Abstract
The abundant compatible solute dimethylsulfoniopropionate (DMSP) is made by many marine algae. Different marine bacteria catabolise DMSP by various mechanisms, some of which liberate the environmentally important gas dimethyl sulfide (DMS). We describe an enzyme, DddY, which cleaves DMSP into DMS plus acrylate and is located in the bacterial periplasm, unlike other DMSP lyases that catalyse this reaction. There are dddY-like genes in strains of Alcaligenes, Arcobacter and Shewanella, in the β-, ɛ- and γ-proteobacteria, respectively. In Alcaligenes, dddY is in a cluster of ddd and acu genes that resemble, but also have significant differences to, those in other bacteria that catabolise both DMSP and acrylate. Although production of DMS and transcription of Alcaligenes dddY are both apparently inducible by pre-growth of cells with DMSP, this substrate must be catabolised to form acrylate, the bona fide coinducer.
Keywords: acrylate, Alcaligenes, dimethyl sulfide, dimethylsulfoniopropionate, periplasmic, Shewanella
Introduction
The volatile dimethyl sulfide (DMS) is the most important biogenic sulfurous compound that is transferred from the oceans to the atmosphere. When DMS or its photochemically oxidised products return to land via precipitation, this represents a major step in the transfer of sulfur from marine to terrestrial environments (Lovelock et al., 1972; Kiene et al., 2000). DMS has other important effects; it is a chemoattractant for marine animals, including seals, copepods and some seabirds (Steinke et al., 2006; DeBose and Nevitt, 2008) and its oxidation products function as cloud condensation nuclei, affecting albedo (Vallina and Simó, 2007).
DMS is made by microbial catabolism of the compatible solute dimethylsulfoniopropionate (DMSP), an anti-stress molecule synthesised in large amounts (∼109 tons worldwide annually) by many phytoplankton, seaweeds and a few angiosperms, including the salt marsh Cordgrass Spartina (see Stefels et al., 2007). There is remarkable biochemical and genetic diversity by which different bacteria catabolise DMSP. Globally, most DMSP is degraded via demethylation, this pathway being prevalent in abundant marine α-proteobacteria known as the Roseobacters and in the ubiquitous SAR11 lineage (Howard et al., 2006). But this pathway liberates no DMS.
We recently identified four wholly different enzymatic gene products that act on DMSP, generating DMS. One of these (DddD) cleaves DMSP into DMS plus 3-hydroxypropionate (3HP) and three ‘DMSP lyases' (DddL, DddP and DddQ) each generate DMS plus acrylate, although they are in wholly different polypeptide families. The dddD gene is mostly found in marine γ-proteobacteria, whereas dddL, dddP and dddQ usually occur in the Roseobacters (Curson et al., 2008, 2010; Todd et al., 2007, 2009, 2010, 2011). Some ddd genes are subject to horizontal gene transfer (HGT), even extending to inter-domain transfer of dddP among bacteria and Ascomycete fungi (Todd et al., 2007, 2009; Kirkwood et al., 2010b).
The dddP and dddQ genes are more abundant than dddD and dddL in the published metagenomes of marine bacteria, most notably the Global Ocean Sampling data set (Rusch et al., 2007), suggesting their widespread occurrence in the environment. These ddd genes, however, are rarer in these metagenomes than dmdA, which encodes the DMSP demethylase (Howard et al., 2008). These ddd and dmdA genes also occur in bacteriophage metagenomes, pointing to phage as likely vehicles for HGT (Raina et al., 2010).
In this study, we describe the genes for another, unusual DMSP lyase called DddY. We identified it in the β-proteobacterium Alcaligenes faecalis M3A, which de Souza and Yoch (1995a) isolated from intertidal sediment containing the DMSP-producing Spartina. A. faecalis M3A grows well on DMSP and acrylate as carbon sources, converting the former to the latter (Ansede et al., 1999), before further conversion of acrylate to 3HP. This DMSP lyase was purified and was located at the bacterial cell surface (de Souza and Yoch, 1995a, 1995b; Yoch et al., 1997), unlike other DMSP lyases, which are cytoplasmic (ARJ Curson, unpublished).
By identifying the dddY gene, we confirmed the novelty of this DMSP lyase in A. faecalis M3A and show that similar enzymes occur in other, unrelated bacteria.
Materials and methods
Strains, plasmids and microbiology
Strains and plasmids are shown in Supplementary Table S1. E. coli, Alcaligenes faecalis M3A, Shewanella oneidensis MR-1 (Venkateswaren et al., 1999), S. putrefaciens CN-32 (Fredrickson et al., 1998) and Pseudomonas putida 1290 (Leveau and Lindow, 2005) were grown routinely on LB medium, Shewanella halifaxensis DSM17350 (Zhao et al., 2006) on Difco marine agar 2216 and Arcobacter nitrofigilis DSM7299 on Columbia blood agar (Oxoid, Basingstoke, UK) supplemented with 5% horse blood or Arcobacter marine agar (McClung et al., 1983). Minimal media were M9 (Sambrook et al., 1989) for E. coli and P. putida, Alcaligenes basal medium (ABM) for A. faecalis (de Souza and Yoch, 1995a) and Shewanella minimal medium (SMM) for Shewanella sp. (Supplementary Materials). E. coli was grown at 37 °C, Alcaligenes and Arcobacter at 30 °C and Shewanella at 22 °C. For Alcaligenes and Pseudomonas, succinate and glucose (10 m) were used, respectively, as carbon sources in minimal media or, where appropriate, DMSP, acrylate or 3HP, each at 5 m.
In vivo and in vitro genetic manipulations
Plasmids were transferred by conjugation in triparental crosses (Figurski and Helinski, 1979) into Alcaligenes and Pseudomonas or by transformation into E. coli (Wexler et al., 2001). Genomic mutations in A. faecalis acu and ddd genes were made by cloning fragments internal to each gene into pBIO1879 (Todd et al., 2010), a derivative of suicide plasmid pK19mob (Schäfer et al., 1994) into which a spectinomycin-resistance cassette had been cloned, to allow counter-selections. These plasmids were mobilised from E. coli into the A. faecalis RifR derivative J481 selecting for transfer of SpcR (200 μg ml−1). Alcaligenes transconjugants were checked for resistance to kanamycin (20 μg ml−1), then by PCR, using primers external to the expected integration site. Promoter fusions in the wide host-range reporter plasmid pMP220 (Spaink et al., 1987) and dddY clones in the expression vector pET21a (Novagen, Darmstadt, Germany) were made by PCR amplification using primers shown in Supplementary Table S2. All clones were sequenced by Genome Enterprise Ltd (Norwich, UK). The genomic library of A. faecalis M3A was made as in Curson et al. (2008). This involved partial EcoRI digestion of A. faecalis M3A genomic DNA, ligated to the wide host-range cosmid pLAFR3 (Staskawicz et al., 1987), also cut with EcoRI, before packaging into Gigapack III XL (Stratagene, Stockport, UK) and transfecting E. coli strain 803. This produced a library of ∼10 000 primary transfectants. Cosmid pBIO1895 was sequenced at the Department of Biochemistry, University of Cambridge.
Enzyme assays
E. coli BL21 containing cloned dddY was grown to OD600 of 0.5 at 37 °C in LB broth containing 200 μ IPTG to induce dddY expression. Cultures were spun at 13 200 rpm and cells were resuspended in 1 ml M9 minimal medium. Then 300 μl was placed in a sealed 2 ml vial (Alltech Associates, Carnforth, UK), DMSP (5 m, pH 6.5) was added and vials were incubated at 22 °C. DMS in the headspace was assayed by gas chromatography after 10 min (Todd et al., 2009). To measure DMS production in other bacteria, strains were grown overnight in minimal medium, with, or without 2 m DMSP, acrylate or 3HP, cells were washed, resuspended in minimal medium and assayed as above. Fractionation of E. coli expressing DddY used the PeriPreps PeriPlasting Kit (Epicentre Biotechnologies, Madison, WI, USA), 10 μl of each fraction being diluted in 50 m Tris-HCl, pH8 and assayed as above. Rates are expressed as pmol DMS μg protein−1 min−1. Protein concentrations were estimated by Bradford assay (Bio-Rad, Hemel Hempstead, UK).
Conversion of [1-13C]DMSP to labelled acrylate was assayed by nuclear magnetic resonance as follows. E. coli strain BL21 with or without cloned dddY was grown overnight in complete medium and cultures were adjusted to equivalent OD600 values. Cells were pelleted, and then resuspended in M9 made with D2O (> 99.9%), with 10 m glycerol as C source, 10 m [1-13C]DMSP and 0.2 m IPTG. Cells were incubated overnight at 28 °C, lysed with perchloric acid (final concentration 5% v/v) and incubated on ice for 10 min. Cell debris was spun down and nuclear magnetic resonance was done as in Todd et al. (2010).
To assay β-galactosidase, Alcaligenes strains containing pMP220 clones were inoculated into ABM with or without inducers (2 m), grown overnight and assayed as in Rossen et al. (1985).
Bioinformatics
NCBI BLAST was used to search for homologues and metagenomes were searched using CAMERA in the ‘GOS: All ORF Peptides (P)' database (version 1.3.2.32). Alignments used Megalign in the DNAStar software package and Genedoc. Signal peptide predictions were from SignalP v3.0 (http://www.cbs.dtu.dk/services/SignalP/). Sequence of the Alcaligenes faecalis M3A ddd/acu region is deposited at Genbank, accession number HQ226120.
Results
The Alcaligenes faecalis gene cluster involved in DMSP catabolism and DMS production
To identify genes involved in DMSP catabolism, a genomic library of A. faecalis M3A was made in the wide host-range cosmid pLAFR3 (Materials and Methods section) and transferred en masse to the heterologous host Pseudomonas putida strain J450. This was the chosen recipient because it has many RNA polymerase sigma factors (Potvin et al., 2008), so, a priori, should be better at expressing heterologous genes. Transconjugants that grew slowly on DMSP as sole carbon source appeared after ∼7-days incubation, at a frequency of ∼10−3 per transconjugant. Two such colonies were purified; these also grew slowly on acrylate as sole carbon source and produced DMS on media containing DMSP (Ddd+ phenotype). Plasmids were isolated from these P. putida transconjugants and transformed into E. coli strain 803 (Wood, 1966). When conjugated back to P. putida, both plasmids conferred slow growth on DMSP and on acrylate and a Ddd+ phenotype to this host. Restriction digests showed that they contained overlapping cloned Alcaligenes genomic DNA. One of these, pBIO1895, was studied further.
Sequencing the ∼30 kb cloned DNA in pBIO1895 revealed eight contiguous genes (Figure 1), five of which had previously been implicated in the ability of other bacteria to grow on DMSP (ddd genes) or acrylate (acu) as sole carbon sources (Todd et al., 2010). Of particular interest, one gene, which we call dddY, encodes a polypeptide that corresponds to the DMSP lyase that was purified from A. faecalis M3A, and whose N-terminal sequence had been identified (de Souza and Yoch, 1995a, 1995b, 1996). This allowed a comparison of the directly sequenced polypeptide with that of the dddY gene product, translated in silico—see below.
Figure 1.
Comparison of the arrangements of acu and ddd genes in Alcaligenes faecalis M3A and Halomonas HTNK1. Locations, names and direction of transcription of the acu and ddd genes in Halomonas HTNK1 (Todd et al., 2010) and Alcaligenes faecalis M3A are shown. Genes specifically involved in DMSP catabolism are shown with vertical lines, those for acrylate breakdown are black and those with a role with both substrates are chequered. Those with diagonal lines are regulatory. Striped arrows above the genes indicate their transcriptional organisation. The dotted lines show the equivalent genes in the two strains, along with the percentage identity of their gene products.
DddY is a periplasmic DMSP lyase
The deduced dddY product has a predicted Mr of 45.5 kDa. It has no overall similarity to any polypeptide with known function, so was hitherto a ‘Domain Of Unknown Function'. However, it was strongly predicted (probability=1.0 on SignalP 3.0) to be a periplasmic protein, with a 21 amino-acid leader (MQKRMLGGMVAGALACFQVQA) that would be cleaved from the holo-polypeptide by SecP protease (Figure 2). Importantly, this generates a processed polypeptide, the N-terminal sequence of which (AQFQCQDDVKPAAISAEE) corresponds very closely to the A. faecalis M3A DMSP lyase purified and sequenced by de Souza and Yoch (1996), although the deduced dddY gene product has a cysteine (underlined above) rather than the histidine that they found (Figure 2). This indicates that DddY is in the periplasm, as confirmed below.
Figure 2.
N-terminal regions of the DddY proteins in Alcaligenes, Arcobacter and Shewanella. The deduced N-terminal DddY holo-polypeptides of strains of Alcaligenes, Shewanella and Arcobacter are shown. The underlined region in the N-terminal region of Alcaligenes faecalis M3A DddY corresponds to that which was directly sequenced, with a cysteine (lower case ‘c') being found here, instead of the histidine residue reported by de Souza and Yoch (1996). The ‘white on black' residues are the N-terminal amino acids of the processed polypeptides, as predicted by SignalP v3.0. Residues with grey backgrounds are those that may correspond in the leader sequences of the two DddY polypeptides of Arcobacter nitrofigilis. ‘Al.' and ‘Ar.' refer to Alcaligenes and Arcobacter strains, respectively, and gene numbers are shown in brackets.
To examine its function, we first made an insertional mutation into the genomic A. faecalis dddY gene. This completely abolished the resultant mutant's (J482) ability to make DMS from DMSP (Figure 3). Interestingly, though, it still grew normally on DMSP as sole carbon source, pointing to the presence of another, unknown, DMSP catabolic pathway in A. faecalis, the identification of which is currently under study.
Figure 3.
Effects of mutations in acu and ddd genes on DMSP-dependent DMS production in Alcaligenes faecalis. Wild-type Alcaligenes faecalis and mutant derivatives with insertions in dddY, acuI, dddZ or acuR, as indicated, were assayed for DMSP-dependent DMS production. Error bars are shown.
We also amplified dddY from A. faecalis M3A genomic DNA and cloned the product into the expression vector pET21a. E. coli strain BL21 (Studier and Moffatt, 1986) harbouring the resultant plasmid (pBIO1912) efficiently generated DMS from DMSP (Table 1). Using [1-13C]DMSP as substrate with E. coli containing pBIO1912, we confirmed by nuclear magnetic resonance that DddY was a DMSP lyase, as the labelled DMSP substrate had been converted to acrylate, in agreement with the earlier findings of Ansede et al. (1999) on the catabolic fate of DMSP in A. faecalis M3A itself.
Table 1. DMSP-dependent DMS production by different bacteria that contain or lack the dddY gene.
| Straina | Genotype/Description | DMS productionb |
|---|---|---|
| Alcaligenes faecalis J481 | Wild type | 60.1 (2.2) |
| Shewanella oneidensis MR-1 | Wild type (no dddY) | 0.05 (0.002) |
| S. putrefaciens CN-32 | Wild type | 1.7 (0.02) |
| S. halifaxensis HAW-EB4S | Wild type | 0.59 (0.2) |
| Arcobacter nitrofigilis DSM 7299 | Wild type | 114.2 (13.7) |
| E. coli BL21:pET21a | Vector only control | 0.05 (0.02) |
| E. coli BL21:pBIO1912 | Cloned dddY of A. faecalis | 559.7 (116) |
| E. coli BL21:pBIO1912 | Cloned dddY of A. faecalis, periplasmic fraction | 966.2 (318.5) |
| E. coli BL21:pBIO1912 | Cloned dddY of A. faecalis, spheroplast fraction | 89.9 (0.1) |
| E. coli BL21:pBIO1913 | Cloned dddY of S. putrefaciens | 12.4 (2.19) |
Abbreviations: DMS, dimethyl sulfide; DMSP, dimethylsulfoniopropionate.
Cells of wild-type Alcaligenes, Shewanella and Arcobacter strains, and E. coli strain BL21 with or without cloned dddY genes, were assayed for DMS production after growth in minimal medium.
Rates of DMS production are expressed in pmol DMS μg protein−1 min−1 (average of two independent experiments) with standard errors shown in brackets.
E. coli containing cloned dddY was also used to show directly that DddY was located in the periplasm. E. coli cells expressing DddY were separated into periplasmic and spheroplastic fractions and these were assayed separately for DMSP-dependent DMS production. Consistent with the predicted location of DddY, the activity was ∼11-fold greater in the periplasmic fraction (Table 1).
DddY homologues in other bacterial genomes and in metagenomes
DddY is not present in the deduced proteomes of any other Alcaligenes species whose genome sequences are available, and it has no close homologues in the current (August 2010) NCBI data base. However, it is ∼35% identical over its entire length to a polypeptide made by some, but not all, species of the γ-proteobacterium Shewanella, namely S. putrefaciens CN-32, S. woodyi ATCC 51908, S. baltica OS155, S. sp. MR-7 and MR-4, S. piezotolerans WP3, S. pealeana ATCC 700345, S. frigidimarina NCIMB 400 and S. halifaxensis HAW-EB4S. It also has a similar level of identity to two polypeptides, encoded by two unlinked genes (Arnit_2767 and Arnit_0113, see http://genome.jgi-psf.org/arcni/arcni.home.html) in the ɛ-proteobacterium Arcobacter nitrofigilis DSM7299 (Pati et al., 2010). The sequences of these polypeptides in the different Shewanella species and in A. nitrofigilis closely resemble each other (∼75% identical in amino acid sequence). Although all the DddY-like polypeptides in Shewanella and one of those (gp Arnit_0113) of Arcobacter are strongly predicted to be periplasmic, their N-terminal leaders are more diverse than the rest of the polypeptides (Figure 2). The sub-cellular location of the other DMSP lyase (gp Arnit_2767) in Arcobacter is uncertain; SignalP 3.0 had a low probability value for its being in the periplasm, although there is some sequence conservation in the N-terminal regions of both DddY polypeptides of this species (Figure 2).
No Shewanella or Arcobacter species were previously known to make DMS—indeed, no DMSP catabolism had been described in any of the ɛ-proteobacteria subphylum. Therefore, we examined the Ddd phenotypes of S. putrefaciens CN-32, S. halifaxensis HAW-EB4S and Arcobacter nitrofigilis DSM7299 and found that all three strains produced DMS from DMSP. Under these conditions, these two Shewanella species produced less DMS than Alcaligenes faecalis, but Arcobacter nitrofigilis made more than either. In contrast, S. oneidensis MR-1, a Shewanella species that lacks a dddY-like gene, made no DMS (Table 1).
To show that dddY of Shewanella is involved in DMS production, the dddY (gene tag Sputcn32_3534; http://genome.jgi-psf.org/shepu/shepu.home.html) of S. putrefaciens CN-32 was cloned into pET21a to form pBIO1913 and an E. coli transformant containing this plasmid had a Ddd+ phenotype (Table 1).
We also searched for DddY polypeptides in metagenomic databases. Only one significant homologue was found, in sewage treatment bacteria (ctg6139; http://www.ncbi.nlm.nih.gov/pubmed/16998472); its polypeptide product was 35% identical to DddY of Shewanella over the 180 C-terminal amino acids that were available in this individual sequence read. Notably, there were no DddY homologues in the Global Ocean Sampling metagenome (Rusch et al., 2007), which is well represented with homologues of the other DMS-emitting enzymes (DddD, DddL, DddP and DddQ) and the DMSP demethylase DmdA (Howard et al., 2008; Raina et al., 2010; Todd et al., 2011).
Comparison of the ddd/acu gene clusters of A. faecalis M3A and Halomonas HTNK1
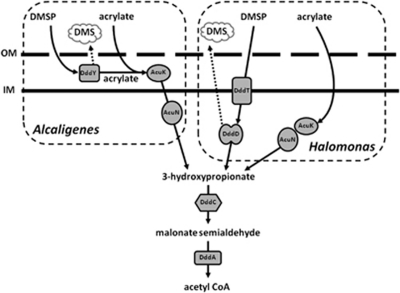
Near dddY of A. faecalis M3A, we noted that there are several ddd and acu genes, which resemble those involved in DMSP and acrylate catabolism in other bacteria that grow on DMSP and which have a Ddd+ phenotype. For example, the γ-proteobacterium Halomonas HTNK1 catabolises acrylate and DMSP using pathways that are initially independent, but which converge on a shared catabolite, 3HP (Todd et al., 2010; Figure 4). DMSP is converted to 3HP via an unusual Class III acyl-CoA transferase encoded by dddD, whereas two other enzymes, a dehydratase (acuK gene product) and a conventional CoA transferase (encoded by acuN) function together to generate 3HP from acrylate. The 3HP is converted to malonate semi-aldehyde via an alcohol dehydrogenase encoded by dddC and then, via the aldehyde dehydrogenase dddA gene product, to acetyl CoA, then into central metabolism.
Figure 4.
Pathways of DMSP and acrylate catabolism in Halomonas HTNK1 and Alcaligenes faecalis M3A. Likely pathways for DMSP and acrylate catabolism in Halomonas HTNK1 and Alcaligenes faecalis M3A are shown separated by dashed boxes (see Todd et al., 2010). In Alcaligenes, the DddY and AcuK polypeptides are in the periplasmic space between the inner and outer membrane (IM and OM, respectively), whereas DddD and AcuK of Halomonas are cytoplasmic. Halomonas also has the DddT BCCT-type transporter in the inner membrane—no such transporter is found with Alcaligenes.
The ddd/acu gene cluster of A. faecalis M3A also contains acuN, acuK, dddA and dddC genes, all of which products are at least 70% identical to the corresponding Halomonas enzymes, although the relative locations and transcriptional organisation of these genes differ in the two strains (Figure 1; see below). One other, significant difference concerns AcuK, which is involved in the initial step in acrylate catabolism (Todd et al., 2010). AcuK of Alcaligenes, but not of Halomonas, has an 18 amino-acid leader that is strongly predicted to be removed by SecP protease.
The most notable difference between the Alcaligenes and Halomonas clusters is that the former uses the periplasmic DddY, whereas Halomonas has a totally different, cytoplasmic, enzyme DddD, which also releases DMS from DMSP (Todd et al., 2007, 2010). Consistent with this, upstream of Halomonas dddD is a gene, dddT, which encodes a BCCT-type transporter that imports DMSP into the cytoplasm. In contrast, DddY requires no transporter and there is no nearby transporter gene.
Three other genes are embedded in the Alcaligenes cluster. Two of these, acuR and dddZ, encode polypeptides in the TetR and LysR super-families of transcriptional regulators, respectively (see below). The third of these ‘extra' genes is acuI, which encodes a member of the highly conserved zinc-containing super-family of alcohol dehydrogenases (PF08240). There are hundreds of close homologues (>72% identical) of A. faecalis AcuI in many, taxonomically diverse bacteria. AcuI-like polypeptides are encoded by genes in ddd clusters of other, known Ddd+ bacteria including Halomonas and Rhodobacter, but the products of these acuI-like genes do not form a particular out-group compared with the mass of other PF08240 family members and their exact functions are unknown.
Effects of mutations in the ddd/acu gene cluster of Alcaligenes faecalis M3A
The phenotype of the DddY− mutant of Alcaligenes faecalis pointed to the existence of another, unknown, pathway for DMSP catabolism in this strain (see above). Further evidence for this came from the effects of mutations in the other structural genes acuI (strain J483), acuN (J488), acuK (J489), dddA (J487), dddC (J485) and the two regulatory genes, acuR (J486) and dddZ (J484). Mutations in two of these genes affected DMSP-dependent DMS production. The AcuI− mutant (J483) produced less DMS than wild type (by ∼four-fold), most likely due to polar effects on dddY expression, which is in the same transcriptional unit (see below). In contrast, the AcuR− mutant J486 produced ∼11-fold more DMS than wild type, consistent with AcuR being a negatively functioning regulator, as predicted from its sequence, and confirmed below.
All the mutants grew as well as the wild type on 5 m DMSP, consistent with the presence of another, unknown pathway for DMSP catabolism in A. faecalis. On acrylate, the AcuI− and DddY− mutants grew normally, but the DddZ− (J484), DddC−(J485), AcuR−(J486), DddA−(J487), AcuN−(J488) and AcuK− (J489) mutants all failed to grow on this substrate. The AcuR− mutant phenotype is likely due to polarity on the downstream dddA, acuN and acuK genes. The DddZ− mutant may be defective on acrylate because this LysR-type regulator is required for expression of genes involved in acrylate catabolism—this will be presented elsewhere.
Using the above experimental and bioinformatic information, we constructed a likely pathway for the catabolism of DMSP and acrylate in A. faecalis (Figure 4). This resembles that which we had elucidated for Halomonas, the key differences being (i) the subcellular locations of the initial enzymatic biotransformations of DMSP and of acrylate and (ii) that in Halomonas, the 3HP intermediate is formed independently from DMSP and from acrylate, but in Alcaligenes, the reactions are sequential, with DMSP being converted to acrylate, then to 3HP.
Regulation of acu and ddd genes of Alcaligenes faecalis M3A
DMS production in A. faecalis M3A was shown to be inducible, not only by the substrate DMSP, but also, more surprisingly, by the catabolites acrylate and 3HP (Yoch, 2002). We confirmed this, showing that pre-growth of A. faecalis in 2 m DMSP, acrylate or 3HP led to increased DMS production by factors of 33-, 27- and 15-fold respectively compared with the controls. We set out to establish whether this induction was due to enhanced expression of dddY.
The A. faecalis dddY gene is predicted to be co-transcribed with the upstream acuI gene, with only a small gap (68 bps) between them. A fragment spanning the proposed promoter region of this operon, upstream of acuI, was cloned into the wide host-range lacZ promoter-probe plasmid pMP220. The resulting acuI-lacZ plasmid, pBIO1905, was mobilized into A. faecalis M3A and the transconjugants were assayed for β-galactosidase activity following growth of the cells in minimal medium and also in media supplemented with DMSP, acrylate or 3HP, each at 2 m. As shown in Figure 5, β-galactosidase activity was significantly enhanced (∼six-fold above background) when the cells were pre-grown with acrylate or with DMSP, but only slightly (<two-fold) with 3HP. In contrast, a fragment that spanned the acuI-dddY intergenic region, cloned into pMP220 to form pBIO1904, did not confer any β-galactosidase activity under any growth conditions, consistent with acuI and dddY being co-transcribed.
Figure 5.
Effects of different co-inducers and mutations in regulatory genes on expression of Alcaligenes faecalis acuI-dddY operon. Wild-type Alcaligenes faecalis and mutant derivatives with insertions in dddY, acuR or dddZ as indicated, and containing the acuI-lacZ transcriptional fusion plasmid pBIO1905, were grown in minimal media with no inducer (no fill), or with DMSP (grey), acrylate (diagonal stripes) or 3HP (stippled), each at 2 mM. Cells were assayed for β-galactosidase activity as described in the text. Errors bars are shown.
In an attempt to identify the gene(s) responsible for regulating transcription of the acuI-dddY operon, pBIO1905 was mobilised into the AcuR− and DddZ− mutant strains (J486 and J484, respectively). Compared with wild type, the acuR mutation caused markedly increased, constitutive expression of the acuI-lacZ fusion, even in the absence of any co-inducer, consistent with the hyper-production of DMS in the AcuR− mutant (see above). In contrast, the dddZ mutation did not affect expression of the fusion under any of the growth conditions. The fusion plasmid was also mobilized into the DddY− mutant strain, J482. In this background, DMSP no longer functioned as a co-inducer, although acrylate retained its co-inducing ability. This suggests that DMSP itself is not a co-inducer, but must first be converted to the likely bona fide co-inducer acrylate by the DddY lyase.
Discussion
More than 10 years ago, Yoch's laboratory demonstrated three features of DMSP catabolism in Alcaligenes faecalis M3A: (i) DMSP cleavage occurred near the bacterial cell surface, not in the cytoplasm (ii) acrylate was the immediate catabolite of DMSP, followed by its conversion to 3HP and (iii) DMSP-dependent DMS production was induced by the DMSP substrate, and by some of its catabolites. The work described here provides a genetic explanation for these biochemically based observations.
From its sequence, and ratified by direct measurement, DddY is indeed a periplasmic enzyme. Apart from its N-terminal leader, it does not resemble any enzyme with known function and has no recognisable functional motifs. Therefore, DddY must use novel mechanisms to cleave DMSP into acrylate plus DMS, compared with the other lyases that effect the same biotransformation, namely DddP, DddL and DddQ (Curson et al., 2008; Kirkwood et al., 2010a; Todd et al., 2009, 2011).
The pathway of DMSP catabolism in Alcaligenes resembles that of Halomonas, but also has some differences, stemming from the different compartments of the primary enzymes, DddD and DddY, which generate DMS from DMSP in the cytoplasm and periplasm, respectively. These different subcellular locations are consistent with (a) the presence of a dedicated DMSP transporter gene, dddT, in Halomonas, but not in Alcaligenes and (b) the deduced periplasmic location of the Alcaligenes AcuK acrylate dehydratase, whereas AcuK of Halomonas is cytoplasmic. AcuK is homologous to E. coli CaiD, a hydratase that functions in concert with the CoA transferase, CaiB, to hydrate crotonobetaine to -carnitine, the CaiD-mediated hydration being followed by transfer of CoA from carnitine via CaiB (Elssner et al., 2001). In Alcaligenes, the second step in the conversion of acrylate to 3HP is predicted to be mediated by AcuN, which is homologous to CaiB, and lacks a leader, so is likely to be cytoplasmic. These bioinformatically based predictions tally with earlier biochemical studies showing that acrylate was converted to 3HP at the Alcaligenes cell surface (de Souza and Yoch, 1995a, 1995b). Genomic mutations in the ddd and acu genes in A. faecalis caused no detectable effects on growth on DMSP as a carbon source. Thus, A. faecalis must have other ways of degrading this substrate. Other bacteria can catabolise DMSP in more than one way—for example, Ruegeria pomeroyi demethylates DMSP via the DmdA demethylase (Howard et al., 2006) and has at least two DMSP lyases, DddP and DddQ (Todd et al., 2009, 2011). We have access to a draft genome sequence of A. faecalis M3A and noted that it lacks dmdA and has no known other ddd genes, so this additional inferred DMSP catabolic pathway must involve some as yet unidentified gene products. In contrast to the lack of effects with DMSP as carbon source, A. faecalis AcuN−, AcuK−, DddA− and DddC− mutants were defective for growth on acrylate, consistent with the pathway shown in Figure 4.
The regulation of the ddd/acu cluster in A. faecalis may be complex, as it contains two predicted regulatory genes, acuR and dddZ, the former being a ratified regulator of acuI-dddY. Being in the TetR family, AcuR is expected to be a repressor (Ramos et al., 2005). Consistent with this, the AcuR− mutant strain overproduces DMS, and has high-level, constitutive expression of the acuI-dddY operon. The repression is relieved by DMSP, but this requires its conversion to acrylate, or some further catabolite, which is the bona fide co-inducer. For reasons that are unknown, induction by DMSP catabolites is a feature of genes involved in DMSP catabolism, not only in A. faecalis, but also in other bacteria.
We have not found a role for dddZ, as the acuI-dddY operon was expressed normally in the DddZ− mutant, as were the dddC, dddZ and acuR-dddA-acuNK transcriptional units (unpublished). However, a DddZ− mutant fails to grow on acrylate, suggesting a role in the expression of some unknown acrylate catabolic genes. Most LysR family members function positively in the presence of a cognate co-inducer to activate expression of their target transcriptional units (Maddocks and Oyston, 2008). Maybe, DddZ requires some co-inducer compound that remains to be identified in order to exert its regulatory properties.
Like other ddd genes, dddY seems to have spread by HGT among distantly related lineages, as close homologues occur in species of Alcaligenes, Shewanella and Arcobacter (β-, γ- and ɛ-proteobacteria, respectively). Although these are only distantly related to each other, members of these three genera are all associated with microaerobic environments. Thus, Shewanella sp. have flexible respiratory abilities and are mostly marine, occurring in sediments and other anoxic conditions, although some strains come from aerobic seawater (Hau and Gralnick, 2007; Fredrickson et al., 2008). The two Shewanella species examined here, S. putrefaciens CN-32 and S. halifaxensis HAW-EB4S, are from anaerobic subsurface shale sandstone and marine sediment, respectively. Interestingly, Arcobacter nitrofigilis DSM7299 is a diazotroph isolated from sediment around Spartina roots, the same type of environment as for A. faecalis. Arcobacter is closely related to Campylobacter (A. nitrofigilis was originally Campylobacter nitrofigilis) and contains pathogens such as A. butzleri, but this genus is also environmentally important, being abundant in hydrothermal vents (Moussard et al., 2006). Further evidence for HGT came from the observation that a DMSP lyase in the marine Chlorophyte Ulva curvata cross-reacted with antibody raised against the A. faecalis DMSP lyase (de Souza et al., 1996). However, the algal polypeptide (78 kDa) was larger than the 48 kDa bacterial lyase, and it will be of interest to compare these two enzymes at a molecular level to see if this represents another case of inter-Domain HGT, as was found with the DddP DMSP lyase (Todd et al., 2009; Kirkwood et al., 2010b).
We noted that the dddY of Shewanella species and one of those in A.nitrofigilis (Arnit_2767) are adjacent to genes, the products of which are membrane-bound cytochromes, raising the question that in these bacteria, DddY may have a role in anaerobic respiration, rather than in food supply, as occurs in A. faecalis. In this connection, Desulfovibrio acrylicus, uses exogenous acrylate as an electron acceptor for anaerobic respiration. It also has a DMSP lyase with some features that resemble DddY (van der Maarel et al., 1996a, 1996b); this enzyme could supply acrylate when these bacteria have access to DMSP.
The association of dddY with bacteria that favour microaerobic environments may also account for the lack of DddY homologues in the published metagenomes of marine bacteria, as these represent the aerobic upper reaches of the oceans. Marine sediments harbour vast numbers of live bacteria, as much as 30% of the world's total (Schippers et al., 2005). The DddY DMSP lyase, though rare in the upper oceans, may be abundant in such environments, particularly where DMSP is available, as occurs in saltmarshes where Spartina can form the predominant flora. Future surveys of the microbial metagenomes of such ecosystems may therefore be instructive in this regard.
Perhaps the most striking feature to emerge from recent genetic work on bacterial DMS production is the remarkable diversity in the genes and hence the enzymes and pathways that are involved in this process in different bacteria. DddY was the first DMSP lyase to be characterised, in 1995, and our analysis of the corresponding gene expands the novelty of this enzyme. Future work on DddY therefore, can range from ecological examinations of its importance in anoxic habitats to molecular enzymology, aimed at elucidating the catalytic mechanisms that this hitherto ‘Domain of Unknown Function' uses to cleave its environmentally important substrate.
Acknowledgments
We thank the BBSRC and NERC for funding, Emily Fowler, Yohan Chan, Colin Hanfrey, Pam Wells and Andrea Hall for technical assistance.
Footnotes
Supplementary Information accompanies the paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ansede JH, Pellechia PJ, Yoch DC. Metabolism of acrylate to beta-hydroxypropionate and its role in dimethylsulfoniopropionate lyase induction by a salt marsh sediment bacterium, Alcaligenes faecalis M3A. Appl Environ Microbiol. 1999;65:5075–5081. doi: 10.1128/aem.65.11.5075-5081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curson ARJ, Rogers R, Todd JD, Brearley CA, Johnston AWB. Molecular genetic analysis of a dimethylsulphoniopropionate lyase that liberates the climate-changing gas dimethyl sulphide in several marine α-proteobacteria and Rhodobacter sphaeroides. Environ Microbiol. 2008;10:757–767. doi: 10.1111/j.1462-2920.2007.01499.x. [DOI] [PubMed] [Google Scholar]
- Curson ARJ, Sullivan MJ, Todd JD, Johnston AWB. Identification of genes for dimethyl sulfide production in bacteria in the gut of Atlantic Herring (Clupea harengus) ISME J. 2010;4:144–146. doi: 10.1038/ismej.2009.93. [DOI] [PubMed] [Google Scholar]
- DeBose JL, Nevitt GA. The use of odors at different spatial scales: comparing birds with fish. J Chem Ecol. 2008;34:867–881. doi: 10.1007/s10886-008-9493-4. [DOI] [PubMed] [Google Scholar]
- de Souza MP, Chen YP, Yoch DC. Dimethylsulfoniopropionate lyase from the marine macroalga Ulva curvata: purification and characterization of the enzyme. Planta. 1996;199:433–438. [Google Scholar]
- de Souza MP, Yoch DC. Purification and characterization of dimethylsulfoniopropionate lyase from an Alcaligenes-like dimethyl sulfide-producing marine isolate. Appl Environ Microbiol. 1995a;61:21–26. doi: 10.1128/aem.61.1.21-26.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Yoch DC. Comparative physiology of dimethyl sulfide production by dimethylsulfoniopropionate lyase in Pseudomonas doudoroffii and Alcaligenes sp. strain M3A. Appl Environ Microbiol. 1995b;61:3986–3991. doi: 10.1128/aem.61.11.3986-3991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Souza MP, Yoch DC.1996N-terminal amino acid sequences and comparison of DMSP lyases from Pseudomonas doudoroffii and Alcaligenes strain M3AIn: Kiene RP, Visscher PT, Keller MD, Kirst GO, (eds).Environmental and Biological Chemistry on Dimethylsulfoniopropionate and Related Sulfonium Compounds Plenum Press: New York, NY; 293–304. [Google Scholar]
- Elssner T, Engemann C, Baumgart K, Kleber HP. Involvement of coenzyme A esters and two new enzymes, an enoyl-CoA hydratase and a CoA-transferase, in the hydration of crotonobetaine to l-carnitine by Escherichia coli. Biochem. 2001;40:11140–11148. doi: 10.1021/bi0108812. [DOI] [PubMed] [Google Scholar]
- Figurski DH, Helinski DR. Replication of an origin-containing derivative of plasmid RK2 dependant on a plasmid function provided in trans. Proc Natl Acad Sci USA. 1979;76:1648–1652. doi: 10.1073/pnas.76.4.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredrickson JK, Romine MF, Beliaev AS, Auchtung JM, Driscoll ME, Gardner TS, et al. Towards environmental systems biology of Shewanella. Nature Rev Microbiol. 2008;6:592–603. doi: 10.1038/nrmicro1947. [DOI] [PubMed] [Google Scholar]
- Fredrickson JK, Zachara JM, Kennedy DW, Dong H, Onstott TC, Hinman NW, et al. Biogenic iron mineralization accompanying the dissimilatory reduction of hydrous ferric oxide by a groundwater bacterium. Geochim Cosmochim Acta. 1998;62:3239–3257. [Google Scholar]
- Hau HH, Gralnick JA. Ecology and biotechnology of the genus Shewanella. Ann Rev Microbiol. 2007;61:237–258. doi: 10.1146/annurev.micro.61.080706.093257. [DOI] [PubMed] [Google Scholar]
- Howard EC, Henriksen JR, Buchan A, Reisch CR, Bürgmann H, Welsh R, et al. Bacterial taxa that limit sulfur flux from the ocean. Science. 2006;314:649–652. doi: 10.1126/science.1130657. [DOI] [PubMed] [Google Scholar]
- Howard EC, Sun S, Biers EJ, Moran MA. Abundant and diverse bacteria involved in DMSP degradation in marine surface waters. Environ Microbiol. 2008;10:2397–2410. doi: 10.1111/j.1462-2920.2008.01665.x. [DOI] [PubMed] [Google Scholar]
- Kiene RP, Linn LJ, Bruton JA. New and important roles for DMSP in marine microbial communities. J Sea Res. 2000;43:4209–4224. [Google Scholar]
- Kirkwood M, Le Brun NE, Todd JD, Johnston AWB. The dddP gene of Roseovarius nubinhibens encodes a novel lyase that cleaves dimethylsulfoniopropionate into acrylate plus dimethyl sulfide. Microbiol. 2010a;156:1900–1906. doi: 10.1099/mic.0.038927-0. [DOI] [PubMed] [Google Scholar]
- Kirkwood M, Todd JD, Rypien KL, Johnston AWB. The opportunistic coral pathogen Aspergillus sydowii contains dddP and makes dimethyl sulfide from dimethylsulfoniopropionate. ISME J. 2010b;4:147–150. doi: 10.1038/ismej.2009.102. [DOI] [PubMed] [Google Scholar]
- Leveau JHJ, Lindow SE. Utilization of the plant hormone indole-3-acetic acid for growth by Pseudomonas putida strain 1290. Appl Environ Microbiol. 2005;71:2365–2371. doi: 10.1128/AEM.71.5.2365-2371.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovelock JE, Maggs RJ, Rasmussen RA. Dimethyl sulphide and the natural sulphur cycle. Nature. 1972;237:452–453. [Google Scholar]
- Maddocks SE, Oyston PC. Structure and function of the LysR-type transcriptional regulator (LTTR) family proteins. Microbiol. 2008;154:3609–3623. doi: 10.1099/mic.0.2008/022772-0. [DOI] [PubMed] [Google Scholar]
- McClung CR, Patriquin DG, Davis RE. Campylobacter nitrofigilis sp.nov., a nitrogen-fixing bacterium associated with roots of Spartina alterniflora Loisel. Int J System Bacteriol. 1983;33:605–612. [Google Scholar]
- Moussard H, Corre E, Cambon-Bonavita MA, Fouquet Y, Jeanthon C. Novel uncultured Epsilonproteobacteria dominate a filamentous sulphur mat from the 13 degrees N hydrothermal vent field, East Pacific Rise. FEMS Microbiol Ecol. 2006;58:449–463. doi: 10.1111/j.1574-6941.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- Pati A, Gronow S, Lapidus A, Copeland A, Del Rio TG, Nolan M, et al. Complete genome sequence of Arcobacter nitrofigilis type strain (CIT) Standards in Genomic Sci. 2010;2:300–308. doi: 10.4056/sigs.912121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin E, Sanschagrin F, Levesque RC. Sigma factors in Pseudomonas aeruginosa. FEMS Microbiol Lett. 2008;32:38–55. doi: 10.1111/j.1574-6976.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- Raina JB, Dinsdale EA, Willis BL, Bourne DG. Do the organic sulfur compounds DMSP and DMS drive coral microbial associations. Trends Microbiol. 2010;18:101–108. doi: 10.1016/j.tim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Ramos JL, Martínez-Bueno M, Molina-Henares AJ, Terán W, Watanabe K, Zhang X, et al. The TetR family of transcriptional repressors. Microbiol Mol Biol Rev. 2005;69:326–356. doi: 10.1128/MMBR.69.2.326-356.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossen L, Shearman CA, Johnston AWB, Downie JA. The nodD gene of Rhizobium leguminosarum is autoregulatory and in the presence of plant exudate induces the nodA,B,C genes. EMBO J. 1985;4:3369–3373. doi: 10.1002/j.1460-2075.1985.tb04092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch DB, Halpern AL, Sutton G, Heidelberg KB, Williamson S, Yooseph S, et al. The Sorcerer II global ocean sampling expedition: Northwest Atlantic through Eastern Tropical Pacific. PloS Biol. 2007;5:398–431. doi: 10.1371/journal.pbio.0050077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T.1989Molecular Cloning: A Laboratory Manual2nd edn.Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA [Google Scholar]
- Schäfer A, Tauch A, Jäger W, Kalinowski J, Thierbach G, Pühler A. Small mobilizable multi-purpose cloning vectors derived from the Escherichia coli plasmids pK18 and pK19: selection of defined deletions in the chromosome of Corynebacterium glutamicum. Gene. 1994;145:69–73. doi: 10.1016/0378-1119(94)90324-7. [DOI] [PubMed] [Google Scholar]
- Schippers A, Neretin LN, Kallmeyer J, Ferdelman TG, Cragg BA, Parkes RJ, et al. Prokaryotic cells of the deep sub-seafloor biosphere identified as living bacteria. Nature. 2005;433:861–864. doi: 10.1038/nature03302. [DOI] [PubMed] [Google Scholar]
- Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ. Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRL1JI. Plant Mol Biol. 1987;9:27–39. doi: 10.1007/BF00017984. [DOI] [PubMed] [Google Scholar]
- Staskawicz B, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefels J, Steinke M, Turner S, Malin G, Belviso S. Environmental constraints on the production of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochem. 2007;83:245–275. [Google Scholar]
- Steinke M, Stefels J, Stamhuis E. Dimethyl sulfide triggers search behavior in copepods. Limnol Oceanograph. 2006;51:1925–1930. [Google Scholar]
- Studier FW, Moffatt BA. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Todd JD, Curson ARJ, Dupont CL, Nicholson P, Johnston AWB. The dddP gene, encoding a novel enzyme that converts dimethylsulfoniopropionate into dimethyl sulfide, is widespread in ocean metagenomes and marine bacteria and also occurs in some Ascomycete fungi. Environ Microbiol. 2009;11:1376–1385. doi: 10.1111/j.1462-2920.2009.01864.x. [DOI] [PubMed] [Google Scholar]
- Todd JD, Curson ARJ, Kirkwood M, Sullivan MJ, Green RT, Johnston AWB.2011DddQ, a novel, cupin-containing, dimethylsulfoniopropionate lyase in marine Roseobacters and in uncultured marine bacteria Environ Microbioldoi: 10.1111/j.1462-2920.2010.02348.x [DOI] [PubMed]
- Todd JD, Curson ARJ, Nikolaidou-Katsaraidou N, Brearley CA, Watmough NJ, Chan Y, et al. Molecular dissection of bacterial acrylate catabolism - unexpected links with dimethylsulfoniopropionate catabolism and dimethyl sulfide production. Environ Microbiol. 2010;12:327–343. doi: 10.1111/j.1462-2920.2009.02071.x. [DOI] [PubMed] [Google Scholar]
- Todd JD, Rogers R, Li YG, Wexler M, Bond PL, Sun L, et al. Structural and regulatory genes required to make the gas dimethyl sulfide in bacteria. Science. 2007;315:666–669. doi: 10.1126/science.1135370. [DOI] [PubMed] [Google Scholar]
- Vallina SM, Simó R. Strong relationship between DMS and the solar radiation dose over the global surface ocean. Science. 2007;315:506–508. doi: 10.1126/science.1133680. [DOI] [PubMed] [Google Scholar]
- van der Maarel MJEC, Aukema W, Hansen TA. Purification and characterization of a dimethylsulfoniopropionate cleaving enzyme from Desulfovibrio acrylicus. FEMS Microbiol Lett. 1996a;143:3241–3245. [Google Scholar]
- van der Maarel MJEC, van Bergeijk S, van Werkhoven AF, Laverman AM, Meijer WG, Stam WT, et al. Cleavage of dimethylsulfoniopropionate and reduction of acrylate by Desulfovibrio acrylicus sp. nov. Arch Microbiol. 1996b;166:109–115. [Google Scholar]
- Venkateswaren K, Moser DP, Dollhopf ME, Lies DPI, Saffarini DA, MacGregor BJ, et al. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int J Syst Bacteriol. 1999;49:705–724. doi: 10.1099/00207713-49-2-705. [DOI] [PubMed] [Google Scholar]
- Wexler M, Yeoman KH, Stevens JB, de Luca NG, Sawers G, Johnston AWB. The Rhizobium leguminosarum tonB gene is required for the uptake of siderophore and haem as sources of iron. Mol Microbiol. 2001;41:801–816. doi: 10.1046/j.1365-2958.2001.02556.x. [DOI] [PubMed] [Google Scholar]
- Wood WB. Host specificity of DNA produced by Escherichia coli: bacterial mutations affecting the restriction and modification of DNA. J Mol Biol. 1966;16:118–133. doi: 10.1016/s0022-2836(66)80267-x. [DOI] [PubMed] [Google Scholar]
- Yoch DC. Dimethylsulfoniopropionate: its sources, role in the marine food web, and biological degradation to dimethylsulphide. Appl Environ Microbiol. 2002;68:5804–5815. doi: 10.1128/AEM.68.12.5804-5815.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoch DC, Ansede JH, Rabinowitz KS. Evidence for intracellular and extracellular dimethylsulfoniopropionate (DMSP) lyases and DMSP uptake sites in two species of marine bacteria. Appl Environ Microbiol. 1997;63:3182–3188. doi: 10.1128/aem.63.8.3182-3188.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao JS, Manno D, Leggiadro C, O'Neill D, Hawari J. Shewanella halifaxensis sp nov., a novel obligately respiratory and denitrifying psychrophile. Int J Syst Evol Microbiol. 2006;56:205–212. doi: 10.1099/ijs.0.63829-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.