located behind this fascia.
Mechanism and spread of anesthesia
A thoracic paravertebral injection of local anaesthetics results in ipsilateral somatic and sympathetic nerve block including the posterior ramus in multiple contiguous thoracic dermatomes21. The spinal nerves in this space are devoid of a fascial sheath, making them exceptionally susceptible to local anaesthetics.
Cheema et al (1995) evaluated the extent of somatic and sympathetic blockade following a single thoracic paravertebral injection using a thermographic imaging technique. They reported a large unilateral somatic (mean of five dermatomes) and sympathetic block (mean of eight dermatomes).5 Apart from strict longitudinal spread, other forms of distribution have also been observed. Lateral and cloud-shaped spread of radio-opaque dye injected in the thoracic paravertebral space, indicating intercostal spreading pattern as reported by Conacher et al in 19876.7
Karmakar et al (2000) reported contralateral spread of contrast anterior to the vertebral bodies after successful paravertebral block for multiple fractured ribs.8 The variability in spread following PVB was explained by Karmakar and Chung (2000).9 They explained the existence of the endothoracic fascia. This assumption was further confirmed by Naja et al (2004) who used a nerve-stimulator guided technique to demonstrate that paravertebral injections ventral to the endothoracic fascia facilitate longitudinal spread while those dorsal to the fascia result in more unpredictable spread.10
Communication of thoracic paravertebral space
The space is continuous with the intercostal space laterally, the epidural space medially and the contralateral paravertebral space through the paravertebral and epidural space. The cranial extension is still not defined but radiolo-gical spread of the contrast medium into the cervical region after thoracic paravertebral injection has been observed. The origin of the psoas major muscle forms the caudal boundary and inferior (lumbar) spread through the Thoracic ParaVertebral Space (TPVS) is thought to be unlikely.
Ipsilateral thoracolumbar anaesthesia, radiologic spread of contrast below the diaphragm, and thoracolumbar spread of colored dye in cadavers have been described, and there is disagreement about the caudal limit of spread. The endothoracic fascia is continuous inferiorly with the fascia transversalis of the abdomen dorsal to the diaphragm through the medial and lateral arcuate ligaments and the aortic hiatus. An injection in the lower TPVS posterior to the endothoracic fascia can spread inferiorly through the medial and lateral arcuate ligaments to the retroperitoneal space behind the fascia transversalis, where the lumbar spinal nerves lie, and are the anatomic basis of the technique of "extended unilateral anaesthesia.
TECHNIQUES
Conventional technique:- Loss of resistance to air The block is performed with the patient in the sitting or lying down position with the neck flexed, back arched, and shoulders dropped forward. Mark a point 2.5 to 3cm lateral to the T4 spine (Figure 2,3). Following strict aseptic precautions the site of injection is infiltrated with 2% lignocaine. Needle is advanced directly posteroanterior, perpendicular to the skin, until contact with the pars intervertebralis, articular column, or transverse process of the particular vertebra was established. Loss-of-resistance syringe is attached to the needle and, while continuously testing for loss of resistance to air the needle is “walked off” the structure in an inferolateral (lateral and caudad) direction and advanced approximately 1 cm (but a maximum of 1.5 cm), ensuring that the bevel of the needle points laterally, away from the medial structures. As the costotransverse ligament is penetrated, a “pop” is felt, and there is a loss of resistance to air. This signifies paravertebral space.
Figure 2.
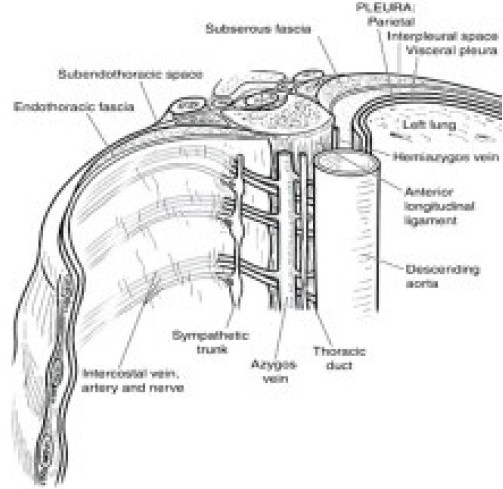
Showing the endothoracic fascia.(Source www.medartist.com/spine_art.html.printed with permission)
Figure 3.
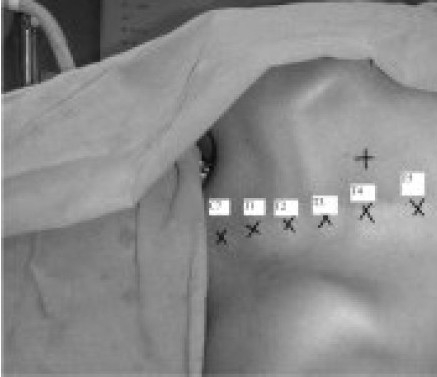
Landmarks for TPVB
Modifications
Conventional technique of paravertebral space localization includes loss of resistance following penetration of superior costotransverse ligament. However, reports of unpredictable spread, failure rate and complications have prompted modifications in this technique to ensure correct localization of the space. Pusch et al (2000) demonstrated that sonographic measurements of the distance from the skin to the transverse process and to the parietal pleura are useful for calculating the required depth of needle insertion in order to avoid unintentional pleural puncture.11
Encouraged by the utility of nerve stimulator guidance in other peripheral nerve-blocks, Wheeler et al (2001) utilized nerve-stimulation technique in performing PVB for breast surgery.12 They recommended twitching of the intercostal muscle (appropriate to the distribution of ventral ramus of the spinal nerve stimulated) at 0.4 mA intensity current as the stimulation end point for the block. Similar technique was also reported by Lang et al in 2002.13
Luyet et al (2009) in a cadaveric imaging study described the successful placement of catheter in the paravertebral space under ultrasound guidance.14 Ultrasound-guided nerve blocks have the potential to improve efficacy and reduce complications via real-time visualization of the intended anatomic space, surrounding structures, and the approaching needle. They found that the superior costotransverse ligament and the paravertebral space were easily identified with a slightly oblique scan using a curved array ultrasound transducer.
Cowie et al (2010) compared a single-versus dual-injection technique for ultrasound-guided paravertebral blockade in a cadaver model, evaluating the spread of contrast dye and location of a catheter. Paravertebral space was easily identified using an ultrasound probe in the transverse plane, a linear transducer and an in-plane needle approach. Contrast dye was seen to surround somatic and sympathetic nerves in the paravertebral, intercostal, and epidural spaces. Contrast dye was present in 19 of 20 paravertebral spaces over 3 to 4 segments (range, 0–10) with no significant differences between single- and dual-injection techniques. They concluded that transverse in-plane ultrasound-guided needle insertion into the thoracic paravertebral space is both feasible and reliable and intercostal and epidural spread contributes significantly to the analgesic efficacy of the paravertebral block.15
Marhofer et al (2010) investigated the anatomy of the lateral paravertebral space using a high-frequency linear ultrasound transducer in twenty women undergoing breast cancer surgery. After identification of the transverse process, internal intercostal membrane (IIM), and pleura at the T3 and T6 levels, an out-of-plane needle guidance technique was used to perform the PVB with 12 ml ropivacaine 0.75% at these two levels in the sitting position and the PVB was successful in all these cases.16
Complications of paravertebral block
PVB is technically easy to learn with a high success rate. The failure rate associated with PVB is not > 13%. Naja and Lonnqvist (2001) prospectively evaluated the failure rate and complications following PVB in 620 adults and 42 children. They reported a failure rate of 6.1% in adults and none in children. Inadvertent vascular puncture (6.8%), hypotension (4%), epidural or intrathecal spread (1%), pleural puncture (0.8%) and pneumothorax (0.5%) were the recorded complications. Likelihood of vascular puncture and pneumothorax was reported to be higher in bilateral compared to unilateral block.17 Pulmonary hemorrhage has been reported after thoracic PVB in a patient with previous thoracic surgery.18 Burlacu et al (2005) reported contralateral harlequin and ipsilateral Horner's syndrome after unilateral paravertebral anaesthesia for breast cancer surgery, attributable to spread to ipsilateral stellate ganglion.19 Postoperative nausea and vomiting are significantly lower in patients given PVB compared to GA.
Applications of Paravertebral block
Thoracic surgery
Paravertebral thoracic block was found to be an accurate, simple and safe method which carries significant advantages over intercostal or epidural block. Continuous paravertebral infusion was compared with extradural infusion of bupivacaine for post-thorocotomy pain relief and found to have comparable results in terms of analgesia.20 Paravertebral bupivacaine was demonstrated superior to epidural bupivacaine in terms of analgesia, pulmonary function, neuroendocrine stress response, side-effects and post operative respiratory morbidity in patients following thoracotomy.21
Continuous PVB was reported to be similar in efficacy to thoracic epidural analgesia with fewer complications in a prospective randomized study by Dhole et al (2001) after minimally invasive coronary artery bypass surgery.22
Hill et al (2006) demonstrated the efficacy of single-dose PVB with bupivacaine in reducing pain after video-assisted thoracoscopic surgery.23
Kotze et al conducted a systematic review and metare-gression on the eficacy and safety of different techniques of paravertebral block for analgesia after thoracotomy. They came to the following conclusions. Higher dose of local anesthetic was found to offer better analgesia. Continuous infusions were better than intermittent boluses. No single adjunct was found to be superior compared to others.24
Liver surgery
Culp et al (2008) have described the use of TPVB for severe pain following radiofrequency ablation of liver mass.25 The block was performed at T6-7 and T8-9 levels with 15ml of 0.25% bupivacaine under nerve stimulator guidance. Sensory block was provided from T4-12 levels. VAS decreased from 10 to 0 in 30 minutes. Culp et al (2005) have also described the use of TPVB for percutaneous transhepatic biliary drainage.26
Inguinal hernia surgery
Wassef et al (1998) compared PVB with Field block for ingunal herniorrhaphy. And they concluded that PVB offers superior anaesthetic efficacy than a field block.27 PVB provides analgesia equivalent to extensive peripheral nerve block for outpatient inguinal herniorrhaphy with fewer side-effects, as reported by Klein et al (2002).28
Ambulatory surgery
Weltz et al (1995) demonstrated the potential of paravertebral block as the sole anaesthetic for ambulatory breast surgery with significant advantages in terms of safety, patient satisfaction, analgesia and cost-savings.29 Hadzic et al (2006) reported that PVB resulted in faster time to home readiness, and was associated with fewer adverse events and better analgesia compared to fast-track general anaesthetic regimen in outpatient inguinal herniorrhaphy.30
Cholecystectomy
Giesecke et al (1988) examined the effect of PVB on the perioperative stress response in patients scheduled for open cholecystectomy. A significant reduction in circulatory and hormonal response to stress was demonstrated.31 Naja et al (2004), in a prospective randomized study design, demonstrated improved pain-relief with bilateral nerve-stimulator guided paravertebral blockade with 0.3 ml/kg of a local anaesthetic mixture containing lidocaine, bupivacaine, fentanyl and clonidine at T5-6 level in patients undergoing laparoscopic cholecystectomy.32 Patients in the PVB group reported less pain scores and required less supplemental analgesics (meperidine and dextropro-poxyphene) compared to the control group for three days postoperatively.
Rib fracture
Karmakar et al (2003) studied the efficacy of a continuous thoracic paravertebral infusion of bupivacaine for pain management in patients with unilateral multiple fractured ribs. A continuous infusion of 0.1 ml/kg/hr was commenced. The block was successful in all patients. Median ipsilateral loss of sensation to cold of >5 dermatomes was demonstrated. In 3 patients contralateral spread was demonstrated. One patient had epidural spread. All patients had improved pain scores. They also had improved respiratory function with decrease in respiratory rate and increase in forced vital capacity. One patient developed Horner's syndrome. Results confirmed that continuous thoracic paravertebral infusion of bupivacaine is a simple and effective method of providing continuous pain relief in patients with unilateral multiple fractured ribs.33 It also produced a sustained improvement in respiratory parameters and oxygenation.
Breast surgery
Weltz et al (1995) conducted a retrospective study from medical records of 15 patients with breast cancer who underwent 16 major operations (simple mastectomy, Wide local excision and MRM) using PVB, as a sole anaesthetic technique. The block (unilateral) was performed from C7-T7. 4ml of 0.5% bupivacaine with 1:400,000 epinephrine was injected at each Paravertebral space. In these patients, anaesthesia was found to be adequate and supplementation was not required in any of the patient. There was postoperative hemorrhage in one patient, seroma in two patients, and superficial wound infection in one patient. Sensory block persisted for 23 hours on an average. None of the patients had any episode of hypotension. Postoperative pain was effectively controlled. Nausea and vomiting afflicted 3 patients. 93% (14 patients) rated the experience as very satisfactory.27
Greengrass et al (1996) studied the effect of PVB in 25 patients undergoing breast surgery. The procedures varied from lumpectomy to MRM. The block (unilateral) was performed from C7-T6 with bupivacaine 0.5% with epinephrine 3-4ml per segment. Out of these, 5 patients had incomplete block. No complications were attributed to the blocks. With regard to postoperative analgesia, of the 17 patients with successful blocks who were available for follow-up, six required no analgesics, two were inadvertently given acetaminophen with 30 mg codeine tablets by nurse, and two patients received plain acetaminophen only. Four patients took one or two acetaminophen with 30 mg codeine tablets for mild pain or “stiffness” and three patients took more than two acetaminophen with 30 mg codeine tablets for mild pain. All patients with incomplete blocks received intravenous Patient Controlled Analgesia (PCA) followed by oral opioids. Patients with successful blocks were all very satisfied. Post operatively, patients with successful block had minimum nausea, vomiting and pain.34
Greengrass et al (1998) compared the safety and efficacy of PVB as a sole anaesthetic technique for intraoperative and post operative management of MRM over GA. A retrospective analysis of 145 patients undergoing 156 breast cancer operations using PVB and 100 patients undergoing GA during a 2 year period was performed. The block was performed at C7-T6 levels. 3-4 ml of 0.5% bupivacaine with 1 in 400,000 epinephrine was injected in each of the above mentioned paravertebral spaces. Surgery was successfully completed in 85% of the cases attempted by using PVB alone and 91% of the cases the surgery was completed by using PVB supplemented with local anaesthetic infilteration. Complications were noted in 2.6% of the patients′. Epidural extension occurred in 2 patients. Pneumothorax occurred in one patient. One patient demonstrated evidence of epinephrine absorption, which responded to labetalol administration.35
Pusch F et al (1999) studied single-injection unilateral PVB given at the level of T4, as a sole anaesthetic technique, for patients undergoing breast surgery for breast malignancy. After written informed consent was obtained, 86 patients were enrolled in this prospective study. Forty-four women were randomly allocated to receive a single-injection PVB at the level of T4, while 42 women received general anaesthesia. The surgical procedures varied from lumpectomy (wide local excision of a tumour) to modified radical mastectomy with axillary dissection. The block was performed using 0.3 ml/kg (maximum dose 150 mg) of bupivacaine 0.5%. The skin and the underlying tissues were infiltrated with local anaesthetic solution two fingers (about 3 cm) from the anatomical midline and level with the cephalad end of the vertebral spine. Time for performance of blocks lasted from 4 to 9 min. Recovery from anaesthesia or sedation was shortened, while postoperative pain scores (VAS), the incidence of vomiting and the requirement for analgesics were lower in the paravertebral group. Less painful restricted movement was observed in the PVB group. PVB was inadequate in 6.8% of patients. Epidural spread with paraparesis and Horner triad was assumed in one patient. Urinary retention was not observed in any patient. None of the patients had any episode of hypotension and the hemodynamic parameters were comparable between the two groups. Patients with axillary dissection had higher postoperative pain scores compared to all others in both the groups.36
Saito et al (2001) undertook a study in volunteers to observe sympathetic changes following unilateral PVB with lidocaine at T11 spine. A total of 22 ml of 1% lidocaine was injected at T11 level. It was demonstrated that PVB provides a reliable, unilateral, somatosensory and sympathetic block without producing hypotension and tachycardia associated with central neuroaxial blocks.37
Terheggen M et al (2002) studied the effect of PVB for minor breast surgical procedures. Though the pain relief in PVB group was superior to the control group (General anesthesia group), the difference was only marginal. Hence they concluded that considering the risk/benefit ratio of PVB, for minor surgical procedures on the breast PVB is not favoured over GA.38
Nerve stimulator guided PVB technique was compared to regular general anaesthesia for breast surgery by Naja et al (2003). Sixty patients were prospectively randomized to receive either PVB or general anaesthesia for breast surgery. The primary end-point of the study was to assess postoperative analgesia (VAS and supplemental opioid requirements); the incidence of postoperative nausea and vomiting and length of hospital stay were considered as secondary outcome measures. VAS both at rest and at movement, as well as the need for supplemental opioid administration during the first 3 days postoperatively, were significantly lower in patients who had been administered PVB as compared to patients who received GA. The number of patients free from nausea and vomiting after operation was significantly higher in the PVB group (93%) compared to the GA group (67%). The use of PVB was also associated with a significantly shorter hospital stay (median 1 day) compared to general anaesthesia (2 days). Both the performance of the block and the intraoperative conditions was well accepted by the vast majority of patients treated by PVB (97%).39
Kairaluoma et al (2006) studied the effect of PVB in the relief of chronic pain after breast surgery. They earlier reported that preincisional PVB provides significant immediate postoperative analgesia after breast cancer surgery. The same patients (n = 60) were followed-up for a year to find out whether PVB could also reduce the prevalence of postoperative chronic pain. The follow-up consisted of a 14-day symptom diary and telephone interviews 1, 6, and 12 months after surgery. 1 month after surgery, the intensity of motion-related pain was lower in the PVB group. Six months after surgery, the prevalence of any pain symptoms was lower in the PVB group. Finally, at 12 months after surgery, in addition to the prevalence of pain symptoms and the intensity of motion-related pain, the intensity of pain at rest was lower in the PVB group. These findings were independent of whether or not axillary dissection had been performed. The incidence of neuropathic pain was low (two and three patients in the PVB and control groups, respectively). In addition to providing acute postoperative pain relief, preoperative PVB seems to reduce the prevalence of chronic pain 1 year after breast cancer surgery.40
Burlacu et al (2006) have shown that adjunctive analgesia with fentanyl or clonidine in combination with low dose levobupivacaine in paravertebral analgesia has superior analgesic efficacy to plain levobupivacaine paravertebral analgesia and to only intravenous morphine PCA for post operative pain relief. Patients were randomly allocated to four groups: Group L received 19 ml bolus levobupivacaine 0.25% plus 1 ml saline followed by an infusion of levobupivacaine 0.1%; Group LF received 19 ml bolus levobupivacaine 0.25% plus fentanyl 50 μg followed by an infusion of levobupivacaine 0.05% with fentanyl 4 μg/ ml); Group LC received 19 ml bolus levobupivacaine 0.25% plus clonidine 150 μg followed by an infusion of levobupivacaine 0.05% with clonidine 3 μg/ml); Group C(Control) received general anaesthesia without paravertebral analgesia. All groups received postoperative i.v. morphine PCA. Although mean postoperative PCA morphine consumption was decreased in LF [7.9 mg] and LC [5.9 mg] vs. L [27.7 mg] or C patients [21.7 mg], p < 0.01, paravertebral fentanyl and clonidine were associated with significantly increased vomiting and hypotension,respectively.41
Exadaktylos et al (2006) carried out a retrospective study, where medical records of 129 patients undergoing mastectomy with axillary clearance were studied. Fifty patients who had surgery under Paravertebral anaesthesia analgesia combined with GA were compared with 79 patients who had GA combined with post operative morphine analgesia. They were followed up for 32±5 months. Recurrence and metastasis-free survival was 94% and 82% at 24 months and at 36 months in Paravertebral and GA groups respectively. Hence the authors concluded that Paravertebral anaesthesia and analgesia for breast cancer surgery reduces the risk of recurrence. The authors speculated that regional anaesthesia might help to maintain perioperative immune function by reducing general anaes-thesia requirements and by sparing postoperative opioids, and thus preventing the dissemination of malignant cells.42
Dabbagh A et al (2007) studied the effect of TPVB in providing post-operative pain relief following breast surgery. 30 patients were given TPVB (study) and compared with 30 patients who received general anaesthesia (control). The study suggested paravertebral block as a suitable alternative to general anesthesia in selected breast surgical patients regarding postoperative pain reduction.43
CONCLUSION
Paravertebral block is a very useful regional anaesthetic technique for surgeries involving thoracic and lumbar dermatom. Nerve stimulation and ultrasound guidance have increased the safety and reliability of the block and hence, may contribute to its ever increasing applications in operative as well as non-operative pain interventions.
REFERENCES
- 1.Richardson J. Fin-de-siecle renaissance of Paravertebral analgesia. Pain Rev. 1997;4:159–71. [Google Scholar]
- 2.Eason MJ, Wyatt R. Paravertebral thoracic block-a reappraisal. Anaesthesia. 1979;34:638–642. doi: 10.1111/j.1365-2044.1979.tb06363.x. [DOI] [PubMed] [Google Scholar]
- 3.Karmakar MK. Thoracic paravertebral block. Anesthesiology. 2001;95:771–80. doi: 10.1097/00000542-200109000-00033. [DOI] [PubMed] [Google Scholar]
- 4.Klein SM, Nielsen KC, Ahmed N, Buckenmaier CC, Steele SM. In situ images of the thoracic paravertebral space. Reg Anesth Pain Med. 2004;29:596–99. doi: 10.1016/j.rapm.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 5.Cheema SPS, Ilsley D, Richardson J, Sabanathan S. A thermographic study of paravertebral analgesia. Anaesthesia. 1995;50:118–121. doi: 10.1111/j.1365-2044.1995.tb15092.x. [DOI] [PubMed] [Google Scholar]
- 6.Conacher ID, Kokri M. Postoperative paravertebral blocks for thoracic surgery: a radiological appraisal. Br J Anaesth. 1987;59:155–61. doi: 10.1093/bja/59.2.155. [DOI] [PubMed] [Google Scholar]
- 7.Conacher ID. Resin injection of thoracic paravertebral spaces. Br J Anaesth. 1988;61:657–61. doi: 10.1093/bja/61.6.657. [DOI] [PubMed] [Google Scholar]
- 8.Karmakar MK, Kwok WH, Kew J. Thoracic paravertebral block: radiological evidence of contralateral spread anterior to the vertebral bodies. Br J Anaesth. 2000;84:263–5. doi: 10.1093/oxfordjournals.bja.a013417. [DOI] [PubMed] [Google Scholar]
- 9.Karmakar MK, Chung DC. Variability of a thoracic paravertebral block: Are we ignoring the endothoracic fascia? (letter) Reg Anesth Pain Med. 2000;25:325–7. doi: 10.1016/s1098-7339(00)90028-2. [DOI] [PubMed] [Google Scholar]
- 10.Naja MZ, Ziade MF, Rajab M, El Tayara K, Lonnqvist PA. Varying anatomical injection points within the thoracic paravertebral space: effect on spread of solution and nerve blockade. Anaesthesia. 2004;59:459–463. doi: 10.1111/j.1365-2044.2004.03705.x. [DOI] [PubMed] [Google Scholar]
- 11.Pusch F, Wildling E, Klimscha W, Weinstabl C. Sonographic measurement of needle insertion depth in paravertebral blocks in women. Br J Anaesth. 2000;85:841–3. doi: 10.1093/bja/85.6.841. [DOI] [PubMed] [Google Scholar]
- 12.Wheeler LJ. Peripheral nerve stimulation end-point for thoracic paravertebral block. Br J Anaesth. 2001;86:598–9. [PubMed] [Google Scholar]
- 13.Lang SA. The use of a nerve stimulator for thoracic paravertebral block. Anesthesiology. 2002;97:521. doi: 10.1097/00000542-200208000-00037. [DOI] [PubMed] [Google Scholar]
- 14.Luyet C, Eichenberger U, Greif R, et al. Ultrasoundguided paravertebral puncture and placemen of catheters in human cadavers: an imaging study. Br J Anaesth. 2009;102:543–9. doi: 10.1093/bja/aep015. [DOI] [PubMed] [Google Scholar]
- 15.Cowie B, McGlade D, Ivanusic J, Barrington MJ. Ultrasound guided thoracic paravertebral blockade : a cadaveric study. Anesth Analg. 2010;110:1735–9. doi: 10.1213/ANE.0b013e3181dd58b0. [DOI] [PubMed] [Google Scholar]
- 16.Marhofer P, Kettner SC, Hajbok L, Dubsky P, Fleischmann E. Lateral ultrasound guided thoracic paravertebral blockade : an anatomical based description of a new technique. Br J Anaesth. 2010 Aug 3; doi: 10.1093/bja/aeq206. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Naja MZ, Lonnqvist PA. Somatic paravertebral nerve blockade: incidence of failed block and complications. Anaesthesia. 2001;56:1184–8. doi: 10.1046/j.1365-2044.2001.02084-2.x. [DOI] [PubMed] [Google Scholar]
- 18.Thomas PW, Sanders DJ, Berrisford RG. Pulmonary haemorrhage after percutaneous paravertebral block. Br J Anaesth. 1999;83:668–9. doi: 10.1093/bja/83.4.668. [DOI] [PubMed] [Google Scholar]
- 19.Burlacu CL, Buggy DJ. Coexisting harlequin and Horner syndromes after high thoracic paravertebral anaesthesia. Br J Anaesth. 2005;95:822–824. doi: 10.1093/bja/aei258. [DOI] [PubMed] [Google Scholar]
- 20.Matthews PJ, Govenden V. Comparison of continuous paravertebral and extradural infusion of bupivacaine for pain relief after thoracotomy. Br J Anaesth. 1989;62:204–205. doi: 10.1093/bja/62.2.204. [DOI] [PubMed] [Google Scholar]
- 21.Richardson J, Sabanathan S, Jones J, Shah RD, Cheema S, Mearns AJ. A prospective randomized comparison of preoperative and continuous balanced epidural or paravertebral bupivacaine on post-thoracotomy pain, pulmonary function and stress responses. Br J Anaesth. 1999;83:387–392. doi: 10.1093/bja/83.3.387. [DOI] [PubMed] [Google Scholar]
- 22.Dhole S, Mehta Y, Saxena H, Juneja R, Trehan N. Comparison of continuous thoracic epidural and paravertebral blocks for postoperative analgesia after minimally invasive direct coronary artery bypass surgery. J Cardiothorac Vasc Anesth. 2001;15:288–292. doi: 10.1053/jcan.2001.23271. [DOI] [PubMed] [Google Scholar]
- 23.Hill SE, Keller RA, Smith MS, Grichnik K, White WD, D'Amico TA, Newman MF. Efficacy of single-dose, multilevel paravertebral nerve blockade for analgesia after thoracoscopic procedures. Anesthesiology. 2006;104:1047–1053. doi: 10.1097/00000542-200605000-00022. [DOI] [PubMed] [Google Scholar]
- 24.Kotze A, Scally A, Howell S, et al. Ef?cacy and safety of different techniques of paravertebral block for analgesia after thoracotomy: a systematic review and metaregression. Br J Anaesth. 2009;103(5):626–36. doi: 10.1093/bja/aep272. [DOI] [PubMed] [Google Scholar]
- 25.Culp W C, Payne M N, Montgomery M L, et al. Thoracic paravertebral block for analgesia following liver mass radiofrequency ablation. Br J Radio. 2008;81:23–25. doi: 10.1259/bjr/61546726. [DOI] [PubMed] [Google Scholar]
- 26.Culp William C, Jr, Culp William C. Thoracic paravertebral block for Percutaneous Transhepatic Biliary Drainage. Journal of Vascular and Interventional Radiology. 2005;10:1397–1400. doi: 10.1097/01.RVI.0000174285.84995.7F. [DOI] [PubMed] [Google Scholar]
- 27.Wassef M, Randazzo T, Ward W. The paravertebral nerve root block for inguinal herniorrhaphy-a comparison with the field block approach. Reg Anesth Pain Med. 1998;23:451–6. doi: 10.1016/s1098-7339(98)90026-8. [DOI] [PubMed] [Google Scholar]
- 28.Klein SM, Pietrobon R, Nielsen KC, Steele SM, Warner DS, Moylan JA, Eubanks WS, Greengrass RA. Paravertebral somatic nerve block compared with perip-heral nerve blocks for outpatient inguinal herniorrhaphy. Reg Anesth Pain Med. 2002;27:476–480. doi: 10.1053/rapm.2002.35147. [DOI] [PubMed] [Google Scholar]
- 29.Weltz CR, Greengrass RA, Lyerly HK. Ambulatory surgical management of breast carcinoma using paravertebral block. Ann Surg. 1995;222:19–26. doi: 10.1097/00000658-199507000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hadzic A, Kerimoglu B, Loreiu D, Karaca PE, Claudio RE, Yufa M, et al. Paravertebral blocks provide superior same-day recovery over general anaesthesia for patients undergoing inguinal hernia repair. Anesth Analg. 2006;102:1076–81. doi: 10.1213/01.ane.0000196532.56221.f2. [DOI] [PubMed] [Google Scholar]
- 31.Giesecke K, Hamberger B, Jarnberg PO, Klingstedt C. Paravertebral block during cholecystectomy: effect on circulatory and hormonal responses. Br J Anaesth. 1988;61:652–6. doi: 10.1093/bja/61.6.652. [DOI] [PubMed] [Google Scholar]
- 32.Naja MZ, Ziade MF, Lonnqvist PA. General anaesthesia combined with bilateral paravertebral blockade (T5-6) vs.general anaesthesia for laparoscopic cholecystectomy: a prospective, randomized clinical trial. Eur J Anaesthesiol. 2004;21:489–95. doi: 10.1017/s026502150400612x. [DOI] [PubMed] [Google Scholar]
- 33.Karmakar Manoj K, Critchley Lester A H, Ho Anthony M -H. Continuous Thoracic Paravertebral Infusion of Bupivacaine for Pain Management in Patients with Multiple Fractured Ribs. Chest. 2003;123:423–31. doi: 10.1378/chest.123.2.424. [DOI] [PubMed] [Google Scholar]
- 34.Greengrass R, O'Brien, Hardman D. Paravertebral block for breast cancer surgery. Can J Anaesth. 1996;43:858–61. doi: 10.1007/BF03013039. [DOI] [PubMed] [Google Scholar]
- 35.Roy Greengrass, Weltz Christina R, Iglehart J Dirk. Use of Paravertebral Block Anaesthesia in Surgical Management of breast Cancer. Annals of surgery. 1998;227:496–501. doi: 10.1097/00000658-199804000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pusch F, Freitag H, Weinstabl C. Single-injection paravertebral block compared to general anaesthesia in breast surgery. Acta Anaesthesiol Scand. 1999 Aug;43:770–4. doi: 10.1034/j.1399-6576.1999.430714.x. [DOI] [PubMed] [Google Scholar]
- 37.Saito T, Den S, Cheema SPS, et al. A single injection multisegmental paravertebral block extension of somatosensory and sympathetic block in volunteers. Acta Anaesthesiol scand. 2001;45:30–3. doi: 10.1034/j.1399-6576.2001.450105.x. [DOI] [PubMed] [Google Scholar]
- 38.Terheggen M, Wille F, Borel R, Ionescu T, Knape J. Paravertebral blockade for minor breast surgery. Anesth Analg. 2002;94:355–9. doi: 10.1097/00000539-200202000-00023. [DOI] [PubMed] [Google Scholar]
- 39.Naja M Z, Ziade M F, Lonqvist P A. Nerve-stimulator guided paravertebral blockade vs general anaesthesia for breast surgery. European Journal of Anesthesiology. 2003;20:897–903. doi: 10.1017/s0265021503001443. [DOI] [PubMed] [Google Scholar]
- 40.Kairaluoma Pekka M, Bachmann Martina S, Rosenberg Per H. Preincisional PVB reduces the prevalence of chronic pain after breast surgery. Anesthesia and Analgesia. 2006;103:703–8. doi: 10.1213/01.ane.0000230603.92574.4e. [DOI] [PubMed] [Google Scholar]
- 41.Burlacu C L, Frizelle H P, Moriarty D C. Fentanyl and clonidine as adjunctive analgesics with levobupivacaine in paravertebral analgesia for breast surgery. Anaesthesia. 2006;61:932–7. doi: 10.1111/j.1365-2044.2006.04793.x. [DOI] [PubMed] [Google Scholar]
- 42.Exadaktylos AK, Buggy DJ, Moriarty DC, Mascha E, Sessler DI. Can anaesthetic technique for primary breast cancer surgery affect recurrence or metastasis? Anesthesiology. 2006;105:660–4. doi: 10.1097/00000542-200610000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dabbagh A, Elyasi H. The role of paravertebral block in decreasing postoperative pain in elective breast surgeries. Med Sci Monit. 2007;13:CR464–7. [PubMed] [Google Scholar]


