Abstract
Background
The metabolic syndrome is an important cluster of coronary heart disease risk factors with common insulin resistance. The extent to which the metabolic syndrome is associated with demographic and potentially modifiable lifestyle factors in the US population is unknown.
Methods
Metabolic syndrome–associated factors and prevalence, as defined by Adult Treatment Panel III criteria, were evaluated in a representative US sample of 3305 black, 3477 Mexican American, and 5581 white men and nonpregnant or lactating women aged 20 years and older who participated in the cross-sectional Third National Health and Nutrition Examination Survey.
Results
The metabolic syndrome was present in 22.8% and 22.6% of US men and women, respectively (P=.86). The age-specific prevalence was highest in Mexican Americans and lowest in blacks of both sexes. Ethnic differences persisted even after adjusting for age, body mass index, and socioeconomic status. The metabolic syndrome was present in 4.6%, 22.4%, and 59.6% of normal-weight, overweight, and obese men, respectively, and a similar distribution was observed in women. Older age, postmenopausal status, Mexican American ethnicity, higher body mass index, current smoking, low household income, high carbohydrate intake, no alcohol consumption, and physical inactivity were associated with increased odds of the metabolic syndrome.
Conclusions
The metabolic syndrome is present in more than 20% of the US adult population; varies substantially by ethnicity even after adjusting for body mass index, age, socioeconomic status, and other predictor variables; and is associated with several potentially modifiable lifestyle factors. Identification and clinical management of this high-risk group is an important aspect of coronary heart disease prevention.
Coronary heart disease (CHD) is the leading cause of death in the United States.1 Factors associated with an increased risk of developing CHD that tend to cluster in individuals include older age, high blood pressure, a low level of high-density lipoprotein (HDL) cholesterol, a high triglyceride level, a high plasma glucose concentration, and obesity.2 These associated risk factors have been called syndrome X,3 the insulin resistance syndrome,4 or the metabolic syndrome.5
The mechanisms underlying the metabolic syndrome are not fully known; however, resistance to insulin-stimulated glucose uptake seems to modify biochemical responses in a way that predisposes to metabolic risk factors.3,6,7 Insulin resistance is thought to be primarily due to obesity or an inherited genetic defect.8 As the prevalence of obesity increases in the United States, the prevalence of the metabolic syndrome may be expected to increase markedly. Estimates of the prevalence of the metabolic syndrome have varied substantially in part because of the variability of evaluated populations and of diagnostic criteria.9
The recent Third Report of the National Cholesterol Education Program Adult Treatment Panel (ATP III) included clinical diagnosis guidelines for the metabolic syndrome.10 Compared with findings from earlier studies3-5 and World Health Organization guidelines, the new ATP III defines criteria readily measured in clinical practice. These consensus-generated guidelines provide the opportunity to assess the overall prevalence of the metabolic syndrome in the US population according to an accepted standard definition. In an initial study, Ford et al11 reported un-adjusted and age-adjusted metabolic syndrome prevalences of 21.8% and 23.7%, respectively, for the US population. The objectives of this study are to examine the prevalence of the metabolic syndrome by ethnicity, age, body mass index (BMI) (calculated as weight in kilograms divided by the square of height in meters), socioeconomic status, and lifestyle factors.
METHODS
STUDY POPULATION
The Third National Health and Nutrition Examination Survey (NHANES III) was conducted in two 3-year phases (October 18, 1988, to October 24, 1991, and September 20, 1991, to October 15, 1994) by the National Center for Health Statistics to assess the health and nutritional status of the noninstitutionalized US population. Conducted at 89 locations, the study used stratified, multistage probability cluster sampling, similar to that used in the 2 previous surveys. Weights indicating the probability of being sampled were assigned to each respondent, enabling results to represent the entire noninstitutionalized US population. The design of NHANES III is described in detail elsewhere.12
The NHANES III staff conducted surveys in households, administering questionnaires to families, adults, and children. Household surveys included demographic, socioeconomic, dietary, and health history questions. Standardized medical examinations were completed at mobile medical centers and included measurements related to metabolic syndrome criteria, including blood pressure, plasma lipid and glucose levels, and waist circumference. All survey instruments were available in English and Spanish.
The sample included non-Hispanic blacks (blacks), Mexican Americans, non-Hispanic whites (whites), and the “other” ethnicity category, aged 20 years or older at the time of NHANES III evaluation for whom anthropometric variables (ie, weight, height, and waist circumference), blood pressure, and blood studies (ie, glucose, total cholesterol, HDL cholesterol, and triglyceride levels) had been measured. Of 14852 individuals, we excluded 1756 who consumed food or beverages other than water within 6 hours of venipuncture. In addition, we also excluded 235 women who were pregnant or lactating at base-line. Of the remaining 12861 individuals, there were 3305 blacks (1494 men and 1811 women), 3477 Mexican Americans (1811 men and 1666 women), 5581 whites (2626 men and 2955 women), and 498 classified as other ethnicities (214 men and 284 women). A total of 5964 individuals aged 20 years or older in NHANES III were not included because of missing anthropometric measurements or blood studies or because they were not fasting. These 5964 individuals had a mean age similar to that of the 12861 individuals who had the required anthropometric measurements and blood studies available and who had not consumed food or beverages for at least 6 hours before venipuncture (men: 43.9 vs 43.0 years; P=.12; women: 46.2 vs 46.3 years; P=.80).
METABOLIC SYNDROME CRITERIA
The ATP III clinical definition of the metabolic syndrome10 requires the presence of 3 or more of the following: (1) abdominal obesity (waist circumference >102 cm in men and >88 cm in women); (2) a high triglyceride level (≥150 mg/dL [≥1.69 mmol/L]); (3) a low HDL cholesterol level (<40 mg/dL [<1.03 mmol/L] for men and <50 mg/dL [<1.29 mmol/L] for women); (4) high blood pressure (systolic ≥130 mm Hg or diastolic ≥85 mm Hg); and (5) a high fasting plasma glucose concentration (≥110 mg/dL). Individuals met the criteria for high blood pressure or high fasting glucose concentration if they were currently using blood pressure medications or oral hypoglycemic diabetes mellitus control. Individuals with a previous physician diagnosis of hypertension or diabetes mellitus who did not report medication use were not allocated to the metabolic syndrome group.
VARIABLE DEFINITION
Normal weight, overweight, and obesity were defined as a BMI less than 25, 25 to 29.9, and 30 or higher, respectively. Education level was divided into 4 categories: less than 8 years, 8 to 12 years, greater than 12 years, and unknown. Economic status was divided into 4 categories according to the participant’s household income for the previous year: $15000 or less, $15001 to $25000, greater than $25000, and unknown. Smoking was categorized as current, past, and never. Past smokers were those who reported that they had smoked at least 100 cigarettes during their lifetime but who did not currently smoke cigarettes. Drinking was categorized as heavy, moderate, never, and unknown. Heavy drinkers were defined as those who ever drank 5 or more alcoholic beverages per day or who drank beer, wine, or hard liquor 1 time per day during the past month. Moderate drinkers had an alcoholic beverage (ie, beer, wine, or hard liquor) less than once per day during the past month. Never drinkers were those who did not drink beer, wine, or hard liquor during the past month. Physical activity level was defined based on the participant’s physical activity density rating scores obtained from participating in one of the following activities during the past month: walking, jogging or running, bicycle riding, swimming, lifting weights, or doing aerobics or aerobic dancing, other dancing, calisthenics, or garden or yard work. Participants in the physically inactive category included those with a total density rating score of 3.5 or less. The point at which the total density rating score equals 3.5 corresponds to approximately the 15th and 25th percentile in the male and female study populations, respectively. Earlier studies13,14 hypothesize a link between dietary composition and metabolic syndrome risk, particularly carbohydrate as an energy source. We selected carbohydrate intake, expressed as a percentage of total kilocalories, as one relevant measure of dietary composition. The percentage of total caloric intake from carbohydrates was evaluated by categorizing intake as high (>60%), middle (40%-60%), and low (<40%). Menopausal status was defined according to self-reported cessation of menstruation at interview.
STATISTICAL METHODS
Sex- and ethnic-specific prevalence rates of the metabolic syndrome were calculated for black, Mexican American, and white participants. Other ethnic groups were included when calculating the prevalence of the metabolic syndrome in the total US adult population. The metabolic syndrome age- and BMI-specific prevalence rates and 95% confidence intervals were also computed. Graphical presentation of prevalence rates for the metabolic syndrome are provided in 10-year increments. The term overall abnormalities is defined as the frequency in participants of a metabolic syndrome risk factor regardless of whether they also had other risk factors. The term isolated abnormalities is defined as the frequency in participants of only 1 risk factor for the metabolic syndrome. The frequencies of overall and isolated components of the metabolic syndrome were calculated for participants in 3 categorical age groups: young (20-34 years), middle aged (35-64 years), and old (≥65 years).
The adjusted Wald χ2 test was used to evaluate the statistical significance of the prevalence rates of the metabolic syndrome and the frequencies of overall abnormalities among blacks, Mexican Americans, and whites, with Bonferroni adjustment for multiple comparisons.
Multiple logistic regression analysis was used for men and women to estimate the odds ratios (ORs) of the metabolic syndrome by age, ethnicity, BMI, smoking and drinking habits, carbohydrate intake, physical activity status, education and household income levels, and menopausal status. The regression model was used to test the interaction between BMI and sex as an independent variable and with the presence of the metabolic syndrome as a dependent variable.
All analyses incorporated sampling weights to produce nationally representative estimates. We used statistical software (Stata, version 7.0 for Windows; Stata Corp, College Station, Tex) to calculate weighted means, percentages, ORs, and SEs to adjust for the complex NHANES III sampling design. Statistical significance was set at P<.05 unless otherwise indicated.
RESULTS
OVERALL PREVALENCE
The anthropometric and sociodemographic characteristics of the participants are summarized in Table 1 and Table 2. The overall percentage of the metabolic syndrome in US adults, including blacks, Mexican Americans, whites, and others, was 22.8% for men and 22.6% for women as defined by the ATP III guidelines (P=.86).
Table 1.
Anthropometric Characteristics of the Study Sample*
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Black (n = 1494) |
Mexican American (n = 1811) |
White (n = 2626) |
Black (n = 1811) |
Mexican American (n = 1666) |
White (n = 2955) |
|
| US population, in millions, No. |
6.1 | 3.8 | 54.2 | 7.6 | 3.2 | 56.9 |
| Age, y | 40.9 (40.2-41.6) | 36.6 (35.9-37.3) | 45.0 (43.9-46.1) | 41.9 (40.8-43.1) | 38.9 (38.2-39.7) | 47.6 (45.9-49.2) |
| Height, cm | 176.4 (176.0-176.7) | 169.9 (169.3-170.5) | 176.4 (176.0-176.8) | 163.1 (162.7-163.5) | 156.9 (156.6-157.3) | 162.3 (161.9-162.7) |
| Weight, kg | 83.2 (82.2-84.2) | 77.6 (76.6-78.7) | 83.3 (82.3-84.2) | 77.0 (75.8-78.1) | 69.2 (68.4-70.0) | 68.7 (68.3-69.1) |
| BMI | 26.7 (26.4-27.0) | 26.8 (26.6-27.1) | 26.7 (26.5-26.9) | 28.9 (28.5-29.3) | 28.1 (27.7-28.4) | 26.1 (25.9-26.3) |
| WC, cm | 92.2 (91.4-93.0) | 93.6 (92.9-94.3) | 96.5 (95.8-97.2) | 93.0 (92.1-93.9) | 91.0 (90.1-91.8) | 88.1 (87.3-88.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); WC, waist circumference.
Data are given as mean (95% confidence interval), except where indicated otherwise.
Table 2.
Sociodemographic Characteristics of the Study Sample*
| Men |
Women |
|||||
|---|---|---|---|---|---|---|
| Black | Mexican American | White | Black | Mexican American | White | |
| Education, y | ||||||
| <8 | 12.2 (10.7-13.7) | 39.0 (36.3-41.7) | 8.1 (6.2-10.0) | 12.0 (10.0-14.0) | 39.3 (36.5-42.1) | 7.7 (6.6-8.8) |
| 8-12 | 56.7 (54.2-59.2) | 41.0 (38.5-43.5) | 43.9 (41.1-46.7) | 55.1 (52.8-57.4) | 43.6 (41.2-46.1) | 50.0 (47.3-52.7) |
| >12 | 30.3 (27.6-33.0) | 19.2 (17.3-21.1) | 47.7 (44.4-51.0) | 32.5 (31.0-34.0) | 16.9 (14.0-19.8) | 41.9 (39.1-44.7) |
| Unknown | 0.8 (0.4-1.2) | 0.8 (0.3-1.3) | 0.2 (0.1-0.3) | 0.4 (0.1-0.7) | 0.2 (0.0-0.4) | 0.5 (0.2-0.8) |
| Household income, $/y | ||||||
| ≤15 000 | 33.2 (28.2-38.2) | 31.0 (24.8-37.2) | 13.2 (10.8-15.6) | 38.0 (33.2-42.8) | 34.7 (28.8-40.6) | 18.7 (16.1-21.3) |
| 15 001-25 000 | 44.7 (39.7-49.7) | 45.2 (40.1-50.3) | 48.4 (42.5-54.3) | 42.0 (37.1-46.9) | 43.1 (37.9-48.3) | 45.1 (39.8-50.4) |
| >25 000 | 14.6 (11.5-17.7) | 13.0 (9.3-16.7) | 33.6 (27.5-39.7) | 11.6 (8.8-14.4) | 11.9 (7.9-15.9) | 30.3 (24.7-35.9) |
| Unknown | 7.5 (0.0-16.9) | 10.8 (0.0-22.2) | 4.8 (0.0-10.9) | 8.4 (0.0-18.0) | 10.2 (0.0-21.1) | 5.9 (0.0-13.5) |
| Smoking | ||||||
| Current | 39.8 (36.3-43.3) | 30.5 (28.1-32.9) | 30.6 (28.3-32.9) | 27.2 (24.7-29.7) | 15.0 (13.5-16.5) | 25.3 (23.0-27.6) |
| Past | 21.0 (19.1-22.9) | 26.4 (23.8-29.0) | 35.2 (33.0-37.4) | 2.6 (11.5-13.7) | 13.6 (11.7-15.5) | 24.1 (22.1-26.1) |
| Never | 39.2 (35.9-42.5) | 43.1 (40.4-45.8) | 34.2 (31.9-36.5) | 60.2 (57.8-62.7) | 71.4 (68.8-74.0) | 50.6 (48.9-52.3) |
| Drinking | ||||||
| Heavy | 24.0 (22.3-25.7) | 21.5 (19.6-23.4) | 23.6 (21.7-25.5) | 7.1 (5.8-8.4) | 4.1 (3.2-5.0) | 9.0 (7.4-10.6) |
| Moderate | 47.2 (45.0-49.4) | 57.0 (54.2-59.8) | 49.8 (47.3-52.3) | 36.2 (33.2-39.2) | 33.6 (30.8-36.4) | 44.7 (41.8-47.6) |
| Never | 28.7 (26.4-31.0) | 21.4 (19.2-23.6) | 26.2 (23.8-28.6) | 56.4 (53.5-59.3) | 62.1 (58.6-65.6) | 46.3 (43.7-48.9) |
| Unknown | 0.1 (0.1-0.3) | 0.1 (0.0-0.3) | 0.4 (0.0-0.9) | 0.3 (0.0-0.7) | 0.2 (0.0-0.5) | 0.0 (0.0-0.1) |
| Carbohydrate intake | ||||||
| Low | 26.8 (24.6-29.0) | 17.9 (15.8-20.0) | 20.8 (19.1-22.5) | 17.1 (15.2-19.0) | 11.7 (10.1-13.3) | 16.0 (14.2-17.8) |
| Moderate | 58.6 (56.6-60.6) | 64.1 (61.7-66.5) | 62.0 (59.9-64.1) | 62.2 (59.5-64.9) | 68.0 (65.8-70.2) | 62.1 (59.8-64.4) |
| High | 11.5 (10.0-13.0) | 11.5 (9.5-13.5) | 14.1 (13.1-15.1) | 17.8 (16.2-19.4) | 18.3 (16.1-20.5) | 19.9 (18.2-21.6) |
| Unknown | 3.1 (2.2-4.0) | 3.0 (2.1-3.9) | 3.1 (2.4-3.8) | 2.9 (2.0-3.8) | 2.0 (1.3-2.7) | 2.1 (1.6-2.6) |
| Physical activity status | ||||||
| Inactive | 22.0 (19.8-24.2) | 29.0 (26.3-31.7) | 13.2 (11.7-14.7) | 39.9 (37.3-42.5) | 43.4 (41.1-45.7) | 23.1 (21.4-24.8) |
| Menopausal status | ||||||
| Postmenopausal | … | … | … | 35.2 (32.2-38.2) | 25.7 (23.9-27.5) | 44.6 (40.5-48.7) |
| Premenopausal | … | … | … | 62.9 (62.2-63.6) | 72.1 (70.0-74.2) | 54.4 (54.1-54.7) |
| Unknown | … | … | … | 1.9 (0.0-5.0) | 2.2 (1.5-2.9) | 1.0 (0.0-5.1) |
Data are given as percentage (95% confidence interval).
ETHNIC-SPECIFIC PREVALENCE
The percentage of participants with the metabolic syndrome was 13.9%, 20.8%, and 24.3%, for black, Mexican American, and white men, respectively. The percentage of men with the metabolic syndrome was higher in Mexican American and white men than in black men (P<.001 and P = .006, respectively; statistical significance set at P<.017); the difference between Mexican American and white men was not statistically significant (P=.06; statistical significance set at P<.017).
The percentage of black and white women with the metabolic syndrome was 20.9% and 22.9%, respectively, and there was no significant between–ethnic group difference (P=.10; statistical significance set at P<.017). The percentage of Mexican American women with the metabolic syndrome was significantly higher, 27.2%, than that of black and white women (P<.001 and P=.002, respectively; statistical significance set at P<.017).
AGE-SPECIFIC PREVALENCE
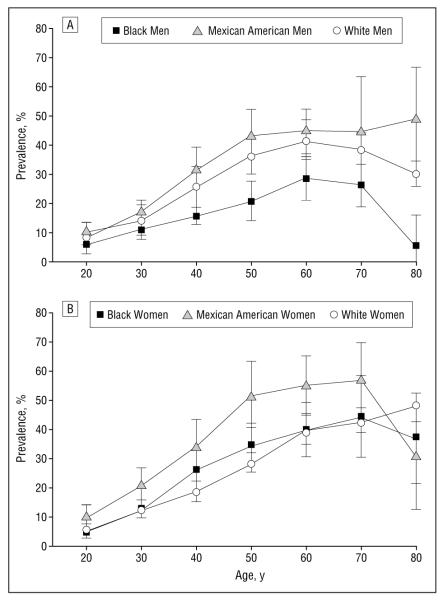
The prevalence of the metabolic syndrome by 10-year age groups is presented in Figure 1. Mexican American men showed the highest prevalence of the metabolic syndrome, followed by white men and then black men. Compared with that for black men, the prevalence of the metabolic syndrome for Mexican American men was significantly higher at 40, 50, 60, and 80 years or older, whereas white men showed a significantly higher prevalence at 40, 50, 70, and 80 years or older (P<.017). There were no statistically significant differences in the prevalence of the metabolic syndrome between Mexican American and white men at any age group.
Figure 1.
The prevalence of the metabolic syndrome by age in men (A) and women (B). Statistical significance is set at P<.017. Error bars represent the 95% confidence interval, expressed as the mean±1.96 SE.
The prevalence of the metabolic syndrome for Mexican American women was highest among the 3 ethnic groups, followed by black women and then white women. Mexican American women showed a significantly higher prevalence for the 10-year age increments between 30 and 60 years compared with white women and at age 30 years compared with black women (P<.017) (Figure 1). There were no statistically significant differences in the prevalence of the metabolic syndrome between black and white women at any age group.
In both sexes, the prevalence of the metabolic syndrome increased steeply after the third decade and reached a peak in men aged 50 to 70 years and in women aged 60 to 80 years.
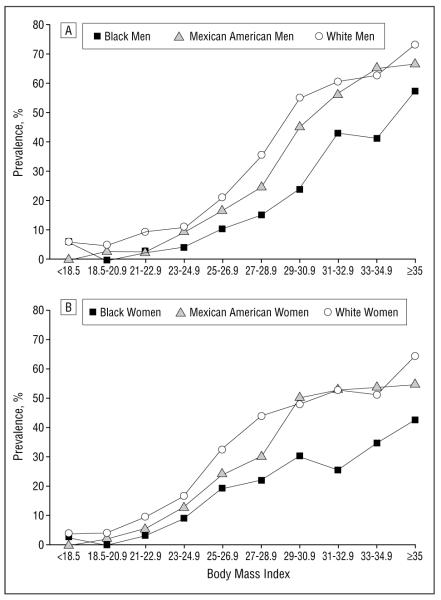
BMI-SPECIFIC PREVALENCE
The association between the prevalence of the metabolic syndrome and BMI, in increments of 2, is presented in Figure 2. A steep rise in the prevalence of the metabolic syndrome is observed in overweight (ie, BMI ≥25 and <30) men and women.
Figure 2.
The prevalence of the metabolic syndrome by body mass index (calculated as weight in kilograms divided by the square of height in meters) in men (A) and women (B).
Overall, 4.6%, 22.4%, and 59.6% of normal-weight, overweight, and obese men, respectively, met the metabolic syndrome diagnostic criteria. Similarly, in women, the corresponding prevalence rates were 6.2%, 28.1%, and 50.0%, respectively.
COMPONENTS OF THE METABOLIC SYNDROME
The overall relative frequency of each component of the metabolic syndrome is given in Table 3 for men and in Table 4 for women. Although the frequencies of abnormal components were highly variable, several patterns are evident. Black men had a significantly higher frequency of high blood pressure (35- to 64-year age group) but lower frequencies of large waist (35- to 64-year age group) and high triglyceride and low HDL cholesterol levels (35- to 64- and ≥65-year age groups) compared with the other 2 ethnic groups. Mexican American women had a significantly higher frequency of elevated triglyceride levels in the young and middle age groups and a low HDL cholesterol level in the young age group compared with the other ethnic groups. Black women had a significantly higher frequency of high blood pressure, whereas white women had a significantly lower frequency of large waist circumference in the young and middle age groups compared with other ethnic groups.
Table 3.
Prevalence of Each Metabolic Syndrome Component in Men*
| Age, y |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20-34 |
35-64 |
≥65 |
|||||||
| Black (n = 551) |
Mexican American (n = 759) |
White (n = 535) |
Black (n = 687) |
Mexican American (n = 784) |
White (n = 1107) |
Black (n = 256) |
Mexican American (n = 268) |
White (n = 984) |
|
| US population, in millions, No. |
2.6 | 2.1 | 18.2 | 2.9 | 1.5 | 27.0 | 6.6 | 2.1 | 9.0 |
|
| |||||||||
| Overall Abnormality, % | |||||||||
| Large waist | 15.8 | 12.1 | 14.1 | 23.5† | 35.4‡ | 36.0 | 34.0 | 41.5 | 46.2 |
| High TG level | 15.4 | 29.8‡ | 25.1 | 25.3† | 50.2‡ | 45.7 | 24.7† | 44.5‡ | 41.5 |
| Low HDL cholesterol level | 22.2 | 29.1 | 30.9 | 22.3† | 39.6‡ | 39.9 | 17.1† | 36.0‡ | 36.7 |
| High BP | 23.2 | 17.4 | 17.1 | 50.1† | 39.2‡ | 40.5 | 75.7 | 80.7 | 70.8§ |
| High glucose level | 3.2 | 4.2 | 3.2 | 11.2 | 18.2‡ | 11.7§ | 27.8 | 37.6 | 25.5 |
|
| |||||||||
| Isolated Abnormality, % | |||||||||
| Large waist | 3.8 | 1.8 | 3.2 | 3.4 | 3.6 | 4.2 | 3.4 | 0.9 | 3.8 |
| High TG level | 3.4 | 8.3 | 5.0 | 3.8 | 6.8 | 6.6 | 1.5 | 0.2 | 0.6 |
| Low HDL cholesterol level | 7.9 | 9.5 | 11.9 | 4.7 | 5.4 | 5.4 | 0.8 | 1.0 | 2.2 |
| High BP | 9.8 | 6.1 | 6.3 | 19.7 | 6.0 | 8.9 | 29.2 | 12.0 | 17.0 |
| High glucose level | 0.5 | 0.9 | 0.6 | 1.8 | 2.1 | 0.9 | 3.3 | 2.1 | 0.5 |
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; TG, serum triglyceride.
Statistically significant differences among black, Mexican American, and white groups were tested using the adjusted Wald test; P was corrected by Bonferroni adjustment in multiple comparisons (p/q,q=15, P<.003).
Statistically significant difference between the black and white groups.
Statistically significant difference between the Mexican American and black groups.
Statistically significant difference between the Mexican American and white groups.
Table 4.
Prevalence of Each Metabolic Syndrome Component in Women*
| Age, y |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 20-34 |
35-64 |
≥65 |
|||||||
| Black (n = 777) |
Mexican American (n = 745) |
White (n = 672) |
Black (n = 1036) |
Mexican American (n = 856) |
White (n = 1393) |
Black (n = 293) |
Mexican American (n = 285) |
White (n = 1169) |
|
| US population, in millions, No. |
3.4 | 1.6 | 17.8 | 4.3 | 1.7 | 31.6 | 1.1 | 0.3 | 13.2 |
|
| |||||||||
| Overall Abnormality, % | |||||||||
| Large waist | 40.7† | 39.2 | 23.6‡ | 68.6† | 71.3 | 49.7‡ | 75.0† | 69.6 | 61.5 |
| High TG level | 6.2 | 22.3§ | 10.7‡ | 15.3† | 38.6§ | 27.8‡ | 32.0 | 46.3§ | 41.9 |
| Low HDL cholesterol level | 31.2† | 45.6§ | 39.2 | 40.6 | 49.5 | 37.9‡ | 29.0 | 36.7 | 35.1 |
| High BP | 8.8† | 4.4 | 3.9 | 40.5† | 29.8§ | 25.3 | 72.4 | 78.8 | 70.3 |
| High glucose level | 2.7 | 2.2 | 0.6 | 14.1† | 16.8 | 7.3‡ | 26.3 | 25.3 | 16.7 |
|
| |||||||||
| Isolated Abnormality, % | |||||||||
| Large waist | 18.6 | 11.3 | 6.1 | 17.0 | 12.2 | 10.7 | 8.2 | 7.0 | 7.5 |
| High TG level | 1.1 | 2.8 | 1.3 | 0.4 | 2.5 | 2.0 | 0.4 | 0.9 | 1.4 |
| Low HDL cholesterol level | 9.3 | 17.1 | 19.8 | 5.4 | 6.0 | 8.5 | 0.3 | 0.9 | 1.0 |
| High BP | 1.9 | 0.9 | 1.0 | 5.8 | 2.4 | 3.8 | 8.8 | 15.0 | 14.2 |
| High glucose level | 0.3 | 0.2 | 0.1 | 0.5 | 0.4 | 0.8 | 0.7 | 0.5 | 0.4 |
Abbreviations: BP, blood pressure; HDL, high-density lipoprotein; TG, serum triglyceride.
Statistically significant differences among black, Mexican American, and white groups were tested using the adjusted Wald test; P was corrected by Bonferroni adjustment in multiple comparisons (p/q,q=15, P<.003).
Statistically significant difference between the black and white groups.
Statistically significant difference between the Mexican American and white groups.
Statistically significant difference between the Mexican American and black groups.
The percentage of participants with each component of the metabolic syndrome who presented with the abnormality in isolated form is summarized in Table 3 for men and in Table 4 for women. In the 35- to 64-year age group, isolated high blood pressure was relatively frequent in black men (19.7%) compared with other isolated components (<10.0%). The proportion of individuals with a large waist was relatively high in the comparably aged women relative to other isolated components.
MULTIPLE LOGISTIC REGRESSION MODELS
Two multiple logistic regression models with the same covariates, except for age, are given in Table 5. Age in model 1 was divided into 3 categories: young, middle aged, and old. Age in model 2 was considered as a continuous variable. After adjusting for age in model 2, BMI, lifestyle-related factors, and socioeconomic status, blacks still showed a significantly lower OR for the metabolic syndrome compared with white men and women. Mexican Americans showed a significantly higher OR only in women. Significantly higher ORs were present in the 35- to 64-year and 65 years and older age groups compared with the 20- to 34-year age group in men and women as derived from model 1 with the same covariates. The ORs were 2.8 and 2.4 in the 35- to 64-year age group and 5.8 and 4.9 in those 65 years and older in men and women, respectively.
Table 5.
Multivariable Adjusted ORs for the Metabolic Syndrome
| Men (n = 5227) |
Women (n = 5591) |
|||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| US population, in millions, No. | 58.5 | 61.1 | ||
|
| ||||
| Model 1 * | ||||
| Age, y | ||||
| 20-34 | 1.0 (Referent) | … | 1.0 (Referent) | … |
| 35-64 | 2.8 (1.8-4.5) | <.001 | 2.4 (1.5-3.7) | <.001 |
| ≥65 | 5.8 (3.9-8.7) | <.001 | 4.9 (3.0-7.8) | <.001 |
|
| ||||
| Model 2 * | ||||
| Ethnicity | ||||
| White | 1.0 (Referent) | … | 1.0 (Referent) | … |
| Black | 0.5 (0.3-0.6) | <.001 | 0.7 (0.5-0.9) | <.01 |
| Mexican American | 1.1 (0.9-1.4) | NS | 1.5 (1.1-2.0) | <.01 |
| BMI | ||||
| <18.5 | 0.4 (0.1-2.0) | NS | 0.3 (0.1-1.2) | .09 |
| 18.5-24.9 | 1.0 (Referent) | … | 1.0 (Referent) | … |
| 25-29.9 | 5.2 (3.9-6.9) | <.001 | 5.4 (3.7-7.9) | <.001 |
| 30-34.9 | 25.2 (19.3-32.9) | <.001 | 14.0 (9.1-21.4) | <.001 |
| ≥35 | 67.7 (40.5-113.3) | <.001 | 34.5 (22.6-52.7) | <.001 |
| Smoking | ||||
| Never | 1.0 (Referent) | … | 1.0 (Referent) | … |
| Current | 1.5 (1.1-2.2) | <.05 | 1.8 (1.2-2.6) | <.01 |
| Past | 1.2 (0.8-1.8) | NS | 1.5 (1.2-2.0) | <.01 |
| Drinking | ||||
| Never | 1.1 (0.8-1.5) | NS | 1.5 (1.2-1.9) | <.01 |
| Slight/moderate | 1.0 (Referent) | … | 1.0 (Referent) | … |
| Heavy | 1.1 (0.8-1.6) | NS | 0.8 (0.6-1.0) | <.05 |
| Carbohydrate intake | ||||
| Low | 0.9 (0.7-1.3) | NS | 1.0 (0.8-1.4) | .74 |
| Middle | 1.0 (Referent) | … | … | … |
| High | 1.7 (1.2-2.5) | <.01 | 1.1 (0.8-1.4) | .60 |
| Physical activity status | ||||
| Inactive | 1.4 (1.0-2.0) | <.05 | 1.2 (1.0-1.4) | .13 |
| Education level, y | ||||
| <8 | 0.8 (0.6-1.3) | NS | 0.9 (0.6-1.3) | .50 |
| 8-12 | 1.0 (0.8-1.3) | NS | 1.1 (0.8-1.4) | .63 |
| >12 | 1.0 (Referent) | … | 1.0 (Referent) | … |
| Household income, $/y | ||||
| ≤15 000 | 1.0 (0.7-1.5) | NS | 1.5 (1.0-2.3) | <.05 |
| 15 001-25 000 | 1.2 (0.8-1.7) | NS | 1.3 (0.9-2.0) | .14 |
| >25 000 | 1.0 (Referent) | … | 1.0 (Referent) | … |
| Menopausal status | ||||
| Postmenopausal | … | … | 1.6 (1.1-2.3) | <.05 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by the square of height in meters); CI, confidence interval; NS, not significant; OR, odds ratio.
Age was inserted in model 1 as young, middle, and older categories and in model 2 as a continuous variable.
The ORs for the metabolic syndrome in the over-weight group relative to the normal-weight group were 5.2 for men and 5.4 for women. The ORs sharply increased to 25.2 and 67.7 for men and 14.0 and 34.5 for women when BMI was 30 to 34.9 and 35 or greater, re-spectively. When both sexes are modeled together using multiple logistic regression, the ORs for the interaction of BMI and sex were significant at BMI 30 to 34.9 (P<.001) and 35 or greater (P<.01).
Currently smoking men and women were at significantly higher risk of having the metabolic syndrome. In men, the ORs were significantly higher in the high carbohydrate intake and physical inactivity groups. In women, significantly higher ORs were observed in previous smokers, nondrinkers, and those with a low house-hold income or who were postmenopausal. Women who were heavy alcohol consumers showed a significantly lower OR than women in the slight or moderate alcohol-consuming group.
COMMENT
Coronary heart disease remains the leading cause of mortality in the United States, accounting for more than 460 000 deaths in 2000.1 The primary target of CHD prevention, according to ATP III guidelines,10 is identification and appropriate treatment of patients with elevated LDL cholesterol levels. A secondary target, the metabolic syndrome, has been recognized for several decades3 but only recently was provided with consensus-generated diagnostic criteria by the ATP III.10 These criteria provided us with a framework for evaluating the main features of the metabolic syndrome in the US adult noninstitutionalized civilian population. Our findings, supporting and extending the initial findings of Ford et al,11 suggest that the metabolic syndrome is widespread among US adults; that prevalence rates are highly variable among ethnic, age, and BMI groups; and that lifestyle factors such as smoking, physical inactivity, and percentage of dietary caloric intake as carbohydrate are linked with the presence of the metabolic syndrome. These observations provide a foundation for CHD prevention initiatives and also raise important questions surrounding the applicability of the metabolic syndrome diagnostic criteria.
PREVALENCE OF THE METABOLIC SYNDROME
The present study results, based on NHANES III, indicate that approximately one fourth of US adults 20 years or older meet the diagnostic criteria for the metabolic syndrome. Prevalence rates were similar in men and women, with relative risk elevated in postmenopausal vs premenopausal women. Our results extend those of earlier studies,7,15-21 based on variable criteria, reporting metabolic syndrome prevalence rates ranging from 2.4% to 35.3%.
Ethnicity
Our findings suggest that metabolic syndrome prevalence rates vary among ethnic groups, ranging from a low of 13.9% in black men to a high of 27.2% in Mexican American women. These ethnic differences persisted even after adjusting for contributing factors such as age, BMI, smoking and drinking habits, socioeconomic status, physical inactivity, and menopausal status among women. Our findings are consistent with those of earlier studies indicating that compared with whites, Mexican Americans are more prone to develop hyperinsulinemia, insulin resistance, and an unfavorable distribution of body fat, which are the central features of the metabolic syndrome.22,23
Blacks are more insulin resistant than whites at a similar degree of adiposity.22-25 Blacks also have the highest overall CHD mortality rate of any ethnic group,26 and black men have a 60% higher incidence of type 2 diabetes mellitus than white men.27 On the other hand, the prevalence of the metabolic syndrome as defined by ATP III criteria in this study was lowest in black men. This lower prevalence in black men was accompanied by significantly lower frequencies of large waist, high triglyceride levels, and low HDL cholesterol levels, but black men had a greater frequency of high blood pressure. Other factors, such as smoking, small LDL particle size, pro-thrombotic state, family history, and environmental risk factors, may occur more frequently in blacks. Brancati et al28 reported that US blacks had a lower education level, were more likely to have a family history of diabetes mellitus, and engaged in less physical activity during leisure time than whites. Other factors related to ethnicity and socioeconomic status may also affect mortality risk, separate from those leading to the metabolic syndrome, such as access to early diagnosis and treatment.
An important question arising from these observations is the validity of the metabolic syndrome criteria when applied across different age, sex, and ethnic groups. Each of the metabolic syndrome criteria are now weighted equally, although some may be more potent CHD risk factors than others. Thus, the power of single components of the metabolic syndrome to predict eventual disease may differ across ethnic groups. The metabolic syndrome “cutoff points” may also vary by ethnic group. For example, for the same waist circumference, blacks have relatively smaller depots of insulin resistance related tovisceral adipose tissue compared with whites.29 Insulin resistance is also associated with blood pressure levels in white but not black Americans.30 Future longitudinal studies are needed to critically test ATP III criteria for the metabolic syndrome, particularly as they apply to the predictive validity for the development of disease across different ethnic groups.
Age
The prevalence of the metabolic syndrome rose with age, reaching peak levels in the sixth decade for men and the seventh decade for women. Prevalence rates declined in the eighth decade for men and women in some ethnic groups. The marked prevalence increase between the third and fifth decades is paralleled by similar increases among US civilians in the prevalence of overweight and obesity,8 key related factors in the development of visceral adiposity, insulin resistance, dyslipidemias, high blood pressure, and impaired glucose metabolism. In addition, aging per se is associated with evolution of insulin resistance, other hormonal alterations, and increases in visceral adipose tissue,31 all of which are important in the pathogenesis of the metabolic syndrome.
Body Mass Index
Although less than 6% of normal-weight adults met the criteria for the metabolic syndrome, rates increased in over-weight participants and reached a prevalence of approximately 60% in moderately obese participants with a BMI of approximately 35. The ORs for the metabolic syndrome increased, beginning in the overweight group, as a function of BMI. The OR increase in men exceeded that in women with a BMI greater than 30, indicating that men may be more sensitive to excessive weight gain than women. Participants with BMI less than 25 meeting the metabolic syndrome criteria may be the “metabolically obese, normal-weight” individuals referred to by Ruderman et al32 who purportedly have insulin resistance as the central feature of their cluster of metabolic abnormalities.
Although BMI serves as a useful marker of obesity and related insulin resistance, stronger correlations are observed between abdominal obesity and metabolic risk factors.20,32-35 The ATP III included waist circumference as a proxy measure of abdominal obesity, and waist circumference is well correlated with visceral adipose tissue36-38 and is a better anthropometric predictor of metabolic risk factors than BMI.35,39,40 Easily measured waist circumference is thus a simple and useful tool for identifying patients who are susceptible to the metabolic syndrome.22
SOCIOECONOMIC AND LIFESTYLE CHARACTERISTICS
Many studies41-43 have reported that low socioeconomic status is associated with a higher mortality rate for cardiovascular disease. A low education level links cardiovascular disease with risk factors such as smoking,41,44-46 hypertension,41,45 impaired glucose tolerance,47 diabetes mellitus,48 physical inactivity,45,46,49 and overweight with associated metabolic disturbances.41,45-47 No significant associations were observed in the present study between education level and the odds of having the metabolic syndrome. In women, however, the OR for the metabolic syndrome was significantly increased in the low household income group. In addition, a variety of lifestyle associations increased the odds of meeting the metabolic syndrome diagnostic criteria. Significantly higher ORs were found in currently smoking men and women than in their nonsmoking counterparts. The association between smoking and the metabolic syndrome remained even after adjusting for other covariates, possibly a reflection of the effect of cigarette smoking on insulin resistance.50 The association of low education levels with elevated risk is likely mediated by other risk factors, such as low household income, smoking, high carbohydrate intake, and physical inactivity.
The prevalence of the metabolic syndrome was elevated in women who abstained from alcohol. Slight and moderate alcohol consumption has been found in epidemiologic studies to be associated with low CHD risk, possibly through beneficial alterations in HDL cholesterol and blood pressure.51-53 There was an additional lowering of the odds of having the metabolic syndrome with high alcohol intake in women. In men, the odds of having the metabolic syndrome were increased in those who ingested a relatively large proportion of their calories from carbohydrates. High carbohydrate intake may predispose individuals to elevated triglyceride and low HDL cholesterol levels, 2 components of the metabolic syndrome.54
The OR was significantly increased for physical inactivity in men. Physical inactivity also imparts an increased risk for CHD and type 2 diabetes mellitus and exacerbates the severity of other risk factors.55 Increased physical activity promotes weight loss and maintenance in obese individuals56 and favorably modifies obesity-associated risk factors, including promoting visceral adipose tissue loss, improving insulin sensitivity, increasing HDL cholesterol levels, and lowering triglyceride levels.45,46,49
STUDY LIMITATIONS
The principal limitation relevant to the interpretation of our results is the use of cross-sectional data; thus, causal pathways underlying the observed relationships cannot be inferred. In addition, our investigation included only one dietary marker outside of alcohol intake: percentage of calories from carbohydrates. Future studies should consider additional dietary variables that are known to affect lipid levels and to be associated with educational status.44,57,58 Finally, the NHANES III database was developed between 1988 and 1994, and the observed prevalence rates may differ from those actually present in the current US population. The NHANES IV database will be available in the near future, allowing for adjustment of the prevalence rates reported in this trial.
CLINICAL IMPLICATIONS
An important advance embodied in the new ATP III criteria for the metabolic syndrome is that all 5 components can be easily evaluated in the clinical setting. Our findings and those of Ford et al11 indicate that more than 1 in 5 patients, and more in some populations, will meet these criteria and harbor what is usually a clinically silent aggregate of CHD risk factors. Although for many obese patients risk is already evident, our findings reveal that the risk of having the metabolic syndrome increases steeply even within the overweight or “pre-obese” range, with approximately 20% of individuals affected between a BMI of 25 and 29.9. Detecting these over-weight individuals and the 6% of normal-weight individuals with the metabolic syndrome and implementing preventive lifestyle interventions—diet education, physical activity, weight control, smoking cessation, and related behavior modification—is a high clinical priority. Support for this recommendation is provided by recent studies demonstrating a slowing in the rate of new type 2 diabetes mellitus onset with lifestyle interventions in individuals with impaired glucose tolerance.59-61
In conclusion, more than 20% of US adults have the metabolic syndrome as defined by ATP III. The present study not only reveals the exceptionally high prevalence of the metabolic syndrome within some specific groups but also brings forth important questions surrounding the mechanisms of between–ethnic group differences and on the validity of a unified set of diagnostic criteria. The increasing number of overweight and obese individuals of all ages combined with the growing number of elderly people promises to make the metabolic syndrome an increasingly common condition amenable to preventive lifestyle interventions.
Acknowledgments
This study was supported by grant DK42618 from the National Institutes of Health, Bethesda, Md, and a donation from Bristol Myers Corp, New York, NY.
REFERENCES
- 1.American Heart Association . 2001 Heart and Stroke Statistical Update. American Heart Association; Dallas, Tex: 2000. [Google Scholar]
- 2.Wilson PWF, Kannel WB, Silbershatz H, D’Agostino RB. Clustering of metabolic factors and coronary heart disease. Arch Intern Med. 1999;159:1104–1109. doi: 10.1001/archinte.159.10.1104. [DOI] [PubMed] [Google Scholar]
- 3.Reaven GM. Role of insulin resistance in human disease. Diabetes. 1988;37:1595–1607. doi: 10.2337/diab.37.12.1595. [DOI] [PubMed] [Google Scholar]
- 4.Haffner SM, Valdez RA, Hazuda HP, Mitchell BD, Morales PA, Stern MP. Prospective analysis of the insulin resistance syndrome (syndrome X) Diabetes. 1992;41:715–722. doi: 10.2337/diab.41.6.715. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM. Hypertriglyceridemia, insulin resistance, and the metabolic syndrome. Am J Cardiol. 1999;83:25F–29F. doi: 10.1016/s0002-9149(99)00211-8. [DOI] [PubMed] [Google Scholar]
- 6.DeFronzo RA, Ferrannini E. Insulin resistance: a multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991;14:173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- 7.Ferrannini E, Haffner SM, Mitchell, Stern MP. Hyperinsulinemia: the key feature of a cardiovascular and metabolic syndrome. Diabetologia. 1991;34:416–422. doi: 10.1007/BF00403180. [DOI] [PubMed] [Google Scholar]
- 8.Liese AD, Mayer-Davis EJ, Haffner SM. Development of the multiple metabolic syndrome: an epidemiologic perspective. Epidemiol Rev. 1998;20:157–172. doi: 10.1093/oxfordjournals.epirev.a017978. [DOI] [PubMed] [Google Scholar]
- 9.Abate N. Obesity and cardiovascular disease: pathogenic role of the metabolic syndrome and therapeutic implications. J Diabetes Complications. 2000;14:154–174. doi: 10.1016/s1056-8727(00)00067-2. [DOI] [PubMed] [Google Scholar]
- 10.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2496. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 12.National Center for Health Statistics Plan and operation of the Third National Health and Nutrition Examination Survey, 1988-1994. Vital Health Stat 1. 1994;32:1–407. [PubMed] [Google Scholar]
- 13.Willett WC. Will high-carbohydrate/low-fat diets reduce the risk of coronary heart disease? Proc Soc Exp Biol Med. 2000;225:187–190. doi: 10.1046/j.1525-1373.2000.22523.x. [DOI] [PubMed] [Google Scholar]
- 14.Wirfält E, Hedblad B, Gullberg B, et al. Food patterns and components of the metabolic syndrome in men and women: a cross-sectional study within the Malmö Diet and Cancer Cohort. Am J Epidemiol. 2001;154:1150–1159. doi: 10.1093/aje/154.12.1150. [DOI] [PubMed] [Google Scholar]
- 15.Bonora E, Kiechl S, Willeit J, et al. Prevalence of insulin resistance in metabolic disorders: the Bruneck Study. Diabetes. 1998;47:1643–1649. doi: 10.2337/diabetes.47.10.1643. [DOI] [PubMed] [Google Scholar]
- 16.Greenlund KJ, Rith-Najarian S, Valdez R, Croft JB, Casper ML. Prevalence and correlates of the insulin resistance syndrome among native Americans: the Intertribal Heart Project. Diabetes Care. 1999;22:441–447. doi: 10.2337/diacare.22.3.441. [DOI] [PubMed] [Google Scholar]
- 17.Abdul-Rahim HF, Husseini A, Bjertness E, Giacaman R, Nahida HG, Jervell J. The metabolic syndrome in the West Bank population. Diabetes Care. 2001;24:275–279. doi: 10.2337/diacare.24.2.275. [DOI] [PubMed] [Google Scholar]
- 18.Rantala AO, Kauma H, Lilja M, Savolainen MJ, Reunanen A, Kesaniemi YA. Prevalence of the metabolic syndrome in drug-treated hypertensive patients and control subjects. J Intern Med. 1999;245:163–174. doi: 10.1046/j.1365-2796.1999.00429.x. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt MI, Duncan BB, Watson RL, Sharrett AR, Brancati FL, Heiss G. A metabolic syndrome in whites and African Americans. Diabetes Care. 1996;19:414–418. doi: 10.2337/diacare.19.5.414. [DOI] [PubMed] [Google Scholar]
- 20.Vanhala MJ, Pitkajarvi TK, Kumpusalo EA, Takala JK. Obesity type and clustering of insulin resistance–associated cardiovascular risk factors in middle-aged men and women. Int J Obes. 1998;22:369–374. doi: 10.1038/sj.ijo.0800597. [DOI] [PubMed] [Google Scholar]
- 21.Wilson PWF, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 22.Okosun IS, Liao Y, Rotimi CN, Prewitt TE, Cooper RS. Abdominal adiposity and clustering of multiple metabolic syndrome in White, Black and Hispanic Americans. Ann Epidemiol. 2000;10:263–270. doi: 10.1016/s1047-2797(00)00045-4. [DOI] [PubMed] [Google Scholar]
- 23.Haffner SM, Stern MP, Hazuda HP, Pugh JA, Patterson JK, Malina RM. Upper body and centralized adiposity in Mexican Americans and non-Hispanic whites: relationship to body mass index and other behavioral and demographic variables. Int J Obes. 1986;10:493–502. [PubMed] [Google Scholar]
- 24.Haffner SM, D’Agostino R, Jr, Saad MF, et al. Increased insulin resistance and insulin secretion in nondiabetic African-Americans and Hispanics compared with non-Hispanic whites: the Insulin Resistance Atherosclerosis Study. Diabetes. 1996;45:742–748. doi: 10.2337/diab.45.6.742. [DOI] [PubMed] [Google Scholar]
- 25.Karter AJ, Mayer-Davis EJ, Selby JV, et al. Insulin sensitivity and abdominal obesity in African-American, Hispanic, and non-Hispanic white men and women: the Insulin Resistance and Atherosclerosis Study. Diabetes. 1996;45:1547–1555. doi: 10.2337/diab.45.11.1547. [DOI] [PubMed] [Google Scholar]
- 26.Gillum RF. Cardiovascular disease in the United States: an epidemiologic overview. In: Saunders E, editor. Cardiovascular Disease in Blacks. FA Davis Co Publishers; Philadelphia, Pa: 1991. pp. 3–16. [PubMed] [Google Scholar]
- 27.Harris MI. Noninsulin-dependent diabetes mellitus in black and white Americans. Diabetes Metab Rev. 1990;6:71–90. doi: 10.1002/dmr.5610060202. [DOI] [PubMed] [Google Scholar]
- 28.Brancati FL, Kao WH Linda, Folsom AR, Watson RL, Szklo M. Incident type 2 diabetes mellitus in African American and white adults. JAMA. 2000;283:2253–2259. doi: 10.1001/jama.283.17.2253. [DOI] [PubMed] [Google Scholar]
- 29.Conway JM, Yanovski SZ, Avila NA, Hubbard VS. Visceral adipose tissue differences in black and white women. Am J Clin Nutr. 1995;61:765–771. doi: 10.1093/ajcn/61.4.765. [DOI] [PubMed] [Google Scholar]
- 30.Saad MF, Lillioja S, Nyomba BL, et al. Racial differences in the relation between blood pressure and insulin resistance. N Engl J Med. 1991;324:733–739. doi: 10.1056/NEJM199103143241105. [DOI] [PubMed] [Google Scholar]
- 31.Boden G, Chen X, DeSantis RA, Kendrick Z. Effects of age and body fat on insulin resistance in healthy men. Diabetes Care. 1993;16:728–733. doi: 10.2337/diacare.16.5.728. [DOI] [PubMed] [Google Scholar]
- 32.Ruderman N, Chisholm D, Pi-Sunyer X, Schneider S. The metabolically obese, normal-weight individual revisited. Diabetes. 1998;47:699–713. doi: 10.2337/diabetes.47.5.699. [DOI] [PubMed] [Google Scholar]
- 33.Vanhala MJ, Kumpusalo EA, Pitkajarvi TK, Takala JK. Metabolic syndrome in a middle-aged Finnish population. J Cardiovasc Risk. 1997;4:291–295. [PubMed] [Google Scholar]
- 34.Vanhala MJ, Kumpusalo EA, Pitkajarvi TK, Notkola IL, Takala JK. Hyperinsu-linemia and clustering of cardiovascular risk factors in middle-aged hypertensive Finnish men and women. J Hypertens. 1997;15:475–481. doi: 10.1097/00004872-199715050-00002. [DOI] [PubMed] [Google Scholar]
- 35.Haffner SM, Ferrannini E, Hazuda HP, Stern MP. Clustering of cardiovascular risk factors in confirmed prehypertensive individuals. Hypertension. 1992;20:38–45. doi: 10.1161/01.hyp.20.1.38. [DOI] [PubMed] [Google Scholar]
- 36.Han TS, Leer EM, Seidell JC, Lean MEJ. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ. 1995;311:1401–1405. doi: 10.1136/bmj.311.7017.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pouliot MC, Despres JP, Lemieux S, et al. Waist circumference and abdominal sagittal diameter: best simple anthropometric indexes of abdominal visceral adipose tissue accumulation and related cardiovascular risk in men and women. Am J Cardiol. 1994;73:460–468. doi: 10.1016/0002-9149(94)90676-9. [DOI] [PubMed] [Google Scholar]
- 38.Ross R, Léger L, Morris D, de Guise J, Guardo R. Quantification of adipose tissue by MRI: relationship with anthropometric variables. J Appl Physiol. 1992;72:787–795. doi: 10.1152/jappl.1992.72.2.787. [DOI] [PubMed] [Google Scholar]
- 39.Wei M, Caskill SP, Haffner SM, Stern MP. Waist circumference as the best predictor of noninsulin dependent diabetes mellitus (NIDDM) compared to body mass index, waist/hip ratio over other anthropometric measurements in Mexican Americans: a 7-year prospective study. Obes Res. 1997;5:16–23. doi: 10.1002/j.1550-8528.1997.tb00278.x. [DOI] [PubMed] [Google Scholar]
- 40.Siedell JC, Oosterlee A, Deurenberg P, Hautvast JGMJ, Ruys JHJ. Abnormal fat depots measured with computed tomograph: effect of obesity, sex and age. Eur J Clin Nutr. 1988;42:805–807. [PubMed] [Google Scholar]
- 41.Pekkanen J, Tuomilehto J, Uutela A, Vartiainen E, Nissinen A. Social class, health behaviors, and mortality among men and women in eastern Finland. BMJ. 1995;311:589–593. doi: 10.1136/bmj.311.7005.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith G Davey, Neaton JD, Wentworth D, Stamler R, Stamler J. Mortality differences between black and white men in the USA: contribution of income and other risk factors among men screened for the MRFIT. Lancet. 1998;351:934–939. doi: 10.1016/s0140-6736(00)80010-0. [DOI] [PubMed] [Google Scholar]
- 43.Kaplan GA, Keil JE. Socieconomic factors and cardiovascular disease: a review of the literature. Circulation. 1993;88:1973–1998. doi: 10.1161/01.cir.88.4.1973. [DOI] [PubMed] [Google Scholar]
- 44.Jacobsen BK, Thelle DS. Risk factors for coronary heart disease and level of education: the Tromso Heart Study. Am J Epidemiol. 1988;127:923–932. doi: 10.1093/oxfordjournals.aje.a114895. [DOI] [PubMed] [Google Scholar]
- 45.Hoeymans N, Smit HA, Verkleij H, Kromhout D. Cardiovascular risk factors in relation to educational level in 36,000 men and women in the Netherlands. Eur Heart J. 1996;17:518–524. doi: 10.1093/oxfordjournals.eurheartj.a014903. [DOI] [PubMed] [Google Scholar]
- 46.Choinniere R, Lafontaine P, Edwards AC. Distribution of cardiovascular disease risk factors by socioeconomic status among Canadian adults. CMAJ. 2000;162(suppl):S13–S24. [PMC free article] [PubMed] [Google Scholar]
- 47.Brunner EJ, Marmot MG, Nanchahal K, et al. Social inequality in coronary risk: central obesity and the metabolic syndrome: evidence from the Whitehall II Study. Diabetologia. 1997;40:1341–1349. doi: 10.1007/s001250050830. [DOI] [PubMed] [Google Scholar]
- 48.Brancati FL, Whelton PK, Kuller LH, Klag MJ. Diabetes mellitus, race, and socioeconomic status: a population-based study. Ann Epidemiol. 1996;6:67–73. doi: 10.1016/1047-2797(95)00095-x. [DOI] [PubMed] [Google Scholar]
- 49.Rosengren A, Wilhelmsen L. Physical activity protects against coronary death and deaths from all causes in middle-aged men. Ann Epidemiol. 1997;7:69–75. doi: 10.1016/s1047-2797(96)00106-8. [DOI] [PubMed] [Google Scholar]
- 50.Attvall S, Fowelin J, Lager I, Von Schenck H, Smith U. Smoking induces insulin resistance: a potential link with the insulin resistance syndrome. J Intern Med. 1993;233:327–332. doi: 10.1111/j.1365-2796.1993.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 51.Jackson R, Stewart A, Beaglehole R, Scragg R. Alcohol consumption and blood pressure. Am J Epidemiol. 1985;122:1037–1044. doi: 10.1093/oxfordjournals.aje.a114185. [DOI] [PubMed] [Google Scholar]
- 52.Gillman MW, Cook NR, Evans DA, et al. Relationship of alcohol intake with blood pressure in young adults. Hypertension. 1995;25:1106–1110. doi: 10.1161/01.hyp.25.5.1106. [DOI] [PubMed] [Google Scholar]
- 53.Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol intake, increased levels of high-density lipoprotein and its subtractions, and decreased risk of myocardial infarction. N Engl J Med. 1993;329:1829–1831. doi: 10.1056/NEJM199312163292501. [DOI] [PubMed] [Google Scholar]
- 54.Liu S, Manson JE, Stampfer MJ, et al. Dietary glycemic load assessed by food-frequency questionnaire in relation to plasma high-density-lipoprotein cholesterol and fasting plasma triacylglycerols in postmenopausal women. AmJClin Nutr. 2001;73:560–566. doi: 10.1093/ajcn/73.3.560. [DOI] [PubMed] [Google Scholar]
- 55.Centers for Disease Control and Prevention. National Center for Chronic Disease Prevention and Health Promotion . Surgeon General’s Report on Physical Activity and Health. Centers for Disease Control and Prevention; Atlanta, Ga: 1996. [Google Scholar]
- 56.National Institutes of Health . The Practical Guide: Identification, Evaluation, and Treatment of Overweight and Obesity in Adults. National Institutes of Health; Bethesda, Md: 2000. NIH publication 00-4084. [Google Scholar]
- 57.Shimakawa T, Sorlie P, Carpenter MA, et al. Dietary intake patterns and sociodemographic factors in the Atherosclerosis Risk in Communities Study. Prev Med. 1994;23:769–780. doi: 10.1006/pmed.1994.1133. [DOI] [PubMed] [Google Scholar]
- 58.Bolton-Smith C, Smith WC, Woodward M, Tunstall-Pedoe H. Nutrient intake of different social-class groups: results from the Scottish Heart Health Study. Br J Nutr. 1991;65:321–335. doi: 10.1079/bjn19910093. [DOI] [PubMed] [Google Scholar]
- 59.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 60.Pan X-R, Cao H-B, Li G-W, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 61.Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med. 2001;345:790–797. doi: 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]