Abstract
Forkhead box L2 (FoxL2) is required for ovarian development and differentiation. FoxL2 is also expressed in the pituitary where it has been implicated in the development and regulation of gonadotropes, which secrete LH and FSH, the endocrine signals that regulate folliculogenesis in the ovary and spermatogenesis in the testis. Here, we show that FoxL2 is not required for the specification of gonadotropes; the pituitaries of Foxl2 mutant mice contain normal numbers of gonadotropes that express glycoprotein α subunit and LHβ. Whereas the specification of gonadotropes and all other hormonal cell types is normal in the pituitaries of Foxl2 mutant animals, FSHβ levels are severely impaired in both male and female animals, suggesting that FoxL2 is required for normal Fshb expression. The size of the pituitary is reduced in proportion to the smaller body size of Foxl2 mutants, with a concomitant increase in the pituitary cellular density. In primary pituitary cultures, activin induces FSH secretion and Fshb mRNA expression in cells from wild-type mice. In cells from Foxl2 mutant mice, however, FSH secretion is not detected, and activin is unable to drive Fshb expression, suggesting that the mechanism of activin-dependent activation of Fshb transcription is impaired. However, a small number of gonadotropes in the ventromedial region of the pituitaries from Foxl2 mutant mice maintain FSHβ expression, suggesting that a FoxL2- and activin-independent mechanism can drive Fshb transcription. These data indicate that, in addition to its role in the ovary, FoxL2 function in the pituitary is required for normal expression of FSH.
Foxl2 (P-frk) encodes a forkhead transcription factor first identified in the ventral portion of the prospective pituitary of embryonic d 10.5 (e10.5) mouse embryos (1). More recently, Foxl2 has been identified as the gene mutated in blepharophimosis/ptosis/epicanthus inversus syndrome, a craniofacial disorder that affects eyelid development and causes premature ovarian failure in a subset of females (2). Forkhead box L2 (FoxL2) is expressed in the developing eyelid and in granulosa cells of the developing and adult ovary, as well as in the developing and adult pituitary (1–3). The role of FoxL2 in the developing ovary has been uncovered from observations in Foxl2 loss-of-function mutant mice in which folliculogenesis fails due to incomplete granulosa cell development, leading to infertility (4, 5). The observation that loss of Foxl2 and Wnt4 leads to the appearance of testicular structures in mutant XX female mice is consistent with the participation of FoxL2 as an antitestis factor (6). More recently, it has been shown that inducible deletion of Foxl2 in the adult ovary is sufficient to induce testicular markers in ovarian cells and reprogram them to testicular lineages, consistent with the requirement for FoxL2 to actively maintain ovarian follicles and prevent ovary to testis sex reversal (7). Although the importance of cell-autonomous FoxL2 action to proper ovarian maturation and function has been established, the contribution of pituitary FoxL2 function to the regulation of the reproductive axis is not understood.
The differential synthesis and secretion of the heterodimeric gonadotropins, LH and FSH, are achieved by the coordinated actions of hypothalamic GnRH, autocrine/paracrine factors such as activin and follistatin, and feedback signals including gonadal steroids and inhibin (for review see Refs. 8 and 9). Whereas the glycoprotein α subunit (αGSU) of FSH is shared by LH in gonadotropes and TSH in thyrotropes, the FSHβ and LHβ subunits are the rate-limiting steps in the production of gonadotropins and define the identity of the terminally differentiated gonadotrope (10). The cyclic variations and differential secretion of LH and FSH are key events that control many aspects of gonadal function in both males and females. In female rodents, the secondary FSH surge late in the estrous cycle is essential for folliculogenesis and largely driven by the actions of activin (11, 12). Deletion of Fshb impairs folliculogenesis, leads to female infertility, and compromises the fertility of mutant males (13).
In the developing pituitary, FoxL2 expression coincides with that of αGSU after e11.5, and in the adult pituitary is restricted to thyrotropes and gonadotropes of both male and female mice (3, 14). Consistent with the colocalization of FoxL2 with αGSU in gonadotropes, Foxl2 is expressed in cell lines representative of this lineage, αT3–1 and LβT2 cells (3, 14). Results from studies with both gonadotrope cell lines show that FoxL2 is involved in the regulation of several key targets such as Follistatin (Fst), Fshb, Gnrhr, and Cga, genes that have been established to directly or indirectly affect ovarian folliculogenesis (3, 14–17). At least three of these, Fst, Fshb and Gnrhr, are also targets of the activin pathway (18–22). The promoter regions of all three genes contain Smad (Sma- and Mad-related protein)-binding elements as well as adjacent Forkhead-binding sites that recruit FoxL2 and mediate their effects on transcription (14, 16, 17, 23). In the case of Fst, FoxL2 is permissive to Smad3-dependent activation of transcription (14). The functional interactions of Smad3 and FoxL2 also activate the murine and human Fshb promoters, although activation of the human promoter by FoxL2 might not be exclusively dependent on the Smad pathway (17). With respect to the Gnrhr promoter, FoxL2 seems to be involved in mediating GnRH effects via activator protein 1 as well as activin effects via Smad3/4 (15). These observations are consistent with the possibility that FoxL2 actions in pituitary gonadotropes work in concert with activin- and GnRH-induced signals to promote cyclic variations and differential patterns of LH and FSH secretion essential for ovarian folliculogenesis and fertility.
In the present study, we address the in vivo importance of FoxL2 for FSHβ expression in pituitary gonadotropes by analyzing pituitary hormone expression in Foxl2 mutant animals. We find that in animals lacking Foxl2, FSHβ expression is severely limited to a small subset of gonadotropes, whereas gonadotrope specification and LHβ expression by gonadotropes remain largely unaffected by the loss of Foxl2. Foxl2 loss of function does not affect the specification of any of the lineages of pituitary hormone-secreting cells. However, the overall size of the pituitary is reduced in Foxl2 mutant animals leading to increased cellular density. Fshb mRNA levels are significantly lower in Foxl2 mutant animals whereas Lhb mRNA levels are not affected but Cga mRNA expression is reduced. FSH secretion from primary cultures of Foxl2 mutant pituitaries is severely impaired and undetectable whereas basal and GnRH-induced LH secretion is equivalent to those measured in cultures of wild-type pituitaries. These findings demonstrate that FoxL2 is required for appropriate expression of FSHβ by pituitary gonadotropes and raise the possibility that pituitary actions of FoxL2 contribute to the established role of this forkhead protein in ovarian development.
Results
Pituitary size is decreased and cellular density is increased in Foxl2 mutant mice
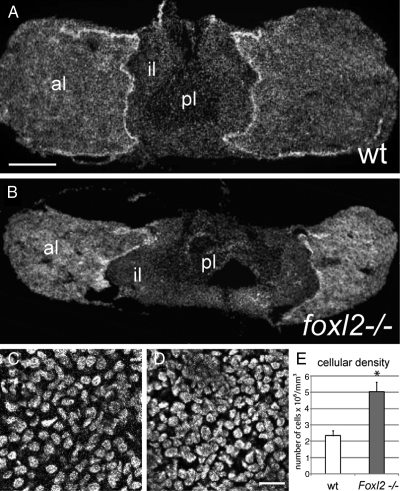
We analyzed the pituitary glands from Foxl2 mutant animals and compared them with wild-type controls. Foxl2 mutant animals were smaller than wild-type or heterozygous littermates and had eyelid deformities characteristic of impaired craniofacial development, as described in previous studies (4, 5). Upon examination of the pituitaries from Foxl2 mutants and wild-type littermates, we immediately noticed that the entire gland of the mutant animals was diminutive, with the intermediate lobe occupying a broader section of the medial region of the gland and the anterior lobes displaced laterally, as shown for representative pituitary cross-sections from male animals (Fig. 1, A and B). The size reduction of the mutant pituitary glands appeared to be proportional to the smaller body size of the mutant animals, although we did not quantify these differences. After sectioning, pituitaries were nissl stained and subjected to unbiased stereology. The cellular densities of Foxl2 mutant pituitaries were almost 2-fold greater when compared with wild-type littermate control pituitaries, as shown for the male (5.05 ± 0.3 × 106 vs. 2.4 ± 0.57 × 106 cells/mm3; n = 3 per genotype; P < 0.05; Fig. 1, C and D). The pituitaries of female Foxl2 mutant animals were similarly smaller with an equivalent increase in cellular densities (data not shown). The volume of the anterior lobes of the Foxl2-/- pituitaries is reduced by greater than 2-fold (1.02 ± 0.11 × 108 vs. 2.86 ± 0.93 × 108 μm3, n = 3 per genotype; P < 0.05). Given the increased density and decreased size of the Foxl2-/- pituitary, the number of cells per mutant pituitary was estimated to be 17% lower than wild type, a difference that failed to reach statistical significance (1.02 ± 0.54 × 106 vs. 1.23 ± 0.21 × 106 cells/mm3; n = 3 per genotype; P = 0.16). These measurements suggest that the smaller size of the Foxl2-/- pituitary leads to an increase in the density of cells in the anterior lobe and a small reduction in the total number of pituitary cells.
Fig. 1.
Foxl2 mutation results in reduced size and increased cellular density of the pituitary. A, Cross-section of a wild-type (wt) pituitary from a male mouse stained with DAPI showing the anterior lobes (al), intermediate lobe (il), and posterior lobe (pl). B, Cross-section of a pituitary from a 3-wk-old male Foxl2 mutant mouse stained with DAPI. Scale bar, 250 μm. C, Representative high-resolution optical section of DAPI-labeled nuclei from the anterior lobe of a wild-type pituitary. D, Representative high-resolution optical section from a Foxl2 mutant pituitary. E, Quantification of the increase in cellular density observed in Foxl2 mutant pituitaries compared with wild-type pituitaries (n = 3 per group; *, P < 0.05; Scale bar, 20 μm).
All hormonal cell types are specified in Foxl2 mutant pituitaries
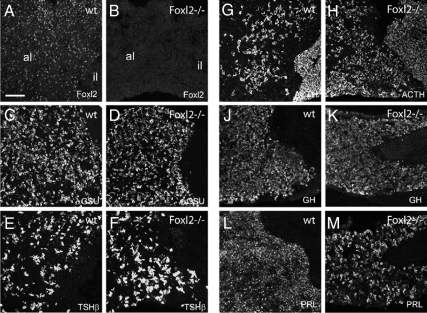
FoxL2 is expressed in all anterior pituitary cells that express αGSU, including thyrotropes and gonadotropes (3, 14), and this expression is absent in Foxl2 mutant pituitaries (Fig. 2, A and B). Therefore we evaluated Foxl2-/- animals to assess whether the number of cells expressing αGSU and all other markers of pituitary cell types is altered in the Foxl2 mutant pituitary. In the first series of experiments, we compared the pituitaries of mutant and wild-type males, as summarized in Figs. 2–4 and Supplemental Table 1 published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org. With few exceptions that are noted below, we observed similar changes in the pituitaries of female Foxl2 mutant animals (data on LHβ and FSHβ staining in female pituitaries shown in Supplemental Fig. 1). We stained for αGSU protein and found that the density of αGSU-positive cells is increased 18% in the male Foxl2 mutant pituitary compared with wild-type pituitary (7.7 ± 2.1 vs. 6.5 ± 2.5 × 103 cells/mm2; Fig. 2, C and D). Given the increase in the density of cells in the Foxl2 mutant pituitary and the decrease in size, we estimate that the total number of αGSU-positive cells is similar between the Foxl2 mutant and wild-type pituitaries. To count gonadotropes, we stained for the gonadotrope marker, LHβ, and again found that Foxl2 mutant pituitaries contain a higher density of LHβ cells (5.9 ± 1.5 vs. 4.7 ± 1.1 × 103 cells/mm 2; Fig. 3, B and E). Similar to αGSU- and LHβ-positive cells, Foxl2 mutant and wild-type pituitaries contain approximately the same density of TSHβ-positive cells localizing to the central region of the anterior lobe (3.3 ± 0.93 vs. 3.1 ± 1.9 × 103 cells/mm 2; Fig. 2, E and F). We also quantified the density of corticotropes, somatotropes, and lactotropes by staining for ACTH, GH, and prolactin (PRL), respectively. The male Foxl2 mutant pituitaries contain a higher density of ACTH-immunoreactive corticotropes (4.7 ± 0.20 vs. 2.8 ± 0.5 × 103 cells/mm2, P < 0.01; Fig. 2, G and H). Female pituitaries contained a similar increase in the number of ACTH-positive corticotropes (data not shown). The GH cell density in pituitaries from male Foxl2 mutant pituitaries was slightly higher compared with male wild-type tissue (7.9 ± 0.5 vs. 5.5 ± 0.5 × 103 cells/mm 2, P < 0.05; Fig. 2, J and K). There was an equivalent increase in GH cell density in the pituitaries of female Foxl2 mutant mice compared with wild type (data not shown). The density of PRL cells in the pituitaries of male Foxl2 mutant animals was not statistically different from wild-type controls (4.76 ± 0.3 vs. 4.2 ± 0.5 × 103 cells/mm2; Fig. 2, L and M) but there were fewer PRL-positive cells in female Foxl2 mutant pituitaries compared with female wild-type tissue (2.4 ± 0.3 vs. 4.3 ± 0.6 × 103 cells/mm2, P < 0.05; data not shown). These observations suggest that FoxL2 is not required for the specification of any of the pituitary hormonal cell types. The overall changes in the size and the cell density and of the pituitary in Foxl2 mutant animals, coupled with changes in the relative numbers of some cell types, however, raise the formal possibility that pituitary development and lineage specification are influenced by Foxl2 loss of function but that none of the cell types fail to be specified.
Fig. 2.
Pituitary hormone expression in the Foxl2 mutant pituitary compared with wild type (wt). A and B, Confocal micrographs of the anterior (al) and intermediate (il) lobes of a pituitary from wild-type (A) or Foxl2 mutant (B) animal stained for FoxL2. C and D, Staining for αGSU in wild-type (C) or Foxl2 mutant (D) pituitary. E and F, Staining for TSHβ in the wild-type (E) or Foxl2 mutant (F) pituitary. G and H, Staining for ACTH in the wild-type (G) or Foxl2 mutant (H) pituitary. J and K, Staining for GH in the wild-type (J) or Foxl2 mutant (K) pituitary. L and M, Staining for PRL in wild-type (L) or Foxl2 mutant (M) pituitary. Images are of pituitary sections from wild-type or Foxl2 mutant male mice. Scale bar, 100 μm.
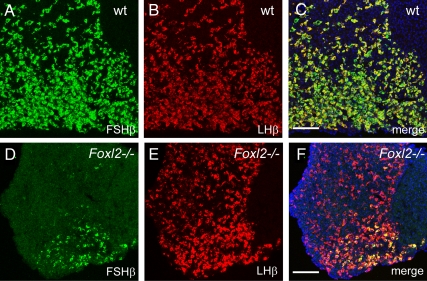
Fig. 3.
FSHβ is absent from most gonadotropes in the Foxl2 mutant pituitary. Confocal micrographs of a 3-wk-old male wild-type (wt) (A–C) or Foxl2 mutant (D–F) pituitary stained for FSHβ, LHβ, and DAPI. Scale bar, 100 μm.
Fig. 4.
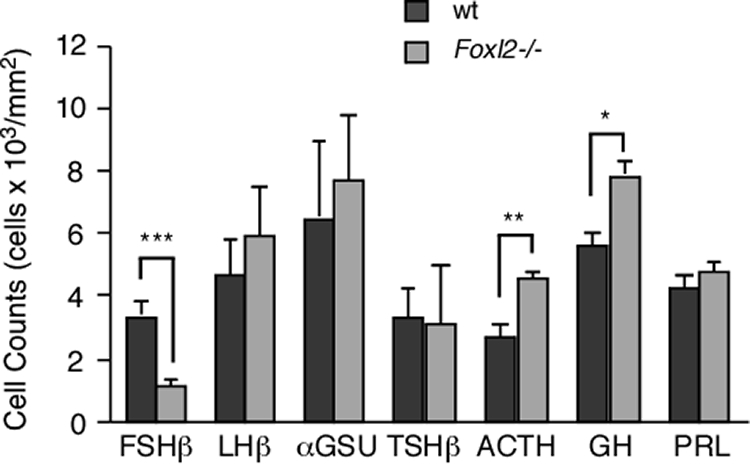
Histogram showing the relative densities of cells expressing each of the anterior pituitary hormonal markers in wild-type (wt) (dark gray bars) and Foxl2 mutant (light gray bars) animals. Values are expressed as averages of cell counts/mm2 ± sem. (*, P < 0.05; **, P < 0.01; ***, P < 0.001). Further details on the number of animals used are presented in Supplemental Table 1.
FSHβ expression is absent in most gonadotropes in Foxl2 mutant pituitaries
Given the importance of FoxL2 in ovarian development and its recently characterized role in regulating the transcription of several key targets, including Fshb, in gonadotrope-derived cell lines, we examined FSHβ protein levels in the pituitaries of Foxl2 mutant and wild-type mice. Using LHβ as a marker for gonadotropes, colabeling for FSHβ revealed a severe reduction in the number of gonadotropes in 3-wk-old male FoxL2 mutant pituitaries that express FSHβ, compared with wild-type male pituitary (1.1 ± 0.2 vs. 3.4 ± 0.5 × 103 cells/mm2, P < 0.001; Figs. 3 and 4). An equivalent reduction in FSHβ staining was also evident in older, 5-wk-old males (data not shown). Interestingly, the gonadotropes that maintained FSHβ expression in male mutant tissue were always found in the ventral portions of the anterior lobes whereas the central portions were largely free of FSHβ-positive gonadotropes. The few remaining FSHβ-positive gonadotropes always occupied the ventromedial area of the anterior pituitary, immediately adjacent to the intermediate lobe (Fig. 3). When we counted the FSHβ-positive cells in the ventromedial quadrant of the Foxl2 mutant pituitary where the most FSHβ-positive gonadotropes remained, we found that only a small fraction of LHβ-positive gonadotropes expressed FSHβ compared with near-complete coexpression of FSHβ and LHβ in the wild-type pituitary (13.3 ± 5.2% vs. 96.8 ± 3.3%). Interestingly, whereas we noted a similar reduction in the number of FSHβ-positive cells in the anterior pituitary of female Foxl2 mutant animals compared with female wild-type littermates, the distribution of these FSHβ-positive cells in the female was less restricted to the ventromedial area, with sparse FSHβ-positive cells located throughout the ventral region of the anterior pituitary (Supplemental Fig. 1). The presence of an equivalent number of LHβ-positive cells coupled with the reduction in the number of these cells positive for FSHβ in the Foxl2 mutant pituitary suggest that FoxL2 is not required for the specification of gonadotropes, but is required for the expression of FSHβ by most gonadotropes. The distribution of the remaining FSHβ-positive cells in the ventromedial quadrant of the anterior pituitary suggests that FoxL2-independent mechanism can drive FSHβ expression in this region of the pituitary.
Pituitary hormone expression levels in Foxl2 mutant pituitaries
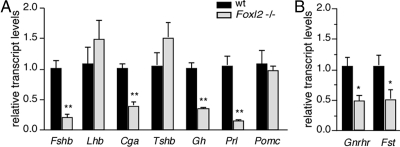
To quantify pituitary hormone expression levels, we isolated RNA from the pituitaries of female Foxl2 mutant and wild-type mice and used quantitative RT-PCR (qRT-PCR) to quantify transcript levels of all major pituitary hormones. In accordance with our findings of severely impaired FSHβ expression in the pituitary sections of both male and female Foxl2 mutant animals, Fshb mRNA expression was significantly lower in the pituitaries of Foxl2 mutant animals compared with wild-type littermates (21% ± 0.06 of wild type; Fig. 5A). Whereas there was no significant difference in the expression level of either Lhb or Tshb mRNA between the two genotypes, consistent with immunohistochemical staining data, Cga mRNA levels were lower in Foxl2 mutant pituitaries (Fig. 5A). Given the immunohistochemical staining data showing that the distribution and number of αGSU cells is not significantly altered in Foxl2 mutant pituitaries (Fig. 2, C and D), we interpret this decrease in Cga mRNA as a change in relative expression levels of αGSU in thyrotropes and gonadotropes. In addition, we quantified Gh, Prl, and Pomc mRNA levels in the pituitaries of female Foxl2 mutant and wild-type littermates. By contrast to the small increase in the number of GH-positive cells in Foxl2 mutant males (Fig. 4 and Supplemental Table 1) and females (data not shown), the expression of Gh mRNA was significantly lower in the mutant pituitaries (Fig. 5A). Prl mRNA levels in female Foxl2 mutant animals were also significantly lower than those in female wild-type animals (Fig. 5A). This is consistent with the reduction in the number of PRL-positive cells in the female (data not shown). Finally, in contrast to the increase in ACTH-positive cells of the anterior lobe of the mutant pituitaries, Pomc mRNA levels extracted from the entire pituitary gland (anterior and intermediate lobes) were not statistically different between Foxl2 mutant and wild-type animals (Fig. 5A). Overall, these findings suggest that loss of Foxl2 has both autonomous and nonautonomous effects, affecting pituitary hormone expression in pituitary cell types that normally express FoxL2 and those that do not, respectively.
Fig. 5.
Pituitary hormone mRNA expression in Foxl2 mutant relative to wild-type (wt) pituitaries. A, qRT-PCR was performed on pituitaries from female Foxl2 mutant mice (light gray bars, n = 3) or wild-type (dark gray bars, n = 4) littermates. Transcript levels were normalized to Gapdh, and calculated values for the expression of each hormone in the mutant pituitaries are reported relative to wild type. B, qRT-PCR analysis of Gnrhr and Fst transcripts in pituitaries from female Foxl2 mutant mice (light gray bars) or wild-type (dark gray bars) littermates; details are as described for panel A. (*, P < 0.05; **, P < 0.01).
The expression of known pituitary targets is lower in Foxl2 mutant pituitaries
In pituitary gonadotrope cell lines, FoxL2 has been shown to regulate the transcription of a limited number of target genes including Fst, Fshb, Gnrhr, and Cga (3, 14–17). As already shown in Fig. 5A, Fshb and Cga mRNA levels in the Foxl2 mutant pituitaries were 21% and 40% of the levels measured in wild-type controls, respectively. Similarly, Gnrhr and Fst mRNA levels in the Foxl2 mutant pituitaries were 49% and 51% of the levels in wild-type mice, respectively (Fig. 5B).
Foxl2 loss of function blocks activin-induced Fshb transcription
Locally produced activin B from gonadotropes has a critical role in driving Fshb transcription and maintaining a releasable pool of FSH for the secondary rise in FSH necessary for follicular development (24, 25). In the gonadotrope-derived LβT2 cell line, activin regulation of Fshb transcription is largely mediated by the actions of Smad3/4 as well as Smad2 (20, 26–28). Consistent with these findings, Fshb mRNA levels are reduced in pituitary cells from Smad3-null mice compared with wild-type pituitary cells (29). Activin regulation of the murine Fshb promoter is also dependent on FoxL2 expression, and it has been shown that the concerted actions of FoxL2 and Smad3 mediate activin induction of Fshb transcription in LβT2 cells (16, 17). We determined whether FoxL2 is required for Fshb expression in primary cultures of pituitaries from female Foxl2 mutant (n = 4) or wild type (n = 4) animals under basal conditions and in response to activin A. Under basal conditions, the expression level of Fshb mRNA was significantly lower in primary cells of Foxl2 mutant pituitaries compared with wild type (0.19 ± 0.08 vs. 0.99 ± 0.06, respectively; Fig. 6). These data are consistent with our immunohistochemical experiments and qRT-PCR analysis of pituitary tissue. In wild-type primary pituitary cultures, treatment with a maximal concentration of activin A for 18 h induced a 2-fold increase in Fshb mRNA expression (0.99 ± 0.06 vs. 2.21 ± 0.31; Fig. 6). By contrast, activin A had no effect on Fshb mRNA expression in Foxl2 mutant pituitary cultures (Fig. 6). Whereas basal Fshb mRNA levels were significantly lower in the mutant cultures, Lhb mRNA levels in primary cultures of Foxl2 mutant pituitaries were not significantly different from those of wild-type cells and, as expected, not affected by activin A (Fig. 6). Compared with pituitary cells from wild-type mice, Foxl2 mutant pituitaries expressed even higher levels of activin type II receptors (Acvr2a and Acvr2b), activin type I receptor (Acvr1b), as well as Smad2 and Smad3, suggesting that the overall sensitivity to activin is not compromised in Foxl2 mutant pituitaries (Fig. 7). The latter is further supported by data showing that small interfering RNA-mediated knockdown of FoxL2 in αT3–1 or LβT2 cells does not compromise activin-induced phosphorylation of Smad2 (14).
Fig. 6.
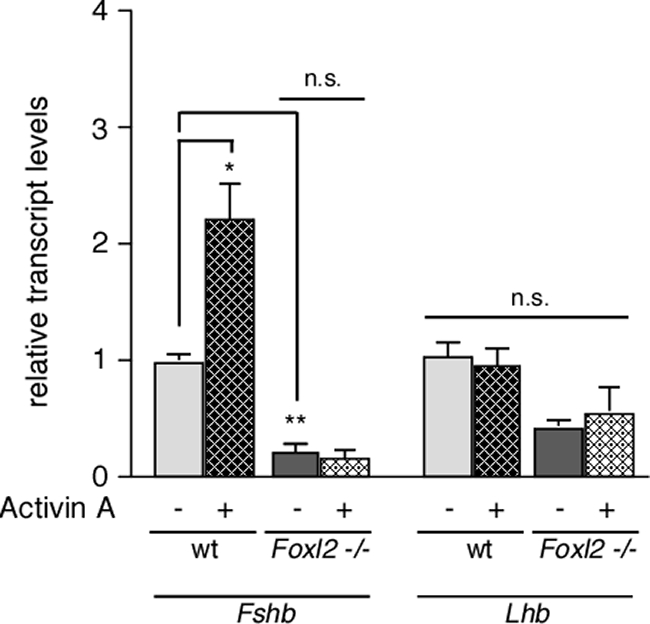
Activin A does not induce Fshb mRNA expression in primary cell cultures of Foxl2 mutant pituitaries. Primary pituitary cultures were prepared from wild-type (wt) (n = 4) and Foxl2 mutant (n = 4) female mice and exposed to 10 nm activin (hatched bars) or vehicle (solid gray bars) for 18 h. Fshb and Lhb mRNA levels were measured by qRT-PCR, normalized to Gapdh mRNA values, and are reported relative to vehicle-treated wild-type cells. (*, P < 0.05; **, P < 0.01; n.s., not significant, P > 0.05).
Fig. 7.
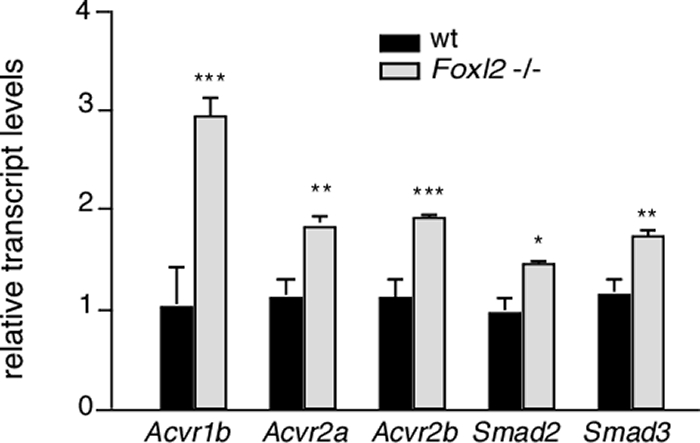
Activin receptors and Smad2/3 are expressed in Foxl2 mutant pituitaries. The mRNA expression levels of activin type II receptors (Acvr2a and Acvr2b), activin type I receptor (Acvr1b), as well as Smad2 and Smad3 in pituitaries from female Foxl2 mutant (light gray bars, n = 3) or wild-type (wt) (dark gray bars, n = 4) mice were quantified by qRT-PCR, as described for Fig. 4. (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
Foxl2 loss of function causes a dramatic reduction in FSH secretion from primary pituitary cultures
Given the dramatic reduction in the number of FSHβ-immunoreactive cells and the level of Fshb mRNA expression in the pituitaries of Foxl2 mutant animals, we evaluated FSH and LH secretion from primary cell cultures of pituitaries from Foxl2 mutant and wild-type females. Consistent with previous findings, a maximal concentration of activin A stimulated FSH secretion from pituitary cells of wild-type animals (2.04 ± 0.2 fold increase from a basal level of 11.9 ± 2.02 ng/ml/18 h; Fig. 8A). By contrast, pituitary cells from Foxl2 mutant females did not secrete measurable FSH levels either under basal conditions or after activin A treatment (Fig. 8A). Basal LH secretion from pituitary cells of Foxl2 mutant animals, on the other hand, was not statistically different from the amount secreted by pituitary cells from wild-type animals (2.93 ± 2.66 vs. 5.15 ± 1.72 ng/ml/18 h, respectively). Moreover, the cultures from both genotypes displayed an equivalent response to a maximal concentration of GnRH (2.17 ± 0.03 vs. 2.56 ± 0.77 fold stimulation for Foxl2 mutant and wild-type cells, respectively; Fig. 8B). These results suggest that, the gonadotrope population in primary cultures of pituitaries from Foxl2 mutant animals retain their ability to secrete LH and respond to GnRH, despite the reduction in Cga mRNA levels, but their ability to produce FSH is dramatically compromised.
Fig. 8.
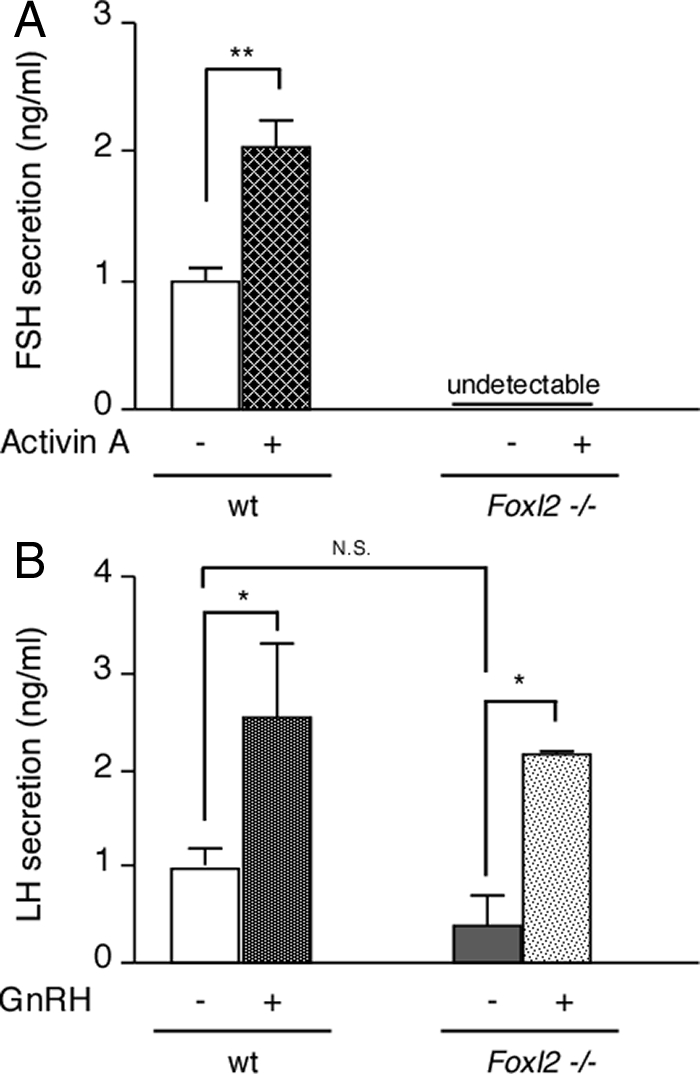
FSH and LH secretion by primary cell cultures of pituitaries from Foxl2 mutant or wild-type (wt) females. Primary cell cultures of pituitaries from wild-type (n = 7) or Foxl2 mutant (n = 8) female mice were treated for 18 h with vehicle, 10 nm activin A (A), or 5 nm GnRH (B). Cumulative FSH or LH secretion was measured by specific RIA by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core. A, Basal FSH secretion from wild-type cells was 11.9 ± 2.02 ng/ml · 18 h. B, Basal LH secretion from Foxl2 mutant and wild-type cells was 2.93 ± 2.66 and 5.15 ± 1.72, respectively. The data are the average (±sem) of normalized data from three independent experiments, with each experiment normalized internally to baseline (vehicle only) secretion from wild-type cells. (*, P < 0.05; **, P < 0.01; n.s., not significant, P > 0.05).
Discussion
To define the role of FoxL2 in the pituitary, we have evaluated the pituitaries of Foxl2 mutant mice by immunohistochemistry and qRT-PCR. We show that FoxL2 is required for normal expression of FSHβ by pituitary gonadotropes. Foxl2 mutant pituitaries are smaller than the pituitaries of their wild-type littermates, with an equal but opposite increase in cellular density. All of the endocrine cell types are present. FoxL2 expression in the adult mouse pituitary is restricted to two cell types, gonadotropes and thyrotropes (3, 14). Immunohistochemical experiments show that αGSU, LHβ, and TSHβ glycoprotein subunit expression is maintained in the anterior pituitary lobes of male and female Foxl2 mutant mice. However, FSHβ is absent from the majority of those cells that otherwise contain LHβ, indicative of impaired FSHβ expression. The few FSHβ-positive cells that remain are LHβ positive and consistently localize to the ventral medial portion of the anterior pituitary in the male. The presence of these remaining FSHβ-positive cells suggests that Fshb expression can be driven by a FoxL2-independent transcriptional mechanism. The nature of this mechanism is not known at this time. The failure of activin to induce Fshb expression in primary cultures of Foxl2 mutant pituitaries, however, suggests that the activin-dependent transcriptional mechanism is insufficient to drive Fshb expression in the absence of FoxL2. The significant reduction in Fshb expression in the Foxl2 mutant pituitaries indicates that, in addition to the role of FoxL2 in the ovary, there is an additional requirement for FoxL2 at the pituitary level to promote Fshb transcription and maintain FSH at levels necessary for normal folliculogenesis and ovarian function. The latter is supported by in vitro data showing that FSH secretion from primary cultures of Foxl2 mutant pituitaries is severely diminished and below measurable levels of the RIA employed (Fig. 8A) as well as by preliminary in vivo observations showing that basal circulating FSH levels of Foxl2 mutant females are significantly lower than those of control littermates (8.03 ± 0.5 vs. 19 ± 0.7; P < 0.05) and, by contrast to wild-type controls, female Foxl2 mutant animals fail to display the Whitten effect and the expected FSH surge (our unpublished preliminary observations).
The dramatic reduction in the number of FSHβ-positive cells, Fshb mRNA levels, and FSH secretion in the Foxl2 mutant pituitaries, that otherwise contain αGSU/LHβ-positive cells equivalent in number to those found in wild-type pituitaries and retain the ability to secrete LH at wild-type levels, suggests that FoxL2 has a role in gonadotropes but is not required for the specification of gonadotropes or any of the other endocrine cell types. This is surprising given that FoxL2 is one of the earliest markers of differentiating pituitary cells and is detectable in the embryonic pituitary by e10.5 (1, 3). The temporal and spatial coincidence of FoxL2 and αGSU expression links FoxL2 to gonadotrope and thyrotrope function. Consistent with this, Foxl2 expression precedes the appearance of factors such as Sf1/Nr5a1 and Egr1 that are implicated in gonadotrope specification and coincides with Gata2 and Isl1, factors known to have roles in gonadotropes and thyrotropes (3). However, given our data showing that the relative proportions of most endocrine cell types, including gonadotropes, are largely unaffected by Foxl2 loss of function suggests that it does not contribute to cell type specification and differentiation or that another factor is able to compensate for its loss. Interestingly, the loss of another early signal, Lhx3, which normally shows a temporal and spatial expression pattern similar to that of FoxL2, results in an almost complete loss of all pituitary cell types, except for a few residual corticotropes, due to increased apoptosis (30). By contrast, the loss of the related factor, Lhx4, has a milder effect leading to a hypoplastic anterior lobe with the majority of endocrine cell types still present (31). Both of these factors are implicated in the regulation of the promoters of Cga, Fshb as well as Prl, Tshb, and Pit1 (32–34). Moreover, it has been suggested that Lhx3 and Lhx4 might also be involved in regulating Foxl2 expression, although whether or not this is a direct action has not been confirmed (3, 31). These observations raise the possibility that the absence of FoxL2 disrupts the Lhx3/4 program and thereby contributes to the dysmorphic and smaller size of the pituitaries in Foxl2 mutant mice. The additional effects of Foxl2 inactivation on lactotropes and, to some extent, somatotropes are consistent with the possibility that Foxl2 inactivation disrupts the establishment of the Pit1 lineage, perhaps by altering the differential expression patterns of factors such as Gata2 and Sf1/Nr5a1 (3). The selective effect of Foxl2 loss of function on Fshb expression in gonadotropes, however, suggests that FoxL2 is required for later stages of gonadotrope differentiation, specifically to drive basal and regulated FSHβ expression and possibly other aspects of gonadotrope differentiation and function.
Alternative transcriptional mechanisms for FSHβ
In animals lacking Foxl2, most gonadotropes fail to express FSHβ; however, a small proportion of gonadotropes maintain FSHβ expression. Interestingly, the remaining FSHβ-positive cells are always localized to the ventral medial portion of the anterior pituitary in males and more diffusely to the ventral half of the anterior pituitary in females, suggesting that these gonadotropes can express FSHβ in the absence of FoxL2 whereas gonadotropes localized in other parts of the anterior pituitary depend on FoxL2 to drive FSHβ expression. One hypothesis to explain this consistent localization of remaining FSHβ-positive cells in the Foxl2 mutant pituitaries is that these cells are exposed to a paracrine signal that drives FoxL2-independent FSHβ expression. One candidate for this signal is BMP-2, which has been shown to drive activin-independent FSHβ expression in LβT2 cells (21, 35, 36). An alternative hypothesis is that gonadotrope-specific transcription factors are able to compensate for FoxL2 and drive FSHβ expression in these cells. Many transcription factors have been reported to be involved in directing the expression of FSHβ including Isl-1, Pitx1 and Pitx2, Prop1, and Lhx3 (34, 37–40). One or a combination of these transcription factors may be able to drive FSHβ expression in the absence of FoxL2, at least in certain cellular contexts. Finally, gonadal steroid receptors have been found to be involved in the transcriptional regulation of Fshb (27, 41). Perhaps gonadotropes in the ventral medial region respond more robustly to progesterone/testosterone and thereby overcome the absolute need for FoxL2 to drive FSHβ expression. These alternative mechanisms are most likely secondary to the FoxL2-dependent mechanism because FSHβ and FSH secretion levels are severely decreased in the absence of FoxL2.
The importance of Foxl2 to nongonadotrope pituitary cell types
The role of FoxL2 in thyrotropes is unclear, and our data suggest that FoxL2 is not a major regulator of TSHβ expression. Immunohistochemical experiments do not show significant differences in the number of TSHβ-positive cells and qRT-PCR analysis indicates that Tshb mRNA levels are not significantly different in the two genotypes. However, whereas the density of αGSU-positive cells is the same in both genotypes, Cga mRNA levels in the Foxl2 mutant animals are approximately 50% of the levels measured in the pituitaries of wild-type littermates. We speculate that this reduction reflects changes in both gonadotropes and thyrotropes, in line with experimental data indicating that FoxL2 is a regulator of the Cga promoter in gonadotropic αT3–1 and LβT2 cells and thyrotrope-like αTSH cells (3). Given that qRT-PCR assays are far more quantitative than are immunohistochemical analyses, it is possible that the decrease in αGSU immunoreactivity is below the limit of immunohistochemical detection. Surprisingly, Gh and Prl mRNA levels are also lower in Foxl2 mutant mice. The mechanism underlying this reduction is likely indirect rather than a direct action on the respective promoters given that FoxL2 is not present in either somatotropes or lactotropes. The decrease in Gh and Prl mRNA expression as well as PRL-positive cells in the females could arise secondary to alterations in feedback signals from the gonads or paracrine interactions within the local pituitary environment (42–47). Gonadal steroids and IGF-I are established feedback regulators of GH and PRL expression (43–47). Foxl2 mutant mice have lower circulating IGF-I levels compared with wild types, and, given the ovarian phenotype of the females, are expected to display hypoestrogenism as well as elevated serum testosterone (4, 5, 7).
FoxL2 functions at multiple levels of the hypothalamic pituitary gonadal (HPG) axis
Gonadotropes secrete LH and FSH in response to GnRH release from the hypothalamus, which, in turn, activate granulosa cell differentiation and growth in the ovary. Activation of granulosa cell differentiation requires FoxL2; in the absence of FoxL2, granulosa cells fail to differentiate leading to sterility (4, 5). The recent observation that somatic reprogramming of the ovarian follicles to testis-like structures occurs upon conditional inactivation of Foxl2 in the ovary of 8-wk-old adult female mice has established FoxL2 as an ovarian maintenance factor (7). However, our data suggest that FoxL2 also functions at the level of the pituitary. In vitro evidence as well as in vivo data presented here altogether demonstrate that FoxL2 is required for transcription of Fshb and FSH secretion, suggesting that in Foxl2 mutants, both the ovary and pituitary are dysfunctional (16, 17). Misfunction in both tissues might contribute to sterility and ovarian anomalies. The Fshb mutant mice are infertile and display a failure in folliculogenesis, as do Foxl2 mutants (13). It remains possible that disruptions of the pituitary functions of FoxL2 to regulate the expression of a variety of targets and maintain FSHβ levels contribute to ovarian anomalies and infertility in Foxl2 mutants.
Materials and Methods
Animals and genetic crosses
The mutant allele of Foxl2 and the mutant Foxl2 line were generated as described elsewhere (5). Heterozygous Foxl2 mutants were rederived by embryo transfer into wild-type C57BL/J mice and outbred to the same strain. Heterozygous Foxl2 animals were interbred to generate mice homozygous null for Foxl2. Animals were genotyped as follows: 5′-GGATCTCTGAGTGCCAACGC and 5′-CACGGGAAAGCAGAGGCCGC forward and reverse primers, respectively, for the wild-type allele and 5′-AGACAATCGGCTGCTCTGAT and 5′-ATACTTTCTCGGCAGGAGCA forward and reverse primers, respectively, for the neomycin cassette. As described previously, the homozygous mutant animals experience a high rate of perinatal mortality, and the majority of surviving mutant animals are lost before or at the time of weaning (4, 5). Hence, the various analyses described below were largely confined to 3-wk-old animals but few 5-wk-old animals were also evaluated. Immunohistochemical experiments were performed on 3-wk-old males and, where indicated, on a limited number of 5-wk-old animals as well as females. For all other experiments, including primary cell preparations and qRT-PCR analyses, pituitary was obtained from female animals. All protocols for animal breeding, handling, and experimental procedures were approved by the Institutional Animal Care and Use Committee of the Salk Institute.
Immunohistochemistry and cell counting
Mutant animals (3 wk of age) and wild-type littermates were transcardially perfused with cold saline followed by 4% paraformaldehyde. The pituitary was dissected out, embedded in tissue freezing medium (O.C.T. compound, Sakura Finetek, Torrance, CA), and sectioned on a cryostat at 20 μm. For Nissl staining, slides were stained with cresyl violet, dehydrated, and mounted with DPX. For immunohistochemsitry, slides were washed three times in Krebs PBS (KPBS) and incubated with primary antibodies overnight at 4 C in KPBS with 2% normal donkey serum and 0.4% Triton X-100. Antibodies from the National Hormone and Peptide Program of National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) included rabbit anti-αGSU (1:1000, NIDDK), guinea pig anti-FSHβ (1:10,000, NIDDK), rabbit anti-LHβ (1:1000, NIDDK), guinea-pig anti-TSHβ (1:5,000, NIDDK), guinea pig anti-GH (1:15,000, NIDDK), and guinea pig anti-PRL (1:12,500, NIDDK). Additional antibodies used were goat anti-FoxL2 (1:500; Imgenex, San Diego, CA) and rabbit anti-ACTH (1:1000; Peptide Biology Laboratories, Salk Institute, La Jolla, CA). After primary antibody incubation, slides were washed three times in KPBS and incubated in fluorophore-conjugated secondary antibodies (1:600, Jackson Immuno Research Laboratories, West Grove, PA) for 1 h, washed three times in KPBS, and cover slipped with Vectastain containing 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA). Slides were imaged on an AOBS confocal microscope (Leica, Mannheim, Germany) and analyzed using Image J. All immunohistochemistry experiments were performed at least three times.
For cell counting to measure cellular density, pituitary volume, and estimated total number of cells, we used Stereo Investigator (MBF Bioscience, Williston, VT) and counted nissl stained sections. The anterior lobe in individual sections was outlined to measure area and multiplied by the section thickness to derive the volume. For density counting, a random array of 25 625-μm2 counting frames generated by the computer program was placed over the outlined area, and each frame was counted for cells within the internal 80% of the thickness of the section. This counting yielded a density of cells for each section. The estimated number of cells per hemisection was computed by multiplying the density by the volume, which was multiplied by 2 to give the total number of cells per section of the anterior pituitary. Because of the irregular shape of the pituitary, the approximation was made that 18 full 20-μm sections could be obtained per pituitary so estimates exclude the small number of cells that are found in the first and last sections. Using the 18 section approximation, a total number of cells per pituitary was estimated. All quantifications are presented ± se.
Counting of hormonal cell types identified by immunohistochemical staining was conducted using confocal micrographs and ImageJ. Optical stacks were Z projected, and a single 165-μm2 area was counted by an observer blind to the genotype in a region of the pituitary containing the most homogenous distribution of cells, usually the central portion of the anterior lobe. Counts were then grouped by genotype and compared using a two-tailed Student's t test to determine statistical significance. Details on the number of male animals used to obtain pituitary cell type counts are presented in Supplemental Table 1. For counting of αGSU-positive cells, three Foxl2-/- and two wild-type pituitaries from 3-wk-old male animals were used. For FSHβ- positive cell counts, pituitary sections from four 3-wk-old and one 5-wk-old male Foxl2-/- and three 3-wk-old and one 5-wk-old wild-type littermates were used. For LHβ cell counts, two wild-type and two Foxl2-/- male animals at 3 wk of age, and two wild-type and one Foxl2-/- male at 5 wk of age were used. For TSHβ counts, two wild-type and one Foxl2-/- male at 3 wk of age and one wild-type and one Foxl2-/- male at 5 wk of age were used. For ACTH cell counts, four wild-type and three Foxl2-/- male animals were used. For PRL staining, two wild-type and two Foxl2-/- male animals were used. For GH-positive cells, two wild-type and two Foxl2-/- males at 3-wk of age were used. No significant differences were seen between counts from 3- and 5-wk-old animals so the data were grouped for statistical analysis. To further assess the pattern of gonadotropin subunit expression in females, one 3-wk- and one 5-wk-old of each genotype, Foxl2-/- vs. wild-type littermate, were also evaluated for αGSU, LHβ, and FSHβ staining.
qPCR analysis of mRNA expression in pituitary tissue and primary cultures of pituitary cells
Female 3-wk-old Foxl2 mutant (n = 3) animals and wild-type littermates (n = 4) were killed by decapitation, and whole pituitaries were removed and rapidly frozen on dry ice. Total RNA was isolated from each pituitary using the RNeasy Micro Kit with on-column deoxyribonuclease treatment to remove genomic DNA contamination (QIAGEN, Valencia, CA). To generate cDNA, the RNA samples were reverse transcribed using oligo (dT) primers and Superscript II Reverse Transcriptase (Invitrogen Life Technologies, Carlsbad, CA). Parallel control samples were processed in the absence of reverse transcriptase. The cDNA products were used as templates for real-time PCR analysis using the SYBR Green PCR Master Mix (Applied Biosystems Life Technologies, Foster City, CA). The ProbeFinder software (Roche Applied Science, Indianapolis, IN) was used for design of primers (Supplemental Table 2). Reactions were carried out on the Roche LightCycler 480 Real Time PCR system (Roche Applied Science). The reactions were run in 10 μl with optimized dilutions of reverse-transcribed cDNA samples. Gapdh mRNA levels were used as internal controls and used for normalization across samples. Assay specificity was checked by careful examination of the melting curves. Samples were analyzed in quadruplicate, and mean values were used to calculate relative transcript levels using the ΔΔCT method. Data were grouped by genotype and compared using a two-tailed Student's t test.
Primary cell culture and activin induction
Whole pituitaries were removed from female Foxl2 mutant and wild-type female littermates at 3–4 wk of age and then dispersed into single cells by incubation with collagenase, as previously described (25). The total number of cells recovered from the entire pituitary of Foxl2 mutant animals was between 55 and 60% of the number recovered from wild-type pituitaries (on the average 2.5 × 105 vs. 4.5 × 105 cells per pituitary, respectively). After plating in 48-well Costar tissue culture plates precoated with poly-d-lysine, the cells were allowed to recover for at least 48 h at 37 C and 7.5% CO2 in culture medium (βPJ) supplemented with growth factors and 2% fetal bovine serum (25). After the addition of fresh medium, duplicate (mutant cells) or triplicate (wild-type cells) wells were treated with vehicle, 10 nm activin A, or 5 nm GnRH for 18 h. The conditioned medium was removed and saved at −20 C for analysis of FSH and LH secretion. The cells were then washed and processed for total RNA extraction, cDNA synthesis, and qRT-PCR analysis as described above. Cumulative LH and FSH secretion was measured using specific RIA, run by the University of Virginia Center for Research in Reproduction Ligand Assay and Analysis Core [which is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development(NICHD)/the National Institutes of Health (NIH) (Specialized Cooperative Centers Program in Reproduction) Grant U54-HD28934]. For FSH and LH secretion experiments, pituitaries from eight Foxl2-/- and seven wild-type females were used. The qRT-PCR data are derived from primary cell preparations of pituitaries from four Foxl2-/- and four wild-type females. Secretion and qRT-PCR data from each independent experiment were internally normalized to baseline values in vehicle-treated cells and are presented as the means ± sem. Statistical comparisons were made using ANOVA with Tukey's post hoc test or two-tailed Student's test, as indicated.
Supplementary Material
Acknowledgments
We thank Alissa N. Blackler for her technical contribution. In addition, we thank Dr. Martin Matzuk for helpful reading and feedback on the manuscript.
This work was supported by Grant 2R01HD046941 awarded by the NICHD. This research was also supported in part by the Intramural Research Program of the NIH, National Institute on Aging. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NICHD or the NIH. The work was supported in part by the Clayton Medical Research Foundation, Inc. W.V. is a Clayton Medical Research Foundation, Inc. Senior Investigator and is the Helen McLoraine Professor of Molecular Neurobiology.
Present Address for N.J.J.: Huffington Center on Aging, Baylor College of Medicine, Houston, Texas.
Disclosure Summary: N.J.J., A.L.B., E.P., D.S., and L.M.B. have nothing to disclose. W.V. is a cofounder, consultant, equity holder, and member of the Board of Directors and Scientific Advisory Board of Acceleron Pharma, Inc. In accordance with Salk Institute policy, W.V. derives patent and licensing income in the activin field.
Footnotes
- Acvr1b
- Activin receptor type I
- Acvr2a
- activin receptor type IIA
- Acvr2b
- activin receptor type IIB
- DAPI
- 4′,6-diamidino-2-phenylindole
- FoxL2
- forkhead box L2
- αGSU
- α-glycoprotein subunit
- Fst
- follistatin
- FSHβ
- FSH β subunit
- KPBS
- Krebs PBS
- PRL
- prolactin
- qRT-PCR
- quantitative RT-PCR
- Smad
- Sma- and Mad-related protein.
References
- 1. Treier M, Gleiberman AS, O'Connell SM, Szeto DP, McMahon JA, McMahon AP, Rosenfeld MG. 1998. Multistep signaling requirements for pituitary organogenesis in vivo. Genes Dev 12:1691–1704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Crisponi L, Deiana M, Loi A, Chiappe F, Uda M, Amati P, Bisceglia L, Zelante L, Nagaraja R, Porcu S, Ristaldi MS, Marzella R, Rocchi M, Nicolino M, Lienhardt-Roussie A, Nivelon A, Verloes A, Schlessinger D, Gasparini P, Bonneau D, Cao A, Pilia G. 2001. The putative forkhead transcription factor FOXL2 is mutated in blepharophimosis/ptosis/epicanthus inversus syndrome. Nat Genet 27:159–166 [DOI] [PubMed] [Google Scholar]
- 3. Ellsworth BS, Egashira N, Haller JL, Butts DL, Cocquet J, Clay CM, Osamura RY, Camper SA. 2006. FOXL2 in the pituitary: molecular, genetic, and developmental analysis. Mol Endocrinol 20:2796–2805 [DOI] [PubMed] [Google Scholar]
- 4. Schmidt D, Ovitt CE, Anlag K, Fehsenfeld S, Gredsted L, Treier AC, Treier M. 2004. The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942 [DOI] [PubMed] [Google Scholar]
- 5. Uda M, Ottolenghi C, Crisponi L, Garcia JE, Deiana M, Kimber W, Forabosco A, Cao A, Schlessinger D, Pilia G. 2004. Foxl2 disruption causes mouse ovarian failure by pervasive blockage of follicle development. Hum Mol Genet 13:1171–1181 [DOI] [PubMed] [Google Scholar]
- 6. Ottolenghi C, Pelosi E, Tran J, Colombino M, Douglass E, Nedorezov T, Cao A, Forabosco A, Schlessinger D. 2007. Loss of Wnt4 and Foxl2 leads to female-to-male sex reversal extending to germ cells. Hum Mol Genet 16:2795–2804 [DOI] [PubMed] [Google Scholar]
- 7. Uhlenhaut NH, Jakob S, Anlag K, Eisenberger T, Sekido R, Kress J, Treier AC, Klugmann C, Klasen C, Holter NI, Riethmacher D, Schütz G, Cooney AJ, Lovell-Badge R, Treier M. 2009. Somatic sex reprogramming of adult ovaries to testes by FOXL2 ablation. Cell 139:1130–1142 [DOI] [PubMed] [Google Scholar]
- 8. Matzuk MM, Lamb DJ. 2002. Genetic dissection of mammalian fertility pathways. Nat Cell Biol 4 Suppl:s41–s49 [DOI] [PubMed] [Google Scholar]
- 9. Bilezikjian LM, Blount AL, Donaldson CJ, Vale WW. 2006. Pituitary actions of ligands of the transforming growth factor-β family: activins and inhibin. Reproduction 132:207–215 [DOI] [PubMed] [Google Scholar]
- 10. Burger LL, Haisenleder DJ, Dalkin AC, Marshall JC. 2004. Regulation of gonadotropin subunit gene transcription. J Mol Endocrinol 33:559–584 [DOI] [PubMed] [Google Scholar]
- 11. Ling N, Ying SY, Ueno N, Shimasaki S, Esch F, Hotta M, Guillemin R. 1986. Pituitary FSH is released by a heterodimer of the β-subunits from the two forms of inhibin. Nature 321:779–782 [DOI] [PubMed] [Google Scholar]
- 12. Vale W, Rivier J, Vaughan J, McClintock R, Corrigan A, Woo W, Karr D, Spiess J. 1986. Purification and characterization of an FSH releasing protein from porcine ovarian follicular fluid. Nature 321:776–779 [DOI] [PubMed] [Google Scholar]
- 13. Kumar TR, Wang Y, Lu N, Matzuk MM. 1997. Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204 [DOI] [PubMed] [Google Scholar]
- 14. Blount AL, Schmidt K, Justice NJ, Vale WW, Fischer WH, Bilezikjian LM. 2009. FoxL2 and Smad3 coordinately regulate follistatin gene transcription. J Biol Chem 284:7631–7645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellsworth BS, Burns AT, Escudero KW, Duval DL, Nelson SE, Clay CM. 2003. The gonadotropin releasing hormone (GnRH) receptor activating sequence (GRAS) is a composite regulatory element that interacts with multiple classes of transcription factors including Smads, AP-1 and a forkhead DNA binding protein. Mol Cell Endocrinol 206:93–111 [DOI] [PubMed] [Google Scholar]
- 16. Lamba P, Fortin J, Tran S, Wang Y, Bernard DJ. 2009. A novel role for the forkhead transcription factor FOXL2 in activin A-regulated follicle-stimulating hormone β subunit transcription. Mol Endocrinol 23:1001–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Corpuz PS, Lindaman LL, Mellon PL, Coss D. 2010. FoxL2 Is required for activin induction of the mouse and human follicle-stimulating hormone β-subunit genes. Mol Endocrinol 24:1037–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bartholin L, Maguer-Satta V, Hayette S, Martel S, Gadoux M, Corbo L, Magaud JP, Rimokh R. 2002. Transcription activation of FLRG and follistatin by activin A, through Smad proteins, participates in a negative feedback loop to modulate activin A function. Oncogene 21:2227–2235 [DOI] [PubMed] [Google Scholar]
- 19. Norwitz ER, Xu S, Jeong KH, Bédécarrats GY, Winebrenner LD, Chin WW, Kaiser UB. 2002. Activin A augments GnRH-mediated transcriptional activation of the mouse GnRH receptor gene. Endocrinology 143:985–997 [DOI] [PubMed] [Google Scholar]
- 20. Suszko MI, Lo DJ, Suh H, Camper SA, Woodruff TK. 2003. Regulation of the rat follicle-stimulating hormone β-subunit promoter by activin. Mol Endocrinol 17:318–332 [DOI] [PubMed] [Google Scholar]
- 21. Huang HJ, Sebastian J, Strahl BD, Wu JC, Miller WL. 2001. Transcriptional regulation of the ovine follicle-stimulating hormone-β gene by activin and gonadotropin-releasing hormone (GnRH): involvement of two proximal activator protein-1 sites for GnRH stimulation. Endocrinology 142:2267–2274 [DOI] [PubMed] [Google Scholar]
- 22. Pernasetti F, Vasilyev VV, Rosenberg SB, Bailey JS, Huang HJ, Miller WL, Mellon PL. 2001. Cell-specific transcriptional regulation of follicle-stimulating hormone-β by activin and gonadotropin-releasing hormone in the LβT2 pituitary gonadotrope cell model. Endocrinology 142:2284–2295 [DOI] [PubMed] [Google Scholar]
- 23. Lamba P, Wang Y, Tran S, Ouspenskaia T, Libasci V, Hébert TE, Miller GJ, Bernard DJ. 2010. Activin A regulates porcine follicle-stimulating hormone β-subunit transcription via cooperative actions of SMADs and FOXL2. Endocrinology 151:5456–5467 [DOI] [PubMed] [Google Scholar]
- 24. DePaolo LV, Bald LN, Fendly BM. 1992. Passive immunoneutralization with a monoclonal antibody reveals a role for endogenous activin-B in mediating FSH hypersecretion during estrus and following ovariectomy of hypophysectomized, pituitary-grafted rats. Endocrinology 130:1741–1743 [DOI] [PubMed] [Google Scholar]
- 25. Corrigan AZ, Bilezikjian LM, Carroll RS, Bald LN, Schmelzer CH, Fendly BM, Mason AJ, Chin WW, Schwall RH, Vale WW. 1991. Evidence for an autocrine role of activin B within rat anterior pituitary cultures. Endocrinology 128:1682–1684 [DOI] [PubMed] [Google Scholar]
- 26. Bernard DJ. 2004. Both SMAD2 and SMAD3 mediate activin-stimulated expression of the follicle-stimulating hormone β subunit in mouse gonadotrope cells. Mol Endocrinol 18:606–623 [DOI] [PubMed] [Google Scholar]
- 27. Thackray VG, Mellon PL. 2008. Synergistic induction of follicle-stimulating hormone β-subunit gene expression by gonadal steroid hormone receptors and Smad proteins. Endocrinology 149:1091–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang Y, Libasci V, Bernard DJ. 2010. Activin A induction of FSHβ subunit transcription requires SMAD4 in immortalized gonadotropes. J Mol Endocrinol 44:349–362 [DOI] [PubMed] [Google Scholar]
- 29. Coss D, Thackray VG, Deng CX, Mellon PL. 2005. Activin regulates luteinizing hormone β-subunit gene expression through Smad-binding and homeobox elements. Mol Endocrinol 19:2610–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sheng HZ, Zhadanov AB, Mosinger B, Jr, Fujii T, Bertuzzi S, Grinberg A, Lee EJ, Huang SP, Mahon KA, Westphal H. 1996. Specification of pituitary cell lineages by the LIM homeobox gene Lhx3. Science 272:1004–1007 [DOI] [PubMed] [Google Scholar]
- 31. Sheng HZ, Moriyama K, Yamashita T, Li H, Potter SS, Mahon KA, Westphal H. 1997. Multistep control of pituitary organogenesis. Science 278:1809–1812 [DOI] [PubMed] [Google Scholar]
- 32. Girardin SE, Benjannet S, Barale JC, Chrétien M, Seidah NG. 1998. The LIM homeobox protein mLIM3/Lhx3 induces expression of the prolactin gene by a Pit-1/GHF-1-independent pathway in corticotroph AtT20 cells. FEBS Lett 431:333–338 [DOI] [PubMed] [Google Scholar]
- 33. Roberson MS, Schoderbek WE, Tremml G, Maurer RA. 1994. Activation of the glycoprotein hormone α-subunit promoter by a LIM-homeodomain transcription factor. Mol Cell Biol 14:2985–2993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. West BE, Parker GE, Savage JJ, Kiratipranon P, Toomey KS, Beach LR, Colvin SC, Sloop KW, Rhodes SJ. 2004. Regulation of the follicle-stimulating hormone β gene by the LHX3 LIM-homeodomain transcription factor. Endocrinology 145:4866–4879 [DOI] [PubMed] [Google Scholar]
- 35. Ho CC, Bernard DJ. 2009. Bone morphogenetic protein 2 signals via BMPR1A to regulate murine follicle-stimulating hormone β subunit transcription. Biol Reprod 81:133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lee KB, Khivansara V, Santos MM, Lamba P, Yuen T, Sealfon SC, Bernard DJ. 2007. Bone morphogenetic protein 2 and activin A synergistically stimulate follicle-stimulating hormone β subunit transcription. J Mol Endocrinol 38:315–330 [DOI] [PubMed] [Google Scholar]
- 37. Wu Y, Luo H, Liu J, Kang D, McNeilly AS, Cui S. 2010. LIM homeodomain transcription factor Isl-1 enhances follicle stimulating hormone-β and luteinizing hormone-β gene expression and mediates the activation of leptin on gonadotropin synthesis. Endocrinology 151:4787–4800 [DOI] [PubMed] [Google Scholar]
- 38. Suszko MI, Antenos M, Balkin DM, Woodruff TK. 2008. Smad3 and Pitx2 cooperate in stimulation of FSHβ gene transcription. Mol Cell Endocrinol 281:27–36 [DOI] [PubMed] [Google Scholar]
- 39. Strahl BD, Huang HJ, Pedersen NR, Wu JC, Ghosh BR, Miller WL. 1997. Two proximal activating protein-1-binding sites are sufficient to stimulate transcription of the ovine follicle-stimulating hormone-β gene. Endocrinology 138:2621–2631 [DOI] [PubMed] [Google Scholar]
- 40. Zakaria MM, Jeong KH, Lacza C, Kaiser UB. 2002. Pituitary homeobox 1 activates the rat FSHβ (rFSHβ) gene through both direct and indirect interactions with the rFSHβ gene promoter. Mol Endocrinol 16:1840–1852 [DOI] [PubMed] [Google Scholar]
- 41. Thackray VG, McGillivray SM, Mellon PL. 2006. Androgens, progestins, and glucocorticoids induce follicle-stimulating hormone β-subunit gene expression at the level of the gonadotrope. Mol Endocrinol 20:2062–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Denef C. 2008. Paracrinicity: the story of 30 years of cellular pituitary crosstalk. J Neuroendocrinol 20:1–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. González-Parra S, Chowen JA, Garcia-Segura LM, Argente J. 1996. In vivo and in vitro regulation of pituitary transcription factor-1 (Pit-1) by changes in the hormone environment. Neuroendocrinology 63:3–15 [DOI] [PubMed] [Google Scholar]
- 44. Ben-Jonathan N, LaPensee CR, LaPensee EW. 2008. What can we learn from rodents about prolactin in humans? Endocr Rev 29:1–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hartman ML, Veldhuis JD, Thorner MO. 1993. Normal control of growth hormone secretion. Horm Res 40:37–47 [DOI] [PubMed] [Google Scholar]
- 46. Stefaneanu L, Powell-Braxton L, Won W, Chandrashekar V, Bartke A. 1999. Somatotroph and lactotroph changes in the adenohypophyses of mice with disrupted insulin-like growth factor I gene. Endocrinology 140:3881–3889 [DOI] [PubMed] [Google Scholar]
- 47. Daftary SS, Gore AC. 2005. IGF-1 in the brain as a regulator of reproductive neuroendocrine function. Exp Biol Med (Maywood) 230:292–306 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.