Abstract
Objective:
Our aim was to test the association of vascular risk factor exposure in midlife with progression of MRI markers of brain aging and measures of cognitive decline.
Methods:
A total of 1,352 participants without dementia from the prospective Framingham Offspring Cohort Study were examined. Multivariable linear and logistic regressions were implemented to study the association of midlife vascular risk factor exposure with longitudinal change in white matter hyperintensity volume (WMHV), total brain volume (TBV), temporal horn volume, logical memory delayed recall, visual reproductions delayed-recall (VR-d), and Trail-Making Test B-A (TrB-A) performance a decade later.
Results:
Hypertension in midlife was associated with accelerated WMHV progression (p < 0.001) and worsening executive function (TrB-A score; p = 0.012). Midlife diabetes and smoking were associated with a more rapid increase in temporal horn volume, a surrogate marker of accelerated hippocampal atrophy (p = 0.017 and p = 0.008, respectively). Midlife smoking also predicted a more marked decrease in total brain volume (p = 0.025) and increased risk of extensive change in WMHV (odds ratio = 1.58 [95%confidence interval 1.07–2.33], p = 0.021). Obesity in midlife was associated with an increased risk of being in the top quartile of change in executive function (1.39 [1.02–1.88], p = 0.035) and increasing waist-to-hip ratio was associated with marked decline in TBV (10.81 [1.44–81.01], p = 0.021). Longitudinal changes in brain structure were significantly correlated with decline in memory and executive function.
Conclusions:
Midlife hypertension, diabetes, smoking, and obesity were associated with an increased rate of progression of vascular brain injury, global and hippocampal atrophy, and decline in executive function a decade later.
Several studies suggest that exposure to vascular risk factors in midlife is associated with an increased risk of dementia.1–3 Whether these risk factors also affect structural brain aging and cognitive performance in individuals without dementia, however, remains unclear. MRI markers of structural brain aging (such as lower total brain volume, hippocampal volume, or increasing white matter hyperintensity load) and performance on neuropsychological tests of memory and executive function are powerful predictors of dementia in the general population.4–8 Evaluating the impact of vascular risk factors on these intermediate markers, therefore, could advance understanding of the mechanisms by which vascular risk factors increase the risk of dementia. Furthermore, knowing if midlife exposure to vascular risk factors predicts an accelerated rate of cognitive decline and structural brain aging in community persons in advance of clinically detectable cognitive impairment could be important from a public health perspective, as most of these risk factors are modifiable by validated treatments and lifestyle changes.
We sought to explore this question in the Framingham Offspring Study, by examining the association of midlife vascular risk factor exposure with subsequent longitudinal change of quantitative MRI markers of brain aging—white matter hyperintensity volume (WMHV), total brain volume (TBV), and temporal horn volume of the lateral ventricles (THV)—as well as longitudinal change in cognitive test scores of verbal memory, visuospatial memory, and executive function.
METHODS
Study population.
The Framingham Heart Study is a single-site, community-based, prospective cohort study that was initiated in 1948 to investigate risk factors for cardiovascular disease. It comprises 3 generations of participants: the original cohort, followed since 19489; their offspring and spouses of the offspring, followed since 1971 (offspring cohort)10; and children from the largest offspring families enrolled in 2000 (gen 3).11 The present study includes participants from the offspring cohort, comprising 5,124 persons examined approximately every 4 years since enrollment.10 As part of a large ancillary study, offspring participants who survived to the 7th examination (1998–2001) and attended at least one evaluation among the 5th, 6th, or 7th examinations or had moved away from Framingham but continued to be followed up offsite (n = 3,623) were invited to take a neuropsychological test battery (NP) and undergo volumetric brain MRI (1999–2005).12 The acceptance rate was 72%. A total of 2,607 subjects underwent NP testing, 2,262 also had a brain MRI. Since 2005, all participants included in the ancillary study were invited to undergo a second NP assessment and brain MRI. Second examinations performed between 2005 and 2007 (n = 1,399) could be used for analysis (data from 2008 to 2010 continue to be tabulated). Participants have been monitored since 1974, using previously described surveillance techniques, for the development of stroke or dementia.13,14 Stroke was defined as an acute onset focal neurologic deficit of presumed vascular etiology, lasting ≥24 hours. Dementia was diagnosed according to the criteria of the DSM-IV.15 We excluded participants with prevalent stroke (n = 16) at the first NP/MRI evaluation (none of the participants had a diagnosis of dementia at this timepoint). We also excluded participants with other neurologic disorders that might confound the assessment of brain volumes (n = 31), at the first or last MRI. Hence the final sample for the present analysis consisted of 1,352 Framingham offspring participants (figure e-1 on the Neurology® Web site at www.neurology.org).
Vascular risk factor and covariate assessment.
We used measures of vascular risk factors, assessed at the 5th offspring examination (examination 5, 1991–1995) and defined as in the Framingham Stroke Risk Profile.14 Body mass index (BMI) was defined as weight (kg) divided by the square of height (m). Standing waist circumference was measured at the level of the umbilicus; hip circumference at the level of the trochanter major. Waist-to-hip ratio was calculated as the ratio of waist to hip circumferences. Other vascular risk factors were defined as follows: hypertension, systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications16; obesity, BMI ≥30 kg/m2; diabetes, fasting glucose ≥7 mmol/L or use of an antidiabetic therapy; hypercholesterolemia, fasting total cholesterol ≥6.20 mmol/L or use of a cholesterol-lowering therapy. Smokers were identified based on current smoking status at examination 5. Participants were categorized according to the presence or absence of ≥1 APOE ϵ4 allele. Educational achievement was studied as a 4-class variable (no high school degree; high school degree, no college; some college; college degree).
Outcome measurement.
Longitudinal change in MRI markers of brain aging.
Brain MRI techniques used in the Framingham Heart Study have been described in detail previously.17 Briefly, participants were evaluated with a 1 or 1.5-T Siemens Magnetom scanner. Three-dimensional T1 and double echo proton density and T2 coronal images were acquired in 4-mm contiguous slices. Centralized reading of all images was performed (QUANTA 6.2, Sun Microsystems Ultra 5 workstation). Semiautomated analysis of pixel distributions based on mathematical modeling of MRI pixel intensity histograms for CSF and brain matter (white and gray matter) were used to determine the optimal threshold of pixel intensity to best distinguish CSF from brain matter. For segmentation of WMH from other brain tissues, the first and second echo images from T2 sequences were summed and a log-normal distribution was fitted to the summed data. A segmentation threshold for WMH was determined as 3.5 SDs in pixel intensity greater than the mean of the fitted distribution of brain parenchyma. WMHV and TBV were computed using a previously validated method. As hippocampal volume at the second MRI was available only in a small subset of participants at this time, change in hippocampal size was estimated using change in THV, as described previously.18 Increasing THV is a surrogate marker of decreasing hippocampal volume.
Longitudinal change in cognitive test scores.
We selected a subset of tests from the NP battery (table e-1) that are representative measures of memory and executive function.19 The delayed recall component of the Logical Memory subtest (LM-d) from the Wechsler Memory Scale provides a savings measure of retention for verbal memory. The delayed recall component of the Visual Reproductions test (VR-d) assesses visuospatial memory. The difference between the score on Trail-Making Tests B and A (TrB-A) is a marker of executive function. We transformed TrB-A so that higher scores reflected better performance.
Statistical analyses.
Our primary analysis consisted of testing the association of vascular risk factor exposure at examination 5 with longitudinal change in WMHV, TBV, THV, LM-d, VR-d, and TrB-A. We studied change both as continuous and dichotomous measures. For continuous measures we used annualized raw change ([value at second NP/MRI − value at first NP/MRI]/time interval between first and second evaluation in years). For dichotomous measures the following thresholds were implemented: >1.34 cm3 for raw change in WMHV (corresponding to an average progression of one unit on the Cardiovascular Health Study semiquantitative scale20); top quartile of annualized change for TBV, THV, LM-d, VR-d, and TrB-A. Pearson correlation coefficients were used to calculate correlations between continuous measures of change. We used multivariable linear regression to relate each vascular risk factor to continuous measures of change and multivariable logistic regression for dichotomous measures of change. All analyses were adjusted for sex, age at the first NP/MRI assessment, time interval between the risk factor assessment and the first NP/MRI assessment, and education for cognitive outcomes. For the dichotomous measure of WMHV change, we also adjusted for the time interval between the first and last NP/MRI evaluation (in the other analyses, this time interval was already accounted for in the calculation of annualized change). As a secondary analysis, we tested whether the associations were similar when additionally adjusting for the baseline measure of the examined outcome variable, because baseline measures are a major predictor of progression, and to account for regression to the mean, a statistical phenomenon whereby unusually large or small measurements tend to be followed by measurements that are closer to the mean. Given the strong association of both elevated blood pressure and WMHV progression with stroke, the association of hypertension and systolic blood pressure with WMHV progression was also adjusted for interim stroke. We tested for interaction with APOE ϵ4 carrier status.
Analyses were performed using Statistical Analyses System software version 9.1 (SAS Institute, Cary, NC).
RESULTS
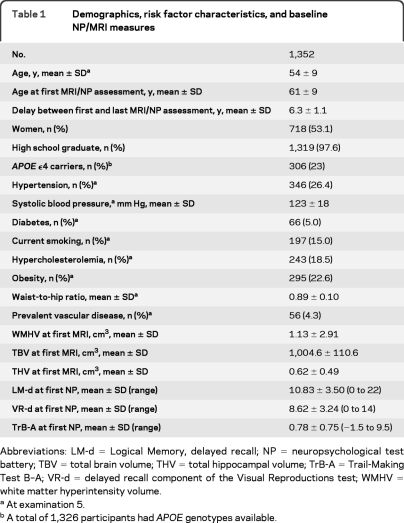
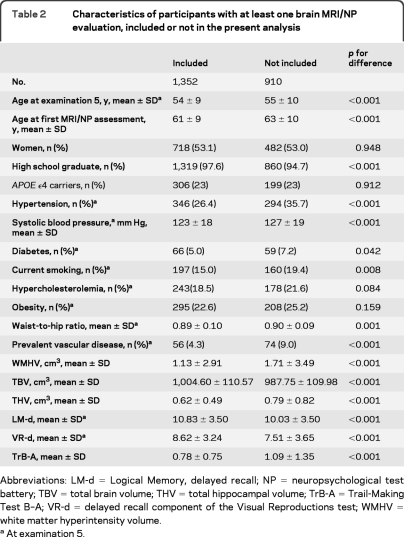
Baseline characteristics of the 1,352 participants are shown in table 1. Among participants with at least one NP/MRI evaluation, those included in the longitudinal analysis were significantly younger and healthier than those for whom no follow-up data were available (table 2). Midlife measurements of vascular risk factors at examination 5 were performed at a mean age of 54 years, 7 years on average before the first NP/MRI assessment. Longitudinal change in cognitive function and brain structure was measured on average between age 61 and 67 (table 1). Between the first and last NP/MRI assessment, 19 participants had an interim stroke and 2 developed dementia.
Table 1.
Demographics, risk factor characteristics, and baseline NP/MRI measures
Abbreviations: LM-d=Logical Memory, delayed recall; NP=neuropsychological test battery; TBV=total brain volume; THV=total hippocampal volume; TrB-A=Trail-Making Test B–A; VR-d=delayed recall component of the Visual Reproductions test; WMHV=white matter hyperintensity volume.
At examination 5.
A total of 1,326 participants had APOE genotypes available.
Table 2.
Characteristics of participants with at least one brain MRI/NP evaluation, included or not in the present analysis
Abbreviations: LM-d=Logical Memory, delayed recall; NP=neuropsychological test battery; TBV=total brain volume; THV=total hippocampal volume; TrB-A=Trail-Making Test B–A; VR-d=delayed recall component of the Visual Reproductions test; WMHV=white matter hyperintensity volume.
At examination 5.
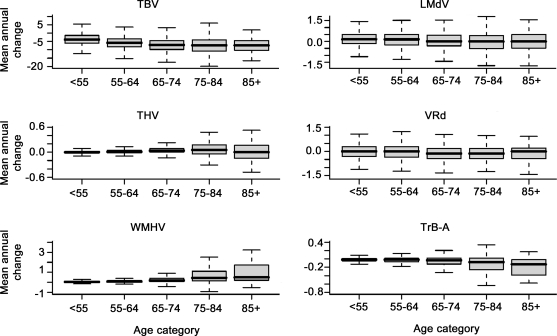
The mean annual decline in TBV and increase in THV were significantly more marked in men than in women (table e-2). Progression of WMHV was significantly more pronounced in participants aged ≥65 years than in younger individuals (table e-2). Overall, all measures of change in brain structure and cognitive function were more marked with increasing age (figure 1). APOE ϵ4 carriers had a more rapid decline in logical memory performance (LMd, estimate [β] ± standard error [SE] −0.15 ± 0.07, p = 0.021) compared to noncarriers, but did not differ significantly for change in other cognitive domains and MRI markers of brain aging (data not shown).
Figure 1. Annual change in quantitative MRI markers of brain aging and cognitive test scores with increasing age.
All measures of change were significantly associated with increasing age category (p for trend < 0.001 for mean annual change in TBV, THV, WMHV, LMd, TrB-A; p for trend = 0.018 for VRd). LM-d = Logical Memory, delayed recall; TBV = total brain volume; THV = total hippocampal volume; TrB-A = Trail-Making Test B–A; VR-d = Visual Reproductions, delayed recall; WMHV = white matter hyperintensity volume.
All measures of change in MRI markers of brain aging were significantly correlated with each other and with change in verbal memory (LM-d) and executive function (TrB-A) (table e-3). Decreasing TBV and increasing WMHV were significantly correlated with change in visuospatial memory (VR-d, table e-3).
Vascular risk factors and structural brain aging.
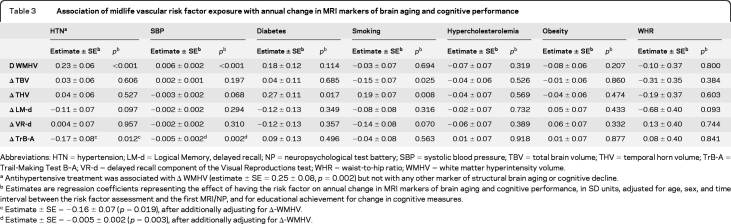
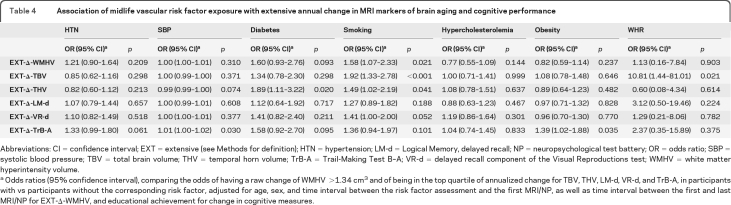
Hypertension and increasing systolic blood pressure in midlife were associated with a more rapid increase in WMHV (table 3). These associations were maintained after adjusting for interim stroke (β ± SE = 0.23 ± 0.06, p < 0.001 for hypertension and 0.006 ± 0.002, p < 0.001 for systolic blood pressure). Midlife diabetes was significantly associated with a greater annual increase in THV (table 3) and a higher risk of being in the top quartile of increase in THV (table 4). Current smoking in midlife was associated with a greater annual increase in THV and decrease in TBV (table 3) and also predicted an increased risk of prominent change in THV, TBV, and WMHV (table 4). Increasing waist-to-hip ratio in midlife was associated with an increased risk of marked decrease in TBV (table 4).
Table 3.
Association of midlife vascular risk factor exposure with annual change in MRI markers of brain aging and cognitive performance
Abbreviations: HTN=hypertension; LM-d=Logical Memory, delayed recall; NP=neuropsychological test battery; SBP=systolic blood pressure; TBV=total brain volume; THV=temporal horn volume; TrB-A=Trail-Making Test B–A; VR-d=delayed recall component of the Visual Reproductions test; WHR=waist-to-hip ratio; WMHV=white matter hyperintensity volume.
Antihypertensive treatment was associated with Δ WMHV (estimate ± SE = 0.25 ± 0.08, p = 0.002) but not with any other marker of structural brain aging or cognitive decline.
Estimates are regression coefficients representing the effect of having the risk factor on annual change in MRI markers of brain aging and cognitive performance, in SD units, adjusted for age, sex, and time interval between the risk factor assessment and the first MRI/NP, and for educational achievement for change in cognitive measures.
Estimate ± SE = −0.16 ± 0.07 (p = 0.019), after additionally adjusting for Δ-WMHV.
Estimate ± SE = −0.005 ± 0.002 (p = 0.003), after additionally adjusting for Δ-WMHV.
Table 4.
Association of midlife vascular risk factor exposure with extensive annual change in MRI markers of brain aging and cognitive performance
Abbreviations: CI=confidence interval; EXT=extensive (see Methods for definition); HTN=hypertension; LM-d=Logical Memory, delayed recall; NP=neuropsychological test battery; OR=odds ratio; SBP=systolic blood pressure; TBV=total brain volume; THV=temporal horn volume; TrB-A=Trail-Making Test B–A; VR-d=delayed recall component of the Visual Reproductions test; WMHV=white matter hyperintensity volume.
Odds ratios (95% confidence interval), comparing the odds of having a raw change of WMHV >1.34 cm3 and of being in the top quartile of annualized change for TBV, THV, LM-d, VR-d, and TrB-A, in participants with vs participants without the corresponding risk factor, adjusted for age, sex, and time interval between the risk factor assessment and the first MRI/NP, as well as time interval between the first and last MRI/NP for EXT-Δ-WMHV, and educational achievement for change in cognitive measures.
Associations of midlife risk factors with change in MRI markers of brain aging were substantially unchanged after adjusting for baseline measures (tables e-4 and e-5). No significant interaction between APOE ϵ4 carrier status and vascular risk factors was detected for associations with structural brain aging.
Vascular risk factors and cognitive decline.
Both hypertension and systolic blood pressure were associated with a more marked decline in TrB-A performance (table 3) and systolic blood pressure with a higher risk of being in the top quartile of decline in TrB-A scores (table 4). These associations remained significant after additionally adjusting for WMHV change (table 3). Midlife obesity predicted a higher risk of being in the top quartile of decline in TrB-A (table 4).
These associations were unaltered after adjusting for baseline cognitive performance (tables e-4 and e-5). No significant interaction between APOE ϵ4 carrier status and vascular risk factors was identified for associations with change in cognitive performance. In particular, the association between APOE ϵ4 and decline in LMd was not modified by vascular risk factors and the association of hypertension and obesity with decline in TrB-A was not modified by APOE ϵ4.
DISCUSSION
In a sample of 1,352 community participants without dementia, midlife hypertension was a significant predictor of WMHV progression and worsening performance in executive function a decade later. Midlife diabetes was associated with a more rapid increase in THV, a surrogate marker of accelerated hippocampal atrophy. Current smokers in midlife were at increased risk of marked expansion of THV and WMHV, and decrease in total brain volume. Midlife obesity was associated with rapid decline in executive function and increasing waist-to-hip ratio with marked decrease in TBV. Moreover, changes in brain structure were significantly associated with decline in both memory and executive function.
Recent data from the Atherosclerosis Risk in Communities (ARIC) Study suggested that midlife blood pressure measurements and cumulative systolic blood pressure are powerful predictors of WMHV progression, measured partly on a semiquantitative scale and partly with an automated procedure.21 Here we extend these findings to another large community-based sample with fully automated quantification of WMHV progression. A few cross-sectional studies have suggested an inverse association of diabetes with hippocampal volume22,23; however, the impact of midlife diabetes on longitudinal change of hippocampal volume had, to our knowledge, not yet been assessed in community participants. A number of cross-sectional associations between smoking and increased WMH load have been described,24,25 and in the Cardiovascular Health Study, cigarette smoking was associated with worsening semiquantitative white matter grade over 5 years.26 Likewise, lower brain volumes have been described cross-sectionally in smokers compared to nonsmokers,27,28 but longitudinal data are lacking. Our study confirms the longitudinal association of midlife smoking with WMHV progression, on a quantitative scale, and additionally shows a longitudinal association with global brain atrophy and a surrogate marker of hippocampal atrophy. The association of waist-to-hip ratio with marked decline in TBV is in agreement with the previously observed inverse association between markers of abdominal fat and brain volume,17 and further suggests that abdominal adiposity may be associated with a dynamic process of accelerated brain atrophy.
A number of studies have assessed the relation of vascular risk factors with cognitive decline.29 Overall, hypertension and diabetes were most frequently associated with a faster decline in executive function and processing speed.30–33 In many of these studies risk factor exposure was ascertained at the time of the first cognitive evaluation and not in midlife. Here we expand published findings by reporting an association of midlife hypertension with a more rapid decline in executive function a decade later. We observed only a trend toward an association of midlife diabetes with decline in executive function; however, given the few diabetic individuals, our power was limited. An inverse relationship between obesity and executive function has been reported cross-sectionally,34,35 but no longitudinal data are available in the literature to our knowledge. Here obesity was also associated with rapid decline in executive function. The association of vascular risk factors with decline in memory performance in the literature is controversial.36–38 In line with recent data from the ARIC study, we did not observe a significant association between midlife vascular risk factors and decline in memory performance, while the latter was significantly associated with APOE ϵ4 carrier status.30 Nonetheless, we found longitudinal effects of most vascular risk factors on structural brain measures that were significantly correlated with change in cognitive performance, including memory, suggesting at least an indirect association.
Although interventional studies are required to formally assess causality, the temporal relationship of midlife risk factor exposure with longitudinal change in structural brain aging and cognitive decline is suggestive. Based on our results, it is tempting to hypothesize that the mechanisms by which different vascular risk factors could potentially impact cognitive aging may be distinct. Thus, hypertension could be associated predominantly with increased small vessel disease load leading to an accelerated rate of WMHV progression and decline in executive function, while diabetes could be related primarily to neurodegenerative mechanisms with accelerated hippocampal atrophy and smoking to both vascular and neurodegenerative lesions. However, caution is warranted, as vascular risk factors are highly correlated with each other, making it difficult to tease out the individual effects of each.
There may be a number of reasons for the lack of direct association between vascular risk factors and memory in our dataset. First, this is a community sample of relatively young subjects, excluding persons with clinical dementia, thus leading to limited variability in cognitive performance. Second, longitudinal differences in brain structure may reflect an earlier effect of exposure to vascular risk than changes in cognition, and in the general population executive function was shown to decline first, before other cognitive domains such as memory.39 Third, measures of change in brain structure are assessed using automated procedures, with less variability and noise than measures of change in cognitive function, which can be influenced by various fluctuating parameters, such as fatigue, mood, or anxiety. Finally, measures of cognitive decline, especially for memory,40 are subject to a learning effect, possibly leading to an underestimation of longitudinal change.
The strengths of this study include the population-based setting, the longitudinal design, and the availability of vascular risk factor exposure data in midlife, several years before the outcome assessment. Vascular risk factor exposure in midlife probably reflects a greater lifetime cumulative exposure than measures recorded later and is less likely to be modified by age-related concomitant disease inducing for instance a drop in blood pressure or weight loss. We were limited by the lack of a direct measure for longitudinal change in hippocampal volume. Furthermore, persons included in this study are not perfectly representative of the general population, as they were almost entirely Caucasian and had fewer vascular risk factors than persons who were unable to undergo or declined brain MRI and NP testing. This may have limited our power to detect associations and therefore these findings likely underestimate true associations in the general population. Finally, we did not perform any correction for multiple testing as we considered our study as exploratory.
The observed associations of midlife vascular risk factors with a more marked change in quantitative imaging and cognitive intermediate markers of dementia have important implications. First, they suggest that vascular risk factors in midlife should be targeted for primary prevention trials of dementia. Second, they provide some evidence that longitudinal intermediate markers based on brain MRI and cognitive testing, which can be measured quantitatively a decade before the age at which dementia commonly presents, could perhaps be useful to screen treatments prior to evaluation in much larger clinical studies using dementia as a clinical endpoint.
Supplementary Material
GLOSSARY
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- LM-d
Logical Memory, delayed recall
- NP
neuropsychological test battery
- TBV
total brain volume
- THV
temporal horn volume
- TrB-A
Trail-Making Test B-A
- VR-d
delayed recall component of the Visual Reproductions test
- WMHV
white matter hyperintensity volume
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Debette: drafting/revising the manuscript, analysis or interpretation of data. Dr. Seshadri: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision, obtaining funding. Dr. Beiser: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis. Dr. Au: drafting/revising the manuscript, acquisition of data. J.J. Himali: analysis or interpretation of data, statistical analysis. Dr. Palumbo: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data. Dr. Wolf: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. DeCarli: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Debette reports no disclosures. Dr. Seshadri serves as an Associate Editor for the Journal of Alzheimer's Disease and on the editorial board of Stroke and receives research support from the NIH (NIA, NINDS, NHLBI). Dr. Beiser receives publishing royalties for Introductory Applied Statistics (Brooks Cole, 2005) and receives research support from the NIH (NIA, NINDS, NHLBI). Dr. Au receives/has received research support from the NIH (NIA, NINDS) and the Wing Tat Lee Fund. J.J. Himali reports no disclosures. Dr. Palumbo serves as a consultant for the NIH/NIDCD; receives salary support from the NIH/NIA; and receives research support from the VA Boston Healthcare System R&D Service (via an IPA to Boston University School of Medicine) to chair the Institutional Review Board. Dr. Wolf receives publishing royalties from the 5th edition of Stroke: Pathophysiology, Diagnosis, and Management (Elsevier, 2008) and receives research support from the NIH (NHLBI, NINDS, NIA). Dr. DeCarli serves as Editor-in-Chief of Alzheimer Disease and Associated Disorders; serves as a consultant for Takeda Pharmaceutical Company Limited and Avanir Pharmaceuticals; and receives research support from Merck Serono and the NIH (NIA, NHLBI).
REFERENCES
- 1. Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol 2006;5:735–741 [DOI] [PubMed] [Google Scholar]
- 2. Tzourio C, Dufouil C, Ducimetiere P, Alperovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly: EVA Study Group: Epidemiology of Vascular Aging. Neurology 1999;53:1948–1952 [DOI] [PubMed] [Google Scholar]
- 3. Alonso A, Mosley TH, Jr, Gottesman RF, Catellier D, Sharrett AR, Coresh J. Risk of dementia hospitalisation associated with cardiovascular risk factors in midlife and older age: the Atherosclerosis Risk in Communities (ARIC) study. J Neurol Neurosurg Psychiatry 2009;80:1194–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jack CR, Jr, Shiung MM, Weigand SD, et al. Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology 2005;65:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004;61:1531–1534 [DOI] [PubMed] [Google Scholar]
- 6. Kuller LH, Lopez OL, Newman A, et al. Risk factors for dementia in the Cardiovascular Health Cognition Study. Neuroepidemiology 2003;22:13–22 [DOI] [PubMed] [Google Scholar]
- 7. Elias MF, Beiser A, Wolf PA, Au R, White RF, D'Agostino RB. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch Neurol 2000;57:808–813 [DOI] [PubMed] [Google Scholar]
- 8. Amieva H, Jacqmin-Gadda H, Orgogozo JM, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population-based study. Brain 2005;128:1093–1101 [DOI] [PubMed] [Google Scholar]
- 9. Dawber TR, Kannel WB. The Framingham Study: an epidemiological approach to coronary heart disease. Circulation 1966;34:553–555 [DOI] [PubMed] [Google Scholar]
- 10. Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med 1975;4:518–525 [DOI] [PubMed] [Google Scholar]
- 11. Splansky GL, Corey D, Yang Q, et al. The Third Generation Cohort of the National Heart, Lung, and Blood Institute's Framingham Heart Study: design, recruitment, and initial examination. Am J Epidemiol 2007;165:1328–1335 [DOI] [PubMed] [Google Scholar]
- 12. Debette S, Beiser A, Decarli C, et al. Association of MRI markers of vascular brain injury with incident stroke, mild cognitive impairment, dementia, and mortality: The Framingham Offspring Study. Stroke 2010;41:600–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Seshadri S, Wolf PA, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease. The impact of mortality on risk estimates in the Framingham Study. Neurology 1997;49:1498–1504 [DOI] [PubMed] [Google Scholar]
- 14. Wolf PA, D'Agostino RB, Belanger AJ, Kannel WB. Probability of stroke: a risk profile from the Framingham Study. Stroke 1991;22:312–318 [DOI] [PubMed] [Google Scholar]
- 15. American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, 4th ed Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 16. Chobanian AV, Bakris GL, Black HR, et al. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003;42:1206–1252 [DOI] [PubMed] [Google Scholar]
- 17. Debette S, Beiser A, Hoffmann U, et al. Visceral fat is associated with lower brain volume in healthy middle-aged adults. Ann Neurol 2010;68:136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Davis PC, Gearing M, Gray L, et al. The CERAD experience, part VIII: neuroimaging-neuropathology correlates of temporal lobe changes in Alzheimer's disease. Neurology 1995;45:178–179 [DOI] [PubMed] [Google Scholar]
- 19. Au R, Seshadri S, Wolf PA, et al. New norms for a new generation: cognitive performance in the Framingham Offspring Cohort. Exp Aging Res 2004;30:333–358 [DOI] [PubMed] [Google Scholar]
- 20. Bryan RN, Manolio TA, Schertz LD, et al. A method for using MR to evaluate the effects of cardiovascular disease on the brain: the cardiovascular health study. AJNR Am J Neuroradiol 1994;15:1625–1633 [PMC free article] [PubMed] [Google Scholar]
- 21. Gottesman RF, Coresh J, Catellier DJ, et al. Blood pressure and white-matter disease progression in a biethnic cohort: Atherosclerosis Risk in Communities (ARIC) study. Stroke 2010;41:3–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Korf ES, van Straaten EC, de Leeuw FE, et al. Diabetes mellitus, hypertension and medial temporal lobe atrophy: the LADIS study. Diabet Med 2007;24:166–171 [DOI] [PubMed] [Google Scholar]
- 23. den Heijer T, Vermeer SE, van Dijk EJ, et al. Type 2 diabetes and atrophy of medial temporal lobe structures on brain MRI. Diabetologia 2003;46:1604–1610 [DOI] [PubMed] [Google Scholar]
- 24. Fukuda H, Kitani M. Cigarette smoking is correlated with the periventricular hyperintensity grade of brain magnetic resonance imaging. Stroke 1996;27:645–649 [DOI] [PubMed] [Google Scholar]
- 25. Longstreth WT, Jr, Arnold AM, Manolio TA, et al. Clinical correlates of ventricular and sulcal size on cranial magnetic resonance imaging of 3,301 elderly people: The Cardiovascular Health Study Collaborative Research Group. Neuroepidemiology 2000;19:30–42 [DOI] [PubMed] [Google Scholar]
- 26. Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke 2005;36:56–61 [DOI] [PubMed] [Google Scholar]
- 27. Ikram MA, Vrooman HA, Vernooij MW, et al. Brain tissue volumes in the general elderly population: The Rotterdam Scan Study. Neurobiol Aging 2008;29:882–890 [DOI] [PubMed] [Google Scholar]
- 28. Brody AL, Mandelkern MA, Jarvik ME, et al. Differences between smokers and nonsmokers in regional gray matter volumes and densities. Biol Psychiatry 2004;55:77–84 [DOI] [PubMed] [Google Scholar]
- 29. Plassman BL, Williams JW, Jr, Burke JR, Holsinger T, Benjamin S. Systematic review: factors associated with risk for and possible prevention of cognitive decline in later life. Ann Intern Med 2010;153:182–193 [DOI] [PubMed] [Google Scholar]
- 30. Knopman DS, Mosley TH, Catellier DJ, Coker LH. Fourteen-year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI Study. Alzheimers Dement 2009;5:207–214 [DOI] [PubMed] [Google Scholar]
- 31. Carmelli D, Swan GE, Reed T, et al. Midlife cardiovascular risk factors, ApoE, and cognitive decline in elderly male twins. Neurology 1998;50:1580–1585 [DOI] [PubMed] [Google Scholar]
- 32. Kuo HK, Jones RN, Milberg WP, et al. Effect of blood pressure and diabetes mellitus on cognitive and physical functions in older adults: a longitudinal analysis of the advanced cognitive training for independent and vital elderly cohort. J Am Geriatr Soc 2005;53:1154–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lu FP, Lin KP, Kuo HK. Diabetes and the risk of multi-system aging phenotypes: a systematic review and meta-analysis. PLoS ONE 2009;4:e4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Walther K, Birdsill AC, Glisky EL, Ryan L. Structural brain differences and cognitive functioning related to body mass index in older females. Hum Brain Mapp 2010;31:1052–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fergenbaum JH, Bruce S, Lou W, Hanley AJ, Greenwood C, Young TK. Obesity and lowered cognitive performance in a Canadian First Nations population. Obesity 2009;17:1957–1963 [DOI] [PubMed] [Google Scholar]
- 36. Waldstein SR, Giggey PP, Thayer JF, Zonderman AB. Nonlinear relations of blood pressure to cognitive function: the Baltimore Longitudinal Study of Aging. Hypertension 2005;45:374–379 [DOI] [PubMed] [Google Scholar]
- 37. van Oijen M, Okereke OI, Kang JH, et al. Fasting insulin levels and cognitive decline in older women without diabetes. Neuroepidemiology 2008;30:174–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Euser SM, Sattar N, Witteman JC, et al. A prospective analysis of elevated fasting glucose levels and cognitive function in older people: results from PROSPER and the Rotterdam Study. Diabetes 2010;59:1601–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. van den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry 2006;77:149–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lamar M, Resnick SM, Zonderman AB. Longitudinal changes in verbal memory in older adults: distinguishing the effects of age from repeat testing. Neurology 2003;60:82–86 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.