Abstract
Objective
To assess the cardiovascular risk of impaired fasting glucose (IFG).
Background
The association between IFG, incident type 2 diabetes mellitus (T2DM) and cardiovascular (CV) events remains unclear.
Methods
The Multi-Ethnic Study of Atherosclerosis (MESA) included participants aged 45–84 free of clinical CV disease at baseline (2000–2002). T2DM was defined as fasting glucose >125mg/dl or anti-diabetes medication at baseline and follow-up exams, IFG as no T2DM and fasting glucose 100–125.mg/dl. Cox proportional hazard analysis was used to assess the association between IFG and incident DM and also with incident CV events.
Results
Of 6753 participants included in these analyses 840 (12.7%) had T2DM, 940 (13.8%) had IFG at the baseline exam. During 7.5 years of follow-up there were 418 adjudicated CV events. T2DM was associated with an increased CV incidence in the univariate [hazard ratio (HR); 2.83(2.25–3.56), p<0.0001] and multivariable models (adjusted for demographics and traditional risk factors) [HR; 1.87(1.47 – 2.37), p<0.0001] compared with subjects without T2DM (IFG + NFG). IFG was associated with increased incidence of T2DM [HR; 13.2 (95%CI 10.8–16.2), p<0.001] that remained after adjusting for demographics, highest educational level, physical activity and BMI [HR; 10.5(8.4–13.1), p<0.001] compared to NFG. IFG was associated with incident CV events in the univariate [HR; 1.64(1.26 – 2.14), p=<0.001] but not in the full multivariable model [HR; 1.16(95% CI 0.88–1.52), p=0.3] compared with NFG.
Conclusion
Having IFG was not independently associated with an increased short-term risk for incident CV events. These data reiterate the importance of intervention in persons with IFG to reduce their incidence of T2DM.
Keywords: Impaired fasting glucose, diabetes mellitus, cardiovascular events, population
Introduction
It has been estimated that more than 44 million adults in the US have impaired fasting blood glucose (IFG) and the numbers will likely continue to increase as a consequence of the ongoing obesity epidemic (1, 2). Individuals with IFG (pre-diabetes) are at an increased risk of developing type 2 diabetes mellitus (T2DM) compared with subjects with normal fasting glucose (NFG) (3, 4). However, the relationship between IFG and clinical cardiovascular events is less well established with some but not all studies suggesting that IFG is an independent CVD risk factor. Furthermore the currently available data concerning IFG and CV events are from cohorts with established CVD (5–7) and/or from either single gender or race/ethnicity groups (8–12), limiting their applicability to general populations.
Several meta-analyses have attempted to address this question but these meta-analyses have limitations such as single race/ethnicity, the use of ICD 9 codes, significant loss to follow of participants and differences in the constituents of the composite outcomes in the studies that were included in these meta-analyses (13–15). Moreover, the degree of adjustment for potential confounders in the studies included in these meta-analyses was variable, limiting the validity of the direct comparison of risk in these studies (13–15). Some of the studies included in these meta-analysis had follow period as long as 21 years, a period by which most individuals with impaired fasting glucose would have developed diabetes mellitus for at least 10 years, making it unclear whether this increased CV risk was due to diabetes mellitus or impaired fasting blood glucose (13,14).
To clarify the associations of IFG with bothT2DM and cardiovascular disease in a more ethnically diverse population, we examined baseline IFG and 7.5 year incident T2DM and cardiovascular events in the Multi-Ethnic Study of Atherosclerosis (MESA).
Methods
Study Population and Data Collection
The study design for MESA has been published elsewhere (16). In brief, MESA is a prospective cohort study to investigate the prevalence, correlates and progression of subclinical CVD in individuals without known CVD at baseline. The cohort includes 6814 women and men aged 45–84 years old recruited from 6 US communities (Baltimore, Md; Chicago, Ill.; Forsyth County, N.C.; Los Angeles County, Calif.; northern Manhattan, N.Y.; and St. Paul, Minn.). MESA cohort participants were 38% white 28% black 22% Hispanic and 12% Chinese. Individuals with a history of physician–diagnosed myocardial infarction, angina, heart failure, stroke, or transient ischemic attack, or who had undergone an invasive procedure for CVD (coronary artery bypass graft, angioplasty, valve replacement, pacemaker placement or other vascular surgeries) were excluded from participation. This study was approved by the Institutional Review Boards of each study site and written informed consent was obtained from all participants.
Demographics, medical history, anthropometric and laboratory data for the present study were taken from the first examination of the MESA cohort (July 2000-August 2002). Current smoking was defined as having smoked a cigarette in the last 30 days. Use of antihypertensive and other medications was based on review of prescribed medication containers. Resting blood pressure was measured 3 times in the seated position, and the average of the second and third readings was recorded. Hypertension was defined as a systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or use of medication prescribed for hypertension. Body mass index was calculated as weight (kg)/height (m2). Total and high-density lipoprotein cholesterol were measured from blood samples obtained after a 12-hour fast. Low-density lipoprotein cholesterol was estimated by the Friedewald equation (17). Fasting blood glucose (serum) was measured by the glucose oxidase method on the Vitros analyzer (Johnson and Johnson Clinical Diagnostics).
Diabetes mellitus was defined as fasting glucose >125 mg/dl or the use of hypoglycemic medications. Among those not reporting use of hypoglycemic medications, we defined IFG glucose between 100 and 125mg/dl and normal fasting glucose (NFG) as fasting blood glucose less than 100 mg/dl.
Definition of Incident Diabetes Mellitus
Fasting blood glucose was measured and data on the use of diabetes medication was collected from MESA participants during exam two, three and four (follow-up through 2007). Incident T2DM in the present study was defined as fasting blood glucose >125 mg/dl or use of hypoglycemic medications during exam two, three or four in participants who did not have T2DM during the baseline MESA examination (2000–2002).
Ascertainment of Cardiovascular Events
Cardiovascular events were adjudicated by a MESA committee which included cardiologists, physician epidemiologists and neurologists. A detailed description of the cardiovascular event adjudication process has already been published (21). For the purposes of this study, we define our composite outcome (composite event) as incident myocardial infarction, definite angina, probable angina (if followed by coronary artery bypass grafting and percutaneous coronary intervention), resuscitated cardiac arrest, stroke, stroke death, CHD death or other CVD death as defined by the MESA protocol.
Statistical Analysis
Descriptive data are presented as mean ± SD for continuous variables or the frequencies of participants for categorical variables. Kaplan Meier analysis was used to assess the univariate association of the three categories of glucose control (NFG, IFG and T2DM) with incident CV events. Cox proportional hazard regression model was use to assess the association of a) Fasting blood glucose (as a continuous variable) with incident cardiovascular event, b) Diabetes mellitus (compared with subjects without T2DM) with incident cardiovascular events and c) IFG (compared with NFG) with incident cardiovascular events in both the univariate analysis and multivariable analysis adjusting for confounders including age, gender, race/ethnicity, systolic blood pressure, BMI, cigarette smoking, total cholesterol, HDL cholesterol, triglycerides, blood pressure medications use and HMG CoA reductase inhibitors use. Potential confounders were selected based on prior evidence of an association with CV events from previous studies. The association between IFG (compared with NFG) and the components of our composite outcome and all cause mortality was also assessed.
Cox proportional hazard analysis was also used to assess the association between IFG (compared with normoglycemia) and the incidence of diabetes mellitus in a univariate analysis and also in a multivariable analysis adjusting for age, gender, race/ethnicity, BMI, physical activity and educational level. Finally traditional CV risk factor profiles and incident CV event rates of the sub-cohort with IFG at baseline who developed T2DM and those who did not developed T2DM were compared. A two-tailed value of p<0.05 was considered significant. All statistical analyses were performed using SAS version 9.2.2 (SAS Institute, Cary, N.C.).
Results
Twenty-nine participants had missing data on fasting blood glucose and 32 participants had no follow-up information, resulting in a final sample of 6753 (840 with T2DM, 940 with IFG and 4973 with NFG). The mean age of the cohort was 62.2 years, 52.9% females, 38.4% Caucasians, 11.8% Chinese, 27.8% African American and 22% were Hispanics. Table 1 shows the demographic characteristics of the three fasting blood glucose categories in the MESA cohort. Subjects with normal fasting glucose (NFG) were relatively younger and generally had better cardiovascular risk profile compared with either subjects with IFG or T2DM. There were 418 adjudicated CV events during the 7.5 years of follow up, 105 in those with DM, 72 in the IFG group and 241 among those with NFG.
Table 1.
Demographic Characteristics of subjects with diabetes mellitus, Impaired fasting glucose and normal fasting glucose in MESA
| Variable | Normoglycemia N=4973(mean SD) | Impaired Fasting Glucose N = 940(mean SD) | Diabetes Mellitus N= 840(mean SD) | P value |
|---|---|---|---|---|
| Age (years) | 61.3 ±10.3 | 64.2 ± 9.8 | 64.7± 9.4 | <0.001 |
|
| ||||
| Male Gender (%) | 44.6 | 56.0 | 52.4 | <0.001 |
|
| ||||
| Race/Ethnicity (%) | <0.001 | |||
| Caucasian | 43.4 | 31.0 | 18.6 | |
| Chinese | 11.2 | 14.5 | 12.3 | |
| African American | 25.5 | 29.7 | 38.4 | |
| Hispanics | 19.9 | 24.9 | 30.7 | |
|
| ||||
| Body mass index(Kg/m2) | 27.6 ±5.2 | 30.1± 5.7 | 30.6 ±5.8 | <0.001 |
|
| ||||
| Cigarette Smoking (%) | 0.85 | |||
| Never | 50.5 | 49.8 | 49.8 | |
| Former | 36.3 | 37.9 | 37.4 | |
| Current | 13.2 | 12.3 | 12.8 | |
|
| ||||
| Systolic BP (mmHg) | 124.5 ±21.2 | 132.0 ±21.2 | 133.1± 22.1 | <0.001 |
|
| ||||
| Diastolic BP (mmHg) | 71.5 ±10.1 | 74.2 ±10.6 | 72.0 ±10.3 | <0.001 |
|
| ||||
| Cholesterol (mg/dl) | ||||
| Total | 195.1±35.0 | 194.5± 35.2 | 188.5 ±40.0 | <0.001 |
| LDL | 117.9± 31.1 | 118.3 ±31.1 | 111.9 ± 33.7 | <0.001 |
| HDL | 52.5 ± 15.2 | 47.2± 12.7 | 46.0 ±12.7 | <0.001 |
| Triglycerides | 123.3 ±73.8 | 147.2 ±91.7 | 163.1 ±142.1 | <0.001 |
|
| ||||
| BP medication use (%) | 31.1 | 56.1 | 64.0 | <0.001 |
|
| ||||
| Statin use (%) | 12.6 | 16.8 | 26.0 | <0.001 |
|
| ||||
| Fasting glucose (mg/dl) | 86.2 ±7.0 | 107.8±7.0 | 151.6 ± 56.4 | <0.001 |
|
| ||||
| Highest educational level | <0.001 | |||
| < high school | 14.9 | 24.0 | 29.7 | |
| High school | 17.7 | 19.4 | 20.0 | |
| >High school | 67.4 | 56.6 | 50.3 | |
Association of Fasting Blood Glucose and Incident Cardiovascular Events
Fasting blood glucose was associated with incident cardiovascular events in both the univariate and the multivariable analysis [hazard ratio/10mg/dl increase; 1.08(1.06 – 1.10), p<0.0001 and 1.05 (1.03 – 1.08), p<0.0001 respectively] (Not shown in figure or table). Figure 1 is the Kaplan Meier adjusted curves showing comparison of the event free survival rates of subjects with NFG, IFG and diabetes mellitus in the MESA cohort. [95.2%, 92.6% and 87.5%, log rank p<0.0001].
Figure 1. CV event free survival of MESA cohort.
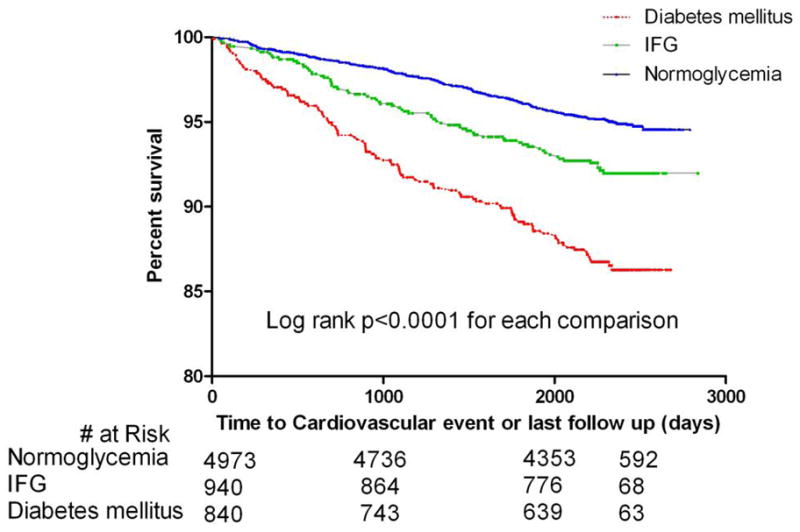
Kaplan Meier curves showing the event free survival of participants categorized as diabetes mellitus, pre-diabetes or impaired fasting glucose (IFG) and normoglycemia in MESA.
Diabetes mellitus was associated with incident cardiovascular events in both the univariate and multivariable models when compared with subjects without diabetes mellitus (IFG +NFG) [hazard ratio; 2.58 (2.07 – 3.22) p<0.0001 and 1.87(1.47 – 2.37) p<0.0001 respectively] (Not shown in figure or table).
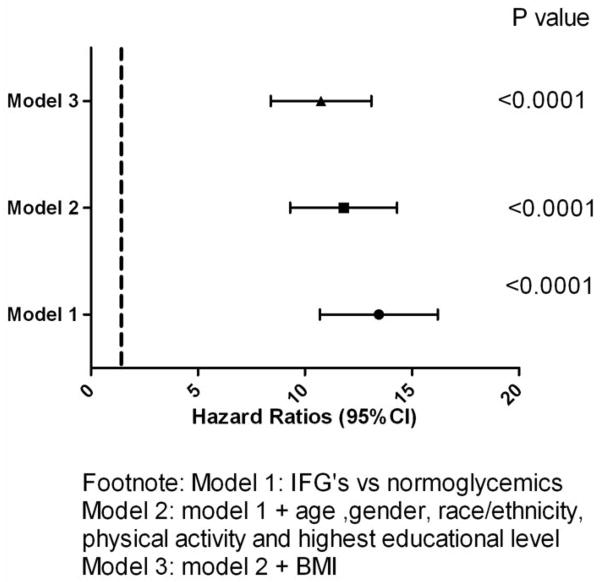
IFG and the Incidence of Diabetes Mellitus
A total of 410 participants without diabetes mellitus at baseline developed diabetes mellitus during the seven years of follow up. IFG (compared with NFG) was associated with an increased incidence of diabetes mellitus in the univariate analysis [hazard ratio; 13.2 (10.7 – 16.2) p<0.0001] and after adjusting for age, gender, race/ethnicity, BMI, physical activity and measures of socioeconomic status such as highest educational level [hazard ratio: 11.5 (9.3 – 14.3) p<0.0001]; and in the full multivariable model [hazard ratio: 10.5 (8.4 – 13.1), p<0.0001] (Figure 2).
Figure 2. Risk for incident diabetes mellitus in MESA.
Hazard ratio and 95% CI of Impaired fasting blood glucose (IFG) compared with normoglycemia for incident diabetes mellitus in Cox proportional hazard models
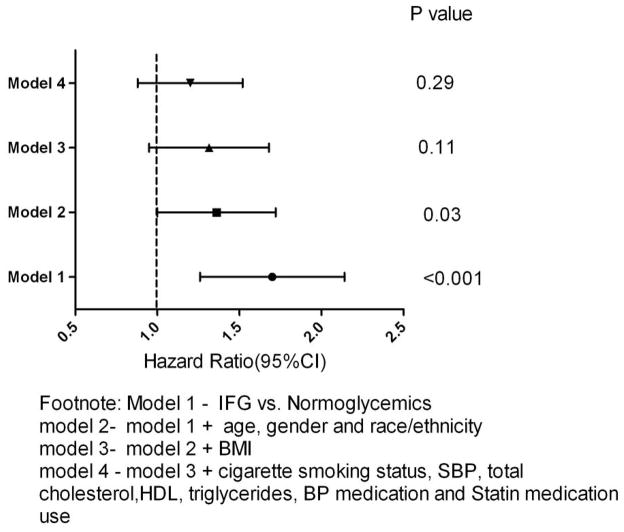
Impaired Fasting Glucose and Incident Cardiovascular Events
Compared with NFG, IFG was associated with incident cardiovascular events in the univariate analysis [hazard ratio; 1.64 (1.26 – 2.14) p=<0.001] and after adjusting for age, gender, race/ethnicity [hazard ratio; 1.31 (1.00 – 1.72), P=0.03]. The association was attenuated in the full multivariable model [hazard ratio; 1.16 (0.88 – 1.52), p=0.3] (Figure 3). Similar hazard ratios and 95% confidence intervals were obtained when individual CV outcomes and all cause mortality were evaluated (Table 2). IFG was not associated with incident cardiovascular events in any gender or race/ethnicity group when our full multivariable model was stratified by gender or race/ethnicity (data not shown).
Figure 3. Risk for incident CV events in MESA.
Hazard ratio and 95% CI of Impaired fasting blood glucose (IFG) compared with normoglycemia for incident cardiovascular event in the stepwise Cox proportional hazard models
Table 2.
Hazard ratio (95% CI) of IFG compared with normoglycemia for the primary outcome (CVD events) and individual CVD outcomes in MESA
| Outcome | # of events | Univariate(95% CI) | P value | Multivariable(95%CI) | P value |
|---|---|---|---|---|---|
| Composite | 418 | 1.64(1.26–2.14) | <0.0001 | 1.16(0.88–1.52) | 0.29 |
| Hard CHD | 283 | 1.41(1.01–1.97) | 0.04 | 1.01(0.72–1.42) | 0.97 |
| MI | 183 | 1.57(1.00–2.48) | 0.05 | 1.21(0.76–1.94) | 0.42 |
| Stroke | 114 | 1.22(0.69–2.13) | 0.49 | 0.85(0.48–1.51) | 0.58 |
| Angina | 211 | 1.95(1.36–2.81) | <0.0001 | 1.44(0.99–2.10) | 0.06 |
| All cause death* | 223 | 1.23(0.88–1.73) | 0.23 | 0.95(0.67–1.35) | 0.78 |
All- cause death was not constituent of our composite outcome.
Hard CHD: myocardial Infarction, Resuscitated cardiac arrest or CHD death Multivariable model was adjusted for age, gender, race/ethnicity, BMI, SBP, total cholesterol, HDL, triglycerides, cigarette smoking, BP medications and Statin use.
Subjects with IFG who developed T2DM and incident cardiovascular event
Two hundred and seventy-three (273) participants with IFG developed diabetes mellitus during the follow up period. When compared with IFG who did not develop diabetes mellitus during follow up (n=600) these participants were younger, more likely female with higher BMI. Variables such as age, lipid profiles, systolic blood pressure Framingham risk score, race/ethnicity, educational level and smoking status were not significantly different between these groups (Table 3). Compared with subjects with IFG who did not develop diabetes mellitus, those who developed T2DM during the follow up period had higher incident CV events in the univariate [hazard ratio: 1.65(1.04 – 2.62), p=0.03 but not in our full multivariable models [hazard ratio: 1.25 (0.78 – 1.99), p= 0.35]. Similarly, participants with normal glucose at baseline (NFG) who developed diabetes mellitus during follow up did not have a significantly higher incident CV events compared with participant with NFG at baseline who did not develop diabetes mellitus during follow up (data not shown).
Table 3.
Demographic characteristics of participants with IFG who did and did not developed diabetes mellitus during the follow up in MESA
| Variables | Developed Diabetes Mellitus N= 273(mean ± SD) | Did Not Develop Diabetes mellitus N= 600(mean ± SD) | P value |
|---|---|---|---|
| Age (years) | 62.1± 10.0 | 65.0± 9.6 | <0.01 |
|
| |||
| Male Gender (%) | 50.6 | 59.8 | 0.01 |
|
| |||
| Race/ethnicity (%) | 0.08 | ||
| Caucasian | 28.1 | 32.5 | |
| Chinese | 11.4 | 16.0 | |
| African American | 33.0 | 27.8 | |
| Hispanics | 27.5 | 23.7 | |
|
| |||
| Systolic BP (mmHg) | 131.9 ± 19.2 | 131.5 ±21.0 | 0.80 |
|
| |||
| Current/former cigarette smoking(%) | 52.8 | 46.7 | 0.50 |
|
| |||
| BMI(Kg/m2) | 31.7 ± 6.1 | 29.3 ± 5.1 | <0.001 |
|
| |||
| Cholesterol(mg/dl) | |||
| Total | 192.2 ± 37.2 | 196.0 ± 33.8 | 0.13 |
| LDL | 115.6 ± 31.8 | 119.9 ± 30.3 | 0.06 |
| HDL | 46.3 ± 11.9 | 47.7 ± 13.1 | 0.15 |
| Triglycerides | 154.5 ± 97.5 | 143. 0 ± 86.4 | 0.08 |
|
| |||
| Framingham Risk Score (%) | 9.6 ± 7.9 | 10.6 ± 8.6 | 0.09 |
Discussion
The goal of this study was to evaluate the hypothesis that IFG is an independent risk factor for cardiovascular events in a population-based sample of adults free of cardiovascular disease at baseline. After 7.5 years of follow up of the largest multi ethnic cohort so far studied on this subject, we observed that: a) The threshold of fasting blood glucose that is independently associated with CV risk may be in the diabetes mellitus range.; b) IFG is an independent risk factor for future T2DM; c) IFG is not an independent cardiovascular risk factor. Since T2DM is an independent CV risk factor, aggressive lifestyle modifications that reduce the incidence of T2DM in individuals with IFG may have significant impact on CV events rates in this population.
Even though current theory supports the association between IFG and incident cardiovascular events, the data are mixed (5–15). Coutinho et al showed in a meta-analysis of 20 studies that the progressive relationship between glucose levels and cardiovascular risk extends below the diabetic threshold (10). Kim et al showed in a cross-sectional study, that IFG may not be associated with increased cardiovascular risk in community-based subjects (9). Tominaga et al used a Japanese population to show that impaired glucose tolerance was a risk factor for cardiovascular disease but IFG was not a risk factor for cardiovascular disease (12). Pankow et al showed in the ARIC study that in addition to the poor agreement between IFG and post challenge glucose levels, neither fasting glucose nor impaired glucose tolerance was associated with adverse cardiovascular risk profile (11). Kanaya et al also showed that IFG is not independently associated with increased risk of cardiovascular events in postmenopausal women with coronary artery disease (7). However, Levitzky et al used the Framingham Offspring participants to show that IFG may be an independent cardiovascular risk factor in women but not in men (8). Sawar et al added a systematic review of western cohorts to the Reykjavik prospective study to show a modest cardiovascular risk in Caucasians with impaired fasting blood glucose (13). The findings of this study (13) were also limited by the incomplete adjustment for confounders in these studies and the use of ICD 9 codes in the ascertainment of outcomes. Ford et al also showed a modest increase cardiovascular risk in their meta-analyses on the association of impaired fasting glucose and cardiovascular outcomes (14). However these authors admitted in their conclusion that depending on the set of studies included in their meta-analysis, their findings could be interpreted as no increase or at most a very modest increase in cardiovascular risk (14). The present study adds to the data suggesting that while IFG is a risk factor for T2DM and diabetes mellitus is an independent cardiovascular risk factor, IFG per se is not an independent cardiovascular risk factor, at least during a relatively short time-frame.
The concept of IFG defined as fasting blood glucose between 110 and 125 mg/dl, was recommended by the ADA expert committee in 1997 as a risk category for further screening using a more definitive test such as a 2-hour post challenge glucose test in subjects at risk of developing T2DM (19). Data from population studies suggested that fasting blood glucose range of 94 to 103 mg/dl predicted persons who would develop T2DM (20). Based on these population based studies and in an attempt to increase the sensitivity of IFG as a screening tool, the fasting glucose criterion was lowered in 2003 by ADA to 100 to 125mg/dl. The implication of the lowering of the fasting glucose criterion was an almost quadrupling of the number of Americans with IFG from 12 million to 44 million (1). The present study found IFG as an independent risk factor for the development of T2DM, supporting the notion that interventions aimed at reducing the incidence of IFG would ultimately result in a reduction in the incidence of T2DM in the population.
Diabetes mellitus is considered a coronary heart disease equivalent (11) and subjects with IFG (pre-diabetes) have an increased risk of developing T2DM. Supported by a meta-regression analysis (10), IFG was postulated to be an independent cardiovascular risk factor and this has been adopted by American Diabetes Association. In the present study, the significant association of IFG and cardiovascular events in our univariate model was explained by traditional cardiovascular risk factors suggesting that the proposed increased cardiovascular risk may be due to the coexistence of traditional cardiovascular risk factors in subjects with IFG.
The subset of IFG who developed T2DM during the follow up period had similar risk for cardiovascular events compared with subjects with IFG who did not develop T2DM during the follow up period. The subjects with IFG who develop T2DM during follow up were younger, more likely to be female and obese. Thus young obese females with IFG were more likely to develop diabetes mellitus in this cohort and would therefore be at relatively high risk of future cardiovascular events.This finding needs replication in other cohorts but have major public health implications with regard to target blood pressure and lipid goals, cost and allocation of resources for the prevention and treatment of cardiovascular diseases. More studies aimed at identifying the subset of subjects with IFG who would develop T2DM and therefore are at risk of cardiovascular events are needed.
The current study has some limitations. The follow up period for the current study was only 7.5 years. We considered defining IFG and diabetes using a time-dependent variable approach (updating the status at each exam), however we had insufficient incident CV events/limited follow-up time post-transition to produce meaningful results. We therefore caution readers to interpret the results of this study within the context of the duration of follow up. It is unclear but possible that given the strong association between IFG and incident T2DM, a longer duration of follow up may reveal a positive association between IFG and CVD events. The sample size of the subjects with IFG was modest. Our investigation of incident CV in the IFG subgroup was limited by the relatively short follow-up time for incident CV after conversion to T2DM. The participants with T2DM had diabetes for a variable time period prior to enrollment in MESA, thus comparing the risk of CV in this group vs. IFG or new T2DM is perhaps not a fair comparison. Our study is observational and although we adjusted for the known confounders in our models, our results may still be affected by residual confounding. Finally, we used a population based cohort free of clinical cardiovascular disease at baseline and our findings do not apply to populations with clinical CVD who are at risk for recurrent CVD.
Conclusion
After 7.5 years of follow up, simply having IFG was not associated with an increased risk for incident cardiovascular events. More studies aimed at identifying subjects with IFG who would develop T2DM and are therefore at increased risk of cardiovascular events are needed.
Acknowledgments
This research was supported by contracts N01- HC-95159 through N01-HC-95165 and N01-HC-95167 and grant NHLBI T32 HL076132.
The authors would like to thank the investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Abbreviations
- IFG
Impaired fasting glucose
- NFG
Normal fasting glucose
- T2DM
Type 2 diabetes mellitus
- CVD
Cardiovascular disease
- BMI
Body mass index
- MESA
Multi Ethnic study of atherosclerosis
- ADA
American diabetes association
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Prevalence of diabetes and impaired fasting glucose in adults—United States, 1999–2000. MMWR Morb Mortal Wkly Rep. 2003;52:833–7. [PubMed] [Google Scholar]
- 2.Davidson MB, Landsman PB, Alexander CM. Lowering the criterion for impaired fasting glucose will not provide clinical benefit. Diabetes Care. 2003;26:3329–30. doi: 10.2337/diacare.26.12.3329. [DOI] [PubMed] [Google Scholar]
- 3.Unwin N, Shaw J, Zimmet P, Alberti KG. Impaired glucose tolerance and impaired fasting glycaemia: the current status on definition and intervention. Diabet Med. 2002;19:708–23. doi: 10.1046/j.1464-5491.2002.00835.x. [DOI] [PubMed] [Google Scholar]
- 4.de Vegt F, Dekker JM, Jager A, et al. Relation of impaired fasting and postload glucose with incident type 2 diabetes in a Dutch population: The Hoorn Study. JAMA. 2001;285:2109–13. doi: 10.1001/jama.285.16.2109. [DOI] [PubMed] [Google Scholar]
- 5.Fisman EZ, Motro M, Tenenbaum A, Boyko V, Mandelzweig L, Behar S. Impaired fasting glucose concentrations in nondiabetic patients with ischemic heart disease: a marker for a worse prognosis. Am Heart J. 2001;141:485–90. doi: 10.1067/mhj.2001.113219. [DOI] [PubMed] [Google Scholar]
- 6.Arcavi L, Behar S, Caspi A, Reshef N, Boyko V, Knobler H. High fasting glucose levels as a predictor of worse clinical outcome in patients with coronary artery disease: results from the Bezafibrate Infarction Prevention (BIP) study. Am Heart J. 2004;147:239–45. doi: 10.1016/j.ahj.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 7.Kanaya AM, Herrington D, Vittinghoff E, et al. Impaired fasting glucose and cardiovascular outcomes in postmenopausal women with coronary artery disease. Ann Intern Med. 2005;142:813–20. doi: 10.7326/0003-4819-142-10-200505170-00006. [DOI] [PubMed] [Google Scholar]
- 8.Levitzky YS, Pencina MJ, D’Agostino RB, et al. Impact of impaired fasting glucose on cardiovascular disease: The Framingham Heart Study. J Am Coll Cardiol. 2008;51:264–70. doi: 10.1016/j.jacc.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 9.Kim SH, Chunawala L, Linde R, Reaven GM. Comparison of the 1997 and 2003 American Diabetes Association classification of impaired fasting glucose: impact on prevalence of impaired fasting glucose, coronary heart disease risk factors, and coronary heart disease in a community-based medical practice. J Am Coll Cardiol. 2006;48:293–7. doi: 10.1016/j.jacc.2006.03.043. [DOI] [PubMed] [Google Scholar]
- 10.Coutinho M, Gerstein HC, Wang Y, Yusuf S. The relationship between glucose and incident cardiovascular events. A metaregression analysis of published data from 20 studies of 95,783 individuals followed for 12.4years. Diabetes Care. 1999;22:233–240. doi: 10.2337/diacare.22.2.233. [DOI] [PubMed] [Google Scholar]
- 11.Pankow JS, Kwan DK, Duncan BB, Couper DJ, Golden S, Ballantyne CM. Cardiometabolic risk in impaired fasting glucose and impaired glucose tolerance. Diabetes Care. 2007;30:325–331. doi: 10.2337/dc06-1457. [DOI] [PubMed] [Google Scholar]
- 12.Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose The Funagata Diabetes Study. Diabetes Care. 1999;22:920–924. doi: 10.2337/diacare.22.6.920. [DOI] [PubMed] [Google Scholar]
- 13.Sawar N, Aspelund T, Eiriksdottir G, et al. Markers of dysglycemia and risk of coronary heart disease in people without diabetes. Reykjavik prospective study and systemic review. PLOS med. 2010;7:e1000278. doi: 10.1371/journal.pmed.1000278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ford ES, Zhao G, Li C. Pre-diabetes and risk of cardiovascular disease. A systemic review of evidence. J Am Col Cardiol. 2010;55:1310 – 1317. doi: 10.1016/j.jacc.2009.10.060. [DOI] [PubMed] [Google Scholar]
- 15.Levitan EB, Song Y, Ford ES, Liu S. Is non diabetic hyperglycemia a risk factor for cardiovascular disease? A meta-analysis of prospective studies. Arch Intern Med. 2004;164:2147–55. doi: 10.1001/archinte.164.19.2147. [DOI] [PubMed] [Google Scholar]
- 16.Bild DE, Bluemke DA, Burke GL, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 17.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 18.Yeboah J, Folsom AR, Burke GL, et al. Predictive value of brachial flow-mediated dilation for incident cardiovascular events in a population-based study: the multi-ethnic study of atherosclerosis. Circulation. 2009;120:502–9. doi: 10.1161/CIRCULATIONAHA.109.864801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20:1183–97. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 20.Genuth S, Alberti KG, Bennett P, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26:3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]