Abstract
The discovery of small noncoding RNA, including P-element-induced wimpy testis-interacting RNA, small interfering RNA, and microRNA, has energized research in reproductive medicine. In the two decades since the identification of small RNA, first in Caenorhabditis elegans and then in other animals, scientists in many disciplines have made significant progress in elucidating their biology. A powerful battery of tools, including knockout mice and small RNA mimics and antagonists, has facilitated investigation into the functional roles and therapeutic potential of these small RNA pathways. Current data indicate that small RNA play significant roles in normal development and physiology and pathological conditions of the reproductive tracts of females and males. Biologically plausible mRNA targets for these microRNA are aggressively being discovered. The next phase of research will focus on elucidating the clinical utility of small RNA-selective agonists and antagonists.
Small RNA pathways play major roles in mammals, reproduction, and cancer biology. These pathways were first described in Caenorhabditis elegans in 1993, and in a 1998 paper (1) for which the 2006 Nobel Prize in Physiology or Medicine was awarded to Drs. Andrew Fire and Craig Mello. Although a comprehensive functional classification system has not yet been determined, small RNA including piRNA [P-element-induced wimpy testis (PIWI)-interacting RNA], small interfereing RNA (siRNA), and microRNA (miRNA) can generally be divided into two general categories based on nucleotide length. Small RNA greater than 24 nucleotides are piRNA, whereas those less than 24 nucleotides are mostly miRNA and siRNA (Table 1). As shown in Table 1 and described in more detail in the sections that follow, these small RNA are synthesized via distinct enzymatic pathways and have clear functional roles in normal and abnormal reproduction in males and females. Because of cell type-specific functions, we will first discuss the role of piRNA in testis physiology and the functions of siRNA in oocytes and early embryogenesis where these small RNA function as the guardians of the germline genome. The majority of this review will focus on the diverse expression and roles of miRNA in reproductive tract development, physiology, and pathology.
Table 1.
Small RNA characteristics and tissue of function in mammals
| piRNA | siRNA | miRNA | |
|---|---|---|---|
| Approximate sizes | 25–30 nt | 18–24 nt | 18–24 nt |
| Major cell type | Male germ cell | Oocyte | Multiple |
| DICER-dependent | No | Yes | Yes |
| Drosha-dependent | No | No | Yes |
| DGCR8-dependent | No | No | Yes |
| Major function | Suppression of transposon synthesis | Cleavage of transposon mRNAs | Cleavage of target mRNA and suppression of translation |
| Estimated number | >10,000 | >10,000 | 600–1000 |
nt, Nucleotides.
piRNA are Required in Male Germ Cells
piRNA were originally identified through their association with Drosophila PIWI family members, and studies in the fruit fly have revealed many of their properties (reviewed in Ref. 2). In 2006, several reports identified piRNA for the first time in the germlines of mice and rats (3–7). piRNA (and their synthesis pathways) are deeply rooted among the animalia kingdom from sponges to humans, have recently been discovered in Tetrahymena (8) and Paramecium (9), but are absent in plantae and fungi that employ siRNA in their stead. The functions of piRNA are nearly exclusive to gametogenesis and are essential to spermatogenesis in mammals through their ability to maintain the integrity of the germline.
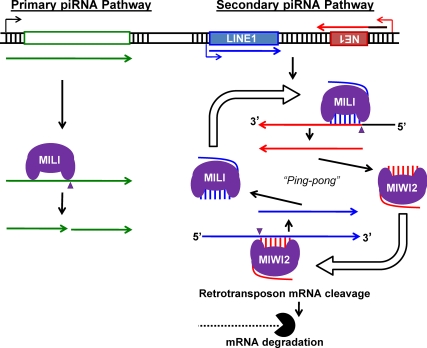
Considering that mammalian piRNA were only discovered 5 yr ago, significant progress has been made in understanding piRNA biosynthesis pathways in mammals in parallel with advances in next generation sequencing technologies and proteomics. A model for how piRNA are believed to be synthesized is shown in Fig. 1. Although many of the piRNA-encoded genomic loci are located at syntenic positions in mammals and are transcribed from the same strand as long primary transcripts, the individual piRNA are not conserved at the sequence level. These precursor RNA are targeted for cleavage by PIWI subfamily Argonaute RNA endonucleases, which generate their 5′-ends. There are three PIWI family members in the mouse (MIWI2, MILI, and MIWI), sequentially expressed in embryonic/mitotic, meiotic, and postmeiotic male germ cells. These PIWI family members generate piRNA that differ slightly in their length (25–27 nucleotides), proportional to the relative distance between the PAZ and MID domains in the three proteins. In Drosophila, the piRNA 3′-end is likely generated by the action of SQUASH and ZUCCHINI, but the 3′-endonuclease remains unknown in mammals. Snapshots of piRNA in embryonic, postnatal d 8, and postnatal d 14 testes have been described as embryonic, prepachytene, and pachytene piRNA that differ in the genomic location and composition relative to repetitive elements that divide piRNA into two classes, repeat-associated and non-repeat-associated piRNA (10–17). For the repeat-associated piRNA, homology between MIWI2-associated antisense piRNA and retrotransposon mRNA or MILI-associated sense piRNA and RNA transcribed antisense to retrotransposons allows a Ping-pong amplification of piRNA synthesis (Fig. 1, right) known as secondary piRNA biogenesis with complementary PIWIs generating the 5′-ends of piRNA from opposing RNA substrates (15). The signature of this synthesis is an enrichment for A at the tenth position of secondary piRNA due to its base pairing with the corresponding initial U on the primary piRNA. The terminal event of biogenesis is methylation of the 3′-end of the mature piRNA by HEN1 2′-O-methylase (18–20), allowing for the preferential binding of the 2′-O-methylated piRNA within the (PAZ) domain pocket of PIWI but not the AGO subfamily (21, 22), presumably making it resistant to the action of uridylation.
Fig. 1.
piRNA biosynthesis. Two major pathways can generate piRNA. In primary piRNA biogenesis the PIWI protein, here MILI, cuts a precursor RNA into piRNA. In secondary piRNA biogenesis piRNAs in a sense orientation to active full-length copies of retrotransposon LINE1 (blue) bound to MILI target cleavage of transcripts containing fragments of LINE1 (3′-terminal fragment depicted as “NE1”) transcribed by antisense-oriented promotion. These new antisense piRNA can be incorporated in complex with MIWI2 to cleave the sense-oriented transcripts of LINE1 to generate more sense piPRNA to repeat the cycle.
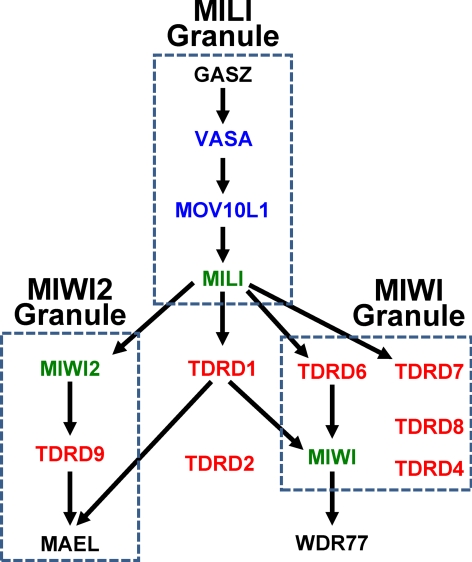
The synthesis of piRNA occurs within cytoplasmic riboprotein-rich granules (nuage or clouds) identified by light microscopy in the 1800s and later by electron microscopy (23) as having an important association with germline specification and functions. As shown genetically (Fig. 2), immunohistochemically, and biochemically (Fig. 1), the formation of granules containing MILI is required for MILI-directed synthesis of primary piRNA, subsequent generation of distinct granules containing MIWI2, and MIWI2-directed synthesis of complementary secondary piRNA. Likewise, MILI granule formation is required for the subsequent genesis of the MIWI-positive chromatoid body that is devoid of MILI and MIWI2. The reproductive importance of these granules and the piRNA pathway is evident by the high evolutionary conservation of these fruit fly and mouse proteins and the findings of sterility in every pathway gene mutated to date (Table 2).
Fig. 2.
Genetic model for nuage assembly and piRNA pathway genes in mice. Many of the proteins involved in piRNA production are conserved between Drosophila and the mouse (Table 2). Overall, two major types of piRNA granules are present: the MILI and MIWI granules are exclusively localized to the cytoplasm whereas the MIWI2 granules shuttle between the nucleus and cytoplasm. Whereas the cytoplasmic granule alone can produce primary piRNA (sense orientation), both granules are necessary for the production of secondary piRNA (antisense orientation) characteristic of the ping-pong amplification cycle. Three protein domains necessary for piRNA granule assembly that recur within the pathway components are the PIWI (green), TUDOR (red), and RNA helicase domains (blue). Mutual dependencies are represented by double-headed arrows. Components with insufficient evidence to assign a position within the pathway are listed at the bottom. See Table 2 for more detailed description of orthologous components.
Table 2.
Conservation of piRNA pathway proteins and functions of piRNA pathway proteins in mice
| Drosophila | M. musculus | Mouse knockout phenotype | Knockout Reference |
|---|---|---|---|
| PIWI | PIWIL1 (MIWI) | Sterile; spermatid block | (13) |
| AUBERGINE | PIWIL2 (MIL1) | Sterile; spermatocyte block | (10, 12) |
| AGO3 | PIWIL4 (MIWI2) | Sterile; spermatocyte block | (12, 14) |
| — | GASZ | Sterile; spermatocyte block | (17) |
| MAELSTROM | MAEL | Sterile; spermatocyte block | (28) |
| VASA | DDX4 (VASA) | Sterile; spermatocyte block | (24) |
| — | DDX25 | Sterile; spermatid block | (207) |
| ARMITAGE | MOV10L1 | Sterile; spermatocyte block | (25, 26) |
| TUDOR | TDRD1 | Sterile; spermatid block | (32) |
| CG7802 | TDRD2 | — | |
| — | TDRD4 (RNF17) | Sterile; spermatid block | (33) |
| TEJAS | TDRD5 | Sterile; spermatid block | (39) |
| KRIMPER | TDRD6 | Sterile; spermatid block (miRNA pathway) | (34) |
| — | TDRD7 | Sterile; spermatid block | (40) |
| — | TDRD8 (STK31) | — | (27) |
| SPN-E | TDRD9 | Sterile; spermatocyte block | |
| — | DNMT3 | Sterile; spermatocyte block and spermatogonia loss | (30, 208) |
| HEN1/PIMET | HEN1 | — | |
| CAPSULEEN | PRMT5 | Early embryonic lethality | (209) |
| VALOIS | WDR77 | Early embryonic lethality | (210) |
| — | — | ||
| ZUCCHINI | MitoPLD | Sterile; spermatocyte block | (211, 212) |
| — | RANBP9 | — |
PIWIL, piwi-like homolog; GASZ, germ-cell ankyrin repeat, sterile α motif, and basic leucine zipper domain; DDX, DEAD (Asp-Glu-Ala-Asp) box; MOV10L1, Moloney leukemia virus 10-like 1; TDRD, tudor domain; RNF17, ring Finger 17; STK31, serine threonine kinase 31; PRMT5, protein arginine N-methyl transferase 5; WDR77, WD repeat 77. Dashes indicate Drosophila homolog and mouse knockout phenotypes for some genes that are unknown.
Although MILI, MIWI2, and MIWI define the various granules, additional proteins are required for the formation and integrity of the granules (Fig. 2). Our group showed that GASZ, which colocalizes with MILI, plays a structural role to initiate the formation of the MILI granules (17). Whereas MILI granules in embryonic/newborn male germ cells are localized to a perinuclear position, absence of GASZ leads to diffuse distribution of mouse VASA homolog (an RNA helicase) and TDRD1 (a TUDOR domain family member), destabilization (loss) of MILI, and suppression of piRNA synthesis. Consistent with these findings, GASZ knockout males are sterile and show a block at the zygote-pachytene stage of meiosis phenocopying VASA knockout (24) and MILI knockout (10, 12) mice. In addition to VASA, knockout of another nuage-associated RNA helicase, MOV10L1, also leads to sterility and a spermatocyte block (25, 26).
MIWI2 is required for secondary piRNA formation, and TDRD9 and MAEL localize to MIWI2 granules (Fig. 2). Moreover, absence of all three of these proteins leads to sterility and zygotene-pachytene spermatocyte arrest (12, 14, 27, 28). Whereas MILI granules are always cytoplasmic, MIWI2-associated proteins shuttle between the cytoplasm and nucleus. Although the functions of non-repeat-associated piRNA are unknown, repeat-associated secondary (antisense) piRNA bound to MIWI2 recognize and target mature active retrotransposon mRNA in the cytoplasm and block expression posttranscriptionally (Fig. 3) (15), potentially by inducing degradation as has been shown in Drosophila (29). Through its ability to shuttle into the nucleus associated with antisense piRNA, MIWI2 also blocks retrotransposon synthesis at the transcriptional level by an incompletely understood mechanism requiring DNA methyltransferase (DNMT) 3A and 3L. Consistent with this transcriptional function of MIWI2, knockout of DNMT3L has a similar spermatocyte block (30). Thus, DNMT3A and DNMT3L not only induce retrotransposon methylation in males but also carry out the mammalian-specific methylation of paternally imprinted genes.
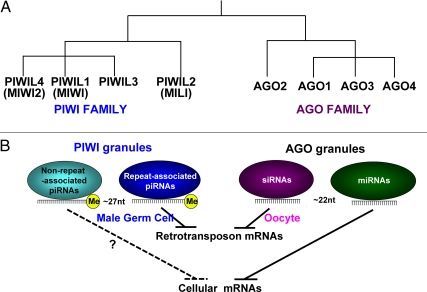
Fig. 3.
A, A phylogenic comparison of the PIWI and AGO families of Argonaute RNA endonucleases in humans. Some mammalian clades (rodents) lack PIWIL3. B, Target overlap among multiple small RNA classes. DICER-dependent AGO-associated small RNA cause the degradation or translational repression of mRNA targets with endogenous siRNA regulating retrotransposons whereas miRNA control cellular mRNA. Small RNA associated with the PIWI subfamily of Argonautes have developed convergent roles to regulate retrotransposons (repeat-associated piRNA) and possibly cellular mRNA (non-repeat-associated piRNA). Genetic studies have delineated a sex-specific division of labor among the small RNA dedicated to retrotransposon surveillance in which endo-siRNA are the major player in the female germ cells whereas the male germ cell genome stability depends upon repeat-associated piRNA. nt, Nucleotides.
Although MIWI, DDX25 (another RNA helicase), and additional TUDOR domain proteins (i.e. TDRD1, TDRD4, and TDRD6) localize to nuage and are implicated in piRNA synthesis, these mouse knockouts show a later spermatogenic block at the round spermatid stage (13, 31–34). Thus, these proteins are unlikely to function in retrotransposon control and are more likely regulating other key aspects of translational control during spermiogenesis.
The assembly of all mammalian germ granules depends upon the association between structural TUDOR domain-containing proteins (reviewed in Ref. 35). TUDOR domains are selective for symmetrical dimethylarginines, and the writer of this posttranslational mark is protein arginine methyltransferase 5 in association with its adaptor protein WD-containing region 77 (27, 36–38). For example, TDRD9 interacts directly with a region of MIWI2 containing symmetrical dimethylarginines and TDRD4, TDRD7, and TDRD8 with MIWI. Other TUDOR domain proteins, such as TDRD6, interact with both MILI and MIWI, whereas TDRD1 and TDRD2 interact with MILI, MIWI2, and MIWI. Despite the interaction of TDRD1 with all three mouse PIWI family proteins, spermatogenesis in TDRD1 knockout mice blocks at the spermatid stage (32). This suggests that TDRD1 and TDRD2 may play redundant structural roles in the male germline. TDRD5 null mice also display spermatid arrest with chromatoid body defects (39). Mutations in human and mouse TDRD7 cause lens fiber cell defects and spermatogenic arrest at the spermatid stage (40). The TDRD7 findings are especially intriguing because they draw parallels between TDRD7-positive granules in lens cells and nuage in spermatids, both cell types that are critically dependent upon stored mRNA for terminal differentiation due to absent and inactive nuclei, respectively. Only the unique function of TDRD2 and TDRD8 remain untested by genetic disruption. Lastly, symmetrical dimethylarginines on MILI1, MIWI2, and MIWI as well as VASA are critical to piRNA granule assembly in multiple organisms and the interactions of these proteins with specific TUDOR family members (37, 41–45). Because all family members mutated thus far in mice are required for male fertility, the lack of redundancy is underscored and suggests that the non-TUDOR domains must contribute to the unique role of each of these TUDOR-domain proteins in nuage functions.
siRNA, but not miRNA, Function to Regulate Oocyte Meiosis
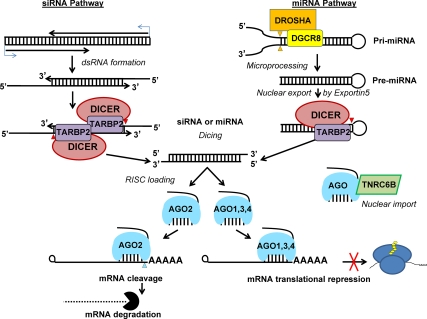
In mammals, miRNA were initially thought to function as the major DICER-dependent small RNA. However, several publications over the last year have uncovered the roles of siRNA in the female germline. Whereas miRNA are synthesized using a pathway that requires Drosha and DGCR8 in the nucleus and DICER in the cytoplasm, siRNA only require the ribonuclease (RNAse) III activity of DICER (Table 1 and Fig. 4). Mice lacking DICER die at the gastrula stage secondary to defects in embryonic stem cell development (46). Likewise, mice lacking argonaute 2 (AGO2), the RNA-induced gene-silencing complex protein that directly slices the target mRNA recognized by the miRNA, die at embryonic d 9.5 (47). To overcome these defects, several investigators have used Cre-loxP-mediated recombination to produce spatiotemporally deleted DICER and AGO2 mouse models to study the roles of small RNA in oocytes (48–52)(Table 3).
Fig. 4.
siRNA and miRNA biosynthesis. siRNA are initially transcribed from promoters within viral genomes, retrotransposons, adjacent genes with a tail-to-tail or overlapping head-to-head (data not shown) orientation of RNA promotion, or from pseudogene mRNA with antisense promotion paired to their functional gene paralogs. The resulting transcripts form a region of dsRNA. By contrast miRNA are transcribed from RNA pol II promoters intronic to other protein-encoding genes or under their own promoters and fold into a hairpin structure containing a region forming a dsRNA stem called a primary miRNA (pri-miRNA). The Microprocessor complex, composed of nuclear type III RNA endonuclease RNASEN/DROSHA and its cofactor DGCR8, excises the pre-miRNA by cleaving at the base of the pri-miRNA stem (orange triangles) and exported from the nucleus by exportin 5. Subsequently, the cytoplasmic type III RNA endonuclease DICER and cofactor TAR RNA binding protein 2 (TARBP2) cleaves the 5′-overhangs of the siRNA precursor or the loop of the pre-miRNA (red triangles). A mature siRNA or miRNA (single strand of the dsRNA) is loaded onto the Argonaute-containing effector complex, RNA-induced silencing complex. In Argonaute cocrystal structures the seed sequence is held rigidly between the PIWI and PAZ domains of the Argonaute allowing base pairing required for targeting. In mammals, AGO2 contains the conserved residues necessary for and demonstrated activity of endonucleolytic cleavage of target mRNA. After mRNA endonucleolytic cleavage, the target mRNA is decapped and exonuclease digestion occurs. The remaining Argonautes (AGO1, AGO3, and AGO4) lack the conserved endonuclease residues but may participate in the alternative outcome of miRNA action, translational repression.
Table 3.
Mouse models created to study siRNA and miRNA function
| Mutant allele | Pathway altered | Phenotype | Reference |
|---|---|---|---|
| Argonaute 2 (null) | miRNA | E9.5 lethality; embryonic defects including neural tube and cardiac defects | (47) |
| Argonaute 2 (flox) Zp3-Cre | siRNA | Female sterility; oocyte meiosis I block | (52) |
| Argonaute 2 (flox) Tnap-Cre | miRNA | Normal male fertility | (178) |
| DICER (null) | miRNA (siRNA?) | E7.5 lethality; defects in ES cells | (46) |
| DICER (hypomorph) | miRNA | Female sterility; defects in vasculature leading to ovarian corpus luteum defects | (213) |
| DICER (flox) Amhr2-Cre | miRNA | Female sterility; oviductal diverticuli and uterine implantation defects | (77–80) |
| DICER (flox) Amh-Cre | miRNA | Male sterility due to defective Sertoli cell differentiation and spermatid loss | (170–172) |
| DICER (flox) Tnap-Cre | miRNA | Male sterility due to impaired spermatogonial proliferation and possible stem cell defects | (178, 179) |
| DICER (flox) Nr5a1-Cre | miRNA | Male sterility due to germ cell apoptosis secondary to altered somatic gonadal cells | (214) |
| DICER (flox) Pitx2-Cre | miRNA | reduced GH, prolactin, and TSHβ; normal proopiomelanocortin and LHβ | (70) |
| DICER (flox) Zp3-Cre | siRNA | Sterile; disorganized spindles, defects in chromosome alignment, and a block at meiosis I | (47, 48) |
| DICER (flox) Arr2pb-Cre | miRNA | Prostate atrophy due to reduced prostatic stem cell proliferation | (192) |
| DGCR8 (flox) Zp3-Cre | miRNA | Normal fertility; confirms that miRNAs are not required in oocytes | (55) |
ES, Embryonic stem.
Initial studies to decipher the roles of DICER in oocyte biology demonstrated that the DICER deletion using zona pellucida 3 (Zp3)-Cre results in infertility (48, 49). The major defects in DICER-deficient oocytes were disorganized spindles, defects in alignment of the chromosomes, and arrest at metaphase of meiosis I. Consistent with these findings, few oocytes (∼10%) progress to meiosis II (i.e. demonstrate a first polar body) and the only embryos that are observed continue to be DICER positive (i.e. fail to show complete recombination of the floxed DICER allele). Phenotypically similar oocyte meiotic arrest and spindle and chromosome defects are observed in oocytes with AGO2 deletion (52). Thus, DICER and AGO2 functions are required for normal oocyte meiosis and female fertility.
Because DICER is required for miRNA and siRNA biosynthesis, the oocyte defects could be attributed to absence of miRNA or siRNA or both. Initial mRNA transcript analysis showed that 18.4% of oocyte-expressed mRNA were altered in their expression in the absence of DICER in one report (48), and about twice as many transcripts were altered in their expression in the other report (49). The transcripts that were up-regulated in the absence of DICER were typically those that are degraded during maturation of the oocyte. Thus, the miRNA or siRNA were not repressing transcription appropriately. One group found that these targets were relatively enriched for targets involved in meiosis and spindle function, an interesting parallel to the piRNA-dependent defects in males (53). Thus, defects in miRNA production or their degradation in mature oocytes from older women could contribute as a possible cause of maternal age-associated aneuploidy. Another interesting finding was that mRNA encoding some repetitive elements (mouse transposon including the MaLR family and SINE) were up-regulated in the absence of DICER (48); however, unlike the findings with absence of the piRNA machinery, LINE1 sequences were not increased. The mouse transposon and RLTR10 retrotransposon mRNA were also up-regulated in the absence of AGO2 (52). Surprisingly, very few of the mRNA that were increased showed a correlation with miRNA that were being lost, suggesting a unique small RNA mechanism of control of oocyte transcripts including retrotransposons. One additional surprise was that the transcripts that were altered in the absence of DICER and AGO2 did not correlate exactly, indicating that additional argonaute proteins may interact with DICER to induce mRNA cleavage.
Follow-up studies have indeed defined the small RNA mechanisms of action in mammalian oocytes. First, Tam et al. (50) and Watanabe et al. (51) discovered that siRNA are synthesized in oocytes, these siRNA are depleted in the DICER null oocytes, and specific mRNA targets of the siRNA are up-regulated in the absence of DICER. Thus, siRNA were discovered for the first time to regulate endogenous targets in a mammalian cell type (Fig. 3).
A further twist to the story came with the recent analysis of DICER activity in oocytes (54) and the knockout of the RNA-binding protein DGCR8 that works with Drosha to recognize the precursor miRNA hairpin (55). Despite the abundance of miRNA in oocytes, Ma et al. (54) showed that these small RNA were incapable of mediating mRNA cleavage or repressing translation. These findings were genetically confirmed by Suh et al. (55), who discovered that absence of DGCR8 in oocytes leads to normal oocyte maturation, fertilization, and offspring, although the number of pups per litter in the DGCR8 conditional knockout (5.5 ± 0.6 pups per litter) was reduced compared with controls (9.5 ± 0.5 pups per litter). These and additional experiments in these reports indicate that DGCR8-independent, DICER-dependent production of siRNA is required for oocyte maturation whereas miRNA and many additional DICER-derived miRNA are dispensable for oocyte function and fertility. It will be interesting to determine whether additional cell types in mammals also show a requirement for siRNA or whether the oocyte is unique in small RNA biology. In particular, because DICER null embryos are lost at E7.5(46) vs. E9.5 in AGO2 null embryos, perhaps there are additional roles of DICER-dependent, but AGO2-independent small RNA in early embryos during this interval. However, the somatic role of siRNA is not likely to extend beyond early embryogenesis because in most somatic cells the large double-stranded RNA (dsRNA) siRNA precursors are recognized by an antiviral response pathway leading to protein kinase R phosphorylation of eIF2A, arrest of cap-dependent translation of mRNA, and subsequent apoptosis. Only because germ cells specifically lack protein kinase R (56) is the formation of siRNA precursor dsRNA tolerated.
Diverse Functions of miRNA in Female Reproduction
To date, 1048 mature miRNA have been identified in humans, and 672 mature miRNA have been discovered in mice (www.miRbase.org). As shown in Fig. 4, miRNA are transcribed from a miRNA locus by RNA polymerase II to first produce the primary miRNA transcript with its characteristic stem loop structure. Some primary miRNA (pri-miRNA) are transcribed by independent promoters, whereas others are intronic to other protein-encoding transcripts. In the former case, a stem-loop structure is cleaved from the larger mRNA by the microprocessor complex (including RNASEN/DROSHA, DGCR8, and DDX17/SAM68). In the latter, the splicing machinery must be involved in the initial cleavage of the intron from the surrounding transcript. In the nucleus, this transcript is processed by Drosha, a nuclear RNase III that initiates the processing of miRNA molecules (57). The precursor miRNA is then exported from the nucleus to the cytoplasm through exportin 5(58). pre-miRNA is recognized, and its loop is removed by the action of DICER to produce the mature approximately 22-nucleotide miRNA (59). Either of these mature miRNA molecules form a complex with the RNA-induced silencing complex (60). DICER in combination with TARBP2 loads the miRNA onto one of the Argonaute family proteins (AGO1–4 in mammals; Fig. 3) which carry out the effect of cleavage of target mRNA and translational repression by binding to complementary sequences in the 3′-untranslated region (UTR) of the mRNA. Recent studies by Bartel and colleagues (61) indicate that miRNA function mainly to lower mRNA levels, and this reduction accounts for nearly all of the expected decrease in protein production.
We will not discuss the roles of miRNA in normal mammary gland development or breast cancer because this area has been extensively reviewed (62), and we will only mention the highlights for ovarian cancer because we have recently reviewed this topic in Endocrine Reviews (63). Although many miRNA expression studies may be correlative because they lack functional confirmation in vitro or in vivo, we have attempted to summarize the data into tables in this review so that the reader is at least aware of the findings for individual miRNA.
miRNA functions in the hypothalamus and pituitary
The miRNA profiles in pituitary and hypothalamus have been described elsewhere (64, 65). Expression of hypothalamic miRNA is responsive to physiological stressors such as sleep deprivation, hyperosmotic stress, and thermoregulation (66–68). Hypothalamic GnRH robustly induces miRNA changes in pituitary gonadotrope cell lines including inducing a primary miRNA containing miR-132 and miR-212 (69). Pituitary-specific deletion of DICER using Pitx2-Cre results in the reduction of GH, prolactin, and TSHβ while sparing proopiomelanocortin and LHβ, due to the loss of miR-26b stimulation of Pit-1 and GH indirectly through miRNA inhibition of Lef-1 (70). Pituitary adenomas display the loss of miRNA with a tumor-suppressive role including miR-15a and miR-16–1 that target inhibition of multiple oncogenes including B-cell lymphoma 2, myeloid cell leukemia sequence 1, cyclin D1, and wingless-type mouse mammary tumor virus integration site family, member 3A (71), let-7 family miRNA (72), and miR-21, miR-141, miR-143, miR-145, and miR-150 (73) as well as elevation of putative oncomirs miR-122 and miR-493 in ACTH-producing adenomas (74). miR-128a, miR-155, and miR-516a-3p targeting WEE1 kinase are elevated in a subset of nonfunctional pituitary adenomas and display correlated translational repression of WEE1(75). Nonfunctional pituitary adenomas may also possess alterations in miRNA predicted to inhibit TGFβ signaling (76).
Roles of miRNA in the ovary and fallopian tube
Strategies to define the global roles of miRNA in the somatic cells of the female reproductive tract have focused on using anti-Mullerian hormone 2 (Amhr2)-driven cre recombinase to delete DICER in the mesenchymal cells (77–80) (Table 3). Our studies published in Molecular Endocrinology (77) and those of others (78–80) have shown that DICER is required for female fertility. Diverticuli form in the fallopian tubes of these mice, and embryos are swept into these diverticuli, preventing transit to the uterus. Because oocyte remnants and embryos are observed at high numbers in the diverticuli, absence of DICER in the granulosa cells of the ovary must not play a major role. Consistent with these findings, four studies (77–80) independently reported that DICER cKO ovaries were observed to show normal stages of folliculogenesis and at most an approximately 50% reduction in oocytes ovulated, but normal fertilization and development. In one report (81), additional follicle defects of unknown significance were also observed. Thus, in the ovary, siRNA are functionally important in the oocyte, whereas miRNA are surprisingly dispensable for ovarian folliculogenesis. In contrast, miRNA play an essential reproductive function in the smooth muscle of the fallopian tube.
Because DICER is an important protein for female reproductive tract function (i.e. fertility) and has been implicated in nonreproductive tract cancers, multiple groups have examined DICER expression levels in ovarian cancers. DICER expression was shown to be decreased in 34 ovarian tumors (82); increased expression is not associated with significantly reduced disease-free survival in serous ovarian adenocarcinomas in one study (83), but an increase in both DICER and Drosha expression is associated with increased median survival in a more recent study (84). Low DICER expression was an independent predictor of reduced survival (84). An independent study showed that low DICER expression in serous ovarian cancers correlated with poor patient survival, global decreased miRNA levels, and decreased expression of estrogen receptor (85). Because serous adenocarcinomas are the major histological type of ovarian cancer, reduced DICER expression and a resultant global decrease in miRNA contribute to poor survival in the majority of women with ovarian cancers and suggest that miRNA must be functioning globally as tumor suppressors rather than as oncogenes.
All reproductive tissues of the female reproductive tract, except for the vagina, have been profiled for miRNA expression either in mice or humans. Although global profiling of miRNA in these tissues and various pathologies of the female reproductive tract has been performed, the functions of only a few of these miRNA in specific disease states have been determined. To identify additional novel miRNA, our group also used next-generation sequencing of miRNA from 100 human female reproductive tract tissues or cell lines and identified seven confirmed novel miRNA and 51 high-confidence miRNA (86). However, the functional roles of these miRNA are not known.
To further identify individual roles of miRNA in ovarian cancer, our group and others have used various strategies to profile miRNA expressed in serous ovarian cancer and cell lines, and the major miRNA and their putative functions in serous adenocarcinoma of the ovary are presented in Table 4. In addition, we also identified several miRNA in clear cell ovarian cancer and performed functional analysis of a few of them including miR-100, which targets mRNA-encoding proteins in the phosphatidylinositol 3-kinase pathway (87). Further details can be found in the original publications or in our Endocrine Reviews article (63). Although miRNA may play similar roles in different histotypes of ovarian cancer, the specific functions of miRNA in other histotypes such as mucinous and endometrioid have not been shown.
Table 4.
miRNA dysregulated or functional in serous ovarian cancer
| miRNA/family | Potential role | Reference |
|---|---|---|
| Let-7 family | Targets KRAS, HRAS, MYC, HMGA2; promotes tumorigenesis | (215, 216) |
| miR-9 | Targets NFKB1; down-regulated in cancer and suppresses cell growth | (217, 218) |
| miR-15a/miR-16 | Target Bmi1; reduces proliferation | (219) |
| miR-22 | Inhibits cell migration and invasion | (220) |
| miR-29b | Down-regulated and correlated with survival | (221) |
| miR-31 | Targets E2F2 and cell cycle; most down-regulated miRNA in serous cancers | (222) |
| miR-34 family | Targets cell cycle genes; loss of p53 suppresses miR-34 | (223) |
| miR-182 | Amplified in 28.9% of ovarian cancers; promotes tumor growth in vivo (i.e. putative oncomir) | (224) |
| miR-185 | Targets Six1; suppresses anchorage-independent growth and cell migration | (225) |
| miR-199a-5p | Targets IKβ; fosters protumor environment | (226) |
| miR-200 family | Represses epithelial-mesenchymal transition | (227) |
| miR-214 | Targets PTEN; overexpression promotes chemoresistance | (228) |
miRNA in normal uterine function
The normal functions of the uterus are to accept an embryo from the fallopian tube, contribute to the formation of a placenta, support the growth of an embryo, and facilitate delivery of the infant. Although little is known about the role of miRNA in the development of the uterus, some insights into the role of miRNA in these specific uterine functions are emerging. Table 5 describes miRNA that participate in these normal uterine functions.
Table 5.
Specific miRNA that play role in normal uterine function and pregnancy
| miRNA | Cell type studied | Function | Disease model | Reference |
|---|---|---|---|---|
| miR-222 | HESF | Decidualization | Normal | (72) |
| miR-29c | HESF | Decidualization; Regulates COL7A1, TFAP2C | Normal | (92) |
| miR-101a | Implantation sites | Regulates Cox2 | Normal | (97) |
| miR-199a | Implantation sites | Regulates Cox2 | Normal | (97) |
| miR-21 | Implantation sites with blastocyst | Targets RECK | Normal | (99) |
| miR-200 family | Term uterus | Targets ZEB1/ZEB2 through progesterone signaling | Normal parturition; preterm labor | (101) |
| miR-338 | Decidual cells | Regulates PLA2G4B | Normal | (102) |
| miR-155 | Trophoblast cell lines | Regulates CYR61; regulates trophoblast migration | Preeclampsia | (109) |
The endometrium is a steroid hormone-responsive tissue. The overall role of estrogen regulation of miRNA expression was reviewed recently (88). However, no study has profiled miRNA through the entire menstrual cycle in humans using both endometrial and stromal components, as has been elegantly done at the mRNA level (89). miRNA have been profiled in the mouse uterus in response to estradiol or estrogen receptor antagonist treatment (90). They have also been profiled using endometrial epithelial cells from late proliferative phase for the maximum estrogen effect and midsecretory phase for maximum progesterone effect of the human menstrual cycle to define the miRNA that may be estrogen- and progesterone-responsive in the endometrium (91).
An important function of the endometrium is decidualization, the differentiation of stromal cells in preparation for pregnancy. In vitro cultures of primary human endometrial stromal fibroblasts (HESF) offer an important system to study decidualization. Qian et al. (72) profiled miRNA in HESF after 4 d of decidualization compared with mock decidualized and found 49 differentially expressed miRNA. MiR-222 inhibition in HESF using antisense oligonucleotides resulted in an increase in endogenous CDKN1C (cyclin-dependent kinase inhibitor 1C also known as p57Kip2). Additionally, miR-222 inhibition treatment led to an increase in luciferase activity from a construct containing the miR-222-binding portion of the 3′-UTR of CDKN1C. In similar experiments, miR-29c was shown to affect endogenous gene expression of genes important in biological adhesion and extracellular matrix formation. Additionally, overexpression of miR-29c in HESF results in a blunted decidualization response (92). Thus, miR-222, miR-29c, and other miRNA are likely to play a role in endometrial decidualization, an important function of the uterine endometrium. Additionally, using in vitro cultures of primary endometrial stromal or epithelial cells from normal women, Pan et al. (93) showed that miR-20a was more highly expressed in stromal cells compared with epithelial cells whereas miR-26a was more highly expressed in epithelial cells. Additionally, miR-20a, miR-21, miR-26a, miR-17-5p, miR542-5p, miR23a, and miR-23b were affected by steroid hormone treatment of stromal and epithelial cells (93, 94). Thus, miRNA are likely regulated by steroid hormones, and miRNA may play a role in cross-talk between the uterine epithelium and stroma.
Work in the rat uterus has shown that let-7a expression is only present in luminal and glandular epithelium, and expression was higher on gestational d 6 to d 7 (95). Additionally, miR-320 is up-regulated in implantation sites and is responsive to steroid hormones (96). Work in the mouse uterus shows that miR-101a and miR-199a regulate Cox2 expression in a temporally important fashion for implantation (97). miRNA microarray profiling using mouse implantation sites suggests that miR-21 is expressed in the uterus but only when a blastocyst is present. Because reversion-inducing cysteine-rich protein with kazal motifs is a predicted target for miR-21 and is important for vascular remodeling of the placenta (98), miR-21 may play an important role in blood vessel remodeling in implantation through signals from the blastocyst (99). Additionally, implantation sites may have additional editing of miRNA as indicated by a next-generation sequencing study of delayed implantation in the mouse (100).
If miRNA play important roles in implantation, then miRNA likely play a role in pregnancy. In both mouse and human term uterus, members of the miR-200 family are highly expressed (101). Additionally, expression is increased in response to signals of preterm labor, suggesting that the miR-200 family is important in parturition. Using chorioamniotic membranes, Montenegro et al. (102) examined miRNA profiles of preterm and term membranes and found a significant number of miRNA that were down-regulated at term compared with preterm, specifically miR-338. By inhibiting expression of miR-338 in vitro using decidual cells, they showed an increased expression of PLA2G4B (phospholipase A2, group IVB), a gene implicated in parturition. Luciferase constructs containing the 3′-UTR of PLA2G4B were down-regulated when transfected with miR-338 constructs suggesting a direct effect of miR-338 on PLA2G4B expression. Additionally, DICER was decreased in the term chorioamniotic membranes. Thus, miRNA likely play a significant role in parturition although the exact function needs further study.
The placenta has an essential role in diseases of the pregnant uterus, specifically intrauterine growth restriction and preeclampsia. Preeclampsia, a perinatal disease characterized by elevated blood pressure and proteinuria, is a top cause of maternal mortality worldwide. Multiple groups have profiled miRNA using placenta from women with severe preeclampsia (103–106), and the biological function of these differentially expressed miRNA is beginning to be determined. CYR61 (cysteine-rich, protein 61), a member of the CCN (cysteine-rich 61/connective tissue growth factor/nephroblastoma overexpressed) family of secreted proteins (107), is implicated as an important angiogenic factor in early pregnancy and is significantly down-regulated in placentas from women with preeclampsia (108). Furthermore, miR-155, a miRNA that potentially targets CYR61, is significantly up-regulated in placentas from preeclamptic pregnancies. Using an in vitro culture system, Zhang et al. (109) demonstrated that forced overexpression of miR-155 decreased endogenous expression of CYR61 at both the mRNA and protein levels in a dose-responsive fashion. Experiments using luciferase constructs containing the 3′-UTR of CYR61 showed a direct and dose-responsive effect of miR-155 in trophoblast cell lines. Importantly, this forced expression of miR-155 slowed trophoblast cell migration. Thus, miR-155 may play an important role in the pathogenesis of preeclampsia, a disease thought to involve issues with placental invasion. Recently, studies have suggested that miRNA can be isolated from serum (110). Mouillet et al. (111) has shown that serum miRNA change with pregnancy demonstrated by increased levels of specific miRNA in the serum of women with fetal intrauterine growth restriction, a common clinical manifestation of severe preeclampsia. Thus, expression of miR-155 in maternal serum may become a clinical marker to suggest pathology of the placenta such as preeclampsia and/or intrauterine growth restriction.
miRNA in uterine pathology
miRNA have been implicated in uterine pathologies, including leiomyomata, endometriosis, and endometrial cancer. Thus, many studies have begun to study these diseases and the functional roles of miRNA in these uterine pathologies. Although many of these studies have been reviewed (112–117), the details of the functional nature of miRNA and their response to steroid hormones has only recently been published. Most of these studies are merely correlative or examine the effect of a particular miRNA on target gene expression. Few focus on biological function in these pathologies.
miRNA in endometriosis
Endometriosis with its characteristic location of endometrium outside the uterine cavity is considered hormone responsive. Although studies have shown that eutopic endometrium from women with endometriosis is resistant to progesterone, this progesterone resistance is most likely due to unopposed estrogen action on endometriotic tissues (89). miRNA have been profiled in different endometriotic tissue types (i.e. endometriomas, peritoneal endometriosis, ovarian endometriosis) and compared with miRNA profiles of eutopic endometrium from paired samples, nonpaired samples, or nonendometriosis control samples (92, 93, 118, 119). Additionally, miRNA have been profiled and compared from eutopic endometrium from women in the secretory phase of the menstrual cycle of women with and without endometriosis (120). Additionally, comparison of eutopic endometrium from women with either mild or severe disease revealed an increased expression of miR-21 and DICER in severe disease (121). Each of these studies of miRNA in endometriosis focused on different tissues and different control groups, and resulted in a unique set of differentially expressed miRNA (Table 6). Predicted targets of these differentially expressed miRNA revealed biologically important pathways in endometriosis (92, 118–120).
Table 6.
Comparison of miRNA profiling studies on endometriosis
| Tissue types | Menstrual cycle phase | Platform | miRBase version | Stage of disease | Down | Up | Reference |
|---|---|---|---|---|---|---|---|
| Four paired eutopic-ectopic, four ectopic | Early/mid secretory | mirVana miRNA probe set | Not listed | Stage 3 | Every miRNA down | Not listed | (93) |
| Eight paired eutopic-peritoneal ectopic | Proliferative and secretory | mirVana miRNA probe set | Not listed | ASRM II-IV | 196b, 20a, 34c, 424, 142-3p, 200b, 141, 200a | 145, 143, 99a, 99b, 126, 100, 125b, 150, 125a, 223, 194, 365, 29c, 1 | (118) |
| Four eutopic endometrium endometriosis, four eutopic non-endometriosis | Early secretory | Exiqon LNA array | miRBase 10 | Moderate-severe | 9, 9*, 34b*, 34c-5P, 34c-3p | None | (120) |
| Sixteen paired eutopic-endometrioma | Proliferative | LC Sciences | miRBase 9.1 | Moderate-severe | 106a, 106b, 130b, 132, 17-5p, 182, 183, 196b, 200a, 200b, 200c, 20a, 25, 375, 425-5p, 486, 503, 638, 663, 671, 768-3p, 768-5p, 93 | 1, 100, 101, 126, 130a, 143, 145, 148a, 150, 186, 199a, 202, 221, 28, 299-5p, 29b, 29c, 30e-3p, 30e-5p, 34a, 365, 368, 376a, 379, 411, 493-5p, 99a | (119) |
| Nine eutopic normal; 10 endometrioma | Varied | Illumina Next Generation Sequencing | miRBase 15 | Moderate-severe | 504, 141, 873, 429, 203, 449b, 200b, 375, 200c, 10a, 200a, 34c-5p | 202, 708, 193-3p, 29c, 509-3-5p, 193a-5p, 574-3p, 100, 485-3p, 720 | (92) |
Eutopic and ectopic endometrial tissues are hormonally responsive. Similar to other studies, ectopic endometriosis has increased expression of CYP19A1, StAR, and COX-2, but the miRNA predicted to target those genes that are important in estrogen synthesis were also up-regulated in ectopic endometriosis (94). Additionally, miRNA may function uniquely in endometriotic tissues from different anatomical locations. Further studies need to examine the actual function of miRNA in normal endometrium as well as different anatomical locations of endometriosis.
miRNA in endometrial cancer
Endometrial cancer is the most common gynecological malignancy (122). Multiple papers have profiled miRNA in endometrial cancer using miRNA microarrays (123–129). Globally, Boren et al. (123) showed that differentially expressed miRNA in endometrial cancers compared with normal endometrium had predicted mRNA targets that were involved in biological pathways important in cancer such as cell death and proliferation. Thus, miRNA are likely to play roles in the pathogenesis of endometrial cancer.
The most common histotype of endometrial cancer is endometrioid, with serous as the second most common. miRNA profiles are distinct between these two different subtypes, suggesting that miRNA play a role in the distinct biological characteristics of these two types of endometrial cancer (126).
Similar to other cancers, endometrial cancer contains a heterogenous group of cell types. Using miRNA profiling data obtained from mostly microdissected epithelial cells from either endometrial cancer or normal endometrium, Chung et al. (124) performed miRNA microarray analysis and determined a list of 30 differentially expressed miRNA with miR-205 being the most overexpressed in tumors. Using clinical characteristics, miR-200a and miR-205 expression correlated with advanced stage disease. MiR-205 also correlated with myometrial invasion and recurrence of disease. MiR-10a, miR-34a, and miR-95 correlated with lymph node metastasis. Using miR-205 inhibitors in an endometrial cancer cell line, an increase in junctophilin 4 protein expression, a predicted target for miR-205, was discovered (124). Junctophilin 4 is down-regulated in endometrial cancer and has been hypothesized to be a candidate tumor suppressor in endometrial cancer (130). Similar studies using formalin-fixed paraffin-embedded sections suggest that overexpression of miR-199a correlates with a longer disease-free interval and overall survival in endometrial cancer (127). Using specimens from only serous endometrial adenocarcinoma showed that lower expression of miR-10b*, miR-29b, or miR-455-5p correlated with vascular invasion. Additionally, low expression of miR-101, miR-10b*, miR-139-5p, miR-152, miR-29b, and miR-455-5p correlated with overall decreased survival. In vitro studies in an endometrial serous adenocarcinoma cell line confirmed that increased expression of miR-101 or miR-152 decreased cellular proliferation (128). Although very little is known as yet about the molecular mechanisms behind many of these miRNA, the role in clinically significant prognostic and outcome factors is apparent (Table 7).
Table 7.
miRNA that play a functional role in endometrial cancer
| miRNA | Cell type studied | Function | Reference |
|---|---|---|---|
| miR-200a, miR-205 | Endometrial cancer | Correlates with advanced stage disease | (124) |
| miR-10a, miR-34a, and miR-95 | Endometrial cancer | Correlates with lymph node status | (124) |
| miR-205 | Endometrial cancer cell lines | Regulates JPH4 | (124) |
| miR-199a | Endometrial cancer | Correlates with disease-free interval and survival | (127) |
| miR-10b*, miR-29b, or miR-455-5p | Endometrial cancer | Low with worsening vascular invasion | (128) |
| miR-101, miR-10b*, miR-139-5p, miR-152, miR-29b, and miR-455-5p | Endometrial cancer | Low with decreased survival | (128) |
| miR-101; miR-152 | Endometrial serous adenocarcinoma cell line | Proliferation | (128) |
| miR-129-2 | Endometrial cancer | Promoter hypermethylation associated with worse survival | (131) |
| miR-196a | Endometrial cell lines | Regulates ANX1 | (133) |
| miR-200c | Endometrial cancer cell lines; endometrial cell lines | Regulates ZEB1, E-cadherin; migration and invasion | (138, 139) |
| miR-200c | Endometrial cancer cell lines | Apoptosis with paclitaxol | (138, 139) |
| miR-200c | Endometrial cancer cell lines | Regulates TUBB3 | (138, 139) |
| miR-9, miR-27, miR-96, miR-153, miR-182, miR-183, and miR-186 | HEC-1B; Ishiwaka | Regulates FOXO1 | (140) |
| miR-199a, miR-101 | HeLa | Regulates COX2 | (97) |
Little is known about the regulation of miRNA, although data are emerging that histone or DNA modifications play a role as noted above. For example, loss of miR-129-2 expression is observed in endometrial cancer samples with increased SOX4, a predicted target of miR-129-2 (131). Histone acetylation and DNA demethylation in vitro lead to hypomethylation of the miR-129-2 promoter and decreased expression of SOX4. Although no direct targeting of miR-129-2 for SOX4 was shown, the hypermethylation of the miR-129-2 promoter correlated with worsening survival in endometrial cancer. Thus, the epigenetic regulation of miRNA may be important in endometrial cancer.
Endometrial cancer is one of the diseases associated with Lynch Syndrome, along with elevated risk of colorectal, gastric, ovarian, urothelial, hepatobiliary, brain, small intestine, pancreatic, and particular skin cancers due to mutations in genes involved in DNA mismatch repair. In spontaneous colon cancer, overexpression of miR-155 correlates with down-regulation of mismatch repair genes, a distinct mechanism to inhibit mismatch repair targeting a common pathway in hereditary and spontaneous colon cancer. Although these studies were not done in endometrial cancer, the authors suggest that overexpression of miR-155 may play a role in initiation or progression of spontaneous endometrial cancer through dysregulation of mismatch repair genes (132). Consistent with this prediction, multiple miRNA that are differentially expressed in endometrial cancers are predicted to target genes important in mismatch repair such as MSH2, MSH6, and MLH1 (127).Thus, miRNA may play a role in the early progression of endometrial cancer through dysregulation of the mismatch repair pathway.
Although multiple studies have shown differentially expressed miRNA in endometrial cancers, several groups are focusing on the functional roles of specific miRNA on gene and protein expression in endometrial cancer. ANXA1 (annexin A1) is an important protein in apoptosis and cellular proliferation, and ANXA1 expression is dysregulated in many cancers, including endometrial cancer. Luthra et al. (133) showed reciprocal expression of ANXA1 and miR-196a and miR-196b in endometrial cell lines. Additionally, miR-196a repressed ANXA1 protein expression in endometrial cell lines. Thus, miR-196a may play a role in endometrial cancers in addition to other cancers potentially through regulation of ANXA1.
Endometrial cancers can be divided into two pathological types. Type I endometrial cancers are low-grade, relatively nonaggressive endometrioid tumors that maintain epithelial characteristics. Type II endometrial cancers are high-grade, aggressive, nonendometrioid that have a poor prognosis. Type II endometrial cancers have lost epithelial characteristics and have gone through the epithelial to mesenchymal transition losing expression of genes such as E-cadherin and gaining expression of ZEB1 and ZEB2 (zinc finger E-box-binding homeobox 1) (134–136). MiR-200c targets ZEB1 (137). In vitro studies have shown that miR-200c expression is high in benign endometrial cells (either epithelial or stroma) with low expression of ZEB1. In aggressive malignant cells, ZEB1 expression is high with low expression of miR-200c and E-cadherin. In aggressive endometrial cancer cell lines, forced overexpression of miR-200c leads to increased E-cadherin expression, decreased ZEB1 expression, and reduced migration and invasion with no effect on cellular proliferation. Forced expression of miR-200c also increased apoptosis response to paclitaxel but not cisplatin, as well as increased cell death response to vincristine and epothilone B but, not DNA-damage chemotherapy agents. Microarray studies with forced miR-200c overexpression revealed several genes downstream of miR-200c. Direct functional studies showed that miR-200c targets the 3′-UTR of TUBB3 (Class III B-tubulin), a gene linked to resistance to microtubule-targeting agents (138, 139). Although the role of miR-200c in the pathogenesis of aggressive endometrial cancer is well characterized, it is unclear whether the loss of miR-200c directly causes the aggressiveness.
The tumor suppressor FOXO1 is down-regulated in endometrial cancer. In vitro studies showed that forced overexpression of miR-9, miR-27, miR-96, miR-153, miR-182, miR-183, and miR-186, miRNA that have decreased expression in endometrial cancer, decreased endogenous FOXO1 expression levels in endometrial cancer cell lines. Inhibition of miR-9, miR-27, miR-96, miR-153, miR-183, and miR-186 induced FOXO1 protein levels in Ishikawa cells, leading to cell cycle arrest and cell death (140). Therefore, specific miRNA acting together may play important roles in regulating tumor suppressor molecules, such as FOXO1, and thus play an integral part in the pathogenesis of endometrial cancer.
More than 50% of endometrial cancers in humans have aberrant expression of phosphatase and tensin analog (PTEN). Conditional deletion of PTEN in the mouse uterus leads to endometrial cancer by 1 month of age with subsequent up-regulation of COX-2. This up-regulation of COX-2 correlates with a down-regulation of miR-199a and miR-101a, which are predicted to target COX-2 (141). In human endometrial serous adenocarcinomas, COX-2 expression is reciprocally related to miR-101 expression (128). In vitro studies using HeLa cells have shown that miR-199a and miR-101a directly targets COX-2 mRNA (97). However, the direct roles of miR-199a and miR-101 in the pathogenesis of endometrial cancer are not known.
miRNA in uterine leiomyomata
Leiomyomata, or uterine fibroids, are the most common benign tumor in women and the most common indication for hysterectomy in the United States. Multiple studies have profiled miRNA in leiomyomata (142–145). Leiomyomata are derived from smooth muscle cells of the uterus, likely the uterine myometrium. Additionally, uterine leiomyomata, uterine leiomyosarcomas, and uterine myometrium have miRNA profiles that cluster independently (145). For yet unknown reasons, African-American women have a higher incidence of uterine leiomyomas (146). Wang et al. (142) determined that certain miRNA in leiomyomata correlate with race. Specifically, miR-23a/b, let-7, miR-145, miR-197, miR-411, and miR-412 were 2-fold overexpressed in leiomyomata from African-American women compared with Caucasians. Other significant differences in miRNA profiles were found associated with phase of the menstrual cycle and age. Additionally, smaller leiomyomata (<3 cm) had higher overexpression of members of the let-7 family compared with leiomyomata larger than 3 cm. HMGA2 (high mobility group AT-hook 2) is a gene highly dysregulated in large uterine leiomyomata, plays a role in cellular growth and transformation, and gives a unique molecular signature to leiomyomata that express HMGA2 compared with those that do not (147). HMGA2 is a predicted target of the let-7 family of miRNA. In vitro studies confirmed that forced overexpression of let-7c led to decreased levels of HMGA2 (142, 148). Interestingly, loss of the let-7 consensus binding site of HMGA2 3′-UTR correlated with stabilization of HMGA2 transcripts (149). Thus, the let-7 family of miRNA likely plays an important role in the pathogenesis of uterine leiomyomata through regulation of HMGA2 expression, although other miRNA may play a role in the clinical differences in leiomyomata between races.
Clinically, uterine leiomyomata are hormone-responsive tumors. Pan et al. (144) have shown that certain miRNA are affected by estrogen treatment of myometrial and leiomyoma smooth muscle lines. They showed that miR-21 has increased expression in leiomyomata compared with myometrium during the proliferative and secretory phase of the menstrual cycle, in response to depot medroxyprogesterone acetate therapy or combined oral contraceptive therapy, but expression of miR-21 was not changed in women on GnRH agonist analog (GnRHa). MiR-21 likely signals through the TGFβ signaling pathway (150). In vitro studies suggest that miR-21 affects endogenous predicted target gene expression of E2F1, PDCD4, PTEN, and TGFBR2 in myometrial and leiomyomata smooth muscle cell lines and spontaneously transformed leiomyomata smooth muscle cell lines, and a human leiomyosarcoma cell line as well as cellular proliferation and apoptosis in a cell line-dependent manner. Thus, miR-21 appears to be regulated by steroid hormones and affects target gene expression and cellular proliferation, but further study is needed to determine the biological function of many of these other miRNA (Table 8).
Table 8.
miRNA that play a functional role in uterine leiomyomata
| miRNA | Cell type studied | Function | Reference |
|---|---|---|---|
| miR-23a/b, miR-145, miR-197, miR-411, miR-412, let-7 | Uterine leiomyomata | Higher expression in uterine leiomyomata of black women | (142) |
| let-7 family | Uterine leiomyomata | Higher expression in uterine leiomyomata <3 cm | (142, 148) |
| let-7c, let-7 family | Uterine leiomyomata; primary leiomyoma cultures | Regulates HMG2A | (142, 148, 149) |
| miR-21 | Myometrial, smooth muscle, spontaneously transformed leiomeiomata, leiomyosarcoma cell lines | Regulates E2F1, PDCD4, PTEN, TGF-BRII; regulates proliferation and apoptosis under steroid hormone regulation | (150) |
miRNA in the cervix
Multiple groups have profiled miRNA in normal cervix, cervical cancer, and cervical cancer cell lines using miRNA microarrays (151–156), cloning (157), or real-time PCR (158, 159). Although some groups have profiled miRNA in the cervix after term labor and delivery (160), most work has focused on cervical cancer and its precursor lesion, cervical intraepithelial neoplasia. The global profiling of miRNA using a cloning approach has identified some novel miRNA in cervical cancer cell lines and tissues (157).
In women, cervical cancer is the second most common cause of cancer death worldwide (161). Although human papillomavirus (HPV) plays a significant role in the pathogenesis of cervical cancer (162) and its precursor lesions are easily identified and treated, efforts to eradicate this disease are largely hampered by economic feasibility in resource-poor countries. Biologically, the pathogenesis of cervical cancer is mediated through the oncogenes E6 and E7 and integration of high-risk HPV subtypes into the genome. Although HPV subtyping has stratified cervical cancer screening, HPV infection alone is not sufficient to induce cancer (163). Recent studies have focused on the role of miRNA in the pathogenesis of this disease as a means to determine which lesions will progress from precancerous lesions to cancer and as a means of early less invasive testing.
Although several different cell lines exist, the cervical cancer line, HeLa, is one of the most popular human immortalized cell lines. These cells have been used extensively to study the function of miRNA with or without correlation to tissue expression in cervical cancer. Table 9 details the functional studies of miRNA in cervical cancer. A majority of these miRNA affect proliferation or tumorigenesis. Additionally, expression of some miRNA (e.g. miR-9, miR-200a) correlates with prognostic factors. Thus, specific miRNA play important roles in the pathogenesis and disease progression of cervical cancer.
Table 9.
Functional miRNA in cervical cancer including precancerous lesions
| miRNA | Cell type studied | Function | Reference |
|---|---|---|---|
| miR-199a | Me-180, SiHa | Proliferation and cisplatinum sensitivity | (159) |
| miR-218 | Cervical cancer cell lines (HPV integrated) | Regulates of LAMB3 | (155) |
| miR-124 | Cervical cancer cell lines | Proliferation and migration | (229) |
| miR-124 | HeLa | Regulates of IGFBP7 | (229) |
| miR-124 | Cervical intraepithelial neoplasia (CIN) | Hypermethylation associated with higher grade lesion | (229) |
| miR-34a | HeLa | Regulates NOTCH1, JAGGED1; cellular invasion | (230) |
| miR-519 | HeLa | Tumorigenesis in nude mice | (231) |
| miR-214 | HeLa | Regulates MEK, JNK1; cellular proliferation | (232) |
| miR-21 | HeLa | Regulates PDCD4; cellular proliferation | (233) |
| miR-143, miR-145, miR-146 | HeLa | Proliferation | (151) |
| miR-122 | HeLa | Proliferation and apoptosis | (234) |
| miR-127, miR-150, miR-185 | HeLa and HuVec | Regulates P2 × 7 | (235) |
| miR-9, miR-200a | Cervical cancer | Important for cancer metastasis | (158) |
Functions of miRNA in the Male Reproductive Tract
The evaluation of male infertility often does not lead to identification of the mechanistic defect. However, there is diagnostic and therapeutic value in distinguishing patients with comorbid conditions vs. those with isolated male infertility when counseling patients about the risks of intracytoplasmic sperm injection or other in vitro fertilization treatment (164). As discussed above, piRNA pathways are important in the function of the male germ line, but miRNAs may prove equally important to the male reproductive tract as well. Androgen-dependent effects are significant drivers of male tract biology, and miRNA are known to be important targets of nuclear hormone receptors including the androgen receptor (165).
Testicular miRNA
Due to the nuclear compaction of what will become the sperm head inherent to spermatid differentiation, translational control during spermiogenesis must take a central role in protein metabolism. Because miRNA could play a central role in spermatid translation control, several groups have characterized the expression of miRNA in the mouse testis (166–168) with special emphasis on X-linked miRNA (167, 169) and those expressed by Sertoli cells (170–172). However, few specific targets of testis-expressed miRNA [ex. miR-122a targeting of protamine 1 mRNA (173)] have been demonstrated by in vitro or in vivo studies. Similar to the mouse, miRNA are present in nonhuman (174) and human (175) primate testes, and there are primate-specific X-linked miRNA (176). Although many miRNA are conserved across multiple phyla, species-specific miRNA are known, many of which display a testis- or germ cell-specific expression pattern, suggesting that there is some reproductive advantage to the development of new miRNA. Yan and colleagues (167, 169) have described a number of species-specific miRNAs on the mouse and human X chromosome, some of which appear to be clustered with intergenic distances similar to the miRNA within the miR-17–92 cluster. Reproductive processes including sperm-egg interactions are known to be rapidly evolving to confer prezygotic barriers to interspecies fertilization. These species-specific miRNA have the potential to target mRNA that modify important reproductive targets especially in the testis. Evidence for a miRNA association with infertility rests on the observation that azoospermic testes display miRNA profile alteration (177). Therefore it will be interesting to determine whether deletions on the X chromosome of men for these human-specific miRNA clusters are associated with infertility.
Testis-specific DICER conditional knockout reveals miRNA roles in proliferation of gonocytes and spermatogonia (178, 179) and Sertoli cell function (171, 172)(Table 2). Lastly, miRNA have also proposed roles in seminomas and carcinoma in situ (180, 181).
miRNA in the epididymis
A number of protein-encoding genes have been implicated in the epididymal maturation of sperm, but the possible role of miRNA mutations as a potential cause of male factor infertility is undefined. A few studies have begun to characterize the expression and possible role of miRNA in the human epididymis. In the first, the miRNA-ome of newborn, adult, and aged human epididymides was described. Similar to the testis, a few robustly-expressed miRNA predominated in the early period (including let-7a, b, c, d, f, miR-125a, miR-125b, miR-143, miR-23b, miR-26a, and miR-347) that were supplanted by a diverse group of less abundant miRNA in the adult has about 40% of the newborn number (182). Only five human epididymal miRNA were significantly associated with aging (hsa-miR-222, hsa-miR-221, hsa-miR-29c, hsa-miR-193b, and hsa-miR-374). In the second, a primate-specific cluster of miRNA on human Xq27.3 resulting from tandem duplications (hsa-mir-890, hsa-mir-888, hsa-mir-892a, hsa-mir-892b, hsa-mir-891b, and hsa-mir-891a) were shown to have robust epididymal expression with predicted targets including sperm-associated antigens 1, 6, and 8 (SPAG1, SPAG6, and SPAG8) as well as transmembrane epididymal protein 1 (183). If essential for sperm maturation, mutations in this cluster of primate-specific miRNA could prove important for understanding normospermic nonobstructive male infertility.
Prostate cancer and the miRNA interface
The majority of studies of miRNA have been performed in prostate cancer cell lines in vitro, and little is known of the role of miRNA in prostate development (184). miRNA have been implicated in various stages of prostate cancer development including the development of androgen independence [e.g. miR-21(185)], perineural invasion, and metastasis. Similar to ovarian cancer, miRNA abundance is reduced in prostatic tumors vs. benign samples (186). Comparison of isotypic tumor-adjacent normal tissue pairs has identified some differentially expressed miRNA that are correlated with cancer recurrence (down-regulated: miR-16, miR-31, miR-125b, miR-145, miR-149, miR-181b, miR-184, miR-205, miR-221, miR-222; upregulated: miR-96, miR-182, miR-182*, miR-183, miR-375) (187).
Two studies demonstrated that specific miRNAs are differentially regulated by androgens, including repression of miR-92 and miR-106a and stimulation of miR-125b, miR-16, miR-21, miR-30c, and miR-100 (188, 189). MiR-331-3p blocks androgen receptor signaling and ERBB2 expression (190). The overall importance of androgen regulation to prostatic miRNA is borne out by knockdown of DICER in prostate cancer cell lines that induced androgen-insensitive behavior (191). Targeted deletion of DICER in the mouse prostate in vivo using a composite rat probasin (Arr2pb) promoter-driven Cre results in hypoplastic prostates that display decreased proliferation potentially implicating miRNA as critical regulators of prostate cancer stem cell renewal (192). MiR-125b expression provides cells with the capacity to survive in the absence of androgens (193). miR-221 and miR-222 are elevated in androgen-independent vs. dependent cell lines (194) and promote tumor growth in xenograft models (195).
Translational Applications And Future Directions
Therapeutic opportunities with small RNA
Small RNA harbor exceptional promise as therapeutic agents for human diseases. Although in vitro studies are promising for miRNA as therapy, in vivo studies are hampered by delivery system limitations and instability of these unique small molecules. Each miRNA as therapy offers the unique ability to target any gene, even genes that are not currently affected by any drugs, small molecule inhibitors, or antibody-directed therapies. Because miRNA target many genes, miRNA as therapy offer the ability to affect many genes in independent signaling pathways. Recently, anti-miR-182 was shown to target liver metastasis in melanoma in a mouse model (196). Additionally, using an adeno-associated virus expression system, miR-26a expression decreases proliferation and tumor burden of hepatocellular carcinoma in a mouse model system (197). Creative delivery systems and studies of reproductive tract diseases are still needed for miRNA as therapy.
Whereas miRNA therapies are limited to preclinical studies, RNA inferference therapy using a nanoparticle delivery system has been done in a Phase I clinical trial with significant success of targeting and limited toxicity (198). The limitations of RNA interference therapy were recently reviewed (199). Important studies in ovarian cancer show promise for gene modulation using liposomal EphA2-siRNA delivery in mouse models (200, 201). Thus, small RNA therapies are likely to be a large part of personalized medicine in the future.
Potential clinical applications of miRNA profiling
miRNA implicated in prostate, breast, and ovarian cancers can be detected in serum (202) and in tumor specimens (203) and thus may be useful as tumor markers (204, 205). Forensic work requires samples to withstand extremes of the environment including time while still giving adequate information to provide evidence. miRNA, due to their small nature, have been hypothesized to be useful for this role. Recently, miRNA profiling of five human secretions was undertaken to determine a miRNA profile for each of the secretions (i.e. semen, vaginal, saliva, whole blood, or menstrual blood). They determined that miRNA were not degraded in semen or blood samples stored for 1 yr. Additionally, the authors were able to determine an miRNA marker for semen and blood but not for the other three body fluids (206).
miRNA variation and its potential functional significance in reproduction
Whereas the focus of this review has been on the expression and potential role of miRNA, several mechanisms exist for cells to antagonize the effects of miRNA including miRNA editing, miRNA uridylation, and RNA helicases. Several proteins involved in these antagonist mechanisms display intriguing expression patterns in reproductive tissues and may be worthwhile candidates to evaluate as possible contributors to reproductive disease. These types of variation may also represent impediments to the therapeutic success of miRNA-based therapies unless their blockade can be incorporated into the therapeutic design.
Acknowledgments
We thank Shirley Baker for expert and tireless help with manuscript formatting.
Current address for G.M.B.: Gamete Biology Group, Laboratory of Reproduction and Developmental Toxicology, National Institute of Environmental Health Sciences, National Institutes of Health (NIH), Research Triangle Park, North Carolina 27709.
Disclosure Summary: The authors have nothing to disclose.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)/NIH through cooperative agreement U54HD0077495 (to S.M.H., M.M.M.) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research (to M.M.M.), National Cancer Institute/NIH R01CA060651 (to M.M.M.), U01HD060496 as part of the Cooperative Program in Male Contraception (to M.M.M.), and R01HD033438 (to M.M.M.); the Women's Reproductive Health Research Program 5K12HD050128 (to S.M.H.), the Herman L. and LeNan Gardner Research Fund in Obstetrics and Gynecology (to S.M.H.); P50-83639 as part of the M.D. Anderson Cancer Center Spore in Ovarian Cancer Career Development Award (to S.M.H.), the Mary Kay Ash Charitable Foundation Innovative/Translational Cancer Research Grant (to M.M.M.), and NICHD/NIH 5T32HD007165 (to G.M.B.).
Footnotes
- AGO2
- Argonaute 2
- ANXA1
- annexin A1
- CYR61
- cysteine-rich, protein 61
- DNMT
- DNA methyltransferase
- dsRNA
- double-stranded RNA
- HMGA2
- high mobility group AT-hook 2
- HPV
- human papillomavirus
- miRNA
- microRNA
- piRNA
- PIWI-interacting RNA
- PIWI
- P-element-induced wimpy testis
- pri-miRNA
- primary miRNA
- PTEN
- phosphatase and tensin analog
- RNase
- ribonuclease
- siRNA
- small interfering RNA
- SPAG
- sperm-associated antigens
- TDRD
- Tudor domain
- UTR
- untranslated region.
References
- 1. Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. 1998. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 391:806–811 [DOI] [PubMed] [Google Scholar]
- 2. Khurana JS, Theurkauf W. 2010. piRNAs, transposon silencing, and Drosophila germline development. J Cell Biol 191:905–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Girard A, Sachidanandam R, Hannon GJ, Carmell MA. 2006. A germline-specific class of small RNAs binds mammalian Piwi proteins. Nature 442:199–202 [DOI] [PubMed] [Google Scholar]
- 4. Lau NC, Seto AG, Kim J, Kuramochi-Miyagawa S, Nakano T, Bartel DP, Kingston RE. 2006. Characterization of the piRNA complex from rat testes. Science 313:363–367 [DOI] [PubMed] [Google Scholar]
- 5. Aravin A, Gaidatzis D, Pfeffer S, Lagos-Quintana M, Landgraf P, Iovino N, Morris P, Brownstein MJ, Kuramochi-Miyagawa S, Nakano T, Chien M, Russo JJ, Ju J, Sheridan R, Sander C, Zavolan M, Tuschl T. 2006. A novel class of small RNAs bind to MILI protein in mouse testes. Nature 442:203–207 [DOI] [PubMed] [Google Scholar]
- 6. Grivna ST, Beyret E, Wang Z, Lin H. 2006. A novel class of small RNAs in mouse spermatogenic cells. Genes Dev 20:1709–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Watanabe T, Takeda A, Tsukiyama T, Mise K, Okuno T, Sasaki H, Minami N, Imai H. 2006. Identification and characterization of two novel classes of small RNAs in the mouse germline: retrotransposon-derived siRNAs in oocytes and germline small RNAs in testes. Genes Dev 20:1732–1743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Couvillion MT, Lee SR, Hogstad B, Malone CD, Tonkin LA, Sachidanandam R, Hannon GJ, Collins K. 2009. Sequence, biogenesis, and function of diverse small RNA classes bound to the Piwi family proteins of Tetrahymena thermophila. Genes Dev 23:2016–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bouhouche K, Gout JF, Kapusta A, Betermier M, Meyer E. 7 January 2011. Functional specialization of Piwi proteins in Paramecium tetraurelia from post-transcriptional gene silencing to genome remodelling. Nucleic Acids Res 10.1093/nar/gkq1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kuramochi-Miyagawa S, Kimura T, Ijiri TW, Isobe T, Asada N, Fujita Y, Ikawa M, Iwai N, Okabe M, Deng W, Lin H, Matsuda Y, Nakano T. 2004. Mili, a mammalian member of piwi family gene, is essential for spermatogenesis. Development 131:839–849 [DOI] [PubMed] [Google Scholar]
- 11. Kuramochi-Miyagawa S, Kimura T, Yomogida K, Kuroiwa A, Tadokoro Y, Fujita Y, Sato M, Matsuda Y, Nakano T. 2001. Two mouse piwi-related genes: miwi and mili. Mech Dev 108:121–133 [DOI] [PubMed] [Google Scholar]
- 12. Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Totoki Y, Toyoda A, Ikawa M, Asada N, Kojima K, Yamaguchi Y, Ijiri TW, Hata K, Li E, Matsuda Y, Kimura T, Okabe M, Sakaki Y, Sasaki H, Nakano T. 2008. DNA methylation of retrotransposon genes is regulated by Piwi family members MILI and MIWI2 in murine fetal testes. Genes Dev 22:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Deng W, Lin H. 2002. miwi, a murine homolog of piwi, encodes a cytoplasmic protein essential for spermatogenesis. Dev Cell 2:819–830 [DOI] [PubMed] [Google Scholar]
- 14. Carmell MA, Girard A, van de Kant HJ, Bourc'his D, Bestor TH, de Rooij DG, Hannon GJ. 2007. MIWI2 is essential for spermatogenesis and repression of transposons in the mouse male germline. Dev Cell 12:503–514 [DOI] [PubMed] [Google Scholar]
- 15. Aravin AA, Sachidanandam R, Girard A, Fejes-Toth K, Hannon GJ. 2007. Developmentally regulated piRNA clusters implicate MILI in transposon control. Science 316:744–747 [DOI] [PubMed] [Google Scholar]
- 16. Aravin AA, Bourc'his D. 2008. Small RNA guides for de novo DNA methylation in mammalian germ cells.[comment]. Genes Dev 22:970–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma L, Buchold GM, Greenbaum MP, Roy A, Burns KH, Zhu H, Han DY, Harris RA, Coarfa C, Gunaratne PH, Yan W, Matzuk MM. 2009. GASZ is essential for male meiosis and suppression of retrotransposon expression in the male germline. PLoS Genet 5:e1000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kirino Y, Mourelatos Z. 2007. The mouse homolog of HEN1 is a potential methylase for Piwi-interacting RNAs. RNA 13:1397–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Saito K, Sakaguchi Y, Suzuki T, Suzuki T, Siomi H, Siomi MC. 2007. Pimet, the Drosophila homolog of HEN1, mediates 2′-O-methylation of Piwi-interacting RNAs at their 3′ ends. Genes Dev 21:1603–1608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kamminga LM, Luteijn MJ, den Broeder MJ, Redl S, Kaaij LJ, Roovers EF, Ladurner P, Berezikov E, Ketting RF. 2010. Hen1 is required for oocyte development and piRNA stability in zebrafish. EMBO J 29:3688–3700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simon B, Kirkpatrick JP, Eckhardt S, Reuter M, Rocha EA, Andrade-Navarro MA, Sehr P, Pillai RS, Carlomagno T. 2011. Recognition of 2′-O-methylated 3′-end of piRNA by the PAZ domain of a Piwi protein. Structure 19:172–180 [DOI] [PubMed] [Google Scholar]
- 22. Tian Y, Simanshu DK, Ma JB, Patel DJ. 2011. Structural basis for piRNA 2′-O-methylated 3′-end recognition by Piwi PAZ (Piwi/Argonaute/Zwille) domains. Proc Natl Acad Sci USA 108:903–910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Eddy EM. 1970. Cytochemical observations on the chromatoid body of the male germ cells. Biol Reprod 2:114–128 [DOI] [PubMed] [Google Scholar]
- 24. Tanaka SS, Toyooka Y, Akasu R, Katoh-Fukui Y, Nakahara Y, Suzuki R, Yokoyama M, Noce T. 2000. The mouse homolog of Drosophila Vasa is required for the development of male germ cells. Genes Dev 14:841–853 [PMC free article] [PubMed] [Google Scholar]
- 25. Frost RJ, Hamra FK, Richardson JA, Qi X, Bassel-Duby R, Olson EN. 2010. MOV10L1 is necessary for protection of spermatocytes against retrotransposons by Piwi-interacting RNAs. Proc Natl Acad Sci USA 107:11847–11852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zheng K, Xiol J, Reuter M, Eckardt S, Leu NA, McLaughlin KJ, Stark A, Sachidanandam R, Pillai RS, Wang PJ. 2010. Mouse MOV10L1 associates with Piwi proteins and is an essential component of the Piwi-interacting RNA (piRNA) pathway. Proc Natl Acad Sci USA 107:11841–11846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, Noce T, Kuramochi-Miyagawa S, Nakano T, Sasaki H, Pillai RS, Nakatsuji N, Chuma S. 2009. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell 17:775–787 [DOI] [PubMed] [Google Scholar]
- 28. Soper SF, van der Heijden GW, Hardiman TC, Goodheart M, Martin SL, de Boer P, Bortvin A. 2008. Mouse maelstrom, a component of nuage, is essential for spermatogenesis and transposon repression in meiosis. Dev Cell 15:285–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gunawardane LS, Saito K, Nishida KM, Miyoshi K, Kawamura Y, Nagami T, Siomi H, Siomi MC. 2007. A slicer-mediated mechanism for repeat-associated siRNA 5′ end formation in Drosophila. Science 315:1587–1590 [DOI] [PubMed] [Google Scholar]
- 30. Bourc'his D, Bestor TH. 2004. Meiotic catastrophe and retrotransposon reactivation in male germ cells lacking Dnmt3L. Nature 431:96–99 [DOI] [PubMed] [Google Scholar]
- 31. Tsai-Morris CH, Sheng Y, Gutti RK, Tang PZ, Dufau ML. 2010. Gonadotropin-regulated testicular RNA helicase (GRTH/DDX25): a multifunctional protein essential for spermatogenesis. J Androl 31:45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chuma S, Hosokawa M, Kitamura K, Kasai S, Fujioka M, Hiyoshi M, Takamune K, Noce T, Nakatsuji N. 2006. Tdrd1/Mtr-1, a tudor-related gene, is essential for male germ-cell differentiation and nuage/germinal granule formation in mice. Proc Natl Acad Sci USA 103:15894–15899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pan J, Goodheart M, Chuma S, Nakatsuji N, Page DC, Wang PJ. 2005. RNF17, a component of the mammalian germ cell nuage, is essential for spermiogenesis. Development 132:4029–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vasileva A, Tiedau D, Firooznia A, Müller-Reichert T, Jessberger R. 2009. Tdrd6 is required for spermiogenesis, chromatoid body architecture, and regulation of miRNA expression. Curr Biol 19:630–639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Siomi MC, Mannen T, Siomi H. 2010. How does the royal family of Tudor rule the PIWI-interacting RNA pathway? Genes Dev 24:636–646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Reuter M, Chuma S, Tanaka T, Franz T, Stark A, Pillai R. 2009. Loss of the Mili-interacting Tudor domain-containing protein-1 activates transposons and alters the Mili-associated small RNA profile. Nat Struct Mol Biol 16:639–646 [DOI] [PubMed] [Google Scholar]
- 37. Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. 2009. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev 23:1749–1762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, Pawson T. 2009. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci USA 106:20336–20341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Yabuta Y, Ohta H, Abe T, Kurimoto K, Chuma S, Saitou M. 2011. TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J Cell Biol 192:781–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL. 2011. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science 331:1571–1576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Anne J, Ollo R, Ephrussi A, Mechler BM. 2007. Arginine methyltransferase Capsuleen is essential for methylation of spliceosomal Sm proteins and germ cell formation in Drosophila. Development 134:137–146 [DOI] [PubMed] [Google Scholar]
- 42. Côté J, Richard S. 2005. Tudor domains bind symmetrical dimethylated arginines. J Biol Chem 280:28476–28483 [DOI] [PubMed] [Google Scholar]
- 43. Kirino Y, Kim N, de Planell-Saguer M, Khandros E, Chiorean S, Klein PS, Rigoutsos I, Jongens TA, Mourelatos Z. 2009. Arginine methylation of Piwi proteins catalysed by dPRMT5 is required for Ago3 and Aub stability. Nat Cell Biol 11:652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kirino Y, Vourekas A, Kim N, de Lima Alves F, Rappsilber J, Klein PS, Jongens TA, Mourelatos Z. 2010. Arginine methylation of vasa protein is conserved across phyla. J Biol Chem 285:8148–8154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kuramochi-Miyagawa S, Watanabe T, Gotoh K, Takamatsu K, Chuma S, Kojima-Kita K, Shiromoto Y, Asada N, Toyoda A, Fujiyama A, Totoki Y, Shibata T, Kimura T, Nakatsuji N, Noce T, Sasaki H, Nakano T. 2010. MVH in piRNA processing and gene silencing of retrotransposons. Genes Dev 24:887–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. 2003. Dicer is essential for mouse development. Nat Genet 35:215–217 [DOI] [PubMed] [Google Scholar]
- 47. Liu H, Dibling B, Spike B, Dirlam A, Macleod K. 2004. New roles for the RB tumor suppressor protein. Curr Opin Genet Dev 14:55–64 [DOI] [PubMed] [Google Scholar]
- 48. Murchison EP, Stein P, Xuan Z, Pan H, Zhang MQ, Schultz RM, Hannon GJ. 2007. Critical roles for Dicer in the female germline. Genes Dev 21:682–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Tang F, Kaneda M, O'Carroll D, Hajkova P, Barton SC, Sun YA, Lee C, Tarakhovsky A, Lao K, Surani MA. 2007. Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21:644–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Tam OH, Aravin AA, Stein P, Girard A, Murchison EP, Cheloufi S, Hodges E, Anger M, Sachidanandam R, Schultz RM, Hannon GJ. 2008. Pseudogene-derived small interfering RNAs regulate gene expression in mouse oocytes. Nature 453:534–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Watanabe T, Totoki Y, Toyoda A, Kaneda M, Kuramochi-Miyagawa S, Obata Y, Chiba H, Kohara Y, Kono T, Nakano T, Surani MA, Sakaki Y, Sasaki H. 2008. Endogenous siRNAs from naturally formed dsRNAs regulate transcripts in mouse oocytes. Nature 453:539–543 [DOI] [PubMed] [Google Scholar]
- 52. Kaneda M, Tang F, O'Carroll D, Lao K, Surani MA. 2009. Essential role for Argonaute2 protein in mouse oogenesis. Epigenetics Chromatin 2:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Liu HC, Tang Y, He Z, Rosenwaks Z. 2010. Dicer is a key player in oocyte maturation. J Assist Reprod Genet 27:571–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ma J, Flemr M, Stein P, Berninger P, Malik R, Zavolan M, Svoboda P, Schultz RM. 2010. MicroRNA activity is suppressed in mouse oocytes. Curr Biol 20:265–270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suh N, Baehner L, Moltzahn F, Melton C, Shenoy A, Chen J, Blelloch R. 2010. MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr Biol 20:271–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Dejucq N, Chousterman S, Jégou B. 1997. The testicular antiviral defense system: localization, expression, and regulation of 2′5′ oligoadenylate synthetase, double-stranded RNA-activated protein kinase, and Mx proteins in the rat seminiferous tubule. J Cell Biol 139:865–873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Rådmark O, Kim S, Kim VN. 2003. The nuclear RNase III Drosha initiates microRNA processing. Nature 425:415–419 [DOI] [PubMed] [Google Scholar]
- 58. Lund E, Güttinger S, Calado A, Dahlberg JE, Kutay U. 2004. Nuclear export of microRNA precursors. Science 303:95–98 [DOI] [PubMed] [Google Scholar]
- 59. Lee Y, Jeon K, Lee JT, Kim S, Kim VN. 2002. MicroRNA maturation: stepwise processing and subcellular localization. EMBO J 21:4663–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. 2004. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell 117:69–81 [DOI] [PubMed] [Google Scholar]
- 61. Guo H, Ingolia NT, Weissman JS, Bartel DP. 2010. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature 466:835–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Greene SB, Herschkowitz JI, Rosen JM. 2010. Small players with big roles: microRNAs as targets to inhibit breast cancer progression. Current drug targets 11:1059–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Edson MA, Nagaraja AK, Matzuk MM. 2009. The mammalian ovary from genesis to revelation. Endocr Rev 30:624–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bak M, Silahtaroglu A, Moller M, Christensen M, Rath MF, Skryabin B, Tommerup N, Kauppinen S. 2008. MicroRNA expression in the adult mouse central nervous system. RNA 14:432–444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Olsen L, Klausen M, Helboe L, Nielsen FC, Werge T. 2009. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS One 4:e7225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Lee HJ, Palkovits M, Young WS., III 2006. miR-7b, a microRNA up-regulated in the hypothalamus after chronic hyperosmolar stimulation, inhibits Fos translation. Proc Natl Acad Sci USA 103:15669–15674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Davis CJ, Bohnet SG, Meyerson JM, Krueger JM. 2007. Sleep loss changes microRNA levels in the brain: a possible mechanism for state-dependent translational regulation. Neurosci Lett 422:68–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kisliouk T, Yosefi S, Meiri N. 2011. MiR-138 inhibits EZH2 methyltransferase expression and methylation of histone H3 at lysine 27, and affects thermotolerance acquisition. Eur J Neurosci 33:224–235 [DOI] [PubMed] [Google Scholar]
- 69. Yuen T, Ruf F, Chu T, Sealfon SC. 2009. Microtranscriptome regulation by gonadotropin-releasing hormone. Mol Cell Endocrinol 302:12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Zhang Z, Florez S, Gutierrez-Hartmann A, Martin JF, Amendt BA. 2010. MicroRNAs regulate pituitary development, and microRNA 26b specifically targets lymphoid enhancer factor 1 (Lef-1), which modulates pituitary transcription factor 1 (Pit-1) expression. J Biol Chem 285:34718–34728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bottoni A, Piccin D, Tagliati F, Luchin A, Zatelli MC, degli Uberti EC. 2005. miR-15a and miR-16-1 down-regulation in pituitary adenomas. J Cell Physiol 204:280–285 [DOI] [PubMed] [Google Scholar]
- 72. Qian K, Hu L, Chen H, Li H, Liu N, Li Y, Ai J, Zhu G, Tang Z, Zhang H. 2009. Hsa-miR-222 is involved in differentiation of endometrial stromal cells in vitro. Endocrinology 150:4734–4743 [DOI] [PubMed] [Google Scholar]
- 73. Amaral FC, Torres N, Saggioro F, Neder L, Machado HR, Silva WA, Jr, Moreira AC, Castro M. 2009. MicroRNAs differentially expressed in ACTH-secreting pituitary tumors. J Clin Endocrinol Metab 94:320–323 [DOI] [PubMed] [Google Scholar]
- 74. Stilling G, Sun Z, Zhang S, Jin L, Righi A, Kovacs G, Korbonits M, Scheithauer BW, Kovacs K, Lloyd RV. 2010. MicroRNA expression in ACTH-producing pituitary tumors: up-regulation of microRNA-122 and -493 in pituitary carcinomas. Endocrine 38:67–75 [DOI] [PubMed] [Google Scholar]
- 75. Butz H, Likó I, Czirják S, Igaz P, Khan MM, Zivkovic V, Bálint K, Korbonits M, Rácz K, Patócs A. 2010. Down-regulation of Wee1 kinase by a specific subset of microRNA in human sporadic pituitary adenomas. J Clin Endocrinol Metab 95:E181–E191 [DOI] [PubMed] [Google Scholar]
- 76. Butz H, Liko I, Czirjak S, Igaz P, Korbonits M, Racz K, Patocs A. 9 November 2010. MicroRNA profile indicates downregulation of the TGFβ pathway in sporadic non-functioning pituitary adenomas. Pituitary 10.1007/s11102-010-0268-x [DOI] [PubMed] [Google Scholar]
- 77. Nagaraja AK, Andreu-Vieyra C, Franco HL, Ma L, Chen R, Han DY, Zhu H, Agno JE, Gunaratne PH, DeMayo FJ, Matzuk MM. 2008. Deletion of DICER in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22:2336–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hong X, Luense LJ, McGinnis LK, Nothnick WB, Christenson LK. 2008. Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207–6212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gonzalez G, Behringer RR. 2009. Dicer is required for female reproductive tract development and fertility in the mouse. Mol Reprod Dev 76:678–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Pastorelli LM, Wells S, Fray M, Smith A, Hough T, Harfe BD, McManus MT, Smith L, Woolf AS, Cheeseman M, Greenfield A. 2009. Genetic analyses reveal a requirement for Dicer1 in the mouse urogenital tract. Mamm Genome 20:140–151 [DOI] [PubMed] [Google Scholar]
- 81. Lei L, Jin S, Gonzalez G, Behringer RR, Woodruff TK. 2010. The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Pampalakis G, Diamandis EP, Katsaros D, Sotiropoulou G. 2010. Down-regulation of dicer expression in ovarian cancer tissues. Clin Biochem 43:324–327 [DOI] [PubMed] [Google Scholar]
- 83. Flavin RJ, Smyth PC, Finn SP, Laios A, O'Toole SA, Barrett C, Ring M, Denning KM, Li J, Aherne ST, Aziz NA, Alhadi A, Sheppard BL, Loda M, Martin C, Sheils OM, O'Leary JJ. 2008. Altered eIF6 and Dicer expression is associated with clinicopathological features in ovarian serous carcinoma patients. Mod Pathol 21:676–684 [DOI] [PubMed] [Google Scholar]
- 84. Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, Deavers MT, Mourad-Zeidan A, Wang H, Mueller P, Lenburg ME, Gray JW, Mok S, Birrer MJ, Lopez-Berestein G, Coleman RL, Bar-Eli M, Sood AK. 2008. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med 359:2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Faggad A, Budczies J, Tchernitsa O, Darb-Esfahani S, Sehouli J, Müller BM, Wirtz R, Chekerov R, Weichert W, Sinn B, Mucha C, Elwali NE, Schäfer R, Dietel M, Denkert C. 2010. Prognostic significance of Dicer expression in ovarian cancer-link to global microRNA changes and oestrogen receptor expression. J Pathol 220:382–391 [DOI] [PubMed] [Google Scholar]
- 86. Creighton CJ, Benham AL, Zhu H, Khan MF, Reid JG, Nagaraja AK, Fountain MD, Dziadek O, Han D, Ma L, Kim J, Hawkins SM, Anderson ML, Matzuk MM, Gunaratne PH. 2010. Discovery of novel microRNAs in female reproductive tract using next generation sequencing. PLoS One 5:e9637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Nagaraja AK, Creighton CJ, Yu Z, Zhu H, Gunaratne PH, Reid JG, Olokpa E, Itamochi H, Ueno NT, Hawkins SM, Anderson ML, Matzuk MM. 2010. A link between mir-100 and FRAP1/mTOR in clear cell ovarian cancer. Mol Endocrinol 24:447–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Klinge CM. 2009. Estrogen regulation of microRNA expression. Curr Genomics 10:169–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC. 2007. Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 148:3814–3826 [DOI] [PubMed] [Google Scholar]
- 90. Nothnick WB, Healy C. 2010. Estrogen induces distinct patterns of microRNA expression within the mouse uterus. Reprod Sci 17:987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Kuokkanen S, Chen B, Ojalvo L, Benard L, Santoro N, Pollard JW. 2010. Genomic profiling of microRNAs and messenger RNAs reveals hormonal regulation in MicroRNA expression in human endometrium. Biol Reprod 82:791–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Hawkins SM, Creighton CJ, Han DY, Zariff A, Anderson ML, Gunaratne PH, Matzuk MM. 2011. Functional microRNAs involved in endometriosis. Mol Endocrinol 25:821–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Pan Q, Luo X, Toloubeydokhti T, Chegini N. 2007. The expression profile of micro-RNA in endometrium and endometriosis and the influence of ovarian steroids on their expression. Mol Hum Reprod 13:797–806 [DOI] [PubMed] [Google Scholar]
- 94. Toloubeydokhti T, Pan Q, Luo X, Bukulmez O, Chegini N. 2008. The expression and ovarian steroid regulation of endometrial micro-RNAs. Reprod Sci 15:993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 95. Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X. 2010. Temporal and spatial regulation of let-7a in the uterus during embryo implantation in the rat. J Reprod Dev 56:73–78 [DOI] [PubMed] [Google Scholar]
- 96. Xia HF, Jin XH, Song PP, Cui Y, Liu CM, Ma X. 2010. Temporal and spatial regulation of miR-320 in the uterus during embryo implantation in the rat. Int J Mol Sci 11:719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Chakrabarty A, Tranguch S, Daikoku T, Jensen K, Furneaux H, Dey SK. 2007. MicroRNA regulation of cyclooxygenase-2 during embryo implantation. Proc Natl Acad Sci USA 104:15144–15149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Chandana EP, Maeda Y, Ueda A, Kiyonari H, Oshima N, Yamamoto M, Kondo S, Oh J, Takahashi R, Yoshida Y, Kawashima S, Alexander DB, Kitayama H, Takahashi C, Tabata Y, Matsuzaki T, Noda M. 2010. Involvement of the Reck tumor suppressor protein in maternal and embryonic vascular remodeling in mice. BMC Dev Biol 10:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, Ma XH, Ni H, Lei W, Yang ZM. 2008. MicroRNA expression and regulation in mouse uterus during embryo implantation. J Biol Chem 283:23473–23484 [DOI] [PubMed] [Google Scholar]
- 100. Su RW, Lei W, Liu JL, Zhang ZR, Jia B, Feng XH, Ren G, Hu SJ, Yang ZM. 2010. The integrative analysis of microRNA and mRNA expression in mouse uterus under delayed implantation and activation. PLoS One 5:e15513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Renthal NE, Chen CC, Williams KC, Gerard RD, Prange-Kiel J, Mendelson CR. 2010. miR-200 family and targets, ZEB1 and ZEB2, modulate uterine quiescence and contractility during pregnancy and labor. Proc Natl Acad Sci USA 107:20828–20833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Montenegro D, Romero R, Kim SS, Tarca AL, Draghici S, Kusanovic JP, Kim JS, Lee DC, Erez O, Gotsch F, Hassan SS, Kim CJ. 2009. Expression patterns of microRNAs in the chorioamniotic membranes: a role for microRNAs in human pregnancy and parturition. J Pathol 217:113–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Hu Y, Li P, Hao S, Liu L, Zhao J, Hou Y. 2009. Differential expression of microRNAs in the placentae of Chinese patients with severe pre-eclampsia. Clin Chem Lab Med 47:923–929 [DOI] [PubMed] [Google Scholar]
- 104. Pineles BL, Romero R, Montenegro D, Tarca AL, Han YM, Kim YM, Draghici S, Espinoza J, Kusanovic JP, Mittal P, Hassan SS, Kim CJ. 2007. Distinct subsets of microRNAs are expressed differentially in the human placentas of patients with preeclampsia. Am J Obstet Gynecol 196:261.e1-6 [DOI] [PubMed] [Google Scholar]
- 105. Zhu XM, Han T, Sargent IL, Yin GW, Yao YQ. 2009. Differential expression profile of microRNAs in human placentas from preeclamptic pregnancies vs normal pregnancies. Am J Obstet Gynecol 200:661.e1-7 [DOI] [PubMed] [Google Scholar]
- 106. Mayor-Lynn K, Toloubeydokhti T, Cruz AC, Chegini N. 2011. Expression profile of microRNAs and mRNAs in human placentas from pregnancies complicated by preeclampsia and preterm labor. Reprod Sci 18:46–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. 1998. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA 95:6355–6360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Gellhaus A, Schmidt M, Dunk C, Lye SJ, Kimmig R, Winterhager E. 2006. Decreased expression of the angiogenic regulators CYR61 (CCN1) and NOV (CCN3) in human placenta is associated with pre-eclampsia. Molecular human reproduction 12:389–399 [DOI] [PubMed] [Google Scholar]
- 109. Zhang Y, Diao Z, Su L, Sun H, Li R, Cui H, Hu Y. 2010. MicroRNA-155 contributes to preeclampsia by down-regulating CYR61. Am J Obstet Gynecol 202:466.e1-7 [DOI] [PubMed] [Google Scholar]
- 110. Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, Benjamin H, Kushnir M, Cholakh H, Melamed N, Bentwich Z, Hod M, Goren Y, Chajut A. 2008. Serum microRNAs are promising novel biomarkers. PLoS One 3:e3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Mouillet JF, Chu T, Hubel CA, Nelson DM, Parks WT, Sadovsky Y. 2010. The levels of hypoxia-regulated microRNAs in plasma of pregnant women with fetal growth restriction. Placenta 31:781–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Pan Q, Chegini N. 2008. MicroRNA signature and regulatory functions in the endometrium during normal and disease states. Semin Reprod Med 26:479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Nothnick WB. 2008. Regulation of uterine matrix metalloproteinase-9 and the role of microRNAs. Semin Reprod Med 26:494–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wei JJ, Soteropoulos P. 2008. MicroRNA: a new tool for biomedical risk assessment and target identification in human uterine leiomyomas. Semin Reprod Med 26:515–521 [DOI] [PubMed] [Google Scholar]
- 115. Guo SW. 2009. Epigenetics of endometriosis. Mol Hum Reprod 15:587–607 [DOI] [PubMed] [Google Scholar]
- 116. Luo X, Chegini N. 2008. The expression and potential regulatory function of microRNAs in the pathogenesis of leiomyoma. Semin Reprod Med 26:500–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Teague EM, Print CG, Hull ML. 2010. The role of microRNAs in endometriosis and associated reproductive conditions. Hum Reprod Update 16:142–165 [DOI] [PubMed] [Google Scholar]
- 118. Ohlsson Teague EM, Van der Hoek KH, Van der Hoek MB, Perry N, Wagaarachchi P, Robertson SA, Print CG, Hull LM. 2009. MicroRNA-regulated pathways associated with endometriosis. Mol Endocrinol 23:265–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Filigheddu N, Gregnanin I, Porporato PE, Surico D, Perego B, Galli L, Patrignani C, Graziani A, Surico N. 2010. Differential expression of microRNAs between eutopic and ectopic endometrium in ovarian endometriosis. J Biomed Biotechnol 2010:369549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Burney RO, Hamilton AE, Aghajanova L, Vo KC, Nezhat CN, Lessey BA, Giudice LC. 2009. MicroRNA expression profiling of eutopic secretory endometrium in women with versus without endometriosis. Mol Hum Reprod 15:625–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Aghajanova L, Giudice LC. 2010. Molecular evidence for differences in endometrium in severe versus mild endometriosis. Reprod Sci 18:229–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, Center MM, Ward E, Wu XC, Eheman C, Anderson R, Ajani UA, Kohler B, Edwards BK. 2008. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100:1672–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Boren T, Xiong Y, Hakam A, Wenham R, Apte S, Wei Z, Kamath S, Chen DT, Dressman H, Lancaster JM. 2008. MicroRNAs and their target messenger RNAs associated with endometrial carcinogenesis. Gynecol Oncol 110:206–215 [DOI] [PubMed] [Google Scholar]
- 124. Chung TK, Cheung TH, Huen NY, Wong KW, Lo KW, Yim SF, Siu NS, Wong YM, Tsang PT, Pang MW, Yu MY, To KF, Mok SC, Wang VW, Li C, Cheung AY, Doran G, Birrer MJ, Smith DI, Wong YF. 2009. Dysregulated microRNAs and their predicted targets associated with endometrioid endometrial adenocarcinoma in Hong Kong women. Int J Cancer 124:1358–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Wu W, Lin Z, Zhuang Z, Liang X. 2009. Expression profile of mammalian microRNAs in endometrioid adenocarcinoma. Eur J Cancer Prev 18:50–55 [DOI] [PubMed] [Google Scholar]
- 126. Ratner ES, Tuck D, Richter C, Nallur S, Patel RM, Schultz V, Hui P, Schwartz PE, Rutherford TJ, Weidhaas JB. 2010. MicroRNA signatures differentiate uterine cancer tumor subtypes. Gynecol Oncol 118:251–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Cohn DE, Fabbri M, Valeri N, Alder H, Ivanov I, Liu CG, Croce CM, Resnick KE. 2010. Comprehensive miRNA profiling of surgically staged endometrial cancer. Am J Obstet Gynecol 202:656.e1-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Hiroki E, Akahira J, Suzuki F, Nagase S, Ito K, Suzuki T, Sasano H, Yaegashi N. 2010. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci 101:241–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Lee JW, Park YA, Choi JJ, Lee YY, Kim CJ, Choi C, Kim TJ, Lee NW, Kim BG, Bae DS. 2011. The expression of the miRNA-200 family in endometrial endometrioid carcinoma. Gynecol Oncol 120:56–62 [DOI] [PubMed] [Google Scholar]
- 130. Wong YF, Cheung TH, Lo KW, Yim SF, Siu NS, Chan SC, Ho TW, Wong KW, Yu MY, Wang VW, Li C, Gardner GJ, Bonome T, Johnson WB, Smith DI, Chung TK, Birrer MJ. 2007. Identification of molecular markers and signaling pathway in endometrial cancer in Hong Kong Chinese women by genome-wide gene expression profiling. Oncogene 26:1971–1982 [DOI] [PubMed] [Google Scholar]
- 131. Huang YW, Jansen RA, Fabbri E, Potter D, Liyanarachchi S, Chan MW, Liu JC, Crijns AP, Brown R, Nephew KP, van der Zee AG, Cohn DE, Yan PS, Huang TH, Lin HJ. 2009. Identification of candidate epigenetic biomarkers for ovarian cancer detection. Oncol Rep 22:853–861 [PMC free article] [PubMed] [Google Scholar]
- 132. Valeri N, Gasparini P, Fabbri M, Braconi C, Veronese A, Lovat F, Adair B, Vannini I, Fanini F, Bottoni A, Costinean S, Sandhu SK, Nuovo GJ, Alder H, Gafa R, Calore F, Ferracin M, Lanza G, Volinia S, Negrini M, McIlhatton MA, Amadori D, Fishel R, Croce CM. 2010. Modulation of mismatch repair and genomic stability by miR-155. Proc Natl Acad Sci USA 107:6982–6987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Luthra R, Singh RR, Luthra MG, Li YX, Hannah C, Romans AM, Barkoh BA, Chen SS, Ensor J, Maru DM, Broaddus RR, Rashid A, Albarracin CT. 2008. MicroRNA-196a targets annexin A1: a microRNA-mediated mechanism of annexin A1 downregulation in cancers. Oncogene 27:6667–6678 [DOI] [PubMed] [Google Scholar]
- 134. Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R. 2005. ΔEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24:2375–2385 [DOI] [PubMed] [Google Scholar]
- 135. Guaita S, Puig I, Franci C, Garrido M, Dominguez D, Batlle E, Sancho E, Dedhar S, De Herreros AG, Baulida J. 2002. Snail induction of epithelial to mesenchymal transition in tumor cells is accompanied by MUC1 repression and ZEB1 expression. J Biol Chem 277:39209–39216 [DOI] [PubMed] [Google Scholar]
- 136. Ohira T, Gemmill RM, Ferguson K, Kusy S, Roche J, Brambilla E, Zeng C, Baron A, Bemis L, Erickson P, Wilder E, Rustgi A, Kitajewski J, Gabrielson E, Bremnes R, Franklin W, Drabkin HA. 2003. WNT7a induces E-cadherin in lung cancer cells. Proc Natl Acad Sci USA 100:10429–10434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Hurteau GJ, Carlson JA, Spivack SD, Brock GJ. 2007. Overexpression of the microRNA hsa-miR-200c leads to reduced expression of transcription factor 8 and increased expression of E-cadherin. Cancer Res 67:7972–7976 [DOI] [PubMed] [Google Scholar]
- 138. Cochrane DR, Spoelstra NS, Howe EN, Nordeen SK, Richer JK. 2009. MicroRNA-200c mitigates invasiveness and restores sensitivity to microtubule-targeting chemotherapeutic agents. Mol Cancer Ther 8:1055–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Cochrane DR, Howe EN, Spoelstra NS, Richer JK. 2010. Loss of miR-200c: a marker of aggressiveness and chemoresistance in female reproductive cancers. J Oncol 2010:821717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Myatt SS, Wang J, Monteiro LJ, Christian M, Ho KK, Fusi L, Dina RE, Brosens JJ, Ghaem-Maghami S, Lam EW. 2010. Definition of microRNAs that repress expression of the tumor suppressor gene FOXO1 in endometrial cancer. Cancer Res 70:367–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Daikoku T, Hirota Y, Tranguch S, Joshi AR, DeMayo FJ, Lydon JP, Ellenson LH, Dey SK. 2008. Conditional loss of uterine Pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res 68:5619–5627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Wang T, Zhang X, Obijuru L, Laser J, Aris V, Lee P, Mittal K, Soteropoulos P, Wei JJ. 2007. A micro-RNA signature associated with race, tumor size, and target gene activity in human uterine leiomyomas. Genes Chromosom Cancer 46:336–347 [DOI] [PubMed] [Google Scholar]
- 143. Marsh EE, Lin Z, Yin P, Milad M, Chakravarti D, Bulun SE. 2008. Differential expression of microRNA species in human uterine leiomyoma versus normal myometrium. Fertil Steril 89:1771–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Pan Q, Luo X, Chegini N. 2008. Differential expression of microRNAs in myometrium and leiomyomas and regulation by ovarian steroids. J Cell Mol Med 12:227–240 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 145. Danielson LS, Menendez S, Attolini CS, Guijarro MV, Bisogna M, Wei J, Socci ND, Levine DA, Michor F, Hernando E. 2010. A differentiation-based microRNA signature identifies leiomyosarcoma as a mesenchymal stem cell-related malignancy. Am J Pathol 177:908–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, Manson JE, Colditz GA, Willett WC, Hunter DJ. 1997. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol 90:967–973 [DOI] [PubMed] [Google Scholar]
- 147. Quade BJ, Wang TY, Sornberger K, Dal Cin P, Mutter GL, Morton CC. 2004. Molecular pathogenesis of uterine smooth muscle tumors from transcriptional profiling. Genes Chromosom Cancer 40:97–108 [DOI] [PubMed] [Google Scholar]
- 148. Peng Y, Laser J, Shi G, Mittal K, Melamed J, Lee P, Wei JJ. 2008. Antiproliferative effects by Let-7 repression of high-mobility group A2 in uterine leiomyoma. Mol Cancer Res 6:663–673 [DOI] [PubMed] [Google Scholar]
- 149. Klemke M, Meyer A, Hashemi Nezhad M, Belge G, Bartnitzke S, Bullerdiek J. 2010. Loss of let-7 binding sites resulting from truncations of the 3′ untranslated region of HMGA2 mRNA in uterine leiomyomas. Cancer Genet Cytogenet 196:119–123 [DOI] [PubMed] [Google Scholar]
- 150. Pan Q, Luo X, Chegini N. 2010. microRNA 21: response to hormonal therapies and regulatory function in leiomyoma, transformed leiomyoma and leiomyosarcoma cells. Mol Hum Reprod 16:215–227 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 151. Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. 2008. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One 3:e2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Hassan SS, Romero R, Tarca AL, Nhan-Chang CL, Mittal P, Vaisbuch E, Gonzalez JM, Chaiworapongsa T, Ali-Fehmi R, Dong Z, Than NG, Kim CJ. 2010. The molecular basis for sonographic cervical shortening at term: identification of differentially expressed genes and the epithelial-mesenchymal transition as a function of cervical length. Am J Obstet Gynecol 203:472.e1–e14 [DOI] [PubMed] [Google Scholar]
- 153. Pereira PM, Marques JP, Soares AR, Carreto L, Santos MA. 2010. MicroRNA expression variability in human cervical tissues. PLoS One 5:e11780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Zhang Y, Dai Y, Huang Y, Ma L, Yin Y, Tang M, Hu C. 2009. Microarray profile of micro-ribonucleic acid in tumor tissue from cervical squamous cell carcinoma without human papillomavirus. J Obstet Gynaecol Res 35:842–849 [DOI] [PubMed] [Google Scholar]
- 155. Martinez I, Gardiner AS, Board KF, Monzon FA, Edwards RP, Khan SA. 2008. Human papillomavirus type 16 reduces the expression of microRNA-218 in cervical carcinoma cells. Oncogene 27:2575–2582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Muralidhar B, Goldstein LD, Ng G, Winder DM, Palmer RD, Gooding EL, Barbosa-Morais NL, Mukherjee G, Thorne NP, Roberts I, Pett MR, Coleman N. 2007. Global microRNA profiles in cervical squamous cell carcinoma depend on Drosha expression levels. J Pathol 212:368–377 [DOI] [PubMed] [Google Scholar]
- 157. Lui WO, Pourmand N, Patterson BK, Fire A. 2007. Patterns of known and novel small RNAs in human cervical cancer. Cancer Res 67:6031–6043 [DOI] [PubMed] [Google Scholar]
- 158. Hu X, Schwarz JK, Lewis JS, Jr, Huettner PC, Rader JS, Deasy JO, Grigsby PW, Wang X. 2010. A microRNA expression signature for cervical cancer prognosis. Cancer Res 70:1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ, Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG, Bae DS. 2008. Altered MicroRNA expression in cervical carcinomas. Clin Cancer Res 14:2535–2542 [DOI] [PubMed] [Google Scholar]
- 160. Hassan SS, Romero R, Pineles B, Tarca AL, Montenegro D, Erez O, Mittal P, Kusanovic JP, Mazaki-Tovi S, Espinoza J, Nhan-Chang CL, Draghici S, Kim CJ. 2010. MicroRNA expression profiling of the human uterine cervix after term labor and delivery. Am J Obstet Gynecol 202:80.e1-8 [DOI] [PubMed] [Google Scholar]
- 161. Pisani P, Bray F, Parkin DM. 2002. Estimates of the world-wide prevalence of cancer for 25 sites in the adult population. Int J Cancer 97:72–81 [DOI] [PubMed] [Google Scholar]
- 162. zur Hausen H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer 2:342–350 [DOI] [PubMed] [Google Scholar]
- 163. Das BC, Sharma JK, Gopalkrishna V, Das DK, Singh V, Gissmann L, zur Hausen H, Luthra UK. 1992. A high frequency of human papillomavirus DNA sequences in cervical carcinomas of Indian women as revealed by Southern blot hybridization and polymerase chain reaction. J Med Virol 36:239–245 [DOI] [PubMed] [Google Scholar]
- 164. Hwang K, Walters RC, Lipshultz LI. 2011. Contemporary concepts in the evaluation and management of male infertility. Nat Rev Urol 8:86–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tessel MA, Krett NL, Rosen ST. 2010. Steroid receptor and microRNA regulation in cancer. Curr Opin Oncol 22:592–597 [DOI] [PubMed] [Google Scholar]
- 166. Yan N, Lu Y, Sun H, Tao D, Zhang S, Liu W, Ma Y. 2007. A microarray for microRNA profiling in mouse testis tissues. Reproduction 134:73–79 [DOI] [PubMed] [Google Scholar]
- 167. Ro S, Park C, Sanders KM, McCarrey JR, Yan W. 2007. Cloning and expression profiling of testis-expressed microRNAs. Dev Biol 311:592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Buchold GM, Coarfa C, Kim J, Milosavljevic A, Gunaratne PH, Matzuk MM. 2010. Analysis of microRNA expression in the prepubertal testis. PLoS One 5:e15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Song R, Ro S, Michaels JD, Park C, McCarrey JR, Yan W. 2009. Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet 41:488–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Papaioannou MD, Lagarrigue M, Vejnar CE, Rolland AD, Kuhne F, Aubry F, Schaad O, Fort A, Descombes P, Neerman-Arbez M, Guillou F, Zdobnov EM, Pineau C, Nef S. 2010. Loss of Dicer in Sertoli cells has a major impact on the testicular proteome of mice. Mol Cell Proteomics 10:M900587MCP200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Papaioannou MD, Pitetti JL, Ro S, Park C, Aubry F, Schaad O, Vejnar CE, Kühne F, Descombes P, Zdobnov EM, McManus MT, Guillou F, Harfe BD, Yan W, Jégou B, Nef S. 2009. Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol 326:250–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Kim GJ, Georg I, Scherthan H, Merkenschlager M, Guillou F, Scherer G, Barrionuevo F. 2010. Dicer is required for Sertoli cell function and survival. Int J Dev Biol 54:867–875 [DOI] [PubMed] [Google Scholar]
- 173. Yu Z, Raabe T, Hecht NB. 2005. MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod 73:427–433 [DOI] [PubMed] [Google Scholar]
- 174. Yan N, Lu Y, Sun H, Qiu W, Tao D, Liu Y, Chen H, Yang Y, Zhang S, Li X, Ma Y. 2009. Microarray profiling of microRNAs expressed in testis tissues of developing primates. J Assist Reprod Genet 26:179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Barad O, Meiri E, Avniel A, Aharonov R, Barzilai A, Bentwich I, Einav U, Gilad S, Hurban P, Karov Y, Lobenhofer EK, Sharon E, Shiboleth YM, Shtutman M, Bentwich Z, Einat P. 2004. MicroRNA expression detected by oligonucleotide microarrays: system establishment and expression profiling in human tissues. Genome Res 14:2486–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Zhang R, Peng Y, Wang W, Su B. 2007. Rapid evolution of an X-linked microRNA cluster in primates. Genome Res 17:612–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Lian J, Zhang X, Tian H, Liang N, Wang Y, Liang C, Li X, Sun F. 2009. Altered microRNA expression in patients with non-obstructive azoospermia. Reprod Biol Endocrinol 7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hayashi K, Chuva de Sousa Lopes SM, Kaneda M, Tang F, Hajkova P, Lao K, O'Carroll D, Das PP, Tarakhovsky A, Miska EA, Surani MA. 2008. MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One 3:e1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Maatouk DM, Loveland KL, McManus MT, Moore K, Harfe BD. 2008. Dicer1 is required for differentiation of the mouse male germline. Biol Reprod 79:696–703 [DOI] [PubMed] [Google Scholar]
- 180. Looijenga LH, Gillis AJ, Stoop H, Hersmus R, Oosterhuis JW. 2007. Relevance of microRNAs in normal and malignant development, including human testicular germ cell tumours. Int J Androl 30:304–314; discussion 314–315 [DOI] [PubMed] [Google Scholar]
- 181. Novotny GW, Nielsen JE, Sonne SB, Skakkebaek NE, Rajpert-De Meyts E, Leffers H. 2007. Analysis of gene expression in normal and neoplastic human testis: new roles of RNA. Int J Androl 30:316–326; discussion 326–327 [DOI] [PubMed] [Google Scholar]
- 182. Zhang J, Liu Q, Zhang W, Li J, Li Z, Tang Z, Li Y, Han C, Hall SH, Zhang Y. 2010. Comparative profiling of genes and miRNAs expressed in the newborn, young adult, and aged human epididymides. Acta Biochim Biophys Sinica 42:145–153 [DOI] [PubMed] [Google Scholar]
- 183. Li J, Liu Y, Dong D, Zhang Z. 2010. Evolution of an X-linked primate-specific micro RNA cluster. Mol Biol Evol 27:671–683 [DOI] [PubMed] [Google Scholar]
- 184. Gandellini P, Folini M, Zaffaroni N. 2009. Towards the definition of prostate cancer-related microRNAs: where are we now? Trends Mol Med 15:381–390 [DOI] [PubMed] [Google Scholar]
- 185. Ribas J, Ni X, Haffner M, Wentzel EA, Salmasi AH, Chowdhury WH, Kudrolli TA, Yegnasubramanian S, Luo J, Rodriguez R, Mendell JT, Lupold SE. 2009. miR-21: an androgen receptor-regulated microRNA that promotes hormone-dependent and hormone-independent prostate cancer growth. Cancer Res 69:7165–7169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Sun R, Fu X, Li Y, Xie Y, Mao Y. 2009. Global gene expression analysis reveals reduced abundance of putative microRNA targets in human prostate tumours. BMC Genomics 10:93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Schaefer A, Jung M, Mollenkopf HJ, Wagner I, Stephan C, Jentzmik F, Miller K, Lein M, Kristiansen G, Jung K. 2009. Diagnostic and prognostic implications of microRNA profiling in prostate carcinoma. Int J Cancer 126:1166–1176 [DOI] [PubMed] [Google Scholar]
- 188. Wang G, Wang Y, Feng W, Wang X, Yang JY, Zhao Y, Liu Y. 2008. Transcription factor and microRNA regulation in androgen-dependent and -independent prostate cancer cells. BMC Genomics 9(Suppl 2):S22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Shi XB, Xue L, Yang J, Ma AH, Zhao J, Xu M, Tepper CG, Evans CP, Kung HJ, deVere White RW. 2007. An androgen-regulated miRNA suppresses Bak1 expression and induces androgen-independent growth of prostate cancer cells. Proc Natl Acad Sci USA 104:19983–19988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Epis MR, Giles KM, Barker A, Kendrick TS, Leedman PJ. 2009. miR-331-3p regulates ERBB-2 expression and androgen receptor signaling in prostate cancer. J Biol Chem 284:24696–24704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Narayanan R, Jiang J, Gusev Y, Jones A, Kearbey JD, Miller DD, Schmittgen TD, Dalton JT. 2010. MicroRNAs are mediators of androgen action in prostate and muscle. PLoS One 5:e13637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Zhang L, Zhang B, Valdez JM, Wang F, Ittmann M, Xin L. 2010. Dicer ablation impairs prostate stem cell activity and causes prostate atrophy. Stem Cells (Dayt) 28:1260–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. DeVere White RW, Vinall RL, Tepper CG, Shi XB. 2009. MicroRNAs and their potential for translation in prostate cancer. Urol Oncol 27:307–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Sun T, Wang Q, Balk S, Brown M, Lee GS, Kantoff P. 2009. The role of microRNA-221 and microRNA-222 in androgen-independent prostate cancer cell lines. Cancer Res 69:3356–3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mercatelli N, Coppola V, Bonci D, Miele F, Costantini A, Guadagnoli M, Bonanno E, Muto G, Frajese GV, De Maria R, Spagnoli LG, Farace MG, Ciafrè SA. 2008. The inhibition of the highly expressed miR-221 and miR-222 impairs the growth of prostate carcinoma xenografts in mice. PLoS One 3:e4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Huynh C, Segura MF, Gaziel-Sovran A, Menendez S, Darvishian F, Chiriboga L, Levin B, Meruelo D, Osman I, Zavadil J, Marcusson EG, Hernando E. 2010. Efficient in vivo microRNA targeting of liver metastasis. Oncogene 30:1481–1488 [DOI] [PubMed] [Google Scholar]
- 197. Kota J, Chivukula RR, O'Donnell KA, Wentzel EA, Montgomery CL, Hwang HW, Chang TC, Vivekanandan P, Torbenson M, Clark KR, Mendell JR, Mendell JT. 2009. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 137:1005–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. 2010. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464:1067–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Pecot CV, Calin GA, Coleman RL, Lopez-Berestein G, Sood AK. 2011. RNA interference in the clinic: challenges and future directions. Nat Rev 11:59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Tanaka T, Mangala LS, Vivas-Mejia PE, Nieves-Alicea R, Mann AP, Mora E, Han HD, Shahzad MM, Liu X, Bhavane R, Gu J, Fakhoury JR, Chiappini C, Lu C, Matsuo K, Godin B, Stone RL, Nick AM, Lopez-Berestein G, Sood AK, Ferrari M. 2010. Sustained small interfering RNA delivery by mesoporous silicon particles. Cancer Res 70:3687–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Mangala LS, Han HD, Lopez-Berestein G, Sood AK. 2009. Liposomal siRNA for ovarian cancer. Methods Mol Biol 555:29–42 [DOI] [PubMed] [Google Scholar]
- 202. Lodes MJ, Caraballo M, Suciu D, Munro S, Kumar A, Anderson B. 2009. Detection of cancer with serum miRNAs on an oligonucleotide microarray. PLoS One 4:e6229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Siva AC, Nelson LJ, Fleischer CL, Majlessi M, Becker MM, Vessella RL, Reynolds MA. 2009. Molecular assays for the detection of microRNAs in prostate cancer. Mol Cancer 8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Bartels CL, Tsongalis GJ. 2009. MicroRNAs: novel biomarkers for human cancer. Clin Chem 55:623–631 [DOI] [PubMed] [Google Scholar]
- 205. Schaefer A, Jung M, Kristiansen G, Lein M, Schrader M, Miller K, Stephan C, Jung K. 2010. MicroRNAs and cancer: current state and future perspectives in urologic oncology. Urol Oncol 28:4–13 [DOI] [PubMed] [Google Scholar]
- 206. Zubakov D, Boersma AW, Choi Y, van Kuijk PF, Wiemer EA, Kayser M. 2010. MicroRNA markers for forensic body fluid identification obtained from microarray screening and quantitative RT-PCR confirmation. Int J Legal Med 124:217–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Tsai-Morris CH, Sheng Y, Lee E, Lei KJ, Dufau ML. 2004. Gonadotropin-regulated testicular RNA helicase (GRTH/Ddx25) is essential for spermatid development and completion of spermatogenesis. Proc Natl Acad Sci USA 101:6373–6378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Hata K, Okano M, Lei H, Li E. 2002. Dnmt3L cooperates with the Dnmt3 family of de novo DNA methyltransferases to establish maternal imprints in mice. Development 129:1983–1993 [DOI] [PubMed] [Google Scholar]
- 209. Tee WW, Pardo M, Theunissen TW, Yu L, Choudhary JS, Hajkova P, Surani MA. 2010. Prmt5 is essential for early mouse development and acts in the cytoplasm to maintain ES cell pluripotency. Genes Dev 24:2772–2777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Zhou L, Wu H, Lee P, Wang Z. 2006. Roles of the androgen receptor cofactor p44 in the growth of prostate epithelial cells. J Mol Endocrinol 37:283–300 [DOI] [PubMed] [Google Scholar]
- 211. Huang H, Gao Q, Peng X, Choi SY, Sarma K, Ren H, Morris AJ, Frohman MA. 2011. piRNA-associated germline nuage formation and spermatogenesis require MitoPLD Profusogenic mitochondrial-surface lipid signaling. Dev Cell 20:376–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Watanabe T, Chuma S, Yamamoto Y, Kuramochi-Miyagawa S, Totoki Y, Toyoda A, Hoki Y, Fujiyama A, Shibata T, Sado T, Noce T, Nakano T, Nakatsuji N, Lin H, Sasaki H. 2011. MITOPLD is a mitochondrial protein essential for nuage formation and piRNA biogenesis in the mouse germline. Dev Cell 20:364–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Otsuka M, Zheng M, Hayashi M, Lee JD, Yoshino O, Lin S, Han J. 2008. Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 118:1944–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Huang CC, Yao HH. 2010. Inactivation of Dicer1 in Steroidogenic factor 1-positive cells reveals tissue-specific requirement for Dicer1 in adrenal, testis, and ovary. BMC Dev Biol 10:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Johnson SM, Grosshans H, Shingara J, Byrom M, Jarvis R, Cheng A, Labourier E, Reinert KL, Brown D, Slack FJ. 2005. RAS is regulated by the let-7 microRNA family. Cell 120:635–647 [DOI] [PubMed] [Google Scholar]
- 216. Mayr C, Hemann MT, Bartel DP. 2007. Disrupting the pairing between let-7 and Hmga2 enhances oncogenic transformation. Science 315:1576–1579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Laios A, O'Toole S, Flavin R, Martin C, Kelly L, Ring M, Finn SP, Barrett C, Loda M, Gleeson N, D'Arcy T, McGuinness E, Sheils O, Sheppard B, O'Leary J. 2008. Potential role of miR-9 and miR-223 in recurrent ovarian cancer. Mol Cancer 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Guo LM, Pu Y, Han Z, Liu T, Li YX, Liu M, Li X, Tang H. 2009. MicroRNA-9 inhibits ovarian cancer cell growth through regulation of NF-κB1. FEBS J 276:5537–5546 [DOI] [PubMed] [Google Scholar]
- 219. Bhattacharya R, Nicoloso M, Arvizo R, Wang E, Cortez A, Rossi S, Calin GA, Mukherjee P. 2009. MiR-15a and MiR-16 control Bmi-1 expression in ovarian cancer. Cancer Res 69:9090–9095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Li J, Liang S, Yu H, Zhang J, Ma D, Lu X. 2010. An inhibitory effect of miR-22 on cell migration and invasion in ovarian cancer. Gynecol Oncol 119:543–548 [DOI] [PubMed] [Google Scholar]
- 221. Flavin R, Smyth P, Barrett C, Russell S, Wen H, Wei J, Laios A, O'Toole S, Ring M, Denning K, Li J, Aherne S, Sammarae D, Aziz NA, Alhadi A, Finn SP, Loda M, Sheppard B, Sheils O, O'Leary JJ. 2009. miR-29b expression is associated with disease-free survival in patients with ovarian serous carcinoma. Int J Gynecol Cancer 19:641–647 [DOI] [PubMed] [Google Scholar]
- 222. Creighton CJ, Fountain MD, Yu Z, Nagaraja AK, Zhu H, Khan M, Olokpa E, Zariff A, Gunaratne PH, Matzuk MM, Anderson ML. 2010. Molecular profiling uncovers a p53-associated role for microRNA-31 in inhibiting the proliferation of serous ovarian carcinomas and other cancers. Cancer Res 70:1906–1915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Corney DC, Hwang CI, Matoso A, Vogt M, Flesken-Nikitin A, Godwin AK, Kamat AA, Sood AK, Ellenson LH, Hermeking H, Nikitin AY. 2010. Frequent downregulation of miR-34 family in human ovarian cancers. Clin Cancer Res 16:1119–1128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zhang L, Volinia S, Bonome T, Calin GA, Greshock J, Yang N, Liu CG, Giannakakis A, Alexiou P, Hasegawa K, Johnstone CN, Megraw MS, Adams S, Lassus H, Huang J, Kaur S, Liang S, Sethupathy P, Leminen A, Simossis VA, Sandaltzopoulos R, Naomoto Y, Katsaros D, Gimotty PA, DeMichele A, et al. 2008. Genomic and epigenetic alterations deregulate microRNA expression in human epithelial ovarian cancer. Proc Natl Acad Sci USA 105:7004–7009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225. Imam JS, Buddavarapu K, Lee-Chang JS, Ganapathy S, Camosy C, Chen Y, Rao MK. 2010. MicroRNA-185 suppresses tumor growth and progression by targeting the Six1 oncogene in human cancers. Oncogene 29:4971–4979 [DOI] [PubMed] [Google Scholar]
- 226. Chen R, Alvero AB, Silasi DA, Kelly MG, Fest S, Visintin I, Leiser A, Schwartz PE, Rutherford T, Mor G. 2008. Regulation of IKKβ by miR-199a affects NF-κB activity in ovarian cancer cells. Oncogene 27:4712–4723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Park SM, Gaur AB, Lengyel E, Peter ME. 2008. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22:894–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Yang H, Kong W, He L, Zhao JJ, O'Donnell JD, Wang J, Wenham RM, Coppola D, Kruk PA, Nicosia SV, Cheng JQ. 2008. MicroRNA expression profiling in human ovarian cancer: miR-214 induces cell survival and cisplatin resistance by targeting PTEN. Cancer Res 68:425–433 [DOI] [PubMed] [Google Scholar]
- 229. Wilting SM, van Boerdonk RA, Henken FE, Meijer CJ, Diosdado B, Meijer GA, le Sage C, Agami R, Snijders PJ, Steenbergen RD. 2010. Methylation-mediated silencing and tumour suppressive function of hsa-miR-124 in cervical cancer. Mol Cancer 9:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Pang RT, Leung CO, Ye TM, Liu W, Chiu PC, Lam KK, Lee KF, Yeung WS. 2010. MicroRNA-34a suppresses invasion through downregulation of Notch1 and Jagged1 in cervical carcinoma and choriocarcinoma cells. Carcinogenesis 31:1037–1044 [DOI] [PubMed] [Google Scholar]
- 231. Abdelmohsen K, Kim MM, Srikantan S, Mercken EM, Brennan SE, Wilson GM, Cabo R, Gorospe M. 2010. miR-519 suppresses tumor growth by reducing HuR levels. Cell Cycle 9:1354–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Yang Z, Chen S, Luan X, Li Y, Liu M, Li X, Liu T, Tang H. 2009. MicroRNA-214 is aberrantly expressed in cervical cancers and inhibits the growth of HeLa cells. IUBMB Life 61:1075–1082 [DOI] [PubMed] [Google Scholar]
- 233. Yao Q, Xu H, Zhang QQ, Zhou H, Qu LH. 2009. MicroRNA-21 promotes cell proliferation and down-regulates the expression of programmed cell death 4 (PDCD4) in HeLa cervical carcinoma cells. Biochem Biophys Res Commun 388:539–542 [DOI] [PubMed] [Google Scholar]
- 234. Ma L, Liu J, Shen J, Liu L, Wu J, Li W, Luo J, Chen Q, Qian C. 2010. Expression of miR-122 mediated by adenoviral vector induces apoptosis and cell cycle arrest of cancer cells. Cancer Biol Ther 9:554–561 [DOI] [PubMed] [Google Scholar]
- 235. Zhou L, Qi X, Potashkin JA, Abdul-Karim FW, Gorodeski GI. 2008. MicroRNAs miR-186 and miR-150 down-regulate expression of the pro-apoptotic purinergic P2X7 receptor by activation of instability sites at the 3′-untranslated region of the gene that decrease steady-state levels of the transcript. J Biol Chem 283:28274–28286 [DOI] [PMC free article] [PubMed] [Google Scholar]