Pain is usually a physiological outcome of injurious stimuli which might be accidental, due to disease or caused by the surgeon's knife. The latter is, in fact, one of the common causes of pain in a hospital setup. The incidence of moderate to severe pain after surgery is quite high which in turn is associated with sleep disturbances, less mobility and prolonged hospital stay.1 In a review conducted on about 20,000 patients, epidural analgesia was noted to be superior to both patient controlled intravenous analgesia and intramuscular administration of analgesics.2 This finding was corroborated by a more recent study.3
The various classes of drugs suitable for epidural administration are (i) Opioids like morphine (ii) Local anaesthetics like bupivacaine (iii) Alpha-2 adrenergic agonists like clonidine (iii) N-type voltage-sensitive calcium channel blockers like ziconotide and (iv) GABA receptor agonists like midazolam and baclofen.4 However, none of the above groups of drugs are free of side effects, particularly at higher doses. For example, morphine is hydrophilic in nature and rapidly spreads from the site of administration to produce side effects like respiratory depression, urinary retention and oversedation. Again, pruritus also occurs in some patients. Considering the above, it is necessary to search for newer drugs, which would have higher efficacy with lower incidence of side effects. However, before that, one needs to have an animal model, where drugs can be infused into the epidural space or intrathecal space. Such a model was first developed by Tony L. Yaksh and T.A. Rudy in 1976.5
In the current work, we report the standardization of a surgical technique for chronic implantation of intrathecal catheters in rats. The reason for this is that the epidural space is rather insignificant in rats in comparison to the intrathecal space. We also investigated the analgesic effect of keterolac, a nonsteroidal anti-inflammatory drug (NSAID), and compared it to morphine, the gold standard and the only drug approved by FDA (USA) for intraspinal delivery.
PATIENTS & METHODS
Experimental subjects
The experiment was conducted on male adult Sprague- Dawley rats, weighing between 260-310 gm. This particular strain of rat was chosen because it has a sphincter at the lower end of the oesophagus, which prevents emesis during anaesthesia. Also, the intrathecal space is considerably larger than that of the commonly used Wistar strain of rats. Permission for experimentation was obtained from the Institutional Animal Ethics Committee of AIIMS. Procedure of intrathecal catheterization
Rats were initially anaesthetized by isoflurane inhalation (4%) in a 1:1 mixture of oxygen and air. The rats were removed from the induction chamber, weighed and the hair on the back of the neck shaved by a battery-operated shaver. The rat was placed in a stereotaxic frame with the head securely held between ear bars. An anaesthetic mask conveying a lower concentration of isoflurane (2-2.5%) was fixed to the nose.
The skin over the nape of the neck was cleaned by ethyl alcohol and Providone iodine and incised for about 1 cm. The muscle on either side of the external occipital crest (Interscutularis) was detached by a periosteal elevator and retracted to expose about 3-4 mm2 of the atlanto-occipital membrane. The membrane was incised by the tip of a bent needle, which led to the escape of CSF. The caudal edge of the cut was lifted and a polyurethane (PE-5) catheter gently inserted into the intrathecal space in the midline, dorsal to the spinal cord. About 8.5 cm of the catheter was inserted which coincided with the placement of the distal end of the catheter in proximity to the lumbar enlargement of the spinal cord. The catheter was purchased from Durect Corporation, USA. The exit end of the catheter was taken out through a separate opening in the skin, between the ears and the opening plugged by a metal wire. The incision site in the skin was apposed by polyamide sutures (4-0) with one of it passing through the underlying muscle. The rat received Ringer-Lactate solution according to the body weight (1 ml per 50 gm) containing meloxicam (an NSAID; 1 mg/kg dose). Then, it was placed in a warm plexiglass chamber till complete recovery from anaesthesia. The motor activity of the rat was examined for any sign of paralysis. Rats with paralysis were euthanized with CO2 inhalation. Rats were allowed to recover for 5 days, when a repeat examination was done to evaluate motor functions. Body weight was also recorded. Rats, which were freely moving and feeding normally, were used for further experimentation (Fig. 1). The placement of the catheter in the intrathecal space was confirmed in few rats (n=3) by injection of a radio opaque dye (200 μL of lomeprol) (Fig. 2).
Figure 1.
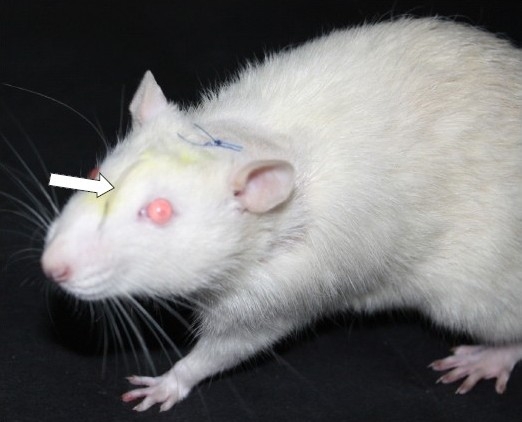
A rat after surgical implantation of the catheter in the intrathecal space (arrow)
Figure 2.
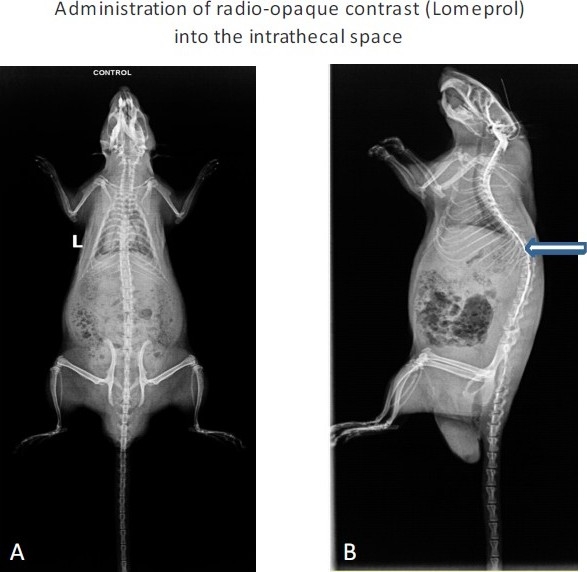
To confirm the exact placement of the catheter, a contrast medium (lomeprol) was injected through the catheter. A = Without contrast B= With contrast. Arrow indicates the spread of the medium in the intrathecal space
Behavioural Pain testing
Basal level of pain sensitivity was estimated in the rats by the Hargreaves paw withdrawal apparatus (UGO Basile) (Fig. 3).6 Each rat was placed on a special glass platform in a separate enclosure. A pinpoint infrared ray of light is focused on the plantar aspect of the foot, when this is placed on the platform. The infrared ray produces a thermal stimulus, which causes the rat to withdraw its paw on feeling pain. The time between exposure of the paw to the stimulus and its withdrawal is called the latency period (measured in sec). Administration of analgesic drugs leads to prolongation of this period. However, a cut-off of 20 sec is fixed for preventing tissue damage to the pain-insensitive paw.
Figure 3.

Hargreaves Paw withdrawal apparatus
Drug administration
During drug administration, the rat was loosely immobilized by a small cloth with the head and neck outside the cloth. Rats with chronic intrathecal catheters (n=6) were administered morphine at doses of 1, 3, 10 and 30 μg in 10 μL volume by a Hamilton syringe. Injections were followed by further administration of 10 μL normal saline for flushing the catheter. A time interval of 7 days was maintained between different doses of the drug to allow effective washout of the drug administered previously. The analgesic response was evaluated at intervals of 15, 30, 60, 90 and 120 min after injection of morphine. Another set of rats (n=6) were injected ketorolac at the same doses as morphine. However, no analgesia was observed. Even at a maximum dose of 300 μg, a submaximal analgesia was observed.
Morphine injection I.P. ampoules (15 mg/ml) was obtained form an approved Government pharmacy after obtaining permission from the narcotics commissioner. Ketorolac (Ketanov®; 30 mg/ml) was purchased from the local Pharmacy. Both the drugs were appropriately diluted in normal saline.
Statistical analysis
The data was analysed by a statistical software “GraphPad Prism” (San Diego, USA). Student's t-test was used for comparing the analgesia produced by morphine and keterolac. Statistical significance was fixed at p< 0.05.
RESULTS
The rats with intrathecal catheters were normal in all aspects. Their motor behavior, as evaluated by the placing and stepping reflexes, were also normal. There was no statistical difference in the values of paw withdrawal latencies, before and after surgery. Administration of 1 and 3 μg morphine produced a dose-dependent analgesic effect, which gradually reached the basal levels by 2 h (Fig. 4). However, higher doses (10 & 30 μg) produced a longer-lasting analgesic effect. Peak analgesia was observed at 15 min after drug administration, which was the earliest time point examined after drug administration.
Figure 4.
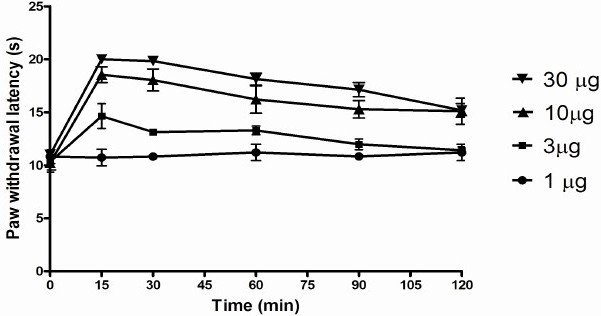
The analgesic effect of different doses of morphine as measured in the Hargreaves Paw Withdrawal Apparatus. Higher latency means greater analgesia.
Administration of ketorolac at the same doses as morphine did not produce any analgesia. However, 300 μg produced a low-level analgesia which was significantly lower than morphine between 15 - 90 min (Fig.5).
Figure 5.
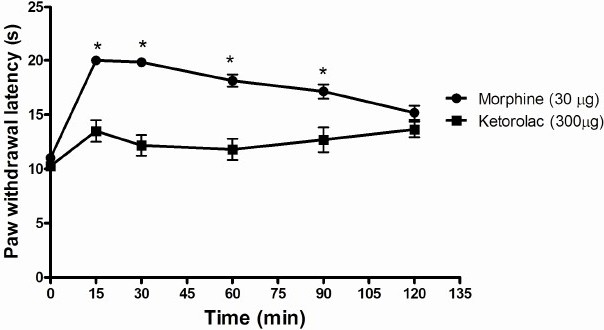
The analgesic effect of ketorolac (300μg) as compared to morphine (30μg). Morphine induced analgesia is significantly higher than ketorolac between 15-90 min (*). Higher latency means greater analgesia. Statistical significance was set at P< 0.05.
DISCUSSION
Intraspinal drug administration through predominantly the epidural route has been routinely used for the treatment of postsurgical and obstetric pain. However, opioid-based intrathecal analgesia has been recommended for chronic cancer pain.4 Thus, intraspinal anaesthesia forms an important part in the relief of pain.
In the present study, we were able to standardize a method of intrathecal catheterization in rats. Further, the analgesic effect of morphine was compared to ketorolac in a model of acute pain. The thermal stimuli produced a sensation of pain but without any actual tissue injury. Also, the rat is free to move without any restriction during this testing period. It was earlier demonstrated by us that mu-opioid receptors are selectively expressed in the superficial laminae (I-II) of the dorsal horn in the rat.7 Nerve fibers mediating painful stimuli from the periphery end in relation to the neurons of this part of the spinal cord. Opioids bind to the receptors and inhibit the onward transmission of pain. Direct administration of morphine into the intrathecal space would facilitate this binding. Ketorolac, being a NSAID, would be effective in an experimental model involving actual tissue injury. Its acts by inhibiting the cyclooxygenase enzyme, which metabolizes arachidonic acid to prostaglandins.8 The latter is a mediator of the inflammatory process. As the present pain model did not result in tissue injury, ketorolac was minimally effective. In fact, in a recent study involving actual tissue injury, ketorolac (30 - 90 μg intrathecal) produced significant pain relief.9 In a clinical phase I trial in healthy human volunteers, intrathecal ketorolac did not produce a high incidence of serious side effects.10
In conclusion, we report the successful standardization of intrathecal catheterization in rats in our laboratory. We plan to use the set-up for novel drug discovery in the future.
Acknowledgments
Financial support for the work was provided by an Extramural research grant from the Indian Council of Medical Research (ICMR), New Delhi (2008-11).
REFERENCES
- 1.Kehlet H, Holte K. Effect of postoperative analgesia on surgical outcome. Br J Anaesth. 2001;87:62–72. doi: 10.1093/bja/87.1.62. [DOI] [PubMed] [Google Scholar]
- 2.Dolin SJ, Cashman JN, Bland JM. Effectiveness of acute postoperative pain management:I.Evidence from published data. Br J Anaesth. 2002;89:409–423. [PubMed] [Google Scholar]
- 3.Guay J. The benefits of adding epidural analgesia to general anesthesia. J Anesth. 2006;20:335–340. doi: 10.1007/s00540-006-0423-8. [DOI] [PubMed] [Google Scholar]
- 4.Cohen SP, Dragovich A. Intrathecal analgesia. Anesthesiology Clin. 2007;25:863–882. doi: 10.1016/j.anclin.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Yaksh TL, Rudy TA. Analgesia mediated by direct spinal action of narcotics. Science. 1976;192:1357–1358. doi: 10.1126/science.1273597. [DOI] [PubMed] [Google Scholar]
- 6.Allen JW, Yaksh TL. Assessment of acute thermal nociception in laboratory animals. In: Luo ZD, editor. Pain research: Methods and Protocol. New Jersey: Humana Press; 2004. pp. 11–23. [DOI] [PubMed] [Google Scholar]
- 7.Ray SB, Gupta H, Gupta YK. Up-regulation of µ-opioid receptors in the spinal cord of morphine tolerant rats. J Biosci. 2004;29:51–56. doi: 10.1007/BF02702561. [DOI] [PubMed] [Google Scholar]
- 8.Tripathi KD. 5th ed. New Delhi: Jaypee Brothers; 2003. Essentials of medical pharmacology; pp. 167–184. [Google Scholar]
- 9.Il-Ok Lee, Youngsun Seo. The effects of intrathecal cyclooxygenase-1, cyclooxygenase-2, or nonselective inhibitors on pain behavior and spinal Fos-like immunoreactivity. Anesth Analg. 2008;106:972–977. doi: 10.1213/ane.0b013e318163f602. [DOI] [PubMed] [Google Scholar]
- 10.Eisenach JC, Curry R, Hood DD, Yaksh TL. Phase I safety assessment of intrathecal ketorolac. Pain. 2002;99:599–604. doi: 10.1016/S0304-3959(02)00208-7. [DOI] [PubMed] [Google Scholar]


