Abstract
The mechanisms of G protein coupling to G protein-coupled receptors (GPCR) share general characteristics but may exhibit specific interactions unique for each GPCR/G protein partnership. The extreme C terminus (CT) of G protein α-subunits has been shown to be important for association with GPCR. Hypothesizing that the extreme CT of Gαs is an essential component of the molecular landscape of the GPCR, human LH receptor (LHR), and β2-adrenergic receptor (β2-AR), a model cell system was created for the expression and manipulation of Gαs subunits in LHR+ s49 ck cells that lack endogenous Gαs. On the basis of studies involving truncations, mutations, and chain extensions of Gαs, the CT was found to be necessary for LHR and β2-AR signaling. Some general similarities were found for the responses of the two receptors, but significant differences were also noted. Computational modeling was performed with a combination of comparative modeling, molecular dynamics simulations, and rigid body docking. The resulting models, focused on the Gαs CT, are supported by the experimental observations and are characterized by the interaction of the four extreme CT amino acid residues of Gαs with residues in LHR and β2-AR helix 3, (including R of the DRY motif), helix 6, and intracellular loop 2. This portion of Gαs recognizes the same regions of the two GPCR, although with differences in the details of selected interactions. The predicted longer cytosolic extensions of helices 5 and 6 of β2-AR are expected to contribute significantly to differences in Gαs recognition by the two receptors.
G protein-coupled receptors (GPCR) comprise the largest gene family in the human genome and mediate a variety of physiological processes after functional coupling with G proteins (1). Although no known sequence homology exists to define the binding of GPCR to specific G protein isoforms, approximately 800–900 GPCR signal through only four families of G proteins, suggesting that charge and conformation are more important than primary sequence identity for the specificity of G protein coupling (2, 3). Prediction algorithms have matched GPCR to their cognate G protein signaling partners with 70–90% accuracy, but these programs often failed to recognize the promiscuity of many GPCR that signal through multiple G protein families (4, 5). Biochemical approaches have identified determinant regions and specific amino acid residues of GPCR and G proteins that are important for functional coupling, although their influence upon the coupling relationship may not be direct (6–9). In general, these studies identified the cytosolic regions of GPCR, specifically residues in the second and third intracellular loops, and residues in the surrounding cytosolic helical extensions.
Less is known about the G protein contribution to the coupling relationship, although the extreme C terminus (CT) of Gα subunits has been recognized as important for binding (7, 10–13), a conclusion, however, that has not been defined for all receptor systems (3). Only 11 or fewer CT residues were sufficient to confer recognition and/or inhibition by Gi/o- and Gq-coupled GPCR (14–16), but many more Gαs residues were required for similar outcomes (17–22). CT fragments of Gαs (93 amino acid residues) and Gαi (76 amino acid residues) were sufficient to inhibit cAMP production mediated by human chorionic gonadotropin (hCG)-activated LH receptor (LHR) (23). It is also recognized that, in addition to the CT domain of G proteins, other regions are important, including the α3/β5 and α4/β6 loops (3, 24, 25). The specific G protein coupling mechanisms of rhodopsin and the β2-adrenergic receptor (β2-AR) have been investigated (26–29). They appear to share some similarities, such as the importance of the Gαs CT in receptor interaction, but they also exhibit significant differences (26–29). Moreover, the Gα CT region is not always sufficient for the coupling of all GPCR/G protein systems (3, 30). Therefore, the mechanisms for GPCR/G protein coupling vary between receptors that couple to different G proteins, and perhaps the coupling relationship is unique for all GPCR/G protein pairs.
Perturbation of Gαs may not only impact the coupling relationship with cognate receptors but may also disturb the structure and stability of the protein and thus the rate of exchange of guanine nucleotides, the affinity for Gβγ, localization, and association with the effector enzyme, adenylyl cyclase. The crystal structures of the Gαs and Gαq subunits suggest that the extreme CT residues extend from the globular protein structure and therefore are unlikely to influence global protein folding (31–35). In the crystal structures, the extreme CT lies approximately 35–40 Å from the nucleotide binding pocket (33, 34), and none of the CT residues has been implicated in the association with Gβγ or adenylyl cyclase (31, 34–37). If Gαs retains its N-terminal palmitoylation and associates with Gβγ, it targets properly to the plasma membrane (38). Therefore, it was hypothesized that the manipulation of the extreme CT is likely to affect only the receptor-binding and functional coupling relationship.
The human LHR is a GPCR that, unlike β2-AR, binds its ligand via the large ectodomain rather than the transmembrane helices. Naturally occurring mutations of LHR have been identified (39), and these and an array of laboratory-engineered mutations have led to a better understanding of the many dynamic molecular relationships between the LHR residues that define receptor function (40). We have recently published the first comprehensive, functional delineation of the individual residues in the intracellular domains of LHR and have established potential switches of receptor activation and a map of the primary receptor recognition sites for Gαs (6).
The inferences from that study were based on a combination of in vitro mutational analysis of selected cytosolic portions of the LHR and in silico predictions of the LHR/Gαs complex. LHR residues important for interacting with Gαs were found in the cytosolic extensions of helices 3 and 4 and in the second and third intracellular loops (IL2 and IL3) (6). The mapping of hot spots onto the computational models of LHR and the LHR/Gαs complexes allowed for a distinction between the receptor sites required for its intramolecular structural changes and those likely to be involved in G protein recognition, including the fully conserved Arg of the DRY motif that is involved in recognizing E392 (E378 in the short isoform) in the CT of Gαs (6).
The predictions from computational modeling emphasized the role of the Gαs CT as the recognition site for LHR. To validate this prediction, a model system was created to determine whether LHR requires the CT of Gαs for activity and whether β2-AR associates with Gαs in the same manner as LHR. Gαs variants with engineered mutations at the CT were introduced into Gαs-deficient cells, thus permitting measurements of a functional response between the Gαs variants and each of the two receptors. The results suggested that the CT is indeed important for the functional interaction of Gαs with LHR and β2-AR, but the two receptors responded differently to certain Gαs variants. This finding shows not only that the mechanisms of coupling differ between GPCR that signal through different families of G proteins but also that they may also vary for receptors that signal through the same G protein isoform. Finally, novel models of the LHR and β2-AR Gαs interactions are presented, thus enhancing our understanding of the molecular details of the interfaces.
Results
Creation and characterization of LHR+ and LHR+-Gαs+ s49 ck cells
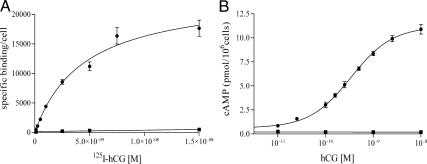
The lack of Gαs mRNA transcription and an inactivating mutation of protein kinase A define the s49 ck (s49 cyc− kin−) murine lymphoma cell line (41, 42). s49 ck cells were transduced with a lentivirus that contained the LHR cDNA and puromycin resistance. A stable, clonal line was isolated, expanded, and characterized for LHR expression by [125I]hCG saturation binding (Fig. 1A). Specific and saturable binding of the transformed cells was measured, and the dissociation constant (Kd) and maximum number of binding sites (Bmax) were 4.5 nm and 24 × 103 receptors per cell, respectively. Although somewhat high, the Kd is within the range of reported values (43).
Fig. 1.
Characterization of the model system of transformed s49 ck cell lines. A, Saturation-binding curve. Specific binding was measured after a 5 h incubation with 125I-hCG at 37 C for the mock (■) and LHR+ s49 ck (●) cells, and the derived Kd and Bmax values for the LHR+ cells are 4.5 ± 0.4 nm and (24 ± 1.2) × 103 receptors per cell, respectively. B, Dose-response curve. The cAMP response was measured after a 30-min incubation with hCG (37 C) of the LHR+/mock (■) and LHR+/Gαs+ (●) transformed cell lines, and the values found for EC50 and Rmax for the LHR+/Gαs+ cell line are 0.34 ± 0.1 nm and 11.2 ± 0.2 pmol/106 cells, respectively. Data are presented as mean ± sem.
The clonal LHR+ s49 ck cell line was then transduced with lentiviral constructs that contained blasticidin resistance and the long splice-variant cDNA of the human Gαs or an empty vector as a mock control. To characterize the different cell lines, LHR+/mock-transfected and LHR+/wild-type Gαs+ cells were incubated with increasing concentrations of hCG, and the resulting cAMP response was measured (Fig. 1B). No enhanced cAMP was generated from the LHR+/mock-transfected cells, but the LHR+/Gαs+ cells responded with cAMP production. The EC50, i.e. the effective concentration for a 50% response from the basal level of cAMP to the maximally stimulated level, was found to be 0.34 nm, consistent with reported values (44). The efficacy (Rmax), i.e. the maximally stimulated level, was 11.2 pmol/106 cells for the LHR+/Gαs+ line.
Design of mutant forms of Gαs
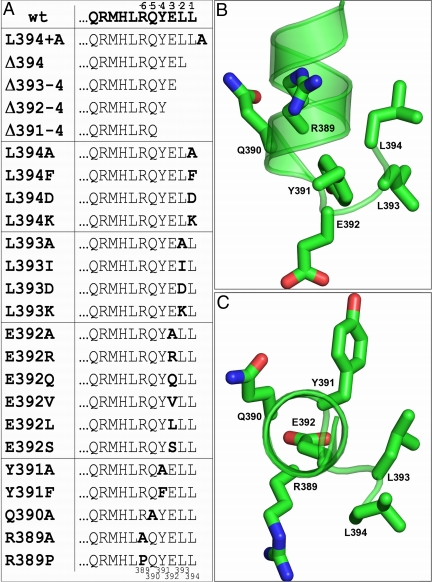
Sequential CT truncations were made at positions −4 through −1 (nos. 391–394), and Ala scanning mutagenesis was performed for each amino acid residue in the −6 through the −1 (389–394) region. The results suggested the design of additional mutations in this portion of Gαs for functional characterization. In all, a total of 26 cell lines, wild-type, mock-transfected, and 24 deletion and replacement mutants, were prepared and characterized (Fig. 2A). Figure 2, B and C, highlights the targeted positions within the crystal structure of Gαs-GTPγS (1AZS) (34). The perspective in Fig. 2C emphasizes the helical orientation of the last amino acids of the protein and places the terminal residue, L394 (disordered in the structure), at the forefront of the image.
Fig. 2.
Gαs CT targets of experimental manipulation. A, List of the last 11 amino acid residues of the wild-type (wt) and manipulated Gαs variants. The variants are grouped according to residue position. The position of the residues with regard to the CT (−1 to −6) is indicated at the top of the chart and the residue number corresponding to the long-splice variant sequence of human Gαs (389–394) is depicted at the bottom. B, Side view perpendicular to the helical main axis of the last 11 amino acids from the computational model of Gαs employed in docking experiments. The backbone is shown in cartoons, and the side chains of the last six amino acids are represented as sticks. C, Same representation as B but in a view parallel to the main axis of the CT helix. The images in B and C were created with PyMOL 0.99rc6.
Expression of wild-type and mutant forms of Gαs
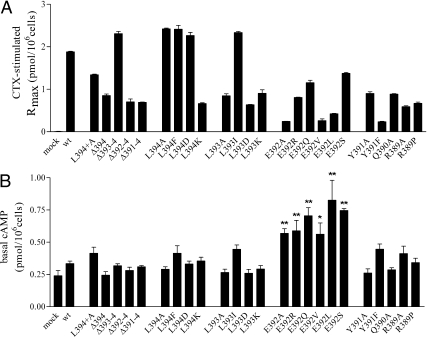
To assess the expression level of functional protein, each of the cell lines expressing wild-type and mutant forms of Gαs was incubated with cholera toxin (CTX), and the resulting increase in cAMP was used as an indicator of intracellular protein concentration relative to that of wild-type Gαs (Fig. 3A and Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://mend.endojournals.org). The extreme CT of Gαs has not been implicated in the binding to and activation of the effector enzyme, adenylyl cyclase (34, 45, 46), and therefore manipulation of this area is assumed not to affect the impact of CTX activation of Gαs. Each of the mutated Gαs proteins responded to CTX stimulation, demonstrating that each expressed in a functional form and that the alterations made at the CT did not abrogate Gαs activity. However, the biological responses to CTX of the mutant proteins were variable. Most levels fell within about 30–130% that of wild-type Gαs; only Y391F and E392A/V/L exhibited apparent lower expression, e.g. 10–25% relative to wild-type Gαs. Thus, due caution must be exercised in attempts to quantitatively interpret potencies and efficacies, particularly for the latter four Gαs mutants.
Fig. 3.
A, Maximal cAMP production (Rmax) of LHR+/Gαs+ wild-type and variant cells stimulated with CTX. Measurements were made after incubating cells for 2 h (37 C) with 2 μg/ml CTX, and the results are given as mean ± sem. One-way ANOVA followed by Dunnett's posttest was used for statistical analysis, and all samples were found to be significantly different from wild-type (wt) Gαs (P < 0.001). B, Basal cAMP values of the transfected s49 ck cells. The cells were incubated for 30 min, and one-way ANOVA followed by Dunnett's posttest was used for statistical analysis. *, P < 0.05; **, P < 0.001.
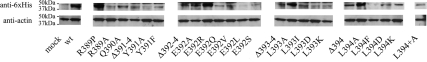
As another monitor of total membrane-associated Gαs expression, albeit more qualitative, crude membranes from each cell line were solubilized, electrophoresed, and probed with an anti-6xHis antibody. The resultant Western blots, measuring total immunoreactive (tagged) Gαs protein, confirmed that all of the deletion and replacement mutants were expressed; however, the apparent levels were, as expected, variable (Fig. 4 and Supplemental Table 1). After normalizing to the respective wild-type values and comparing the results of the Western blots and CTX stimulation, there was agreement to within about 25–30% for several of the mutants: L394+A, Δ393–394, Δ392–394, L394A, L393I, E392Q, and Y391A (most of these being within 10% of each other). In many cases, the Westerns gave higher values (2- to 5-fold) than CTX stimulation, suggesting that some of the immunodetected protein was not functional; for a few mutants, the Westerns suggested a lower level of expression than CTX stimulation (Supplemental Table 1). The differences could arise from the (often) nonquantitative nature of Western blots, particularly of membrane-associated proteins such as Gαs, and of course the concentration of functional protein in transfected cells can be quite different, generally less, than that of total protein. Although the Western blots were made on membrane fractions, they could contain inactive Gαs proteins. Although the expression levels cannot be quantified precisely, both independent approaches demonstrated that all of the mutant forms of Gαs were expressed.
Fig. 4.
Western blots of membrane protein from the Gαs-transformed cell lines. As an estimate of total LHR expression, 100 μg crude membrane protein was probed with anti-6xHis (top panels), along with an anti-actin probe to serve as a control for protein recovery and loading (bottom panels). wt, Wild type.
Basal signaling in and functional analysis of cells expressing LHR, β2-AR, and engineered forms of Gαs
Basal cAMP values were determined for wild-type and Gαs mutants, and only mutants harboring replacements of E392 exhibited slightly elevated basal activity (Fig. 3B). Although a negatively charged side chain at position −3 (no. 392) may function in restricting the protein to its inactive form, the increased basal activity was not observed in the deletion mutants Δ392–394 and Δ391–394. Thus, other factors, e.g. a localized conformational change, altered cAMP transport, or additional signaling mechanisms, may be involved.
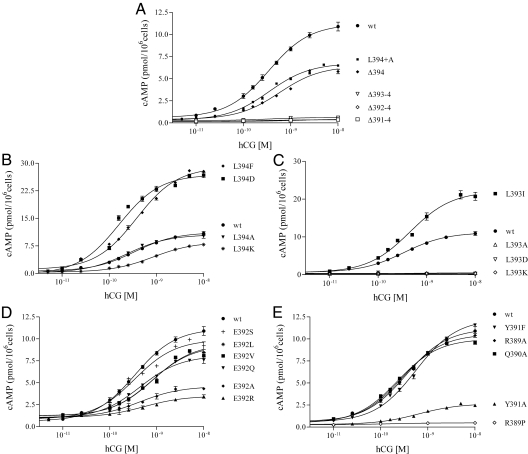
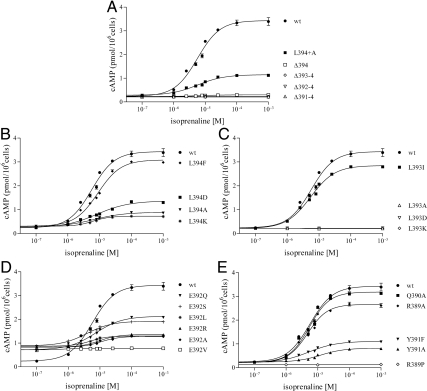
Figures 5 and 6 show, respectively, the dose-response curves of the various cell lines with increasing concentrations of the appropriate agonist, hCG to LHR and isoprenaline (racemic mixture of isoproterenol) to β2-AR. The signaling parameters of EC50 and Rmax for wild-type and each mutant form of Gαs are given in Table 1. Of the 24 deletion and replacement mutants investigated, seven and eight Gαs variants were found to be nonfunctional in response to LHR and β2-AR activation, respectively. For both receptors, the inactive forms of Gαs are Δ393–394, Δ392–394, Δ391–394, L393A/D/K, and R389P; the additional inactive form of Gαs in β2-AR signaling was the Δ394 deletion mutant. As judged by CTX stimulation of cAMP biosynthesis and by Western blots, all inactive mutants were expressed at sufficient levels to engender confidence in the results.
Fig. 5.
Dose-response isotherms of cAMP concentrations after hCG stimulation of LHR in the transfected s49 ck cell lines. All panels include data from LHR+/Gαs+ wild-type (wt) cells. A, Data from the CT Ala extension, L394+A, and the truncation mutations; B–E, data from the point mutations at positions −1 (394), −2 (393), −3 (392), and −4/−5/−6 (391/390/389), respectively. The results show mean ± sem; the numerical values are given in Table 1.
Fig. 6.
Dose-response isotherms of cAMP concentrations after isoprenaline stimulation of β2-AR in the transfected s49 ck cell lines. All panels include data from LHR+/Gαs+ wild-type (wt) cells. A, Data from the CT Ala extension, L394+A, and the truncation mutations; B–E, data from the mutations positions −1 (394), −2 (393), −3 (392), and −4/−5/−6 (391/390/389), respectively. The results show mean ± sem; the numerical values are given in Table 1.
Table 1.
EC50 and Rmax values determined after ligand stimulation of LHR+ s49 cells
| hCG |
isoprenaline |
|||
|---|---|---|---|---|
| EC50 (nM) | Rmax (pmol/106 cells) | EC50(μM) | Rmax (pmol/106 cells) | |
| mock | —a | 0.19 ± 0.06c | —a | 0.14 ± 0.01c |
| wt | 0.34 ± 0.11 | 11.2 ± 0.2 | 5.7 ± 1.1 | 3.44 ± 0.07 |
| L394+A | 0.35 ± 0.11 | 6.76 ± 0.08c | 5.8 ± 1.1 | 1.15 ± 0.02c |
| Δ394 | 0.58 ± 0.11 | 6.44 ± 0.17c | —a | 0.30 ± 0.00c |
| Δ393-4 | —a | 0.63 ± 0.03c | —a | 0.21 ± 0.01c |
| Δ392-4 | —a | 0.42 ± 0.02c | —a | 0.22 ± 0.00c |
| Δ391-4 | —a | 0.35 ± 0.03c | —a | 0.23 ± 0.01c |
| L394A | 0.24 ± 0.11 | 10.5 ± 0.24 | 6.7 ± 1.1 | 0.89 ± 0.01c |
| L394F | 0.38 ± 0.11 | 28.9 ± 0.3c | 8.6 ± 1.2c | 3.09 ± 0.04c |
| L394D | 0.16 ± 0.11 | 27.0 ± 0.88c | 8.1 ± 1.1b | 1.37 ± 0.03c |
| L394K | 0.84 ± 0.10c | 8.54 ± 0.09b | 6.1 ± 1.1 | 0.73 ± 0.01c |
| L393A | —a | 0.47 ± 0.05c | —a | 0.22 ± 0.00c |
| L393I | 0.45 ± 0.11 | 22.1 ± 0.5c | 5.4 ± 1.0 | 2.85 ± 0.03c |
| L393D | —a | 0.30 ± 0.05c | —a | 0.27 ± 0.00c |
| L393K | —a | 0.15 ± 0.39c | —a | 0.22 ± 0.01c |
| E392A | 0.49 ± 0.11 | 5.38 ± 0.25c | 11 ± 1.1c | 1.28 ± 0.01c |
| E392R | 0.75 ± 0.12b | 3.50 ± 0.14c | 9.5 ± 1.1c | 1.36 ± 0.03c |
| E392Q | 0.44 ± 0.11 | 8.09 ± 0.13c | 7.2 ± 1.1 | 2.13 ± 0.02c |
| E392V | 0.80 ± 0.11b | 9.29 ± 0.19b | — | 0.77 ± 0.00c |
| E392L | 0.83 ± 0.11b | 9.44 ± 0.12b | 5.0 ± 1.1 | 1.30 ± 0.01c |
| E392S | 0.21 ± 0.15 | 9.94 ± 0.76 | 3.8 ± 1.0 | 1.92 ± 0.01c |
| Y391A | 0.62 ± 0.11 | 2.66 ± 0.06c | 12 ± 1.1c | 0.82 ± 0.01c |
| Y391F | 0.55 ± 0.11 | 12.3 ± 0.2 | 5.2 ± 1.1 | 1.11 ± 0.02c |
| Q390A | 0.23 ± 0.11 | 10.0 ± 0.1 | 5.4 ± 1.1 | 3.18 ± 0.06c |
| R389A | 0.28 ± 0.11 | 10.6 ± 0.1 | 4.8 ± 1.1 | 2.66 ± 0.05c |
| R389P | —a | 0.47 ± 0.04c | —a | 0.13 ± 0.01c |
indicates zero or insufficient response to ligand for determination of EC50.
P < 0.05.
P < 0.001.
Relative to wild-type Gαs, several other mutants exhibited diminished potencies, i.e. higher EC50, and none were found to have increased potencies. The efficacies varied somewhat for the two receptors, perhaps reflecting to a large extent differences in levels of expression as well as overall coupling efficiency. When different from that of wild-type Gαs, most exhibited reduced Rmax values, the exceptions being L394F/D and L393I for LHR. For these mutants, it is possible that the rate constants for binding, nucleotide exchange, and dissociation may be altered, resulting in increased coupling productivity. The level of β2-AR expression appeared lower than that of LHR as judged by the Rmax values for wild-type Gαs, e.g. over 3.0-fold greater with LHR activation, although this could be influenced by receptor coupling efficiency. Also, competition with other G proteins may be affected by the mutations, thus altering the GPCR-mediated production of cAMP.
In an effort to obtain a more quantitative comparison of the Rmax values, corrected for the expression level of Gαs, the hCG-mediated Rmax values in Table 1 were evaluated by obtaining the ratio of [(Rmax − basal cAMP)mutant/(Rmax − basal cAMP)wild-type]. The ratio for each mutant was then corrected for Gαs expression as estimated by CTX stimulation (Fig. 3A) and by the relative intensities from Western blots (Fig. 4). A similar analysis was also done for β2-AR, but the apparent lower expression of β2-AR, as judged by the reduced magnitudes of Rmax for wild-type receptor, compromised some of the numbers. The results for both receptors are presented in Supplemental Table 1. It is noteworthy that, with but a few exceptions, a qualitative, and sometimes quantitative, ranking of mutants depending upon their Rmax values were similar when no corrections were made for expression (Table 1) or when corrections were achieved by normalizing to Westerns or CTX stimulation. However, in view of the varying levels of expression of wild-type and mutant forms of Gαs, and the impact of that on Rmax values, most of the emphasis hereafter will be given to the potencies of the Gαs mutants, although some attention will be directed to the Rmax values. Detailed analyses of the effects of elongation at and deletion of the CT of Gαs, along with replacements at positions 389–394, are presented in following sections.
Functional effects of elongation and deletion of the CT region of Gαs
When Gαs was extended at the CT by addition of an Ala (Gαs L394+A), there was no effect on the potencies associated with both receptors, whereas the efficacies were reduced. Stepwise deletion from the CT of Gαs, resulting in removal of L394 (Δ394), L393/L394 (Δ393–394), E392/L393/L394 (Δ392-394), and Y391/E392/L393/L394 (Δ391–394), indicated differences and similarities in receptor coupling. Deletion of L394 had no major effect on the parameters associated with LHR signaling (Fig. 5A and Table 1). Thus, neither the location of the α-carboxyl group nor the presence of Leu at position 394 is absolutely essential for LHR-mediated signaling through Gαs. In contrast to LHR, signaling by β2-AR was abolished upon removal of L394 (Fig. 6A and Table 1). This finding demonstrates that the chemical nature of the CT residue and the placement of the α-carboxylate are much more important for β2-AR/Gαs signaling than for LHR/Gαs signaling. Further truncations abrogated signaling in both receptors. Therefore, most of the extreme CT of Gαs is necessary for effective coupling to LHR, which is consistent with previous (6) and present predictions by computational modeling (see below); moreover, the β2-AR also required this region for functionality, in agreement with previous reports (21, 47).
Ala scanning mutagenesis of amino acid residues 389–394 in Gαs
Ala scanning mutagenesis at each of the positions −6 through −1 (389–394) showed that, for hCG activation of LHR, AL replacements at positions R389, Q390, Y391, E392, and L394 had no significant effect on the potencies, whereas signaling was abrogated with Ala at position L393 (Fig. 5, B–E, and Table 1). With isoprenaline stimulation of β2-AR, AL replacements in Gαs resulted in functional responses similar to those observed with hCG/LHR, e.g. a loss of signaling with L393A, but there were also some differences. The potencies were decreased with E392A and Y391A (Fig. 6, B–E, and Table 1).
Extended functional analysis at positions 389–394 in Gαs
To further characterize the nature of the side chains that are required for bioactivity at the CT of Gαs, additional mutations were made with a focus on residues 392–394. Also, the R389P variant, identified in the s49 unc− line, was included as a negative control because this mutation is known to uncouple the G protein from the β2-AR (48). The results are presented for hCG/LHR-mediated and isoprenaline/β2-AR-mediated signaling in Figs. 5, B–E, and 6, B–E, respectively, with the results summarized in Table 1.
The results of Ala scanning mutagenesis suggested that the L394A mutant signaled similarly to wild-type Gαs when coupled to activated LHR; consequently, the effects of replacement with aromatic (L394F) and charged (L394D and L394K) residues were probed (Fig. 5B and Table 1). The potencies of the F/D replacements were comparable to that of wild-type Gαs, whereas the potency of the K replacement was reduced about 2.5-fold relative to wild-type protein. The apparent efficacy was increased some 2- to 3-fold with L394F/D, an interesting observation reflecting enhanced coupling. LHR signaling thus appears to tolerate various amino acid side chains at the −1 position of Gαs. With β2-AR-mediated signaling, these same substitutions were tolerated, although the potencies were somewhat reduced for the F/D replacements (Fig. 6B and Table 1).
Because an Ala replacement at L393 abrogated signaling with both receptors, a larger hydrophobic side chain, Ile, was tested. When coupled to ligand-activated LHR and ligand-activated β2-AR, the potency was equivalent to that of wild-type Gαs; however, replacements with the ionizable side chains, Asp and Lys, abolished signaling in both receptors (Figs. 5C and 6C and Table 1). As with L394F/D, the apparent efficacy was also increased some 2- to 3-fold with L393I. Thus, a relatively large hydrophobic side chain appears to be required at position −2 (393) for wild-type-like signaling in the two receptors.
Replacement with Ala at position −3 (392) was somewhat tolerated for both receptors, and thus a series of additional replacements, R/Q/V/L/S, were probed. None of the replacements entirely abrogated signaling through LHR, although the potencies were reduced some 2- to 3-fold with R/V/L (Fig. 5D and Table 1). With β2-AR, the potencies of the various mutants were not affected by replacements with Q/L/S; those of the A/R replacements were significantly less. The V replacement signaled poorly (Fig. 6D and Table 1), but the combined low expressions of β2-AR and Gαs render this result unreliable.
Knowing that Y391A led to reduced signaling for β2-AR, but not LHR, the Tyr was replaced with another aromatic side chain, Phe. With both receptors, the Y391F potencies were about the same as wild-type Gαs (Figs. 5E and 6E and Table 1), but the low expression of this mutant prohibits a firm interpretation of the results. As discussed above, AL substitutions of Q390 and R389 had minimal effects on signaling of the two receptors. The R389P mutant, as expected (21), abolished signaling (Figs. 5E and 6E and Table 1).
Mapping the Gαs CT hot spots into the structural models of the LHR/Gαs and β2-AR/Gαs complexes
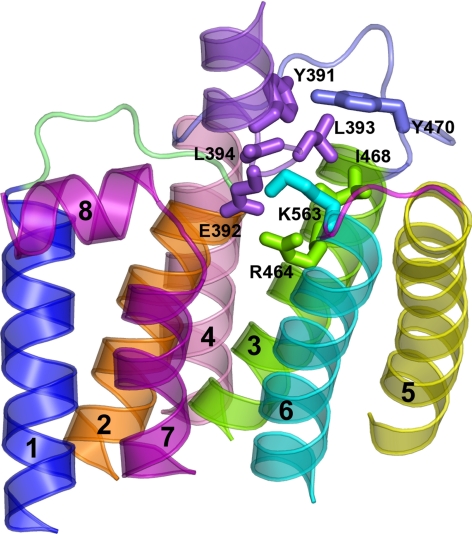
Using a novel model of a mutation-induced, constitutively active form of LHR (D564G), the structure of constitutively active opsin (49), and rigid body docking, a model was developed for an LHR-Gαs complex. This predicted complex emphasized the role of the Gαs CT in the recognition of the cytosolic extensions of helices 3 and 6 of LHR (Fig. 7). Other Gαs regions, like the CT end of αN and the α4/β6 loop, make contacts, respectively, with IL2 and helix 8 of the LHR. Two LHR-Gαs complexes were predicted to be reliable. One was similar to the one recently predicted (6), which employed a computational model of the D564G mutant that was based upon the dark rhodopsin structure, whereas the other resembles the crystallographic complex between opsin and the CT from transducin Gα (12). The latter is characterized by a 180° rotation around the CT main axis in the membrane plane and is consistent with the results of the mutational analyses of both the LHR cytosolic regions (6) and of the Gαs CT (this study). It must be emphasized that these models reflect a predicted conformation of a constitutively active LHR and not that of wild-type LHR activated by ligand.
Fig. 7.
Complex between Gαs and the D564G constitutively active LHR mutant. The side view, in a direction parallel to the membrane plane, of the selected complex between the Gαs CT (violet) and the cytosolic half of the D564G mutant is shown. The amino acid side chains involved in pairwise interactions are represented by sticks. Helices 1, 2, 3, 4, 5, 6, and 7 are blue, orange, green, pink, yellow, cyan, and purple, respectively (helix 8 is purple as well), whereas IL1, IL2, and IL3 are lime, slate, and magenta, respectively. As for Gαs, the numbering of the long form, employed in the in vitro experiments, is shown in this figure, although the structural model refers to the short variant. In this respect, the amino acids Y391, E392, L393, and L394 in the long form correspond, respectively, to Y377, E378, L379, and L380 in the structural model. Drawings were created with PyMOL 0.99rc6.
The docking of LHR D564G to the Gαs model with a CT similar to that of transducin scored the highest. In that model, of the 10 CT Gαs amino acids, only four contribute significantly to the LHR interaction (Fig. 7 and Supplemental Table 2). These include L394, L393, E392, and Y391, corresponding, respectively, to L380, L379, E378, and Y377 of the short Gαs variant (Fig. 7). In this respect, L394 uses primarily the free α-carboxylate, rather than its side chain, for interaction with LHR through the formation of a salt bridge with K563 in helix 6. In contrast to the predicted salt bridge between the free α-carboxylate of Gαs L394 and the side chain of LHR K563, L393, E392, and Y391 of Gαs interact with LHR through their side chains. In detail, 1) L393 interacts primarily with I468 at the cytosolic end of helix 3, 2) E392 makes a salt bridge with R464 of the DRY motif, and 3) Y391 interacts with Y470 in IL2 (Fig. 7).
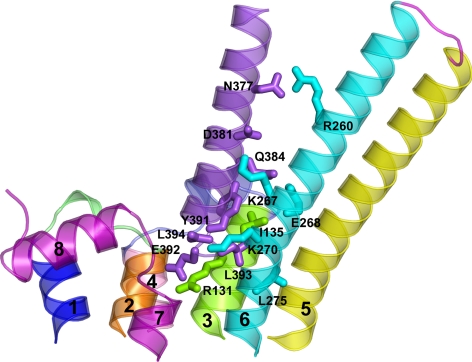
The best scored and reliable Gs docking modes onto the LHR resulted to be the top ones also for the β2-AR. For the latter, a singular docking mode was also found, which was characterized by the docking of Gαs CT in between helices 6 and 7, the E/DRY Arg not being involved in any intermolecular contact. However, similar to the LHR, the β2-AR-Gαs complex, which seems to better fit the indications from mutational analysis, is characterized by the docking of the Gαs CT between helices 3, 5, and 6, as well as by contacts between the α4/β6, α3/β5, and α2/β4 loops of Gαs and helix 8, IL1, and IL2 of the β2-AR, respectively (Figs. 7 and 8 and Supplemental Table 2). IL2 is also involved in contacts with the C-terminal part of αN. The most significant similarities (at least in the physicochemical character of selected interactions) between the complexes involving the two GPCR concern just the extreme CT of Gαs. In this respect, 1) the C-terminal carboxylate of Gαs makes a salt bridge with K270 in helix 6 of the β2-AR, 2) L393 docks into an hydrophobic pocket formed by I135 (helix 3) and L275 (helix 6), 3) E392 makes a salt bridge with R131 of the E/DRY motif in helix 3; and 4) Y391 makes contacts with I135 (helix 3) (Fig. 8). Different from the predicted complex with the LHR, not only the carboxylate group but also the side chain of L394 seems to be involved in β2-AR recognition by establishing contacts with both K270 and A271 in helix 6. This is in line with L394 substitutions affecting β2-AR recognition more than LHR recognition. Supplemental Table 2 delineates the major interactions of the Gαs CT with LHR and β2-AR, showing the similarities and differences. The predicted longer cytosolic extensions of helices 5 and 6 of the β2-AR compared with the LHR is expected to contribute the most to differences in Gαs recognition by the two receptors. Because of these structural differences between the two GPCR in the predicted complex of β2-AR and Gαs, the α5 helix of the G protein makes contacts with helix 6 of the receptor, which are distributed along the entire length of the helix (see Fig. 8 for details of the interactions) rather than being essentially limited to the last 10 amino acids as in the case of the Gs-LHR complex (Figs. 7 and 8).
Fig. 8.
Complex between Gαs and an activated state of the β2-AR. The side view, in a direction parallel to the membrane plane, of the selected complex between the Gαs CT (violet) and the cytosolic half of the receptor is shown. The amino acid side chains involved in pairwise interactions are represented by sticks. Helices 1, 2, 3, 4, 5, 6, and 7 are blue, orange, green, pink, yellow, cyan, and purple, respectively (helix 8 is purple as well), whereas IL1, IL2, and IL3 are lime, slate, and magenta, respectively. As for Gαs, the numbering of the long form, employed in the in vitro experiments, is shown in this figure, although the structural model refers to the short variant. In this respect, the amino acids Y391, E392, L393, and L394 in the long form correspond, respectively, to Y377, E378, L379, and L380 in the structural model. Drawings were created with PyMOL 0.99rc6.
Discussion
The most important findings of this study are that 1) seven common CT Gαs deletion and replacement mutants abrogated signaling in both LHR and β2-AR, 2) the CT Leu is required for β2-AR signaling, in agreement with other reports (21, 50), but not for LHR signaling, and 3) new models are proposed for the LHR and β2-AR interfaces with the CT of Gαs. Thus, although we are unable to distinguish physical association of the Gαs to receptor from receptor activation of the G protein per se, it is possible to conclude that there are both similarities and differences in the functional coupling of Gαs to these receptors. Our finding that the amino acid residues in the extreme CT of Gαs are necessary for functional coupling to LHR, as assessed by changes in cAMP production, is consistent with previous studies (6) and the present predictions by computational modeling. The novel computational model of the LHR/Gs interaction presented in this study suggests that, of the 10 CT amino acids of Gαs, only four bind with LHR IL2 and the CT ends of helices 3 and 6. Of these four predicted interactions, it seems that L393, predicted to interact with LHR I468 of helix 3, is the most important for signaling. Due to the stronger agreement with the results of mutational analysis done in this study, this newly predicted LHR-Gαs complex is likely to hold improved resolution over the previous one (6) and thus represents an important advance in the structural characterization of the LHR/Gαs interface.
Numerous biochemical studies have indicated that the coupling of many G protein isoforms to GPCR involves the extreme CT of Gα subunits (3, 14–23). This region, however, is not always sufficient for the coupling of GPCR/G protein systems (3, 30), because, in line with our computational experiments, the α3/β5 and α4/β6 loops of Gαs also seem to contribute (24–26). A study using chemical cross-linking to define contact sites of Gαq and the M3 muscarinic acetylcholine receptor showed that the three CT residues and the α4/β6 loop of Gαq are in close contact with helices 6 and 8 of the receptor after binding of agonist (7). The interaction model between the M3 receptor and Gαq as inferred from that study seems to be similar to our predicted models between the two GPCR considered in this study and Gαs. Despite the suggested similarities in the gross recognition modes between the two different receptor/G protein systems, the details of the intermolecular interactions are predicted to vary even for two different receptors recognizing the same G protein as found herein from the results of in vitro mutational analysis and computational experiments. Differences in Gαs recognition by LHR and β2-AR may be due, at least in part, to the cytosolic extensions of helices 5 and 6, which participate in the receptor-G protein interface and are predicted to be significantly longer in β2-AR than in LHR.
The recent solution of opsin and metarhodopsin II in complex with a peptide corresponding to the last 11 amino acid residues of transducin (Gαt) illustrates the relationships between the terminal Gαt residues and the G protein binding pocket of the GPCR (12, 51). The conserved Gly at position −3 in the Gαi/o family of G proteins permits the formation of an open reverse turn, an αL-type C-capping motif (12); because there is not a Gly in this position of wild-type Gαs, it is unlikely that it can form this exact conformation. Thus, Gs-coupled receptors may require a different G protein conformation at the extreme CT for coupling. Interestingly, peptides that correspond to the extreme CT of Gαs form complete helices in solution without a capping motif (50).
However, the results of docking experiments shown herein refer to a Gαs CT with a conformation similar to that of the CT peptide from transducin, i.e. that of a canonical α-helix with the exception of the last three amino acids. Simulations were also performed with a Gαs CT in a complete α-helix. Solutions from the transducin-based Gαs CT model were the most reliable in this study and exhibited a gross docking mode of the Gαs CT. The docking scores were lower for the fully α-helical conformation because this fold emphasizes the role of L394 in binding to a hydrophobic pocket that is contributed by V392, V467, and L478 of LHR and neglects the role of Gαs Y391 and L393 in receptor coupling. These results were less consistent with the in vitro data. Collectively, the results of computational experiments suggest that the CT of Gαs and Gαt assume similar conformations in their receptor-bound states.
A patient with Albright hereditary osteodystrophy was determined to have an Arg to His mutation within the CT of Gαs at position 385 that impaired Gαs function (52). No other naturally occurring mutations within this region have been reported, which suggests that the CT domain has experienced selective pressure during evolution. The results presented here imply that although the region may tolerate substitution during the measurement of one type of receptor-coupling event, the wild-type sequence must remain intact for recognition by all Gs-coupled receptors.
In crystal structures, the α5 helix of Gαs bends at position −17 from the CT, which brings the CT toward the globular protein structure and specifically near the α2/β4, α3/β5, and α4/β6 loops. The α3/β5 loop has been implicated as important for β2-AR/Gs coupling (26). Conversely, the α5 helix of Gαi/o proteins remains straight in crystal structures, and evidence indicates that the extreme CT of these proteins is sufficient for receptor coupling. A recent model of the activation of Gαi1 by rhodopsin suggests that a rigid-body rotation and translation of the Gα α5 helix destabilizes the β6/α5 loop and thus promotes GDP release and G protein activation (53). This loop was also predicted to participate in the GDP exit route after molecular dynamics (MD) simulations on a different receptor-G protein system, i.e. the complex between the thromboxane A2 receptor and heterotrimeric Gαq (54). Computational experiments suggested that the interaction between the CT of Gαq and helices 3 and 6 of the thromboxane A2 receptor triggers the formation of a putative GDP exit route involving αF, α5, and the β6/α5 loop following motions that propagate from α5 to the α-helical domain. The latter undergoes concerted motions with respect to the Ras-like domain, which are intrinsic to the fold of the Gα proteins but are amplified by receptor.
Perhaps the mechanism of activation, an event that is initiated at the receptor/G protein binding interface and subsequently translated to the nucleotide pocket, is conserved in general for all isoforms of Gα proteins, but the specific G protein interface for each receptor could be slightly different. Our in vitro and in silico experiments suggest that general points of contact may be shared for GPCR that couple to the same families of G proteins, but for each receptor-G protein pair, the precise determinants and the strength of their contributions may vary (3, 30). If the coupling interface is as unique for each GPCR-G protein pair as these studies suggest, drug discovery applications could potentially target these relationships to improve the specificity of engineered therapeutics.
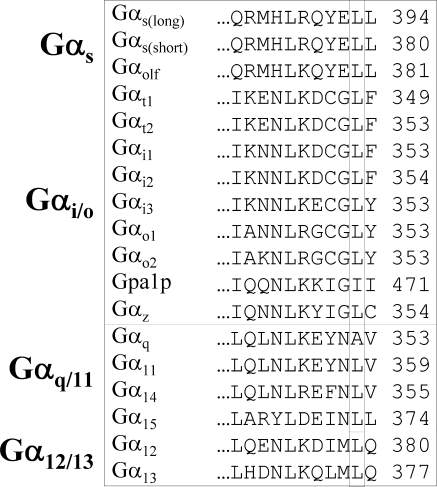
In terms of the proposed model, the decrease in potency found with Gαs L394K may indicate charge repulsion with LHR K563. For β2-AR, the decrease in potency after L394 replacements by Asp or Phe may be due to the steric hindrance, which may compromise coupling efficiency. The predicted hydrophobic side chain interaction of Gαs L393 with I468 at the cytosolic end of LHR helix 3 is fully consistent with our experimental findings. Interestingly, the Leu at this position is invariant in the Gα proteins (Fig. 9), suggestive of an important structural and/or functional role in G proteins that may be necessary for all Gs/receptor coupling. A study that monitored the inhibition of A2A-adenosine receptor signaling with dominant-negative Gαs CT synthetic peptides also found the −2 position to be sensitive to substitutions (55). A salt bridge is predicted between Gαs E392 and the Arg of the DRY motif of both LHR and β2-AR, yet some degree of coupling between the various E392 mutants and the two receptors suggests that this predicted salt bridge is not critical for functional coupling. Replacement of this residue may lead to a conformational change within the Gαs subunit that alters its affinity for GDP, thereby promoting an increase in the rate of nucleotide release and the level of basal activity, GDP release being the rate-limiting step for G protein activation (56). Alternatively, if a receptor and G protein are precoupled within a signalosome, the loss of Gαs E392 may affect the conformation of the receptor and promote the conversion of the ligand-free receptor to an active state that then is able to activate the altered G protein. The predicted interaction of Gαs Y391 with Y470 in IL2 of LHR or with I135 in the cytosolic extension of helix 3 in the β2-AR is consistent with our observations. The Q390A and R389A substitutions had no effect on potency in either receptor, consistent with the proposed model indicating that they do not interact directly with either LHR or β2-AR. Because the loss of the positive charge at position −6 does not impair receptor signaling, the R389P mutation most likely places the extreme CT into an unfavorable conformation for receptor coupling; thus, the maintenance of an α-helical backbone is important for Gs-receptor coupling.
Fig. 9.
CLUSTALW alignment of the last 11 amino acids of the human isoforms of the Gα protein and the Saccharomyces cerevisiae Gpa1p homolog. The isoforms are grouped into families according to sequence homology, and the numbers of the terminal amino acids are indicated to the right of the sequences.
Structural alteration of Gαs may not only impact the coupling relationship with cognate receptors but may also disturb the conformation and stability of the protein and thus the binding, exchange, and release of guanine nucleotides; the affinity for Gβγ localization; and association with the effector enzyme, adenylyl cyclase. The crystal structures of the Gαs (25) and Gαq (32) subunits suggest that the −1 to −3 positions extend from the globular protein structure and are therefore unlikely to influence global protein folding. The extreme CT is located approximately 35–40 Å from the nucleotide binding pocket (33, 34), and none of the CT residues has been implicated in the association with Gβγ or adenylyl cyclase (31, 34–37). If Gαs retains its N-terminal palmitoylation and associates with Gβγ, it targets properly to the plasma membrane (38). Therefore, it is concluded that the manipulation of the extreme CT is likely to only affect the receptor-coupling relationship.
Conclusions
For these studies, functional LHR and Gαs were successfully incorporated into β2-AR+ s49 ck cells, thus enabling studies of GPCR/Gs coupling in an intact cellular background lacking endogenous Gαs activity (41, 42). This unique genetically engineered cell line thus serves as a model system to monitor hCG/LHR-mediated and isoprenaline/β2-AR-mediated cAMP production. In agreement with the computational results, our experimental findings demonstrated that LHR and β2-AR signaling requires amino acid residues at the CT of Gαs and that not only may the details of interaction between receptor and the CT of Gα vary between receptors that recognize different families of G proteins, but they also vary between receptors that couple to the same isoform of G protein. This is expected to occur despite similarities in the overall architecture of the different receptor/G protein complexes, a concept supported by the lack of primary sequence determinants for GPCR/G protein coupling (2–5). The novel computational models of the LHR/Gαs and β2-AR/Gαs interaction indicates that, of the 10 CT amino acid residues of Gαs, only four bind to receptor IL2 and the CT ends of helices 3 and 6. The proposed models of LHR/β2-AR-Gαs complexes should prove invaluable in guiding future experiments aimed at elucidating the full range of molecular contacts and the mechanism of activation. The overall results presented herein are consistent with the prevailing model that ligand binding to GPCR leads to a conformational change that exposes amino acid residues on the inner membrane face of the receptor, permitting interaction of these residues and those on the intracellular loops with the Gαs CT and other regions, e.g. the CT of αN and the α2/β4, α3/β5, and α4/β6 loops (3, 24, 25, 46, 57).
Materials and Methods
Materials
The s49 ck (cyc− kin−) cells were obtained from the University of California, San Francisco, Cell Culture Facility, San Francisco, CA (41). The Virapower Lentivirus Expression System, HEK293FT cells, and Lipofectamine 2000 were from Invitrogen (Carlsbad, CA). DMEM and Weymouth's medium were products of Cellgro Mediatech Inc. (Herndon, VA). PBS, HEPES, MEM nonessential amino acids, hexadimethrine bromide (Polybrene), purified hCG (no. C0434), crude hCG (no. CG10), isoprenaline (no. I5627), BSA (no. A7906), and isobutylmethylxanthine (IBMX) were purchased from Sigma Chemical Co. (St. Louis, MO). Iodo-Gen was from Pierce Biotechnology (Rockford, IL). Heat-inactivated horse serum, fetal bovine serum, and OPTI-MEM were obtained from GIBCO (Carlsbad, CA). Blasticidin and puromycin were products of Invivogen (San Diego, CA). Penicillin, streptomycin, and amphotericin B and EDTA-free trypsin were from Life Science Technologies, (Gaithersburg, MD), and cholera toxin (no. 227036) was from Calbiochem (Gibbstown, NJ).
cDNA plasmids
The long splice variant of human Gαs cDNA was purchased from American Type Culture Collection (Manassas, VA). The insertions of the internal 6x-His tag into the long splice variant of Gαs (after codon 76) and the N-terminal c-myc epitope tag (EQKLISEEDL) into LHR (after codon 24) were prepared with the method of overlap PCR (58). The final PCR products were ligated into the pLenti6 Topo vector (Invitrogen). The c-myc_LHR/pLenti6 vector was modified to replace the EM7 promoter and the blasticidin-resistance cDNA with the cDNA encoding puromycin resistance with the use of standard molecular cloning techniques. An AgeI site was introduced upstream of the PSV40 sequence in the puromycin cassette (pRS vector from Origene, Rockville, MD), and the replacement was performed with Age1 and KpnI sites. Single site-directed mutations were created with the use of the Stratagene QuikChange method (La Jolla, CA). The entire reading frames of manipulated cDNA were sequenced (Sequencing and Synthesis Facility, University of Georgia, Athens, GA; Genewiz, South Plainfield, NJ).
Cell culture
The s49 ck cells (41, 42) were maintained in a stationary suspension of DMEM that was supplemented with 10% (vol/vol) heat-inactivated horse serum at 37 C in 10% CO2 as described (59). Conditioned medium for s49 cell variants was produced as described (59) by seeding growth medium at a density of 1 × 105 cells/ml and harvesting the supernatant after 48 h. HEK293FT cells were maintained in DMEM that was supplemented with 10% (vol/vol) fetal bovine serum, 2 mm l-glutamate, 5 mm MEM nonessential amino acids, and penicillin, streptomycin, and amphotericin B at 37 C in 5% CO2.
Lentiviral production, viral transduction of s49 ck cells, and selection of stable transformants
Lentivirus production in HEK293FT cells was performed with the ViraPower Lentivirus Expression System protocol (Invitrogen) in 10-cm dishes. Virus-containing medium was removed and filtered 60–72 h after transfection. Immediately after harvest of the virus, transduction was performed in the presence of 6 μg/ml hexadimethrine bromide (Polybrene). HEK293 cells at 40% confluency in six-well plates were transduced for viral titering.
For each transduction of s49 ck cells, 5 × 105 s49 cells were resuspended in 2 ml virus-containing medium (average titer of 5 × 105 transducing U/ml) and plated in a six-well plate. After 6 h, the cells were pelleted and resuspended in fresh growth medium. Then, 48 h after transduction, the cells were resuspended in 2 ml growth medium that was supplemented with 50 μg/ml blastidicin or 2.5 μg/ml puromycin. Two weeks after the addition of selective medium, the population of transformed cells was diluted to a density of 1 cell/200 μl conditioned and selective medium per well in 96-well plates for clonal selection. After 2 wk, clonal lines were expanded and assayed for recombinant protein expression.
[125I]hCG saturation binding
Radioiodination of hCG was performed with the Iodo-Gen reagent (Pierce Biotechnology) and Na125I (GE Life Sciences, Piscataway, NJ). Transformed s49 ck cells were washed twice in assay buffer (Weymouth's medium supplemented with 0.1%, wt/vol, BSA). Cells were resuspended in assay buffer with increasing amounts of [125I]hCG with or without 100 IU crude hCG at a density of 4 × 106 cells/ml. A 0.5-ml volume was plated per well in 12-well plates, and the cells were incubated at 37 C for 5 h. Cells were washed twice with 0.5 ml PBS, lysed in 1 ml 1 n NaOH, and counts were measured in a γ-counter (PerkinElmer, Waltham, MA). Prism version 3.0 (GraphPad Software, Inc., La Jolla, CA) was used to analyze saturation binding data with nonlinear regression curve fitting.
CTX stimulation of cAMP
Transformed s49 ck cells were pelleted and washed twice with cAMP medium (Weymouth's medium that was supplemented with 0.1% BSA). Cells (4 × 106) were added to 0.5 ml cAMP medium that was supplemented with 0.8 mm IBMX per well of a 12-well plate at 37 C. After 15 min, medium was replaced with cAMP medium plus 0.8 mm IBMX with or without CTX (2 μg/ml). The cells were incubated for 2 h at 37 C and then stored in 0.5 ml/well 100% ethanol at −20 C overnight. The samples were dried in a SpeedVac (ThermoFisher, Waltham, MA) and resuspended in 200 μl cAMP buffer. The [125I]cAMP RIA was used to determine cAMP concentrations (PerkinElmer). cAMP values from nonstimulated cells were subtracted from the values for stimulated cells to represent the amount of cAMP that was produced solely from the CTX treatment. Prism version 3.0 (GraphPad Software) was used to analyze the data with the one-way ANOVA that was followed by Dunnett's posttest.
Collection of cellular membranes, SDS-PAGE, and Western blotting
Transformed s49 ck cells (4 × 108) were pelleted, washed twice with 50 ml cold PBS, frozen at −80 C, and thawed in 10 ml cold membrane buffer [50 mm Tris-Cl (pH 7.4), 250 mm sucrose, 1 mm phenylmethylsulfonyl fluoride, 5 nm N-ethylmaleimide, and 2 mm EDTA (pH 8.0)]. All subsequent steps were performed on ice or at 4 C (60). The suspension was homogenized with a Tekmar instrument for 2 min with the small probe (power level of 25) and then was centrifuged at 1000 × g for 5 min. The supernatant was collected, and the pellet was resuspended and homogenized again. The combined supernatants were centrifuged at 48,000 × g for 45 min at 4 C. The membrane-containing pellet was suspended in membrane buffer without glucose and frozen at −80 C. Protein concentrations were determined with the Bradford method (Bio-Rad, Hercules, CA).
Crude membranes (100 μg) in reducing Laemmli sample buffer were resolved on 4–20% Tris-Cl denaturing gels (Bio-Rad) and were transferred to Immobilon polyvinylidene difluoride membranes (Millipore, Billerica, MA). Western blotting was performed in 5% blocking buffer [20 mm Tris-Cl (pH 7.4), 150 mm NaCl, 0.05% Tween 20, 5% nonfat milk]. Antibody dilutions were the following in 1.5% blocking buffer: 10,000× for anti-6xHis (6219-1; BD Biosciences, San Jose, CA), 50× for anti-actin (sc-1616; Santa Cruz Biotechnology, Santa Cruz, CA), 10,000× for sheep antimouse (GE Life Sciences), and 20,000× for donkey antigoat horseradish peroxidase (Santa Cruz). Incubations with primary antibodies were performed at 4 C overnight, and the incubation for secondary antibodies was 1 h at room temperature. The Western Lightning ECL kit was used to visualize chemiluminescence (PerkinElmer).
Dose-response cAMP assays
After 15 min, the cAMP assay medium was replaced with 0.5 ml/well cAMP medium plus 0.8 mm IBMX that was supplemented with the appropriate concentration of purified hCG or isoprenaline at 37 C. After 30 min, the medium was replaced with 0.5 ml/well 100% ethanol (Pharmco-AAPER, Shelbyville, KY), and the plates were stored at −20 C overnight. The samples were dried, resuspended, measured, and analyzed using nonlinear regression curve fitting in Prism version 3.0. One-way ANOVA that was followed by Dunnett's posttest was performed for the comparison of basal cAMP levels.
Computational modeling
A novel model of the constitutively active D564G LHR was achieved through the combination of comparative modeling of the template, the crystal structure of the constitutively active opsin (49), with 10-nsec MD simulations. The selected model was mutated and subjected to energy minimization and 10-nsec MD simulations in implicit water/membrane (61). Minimizations were carried out with 1500 steps of steepest descent, followed by adopted basis Newton-Raphson (ABNR) minimization until the root mean square gradient was less than 0.001 kcal/mol Å. With respect to the setup of MD simulations, the lengths of the bonds that involved the hydrogen atoms were restrained by the SHAKE algorithm, which allows for an integration time step of 0.002 psec. The systems were heated to 300 K with 7.5 K rises every 2.5 psec per 100 psec with the random assignment of velocities from a Gaussian distribution. After heating, the system was allowed to equilibrate for 100 psec. The temperature of the systems was kept constant through the duration of the production phase. The structures that were averaged over the first 100 psec and last 100 psec of the equilibrated MD trajectory were energy minimized and employed for the prediction of a putative complex with heterotrimeric Gs, in accordance with a rigid body docking protocol that was previously described (6).
The same heterotrimeric Gs was also employed in docking experiments with a computational model of activated β2-AR. The latter model was achieved by completing the gap between helices 5 and 6 in the recent crystallographic structure of a nanobody-stabilized active state of the β2-AR (62). In this respect and consistent, at least in part, with the latest structure determinations of the β1-AR (63), the cytosolic ends of helices 5 and 6 were elongated by four turns and connected by four amino acids in random coil conformation by means of the MODELLER software (64). Based on quality checks, the three best models were selected for docking simulations, and six independent docking simulations by of β2-AR gave overlapping results.
For both receptors, the results presented in this study concern docking simulations in which the last 11 amino acids of Gαs hold the same conformation as the Gαt CT from the crystallographic complex with opsin and (12). This conformation is such that only the last three amino acids are not in a canonical α-helix conformation. A Gαs variant with the entire CT in canonical α-helix conformation was probed as well in docking simulations with LHR.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Health DK33973 (to D.P.) and the Telethon-Italy Grant S00068TELU (to F.F.).
Present address for G.D.: Department of Biology, College of Science and Technology, Science Center, Armstrong Atlantic State University, 11935 Abercorn Street, Savannah, Georgia 31419-1997.
Disclosure Summary: The authors have no conflicts to declare.
Footnotes
- β2-AR
- β2-Adrenergic receptor
- CT
- C terminus
- CTX
- cholera toxin
- GPCR
- G protein-coupled receptor
- hCG
- human chorionic gonadotropin
- IBMX
- isobutylmethylxanthine
- IL
- intracellular loop
- LHR
- LH receptor
- MD
- molecular dynamics.
References
- 1. Lefkowitz RJ. 2007. Seven transmembrane receptors: something old, something new. Acta Physiol (Oxf) 190:9–19 [DOI] [PubMed] [Google Scholar]
- 2. Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. 2003. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol 63:1256–1272 [DOI] [PubMed] [Google Scholar]
- 3. Cabrera-Vera TM, Vanhauwe J, Thomas TO, Medkova M, Preininger A, Mazzoni MR, Hamm HE. 2003. Insights into G protein structure, function, and regulation. Endocr Rev 24:765–781 [DOI] [PubMed] [Google Scholar]
- 4. Cao J, Panetta R, Yue S, Steyaert A, Young-Bellido M, Ahmad S. 2003. A naive Bayes model to predict coupling between seven transmembrane domain receptors and G-proteins. Bioinformatics 19:234–240 [DOI] [PubMed] [Google Scholar]
- 5. Sgourakis NG, Bagos PG, Hamodrakas SJ. 2005. Prediction of the coupling specificity of GPCR to four families of G-proteins using hidden Markov models and artificial neural networks. Bioinformatics 21:4101–4106 [DOI] [PubMed] [Google Scholar]
- 6. Angelova K, Fanelli F, Puett D. 2008. Contributions of intracellular loops 2 and 3 of the lutropin receptor in Gs coupling. Mol Endocrinol 22:126–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hu J, Wang Y, Zhang X, Lloyd JR, Li JH, Karpiak J, Costanzi S, Wess J. 2010. Structural basis of G protein-coupled receptor-G protein interactions. Nat Chem Biol 6:541–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kristiansen K. 2004. Molecular mechanisms of ligand binding, signaling, and regulation within the superfamily of G-protein-coupled receptors: molecular modeling and mutagenesis approaches to receptor structure and function. Pharmacol Ther 103:21–80 [DOI] [PubMed] [Google Scholar]
- 9. Schöneberg T, Schultz G, Gudermann T. 1999. Structural basis of G protein-coupled receptor function. Mol Cell Endocrinol 151:181–193 [DOI] [PubMed] [Google Scholar]
- 10. Ayoub MA, Trinquet E, Pfleger KD, Pin JP. 2010. Differential association modes of the thrombin receptor PAR1 with Gαi1, Gα12, and β-arrestin 1. FASEB J 24:3522–3535 [DOI] [PubMed] [Google Scholar]
- 11. Bourne HR. 1997. How receptors talk to trimeric G proteins. Curr Opin Cell Biol 9:134–142 [DOI] [PubMed] [Google Scholar]
- 12. Scheerer P, Park JH, Hildebrand PW, Kim YJ, Krauss N, Choe HW, Hofmann KP, Ernst OP. 2008. Crystal structure of opsin in its G-protein-interacting conformation. Nature 455:497–502 [DOI] [PubMed] [Google Scholar]
- 13. Wess J. 1997. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J 11:346–354 [PubMed] [Google Scholar]
- 14. Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. 1993. Substitution of three amino acids switches receptor specificity of Gqα to that of Giα. Nature 363:274–276 [DOI] [PubMed] [Google Scholar]
- 15. Gilchrist A, Mazzoni MR, Dineen B, Dice A, Linden J, Proctor WR, Lupica CR, Dunwiddie TV, Hamm HE. 1998. Antagonists of the receptor-G protein interface block Gi-coupled signal transduction. J Biol Chem 273:14912–14919 [DOI] [PubMed] [Google Scholar]
- 16. Gilchrist A, Bünemann M, Li A, Hosey MM, Hamm HE. 1999. A dominant-negative strategy for studying roles of G proteins in vivo. J Biol Chem 274:6610–6616 [DOI] [PubMed] [Google Scholar]
- 17. Palm D, Münch G, Malek D, Dees C, Hekman M. 1990. Identification of a Gs-protein coupling domain to the β-adrenoceptor using site-specific synthetic peptides. Carboxyl terminus of Gsα is involved in coupling to β-adrenoceptors. FEBS Lett 261:294–298 [DOI] [PubMed] [Google Scholar]
- 18. Conklin BR, Herzmark P, Ishida S, Voyno-Yasenetskaya TA, Sun Y, Farfel Z, Bourne HR. 1996. Carboxyl-terminal mutations of Gqα and Gsα that alter the fidelity of receptor activation. Mol Pharmacol 50:885–890 [PubMed] [Google Scholar]
- 19. D'Ursi AM, Giusti L, Albrizio S, Porchia F, Esposito C, Caliendo G, Gargini C, Novellino E, Lucacchini A, Rovero P, Mazzoni MR. 2006. A membrane-permeable peptide containing the last 21 residues of the Gαs carboxyl terminus inhibits Gs-coupled receptor signaling in intact cells: correlations between peptide structure and biological activity. Mol Pharmacol 69:727–736 [DOI] [PubMed] [Google Scholar]
- 20. Mazzoni MR, Taddei S, Giusti L, Rovero P, Galoppini C, D'Ursi A, Albrizio S, Triolo A, Novellino E, Greco G, Lucacchini A, Hamm HE. 2000. A Gαs carboxyl-terminal peptide prevents Gs activation by the A2A adenosine receptor. Mol Pharmacol 58:226–236 [DOI] [PubMed] [Google Scholar]
- 21. Rasenick MM, Watanabe M, Lazarevic MB, Hatta S, Hamm HE. 1994. Synthetic peptides as probes for G protein function. Carboxyl-terminal Gαs peptides mimic Gs and evoke high affinity agonist binding to β-adrenergic receptors. J Biol Chem 269:21519–21525 [PubMed] [Google Scholar]
- 22. Feldman DS, Zamah AM, Pierce KL, Miller WE, Kelly F, Rapacciuolo A, Rockman HA, Koch WJ, Luttrell LM. 2002. Selective inhibition of heterotrimeric Gs signaling. Targeting the receptor-G protein interface using a peptide minigene encoding the Gαs carboxyl terminus. J Biol Chem 277:28631–28640 [DOI] [PubMed] [Google Scholar]
- 23. Lee C, Ji I, Ji TH. 2004. Distinct mechanisms of cAMP induction by constitutively activating LH receptor and wild-type LH receptor activated by hCG. Endocrine 25:111–115 [DOI] [PubMed] [Google Scholar]
- 24. Birnbaumer L. 2010. Signal transduction by G proteins: basic principles, molecular diversity, and structural basis of their action. In: Bradshaw RA, Dennis EA. eds. Handbook of cell signaling. Burlington, MA: Academic Press/Elsevier; 1597–1614 [Google Scholar]
- 25. Sprang SR. 2010. Structures of heterotrimeric G proteins and their complexes. In: Bradshaw RA, Dennis EA. eds. Handbook of cell signaling. 2nd ed Burlington, MA: Academic Press/Elsevier; 119–128 [Google Scholar]
- 26. Grishina G, Berlot CH. 2000. A surface-exposed region of Gsα in which substitutions decrease receptor-mediated activation and increase receptor affinity. Mol Pharmacol 57:1081–1092 [PubMed] [Google Scholar]
- 27. Herrmann R, Heck M, Henklein P, Kleuss C, Wray V, Hofmann KP, Ernst OP. 2006. Rhodopsin-transducin coupling: role of the Gα C-terminus in nucleotide exchange catalysis. Vision Res 46:4582–4593 [DOI] [PubMed] [Google Scholar]
- 28. Marsh SR, Grishina G, Wilson PT, Berlot CH. 1998. Receptor-mediated activation of Gsα: evidence for intramolecular signal transduction. Mol Pharmacol 53:981–990 [PubMed] [Google Scholar]
- 29. Onrust R, Herzmark P, Chi P, Garcia PD, Lichtarge O, Kingsley C, Bourne HR. 1997. Receptor and βγ binding sites in the α subunit of the retinal G protein transducin. Science 275:381–384 [DOI] [PubMed] [Google Scholar]
- 30. Blahos J, 2nd, Mary S, Perroy J, de Colle C, Brabet I, Bockaert J, Pin JP. 1998. Extreme C terminus of G protein α-subunits contains a site that discriminates between Gi-coupled metabotropic glutamate receptors. J Biol Chem 273:25765–25769 [DOI] [PubMed] [Google Scholar]
- 31. Lambright DG, Sondek J, Bohm A, Skiba NP, Hamm HE, Sigler PB. 1996. The 2.0 A crystal structure of a heterotrimeric G protein. Nature 379:311–319 [DOI] [PubMed] [Google Scholar]
- 32. Nishimura A, Kitano K, Takasaki J, Taniguchi M, Mizuno N, Tago K, Hakoshima T, Itoh H. 2010. Structural basis for the specific inhibition of heterotrimeric Gq protein by a small molecule. Proc Natl Acad Sci USA 107:13666–13671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sunahara RK, Tesmer JJ, Gilman AG, Sprang SR. 1997. Crystal structure of the adenylyl cyclase activator Gsα. Science 278:1943–1947 [DOI] [PubMed] [Google Scholar]
- 34. Tesmer JJ, Sunahara RK, Gilman AG, Sprang SR. 1997. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsα.GTPγS. Science 278:1907–1916 [DOI] [PubMed] [Google Scholar]
- 35. Wall MA, Coleman DE, Lee E, Iñiguez-Lluhi JA, Posner BA, Gilman AG, Sprang SR. 1995. The structure of the G protein heterotrimer Giα1β1γ2. Cell 83:1047–1058 [DOI] [PubMed] [Google Scholar]
- 36. Grishina G, Berlot CH. 1997. Identification of common and distinct residues involved in the interaction of αi2 and αs with adenylyl cyclase. J Biol Chem 272:20619–20626 [DOI] [PubMed] [Google Scholar]
- 37. Berlot CH, Bourne HR. 1992. Identification of effector-activating residues of Gsα. Cell 68:911–922 [DOI] [PubMed] [Google Scholar]
- 38. Evanko DS, Thiyagarajan MM, Wedegaertner PB. 2000. Interaction with Gβγ is required for membrane targeting and palmitoylation of Gαs and Gαq. J Biol Chem 275:1327–1336 [DOI] [PubMed] [Google Scholar]
- 39. Ascoli M, Puett D. 2009. The gonadotropins and their receptors. In: Strauss JF, III, Barbieri R. eds. Yen and Jaffee's reproductive endocrinology. 6th ed Philadelphia: Elsevier; 35–55 [Google Scholar]
- 40. Van Durme J, Horn F, Costagliola S, Vriend G, Vassart G. 2006. GRIS: glycoprotein-hormone receptor information system. Mol Endocrinol 20:2247–2255 [DOI] [PubMed] [Google Scholar]
- 41. Insel PA, Bourne HR, Coffino P, Tomkins GM. 1975. Cyclic AMP-dependent protein kinase: pivotal role in regulation of enzyme induction and growth. Science 190:896–898 [DOI] [PubMed] [Google Scholar]
- 42. Bourne HR, Coffino P, Tomkins GM. 1975. Selection of a variant lymphoma cell deficient in adenylate cyclase. Science 187:750–752 [DOI] [PubMed] [Google Scholar]
- 43. Ascoli M, Fanelli F, Segaloff DL. 2002. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr Rev 23:141–174 [DOI] [PubMed] [Google Scholar]
- 44. Angelova K, Narayan P, Puett D. 2003. The luteinizing hormone receptor: influence of buffer composition on ligand binding and signaling of wild type and mutant receptors. Mol Cell Endocrinol 204:1–9 [DOI] [PubMed] [Google Scholar]
- 45. Freissmuth M, Gilman AG. 1989. Mutations of GSα designed to alter the reactivity of the protein with bacterial toxins. Substitutions at ARG187 result in loss of GTPase activity. J Biol Chem 264:21907–21914 [PubMed] [Google Scholar]
- 46. Lambright DG. 2010. Heterotrimeric G-protein signaling at atomic resolution. In: Bradshaw RA, Dennis EA. eds. Handbook of cell signaling. 2nd ed Burlington, MA: Academic Press/Elsevier; 1615–1619 [Google Scholar]
- 47. Pantaloni C, Audigier Y. 1993. Functional domains of the Gsα subunit: role of the C-terminus in the receptor-dependent and receptor-independent activation. J Recept Res 13:591–608 [DOI] [PubMed] [Google Scholar]
- 48. Sullivan KA, Miller RT, Masters SB, Beiderman B, Heideman W, Bourne HR. 1987. Identification of receptor contact site involved in receptor-G protein coupling. Nature 330:758–760 [DOI] [PubMed] [Google Scholar]
- 49. Park JH, Scheerer P, Hofmann KP, Choe HW, Ernst OP. 2008. Crystal structure of the ligand-free G-protein-coupled receptor opsin. Nature 454:183–187 [DOI] [PubMed] [Google Scholar]
- 50. Dursi AM, Albrizio S, Greco G, Mazzeo S, Mazzoni MR, Novellino E, Rovero P. 2002. Conformational analysis of the Gαs protein C-terminal region. J Pept Sci 8:476–488 [DOI] [PubMed] [Google Scholar]
- 51. Choe HW, Kim YJ, Park JH, Morizumi T, Pai EF, Krauss N, Hofmann KP, Scheerer P, Ernst OP. 2011. Crystal structure of metarhodopsin II. Nature 471:651–655 [DOI] [PubMed] [Google Scholar]
- 52. Schwindinger WF, Miric A, Zimmerman D, Levine MA. 1994. A novel Gsα mutant in a patient with Albright hereditary osteodystrophy uncouples cell surface receptors from adenylyl cyclase. J Biol Chem 269:25387–25391 [PubMed] [Google Scholar]
- 53. Oldham WM, Van Eps N, Preininger AM, Hubbell WL, Hamm HE. 2006. Mechanism of the receptor-catalyzed activation of heterotrimeric G proteins. Nat Struct Mol Biol 13:772–777 [DOI] [PubMed] [Google Scholar]
- 54. Raimondi F, Seeber M, Benedetti PG, Fanelli F. 2008. Mechanisms of inter- and intramolecular communication in GPCR and G proteins. J Am Chem Soc 130:4310–4325 [DOI] [PubMed] [Google Scholar]
- 55. Grieco P, Albrizio S, D'Ursi AM, Giusti L, Mazzoni MR, Novellino E, Rovero P. 2003. A structure-activity relationship study on position-2 of the Gαs C-terminal peptide able to inhibit Gs activation by A2A adenosine receptor. Eur J Med Chem 38:13–18 [DOI] [PubMed] [Google Scholar]
- 56. Gilman AG. 1987. G proteins: transducers of receptor-generated signals. Annu Rev Biochem 56:615–649 [DOI] [PubMed] [Google Scholar]
- 57. Oldham WM, Hamm HE. 2008. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat Rev Mol Cell Biol 9:60–71 [DOI] [PubMed] [Google Scholar]
- 58. Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR. 1989. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77:61–68 [DOI] [PubMed] [Google Scholar]
- 59. Berlot CH. 2002. Expression and functional analysis of G protein α subunits in S49 lymphoma cells. Methods Enzymol 344:261–277 [DOI] [PubMed] [Google Scholar]
- 60. Wu C, Narayan P, Puett D. 1996. Protein engineering of a novel constitutively active hormone-receptor complex. J Biol Chem 271:31638–31642 [DOI] [PubMed] [Google Scholar]
- 61. Im W, Feig M, Brooks CL., 3rd 2003. An implicit membrane generalized born theory for the study of structure, stability, and interactions of membrane proteins. Biophys J 85:2900–2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Rasmussen SG, Choi HJ, Fung JJ, Pardon E, Casarosa P, Chae PS, Devree BT, Rosenbaum DM, Thian FS, Kobilka TS, Schnapp A, Konetzki I, Sunahara RK, Gellman SH, Pautsch A, Steyaert J, Weis WI, Kobilka BK. 2011. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469:175–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Warne T, Moukhametzianov R, Baker JG, Nehmé R, Edwards PC, Leslie AG, Schertler GF, Tate CG. 2011. The structural basis for agonist and partial agonist action on a β1-adrenergic receptor. Nature 469:241–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sali A, Blundell TL. 1993. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234:779–815 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.