SUMMARY
Post-implantation facial nerve stimulation is one of the best known and most frequent complications of the cochlear implant procedure. Some conditions, such as otosclerosis and cochlear malformations, as well as high stimulation levels that may be necessary in patients with long auditory deprivation, expose patients to a higher risk of developing post-implant facial nerve stimulation. Facial nerve stimulation can frequently be resolved with minimal changes in speech processor fitting but, in some cases, this can lead to a reduction in the outcome. A retrospective review has been made of the clinical features of 11 patients (out of 119 patients consecutively implanted, from 1999 to 2007, at the ENT Clinic of the University of Pisa) who developed post-implantation facial nerve stimulation.
KEY WORDS: Facial nerve, Cochlear implants, Otosclerosis
RIASSUNTO
La stimolazione del nervo facciale è una delle complicanze più comuni e meglio conosciute della procedura di impianto cocleare. Alcune condizioni come l'otosclerosi, le malformazioni cocleari ma anche gli elevati livelli di stimolazione, che possono essere necessari in pazienti con lunga deprivazione uditiva, sottopongono i pazienti ad un maggior rischio di sviluppare la stimolazione del nervo facciale. Questa può essere trattata mediante minime modifiche nel fitting post-operatorio con talvolta una riduzione delle performance uditive. In questo articolo abbiamo analizzato retrospettivamente le caratteristiche cliniche di 11 pazienti (su un gruppo di 119 impiantati dal 1999 al 2007 presso la Clinica ORL dell'Università di Pisa) che hanno sviluppato la stimolazione del nervo facciale.
Introduction
Cochlear Implant (CI) has developed into a commonly performed procedure for severe to profound deafness in patients who derive minimal benefit from conventional acoustic amplification. One of the most frequent complications in the CI procedure is inadvertent facial nerve stimulation (FNS). The electric current, passing through the electrode to the spiral ganglion cell, could spread to the nearby facial nerve causing symptoms ranging from simple awareness to severe facial spasm 1. Kelsall et al. 1 proposed a grading system to document subjective facial nerve stimulation. This grading scale is based upon six degrees ranging from Grade I corresponding to "no stimulation" to Grade VI corresponding to "total stimulation: severe gross motion of total facial musculature and/or severe pain" 1. Some scientific papers reported a variable FNS rate in CI users raging between 1% and 14.9% 1-6. Cochlear malformations, otosclerosis, cochlear ossification (post-meningitis, otosyphilis), temporal bone fracture and osteoporosis have been identified as predisposing factors to FNS 1-4 7 8. A lower rate of post-implant facial nerve stimulation is reported in children, probably due to a certain percentage of misdiagnosed cases 9. The FNS onset can be immediate or delayed. It usually occurs within the first year after implantation but it could develop up to 10 years later.
Several hypotheses have been advanced to explain the pathogenesis of FNS following CI. First the close proximity of the facial nerve to the lateral wall of the cochlea (in the superior segment of the basal turn) could explain why the mid-array (corresponding to electrodes 16-17 in 22 electrodes straight arrays and to electrodes 16 to 18 in 22 electrodes perimodiolar arrays) is most frequently involved in FNS 1 8. Other factors responsible for FNS could be a low impedance pathway at the modiolar base, high stimulation levels necessary to stimulate hypoplasic acoustic nerves or malfunctioning electrodes. Finally, in patients with otosclerosis, the new soft and remodelled bone has a lower impedance and this could be responsible for the high rate of FNS in these patients 10. Some Authors also hypothesized that the array of electrodes could erode the bony layer between the scala tympani and facial nerve 8. Various methods have been proposed to eliminate FNS, such as changing the programming strategy and/or stimulation mode, turning off some electrodes, reducing the C-levels under FNS thresholds and also a re-implantation with a new device.
The aim of this study was to analyse the clinical features of patients who developed post-implantation FNS, in a group of 119 patients consecutively implanted at the ENT Clinic of the University of Pisa.
Material and methods
A retrospective chart review was used for 119 patients, 60 males (50.4%) and 59 females (49.6%), who had been consecutively implanted at the ENT Clinic of the University of Pisa between January 1999 and January 2007. Only those patients implanted with the Nucleus 24 CI, manufactured by Cochlear Corporation (Sydney, Australia) were included in the study. Overall, 99 patients (83.2%) received the Nucleus Contour or Contour Advance electrode arrays, 19 (16%) the straight Nucleus electrode arrays and one patient (0.8%) received a double array. Of these patients, 63 were adults (age ≥ 18 years) and 56 were children. In the group of adult patients, 25/63 (39.7%) were pre-lingually deafened, and 38/63 (60.3%) were post-lingually deafened. All the patients were operated upon by the same surgical team and the final insertion of the CI was performed by the same senior surgeon, in all the patients. In all cases, the array of electrodes was fully inserted. A post-operative X-ray of the skull was performed to confirm correct placement of the CI. FNS was defined as the presence of any facial movement at or below the maximum comfortable level (C-level) on one or more electrodes. FNS was managed by modifying CI fitting parameters, as follows: decreasing stimulation levels, switching off the offending electrodes and changing stimulation modes and/or the coding strategies, if necessary. The duration of follow-up is > 24 months for all the patients. A retrospective analysis was performed on the clinical charts, programming records, surgical reports, and imaging reports.
Results
Of the 119 patients reviewed, 11 (9.2%) patients were identified with FNS, mean age 41 years and 5 months (range 27 months - 67 years); of these, 7/11 patients (63.6%) were adult and 4/11 (36.4%) were children. FNS occurred in 11% of the adult CI recipients and in 7.1% of the paediatric recipients. The onset of FNS was immediate in 4/11 patients (36.4%), within the first year after activation in one patient (9%), and 1 year or more after activation in 6/11 patients (54.6%). In the group of patients with FNS, 4/11 had some inner ear and cochlear nerve anomalies: 1 child had a malformed cochlea with hypoplasia of the cochlear nerve, 2 adult patients had a cochlear otosclerosis and one adult patient had a cochlear ossification due to meningitis. Among the remaining 7 patients with FNS, one adult patient had a Central Nervous System Superficial Siderosis (CNSSS), 2 children had a genetic deafness with a homozygous mutation of the gene codifying for Connexin 26, 3 adult patients had an idiopathic pre-lingual deafness, and one child had a history of high grade prematurity (Fig. 1). No cases of extrusion, migration or failure of the electrodes were observed in any of the patients.
Fig. 1.
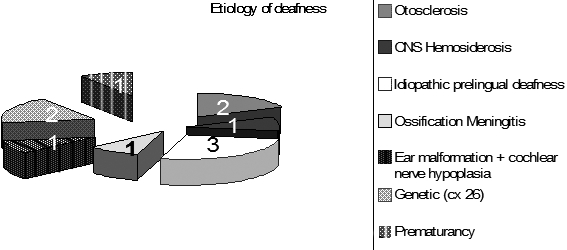
Aetiology of deafness in the present series.
In all the patients, FNS was successfully eliminated and none of the patients stopped using the implant due to FNS. Explantation or reintervention was not necessary in any of these cases. In 6/11 patients, FNS was eliminated with success, minimally reducing stimulation levels (C-level) and without any significant deterioration of the outcome.
In the remaining 5 patients, exclusion of the stimulating electrodes and an important reduction in stimulation levels were necessary, leading to a deterioration in hearing: in two patients, the results remained reasonably good, while in 3 cases they became unsatisfactory. As far as concerns the three latter patients (one with otosclerosis, one with CNSSS and one with malformed cochlea), one is able only to discriminate words in closed set (70% of identification of words in closed set), one patient is only able to detect sounds, while the patient with malformed cochlea has a very low hearing sensation.
Discussion
FNS has long been recognized as a complication of the CI procedure. In the last 20 years, several reports regarding FNS have been published. The reported rate of FNS with multi-channel cochlear implants varies considerably, ranging from 0.9% to 14.6% as reported in Table I . The incidence of FNS, in our group of CI recipients, is 9.2% and is similar to that reported in other reports in the literature. In paediatric CI recipients, the reported FNS rate is generally slightly lower in comparison to that in adults (3% reported by Kempf et al. 4 and 0.9% reported by Hoffman and Cohen 11) and this could be due to the difficulty in diagnosing mild FNS. In this regard, Cushing et al. 9 recently supposed that children may not be able to comment on the presence of facial twitching and that it is possible that, inspecting facial movements, not all facial stimulations are identified. In the above-mentioned publication. the Authors reported a very high incidence of FNS (59% in a prospective group and 34% in a retrospective group) defined as electromyographic responses of the facial musculature. In our series, FNS, observed by inspecting facial movements, occurred in 7.1% of the implanted children. There were no significant sex differences, as reported in literature 12. The onset of FNS is often variable as shown in Table I. Only a few papers have reported the time between CI activation and the onset of FNS 1 2 8 12-14. Reviewing these papers, of the 81 patients with FNS, in which the onset was timed, 32 had an immediate onset and 49 had a delayed onset. Thus delayed onset of FNS is not as rare as previously hypothesized 15. With regard to delayed FNS pathogenesis in CI users, some Authors hypothesized that the delayed onset could be due to formation of adhesions following an inflammatory process within the temporal bone 14. Others suggest a change in current pathway or tissue impedance or in facial nerve sensitivity 12. Bigelow et al. 8 hypothesized that the array of electrodes could erode the thin bony layer between the scala tympani and the facial nerve. In our series, 4/11 (36.4%) patients had an immediate onset of FNS and 7/11 (63.6%) had a delayed onset. An interesting finding was that 6/11 (54.1%) patients developed FNS one year after CI activation or much later. This delay, in some cases, could be related to an increase in stimulation levels over time.
Table I.
Reported rate of FNS with multi-channel cochlear implants.
| Investigators | Year | Incidence | Onset |
|---|---|---|---|
| Cohen et al. (5) | 1988 | 4/459 (0.9%) | Not reported |
| Niparko et al. (2) | 1991 | 12/82 (14.6%) | 2/12 (16.7%) immediate 4/12 (33.3%) < 3 months 6/12 (50%) 3 - 18 months |
| Bachor et al. (13) | 1993 | 3/53 (5.6%) | Not reported |
| Shea and Domico (14) | 1994 | 8/109 (7.3%) | 6/8 (75%) immediate 2/8 (25%) < 1 year |
| Muckle et al. (3) | 1994 | 4/38 (10.5%) | Not clearly reported |
| Hoffman and Cohen (11) | 1995 | 101/4969 (2%) | Not reported |
| Kelsall et al. (1) | 1997 | 14/200 (7%) | 7/14 (50%) immediate 7/14 (50%) < 1 year |
| Bigelow et al. (8) | 1998 | 8/58 (13.8%) | 3/8 (37.5%) immediate 4/8 (50%) < 1 year 1/8 (12.5%) >1 year |
| Kempf et al. (4) | 1999 | 38/667 (5.7%) | Not reported |
| Broomfield et al. (30) | 2000 | 20/163 (12.3%) | Not reported |
| Rayner et al. (6) | 2003 | 12/147 (8.1%) | Not reported |
| Smullen et al. (12) | 2005 | 39/600 (6.5%) | 14/39 (35.9%) immediate 19/39(48.7%) <1 year 6/39 (15.4%) >1 year |
| Ahn et al. (25) | 2009 | 23/394 (5.8%) | Not reported |
There are several cochlear disorders (otosclerosis, cochlear malformations, cochlear ossification, closed head injury and temporal bone fractures) that have been associated with a higher incidence of FNS 1-4 7 8.
In the study outlined herewith, 4/11 patients with FNS presented cochlear anomalies that probably contributed to the stimulation. There were 2 patients with otosclerosis, one patient with labyrinthitis ossificans due to meningitis and one patient with inner ear malformations and bilateral hypoplasia of the cochlear nerve.
With regard to otosclerosis, 6 patients out of 119 (5%) from our series were otosclerotic and two patients in this group had FNS. One of the two patients was implanted with a perimodiolar electrode and developed an immediate FNS. The other patient was implanted with a straight electrode and presented a delayed and progressive FNS. The incidence of FNS, in our series of implanted otosclerotic patients, is 33% (2 out of 6), while in previous publications 1 3 6 7 16 an incidence between 17 and 75% is reported. This higher incidence is thought 10-17 to be due to extracochlear current spread due to reduced electric resistance exerted by the otospongiotic labyrinthine bone. Another hypothesis is that FNS could result from the decrease in distance from the electrode's array to the facial nerve due to loss of bone and cavity formation 18. FNS, in otosclerotic patients, is reported 7-16 to be more frequent in those patients with the most severe confluent disease (Grade III ) revealed in CT scans. Thus CT scans could be helpful in predicting FNS and may be decisive in establishing the side of implantation 7. The two patients with otosclerosis and FNS after CI, collected from our series, had a Grade III disease, confirmed radiologically; indeed, the patients had a diffuse confluent retro-fenestral involvement of the otic capsule also with fenestral involvement (Fig. 2).
Fig. 2.
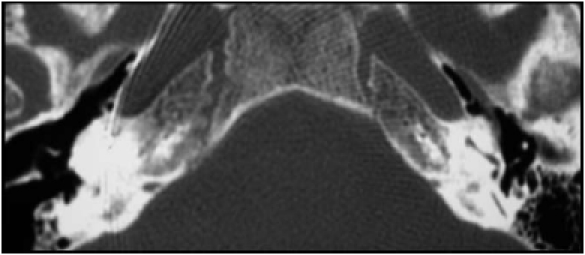
Pre-operative CT shows the confluent retro-fenestral involvement of bilateral otic capsule.
The anomalous cochleo-vestibular anatomy, as mentioned above, has been associated with a higher incidence of FNS. Papsin 19 reported a high incidence of FNS, at normal stimulation levels, in patients with anomalous cochlear anatomy. The Author also reported a higher incidence, in these patients, of an anomalous course of the facial nerve 19. Cushing et al. 9 reported 8 children with FNS out of 10 implanted with a malformed cochlea (80%). The percentage of FNS in anomalous cochlea, in our series, is lower than in previous reports. Overall, 7/119 patients (5.8%) from our series had some cochlear anomalies (one had a common cavity, 2 a Mondini anomaly, 3 an enlarged vestibular aqueduct and one a VIII nerve hypoplasia), but only one patient developed FNS. This child presented a malformed temporal bone with bilateral agenesis of the lateral semicircular canal, no identifiable bone layer between the basal and second turn of the cochlea and a narrowed internal auditory meatus with hypoplasic cochlear nerve bilaterally (Fig. 3). The very low number of cochlear nerve fibres in this patient led us to increase stimulation levels. Unfortunately, a facial twitching appeared and it was necessary to switch off the offending electrodes and reduce the stimulation levels of the remaining electrodes. This was not sufficient to reach an adequate hearing sensation and, with the CI, the child has only a low hearing sensation, not sufficient for speech discrimination.
Fig. 3.
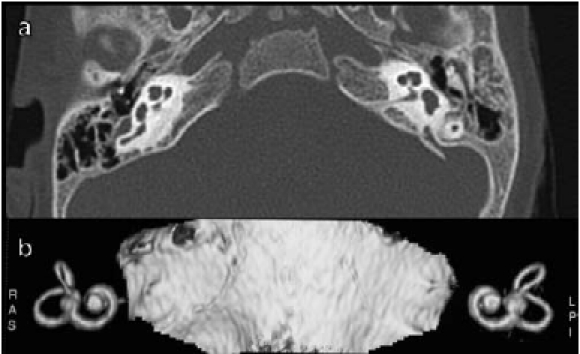
a) CT shows the anomalous anatomy of the patient. There is bilateral agenesis of lateral semicircular canal, no identifiable bone layer between basal and second turn of the cochlea and a narrowed internal auditory canal; b) same anomalies at MRI 3D reconstruction.
A significant percentage of the patients reported herein, i.e., 3 out of 11 patients, were prelingually deafened adults. Prelingually deafened adult patients are generally used to very high stimulations, mostly in the low frequencies range. This is probably the reason why they frequently ask for high stimulation levels with CI. Moreover, some of them may have a hypoplasic cochlear nerve for the long auditory deprivation, requiring high stimulation levels to gain an adequate hearing sensation with the CI. This could explain why, in our series, a relatively high percentage of patients developed a progressive FNS, one year or more after implantation.
One patient in this series was affected by CNSSS. This disorder is characterized by intracellular and extracellular deposits of hemosiderin in the leptomeninges, subpial tissue, spinal cord, and cranial nerves 20. CNSSS symptoms are progressive hearing loss, ataxia, pyramidal signs and dementia 20. CI would appear to play a possible role in the treatment of patients affected by CNSSS 20-23. A more recent report 24 described two patients affected by CNSSS who gained no benefit from the CI procedure. They hypothesized that poor results could be due to an ongoing neural deterioration of the retrocochlear pathways. This is probably what occurred in the patient reported here, who needed high stimulation levels (to reach an adequate hearing sensation) due to the deterioration of the retrocochlear pathways determining FNS.
Some Authors 7 25-27 described a higher incidence of FNS in patients with anomalous cochleas implanted with straight electrodes rather than patients implanted with perimodiolar electrodes. Perimodiolar electrodes with contacts facing towards the modiolus have a less current flow towards the outer wall of the cochlea 26. In the literature, no difference in the incidence of FNS, between perimodiolar and straight electrodes, in patients with normal cochleas, has been reported 7 12 25. Ahn et al. 25 reported a difference in the incidence of FNS between the devices with perimodiolar electrodes; it was significantly lower in patients with the Nucleus Contour Advance Soft Tip than in patients with the Nucleus Contour. This could be due to the design of this array studied in order to preserve the delicate structure and minimize lateral wall forces on the cochlea during the insertion. In our series, only one patient out of 19 implanted with straight electrode arrays developed FNS. This patient was the only one affected by otosclerosis in the group of 19 implanted with this type of array.
FNS, with deterioration of hearing, can also be the initial manifestation of a ‘soft failure' of the device. For that reason, it has been included in the check-list of non-auditory symptoms suggesting the "soft failure" of the device by some Authors 28. Various methods have been suggested by the Authors to eliminate FNS. Changing mapping strategies and stimulation modes, lowering the current amplitude, increasing the pulse width have been proposed as possible solutions 1 29. If these MAP modifications are not effective, as described by Shea and Domico 14, it might be necessary to switch off the offending electrodes. This technique could lead to a significant deterioration in speech perception 26. In other cases of refractory FNS, a revision surgery has been performed 26 29. In our series, in 6/11 patients, FNS was eliminated with success, minimally reducing stimulation levels (C-level) without any deterioration in speech perception. In the remaining 5 patients, it was necessary both to switch off the stimulating electrodes and to reduce the stimulation levels. In these patients, a deterioration in the hearing outcome was observed: in 2 patients, the results remained good, while in 3 they became unsatisfactory.
Conclusions
FNS is one of the well-known and most frequent minor complications of the CI procedure. It can produce significant discomfort, in some cases affecting the outcome and limiting the use of an implant. FNS can occur immediately upon CI activation but more frequently can be delayed, as confirmed by our results. Some conditions (otosclerosis, cochlear malformation, trauma, etc.) predispose patients to the development of post-implant FNS: these have to be taken into account for proper preoperative counselling and surgical planning, including the choice of the device. The management of FNS requires familiarity with programming techniques and, in some cases, surgical explantation or re-implantation. Generally, FNS can be resolved minimally by modifying the MAPs, but this can lead to a reduction in hearing outcome in some patients
References
- 1.Kelsall DC, Shallop JK, Brammeier TG, et al. Facial nerve stimulation after Nucleus 22-channel cochlear implantation. Am J Otol. 1997;18:336–341. [PubMed] [Google Scholar]
- 2.Niparko JK, Oviatt DL, Coker NJ, et al. Facial nerve stimulation with cochlear implantation. VA Cooperative Study Group on Cochlear Implantation. Otolaryngol Head Neck Surg. 1991;104:826–830. doi: 10.1177/019459989110400610. [DOI] [PubMed] [Google Scholar]
- 3.Muckle RP, Levine SC. Facial nerve stimulation produced by cochlear implants in patients with cochlear otosclerosis. Am J Otol. 1994;15:394–398. [PubMed] [Google Scholar]
- 4.Kempf HG, Tempel S, Johann K, et al. Complications of cochlear implant surgery in children and adults. Laryngorhinootologie. 1999;78:529–537. doi: 10.1055/s-1999-8753. [DOI] [PubMed] [Google Scholar]
- 5.Cohen NL, Hoffman RA, Stroschein M. Medical or surgical complications related to the Nucleus multichannel cochlear implant. Ann Otol Rhinol Laryngol Suppl. 1988;135:8–13. doi: 10.1177/00034894880975s202. [DOI] [PubMed] [Google Scholar]
- 6.Rayner MG, King T, Djalilian HR, et al. Resolution of facial stimulation in otosclerotic cochlear implants. Otolaryngol Head Neck Surg. 2003;129:475–480. doi: 10.1016/S0194-59980301444-X. [DOI] [PubMed] [Google Scholar]
- 7.Rotteveel LJ, Proops DW, Ramsden RT, et al. Cochlear implantation in 53 patients with otosclerosis: demographics, computed tomographic scanning, surgery, and complications. Otol Neurotol. 2004;25:943–952. doi: 10.1097/00129492-200411000-00014. [DOI] [PubMed] [Google Scholar]
- 8.Bigelow DC, Kay DJ, Rafter KO, et al. Facial nerve stimulation from cochlear implants. Am J Otol. 1998;19:163–169. [PubMed] [Google Scholar]
- 9.Cushing SL, Papsin BC, Gordon KA, et al. Incidence and characteristics of facial nerve stimulation in children with cochlear implants. Laryngoscope. 2006;116:1787–1791. doi: 10.1097/01.mlg.0000231303.85828.20. [DOI] [PubMed] [Google Scholar]
- 10.Weber BP, Lenarz T, Battmer RD, et al. Otosclerosis and facial nerve stimulation. Ann Otol Rhinol Laryngol Suppl. 1995;166:445–447. [PubMed] [Google Scholar]
- 11.Hoffman RA, Cohen NL. Complications of cochlear implant surgery. Ann Otol Rhinol Laryngol Suppl. 1995;166:420–422. [PubMed] [Google Scholar]
- 12.Smullen JL, Polak M, Hodges AV, et al. Facial nerve stimulation after cochlear implantation. Laryngoscope. 2005;115:977–982. doi: 10.1097/01.MLG.0000163100.37713.C6. [DOI] [PubMed] [Google Scholar]
- 13.Bachor E, Laszig R, Battmer RD, et al. Stimulus-inadequate sensations in cochlear implant patients. Acta Otolaryngol. 1993;113:585–590. doi: 10.3109/00016489309135868. [DOI] [PubMed] [Google Scholar]
- 14.Shea JJ, 3rd, Domico EH. Facial nerve stimulation after successful multichannel cochlear implantation. Am J Otol. 1994;15:752–756. [PubMed] [Google Scholar]
- 15.Morris DP, Maessen H, Creaser C, et al. Refractory severe facial nerve cross-stimulation and loss of auditory sensation after ten years of uneventful cochlear implant use. A rare and challenging case. Cochlear Implants Int. 2004;5:117–124. doi: 10.1179/cim.2004.5.3.117. [DOI] [PubMed] [Google Scholar]
- 16.Marshall AH, Fanning N, Symons S, et al. Cochlear implantation in cochlear otosclerosis. Laryngoscope. 2005;115:1728–1733. doi: 10.1097/01.mlg.0000171052.34196.ef. [DOI] [PubMed] [Google Scholar]
- 17.Mens LH, Oostendorp T, Broek P. Cochlear implant generated surface potentials: current spread and side effects. Ear Hear. 1994;15:339–345. doi: 10.1097/00003446-199408000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Ramsden R, Bance M, Giles E, et al. Cochlear implantation in otosclerosis: a unique positioning and programming problem. J Laryngol Otol. 1997;111:262–265. doi: 10.1017/s0022215100137028. [DOI] [PubMed] [Google Scholar]
- 19.Papsin BC. Cochlear implantation in children with anomalous cochleovestibular anatomy. Laryngoscope. 2005;115:1–26. doi: 10.1097/00005537-200501001-00001. [DOI] [PubMed] [Google Scholar]
- 20.Kim CS, Song JJ, Park MH, et al. Cochlear implantation in superficial siderosis. Acta Otolaryngol. 2006;126:892–896. doi: 10.1080/00016480500529330. [DOI] [PubMed] [Google Scholar]
- 21.Irving RM, Graham JM. Cochlear implantation in superficial siderosis. J Laryngol Otol. 1996;110:1151–1153. doi: 10.1017/s0022215100135996. [DOI] [PubMed] [Google Scholar]
- 22.Dhooge IJ, Vel E, Urgell H, et al. Cochlear implantation in a patient with superficial siderosis of the central nervous system. Otol Neurotol. 2002;23:468–472. doi: 10.1097/00129492-200207000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Hathaway B, Hirsch B, Branstetter B. Successful cochlear implantation in a patient with superficial siderosis. Am J Otolaryngol. 2006;27:255–258. doi: 10.1016/j.amjoto.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 24.Wood VH, Bird PA, Giles EC, et al. Unsuccessful cochlear implantation in two patients with superficial siderosis of the central nervous system. Otol Neurotol. 2008;29:622–625. doi: 10.1097/MAO.0b013e3181758e7e. [DOI] [PubMed] [Google Scholar]
- 25.Ahn JH, Oh SH, Chung JW, et al. Facial nerve stimulation after cochlear implantation according to types of Nucleus 24- channel electrode arrays. Acta Otolaryngol. 2009;129:588–591. doi: 10.1080/00016480802325965. [DOI] [PubMed] [Google Scholar]
- 26.Battmer R, Pesch J, Stöver T, et al. Elimination of facial nerve stimulation by reimplantation in cochlear implant subjects. Otol Neurotol. 2006;27:918–922. doi: 10.1097/01.mao.0000235374.85739.c6. [DOI] [PubMed] [Google Scholar]
- 27.Matterson AG, O'Leary S, Pinder D, et al. Otosclerosis: selection of ear for cochlear implantation. Otol Neurotol. 2007;28:438–446. doi: 10.1097/MAO.0b013e31803115eb. [DOI] [PubMed] [Google Scholar]
- 28.Balkany TJ, Hodges AV, Buchman CA, et al. Cochlear implant soft failures consensus development conference statement. Otol Neurotol. 2005;26:815–818. doi: 10.1097/01.mao.0000178150.44505.52. [DOI] [PubMed] [Google Scholar]
- 29.Polak M, Ulubil SA, Hodges AV, et al. Revision cochlear implantation for facial nerve stimulation in otosclerosis. Arch Otolaryngol Head Neck Surg. 2006;132:398–404. doi: 10.1001/archotol.132.4.398. [DOI] [PubMed] [Google Scholar]
- 30.Broomfield S, Mawman D, Woolford TJ, et al. Non-auditory stimulation in adult cochlear implant users. Cochlear Implant Int. 2000;1:55–66. doi: 10.1179/cim.2000.1.1.55. [DOI] [PubMed] [Google Scholar]


