Abstract
Duchenne muscular dystrophy (DMD) is characterized by increased muscle damage and an abnormal blood flow after muscle contraction leading to a state of functional ischemia. Abundant evidence suggests that endothelial circulating progenitor cells (EPCs) play an important role in mediating vascular and muscle repair mechanisms and that the stromal cell-derived factor (SDF)-1 α chemokine is responsible for both progenitor cell mobilization from the bone marrow to peripheral blood and homing to the sites of vascular and tissue injury. Since normal neovascularization is disrupted in DMD pathogenesis and may contribute ultimately to heart failure and sudden death, the aim of the present study is to investigate whether the (SDF)-1 α, and EPCs surface receptors in terms of CD34, CD133 and kinase domain receptor (KDR) are involved in DMD pathophysiology. In the present study, peripheral blood concentrations of circulating CD34, CD133, and CD34/ CD 133 progenitor cells were measured by flow cytometry, together with serum levels of (SDF)-1α and hypoxia inducible factor (HIF-1α.), in 28 DMD patients vs. 20 healthy age and socioeconomic matching controls. Results showed a significant increase in the number of mononuclear cells bearing EPC markers, HIF-1α mRNA expression and serum (SDF)-1 α, indicating that regeneration is an ongoing process in these patients. However, this regeneration cannot counterbalance the damage induced by dystrophine mutation.
Key words: Duchenne dystrophy, stromal cell-derived factors, EPCs surface receptors
Introduction
Duchenne muscular dystrophy (DMD) represents an X-linked recessive disorder related to mutations in the dystrophin gene which is located on chromosome Xp21.1 (1). It is the most common and severe form of dystrophinopathies, characterized by progressive and disabling muscle weakness affecting approximately 1 in 3000 to 4000 male births (2). The disease is characterized by ongoing degeneration and regeneration of skeletal muscle that leads to replacement of muscle by connective tissue and fat (3). In addition to the profound skeletal muscle lesions, a distinctive cardiomyopathy has been recognized in DMD patients (4, 5). Cardiac lesions, characterized by subepicardial fibrosis, particularly of the posterobasal portion of the left ventricle (LV), occur in later stages of the disease and are apparently progressive (6-8), while severe cardiomyopathy develops in the later stages of the disease in a large percentage of patients (8).
Cardiomyopathy is a cardiocytic disease that could be followed by a vascular disease. Previous studies suggested that endothelium-mediated relaxation was attenuated in both coronary and peripheral vessels in cardiomyopathy patients (9, 10), which means that such patients suffer from endothelial dysfunction. Recent studies have identified a population of presumably bone marrow–derived cells, called circulating endothelial progenitor cells (EPCs) that can be isolated from bone marrow or circulating mononuclear cells (11). These EPCs express a variety of endothelial surface markers including CD34, Cd133 and KDR (12). They can incorporate into sites of neovascularization (13) and home to sites of endothelial denudation (14). Initial clinical studies demonstrated that risk factors for atherosclerosis are associated with reduced levels of circulating EPCs (15) and that the functional integrity of the endothelium correlates with the activities of EPCs (16). The number of circulating progenitor cells is thought to be a marker of vascular function and repair capacity and is known to decreases with age (17, 18).
Stromal cell-derived factor (SDF)-1α plays an important role in neovascularization. (SDF)-1α is an EPC chemokine known to be responsible for both progenitor cell mobilization from the bone marrow to peripheral blood and homing to the sites of vascular and tissue injury (19, 20). Recently, SDF-1α and its receptor were identified as essential in bone marrow retention of hematopoietic stem cells, cardiogenesis, angiogenesis, and recruitment of EPCs into ischemic tissue (21, 22). The production of SDF-1and other angiogenic factors is mediated by HIF-1α, a transcriptional activator that functions as a master regulator of responses to tissue hypoxia/ ischemia (23). HIF-1 is a heterodimer composed of a constitutively expressed HIF-1β subunit and an O2-regulated HIF-1α subunit (24).
Since it was suggested lately that normal neovascularization is disrupted in DMD pathogenesis (25), and it is well known that endothelial progenitor cells (EPC) play an important role in mediating vascular repair mechanisms, we assessed the number of EPCs with surface markers KDR, CD133, and CD34 progenitor cells in DMD patients vs. healthy control subjects. Plasma levels of SDF-1 and HIF-1α were additionally investigated.
Subjects and methods
Subjects were 24 boys diagnosed clinically and at the molecular level as having DMD (mean of age (8.1 ± 1.9), versus 20 age and socioeconomic matching healthy boys (mean of age 8.2 ± 2.2). Patients and controls were chosen to be free from any infection and receiving no therapeutic treatment known to increase the oxidative stress. Blood samples were drawn after their parents' consent.
Methods
Peripheral blood mononuclear cell isolation and flow cytometry
Mononuclear cells were isolated using a Ficoll density gradient (Biocoll, Biochrom, Berlin, Germany) according to standard protocols as previously described (26). For FACS analysis, mononuclear cells were resuspended in 100 μl of PBS. Immunofluorescence cell staining was performed in duplicate with the use of the fluorescent conjugated antibodies CD34– FITC (Becton Dickinson, San Jose, USA; clone 8G12) and CD133-APC (Becton Dickinson, San Jose, USA; clone AC133). Cell fluorescence was measured immediately after staining, and data were analyzed with the help of CellQuest software (FACSCalibur, Becton Dickinson, Heidelberg, Germany). Units of all measured components are absolute cell counts obtained after the measurement of 250,000 events in a lymphocyte gate.
Reverse Transcriptase-polymerase Chain Reaction (RT-PCR) Analysis for HIF-1α
Total RNA was extracted from lymphocytes using QIAGEN RNA extraction kit (QIAGEN Inc, USA). The RNA samples were reverse transcribed using superscript reverse transcriptase, using QIAGEN OneStep RT-PCR kit (QIAGEN Inc USA, Clini Lab). Primer sequences were: HIF-1α, forward: 5'- CTGTGATGAGGCTTACCATCAGC- 3'; reverse: 5'-CTCGGCTAGTTAGGGTACACTTC- 3'; β-actin forward: 5'-GTG GGG CGC CCC AGG CAC CA-3'; and reverse: 5'-CTC CTT AAT GTC ACG CAC GAT TTC-3'. 5 μl of RT reaction of each cDNA were processed for PCR. Ten μL from each PCR reaction product were separated on a 2% agarose gel then stained with ethidium bromide. The appearance of specific bands at 283 and 540 bp for HIF-1α and β-actin respectively were evaluated under ultraviolet light and photographed. Photoes were scanned and quantification of each band was carried out using gene tools version 4. Each quantified data point was related to its individual β-actin (27).
Enzyme-linked immunosorbent assay (ELISA)
Plasma levels of SDF-1 were measured using a commercially available ELISA kit according to the manufacturer's guidelines (R&D Systems, Minneapolis, USA). EDTA plasma probes were centrifuged for 15 min at 10,000 g within 30 min of collection. Probes were aliquoted and stored at −20°C before analysis. The lower detection limit of this assay is 18 pg/ml (28).
Statistical analysis
Each experimental condition was performed and expressed as mean ± SD. Comparisons were made by Student's t-test (two-tailed for independent samples).
Results
The results are listed in tables and figures. There was a significant increase in the number of mononuclear cells bearing EPC markers in DMD patients compared to controls. CD 34 (75 ± 6.2 vs. 60 ± 4.8), CD133 (86 ± 4.7 vs. 75 ± 5.3), KDR (61 ± 4.5 vs. 45 ± 5.6) (table 1 & figure 1). Also, cells bearing CD34 CD 133 (44 ± 7.2 vs. 34 ± 4.2), CD34 KDR (36.1 ± 6.5 vs. 21.5 ± 8.3) and CD 133 KDR (35.4 ± 5.7 vs. 25.5 ± 3.8) were significantly increased among DMD patients compared to controls (table 1 & figure 1). There was a significant increase in SDF-1 serum level (506.5 ± 75.9 vs. 435 ± 82.6) and HIF-ά mRNA relative expression (3.3 ± 1.2 vs. 1.8 ± 0.6) among DMD patients compared to controls.
Table 1.
Endothelial progenitor cells surface markers in blood of DMD patients compared to controls.
| EPCs | DMD patients | Controls | t value | P value |
|---|---|---|---|---|
| CD34 | 75 ± 6.2 | 60 ± 4.8 | 9.1 | P < 0.0001 |
| CD133 | 86 ± 4.7 | 75 ± 5.3 | 7.2 | P < 0.0001 |
| KDR | 61 ± 4.5 | 45 ± 5.6 | 10 | P < 0.0001 |
| CD34 /CD133 | 44 ± 7.2 | 34 ± 4.2 | 6 | P < 0.001 |
| CD 34 / KDR | 36.1 ± 6.5 | 21.5 ± 8.3 | 6.5 | P < 0.001 |
| CD 133/ KDR | 35.4 ± 5.7 | 25.5 ± 3.8 | 8.3 | P < 0.0001 |
Figure 1.
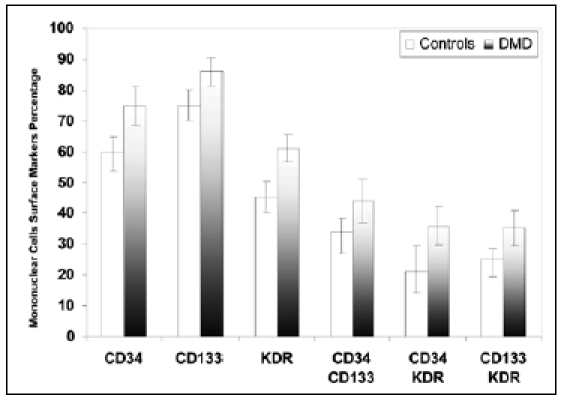
Endothelial progenitor cells surface markers in blood of DMD patients compared to controls.
Figure 2.
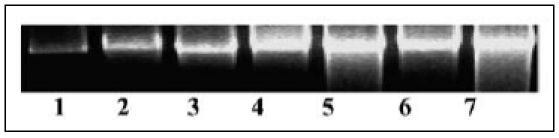
HIF-ά mRNA Relative Expression among DMD (3-7) compared to Controls (1-2).
Figure 3.
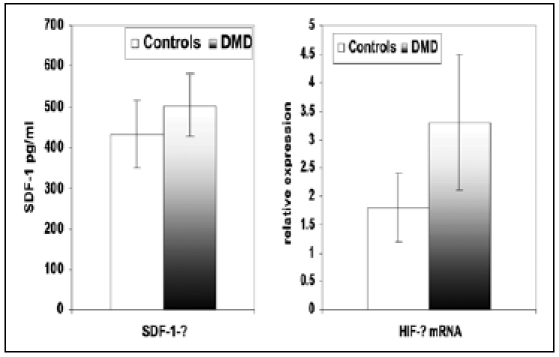
SDF-1 and HIF-ά mRNA relative expression among DMD compared to controls.
Table 2.
SDF-1 and HIF-ά mRNA relative expression in DMD patients compared to controls.
| DMD | Controls | t | ||
|---|---|---|---|---|
| SDF-1 pg/ml | 506.5 ± 75.9 | 435 ± 82.6 | 3.1 | P < 0.01 |
| HIF-ά mRNA relative expression | 3.3 ± 1.2 | 1.8 ± 0.6 | 5 | P < 0.001 |
Discussion
In the present study the number of mononuclear cells bearing EPC markers and HIF-ά together with serum SDF-1 were significantly increased indicating that regeneration is an ongoing process in DMD patients. CD34 has been shown to significantly higher among DMD patients compared to controls. To our knowledge circulating CD34 positive cells in blood of DMD patients has not been previously measured neither in blood of DMD patients nor in animal models. However it can be expected that CD34 cells are present in DMD patients for tissue regeneration, but their capacity for muscle regeneration is hindered. The latter assumption can be confirmed by the study of Li et al., 2008 (29), who reported different proliferative and myogenic abilities of mesenchymal stem cells (MSCs) in mdx (mice model for DMD compared to the normal C57BL/10 mice). mdx-MSCs exhibited increased heterochromatin, larger vacuoles, and more lysosomes under electron microscopy compared to C57BL/10-MSCs. C57BL/10-MSCs formed a few myotubes, while mdx-MSCs did not. They indicated that at passage 21, mdx-MSCs but not C57BL/10-MSCs had gradually lost their proliferative ability. In addition, there was a significant difference in the expression of CD34. They suggested that the changes in mouse MSC behaviour may be influenced by lack of dystrophin protein in mdx mouse. CD34 is a very interesting stem cell marker. It is an EPC/hematopoietic population, which is capable of differentiation into cardiomyocytes in vitro (30) and into cardiomyocytes and smooth muscle cells in vivo (31). Its pattern and level of expression in muscle stem cells change as these cells differentiate into myotubes (32).
In the present study CD133 level was also significantly higher among DMD patients compared to controls. This is in agreement with Marchesi et al (2008) (33), who found that there was an increase in CD133 cells in the blood of DMD patients compared with healthy controls and that the mean levels of CD133 cells in DMD subjects showed a tendency to decrease with advancing age. It was also suggested that the high level of CD133 cells in blood of DMD patients may indicate that these cells probably receive more specific signals for endothelial differentiation from DMD tissues such as a variety of inflammatory cytokines (34). CD133-positive cells fraction are mesennchymal stem cells (MSCs) with high proliferative potential. When placed in appropriate conditions, these cells proved their capacity to differentiate into adipocytes, osteocytes, chondrocytes, and neuronal/glial cells (35). Also blood-derived CD133- positive cells were shown to promote the repair of spinal cord injury and peripheral nerve defects (36). A subpopulation of human circulating stem cells expressing the CD133 antigen that can differentiate into endothelial and muscle cell types were identified (37).
KDR is a cell surface receptor and is also a term given to vascular endothelial growth factor receptor VEGFR-2. In the present study the mononuclear cells bearing KDR were significantly increased among DMD patients compared to controls. Data to support this finding are scarce. However, it has been recently indicated that there is an increased angiogenesis in the brain of dystrophin-deficient mdx mouse, and that this was related to increased levels of VEGF and VEGFR (38). In the present study there is also a significant increase in CD34 KDR and CD133 KDR mononuclear cells compared to controls. It ought to be mentioned that EPCs are mobilized from the bone marrow and contribute to postnatal vasculogenesis and vascular homeostasis, and that the number and the in vitro function of circulating EPCs are related to endothelial function (39). It is believed that EPCs are recruited at high frequency by the overexpression of SDF-1 (40), which has been shown to be elevated in the present study.
SDF-1 has been shown to be significantly increased in blood of DMD patients compared to controls. SDF-1 is known to play a key role in CD34-positive cell trafficking (41), and to mediate mobilization of haematopiotic stem cells and progenitor cells from the bone marrow by chemotaxis (42, 43). SDF-1 is known to be induced by hypoxia and to exert angiogenic effects (44-46). It has been demonstrated that SDF-1 is overexpressed in dystrophic muscle, enhances the extravasation of these cultured progenitor cells into skeletal muscle after intraarterial transplantation (47). This provides proof to the finding of the present study.
HIF-1α in the present study was also significantly higher among DMD patients compared to controls indicating that a hypoxic condition predominates in DMD. Induction of SDF-1 after ischemia is mediated by hypoxia- inducible factor-1 (HIF-1) as a transcription factor and the central mediator of cellular responses to hypoxic conditions (48). SDF-1 is readily degraded in normoxic cells and HIF-2 has been shown to be an important mediator of adaptive responses after ischemia (49). HIF-1 regulates the expression of hundreds of genes, including those encoding angiogenic cytokines such as stromal-derived factor (SDF)-1 (50-52). HIF-1 also mediates cell-autonomous responses to hypoxia in endothelial cells (53-55). These data suggest an activation of HIF-1alpha in the brain of dystrophic mice (38).
We conclude that hypoxic and/or ischemic conditions in muscle tissue of DMD patients initiate regenerative processes, which include secretion of (SDF)-1α that mobilizes EPC from the bone marrow to peripheral blood, homing it to the sites of vascular and tissue injury. However, the regeneration process does not balance the ongoing degeneration caused by dystrophin gene mutations in DMD patients. The variability in this balance may control the severity of phenotypic expression in DMD patients.
References
- 1.Emery A. The muscular dystrophies. Lancet. 2002;359:687–695. doi: 10.1016/S0140-6736(02)07815-7. [DOI] [PubMed] [Google Scholar]
- 2.Moser H. Duchenne muscular dystrophy: Pathogenetic aspects and genetic prevention. Hum Genet. 1984;66:17–40. doi: 10.1007/BF00275183. [DOI] [PubMed] [Google Scholar]
- 3.Cullen M, Mastaglia F. Morphological changes in dystrophic muscle. Br Med Bull. 1980;36:145–152. doi: 10.1093/oxfordjournals.bmb.a071630. [DOI] [PubMed] [Google Scholar]
- 4.Finsterer J, Stollberger C. The heart in human dystrophinopathies. Cardiology. 2003;99:1–19. doi: 10.1159/000068446. [DOI] [PubMed] [Google Scholar]
- 5.Yotsukura M, Fujii K, Katayama A, et al. Nine-year follow-up study of heart rate variability in patients with Duchenne-type progressive muscular dystrophy. Am Heart J. 1998;136:289–296. doi: 10.1053/hj.1998.v136.89737. [DOI] [PubMed] [Google Scholar]
- 6.Yotsukura M, Sasaki K, Kachi E, et al. Circadian rhythm and variability of heart rate in Duchenne-type progressive muscular dystrophy. Am J Cardiol. 1995;76:947–951. doi: 10.1016/s0002-9149(99)80267-7. [DOI] [PubMed] [Google Scholar]
- 7.Hunsaker R, Fulkerson P, Barry F, et al. Cardiac function in Duchenne's muscular dystrophy: Results of 10-year follow-up study and noninvasive tests. Am J Med. 1982;73:235–238. doi: 10.1016/0002-9343(82)90184-x. [DOI] [PubMed] [Google Scholar]
- 8.Kaspar R, Allen H, Montanaro F. Current understanding and management of dilated cardiomyopathy in Duchenne and Becker muscular dystrophy. J Am Acad Nurse Pract. 2009;21:241–249. doi: 10.1111/j.1745-7599.2009.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marti V, Aymat R, Ballester M, et al. Coronary endothelial dysfunction and myocardial cell damage in chronic stable idiopathic dilated cardiomyopathy. Int J Cardiol. 2002;82:237–245. doi: 10.1016/s0167-5273(02)00003-7. [DOI] [PubMed] [Google Scholar]
- 10.Roura S, Planas F, Prat-Vidal C, et al. Idiopathic dilated cardiomyopathy exhibits defective vascularization and vessel formation. Eur J Heart Fail. 2007;9:995–1002. doi: 10.1016/j.ejheart.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Lin Y, Weisdorf D, Solovey A, et al. Origins of circulating endothelial cells and endothelial outgrowth from blood. J Clin Invest. 2000;105:71–77. doi: 10.1172/JCI8071. http://circ.ahajournals.org/cgi/external_ref?access_num=10619863&link_type=MED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peichev M, Naiyer AJ, Pereira D, et al. Expression of VEGFR-2 and AC133 by circulating human CD34+ cells identifies a population of functional endothelial precursors. Blood. 2000;95:952–958. [PubMed] [Google Scholar]
- 13.Urbich C, Heeschen C, Aicher A, et al. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108:2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 14.Fujiyama S, Amano K, Uehira K, et al. Bone marrow monocyte lineage cells adhere on injured endothelium in a monocyte chemoattractant protein-1-dependent manner and accelerate reendothelialization as endothelial progenitor cells. Circ Res. 2003;10:980–989. doi: 10.1161/01.RES.0000099245.08637.CE. [DOI] [PubMed] [Google Scholar]
- 15.Vasa M, Fichtlscherer S, Aicher A, et al. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:e1–e7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 16.Hill J, Zalos G, Halcox J, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593–600. doi: 10.1056/NEJMoa022287. [DOI] [PubMed] [Google Scholar]
- 17.Taguchi A, Matsuyama T, Moriwaki H, et al. Circulating CD34- positive cells provide an index of cerebrovascular function. Circulation. 2004;109:2972–2975. doi: 10.1161/01.CIR.0000133311.25587.DE. [DOI] [PubMed] [Google Scholar]
- 18.Thijssen D, Vos J, Verseyden C, et al. Haematopoietic stem cells and endothelial progenitor cells in healthy men: effect of aging and training. Aging Cell. 2006;5:495–503. doi: 10.1111/j.1474-9726.2006.00242.x. [DOI] [PubMed] [Google Scholar]
- 19.Kucia M, Reca R, Miekus K, et al. Trafficking of normal stem cells and metastasis of cancer stem cells involve similar mechanisms: Pivotal role of SDF- 1-CXCR4 axis. Stem Cells. 2005;23:879–894. doi: 10.1634/stemcells.2004-0342. [DOI] [PubMed] [Google Scholar]
- 20.Hattori K, Heissig B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 21.Tomita S, Mickle D, Weisel R, et al. Improved heart function with myogenesis and angiogenesis after autologous porcine bone marrow stromal cell transplantation. J Thorac Cardiovasc Surg. 2002;123:1132–1140. doi: 10.1067/mtc.2002.120716. [DOI] [PubMed] [Google Scholar]
- 22.Yamaguchi J, Kusano KF, Masuo O, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 23.Semenza G. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda) 2009;24:97–106. doi: 10.1152/physiol.00045.2008. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Jiang B, Rue EA, Semenza G. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510–5514. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Straino S, Germani A, Carlo A, et al. Enhanced arteriogenesis and wound repair in dystrophin-deficient mdx mice. Circulation. 2004;110:3341–3348. doi: 10.1161/01.CIR.0000147776.50787.74. [DOI] [PubMed] [Google Scholar]
- 26.Stellos K, Bigalke B, Langer H, et al. Expression of stromal-cellderived factor on circulating platelets is increased in patients with acute coronary syndrom and correlates with the number of CD34+ progenitor cells. Eur Heart J. 2009;30:584–593. doi: 10.1093/eurheartj/ehn566. [DOI] [PubMed] [Google Scholar]
- 27.Nakamura H, Makino Y, Okamoto K, et al. TCR engagement increases hypoxia-inducible factor-1 alpha protein synthesis via rapamycin-sensitive pathway under hypoxic conditions in human peripheral T cells. J Immunol. 2005;174:7592–7599. doi: 10.4049/jimmunol.174.12.7592. [DOI] [PubMed] [Google Scholar]
- 28.Jin CZ, Zhao Y, Zhang FJ, et al. Different plasma levels of interleukins and chemokines: comparison between children and adults with AIDS in China. Chin Med J (Engl) 2009 Mar 05;122(5):530–535. [PubMed] [Google Scholar]
- 29.Li Y, Zhang C, Xiong F, et al. Comparative study of mesenchymal stem cells from C57BL/10 and mdx mice. BMC Cell Biol. 2008;9:24–35. doi: 10.1186/1471-2121-9-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badorff C, Brandes R, Popp R, et al. Transdifferentiation of bloodderived human adult endothelial progenitor cells into functionally active cardiomyocytes. Circulation. 2003;107:1024–1032. doi: 10.1161/01.cir.0000051460.85800.bb. [DOI] [PubMed] [Google Scholar]
- 31.Iwasaki H, Kawamoto A, Ishikawa M, et al. Dose-dependent contribution of CD34-positive cell transplantation to concurrent vasculogenesis and cardiomyogenesis for functional regenerative recovery after myocardial infarction. Circulation. 2006;113:1311–1325. doi: 10.1161/CIRCULATIONAHA.105.541268. [DOI] [PubMed] [Google Scholar]
- 32.Jankowski R, Deasy B, Cao B, et al. The role of CD34 expression and cellular fusion in the regeneration capacity of myogenic progenitor cells. J Cell Sci. 2002;115:4361–4374. doi: 10.1242/jcs.00110. [DOI] [PubMed] [Google Scholar]
- 33.Marchesi C, Belicchi M, Meregalli M, et al. Correlation of Circulating CD133+ Progenitor Subclasses with a Mild Phenotype in Duchenne Muscular Dystrophy Patients. PLoS ONE. 2008;3:e2218–e2218. doi: 10.1371/journal.pone.0002218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tondreau T, Meuleman N, Delforge A, et al. Mesenchymal Stem Cells Derived from CD133-Positive Cells in Mobilized Peripheral Blood and Cord Blood: Proliferation, Oct4 Expression, and Plasticity. Stem Cells. 2005;23:1105–1112. doi: 10.1634/stemcells.2004-0330. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki H, Ishikawa M, Tanaka N, et al. Administration of human peripheral blood-derived CD133+ cells accelerates functional recovery in a rat spinal cord injury model. Spine. 2009;34:249–254. doi: 10.1097/BRS.0b013e3181913cde. [DOI] [PubMed] [Google Scholar]
- 36.Kijima Y, Ishikawa M, Sunagawa T, et al. Regeneration of peripheral nerve after transplantation of CD133+ cells derived from human peripheral blood. J Neurosurg. 2009;110:758–767. doi: 10.3171/2008.3.17571. [DOI] [PubMed] [Google Scholar]
- 37.Torrente Y, Belicchi M, Sampaolesi M, et al. Human circulating AC133(+) stem cells restore dystrophin expression and ameliorate function in dystrophic skeletal muscle. J Clin Invest. 2004;114:182–195. doi: 10.1172/JCI20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nico B, Mangieri D, Crivellato E, et al. HIF activation and VEGF overexpression are coupled with ZO-1 up-phosphorylation in the brain of dystrophic mdx mouse. Brain Pathol. 2007;17:399–406. doi: 10.1111/j.1750-3639.2007.00090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shantsila E, Watson T, Lip G. Endothelial progenitor cells in cardiovascular disorders. J Am Coll Cardiol. 2007;49:741–752. doi: 10.1016/j.jacc.2006.09.050. [DOI] [PubMed] [Google Scholar]
- 40.Csaky KG, Baffi JZ, Byrnes GA, et al. Recruitment of marrowderived endothelial cells to experimental choroidal neovascularization by local expression of vascular endothelial growth factor. Exp Eye Res. 2004;78:1107–1116. doi: 10.1016/j.exer.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Hattori K, Heissiq B, Tashiro K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 42.Gupta S, Lysko P, Pillarisetti K, et al. Chemokine receptors in human endothelial cells. Functional expression of CXCR4 and its transcriptional regulation by inflammatory cytokines. J Biol Chem. 1998;273:4282–4287. doi: 10.1074/jbc.273.7.4282. [DOI] [PubMed] [Google Scholar]
- 43.Ceradini DJ, Kulkarni AR, Callaghan MJ, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 44.Ben-Shoshan J, Schwartz S, Luboshits G, et al. Constitutive expression of HIF-1alpha and HIF-2alpha in bone marrow stromal cells differentially promotes their proangiogenic properties. Stem Cells. 2008;26:2634–2643. doi: 10.1634/stemcells.2008-0369. [DOI] [PubMed] [Google Scholar]
- 45.Du R, Lu KV, Petritsch C, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Unoki N, Murakami T, Nishijima K, et al. SDF-1/CXCR4 contributes to the activation of tip cells and microglia in retinal angiogenesis. Invest Ophthalmol Vis Sci. 2010;51:3362–3371. doi: 10.1167/iovs.09-4978. [DOI] [PubMed] [Google Scholar]
- 47.Perez A, Bachrach E, Illigens B, et al. CXCR4 enhances engraftment of muscle progenitor cells. Muscle Nerve. 2009;40:562–572. doi: 10.1002/mus.21317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ceradini D, Kulkami A, Callaghan M, et al. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 49.Iyer N, Kotch L, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kelly B, Hackett S, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 51.Iyer N, Kotch L, Agani F, et al. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1. Genes Dev. 1998;12:149–162. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kelly B, Hackett S, Hirota K, et al. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in non-ischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–1081. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 53.Manalo D, Rowan A, Lavoie T, et al. Transcriptional regulation of vascular endothelial cell responses to hypoxia by HIF 1. Blood. Blood;105:659–669. doi: 10.1182/blood-2004-07-2958. [DOI] [PubMed] [Google Scholar]
- 54.Tang N, Wang L, Esko J, et al. Loss of HIF-1 in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–495. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 55.Calvani M, Rapisarda A, Uranchimeg B, et al. Hypoxic induction of an HIF-1-dependent bFGF autocrine loop drives angiogenesis in human endothelial cells. Blood. 2006;107:2705–2712. doi: 10.1182/blood-2005-09-3541. [DOI] [PMC free article] [PubMed] [Google Scholar]


