Abstract
The growth of many human breast tumors requires the proliferative effect of estrogen acting via the estrogen receptor α (ERα). ERα signaling is therefore a clinically important target for breast cancer prevention and therapeutics. Although extensively studied, the mechanism by which ERα promotes proliferation remains to be fully established. We observed an up-regulation of transcript encoding the pH-sensitive two-pore domain potassium channel KCNK5 in a screen for genes stimulated by 17β-estradiol (E2) in the ERα+ breast cancer cell lines MCF-7 and T47D. KCNK5 mRNA increased starting 1 h after the onset of E2 treatment, and protein levels followed after 12 h. Estrogen-responsive elements are found in the enhancer region of KCNK5, and chromatin immunoprecipitation assays revealed binding of ERα to the KCNK5 enhancer in E2-treated MCF-7 cells. Cells treated with E2 also showed increases in the amplitude of pH-sensitive potassium currents, as assessed by whole-cell recordings. These currents are blocked by clofilium. Although confocal microscopy suggested that most of the channels are located in intracellular compartments, the increase in macroscopic currents suggests that E2 treatment increases the number of active channels at the cell surface. Application of small interfering RNA specific for KCNK5 decreased pH-sensitive potassium currents and also reduced the estrogen-induced proliferation of T47D cells. We conclude that E2 induces the expression of KCNK5 via ERα+ in breast cancer cells, and this channel plays a role in regulating proliferation in these cell lines. KCNK5 may therefore represent a useful target for treatment, for example, of tamoxifen-resistant breast cancer.
Lifetime exposure to estrogen is an important risk factor for breast cancer, and most primary breast tumors are sensitive to estrogen. For this reason, estrogen signaling is an important target for breast cancer therapeutics. Estrogen receptors (ER) are part of the steroid receptor family of nuclear receptors that classically influence gene expression by direct binding to estrogen response elements in the promoter or enhancer regions of target genes. Two types of ER have been described and are referred to as ERα and ERβ. Increases in ERα signaling are correlated with breast carcinogenesis, and this receptor is currently used as a prognostic and therapeutic marker in breast cancer. ERα is known to induce proliferation in breast cancer cells. The mechanism is not fully known, but it involves up-regulation of the early cell cycle gene MYC through binding by ERα to its promoter (1). By contrast, there is evidence that suggests that ERβ suppresses tumor proliferation by opposing ERα effects (2).
We recently reported a microarray study aimed at understanding the interactions of ERα and ERβ at the level of gene regulation in breast cancer cells (3). In this screen, one of the genes most highly induced by estrogen was the two-pore domain potassium channel K2P5.1, more commonly known as KCNK5 or TASK-2. Interestingly, in T47D cells overexpressing ERβ, we found down-regulation of KCNK5, suggesting that this gene is regulated in opposite ways by both types of ER (3).
KCNK5 is a pH-sensitive potassium channel expressed in many different tissues including liver, pancreas, small intestine, and kidney (4). KCNK5 currents are activated by alkaline intra- or extracellular pH and inhibited by acidic pH on either side of the membrane (5, 6). KCNK5 channels are insensitive to the classical potassium channel blockers tetraethylammonium (TEA) and 4-aminopyridine but are inhibited by quinidine (4) and the antiarrhythmic agent clofilium (7). The expression and function of KCNK5 channels in cells derived from mammary epithelium have not been previously studied. Aside from important functions of this ion channel in the control of HCO3− excretion by the kidney (8), KCNK5 is required for the regulatory volume decreases in response to hypotonicity (7, 9) and during apoptosis (10). Recently, a role for this channel in central chemoreception has been described in mice (11).
Potassium channels have emerged as potential targets for cancer therapeutics (12) owing to their effects on proliferation, sensitivity to growth signals, evasion of apoptosis, angiogenesis, and metastasis and invasion (13). The mechanisms underlying the role of potassium channels in cell proliferation are poorly understood and could result from effects on membrane potential, calcium homeostasis, and/or cell volume regulation, all of which can influence proteins directly involved in the cell cycle (13–19). Potassium channels can also control cell proliferation by regulating the activity of transporters involved in pH control (20–23). In the present study we describe the induction of KCNK5 by 17β-estradiol (E2) in ERα+ breast cancer cell lines and provide evidence that this channel is required for estrogen-induced proliferation.
Results
Estrogen induced an increase in KCNK5 at the mRNA and protein levels in T47D and MCF-7 cells
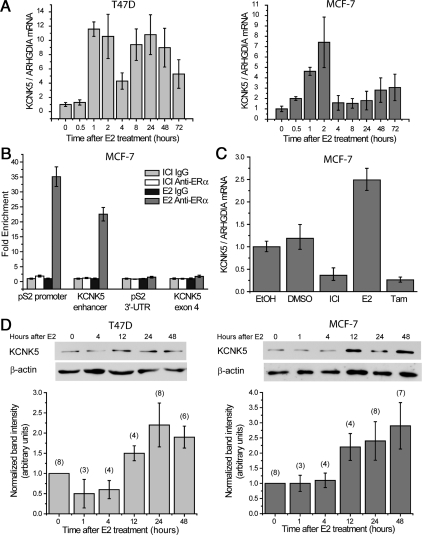
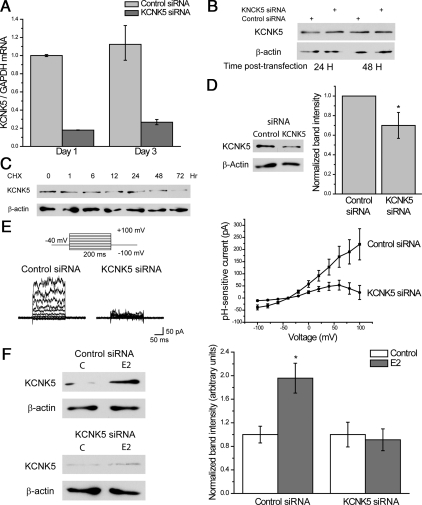
We have previously reported that KCNK5 mRNA is highly regulated by E2 in T47D cells as detected by microarray analysis (3). To confirm this result, we measured KCNK5 mRNA levels in cells treated with E2 using quantitative RT-PCR. An analysis carried out over 72 h showed that the transcription of KCNK5 is rapidly induced by E2 in T47D cells and MCF-7 cells (Fig. 1A). This effect appears to be a consequence of direct genomic regulation by ERα. At least three prior genome-scale studies of ERα binding in breast cancer cell lines have found the receptor to bind in the enhancer region upstream of the transcription start site of KCNK5 (24–26). Here we verified that E2 treatment causes ERα to bind to this region of the genome by chromatin immunoprecipitation (ChIP)-quantitative PCR (qPCR). We found that ERα binding is enriched in the region, as well as on the promoter of the prototypical E2-target gene pS2 (TFF1), but not in exonic or 3′-untranslated regions of these genes (Fig. 1B). Moreover, ERα binding to the enhancer of KCNK5 was not observed in MCF-7 cells pretreated with the ERα-antagonistic compound ICI 182780 (Fig. 1B). To confirm further the effects of ERα stimulation on the transcription of KCNK5 in MCF-7 cells, we treated cells growing in regular medium with antiestrogens. Pretreating the cells with ICI 182780 or the ERα antagonist tamoxifen for 24 h reduced the basal levels of KCNK5 transcription (Fig. 1C). To test whether E2 also increased KCNK5 proteins, we carried out immunoblot analyses of extracts obtained from synchronized MCF-7 and T47D cells at different time points after the onset of E2 treatment. We found increases in KCNK5 in both cell lines starting 12 h after the onset of treatment and lasting for 48–72 h (Fig. 1D).
Fig. 1.
Estrogen induces the expression of KCNK5 in ERα+ breast cancer cells. A, Time course of the induction of KCNK5 mRNA by 10 nm E2 in T47D cells (left) and MCF-7 cells (right) synchronized before treatment. The qPCR data were normalized to the reference gene ARHGDIA (n = 2). B, In MCF-7 cells, ERα binds to the promoter and enhancer element of the genes pS2 and KCNK5, respectively. ChIP-qPCR results are shown for treatment with 10 nm ICI 182780 and 10 nm E2, and with unspecific IgG and specific anti-ERα immunoprecipitation (n = 3). ERα binding is enriched neither in the 3′-untranslated region of pS2 nor the fourth exon of KCNK5. C, Data from qPCR show the repressive effects of 10 nm ICI 182780 and 10 nm tamoxifen (Tam) on basal levels of KCNK5 mRNA in MCF-7 cells growing in serum-containing complete medium. Application of 10 nm E2 increased the level of KCNK5 in cells growing in these conditions. KCNK5 levels in cells treated with the vehicles EtOH and dimethylsulfoxide (DMSO) are presented as controls. Cells were treated for 24 h without previous synchronization. D, Time course of the increase in KCNK5 protein induced by 10 nm E2 in T47D (left) and MCF-7 cells (right). Immunoblots show an increase in KCNK5 in extracts obtained from synchronized T47D and MCF-7 cells treated with 10 nm E2 for 12 h or more. Bar graphs show the quantification of immunoblots normalized to β-actin and control (0 time point). The number of replicates for each time point is indicated above each bar. In this and subsequent figures, error bars represent mean ± sem.
Estrogen-induced increases in KCNK5 currents in T47D and MCF-7 cells
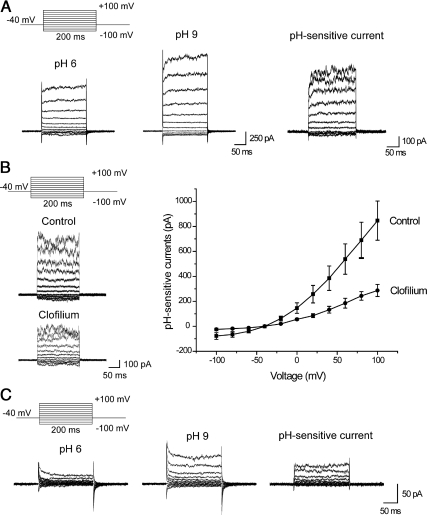
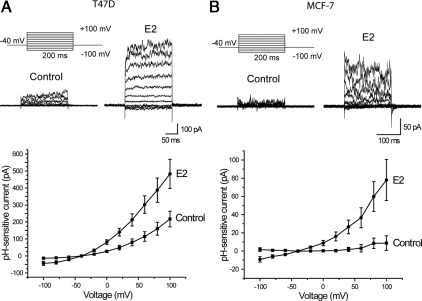
E2 treatment also caused changes in the physiological behavior of T47D and MCF-7 cells consistent with increased expression of KCNK5 at the plasma membrane. To establish this, we measured pH-sensitive currents using whole-cell recordings in MCF-7 and T47D cells treated with E2 for 24 h. Recordings of currents at different membrane potentials were made in the presence of 10 mm TEA to block voltage-activated potassium channels and were analyzed by subtracting the currents recorded at external pH 6.0 from the currents measured in the same cell at an external pH of 9.0. T47D cells showed pH-sensitive currents, which were observed in 58 of 60 cells examined, regardless of whether they were synchronized or growing in complete medium (Fig. 2A). The subtracted currents showed some outward rectification and were almost instantaneous at most membrane potentials (Fig. 2B), suggesting that their gating is not highly voltage sensitive. The pH-sensitive currents in T47D cells were partially blocked by 25 μm clofilium (Fig. 2B), which is reported to inhibit KCNK5 channels (7). It was not possible to examine higher concentrations of clofilium because the recordings became somewhat unstable under those conditions. In contrast to T47D cells, pH-sensitive currents were substantially smaller and were not observed in every MCF-7 cell examined (Fig. 2C). A 24-h exposure to E2 increased the amplitude of pH-sensitive currents in both T47D and MCF-7 cells (Fig. 3).
Fig. 2.
pH-sensitive currents can be detected in ERα+ breast cancer cells. A, Traces from a representative T47D cell showing whole-cell currents measured at pH 6 (left), pH 9 (middle), and the current at pH 6 subtracted from the current at pH 9 (right). External solutions contained 10 mm TEA throughout this experiment. B, Clofilium blocks pH-sensitive currents in T47D cells. Representative traces of pH-sensitive currents (left) and current-voltage (I–V) plots (right) showing a reduction in the pH-sensitive currents when 25 μm clofilium is applied in the bath. The I–V plots were constructed from pH-sensitive currents from seven control cells and six cells treated with clofilium. C, MCF-7 cells show pH-sensitive currents that are somewhat smaller in amplitude.
Fig. 3.
Increase in pH-sensitive currents in T47D and MCF-7 cells treated with E2. A, Representative traces (top) and I–V plots (bottom) showing an increase in the pH-sensitive currents in synchronized T47D cells treated with 10 nm E2 for 24 h. The I–V plot was constructed from 13 cells in both treatment groups. B, pH-sensitive currents were also increased in MCF-7 cells treated with E2. I–V plot was constructed from eight control and 10 E2 treated cells.
Estrogen does not change the localization of KCNK5 channels in T47D or MCF-7 cells
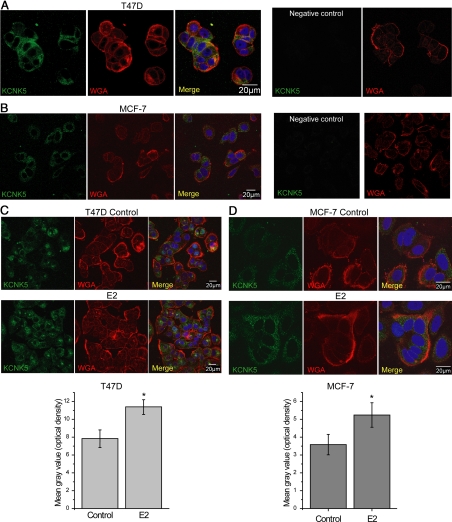
To further characterize estrogen regulation of KCNK5 channels, we performed immunofluorescence in T47D and MCF-7 cultures synchronized and treated with E2 for 24 h. In both cell lines, KCNK5 localized predominantly in intracellular compartments and colocalized poorly with wheat-germ agglutinin (WGA), a marker of the plasma membrane (Fig. 4, A and B). Although a similar pattern was observed in both cell lines, the signal for KCNK5 in MCF-7 cells was consistently less intense. The qualitative pattern of localization of KCNK5 was not changed by treatment of T47D and MCF-7 cells with E2, although there was a modest but significant increase in the intensity of the KCNK5 signal in cells treated with E2 (Fig. 4, C and D).
Fig. 4.
KCNK5 localization is predominantly intracellular in T47D and MCF-7 cells. A, Representative confocal images of T47D cells showing a predominant intracellular localization of the channel with small areas of overlap with the plasma membrane marker WGA. Some diffusion of WGA into the cells during the staining was observed. The nuclear stain 4′,6-diamidino-2-phenylindole is included in overlay images. No signal was observed in T47D cells stained in the absence of primary antibody (negative control). B, Representative images showing KCNK5 localization in MCF-7 cells. Signal was less intense, and there was limited colocalization with WGA. C, Representative images of control (top) or synchronized T47D cells treated with 10 nm E2 for 24 h (bottom) showing no qualitative change in the localization of the channel. The bar graph shows the quantification of the mean optical density in control and E2-treated cells (n = 25). A significant (P < 0.05) increase in the intensity of the signal can be observed in cells treated with E2. D, Similar observations in synchronized MCF-7 cells. The bar graph shows summary quantifications for nine cells (P < 0.05). In panels C and D, cells were processed in parallel, and images were obtained using constant settings on the confocal microscope to facilitate comparisons of signal intensity.
KCNK5 is a long-lived protein in T47D cells
We tested several protocols for small interfering RNA (siRNA) knockdown of KCNK5 in T47D cells. A single treatment with siRNA markedly decreased KCNK5 transcripts within 24 h, and this effect lasted for at least 3 d after a single transfection (Fig. 5A). However, we could not obtain a consistent and robust knockdown of the protein at any time after a single siRNA transfection (Fig. 5B). This suggests that KCNK5 proteins have a long half-life in breast cancer cell lines. In support of this, we observed that KCNK5 levels decreased only after 48 h of treatment of T47D cultures with the protein synthesis inhibitor cycloheximide (Fig. 5C). To obtain a more robust reduction of KCNK5 protein, we transfected cells repeatedly with siRNA. Cells were transfected three times at 48-h intervals and were harvested for analysis 6 d after the first transfection. With this protocol we obtained a consistent reduction in KCNK5 protein in T47D cells as evaluated by immunoblot analysis (Fig. 5D). A similar pattern was seen with MCF-7 cells (data not shown). Transfection of T47D cells with siRNAs targeting KCNK5 also reduced the pH-sensitive currents (Fig. 5E). In addition, we observed that treatment with siRNA completely blocked the increase in KCNK5 expression induced by E2 as measured by immunoblot (Fig. 5F).
Fig. 5.
KCNK5 proteins have a long half-life in T47D cells. A, siRNA treatment of T47D cells reduced mRNA levels of KCNK5 effectively over 3 d, as compared with cells treated with control siRNA. B, Representative immunoblot showing the lack of an efficient reduction in KCNK5 protein 1–2 d after KCNK5 siRNA transfection in T47D cells. A single transfection with KCNK5 siRNA was insufficient to obtain a robust decrease in KCNK5 proteins in T47D cells. Similar results were observed after 3–4 d of transfection (data not shown). C, KCNK5 protein has a long half-life. Immunoblot showing the effect of treatment with the protein synthesis blocker cyclohemixide (CHX, 100 μg/ml) for different periods of time on the level of KCNK5 in T47D cells. D, Three cycles of transfection with KCNK5 siRNA gave a significant reduction of the channel at the protein level. A representative immunoblot is shown to the left, and the bar graph shows the densitometric analysis of the effect of KCNK5 siRNA (n = 6). E, KCNK5 siRNA blocked most of the pH-sensitive currents in T47D cells. Representative traces (left) and I–V plot (right) showing a reduction in the pH-sensitive currents in cells transfected with KCNK5 siRNA compared with cells transfected with control siRNA. The I–V plot is constructed from mean current amplitudes recorded from 11 cells in each group. F, Representative immunoblots showing KCNK5 in cells transfected with control siRNA or KCNK5 siRNA for 6 d and treated with either vehicle or 10 nm E2 for 5 d starting 1 d after the first transfection (left). E2 induced an increase in KCNK5 in cells transfected with control siRNA but not in KCNK5 siRNA. A reduction in the basal level of KCNK5 was obtained in experimental cultures. The bar graph to the right summarizes the level of KCNK5 normalized to actin and the respective control in cells transfected and treated with E2 for three repetitions of this experiment. GAPDH, Glyceraldehyde-3-phosphate dehydrogenase.
Knockdown of KCNK5 reduces proliferation of T47D cells
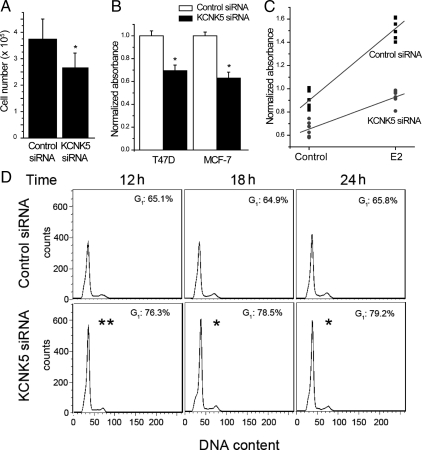
In cultures transfected with siRNA targeting KCNK5 we observed a significant decrease in the number of T47D cells after 6 d using the multiple-transfection protocol described above (Fig. 6A). We did not observe obvious morphological differences compared with control in the cells that were present, and there did not appear to be an increase in the amount of floating cells in cultures transfected with KCNK5 siRNA compared with controls. The percentage of nonviable cells as measured by trypan blue exclusion in cells transfected with KCNK5 siRNA was not significantly different from that of cells transfected with control siRNA (29 ± 6% in control siRNA vs. 36 ± 9% in KCNK5 siRNA; n = 6, P = 0.377 by Student's unpaired t test). These data suggest that the decrease in cell number caused by KCNK5 knockdown cannot be attributed to cell death. Therefore, we examined the effect of KCNK5 siRNA transfection on proliferation of T47D cells. Cells growing in complete medium were transfected with KCNK5 or control siRNA using the multiple transfection protocol described above, and proliferation was assessed 6 d after the first transfection using the MTS assay. T47D cells transfected with KCNK5 siRNA showed significant reductions in basal proliferation (Fig. 6B). This was also observed in MCF-7 cells.
Fig. 6.
Knocking down KCNK5 decreases proliferation in T47D and MCF-7 cells. A, Bar graph showing total cell counts in cultures transfected with control or KCNK5 siRNA. A significant decrease was observed in cells treated with KCNK5 siRNA (n = 8). B, Transfected cultures were incubated with the MTS reagent to evaluate cell proliferation. Bar graphs show the absorbance in cells treated with KCNK5 siRNA normalized to the absorbance of the respective control siRNA cells within each experiment (n = 5 for T47D cells and n = 3 for MCF-7 cells). Proliferation was significantly reduced in T47D and MCF-7 cells transfected with KCNK5 siRNA for 6 d. C. Graph showing the normalized absorbance in synchronized cultures transfected with control or KCNK5 siRNA and treated with 10 nm E2 or vehicle for 5 d (n = 6 experiments in each group). Absorbances were normalized to the mean of each experiment. A significantly smaller increase induced by E2 was observed in cells transfected with KCNK5 siRNA. Two-way ANOVA revealed significant effects of E2 treatment, KCNK5 siRNA, and a significant interaction effect between these treatments (see text). D, Analysis of cell cycle by flow cytometry was performed to investigate the reduced proliferation caused by siRNAs targeting KCNK5. Numbers in the histograms show the percentage of cells in G1 phase of the cell cycle. The higher percentage of KCNK5 siRNA cells in G1 phase compared with the control siRNA cells suggests that knockdown of KCNK5 keeps the cells in G1/S cell-cycle arrest. The asterisk indicates a statistically significant difference (P < 0.05) between cells transfected with control and KCNK5 siRNA various times after E2 treatment.
We also used the MTS assay to determine whether KCNK5 plays a role in E2-induced proliferation (Fig. 6C). This experimental design has two independent variables (siRNA and E2 treatment) and a single dependent variable (cell proliferation). As expected, we observed a highly significant effect of E2 on cell proliferation as established by two-way ANOVA (F = 209.40728; n = 6; P = 4.66 × 10−2). In addition, we observed a highly significant effect of KCNK5 siRNA on proliferation (F = 183.36383; P = 1.56 × 10−1). Most importantly, we also observed a robust and statistically significant interaction between the effects of E2 and the KCNK5 siRNA (F = 30.86874; P = 1.94 × 10−5). Post hoc analysis using Tukey's test confirmed the effects of E2 and KCNK5 siRNA. These results indicate that KCNK5 knockdown caused a modest reduction in basal proliferation of T47D cells but caused a proportionately greater attenuation of the proliferation response that occurs in response to E2 (Fig. 6C). To assess this effect in more detail, we carried out cell cycle analysis by flow cytometry. Cells transfected with siRNA directed against KCNK5 were compared with cells transfected with control siRNA during E2-stimulated proliferation. We observed that KCNK5 knockdown caused a significantly higher proportion of the cells to remain in the G1 phase compared with controls (P = 0.0015 at 12 h of E2; P = 0.012 at 18 h; and P = 0.032 at 24 h; Student's two-tailed t test), as illustrated in Fig. 6D. These data indicate that knockdown of KCNK5 induces G1/S cell cycle arrest, thereby reducing the proliferative response to E2.
Discussion
In this study we have shown that activation of ERα+ by E2 causes induction of KCNK5 channels in two different breast cancer cell lines. Consistent with this, treatment of MCF-7 cells growing in regular medium with ERα antagonists induced a significant decrease in the transcription of KCNK5, and ERα was observed to bind the KCNK5 enhancer in MCF-7 cells treated with E2 but not in cells pretreated with ICI 182780. Confocal analyses suggested that the majority of KCNK5 channels are localized in intracellular compartments, possibly including nuclei, although whole-cell recordings indicated that functional KCNK5 channels are also present at the cell surface and increased by E2 treatment. This pattern is also observed in astrocytes (27), and we cannot exclude that KCNK5 channels may also have a functional role in intracellular compartments. A recent study in glioma cells has suggested that mistargeting of K+ channels to nuclei may play a role in their abnormal growth and invasiveness compared with normal astrocytes (28). In this regard, in whole-cell recordings, we used the sensitivity of KCNK5 to extracellular pH as an indication of the activity of the channel. The pH-sensitive currents showed outward rectification and no inactivation, as has been described for KCNK5 channels in heterologous expression systems (4). The pH-sensitive outward currents in breast cancer cell lines were also inhibited by micromolar concentrations of clofilium, and more importantly, by siRNAs targeting KCNK5.
We observed an increase in pH-sensitive currents in T47D and MCF-7 cells treated with E2 for 24 h. No obvious qualitative change in the pattern of KCNK5 localization as a result of E2 treatment could be discerned by confocal microscopy. This suggests that the increase in the current is caused by an increase in the total KCNK5 protein and a corresponding mass-action increase in the number of channels that traffic to the cell surface.
When T47D cells were transfected with siRNA targeting KCNK5, we observed a decrease in the rate of proliferation in cells growing in complete medium and a proportionately larger effect on the proliferation of synchronized cells treated with E2. Analysis of the cell cycle suggested that this effect is caused by arrest of the cell cycle at the G1 phase. Other potassium channels have been shown to regulate cell proliferation through the regulation of cell membrane potential, intracellular free calcium concentration, cell volume, and possibly intracellular pH (13–23). Some of these functions have been previously described for KCNK5 in other cellular contexts (8, 9, 29), and they represent plausible roles for KCNK5 channels in breast epithelium. However, we did not observe an increase in cell death in cultures treated with KCNK5 siRNA. Instead, the data suggest that the increase in KCNK5 induced by E2 is necessary for the cells to progress past the G1 phase into S1, and thereby allowing for E2-mediated cell proliferation. Six days of transfection with KCNK5 siRNA led to a consistent decrease in KCNK5 protein and macroscopic currents and a robust attenuation of E2-induced proliferation. KCNK5 proteins appear to be unusually stable, and siRNA-evoked reduction of KCNK5 was slow and not complete; for this reason, it is possible that the remaining E2-evoked proliferation was due to residual KCNK5.
KCNK5 channels may contribute to the pathogenesis of cancer. For example, a recent study of a large number of primary human cancers and cancer cell lines identified a novel amplicon located on 6p21.2 that contained eight genes including KCNK5, as well as the closely related genes KCNK16 and KCNK17 (30). Moreover, they found up-regulation of KCNK5 transcripts in several of the cancer cell lines that they examined. Other channels in the two-pore domain potassium channels family have also been implicated in cancer. For example, the KCNK9 gene, which encodes a potassium channel that is also inhibited by extracellular acidification, is significantly amplified in 10% of breast tumors, and its transcript is markedly overexpressed in 44% of breast tumors (31). Cells over-expressing KCNK9 are resistant to hypoxia. Given the similarities in function, it is possible that multiple members of this family of channels may play a role in allowing cells to adapt to the relatively anoxic microenvironment of solid tumors. In this regard, KCNK5 and KCNK9 have been reported to play a similar role in protecting cells from stress (32), and the potential mechanisms whereby these channels may regulate proliferation and apoptosis have been reviewed (33).
The present results therefore offer an enhanced view of the mechanisms whereby ERα induces proliferation. In addition to the known direct up-regulation of the protooncogene MYC, a transcription factor that subsequently up-regulates cyclins and down-regulates p21, ERα induces the KCNK5 ion channel, which is necessary for normal estrogen-induced proliferation. The effect of KCNK5 on proliferation suggests that this channel and other members of its family could be useful pharmacological targets for treatment of ERα+ breast cancer. ERα+ tumors account for the majority of breast tumor diagnoses. At present, the only targeted therapy is treatment with antiestrogens or aromatase inhibitors. The side effects of these treatments and the development of resistance over time suggest the importance of finding new strategies to target ERα+ tumors. Targeting KCNK5 would thus specifically block the proliferation-inducing ability of ERα, while preserving more beneficial activities, such as the differentiation- and apoptosis-inducing effects of ERα. Such a targeted therapy would be an alternative also for refractory ERα+ cancers. Unfortunately specific blockers for KCNK5 are not currently available, and the currently available inhibitors block other types of channels and have high toxicity in vivo at the doses required to block KCNK5 (34).
In summary, we have shown that KCNK5, a pH-sensitive potassium channel in the two-pore superfamily, is induced by estrogen in ERα+ breast cancer cell lines, and that this channel is required for normal E2-evoked proliferation in these cells. KCNK5 and related channels may therefore represent potential targets for breast cancer therapeutics.
Materials and Methods
Drugs and reagents
All cell culture reagents were from Invitrogen (Carlsbad, CA). E2, clofilium tosylate, tamoxifen, and cycloheximide were from Sigma (St. Louis, MO). ICI 182780 was from Tocris Bioscience (Ellisville, MO).
Cell culture, estrogen treatment, and transfection
T47D and MCF-7 cells were obtained from the American Type Culture Collection (Manassas, VA). All experiments were performed between passages 9 and 30. T47D cells were grown in DMEM/Nutrient Mixture F-12 (DMEM/F12) supplemented with 5% heat-inactivated fetal bovine serum (FBS) and 100 U/ml penicillin-100 μg/ml streptomycin (Pen/Strep). MCF-7 cells were grown in DMEM supplemented with 5% heat-inactivated FBS and Pen/Strep. Cells were propagated in a humidified incubator at 37 C in 5% CO2. T47D and MCF-7cells were synchronized before estrogen treatment by incubation for 24 h in the respective medium without phenol red supplemented with 5% dextran-coated charcoal-stripped FBS (DCC-FBS) and Pen/Strep, followed by 48 h incubation in medium supplemented with 0.5% DCC-FBS. After synchronization, cells were treated with 10 nm E2 in 0.5% DCC-FBS supplemented medium. A pool of four siRNAs targeting KCNK5 was used (ON-TARGETplus SMARTpool from Dharmacon, Thermo Scientific, Waltham, MA). A nontargeting pool from the same company was used as a control in all the experiments. Transient transfections were performed using DharmaFECT 1 Reagent from Thermo Scientific following the manufacturer's instructions. T47D cells were transfected in antibiotic-free DMEM/F12 medium supplemented with 5% heat-inactivated FBS or 0.5% DCC-FBS. MCF-7 cells were transfected in antibiotic-free DMEM supplemented with 5% heat-inactivated FBS.
RNA extraction, cDNA synthesis, and qPCR
RNA was extracted from cultured cells with Qiazol and purified with RNeasy Mini Kit, according to the manufacturer's instructions, including on-column DNA digestion with deoxyribonuclease I, all from QIAGEN (Germantown, MD). Synthesis of cDNA was carried out in a total volume of 20 μl using 0.4–1 μg of RNA to which 0.1 nmol of random-hexamer primers were added. The mixture was incubated for 10 min at 70 C and 5 min on ice after which 1× first-strand buffer, 5 mm dithiothreitol (both from Invitrogen), and 0.5 mm deoxynucleotide triphosphates (Sigma) were added. After addition of 200 U of Superscript III (Invitrogen), the reaction was started at 25 C for 10 min, and continued at 46 C for 1 h. The enzyme was deactivated at 70 C for 15 min, and 10 ng of cDNA were used for each real-time PCR, with 1 pmol of forward and reverse primer and 1× Fast SYBR Green Master Mix (Applied Biosystems, Foster City, CA). Samples were run in duplicates or triplicates with no-template control on a 7500 Fast Real-Time PCR (Applied Biosystems). Melt-curve analysis was performed to ascertain specific amplification. Primers for cDNA were designed to span introns to avoid amplification of genomic DNA. Primer sequences can be provided upon request. Analysis of the expression level of KCNK5 was made using the ΔΔCT method, thereby determining differences in fold change and standard deviation in transcript levels. CT values were obtained from the linear phase of the logarithmic amplification using the 7500 software version 2.0.1. Gene expression was normalized to glyceraldehyde-3-phosphate dehydrogenase ARHGDIA mRNA.
ChIP-qPCR
ChIP was carried out using 2 μg anti-ERα antibody HC-20X or normal rabbit IgG (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), bound to Dynabeads Protein G from Invitrogen, according to Schmidt et al. (35). Synchronized cells were treated with 10 nm E2 for 45 min or 10 nm ICI 182780 (ICI) for 24 h. Cells were cross-linked with 1.5% formaldehyde in PBS. Cross-linking was stopped with 125 mm glycine after which cells were scraped in 100 mm Tris-HCl (pH 9.4) and 10 mm dithiothreitol. Nuclei were isolated by sequential resuspension in NCP I (10 mm EDTA; 0.5 mm EGTA; 10 mm HEPES, pH 6.5; 0.25% Triton X-100), NCP II (10 mm EDTA; 0.5 mm EGTA; 10 mm HEPES, pH 6.5; 200 mm NaCl), and lysis buffer [10 mm EDTA, 20 mm Tris-HCl (pH 8.0), 0.5% Empigen BB, 1% sodium dodecyl sulfate, 1× complete protease inhibitor from Roche (Mannheim, Germany)]. Chromatin was sonicated to an approximate fragment size of 200-1000 bp and incubated overnight at 4 C with bead-bound antibody in 2 mm EDTA (pH 8.0), 20 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% Triton X-100, and 1× complete protease inhibitor. The beads were washed before elution with different buffers, and samples were eluted with 0.1 mm NaHCO3, 1% sodium dodecyl sulfate at 65 C. Cross-linking was reversed overnight at 65 C, and DNA was purified using MinElute PCR Purification Kit (QIAGEN). Input samples were diluted 1:50 before qPCR with primers designed for promoter/enhancer regions and negative control regions. Enrichment was calculated as percentage of input (36).
Cell counts, viability, and proliferation assays
Cells were counted using a hemacytometer. In some experiments, proliferation was assessed using Celltiter 96 Aqueous One Solution Cell Proliferation Assay from Promega Corp. (Madison, WI) following the manufacturer's instructions. In experiments with synchronized T47D cells, 5000 cells per well were seeded in 96-well plates and were allowed to attach for 1 d before synchronization. For experiments using unsynchronized T47D and MCF-7 cells, 10,000 cells were plated per well 1 d before transfection. At the end of the treatments, cells were incubated in the MTS solution for 4 h in a 5% CO2 incubator at 37 C, and absorbance was measured at 492 nm. For cell cycle analysis by flow cytometry, T47D cells were synchronized as described above and transfected with KCNK5 siRNAs 48 h before and at time point zero. After treatment with 10 nm E2 for the indicated times, cells were harvested and fixed in ethanol overnight at −20 C. Cells were stained with propidium iodide, washed, and analyzed for DNA content by flow cytometry using a FACSAria II cell sorter (BD Biosciences, Palo Alto, CA). The percentage of cells in G1 phase of the cell cycle was then calculated.
Confocal microscopy
Cells were grown on noncoated glass coverslips for at least 1 d before staining. Cells were fixed in 4% paraformaldehyde, permeabilized using 0.2% Tween 20, and blocked in 1% BSA, all prepared in PBS. Cells were incubated with unconjugated anti-KCNK5 antibody from Alomone Laboratories (Jerusalem, Israel) overnight, followed by Alexa Fluor 488-conjugated secondary antibody from Invitrogen. To label the plasma membrane, cells were stained with fluorescent WGA before permeabilization. Images were collected using a Fluoview 1000 inverted confocal microscope (Olympus, Tokyo, Japan) equipped with an Olympus Plan Apo N 60× 1.42 numerical aperture oil immersion objective. The fluorescence intensity per cell was quantified using ImageJ software (National Institutes of Health, Bethesda, MD).
Immunoblot
Cells were lysed using radioimmune precipitation buffer (Sigma) supplemented with 1 mm phenylmethylsulfonylfluoride and protease-inhibitor cocktail (both from Sigma). Proteins were separated by 10% SDS-PAGE and transferred to nitrocellulose membranes. Blots were blocked and incubated overnight at 4 C in primary antibody against KCNK5 (Alomone Laboratories) or β-actin (Santa Cruz Biotechnology). Immunoreactive bands were detected using SuperSignal West Pico Chemiluminescent Substrate (Thermo Scientific) on x-ray autoradiography films. Films were analyzed using ImageJ software, and the intensity of the bands was normalized to β-actin as a loading control within the same blot.
Electrophysiology
Cells were cultured on glass coverslips for at least 1 d before electrophysiological recordings. All recordings were made at room temperature. Currents were recorded using an Axopatch 1D patch-clamp amplifier from Molecular Devices (Sunnyvale, CA) in the whole-cell configuration using Clampex version 9.2 data-acquisition software (Molecular Devices). Cells were perfused with an external solution containing in millimolar concentration: 140 NaCl, 4 KCl, 2 CaCl2, 1 MgCl2, 11.1 glucose, and 10 HEPES (pH 7.4 with NaOH). Pipette electrodes were filled with a solution containing in millimolar concentration: 125 K-gluconate, 20 KCl, 0.7 CaCl2, 1 MgCl2, 1 Mg-ATP, 1 EGTA, 10 HEPES (pH 7.2 with KOH). Whole-cell recordings were low-pass filtered at 1 kHz (eight-pole Bessel) and sampled at 5 kHz. Currents were evoked by a series of 11 200-msec pulses of 20-mV increments from a holding potential of −40 mV. To measure pH-sensitive currents, cells were perfused in a solution containing (in millimolar concentration): 140 NMDG-Cl, 4 KCl, 2 CaCl2, 1 MgCl2, 11.1 glucose, 10 2-(N-morpholino) ethanesulfonic acid, 10 mm TEA-Cl (pH 6.0). After a stable response was obtained, cells were switched to a solution containing 140 NMDG-Cl, 4 KCl, 2 CaCl2, 1 MgCl2, 11.1 Glucose, 10 Tris, 10 mm TEA-Cl (pH 9.0). Traces obtained at pH 6 were subtracted from those at pH 9 to obtain the pH-sensitive currents. For all analyses the steady-state current in the last 50 msec of the pulses was quantified using Clampfit version 9.2 from Molecular Devices.
Statistics
Statistical analyses were performed using Origin 7.0 software (Origin Lab, Northampton, MA). Data were analyzed using Student's t test or by two-way ANOVA followed by post hoc analysis using Tukey's test. The significant P value was set to < 0.05 in all the analyses. Data shown are mean ± sem.
Acknowledgments
We thank Karin Edvardsson and Anne Katchy for assistance in the laboratory.
This work was supported in part by the Texas Emerging Technology Fund, under Agreement 300-9-1958 to the Center for Nuclear Receptors and Cell Signaling and National Institutes of Health Grant RO1-DK82529 (to S.E.D.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ChIP
- Chromatin immunoprecipitation
- DCC
- dextran-coated charcoal
- E2
- 17β-estradiol
- ER
- estrogen receptor
- FBS
- fetal bovine serum
- qPCR
- quantitative PCR
- siRNA
- small interfering RNA
- TEA
- tetraethylammonium
- WGA
- wheat-germ agglutinin.
References
- 1. Dubik D, Shiu RP. 1992. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene 7:1587–1594 [PubMed] [Google Scholar]
- 2. Ström A, Hartman J, Foster JS, Kietz S, Wimalasena J, Gustafsson JA. 2004. Estrogen receptor β inhibits 17β-estradiol-stimulated proliferation of the breast cancer cell line T47D. Proc Natl Acad Sci USA 101:1566–1571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams C, Edvardsson K, Lewandowski SA, Ström A, Gustafsson JA. 2008. A genome-wide study of the repressive effects of estrogen receptor β on estrogen receptor α signaling in breast cancer cells. Oncogene 27:1019–1032 [DOI] [PubMed] [Google Scholar]
- 4. Reyes R, Duprat F, Lesage F, Fink M, Salinas M, Farman N, Lazdunski M. 1998. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J Biol Chem 273:30863–30869 [DOI] [PubMed] [Google Scholar]
- 5. Morton MJ, Abohamed A, Sivaprasadarao A, Hunter M. 2005. pH sensing in the two-pore domain K+ channel, TASK2. Proc Natl Acad Sci USA 102:16102–16106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niemeyer MI, Cid LP, Peña-Münzenmayer G, Sepúlveda FV. 2010. Separate gating mechanisms mediate the regulation of K2P potassium channel TASK-2 by intra- and extracellular pH. J Biol Chem 285:16467–16475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Niemeyer MI, Cid LP, Barros LF, Sepúlveda FV. 2001. Modulation of the two-pore domain acid-sensitive K+ channel TASK-2 (KCNK5) by changes in cell volume. J Biol Chem 276:43166–43174 [DOI] [PubMed] [Google Scholar]
- 8. Warth R, Barrière H, Meneton P, Bloch M, Thomas J, Tauc M, Heitzmann D, Romeo E, Verrey F, Mengual R, Guy N, Bendahhou S, Lesage F, Poujeol P, Barhanin J. 2004. Proximal renal tubular acidosis in TASK2 K+ channel-deficient mice reveals a mechanism for stabilizing bicarbonate transport. Proc Natl Acad Sci USA 101:8215–8220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Niemeyer MI, Cid LP, Sepúlveda FV. 2001. K+ conductance activated during regulatory volume decrease. The channels in Ehrlich cells and their possible molecular counterpart. Comp Biochem Physiol A Mol Integr Physiol 130:565–575 [DOI] [PubMed] [Google Scholar]
- 10. L'Hoste S, Poet M, Duranton C, Belfodil R, é Barriere H, Rubera I, Tauc M, Poujeol C, Barhanin J, Poujeol P. 2007. Role of TASK2 in the control of apoptotic volume decrease in proximal kidney cells. J Biol Chem 282:36692–36703 [DOI] [PubMed] [Google Scholar]
- 11. Gestreau C, Heitzmann D, Thomas J, Dubreuil V, Bandulik S, Reichold M, Bendahhou S, Pierson P, Sterner C, Peyronnet-Roux J, Benfriha C, Tegtmeier I, Ehnes H, Georgieff M, Lesage F, Brunet JF, Goridis C, Warth R, Barhanin J. 2010. Task2 potassium channels set central respiratory CO2 and O2 sensitivity. Proc Natl Acad Sci USA 107:2325–2330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Felipe A, Vicente R, Villalonga N, Roura-Ferrer M, Martínez-Mármol R, Solé L, Ferreres JC, Condom E. 2006. Potassium channels: new targets in cancer therapy. Cancer Detect Prev 30:375–385 [DOI] [PubMed] [Google Scholar]
- 13. Prevarskaya N, Skryma R, Shuba Y. 2010. Ion channels and the hallmarks of cancer. Trends Mol Med 16:107–121 [DOI] [PubMed] [Google Scholar]
- 14. Lang F, Föller M, Lang K, Lang P, Ritter M, Vereninov A, Szabo I, Huber SM, Gulbins E. 2007. Cell volume regulatory ion channels in cell proliferation and cell death. Methods Enzymol 428:209–225 [DOI] [PubMed] [Google Scholar]
- 15. Wang Z. 2004. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch 448:274–286 [DOI] [PubMed] [Google Scholar]
- 16. Dubois JM, Rouzaire-Dubois B. 2004. The influence of cell volume changes on tumour cell proliferation. Eur Biophys J 33:227–232 [DOI] [PubMed] [Google Scholar]
- 17. Ouadid-Ahidouch H, Ahidouch A. 2008. K+ channel expression in human breast cancer cells: involvement in cell cycle regulation and carcinogenesis. J Membr Biol 221:1–6 [DOI] [PubMed] [Google Scholar]
- 18. Shen Z, Yang Q, You Q. 2009. Researches toward potassium channels on tumor progressions. Curr Top Med Chem 9:322–329 [DOI] [PubMed] [Google Scholar]
- 19. Wonderlin WF, Strobl JS. 1996. Potassium channels, proliferation and G1 progression. J Membr Biol 154:91–107 [DOI] [PubMed] [Google Scholar]
- 20. Ikuma M, Binder HJ, Geibel J. 1998. Role of apical H-K exchange and basolateral K channel in the regulation of intracellular pH in rat distal colon crypt cells. J Membr Biol 166:205–212 [DOI] [PubMed] [Google Scholar]
- 21. Schreiber R. 2005. Ca2+ signaling, intracellular pH and cell volume in cell proliferation. J Membr Biol 205:129–137 [DOI] [PubMed] [Google Scholar]
- 22. Shrode LD, Tapper H, Grinstein S. 1997. Role of intracellular pH in proliferation, transformation, and apoptosis. J Bioenerg Biomembr 29:393–399 [DOI] [PubMed] [Google Scholar]
- 23. Gillies RJ, Martinez-Zaguilan R, Martinez GM, Serrano R, Perona R. 1990. Tumorigenic 3T3 cells maintain an alkaline intracellular pH under physiological conditions. Proc Natl Acad Sci USA 87:7414–7418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Welboren WJ, van Driel MA, Janssen-Megens EM, van Heeringen SJ, Sweep FC, Span PN, Stunnenberg HG. 2009. ChIP-Seq of ERα and RNA polymerase II defines genes differentially responding to ligands. EMBO J 28:1418–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu Y, Gao H, Marstrand TT, Ström A, Valen E, Sandelin A, Gustafsson JA, Dahlman-Wright K. 2008. The genome landscape of ERα- and ERβ-binding DNA regions. Proc Natl Acad Sci USA 105:2604–2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cicatiello L, Mutarelli M, Grober OM, Paris O, Ferraro L, Ravo M, Tarallo R, Luo S, Schroth GP, Seifert M, Zinser C, Chiusano ML, Traini A, De Bortoli M, Weisz A. 2010. Estrogen receptor α controls a gene network in luminal-like breast cancer cells comprising multiple transcription factors and microRNAs. Am J Pathol 176:2113–2130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Rusznák Z, Pocsai K, Kovács I, Pór A, Pál B, Biró T, Szücs G. 2004. Differential distribution of TASK-1, TASK-2 and TASK-3 immunoreactivities in the rat and human cerebellum. Cell Mol Life Sci 61:1532–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Olsen ML, Sontheimer H. 2004. Mislocalization of Kir channels in malignant glia. Glia 46:63–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barriere H, Belfodil R, Rubera I, Tauc M, Lesage F, Poujeol C, Guy N, Barhanin J, Poujeol P. 2003. Role of TASK2 potassium channels regarding volume regulation in primary cultures of mouse proximal tubules. J Gen Physiol 122:177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Santarius T, Bignell GR, Greenman CD, Widaa S, Chen L, Mahoney CL, Butler A, Edkins S, Waris S, Thornalley PJ, Futreal PA, Stratton MR. 2010. GLO1-A novel amplified gene in human cancer. Genes Chromosomes Cancer 49:711–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mu D, Chen L, Zhang X, See LH, Koch CM, Yen C, Tong JJ, Spiegel L, Nguyen KC, Servoss A, Peng Y, Pei L, Marks JR, Lowe S, Hoey T, Jan LY, McCombie WR, Wigler MH, Powers S. 2003. Genomic amplification and oncogenic properties of the KCNK9 potassium channel gene. Cancer Cell 3:297–302 [DOI] [PubMed] [Google Scholar]
- 32. Liu C, Cotten JF, Schuyler JA, Fahlman CS, Au JD, Bickler PE, Yost CS. 2005. Protective effects of TASK-3 (KCNK9) and related 2P K channels during cellular stress. Brain Res 1031:164–173 [DOI] [PubMed] [Google Scholar]
- 33. Patel AJ, Lazdunski M. 2004. The 2P-domain K+ channels: role in apoptosis and tumorigenesis. Pflugers Arch 448:261–273 [DOI] [PubMed] [Google Scholar]
- 34. Farkas A, Dempster J, Coker SJ. 2008. Importance of vagally mediated bradycardia for the induction of torsade de pointes in an in vivo model. Br J Pharmacol 154:958–970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schmidt D, Wilson MD, Spyrou C, Brown GD, Hadfield J, Odom DT. 2009. ChIP-seq: using high-throughput sequencing to discover protein-DNA interactions. Methods 48:240–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Haring M, Offermann S, Danker T, Horst I, Peterhansel C, Stam M. 2007. Chromatin immunoprecipitation: optimization, quantitative analysis and data normalization. Plant Methods 3:11. [DOI] [PMC free article] [PubMed] [Google Scholar]