Abstract
Background
High-density lipoprotein cholesterol (HDL-C) levels in premenopausal and postmenopausal women are differentially affected by exogenous sex hormones depending on their apolipoprotein E (apo E) genotype. Because endogenous sex hormones markedly increase during pregnancy, we examined whether HDL-C declines after a first birth varied by apo E polymorphisms.
Methods
In 1147 nulliparas (416 black, 731 white), fasting blood samples (nonpregnant) were drawn at baseline and at follow-up years 5, 7, and 10. Time-dependent pregnancy groups included 0 pregnancies (P0), 1+ short pregnancy (P1+), 1 birth (B1), 2 or more births (B2+). ApoE groups by alleles identified with a phenotype method included E4 (4/3 and 4/4), E3 (3/3), and E2 (2/2 and 3/2). Differences in adjusted mean HDL-C changes among pregnancy groups and ApoE groups were examined using repeated measures multiple linear regression.
Results
HDL-C declines associated with parity (one or more births) depended on ApoE group (ApoE*Pregnancy Interaction; p < 0.002). For B1 and B2+ vs. P0, HDL-C declines were −2.4 to −2.7 mg/dl in E4 and −3.4 to −4.1 mg/dl in E3. In E2, HDL-C declines were −6.6 mg/dl for one birth, and −11.5 mg/dl for two or more births, each relative to the 0 pregnancies (P0) group (linear trend, p < 0.001).
Conclusions
The degree to which childbearing adversely affects long-term HDL-C declines varies by apo E phenotype, based on a method that accurately classifies genotype. Our findings show that 2/2 and 3/2 genotypes are associated with larger parity-related HDL-C declines than 3/3, 4/3, and 4/4 genotypes.
INTRODUCTION
Cross-sectional studies have reported an inverse association between parity and high-density lipoprotein cholesterol (HDL-C) or lower HDL-C levels at a threshold of high parity (e.g., 5 or more vs. 4 or fewer births) in both premenopausal and postmenopausal women.1–3 However, few studies have prospectively examined longitudinal changes in blood lipids from preconception to beyond 12 months postpartum or have controlled for the effects of aging and secular trends on lipid changes by comparison with nongravid women. A single longitudinal study of pregnant women (n = 22) found lower HDL-C levels at 1 year postpartum than 1 year before conception.4 Two large prospective studies, including the Coronary Artery Risk Development in Young Adults (CARDIA) Study, found HDL-C declines of −2.4 mg/dl per birth or a net decline of −5.1 mg/dl after a first birth compared with no births in 2 or 3 years of follow-up.5,6 CARDIA has also reported −3 to −4 mg/dl declines in HDL-C, but not low-density lipoprotein cholesterol (LDL-C) or triglyceride (TG) levels, among primiparas compared with nongravidas in 10 years of follow-up independent of changes in behaviors, body weight, and central adiposity.7
HDL-C levels are strongly inversely related to risk of cardiovascular disease (CVD) in women8,9 and are influenced by variations in apolipoprotein E (apo E-protein) polymorphisms, the gene expressing apolipoprotein (apo E-gene), a glycoprotein that mediates the binding of certain lipid particles to specific lipoprotein receptors. Any ε4 allele increases the risk of atherosclerosis, coronary artery disease (CAD), stroke, and ischemic heart disease, whereas ε2 allele lowers the risk.10–13 Moreover, the ε4 allele is associated with lower apo E-protein concentrations, resulting in lower HDLs, higher LDLs, and higher TGs, whereas the opposite is true for ε2 alleles.14,15 Evidence from animal models indicates that apo E genotype and estrogen act in concert to influence lipid profiles16 and that apo E genotype expression itself may be regulated in part by estrogen.17 Both estrogen and progesterone interact with apo E genotypes to affect lipids in animals.18 In humans, both oral contraceptive (OC) use and hormone replacement therapy (HRT) modify the association between apo E genotype and lipoprotein levels. In OC users vs. non-OC users, significantly lower apo E and apo E-Lp-non-B (a lipoprotein class that does not contain apo B) concentrations were found for those carrying the ε2 and ε3 alleles, but no difference was found for those with ε4 alleles.19 In contrast, in postmenopausal women, HRT use was associated with higher HDL-C levels for those carrying ε2 or ε3 alleles, but little difference in HDL-C levels for those carrying the ε4 allele.20–22 To our knowledge, effect modification by apo E phenotype or genotype in the association between a first birth and long-term HDL-C declines has not been examined.
The objective of this study was to determine if the association of childbearing with long-term HDL-C declines varies by apo E genotype and if gains in central adiposity and body weight mediate the differences within the stratum of apo E genotype. We hypothesized that the association of parity with HDL-C would be stronger for ε2 allele genotypes (2/2, 2/3) and homozygous ε3 allele genotype (3/3) and weaker for ε4 allele genotypes (4/4, 4/3), based on our previous findings and more similar biological mechanisms of action for OC use and pregnancy on apolipoprotein E in premenopausal women.
MATERIALS AND METHODS
The CARDIA Study is a multicenter, longitudinal, observational study designed to describe the development of risk factors for coronary heart disease (CHD) in young black and white men and women. Descriptions of the study design, methodology, and cohort characteristics have been reported previously.23,24 The study population was recruited from four geographic areas: Birmingham, Alabama, Chicago, Illinois, Minneapolis, Minnesota, and Oakland, California. From 1985 through 1986, baseline data were collected on a total of 5115 subjects (2787 women) aged 18–30 years, of whom 52% were black and 48% were white. Baseline and follow-up examinations included a variety of physiological and self-reported measures,23,25 and retention rates were 91%, 86%, 81%, and 79% of the surviving cohort at years 2, 5, 7, and 10, respectively.25
Sample selection criteria
Of 2787 women enrolled at baseline, we selected women attending one or more follow-up examinations. To examine the effect modification by apo E polymorphisms, we used the same sample of nulliparas for which we observed that HDL-C declines with childbearing over 10 years.7 Briefly, women were excluded if at baseline they had had a hysterectomy or removal of both ovaries, reported a pregnancy 3 months prior to the examination, were currently pregnant or breastfeeding, or were nonfasting (n = 109). None had taken lipid-lowering medications at baseline. At follow-up, measurements were excluded for women who had taken lipid-lowering medications, had a plasma TG >400 mg/dl (n = 23), were nonfasting (<8 hours) prior to venipuncture (n = 85), were pregnant or lactating at follow-up examinations, or were <12 months postpartum at year 10. Women who were missing covariates (n = 18), missing apo E phenotype (n = 213), or had the 2/4 apo E phenotype (n = 55) also were excluded. Because HDL-C declines were associated with primiparity in our previous study,7 we selected women who were nulliparous at baseline for a final analytical sample of 1147 women (n = 416 blacks, n = 731 whites). Plasma HDL-C measurements were available for all four examinations in 758 women (66%) and three of four examinations in 320 women (28%). Institutional Review Boards at each participating study center approved the study. Written, informed consent was obtained from subjects for all study procedures.
Data collection methods
A description of the methodology used to recruit subjects and perform data collection is detailed elsewhere.23,24 Participants were asked to fast prior to each examination and reported the number of hours since their last intake of food or beverages prior to the blood draw. Blood samples were drawn in the morning after an overnight fast using a Vacutainer tube containing EDTA.26 Procedures followed in the collection and storage of plasma samples, laboratory quality control procedures, and methodology used to determine concentrations of plasma TG, HDL-C, LDL-C, and total cholesterol (TC) have been reported in detail elsewhere.26
The apo E phenotype was determined from plasma samples collected in 1993–199427 by a modification of the methods of Kamboh et al.28 The procedure, which involves isoelectric focusing and immunoblotting techniques, has been previously described.29 There was 96% concordance of apo E phenotype with genotype in a set of 400 samples using this method.29 The method accurately identifies the apo E genotype, and the ApoE groups for defined analyses are interpreted to represent apo E genotypes.
Women were categorized into three distinct ApoE groups: E3 (3/3), E2 (2/2 and 2/3), and E4 (4/4 and 4/3). The 2/4 allele combination (n = 55) was excluded, as ε2 and ε4 alleles have opposite influences on apoE phenotypes, and 2/4 is a relatively rare genotype.14,15
Measurements of weight, height, and waist circumference (waist girth) were obtained at each examination according to standardized protocol described previously.30 Body mass index (BMI) was computed as weight in kilograms divided by squared height in meters (kg/m2).
Sociodemographic and behavioral data (medication use, alcohol intake [ml/day], cigarette smoking, education, marital status, employment status, OC use, and physical activity) were collected at each examination using self-administered and interviewer-administered questionnaires. Categorical variables were defined as smoking: never, former, or current; years of education: 12 or less, 13–15, and 16 or more; marital status: never married, widowed, divorced or separated, or married; and employment outside the home: none, part-time, or full-time. Physical activity score was derived from the CARDIA Physical Activity History.31 The CARDIA Dietary History assessed dietary intake: percentage of kilocalories from total fat, saturated fat, protein, and carbohydrate at baseline.32 OC use was self-reported at each examination and was categorized as never, past, or current. Lifetime history of OC use (in months) was self-reported from age at menarche through age at the year 10 examination.
Reproductive history was obtained by self-report of the number of pregnancies and births at each examination. Participants were asked whether they were currently pregnant or breast-feeding; number of times they had been pregnant, including abortions, miscarriages, and live or stillbirths since the previous examination; duration of gestation; and dates of delivery. The number of pregnancies ending in miscarriages, abortions, or <20 weeks gestation were counted as short pregnancies (pregnancy losses). Pregnancies >20 weeks gestation were counted as births classified as 0, 1, and 2 or more births.
Variables
Repeated outcome measures
Changes in HDL-C were computed for three time intervals, each starting from baseline (0–5, 0–7, and 0–10 years) by subtracting the baseline from each follow-up measurement.
Time-dependent follow-up pregnancy groups
Four time-dependent categories were constructed based on number of pregnancies and births, and women were assigned to one of four groups within each follow-up time interval: P0 (0 pregnancies; nongravid), P1+ (pregnancy loss; one or more miscarriages or abortions, short pregnancies), B1 (one birth), and B2+ (two or more births). The P0 group was not pregnant during current or any prior intervals. The P1+ group was pregnant but did not give birth during current and any prior intervals. The B1 group gave birth one time, and the B2+ group gave birth two or more times during current or prior intervals. Group assignments continued into subsequent intervals unless new pregnancies or births (or both) occurred since the last examination. For example, a woman reporting a miscarriage between years 0 and 5, and one birth between years 5 and 7 would be classified as P1+ in the year 0–5 and B1 in the year 0–7 intervals. If no further births were reported, classification as B1 would remain for the subsequent interval, year 0–10, regardless of any subsequent miscarriages.
The total number of short pregnancies was also included as a separate, time-dependent covariate to control for short pregnancies across the birth groups. Other time-dependent covariates included age, changes in weight, waist girth, OC use (in months), physical activity, and alcohol intake constructed by subtraction of the baseline from the follow-up measurement, and categorical variables (smoking, marital, employment, and education status).
Statistical methods
Preliminary analyses involved description of frequency and percent of pregnancies and births among ApoE groups as well as mean (SD) for baseline anthropometric and lipid profiles among ApoE groups stratified by race. Baseline sociodemographic, behavioral, and anthropometric characteristics were examined by follow-up pregnancy groups for races combined. Multiple linear regression methods (analysis of variance [ANOVA]) were used to assess baseline differences in plasma lipoproteins and TGs, age, height, weight, BMI, and waist girth among ApoE groups (E2, E3, E4) and dietary intake among follow-up pregnancy and birth groups (P0, P1+, B1, B2+). Differences in means for log-transformation of TGs were also examined but were not significant. Chi-square tests were used to assess associations with baseline sociodemographic and behavioral categorical variables. P values were obtained from two-sided tests (significance <0.05). Kruskal-Wallis one-way test was used to assess differences in alcohol intake and physical activity due to skewedness in the distributions.
Plasma HDL-C measurements from years 0, 5, 7, and 10 were assembled along with fixed variables, race, study center, and ApoE groups as well as time-dependent follow-up pregnancy groups. Repeated measures linear regression methods were used (SAS, PROC MIXED 8.2, SAS Institute, Carey, NC) to assess the association of follow-up pregnancy and birth groups with plasma HDL-C changes over three intervals. Two-way interaction terms were examined simultaneously (ApoE groups, race, OC use) to assess the heterogeneity in the associations of HDL-C changes with follow-up pregnancy groups by including appropriate cross-product terms in the models. Based on these analyses, races were combined, and mean changes in HDL-C were contrasted among follow-up pregnancy groups within ApoE group strata. Covariates included in stepwise models as confounders were selected based on their association with outcome measures independent of association with follow-up groups. Model fit was assessed by comparison of AIC (Akaike Information Criterion) statistics.
Model 1 (minimally adjusted) is adjusted for race, study center, time, baseline covariates (plasma HDL-C, BMI, waist circumference, alcohol intake, smoking, education level, physical activity score, OC use categories), as well as age and number of short pregnancies as time-dependent variables. Model 2 (fully adjusted) includes all variables in Model 1 plus time-dependent covariates (education, smoking, months of OC use, changes in alcohol intake, physical activity score after baseline). To determine if the decline in HDL-C was mediated by increased waist girth or weight gain or both, these covariates were evaluated separately by stepwise addition to Model 2.
Differences in adjusted mean HDL-C changes among time-dependent follow-up pregnancy groups were tested overall (global p value) and through pairwise comparisons of P1+, B1, and B2+ groups with the referent group, P0 (nongravid), and of B1 with B2 within each stratum of the ApoE group. We assumed the covariance structure was compound symmetry in the repeated regression models; analyses based on the assumption of unstructured variance-covariance matrix yielded similar results.
RESULTS
In the analytical sample of 1147 women, follow-up pregnancy groups included 599 (52.2%) with no pregnancies (P0), 140 (12.2%) with one or more short pregnancies and no births (P1+), 222 (19.4%) with one birth (B1), and 186 (16.2%) with two or more births (B2+) during follow-up.
The distribution of pregnancies and births was not associated with ApoE group in either race (Table 1). Baseline characteristics that differed among ApoE groups for both races included higher plasma HDL-C and lower LDL-C and TC levels for the E2 group compared with the E3 group and lower HDL-C levels and higher levels of other lipids for the E4 group (Table 2). No significant two-way interactions were found for race or OC use within the association of follow-up pregnancy groups with HDL-C changes. Baseline characteristics for races combined (Table 3) that differed among the follow-up pregnancy groups included OC use, marital status, education level, and study center. Baseline plasma HDL-C levels did not differ among follow-up pregnancy groups within any of the ApoE groups (Table 4). Unadjusted mean HDL-C levels at the end of the follow-up period differed significantly with pregnancies and births for the E2 and E3 groups.
Table 1.
Distribution of apo E Polymorphisms by Follow-Up Pregnancy Groups Stratified by Race
|
ApoE groupsa |
||||||
|---|---|---|---|---|---|---|
|
Follow-up pregnancy groups by racea |
E2 (2/2, 3/2) |
E3 (3/3) |
E4 (4/3, 4/4) |
|||
| n | % | n | % | n | % | |
| Blacks (all)b | 71 | 17.0 | 186 | 44.7 | 159 | 38.2 |
| P0 | 30 | 15.0 | 90 | 45.0 | 80 | 40.0 |
| P1+ | 13 | 21.0 | 28 | 45.2 | 21 | 33.9 |
| B1 | 16 | 16.0 | 47 | 47.0 | 37 | 37.0 |
| B2+ | 12 | 22.2 | 21 | 38.9 | 21 | 38.9 |
| Whites (all)b | 108 | 14.8 | 467 | 63.9 | 156 | 21.3 |
| P0 | 62 | 15.5 | 255 | 63.9 | 82 | 20.6 |
| P1+ | 11 | 14.1 | 59 | 75.6 | 8 | 10.3 |
| B1 | 18 | 14.8 | 71 | 58.2 | 33 | 27.1 |
| B2+ | 17 | 12.9 | 82 | 62.1 | 33 | 25.0 |
Follow-up pregnancy groups and ApoE groups stratified by race.
Overall association: blacks p value = 0.81; whites p value = 0.12.
Table 2.
Baseline Characteristics for ApoE Groups Stratified by Race
| ApoE group | |||||||
|---|---|---|---|---|---|---|---|
| Baseline characteristics by race |
E2 (2/2, 3/2) |
E3 (3/3) |
E4 (4/3, 4/4) |
||||
| Mean | SD | Mean | SD | Mean | SD | p value | |
| Blacks | n = 71 | n = 186 | n = 159 | ||||
| Age (years) | 22.8 | 3.7 | 22.9 | 3.4 | 23.3 | 3.6 | 0.49 |
| BMI (kg/m2) | 24.9 | 6.3 | 25.0 | 5.3 | 24.9 | 6.0 | 0.97 |
| Waist circumference (cm) | 73.9 | 12.9 | 74.3 | 10.9 | 73.9 | 12.8 | 0.93 |
| Plasma (mg/dl) | |||||||
| HDL-C | 59.7 | 12.9 | 57.3 | 13.3 | 54.2 | 11.1 | 0.004 |
| LDL-C | 90.8 | 34.9 | 113.8 | 28.5 | 117.6 | 28.8 | 0.001 |
| Total cholesterol | 161.3 | 36.7 | 183.4 | 30.6 | 183.7 | 31.2 | 0.001 |
| Triglycerides | 53.9 | 22.2 | 61.1 | 28.0 | 59.7 | 28.7 | 0.17 |
| Whites | n = 108 | n = 467 | n = 156 | ||||
| Age (years) | 25.1 | 3.5 | 25.4 | 3.4 | 25.4 | 3.4 | 0.83 |
| BMI (kg/m2) | 23.1 | 3.9 | 22.8 | 4.0 | 23.0 | 4.1 | 0.66 |
| Waist circumference (cm) | 71.6 | 7.7 | 70.8 | 8.6 | 71.2 | 8.4 | 0.66 |
| Plasma (mg/dl) | |||||||
| HDL-C | 59.7 | 14.3 | 58.3 | 12.3 | 54.8 | 12.7 | 0.003 |
| LDL-C | 88.2 | 22.6 | 106.4 | 27.6 | 114.1 | 28.3 | 0.001 |
| Total cholesterol | 161.3 | 26.2 | 177.8 | 29.8 | 182.7 | 31.0 | 0.001 |
| Triglycerides | 67.2 | 32.4 | 65.2 | 30.2 | 69.0 | 34.6 | 0.39 |
Table 3.
Baseline Characteristics among Follow-Up Pregnancy Groups for Races Combined
| Follow-up pregnancy groups at end of follow-up |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
|
P0 (0 pregnancies) n = 599 |
P1+ (1+ short pregnancies) n = 140 |
B1 (1 birth) n = 222 |
B2+ (2+ births) n = 186 |
||||||
| Baseline characteristics | n | % | n | % | n | % | n | % | p value |
| Education | |||||||||
| High school or less | 137 | 47.4 | 40 | 13.8 | 73 | 25.3 | 39 | 13.5 | 0.001 |
| Some college | 212 | 53.5 | 54 | 13.6 | 81 | 20.5 | 49 | 12.4 | |
| 4 years college or more | 250 | 54.1 | 46 | 10.0 | 68 | 14.7 | 98 | 21.2 | |
| Marital status | |||||||||
| Never married | 514 | 55.8 | 120 | 13.0 | 168 | 18.2 | 119 | 12.9 | 0.001 |
| Separated, divorced | 26 | 44.8 | 8 | 13.8 | 13 | 22.4 | 11 | 19.0 | |
| Married | 59 | 35.1 | 12 | 7.1 | 41 | 24.4 | 56 | 33.3 | |
| Smoking | |||||||||
| Never | 381 | 52.8 | 71 | 9.9 | 143 | 19.8 | 126 | 17.5 | 0.07 |
| Former | 84 | 52.5 | 26 | 16.3 | 30 | 18.8 | 20 | 12.5 | |
| Current | 134 | 50.4 | 43 | 16.2 | 49 | 18.4 | 40 | 15.0 | |
| Oral contraceptive (OC) use | |||||||||
| Never | 221 | 66.4 | 32 | 9.6 | 46 | 13.8 | 34 | 10.2 | 0.001 |
| Former | 210 | 47.8 | 71 | 16.2 | 86 | 19.6 | 72 | 16.4 | |
| Current | 168 | 44.8 | 37 | 9.9 | 90 | 24.0 | 80 | 21.3 | |
| Study center | |||||||||
| Alabama | 108 | 49.3 | 19 | 8.7 | 57 | 26.0 | 35 | 16.0 | 0.001 |
| Chicago | 125 | 51.2 | 13 | 5.3 | 45 | 18.4 | 61 | 25.0 | |
| Minneapolis | 164 | 56.6 | 38 | 13.1 | 47 | 16.2 | 41 | 14.1 | |
| Oakland | 202 | 51.3 | 70 | 17.8 | 73 | 18.5 | 49 | 12.4 | |
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
|
|
|||||||||
| Height (cm) | 164.7 | 6.6 | 164.7 | 6.5 | 164.4 | 7.4 | 164.6 | 6.2 | 0.92 |
| Weight (kg) | 64.5 | 14.3 | 64.5 | 14.7 | 63.4 | 14.5 | 62.4 | 11.0 | 0.29 |
| BMI (kg/m2) | 23.8 | 4.9 | 23.8 | 5.4 | 23.5 | 4.8 | 23.1 | 4.0 | 0.34 |
| Waist girth (cm) | 72.6 | 10.5 | 72.0 | 10.7 | 71.7 | 9.0 | 71.3 | 8.7 | 0.39 |
| Age (years) | 24.7 | 3.7 | 23.9 | 3.4 | 24.2 | 3.6 | 24.7 | 3.6 | 0.07 |
| Physical activity (intensity score)a |
328.0 | 347.0 | 302.5 | 297.0 | 299.5 | 310.0 | 364.5 | 309.0 | 0.20 |
| Alcohol intake (ml/day)a | 2.4 | 9.7 | 4.8 | 11.1 | 2.4 | 9.6 | 2.7 | 12.1 | 0.11 |
| Dietary carbohydrate (% kcal) |
48.0 | 7.1 | 46.6 | 6.8 | 47.2 | 7.7 | 47.2 | 7.3 | 0.13 |
| Dietary fat (% kcal) | 36.1 | 6.0 | 37.0 | 6.0 | 36.9 | 6.4 | 36.6 | 5.8 | 0.17 |
Median (interquartile range); Kruskal-Wallis Test: nonparametric one-way tests used for physical activity and alcohol intake to assess differences among groups.
Table 4.
Baseline and End of Follow-Up Unadjusted HDL-C (mg/dl) Levels for Follow-Up Pregnancy Groups by ApoE Groups
| Follow-up pregnancy groups HDL-C levels |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P0 (0 pregnancies) |
P1+ (1+ short pregnancies) |
B1 (1 birth) |
B2+ (2 births) |
||||||||||
| ApoE group | na | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | p valueb |
| E2 | 92 | 24 | 34 | 29 | |||||||||
| Baseline | 60.9 | 12.5 | 59.7 | 16.8 | 57.6 | 13.0 | 58.1 | 15.7 | 0.58 | ||||
| Follow-up | 61.0 | 15.3 | 55.5 | 17.1 | 53.3 | 11.5 | 48.5 | 15.2 | 0.001 | ||||
| E3 | 345 | 87 | 118 | 103 | |||||||||
| Baseline | 58.0 | 12.1 | 59.3 | 13.4 | 57.5 | 13.5 | 57.8 | 12.5 | 0.79 | ||||
| Follow-up | 57.4 | 13.6 | 57.5 | 13.2 | 53.8 | 13.9 | 54.3 | 11.9 | 0.03 | ||||
| E4 | 162 | 29 | 70 | 54 | |||||||||
| Baseline | 54.6 | 12.3 | 53.1 | 13.8 | 55.6 | 10.4 | 53.2 | 11.4 | 0.65 | ||||
| Follow-up | 54.4 | 13.2 | 52.4 | 12.5 | 52.7 | 10.9 | 50.5 | 12.0 | 0.23 | ||||
n, number of subjects; reflects the sample size within pregnancy and birth groups at the end of follow-up. In the multivariate models of repeated outcome measures, (see Table 5), subjects contribute measurements at multiple follow-up examinations given the time-dependent classification of subjects into pregnancy and birth groups for each time interval.
p values for test of differences in HDL-C among follow-up pregnancy groups.
The association of follow-up pregnancy groups with adjusted HDL-C changes varied by ApoE group (pregnancy and birth-ApoE group interaction, p < 0.002). Adjusted mean (95% CI) HDL-C changes among follow-up pregnancy and birth groups (Table 5) are shown stratified by ApoE groups. For each ApoE group, HDL-C declines were found in B1 and B2+ groups (Model 1). In E2 and E3 groups, HDL-C also declined in the P1+ group (Model 1). HDL-C levels remained stable for the P0 group in E3 and E4 groups, but HDL-C levels increased by 3 mg/dl on average for the E2 group in both minimally and fully adjusted models.
Table 5.
Minimally and Fully Adjusted Mean (95% CI) Changes in HDL-C (Δ HDL-C) for Pregnancy and Birth Groups Stratified by ApoE Groups
| Follow-up pregnancy groups: ΔHDL-C (mg/dl) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| P0: 0 pregnancies (referent) (n = 599)a |
P1+: 1+short pregnancies (n = 140) |
B1: 1 birth (n = 222) |
B2+: 2 or more births (n = 186) |
Global p value |
|||||
| ApoE groupb | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | Mean | 95% CI | |
| E2 (2/2, 3/2) | |||||||||
| Model 1 (minimally adjusted) |
3.4 | (1.8, 5.0) | −1.1* | (−4.4, 2.2) | −3.8*** | (−6.3, −1.4) | −9.5*** | (−12.4, −6.5) | <0.001 |
| Model 2 (fully adjusted) | 3.2 | (1.6, 4.7) | −1.9** | (−5.1, 1.3) | −3.4*** | (−5.9, −1.0) | −8.3*** | (−11.3, −5.4) | <0.001 |
| Model 2 + waist girth | 2.9 | (1.5, 4.4) | −2.2** | (−5.3, 0.8) | −1.0** | (−3.5, 1.4) | −7.4*** | (−10.2, −4.6) | <0.001 |
| E3 (3/3) | |||||||||
| Model 1 (minimally adjusted) |
1.1 | (0.2, 1.9) | −1.3* | (−3.1, 0.5) | −2.6*** | (−3.8, −1.3) | −3.6*** | (−5.2, −2.0) | <0.001 |
| Model 2 (fully adjusted) | 1.0 | (0.2, 1.8) | −1.5* | (−3.3, 0.3) | −2.4*** | (−3.6, −1.1) | −3.1*** | (−4.7, −1.6) | <0.001 |
| Model 2 + waist girth | 0.7 | (−0.1, 1.4) | −1.8* | (−3.5, 0) | −2.4*** | (−3.6, −1.1) | −2.4*** | (−3.9, −10.8) | <0.001 |
| E4 (3/4, 4/4) | |||||||||
| Model 1 (minimally adjusted) |
−0.6 | (−1.8, 0.6) | −1.1 | (−4.0, 1.8) | −3.5** | (−5.1, −1.9) | −3.5* | (−5.8, −1.3) | 0.015 |
| Model 2 (fully adjusted) | −0.7 | (−1.9, 0.5) | −1.1 | (−4.0, 1.8) | −3.2** | (−4.8, −1.6) | −3.4* | (−5.6, −1.2) | 0.04 |
| Model 2 + waist girth | −0.8 | (−1.9, 0.3) | −0.9 | (−3.7, 1.8) | −2.5 | (−4.1, −0.9) | −2.7 | (−4.9, −0.5) | 0.23 |
n, number at end of follow-up within group (repeated measures analysis includes three follow-up measurements per subject for 94% of subjects in the sample).
Model 1, minimally adjusted means; model includes race, study center, time, baseline characteristics (plasma HDL-C, BMI, waist circumference, smoking status, OC use categories, alcohol intake, physical activity score) and time-dependent age and number of short pregnancies. Model 2, fully adjusted means includes covariates in Model 1 plus time-dependent covariates (smoking, education, months of OC use, and changes in physical activity score and alcohol intake). Model 2 + waist girth; waist girth change added to Model 2 as a mediator of parity-associated HDL-C declines.
Pairwise comparisons of P1+, B1, and B2+ to the referent group (P0): p < 0.05;
p < 0.001;
p < 0.001.
The magnitude of the absolute HDL-C declines associated with births varied across ApoE groups (Table 5). In minimally and fully adjusted models, the E2 group compared with E3 and E4 groups had the largest absolute declines among women who had one birth and two or more births during follow-up. HDL-C levels for the B1 and B2+ groups, respectively, decreased by −3.8 and −9.5 mg/dl for E2 and by −2.6 and −3.6 mg/dl for E3 (Model 1). The E4 group had absolute HDL-C declines in B1 and B2+ groups of −3.5 mg/dl.
The addition of time-dependent follow-up behavioral covariates to the model (Model 2) attenuated the HDL-C declines across all ApoE groups but the strong association with pregnancy groups remained for E2 and E3 groups and a modest association for E4. Addition of waist girth change, a potential mediator of parity-related HDL-C declines, to the fully adjusted model (Model 2+waist girth) moderately attenuated HDL-C changes across the pregnancy groups (P0, P1+, B1, and B2). However, differences among the pregnancy groups remained highly significant for E2 and E3 ApoE groups. For the E4 group, waist girth attenuated HDL-C declines by 10%–20% and overall differences between groups were no longer statistically significant by pregnancy group (p = 0.23), potentially related to the smaller number of women with ε4 alleles. Inclusion of weight change in the models had little impact on the estimates (data not shown).
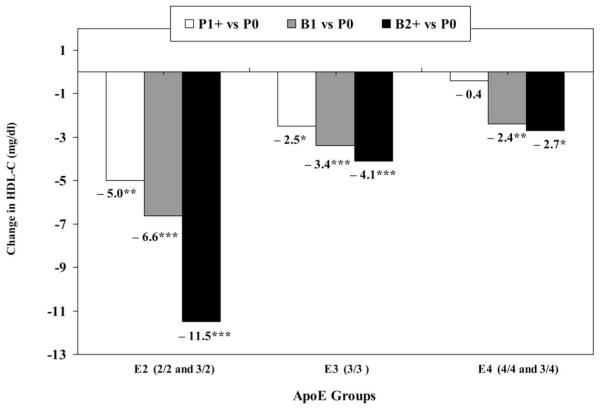
Net differences in fully adjusted mean HDL-C changes for P1+, B1, and B2+ groups relative to their respective referent P0 groups are shown by ApoE group (Fig. 1). For E4, net differences in HDL-C declines were smallest (−2.4 to −2.7 mg/dl) for B1 and B2+ vs. the P0 group (p < 0.01). For E3, the relative HDL-C decline was somewhat larger at −3.4 and −4.1 mg/dl for B1 and B2+ groups, respectively, vs. the P0 group (p < 0.001). The E2 group had the largest net HDL-C declines for P1+, B1, and B2 vs. P0 and a significant linear trend (p trend < 0.001) with number of births in fully adjusted models. The net differences were −5.0 mg/dl for P1+ (p < 0.01), −6.6 mg/dl for B1 (p < 0.001), and −11.5 mg/dl for B2+ (p < 0.001) vs. P0.
FIG. 1.
Net differences in fully adjusted mean change in HDL-C levels (mg/dl) between follow-up pregnancy groups vs. the referent group by ApoE groups. P0, no pregnancies (referent]); P1+, 1 or more short pregnancies (no births); B1, one birth; B2+, two or more births. Pairwise comparisons to referent group (P0); significance levels: *p < 0.05; **p < 0.01; ***p < 0.001).
DISCUSSION
This is the first study, to our knowledge, to evaluate effect modification by apo E phenotype (genotype) in the association of HDL-C declines with childbearing. The E2 (2/2, 3/2) ApoE group showed the greatest declines in HDL-C associated with childbearing and a strong dose-response relationship with the number of births. The E3 (3/3) ApoE group, which was the most prevalent, showed an intermediate decline in HDL-C associated with childbearing that did not increase with number of births. Finally, the E4 (4/4 and 4/3) ApoE group showed the smallest HDL-C decline associated with a first birth. The parity-related HDL-C declines for all ApoE groups were modestly attenuated by inclusion of waist girth change in the models, but pregnancy group differences were still statistically significant within E2 and E3 groups but not for E4. Central obesity is known to be an important predictor of HDL-C levels in women. Our findings show that parity-associated HDL-C declines are not explained by increased waist girth or weight gain and that effect modification by apo E polymorphisms was not attributable to confounding by parity-related gains in central adiposity. In this same cohort of CARDIA women, we previously reported that the parity-related decline in HDL-C is not primarily mediated by abdominal adiposity (central), weight gain, dietary intake, changes in OC use, physical activity, alcohol intake, cigarette smoking, and other behaviors.7
It is well established that lipid profiles differ by apo E genotype in human populations.33 The three major isoforms, ε2, ε3, and ε4, result from different amino acid substitutions that are encoded from separate alleles. Any ε4 increases the risk of atherosclerosis, CAD, stroke, and ischemic heart disease, whereas ε2 lowers the risk.10–13 Moreover, ε4 is associated with lower HDL-C and higher LDL-C and higher TG, whereas the inverse is true for ε2.14,15 At baseline, lipid profiles of nulliparous CARDIA women in our sample reflected the known effects of apo E polymorphisms on HDL-C and LDL-C plasma concentrations.
Evidence that exogenous sex hormone exposures modulate lipid profiles depending on apo E genotype19 led to our hypothesis that pregnancy may differentially influence lipid profiles by apo E genotype. Cross-sectional studies of the effects of OC use on lipid profiles have been inconsistent.34–38 The variations across studies may be explained by the opposing actions of estrogen and progestogens on lipid metabolism that are influenced by differences in OC formulations, as well as the interaction between apo E polymorphisms and OC use. This evidence showing that OC use decreases apo E concentrations for ε2 and ε3/ε3 alleles compared with ε4 alleles19 is consistent with our study findings of greater parity-associated decrements in HDL-C concentrations for these same apo E genotypes—carriers of ε2 and ε3/ε3 alleles.
In postmenopausal women, the effects of HRT on HDL-C levels by apo E genotype are the opposite of the effects related to OC use and our findings for pregnancy in premenopausal women. Both a randomized trial of 5-year HRT use (continuous estrogen and cyclic progestin) vs. placebo and cross-sectional studies in postmenopausal women showed increased or higher HDL-C levels for those carrying ε2 or ε3 alleles and unaltered levels or no improvement for those with the ε4 allele.20–22,39
One of the plausible mechanisms to explain our findings is that much higher gestational levels of estrogen and progesterone lead to decreased apo E-protein levels for the ε2 and ε3/ε3 alleles. Evidence from OC users and animal models is consistent with lowering of plasma HDL-C levels. In mice, administration of supraphysiological doses of estradiol were found to decrease plasma HDL-C levels by 30%40 and to cause a pronounced shift toward apo E-based lipoprotein metabolism in the placenta during pregnancy.41 Endocrine-associated changes in pregnancy may modulate the expression of the ε2 allele to produce the detrimental effects on HDL-C levels. Although the findings for HRT use in postmenopausal women, an estrogen-deficient state, are opposite the effects found for OC use and in the hyperestrogenic state of pregnancy, they are consistent in confirming the beneficial effects of estrogen use at physiological levels where apo E-protein levels are highest in carriers of ε2 and lowest in carriers of ε4.39
Our finding that the apo E phenotype modifies the association between pregnancy and HDL-C declines is also consistent with previous reports of apoE genotype influencing the association between environmental factors and lipids. In the Bogalusa Heart Study,42 subjects with ε4 had greater increases in LDL-C levels over a 15-year period than those with ε3, and those with ε2 were more responsive to the effect of obesity and dietary fat intake on LDL-C levels. Similarly, others have shown that those with ε2 alleles are more responsive to lipid-lowering interventions and more susceptible to the effect of saturated fat on HDL-C and very low density lipoprotein (VLDL) levels compared with those with ε4.43,44 Consistent with our results, these findings illustrate that although those with ε2 have lower LDL-C and higher HDL-C (in general, a better lipid profile), they are also more susceptible to the influence of both positive and negative external factors, such as pregnancy, obesity, diet, OC use, and HRT.
Pregnancy is known to cause 100-fold increases in endogenous reproductive hormones, including progesterone and estrogens (estriol, estrone, and 17-β estradiol),45 as well as production of human placental lactogen.46 During gestation, TC and LDL-C levels increase by 40%–50%, TG levels increase by more than 2-fold,47,48 and HDL-C increases by 0–25%. During the puerperium, TG returns rapidly to baseline,49,50 whereas TC and LDL-C49 have a slower decline throughout the first year postpartum. By 1 year postpartum, HDL-C levels decline below preconception levels,4,50 and larger HDL-C declines have been reported in primiparas compared with nulliparas over several years.5 One study of 250 healthy pregnant women found lower third-trimester TC and LDL-C levels for ε2 alleles compared to ε3/ε3 genotype but no significant differences in plasma TG or HDL-C levels.51 We are unaware of any studies that have examined whether the long-term HDL-C declines subsequent to pregnancy vary by apo E phenotype or genotype.
Ninety-four percent of women included in this analysis had HDL-C levels measured at least three times during the 10-year follow-up period. Repeated measures linear regression methods enabled estimates of longitudinal changes in HDL-C levels. Our analyses were conducted using single-inference, rather than multiple-inference, procedures (single association p values and 95% CI) as recommended by Greenland and Rothman.52 Thus, it is possible that some of our estimates achieved statistical significance at the 0.05 level by chance alone. However, there was strong evidence for effect modification, given that the statistical test for interaction was associated with a p value <0.002, and for E2 and E3, the two pairwise comparisons of each birth group to the nongravid reference group had p values <0.001, suggesting that our findings are not likely to be the result of a type 1 error. The ApoE groups in our study closely represent apo E genotypes, given the high accuracy of genotype categorization with the method.29
A limitation of this study is that we were unable to examine whether HDL-C decrements would have been even greater with higher numbers of births because few women in the sample (n = 23) had delivered at least three children.
CONCLUSIONS
The higher HDL-C levels commonly associated with 2/2 and 2/3 compared to 3/3 apo E genotypes are abolished by the 2–3-fold greater HDL-C declines associated with increasing parity for 2/2 and 2/3 apo E genotypes. Thus, HDL-C declines associated with childbearing for women carrying ε2 alleles compared with the homozygous ε3 allele genotype may result in more similar HDL-C levels across the apo E genotypes, which may explain the conflicting findings for HDL-C and apo E genotype associations among women in some epidemiological studies. Based on our multivariate models, increased risk of CHD may range from 10% to 18% or more due to pregnancy-related HDL-C declines depending on apo E genotype.53 Just as weight gain and increased waist girth attenuate pregnancy-related HDL-C declines but are not responsible for the long-term changes associated with childbearing, the differences in HDL-C changes associated with childbearing for apo E polymorphisms are not explained by central or overall adiposity.
The evidence points to hormonal alterations due to pregnancy that influence lipoprotein levels, which are in turn modified by genetic factors, such as apo E polymorphisms. Women who start with more favorable HDL-C levels are not exempt from declining levels, and monitoring of lipoprotein profiles is necessary among women of reproductive age. All women need to make lifestyle modifications as they age and bear children to maintain maximum HDL-C levels. Further elucidation of hormonal exposures, both endogenous and exogenous, their influence on risk factors for CVD, and genetic characteristics that modify these relationships in premenopausal women may provide a better understanding of determinants of disease risk in women over the life-span.
Acknowledgments
This work was supported by Contracts N01-HC-48047, N01-HC-48048, N01-HC-48049, N01-HC-48050, and N01-HC-95095, from the National Heart, Lung and Blood Institute and by Career Development Award Grant number 1 K01 DK059944 from the National Institute of Diabetes, Digestive and Kidney Diseases.
REFERENCES
- 1.Humphries KH, Westendorp IC, Bots ML, et al. Parity and carotid artery atherosclerosis in elderly women: The Rotterdam Study. Stroke. 2001;32:2259. doi: 10.1161/hs1001.097224. [DOI] [PubMed] [Google Scholar]
- 2.Kritz-Silverstein D, Barrett-Connor E, Wingard DL. The relationship between multiparity and lipoprotein levels in older women. J Clin Epidemiol. 1992;45:761. doi: 10.1016/0895-4356(92)90053-p. [DOI] [PubMed] [Google Scholar]
- 3.Ness RB, Cosmatos I, Flegal KM. Gravidity and serum lipids among Hispanic women in the Hispanic Health and Nutrition Examination Survey. J Womens Health. 1995;4:149. [Google Scholar]
- 4.van Stiphout WA, Hofman A, de Bruijn AM. Serum lipids in young women before, during, and after pregnancy. Am J Epidemiol. 1987;126:922. doi: 10.1093/oxfordjournals.aje.a114729. [DOI] [PubMed] [Google Scholar]
- 5.Lewis CE, Funkhouser E, Raczynski JM, Sidney S, Bild DE, Howard BV. Adverse effect of pregnancy on high-density lipoprotein (HDL) cholesterol in young adult women. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1996;144:247. doi: 10.1093/oxfordjournals.aje.a008919. [DOI] [PubMed] [Google Scholar]
- 6.Haertel U, Heiss G, Filipiak B, Doering A. Cross-sectional and longitudinal associations between high-density lipoprotein cholesterol and women’s employment. Am J Epidemiol. 1992;135:68. doi: 10.1093/oxfordjournals.aje.a116203. [DOI] [PubMed] [Google Scholar]
- 7.Gunderson EP, Lewis CE, Murtaugh MA, Quesenberry CP, Smith WD, Sidney S. Long-term plasma lipid changes associated with a first birth: The Coronary Artery Risk Development in Young Adults Study. Am J Epidemiol. 2004;159:1028. doi: 10.1093/aje/kwh146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High-density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977;62:707. doi: 10.1016/0002-9343(77)90874-9. [DOI] [PubMed] [Google Scholar]
- 9.Kannel WB. High-density lipoproteins: Epidemiologic profile and risks of coronary artery disease. Am J Cardiol. 1983;52:9B. doi: 10.1016/0002-9149(83)90649-5. [DOI] [PubMed] [Google Scholar]
- 10.Davignon J, Gregg RE, Sing CF. Apolipoprotein E polymorphism and atherosclerosis. Arteriosclerosis. 1988;8:1. doi: 10.1161/01.atv.8.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Eichner JE, Kuller LH, Orchard TJ, et al. Relation of apolipoprotein E phenotype to myocardial infarction and mortality from coronary artery disease. Am J Cardiol. 1993;71:160. doi: 10.1016/0002-9149(93)90732-r. [DOI] [PubMed] [Google Scholar]
- 12.Wilson PW, Myers RH, Larson MG, Ordovas JM, Wolf PA, Schaefer EJ. Apolipoprotein E alleles, dyslipidemia, and coronary heart disease. The Framingham Offspring Study. JAMA. 1994;272:1666. [PubMed] [Google Scholar]
- 13.McCarron MO, Delong D, Alberts MJ. APOE genotype as a risk factor for ischemic cerebrovascular disease: A meta-analysis. Neurology. 1999;53:1308. doi: 10.1212/wnl.53.6.1308. [DOI] [PubMed] [Google Scholar]
- 14.Hagberg JM, Wilund KR, Ferrell RE. APOE gene and gene-environment effects on plasma lipoprotein-lipid levels. Physiol Genomics. 2000;4:101. doi: 10.1152/physiolgenomics.2000.4.2.101. [DOI] [PubMed] [Google Scholar]
- 15.Eto M, Watanabe K, Ishii K. Reciprocal effects of apolipoprotein E alleles (epsilon 2 and epsilon 4) on plasma lipid levels in normolipidemic subjects. Clin Genet. 1986;29:477. doi: 10.1111/j.1399-0004.1986.tb00547.x. [DOI] [PubMed] [Google Scholar]
- 16.Srivastava RA, Bhasin N, Srivastava N. Apolipoprotein E gene expression in various tissues of mouse and regulation by estrogen. Biochem Mol Biol Int. 1996;38:91. [PubMed] [Google Scholar]
- 17.Boffelli D, Zajchowski DA, Yang Z, Lawn RM. Estrogen modulation of apolipoprotein(a) expression. Identification of a regulatory element. J Biol Chem. 1999;274:15569. doi: 10.1074/jbc.274.22.15569. [DOI] [PubMed] [Google Scholar]
- 18.Kushwaha RS, Foster DM, Barrett PH, Carey KD, Bernard MG. Metabolic regulation of plasma apolipoprotein E by estrogen and progesterone in the baboon (Papio sp) Metabolism. 1991;40:93. doi: 10.1016/0026-0495(91)90198-6. [DOI] [PubMed] [Google Scholar]
- 19.Starck M, Schiele F, Herbeth B, et al. Apolipoproteins E and C-III in apo B- and non-apo B-containing lipoproteins in middle-aged women from the Stanislas cohort: Effect of oral contraceptive use and common apolipoprotein E polymorphism. Atherosclerosis. 2001;155:509. doi: 10.1016/s0021-9150(00)00600-6. [DOI] [PubMed] [Google Scholar]
- 20.von Muhlen D, Barrett-Connor E, Kritz-Silverstein D. Apolipoprotein E genotype and response of lipid levels to postmenopausal estrogen use. Atherosclerosis. 2002;161:209. doi: 10.1016/s0021-9150(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 21.Heikkinen AM, Niskanen L, Ryynanen M, et al. Is the response of serum lipids and lipoproteins to postmenopausal hormone replacement therapy modified by ApoE genotype? Arterioscler Thromb Vasc Biol. 1999;19:402. doi: 10.1161/01.atv.19.2.402. [DOI] [PubMed] [Google Scholar]
- 22.Garry PJ, Baumgartner RN, Brodie SG, et al. Estrogen replacement therapy, serum lipids, and polymorphism of the apolipoprotein E gene. Clin Chem. 1999;45:1214. [PubMed] [Google Scholar]
- 23.Cutter GR, Burke GL, Dyer AR, et al. Cardiovascular risk factors in young adults. The CARDIA baseline monograph. Control Clin Trials. 1991;12:1S. doi: 10.1016/0197-2456(91)90002-4. [DOI] [PubMed] [Google Scholar]
- 24.Friedman GD, Cutter GR, Donahue RP, et al. CARDIA: Study design, recruitment, and some characteristics of the examined subjects. J Clin Epidemiol. 1988;41:1105. doi: 10.1016/0895-4356(88)90080-7. [DOI] [PubMed] [Google Scholar]
- 25.Lewis CE, Jacobs DR, Jr, McCreath H, et al. Weight gain continues in the 1990s: 10-year trends in weight and overweight from the CARDIA study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 2000;151:1172. doi: 10.1093/oxfordjournals.aje.a010167. [DOI] [PubMed] [Google Scholar]
- 26.Bild DE, Jacobs DR, Liu K, et al. Seven-year trends in plasma low-density lipoprotein-cholesterol in young adults: The CARDIA Study. Ann Epidemiol. 1996;6:235. doi: 10.1016/1047-2797(96)00005-1. [DOI] [PubMed] [Google Scholar]
- 27.Howard BV, Gidding SS, Liu K. Association of apolipoprotein E phenotype with plasma lipoproteins in African-American and white young adults. The CARDIA Study. Coronary Artery Risk Development in Young Adults. Am J Epidemiol. 1998;148:859. doi: 10.1093/oxfordjournals.aje.a009711. [DOI] [PubMed] [Google Scholar]
- 28.Kamboh MI, Ferrell RE, Kottke B. Genetic studies of human apolipoproteins. V. A novel rapid procedure to screen apolipoprotein E polymorphism. J Lipid Res. 1988;29:1535. [PubMed] [Google Scholar]
- 29.Kataoka S, Paidi M, Howard BV. Simplified isoelectric focusing/immunoblotting determination of apoprotein E phenotype. Clin Chem. 1994;40:11. [PubMed] [Google Scholar]
- 30.Lewis CE, Smith DE, Wallace DD, Williams OD, Bild DE, Jacobs DR., Jr. Seven-year trends in body weight and associations with lifestyle and behavioral characteristics in black and white young adults: The CARDIA study. Am J Public Health. 1997;87:635. doi: 10.2105/ajph.87.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anderssen N, Jacobs DR, Jr, Sidney S, et al. Change and secular trends in physical activity patterns in young adults: A seven-year longitudinal follow-up in the Coronary Artery Risk Development in Young Adults Study (CARDIA) Am J Epidemiol. 1996;143:351. doi: 10.1093/oxfordjournals.aje.a008749. [DOI] [PubMed] [Google Scholar]
- 32.McDonald A, Van Horn L, Slattery M, et al. The CARDIA dietary history: Development, implementation, and evaluation. J Am Diet Assoc. 1991;91:1104. [PubMed] [Google Scholar]
- 33.Eichner JE, Dunn ST, Perveen G, Thompson DM, Stewart KE, Stroehla BC. Apolipoprotein E polymorphism and cardiovascular disease: A HuGE review. Am J Epidemiol. 2002;155:487. doi: 10.1093/aje/155.6.487. [DOI] [PubMed] [Google Scholar]
- 34.Greenlund KJ, Webber LS, Srinivasan S, Wattigney W, Johnson C, Berenson GS. Associations of oral contraceptive use with serum lipids and lipoproteins in young women: The Bogalusa Heart Study. Ann Epidemiol. 1997;7:561. doi: 10.1016/s1047-2797(97)00119-1. [DOI] [PubMed] [Google Scholar]
- 35.Crook D. Multicenter study of endocrine function and plasma lipids and lipoproteins in women using oral contraceptives containing desogestrel progestin. U.K. Desogen Study Group. Contraception. 1997;55:219. doi: 10.1016/s0010-7824(97)00010-3. [DOI] [PubMed] [Google Scholar]
- 36.Fotherby K. Oral contraceptives and lipids. BMJ. 1989;298:1049. doi: 10.1136/bmj.298.6680.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lobo RA, Skinner JB, Lippman JS, Cirillo SJ. Plasma lipids and desogestrel and ethinyl estradiol: A meta-analysis. Fertil Steril. 1996;65:1100. [PubMed] [Google Scholar]
- 38.Croft JB, Freedman DS, Cresanta JL, et al. Adverse influences of alcohol, tobacco, and oral contraceptive use on cardiovascular risk factors during transition to adulthood. Am J Epidemiol. 1987;126:202. doi: 10.1093/aje/126.2.202. [DOI] [PubMed] [Google Scholar]
- 39.Somekawa Y, Wakabayashi A. Relationship between apolipoprotein E polymorphism, menopausal symptoms, and serum lipids during hormone replacement therapy. Eur J Obstet Gynecol Reprod Biol. 1998;79:185. doi: 10.1016/s0301-2115(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 40.Srivastava N, Chowdhury PR, Averna M, Srivastava RA. Estrogen increases hepatic lipase levels in inbred strains of mice: A possible mechanism for estrogen-dependent lowering of high-density lipoprotein. Mol Cell Biochem. 2001;220:87. doi: 10.1023/a:1010845032399. [DOI] [PubMed] [Google Scholar]
- 41.Overbergh L, Lorent K, Torrekens S, Van Leuven F, Van den BH. Expression of mouse alpha-macroglobulins, lipoprotein receptor-related protein, LDL receptor, apolipoprotein E, and lipoprotein lipase in pregnancy. J Lipid Res. 1995;36:1774. [PubMed] [Google Scholar]
- 42.Srinivasan SR, Ehnholm C, Elkasabany A, Berenson G. Influence of apolipoprotein E polymorphism on serum lipids and lipoprotein changes from childhood to adulthood: The Bogalusa Heart Study. Atherosclerosis. 1999;143:435. doi: 10.1016/s0021-9150(98)00304-9. [DOI] [PubMed] [Google Scholar]
- 43.Pedro-Botet J, Schaefer EJ, Bakker-Arkema RG, et al. Apolipoprotein E genotype affects plasma lipid response to atorvastatin in a gender-specific manner. Atherosclerosis. 2001;158:183. doi: 10.1016/s0021-9150(01)00410-5. [DOI] [PubMed] [Google Scholar]
- 44.Campos H, D’Agostino M, Ordovas JM. Gene-diet interactions and plasma lipoproteins: Role of apolipoprotein E and habitual saturated fat intake. Genet Epidemiol. 2001;20:117. doi: 10.1002/1098-2272(200101)20:1<117::AID-GEPI10>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 45.Kalkhoff RK, Kissebah AH, Kim HJ. Carbohydrate and lipid metabolism during normal pregnancy: Relationship to gestational hormone action. Semin Perinatol. 1978;2:291. [PubMed] [Google Scholar]
- 46.Tulchinsky D, Hobel CJ, Yeager E, Marshall JR. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol. 1972;112:1095. doi: 10.1016/0002-9378(72)90185-8. [DOI] [PubMed] [Google Scholar]
- 47.Piechota W, Staszewski A. Reference ranges of lipids and apolipoproteins in pregnancy. Eur J Obstet Gynecol Reprod Biol. 1992;45:27. doi: 10.1016/0028-2243(92)90190-a. [DOI] [PubMed] [Google Scholar]
- 48.Martin U, Davies C, Hayavi S, Hartland A, Dunne F. Is normal pregnancy atherogenic? Clin Sci (Lond) 1999;96:421. doi: 10.1042/cs0960421. [DOI] [PubMed] [Google Scholar]
- 49.Jimenez DM, Pocovi M, Ramon-Cajal J, Romero MA, Martinez H, Grande F. Longitudinal study of plasma lipids and lipoprotein cholesterol in normal pregnancy and puerperium. Gynecol Obstet Invest. 1988;25:158. doi: 10.1159/000293765. [DOI] [PubMed] [Google Scholar]
- 50.Darmady JM, Postle AD. Lipid metabolism in pregnancy. Br J Obstet Gynaecol. 1982;89:211. doi: 10.1111/j.1471-0528.1982.tb03616.x. [DOI] [PubMed] [Google Scholar]
- 51.McGladdery SH, Frohlich JJ. Lipoprotein lipase and apoE polymorphisms: Relationship to hypertriglyceridemia during pregnancy. J Lipid Res. 2001;42:1905. [PubMed] [Google Scholar]
- 52.Greenland S, Rothman KJ. Fundamental of epidemiologic data analysis. In: Rothman KJ, Greenland S, editors. Modern epidemiology. 2nd ed Lippincott-Raven; Philadelphia: 1998. [Google Scholar]
- 53.Wilson PW, Anderson KM, Castelli WP. Twelve-year incidence of coronary heart disease in middle-aged adults during the era of hypertensive therapy: The Framingham offspring study. Am J Med. 1991;90:11. doi: 10.1016/0002-9343(91)90500-w. [DOI] [PubMed] [Google Scholar]