Abstract
We have previously described the development of a live, fully attenuated Mycobacterium tuberculosis (Mtb) vaccine candidate strain with two independent attenuating auxotrophic mutations in leucine and pantothenate biosynthesis. In the present work, those studies have been extended to include testing for protective efficacy in a long-term guinea pig survival model and safety testing in the highly tuberculosis susceptible Rhesus macaque. To model the safety of the ΔleuD ΔpanCD strain in HIV-infected human populations, a Simian Immunodeficiency Virus (SIV)-infected Rhesus macaque group was included. Immunization with the non-replicating ΔleuD ΔpanCD conferred long-term protection against challenge with virulent M. tuberculosis equivalent to that afforded by BCG as measured by guinea pig survival. In safety studies, clinical, hematological and bacteriological monitoring of both SIV-positive and SIV-negative Rhesus macaques immunized with ΔleuD ΔpanCD, revealed no vaccine-associated adverse effects. The results support the further development of the ΔleuD ΔpanCD strain as a viable tuberculosis (TB) vaccine candidate.
Keywords: Tuberculosis, vaccine, auxotroph, SIV, Rhesus macaque, guinea pig
1. Introduction
Infection with Mycobacterium tuberculosis (Mtb) represents a substantial threat to global health, with approximately 9.8 million new cases estimated in 2010 [1]. The situation is exacerbated by the concomitant HIV epidemic, which significantly increases the risk of progression to active disease in patients with sub-clinical M. tuberculosis infection [2]. HIV infection may present additional complications for TB vaccination efforts, both in terms of limited vaccine efficacy as well as possible adverse effects to vaccination [3, 4].
The only currently licensed TB vaccine is the attenuated strain of Mycobacterium bovis, Bacille Calmette Guérin (BCG). Although this is the most widely used vaccine worldwide, given to over 100 million people [5], it elicits variable protective efficacy [6] and is increasingly associated with adverse effects in HIV positive children. There are a number of hypotheses to explain the variable efficacy of BCG vaccines. One possible explanation is the limited antigenic repertoire of M. bovis BCG. Whole genome comparisons have revealed that BCG has 16 major genomic deletions, and many smaller mutations relative to Mtb, including genes that encode key antigens [7]. Furthermore, the HIV epidemic has negatively impacted the historically good safety record of BCG. Multiple recent reports have documented the risk of disseminated BCG infection and other complications of vaccination in HIV-infected children [4, 8, 9]. Revised WHO guidelines now advise against immunization of HIV-positive neonates with M. bovis BCG, as the risks outweigh the potential benefits [10], although in practical terms, relatively few children immunized at the time of birth are tested for HIV infection [11].
New safe and effective TB vaccines are urgently needed, and numerous candidates are currently in preclinical and clinical development [12]. These include recombinant live, viral vectored and recombinant protein candidates. Recombinant live vaccines hold the advantage of potentially eliciting a more sustained protective immune response. Two recombinant live attenuated vaccine candidates based on M. bovis BCG have completed Phase I clinical trials [13–15], and further such strains are presently in pre-clinical development [13, 16]. As these are all based on recombinant forms of M. bovis BCG, they may share some of the potential risks of the parent strain in HIV-infected populations.
In addition to M. bovis BCG-based candidates, other live attenuated vaccine candidates in preclinical development include strains based on genetically attenuated M. tuberculosis [17–19]. The latter approach may address some of the limitations of candidates derived from M. bovis BCG. For example, strains based on M. tuberculosis would be expected to have a greater antigenic repertoire and therefore elicit a qualitatively broader immune response. Furthermore, rationally targeted gene deletions can render strains substantially safer for use than BCG, even in immune-compromised populations. However, any attenuated strains based on M. tuberculosis would obviously require rigorous pre-clinical efficacy assessment and safety testing in multiple animal models prior to entry into clinical trials.
In this study, we report further assessment of a live, attenuated strain of M. tuberculosis as a vaccine candidate. This strain was constructed with 2 independent attenuating deletions of essential genes, which render it auxotrophic for the amino acid leucine and the vitamin pantothenate. We previously demonstrated that the ΔleuD ΔpanCD strain is highly attenuated in the immunodeficient SCID mouse M. tuberculosis model [18]. In the guinea pig M. tuberculosis challenge model, the double auxotroph elicits mycobacterial antigen-specific immune responses and performs as well as M. bovis BCG in short-term protection experiments [18]. Here, we expand upon previous work and show that immunization with the ΔleuD ΔpanCD strain confers long term protection of guinea pigs. We also assess the immunogenicity and safety of the strain in the highly susceptible Rhesus macaque model. Importantly, these studies also included SIV-infected animals, perhaps the best model for HIV-positive human populations, providing valuable safety data supporting possible future development of this strain as a live attenuated vaccine candidate that could safely be used in HIV-positive individuals.
2. Materials and Methods
2.1 Bacteria
M. tuberculosis ΔleuD ΔpanCD was constructed as described previously [18]. Briefly, using allelic replacement, we generated an unmarked leuD deletion and a hygromycin-marked panCD deletion in the M. tuberculosis H37Rv 102J23 parental strain. Laboratory stocks of M. bovis BCG Pasteur (BCG-P) (originally obtained from the Trudeau Institute) and M. tuberculosis H37Rv were used for guinea pig studies. Aliquots of all strains were stored at −80°C. For immunization or infection, thawed aliquots of strains were diluted in sterile phosphate-buffered saline (PBS) (or Hanks Balanced salt solution with 50 μg/ml leucine and 24 μg/ml pantothenate for ΔleuD ΔpanCD) and briefly sonicated prior to administration. Aliquots of diluted infection and immunization stocks were plated to confirm doses given.
2.2 Experimental Animals
Female Hartley guinea pigs (340g – 540g) (Elm Hill, Chelmsford, MA, USA) were housed according to institutional protocols. Guinea pigs were visually monitored for signs of morbidity daily during the course of the experiment and total body weights were obtained weekly. The main clinical sign of morbidity was reduced mobility and feeding leading to weight loss. When animals exhibited a 20% loss of weight from peak body weight, they were euthanized according to a protocol approved by the Institutional Animal Care and Use Committee of the University of Texas Health Science Center at San Antonio.
A total of 8 Rhesus macaques (Macacca mulatta) (male and female, aged between 3 and 7 years were randomly assigned to study groups (Table S1). All animals were born and raised in captivity, and housed at the New England Regional Primate Research Centre in a centralized animal biosafety level 3 containment facility in HEPA filtered isolation cubicles. Macaques were housed in accordance with standards of the Association for Assessment and Accreditation of Laboratory Animal Care and Use and Harvard Medical School s Animal Care and Use Committee. Animals were tested and shown to be free of simian retrovirus type-D, simian T lymphotrophic virus-1, and herpes B virus before assignment to experimental protocols. Procedures were performed under 10 mg/kg of ketamine HCl, or 5 mg/kg of Telazol if the procedure coincided with biopsies or tracheal intubation. Macaques received commercial monkey chow and autoclaved water ad libitum. The animals were observed daily for signs such as increased respiratory effort, cough, diarrhea, decreased appetite, unkempt hair coat, decreased stool/urine output. When sedated for phlebotomy they were given a full physical exam including weight assessment, pulmonary auscultation and abdominal palpation. Animals were euthanized when moribund or deemed necessary by the veterinary staff.
2.3 Study Design
2.3.1 Long term protective efficacy in guinea pigs
See Table 1. To assess the capacity of the ΔleuD ΔpanCD strain to enhance guinea pig survival after challenge with virulent M. tuberculosis, one group of animals received a single immunization with 3.1 × 105 CFU ΔleuD ΔpanCD and a positive control group received 5.3 × 106 CFU BCG-P, for comparison to the currently available M. tuberculosis vaccine (BCG). In the present study, we aimed to use immunization doses comparable to those utilized in our previously published work. As we always confirm the actual dose administered by serial dilution plating, it was revealed an approximately 10-fold lower dose of the double auxotroph had been administered. However, as we subsequently showed that even this reduced dose elicited statistically equivalent DTH responses to PPD as the administered BCG dose, we proceeded with the experiment.
Table 1.
Guinea pig long term protection study design
| Procedure | Week | ||||
|---|---|---|---|---|---|
| −7 | 0 | 5 | Continuation | 65 | |
| Immunizationa | X | ||||
| Challengeb | X | ||||
| DTHc | X | ||||
| CFU determinationd | X | ||||
| Weigh (weekly) | X | X | X | X | X |
| Clinical monitoring (weekly) | X | X | X | X | X |
| Terminate experimente | X | ||||
Intradermal immunization with 5 × 106 CFU BCG or 3 × 105 CFU ΔleuD ΔpanCD
Aerosol challenge with 33 CFU M. tuberculosis H37Rv
Intradermal injection of rESAT-6 and PPD; DTH reading was done 24h later
Lungs and spleens from 4 animals/group were harvested for determination of bacterial load by serial dilution plating of organ homogenates.
Surviving animals (n=7 and n=5 from BCG- and ΔleuD ΔpanCD-immunized groups respectively) were euthanized at 65 weeks post-challenge.
An unimmunized group was included as a negative control (n = 16 for all groups). Immunizations were administered intradermally, and distributed over 5 sites on the shaved flank. DTH skin testing was performed on 5 animals from each group on the day of challenge with M. tuberculosis H37Rv; 40 TU PPD (FDA/CBER) and 1 μg rESAT-6 (Mycos Research, Loveland, CO, USA) were injected intradermally into a shaved flank, and the diameter of induration was measured 24 hours later. Guinea pigs were challenged at seven weeks after immunization by aerosol with a dose of 20 to 50 CFU (mean 33 CFU) of M. tuberculosis H37Rv per animal, using a Madison Aerosol Exposure Chamber (University of Wisconsin, Madison, WI, USA). Three animals (one DTH skin-tested animal from each experimental group) were euthanized 1 day after aerosol challenge, and the entirety of their lung homogenate was plated to confirm the challenge dose. At 5 weeks post-challenge, the 4 remaining DTH skin-tested animals from each group were euthanized and right caudal lung and spleen bacterial burdens were determined by dilution plating of organ homogenates. The remaining (non-skin tested) animals were monitored closely to establish survival times.
2.3.2 Non-human primate safety study
See Table 2. The safety and immunogenicity of the ΔleuD ΔpanCD strain was assessed in Rhesus macaques. Immunologically normal animals (n = 4) were compared to animals inoculated intravenously with SIVmac251 (25 ng p27, n = 4). Immunization was performed 2 weeks after SIV infection to coincide with the period of peak viremia [20] and a time of reduced CD4 T-cell numbers. 6–7 × 105 CFU ΔleuD ΔpanCD was administered intradermally, over 5 sites on shaved abdomens. Animals were anesthetized for immunization, and therefore, for pragmatic reasons, they were immunized in 2 groups over 2 consecutive days. SIV-infected and non-infected animals were spread evenly between the 2 immunization groups.
Table 2.
Non-human primate safety study design
| Procedure | Week | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| −5 | −3 | −2 | 0 | 2 | 4 | 5 | 9 | 12 | 16 | 18 | 26 | 41 | 58 | |
| SIV infectiona | X | |||||||||||||
| Immunizationb | X | |||||||||||||
| Phlebotomyc | X | X | X | X | X | X | X | X | X | X | X | X | ||
| DTHd | X | X | X | X | X | |||||||||
| Bacteriological culturee | X | X | X | X | X | X | X | |||||||
| Weigh | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Clinical monitoring | X | X | X | X | X | X | X | X | X | X | X | X | X | |
| Terminate experimentf | X | |||||||||||||
One group (n=4) of macaques were infected with 25 ng p27 SIVMac251, the remainder (n=4) were not infected
All macaques were immunized with 6–7 × 105 CFU ΔleuD ΔpanCD
Blood was drawn under anesthesia for bacteriological culture, ELISPOT assays, viral load determination, hematology and blood chemistry assays
DTH skin testing was performed by implantation of MOT in palpebral area, followed by scoring of reaction at 48 hours
Peripheral blood was assessed for the presence of mycobacteria by serial dilution plating, acid-fast staining and BACTEC culture.
Surviving SIV-infected macaques were euthanized at 58 weeks post-immunization..
All animals were bled before SIV inoculation and at multiple time points during the experimental period. Blood was processed for complete blood counts, serum chemistries, acid-fast stains, BACTEC culture, immunohistochemistry, SIV isolation, lymphocyte subset analysis, Primagam whole-blood IFN-γ assay and ELISPOT. Tuberculin skin testing was performed at time points as detailed in Table 2 (see below for details of assays).
Two SIVmac251-infected macaques were euthanized due to progressive wasting at 9 w and 52 w post-immunization infection, respectively. The remaining two SIV-infected animals remained healthy up to the end of the study, when they were euthanized (58 w post-SIV infection). Necropsy samples were taken from all SIV-infected animals at the time of euthanasia, and organ homogenates were plated to determine whether any ΔleuD ΔpanCD bacilli had persisted in these animals.
2.4 Tuberculin skin testing of Rhesus macaques
Tuberculin skin testing was performed on the palpebral area. Following anesthesia at the time of phlebotomy, intradermal skin testing was performed with 0.1 ml mammalian old tuberculin (MOT, Synbiotics Inc, San Diego, CA). The skin was cleaned with 1% alcohol and 0.1 ml of antigen was injected intradermally into the palpebral area using a 25g needle and 1.0 ml syringe. Palpebral reactions were graded at 24, 48 and 72h, using a standard scoring system (Richter et al, 1984). On this scale, 0 = no reaction; 1+ = bruise; 2+ = erythema without swelling; 3+ = various degrees of erythema with minimum swelling or slight swelling without erythema; 4+ = obvious palpebral swelling with drooping of eyelid and various degrees of erythema; 5+ = swelling and/or necrosis with eyelid closed. Scores above 3 were considered to be positive.
2.5 PBMC and plasma preparation
Rhesus macaque blood samples collected into EDTA-containing tubes were used for the isolation of cell-free plasma and peripheral blood mononuclear cells (PBMCs). Briefly, blood samples were centrifuged to separate plasma and cells. Plasma was re-centrifuged and carefully but rapidly removed from any residual cell pellet, mixed, aliquoted and stored at −80°C if not immediately further processed. Cells were reconstituted with RPMI 1640 containing 10% heat-inactivated fetal calf serum (R10), mixed carefully, and layered onto LSM Lymphocyte Separation Medium (MP Biomedicals) for density gradient separation. PBMCs were recovered after centrifugation, washed with PBS and incubated with Red Blood Cell Lysing Buffer (SIGMA) to lyse residual red blood cells. PBMCs were washed twice with R10, counted, resuspended to 6 × 106 cells/ml in R10 and stored briefly on ice until use.
2.6 ELISPOT
Rhesus macaque PBMCs producing IFN-γ in response to PPD (Mycos Research) stimulation were enumerated with IFN-γ ELISPOT assays. The assay was performed using MultiScreen Immobilon-P plates (Millipore), ELISPOT Monkey IFN-gamma alkaline phosphatase kit (Mabtech) and alkaline phosphatase conjugate substrate kit (Biorad), according to manufacturer s instructions. Briefly, either 1 × 105 or 3 × 105 cells were added in 100 μl culture medium per pre-wetted, coated and blocked well with either no antigen, 5 μg/well PPD (Mycos Research) or the positive control, 100 ng/ml LPS (SIGMA). After overnight incubation and spot development, the number of spot-forming cells in each well were evaluated in high resolution (pixel size <5μm) using an automated Elispot reader system (Carl Zeiss) with KS Elispot Software 4.8 (Zellnet consulting).
2.7 Hematology and Blood chemistry
Rhesus macaque hematological and blood chemistry assays were performed by Brigham and Women s Hospital Chemistry and Hematology laboratories (Boston, MA, USA).
2.8 Viral isolation and quantitation
Plasma simian immunodeficiency virus (SIV) RNA viral loads were measured by quantitative real time RT-PCR. Samples were prepared as previously described by Cline et al [21]. Briefly, virus was concentrated from plasma by centrifugation, then subjected to Proteinase K digestion in the presence of GuHCl and CaCl2 to release RNase-free RNA. RNA was recovered by isopropanol precipitation after treatment with GuSCN and glycogen as a carrier. Dried RNA pellets were sent to SAIC-Frederick (Science Applications International Corporation, Frederick, MD, USA) for viral load determination according to established protocols [21].
2.9 Determination of bacterial load
Bacterial loads in guinea pig and macaque tissue samples were measured by serial dilution plating onto 7H10 plates (supplemented with 10% OADC, 0.5% glycerol, 100 μg/ml cycloheximide) after homogenization of the organs in PBS-0.05% Tween-80. For guinea pig samples, plates contained 2 μg/ml of thiophene-2-carboxylic acid hydrazide (SIGMA), to prevent growth of any residual BCG-P. Non-human primate samples were decontaminated with BBL Mycoprep (Becton Dickinson) prior to plating. Peripheral blood samples from Rhesus macaques were also assessed by serial dilution plating for CFU, acid-fast staining and BACTEC culture (Focus Diagnostics, Cypress, CA, USA). When testing for the presence of ΔleuD ΔpanCD media (including BACTEC medium) were supplemented with 50 μg/ml leucine and 24 μg/ml pantothenate where appropriate.
2.10 Primagam
Non-human primate whole-blood IFN-γ production in response to PPD stimulation was measured using the Primagam kit (Prionics, Switzerland) according to the manufacturer s instructions. Briefly, whole blood samples were collected in heparinized tubes, then incubated overnight with nil antigen control, avian PPD or bovine PPD preparations provided with the kit. Plasma was collected and IFN-γ release was measured using an ELISA-based assay, with reagents provided in the kit. Readings were expressed as OD450 – Nil antigen control well, with values >0.1 considered to be positive.
2.11 Morphometric analysis
Hematoxylin and eosin-stained tissue sections were scanned and Image-Pro® Plus (MediaCybernetics) was used to measure the area of the entire magnified image as well as that of the diseased areas. This allowed quantification of lung involvement as a percentage of diseased tissue.
2.12 Statistical analysis
Statistical analyses were performed using GraphPad Prism 4 software. DTH, CFU, and morphometric analysis data was analyzed using one-way ANOVA applying Bonferroni s post-test. Survival data was analysed using Logrank test comparisons. P values <0.05 were considered significant.
3. Results
3.1 Immunogenicity and long-term protective efficacy in guinea pigs
We had earlier demonstrated that the ΔleuD ΔpanCD strain conferred short-term protective immunity against challenge with virulent M. tuberculosis in the highly sensitive guinea pig model [18]. In this study, we sought to determine whether this would translate into longer-term protective efficacy. As confirmation of the immunogenicity of the ΔleuD ΔpanCD strain, we measured cutaneous delayed-type hypersensitivity (DTH) responses in animals immunized with the double auxotroph or BCG and compared responses to that of non-immunized controls. PPD (40 TU) was planted intradermally, and the diameter of induration was measured 24 h later (Fig. 1). As seen previously, ΔleuD ΔpanCD-immunized guinea pigs mounted a significant DTH response to PPD (9.0 ± 3.7 mm). A larger DTH response was observed in BCG-immunized animals (16.2 ± 1.8 mm) likely because the animals received more BCG than ΔleuD ΔpanCD. Nonetheless, despite a lower immunization dose of ΔleuD ΔpanCD, a robust DTH response to PPD was engendered. We also assessed immune responses to ESAT-6, a key antigen recognized by human T cells [22] encoded by the esx-1 locus, which is deleted in all BCG strains [7]. As predicted, BCG-immunized and non-immunized control animals showed no detectable responses to rESAT-6. However, animals immunized with ΔleuD ΔpanCD demonstrated ESAT-6-specific DTH responses similar in magnitude to those elicited by PPD (7.8 ± 1.1 mm). The latter underscores the possibility that the ΔleuD ΔpanCD strain may produce a greater repertoire of potentially protective antigens in comparison to BCG. Importantly this also demonstrates the ability of the double auxotroph to sensitize to secreted antigens.
FIG. 1. Mycobacterial antigen-specific DTH Response in guinea pigs.
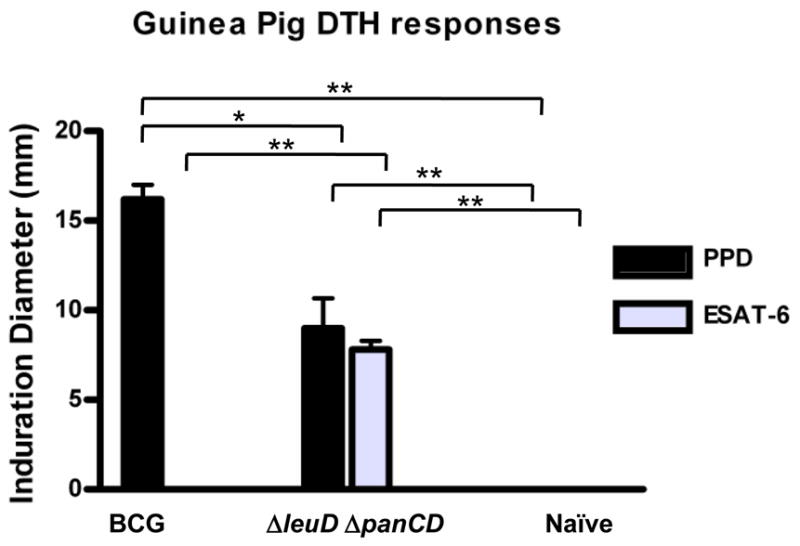
Guinea pigs were immunized with 5.3 × 106 CFU BCG-P or 3.1 × 105 CFU ΔleuD ΔpanCD or remained unimmunized. Seven weeks after immunization, DTH responses were determined by intradermal injection of 40 TU PPD or 1 ug rESAT-6, followed by measurement of the diameter of induration 24h later. Mean induration diameter (±SD) elicited by PPD injection was 16.2 ± 1.8 mm for BCG-P-immunized guinea pigs and 9.0 ± 3.7 mm for ΔleuD ΔpanCD–immunized animals. In response to rESAT-6, the mean induration in ΔleuD ΔpanCD-immunized animals was 7.8 ± 1.1 mm, whereas BCG-P immunized animals did not show any positive responses. No response to either antigen preparation was observed in naïve animals (n= 5 animals per group). *, p<0.01, and **, p<0.001, as determined by one-way ANOVA applying Bonferroni s post-test.
We compared the protective effectiveness of the Mtb auxotroph and BCG in short and long-term protection experiments following virulent Mtb infection in the sensitive guinea pig model. The ΔleuD ΔpanCD-, BCG- and non-immunized groups were all challenged by aerosol at 7 weeks post-immunization with 33 CFU virulent M. tuberculosis. To assess the short-term protective efficacy of the double auxotroph, lung bacterial organ burdens were determined by serial dilution plating of organ homogenates at 5 weeks post-challenge. ΔleuD ΔpanCD conferred protection against challenge in the short term, with modest, but statistically significant reductions in lung organ burden to levels comparable to that observed in BCG-immunized animals (Fig. S1).
Having confirmed the immunogenicity and short-term protective efficacy of the ΔleuD ΔpanCD strain, we investigated whether immunization would also confer longer-term protection. In a more stringent test of efficacy, the consequences of M. tuberculosis challenge were followed over many months to allow the manifestation of clinical signs of disease, comparing ΔleuD ΔpanCD-immunized, BCG-immunized and non-immunized animals (Fig. 2). We monitored body weight on a weekly basis and found that unimmunized guinea pigs gained less weight over time relative to immunized animals. At 3 months post challenge, mean body weight of the unimmunized guinea pigs was 642 g ± 88.5 g (mean ± SD) whereas that of the ΔleuD ΔpanC- and BCG-immunized animals was 778 g ± 54 g and 706 g ± 103 g, respectively. Mean weights of the ΔleuD ΔpanCD- and BCG- immunized groups were statistically equivalent throughout the course of infection (as assessed by t-test, 2-tailed P value <0.05). Similar weight gains were observed for the 2 groups; for example, at 32 weeks post-infection, BCG-immunized animals demonstrated a fold-increase over pre-challenge weight of 1.79 ± 0.29, compared to 1.85 ± 0.36 for the ΔleuD ΔpanCD-immunized group. Following challenge, all unimmunized animals succumbed by 300 days post-infection (Median survival time (MST) = 154 days). In contrast, the ΔleuD ΔpanCD-immunized guinea pigs showed significantly prolonged survival, statistically comparable to that of the BCG immunized group (Fig.2). When the experiment was terminated at 54 weeks post-challenge, 7/11 BCG-immunized and 5/11 ΔleuD ΔpanCD-immunized guinea pigs remained alive.
FIG. 2. Protection of guinea pigs against aerosol challenge with M. tuberculosis.
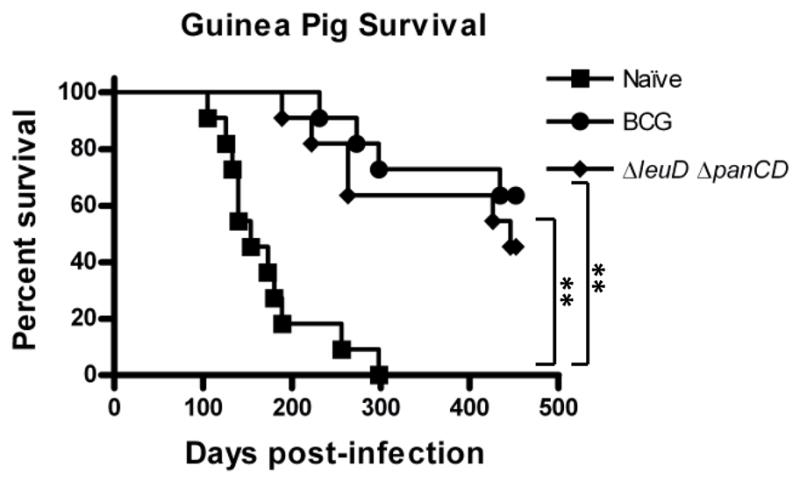
Seven weeks following immunization with BCG-P or ΔleuD ΔpanCD, immunized animals and naive controls were challenged with 33 CFU M. tuberculosis. Survival of infected guinea pigs was followed for up to 452 days post-challenge. Naive animals succumbed to challenge with a median survival time (MST) of 154 days, whereas ΔleuD ΔpanCD- and BCG-immunized guinea pigs survived significantly longer (MST 446 d and 452 d, respectively). *, p<0.0001 as determined by Logrank test comparisons of survival curves, n=11 animals per experimental group.
Both gross (data not shown) and microscopic (Table S2 and S3) analysis of experimental end-point lung and spleen sections indicated that the overall character of the lesions was similar between the BCG- and ΔleuD ΔpanCD-immunized animals. A trend towards greater severity of lesions (in terms of number and size of lesions) was observed in the BCG-immunized group, although there was large animal to animal variability in the extent of lesion involvement within groups. Morphometric analysis of lung sections revealed no significant differences in the extent of inflammation or pulmonary involvement between the immunized groups; the total percentage diseased lung was 8.82 % ± 3.78 (mean ± standard deviation) and 10.53 % ± 8.88 in BCG- and ΔleuD ΔpanCD-immunized groups, respectively.
3.2 Safety assessment of the ΔleuD ΔpanCD strain in SIV-positive and SIV-negative non-human primates
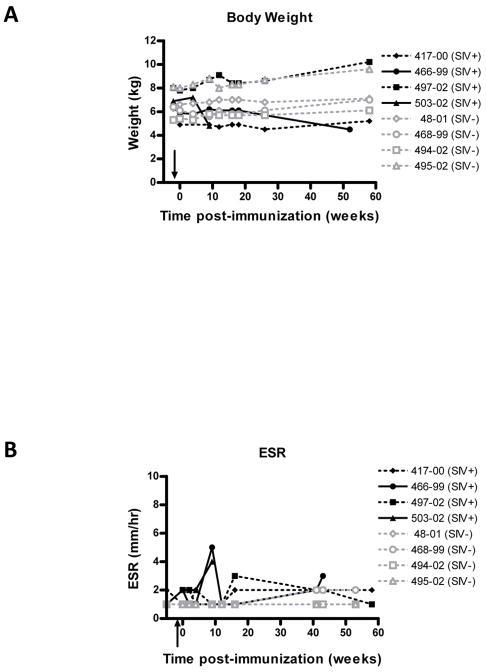
If any vaccine against tuberculosis is to be widely used, it will have to be safe in HIV-positive and immunodeficient children and adults in many countries, in circumstances where HIV testing in not routinely available. Thus we sought to rigorously assess the safety of the double auxotrophic strain in the highly susceptible Rhesus macaque. Furthermore, to model the effects of vaccination in HIV-infected human populations, we had the opportunity to immunize SIV-infected Rhesus macaques (n = 4) (Table 2). ΔleuD ΔpanCD immunization was administered at 2 weeks post-SIV infection, at which time peak viremia is expected [20, Supporting Information] and when CD4+ T-cell numbers are reduced. By administering the vaccine at 2 weeks post SIV infection, it was insured that the vaccine was delivered at time of heightened susceptibility thus providing greater stringency to assessment of its safety. For comparison, a group of SIV negative macaques were immunized with the same dose of ΔleuD ΔpanCD. We observed a small degree of erythema at the immunization sites in both SIV-negative and SIV-positive animals, however this resolved within 48 hours. We subsequently monitored a variety of clinical parameters for up to 58 weeks post-immunization (Table 2). To determine whether immunization with ΔleuD ΔpanCD had any adverse effect on SIV viral load, we monitored plasma viral RNA for 58 weeks post-immunization (Fig. 3). We also monitored body weight (Fig. 4A) and erythrocyte sedimentation rate (ESR, Fig. 4B) as non-specific clinical indicators of overall health and inflammation, respectively. For 3/4 macaques, a similar trend was observed: peak viremia was evident at 2 weeks post-SIV infection (mean viral copy equivalents 2.3 × 107), dropping between 1 and 2 logs by 4 weeks post-SIV administration, with some minor fluctuations observed around this set point in subsequent weeks. The fourth animal (Mm503-02) demonstrated a significantly higher initial (pre-immunization) viral load (1.1 × 108 viral copy equivalents at 2 weeks post-SIV infection), which subsequently remained elevated (Fig. 3). It has previously been shown that high viral setpoints associate with poor clinical prognosis [23], and consistent with this, at 9 weeks post-immunization, Mm503-02 showed a slightly elevated ESR and significant wasting was apparent (Fig. 4), and thus had to be euthanized for humane reasons. A second macaque, Mm466-99, exhibited stable viral titres, ESR and body weight for the majority of the experimental period. However, by 52 weeks post-immunization, this animal had developed intermittent diarrhea and was euthanized due to significant weight loss (Fig. 4). Wasting is one of the most common clinical features of simian AIDS and is observed in the majority of animals with disease progression [24, Supporting Information]. There was no indication that wasting in these two animals was the result of survival or replication of the attenuated M. tuberculosis strain. The short time to death of Mm503-02, is consistent with reported acute SIV disease progression in Rhesus macaques and the longer-term wasting of Mm466-99 is consistent with chronic SIV-infection [24–28, Supporting Information]. All other animals (SIV-infected and SIV-negative), maintained stable body weights and remained healthy over the experimental period (Fig. 4) and there was no evidence that immunization with the auxotrophic Mtb vaccine had any influence on viral replication in the macaques.
FIG.3. SIV RNA Viral load in SIV-infected, ΔleuD ΔpanCD-immunized Rhesus macaques.
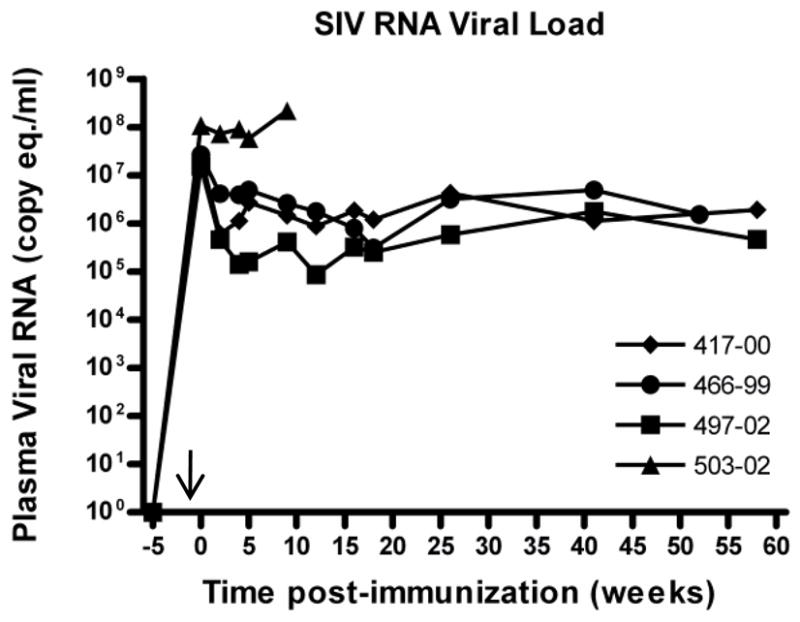
A group of 4 Rhesus macaques were infected with SIVmac251 (time indicated by arrow on X-axis), prior to immunization with ΔleuD ΔpanCD 2 weeks later (Week 0 on graph). SIV viral RNA was extracted from plasma at multiple time points following infection, and genomic copy equivalents (copy eq.) were enumerated using quantitative real-time RT-PCR. Individual macaque identification numbers are indicated on graph.
FIG. 4. Clinical evaluation of ΔleuD ΔpanCD-immunized SIV-positive and SIV-negative Rhesus macaques.
SIVmac251-positive (filled black symbols) and SIVmac251-negative (open grey symbols) macaques were immunized with ΔleuD ΔpanCD and monitored for 1 year post-immunization. (A) Body weight over time. (B) Erythrocyte sedimentation rate (ESR) over time. Arrows on X-axes indicate time of SIV administration. Solid lines highlight 2 SIV-infected macaques Mm503-02 and Mm466-99 euthanized due to excessive weight loss at 9 weeks and 52 weeks post-immunization, respectively.
Published reports indicate that M. bovis BCG immunization can result in disseminated mycobacterial infection in BCG-exposed, SIV-infected Rhesus macaques [29–32] as well as in immunocompromised human hosts [4, 8, 9]. To confirm that the auxotroph did not pose a similar risk, we investigated whether the ΔleuD ΔpanCD strain was present in blood samples drawn at multiple time points post-immunization (Table 2), as well as in a variety of tissue samples taken at necropsy. Samples were analyzed using BACTEC and plate culture assays (with appropriate supplementation of media with leucine and pantothenate), as well as by AFB smear tests, and no acid-fast bacilli were cultured or observed at any time point.
Several indicators of overall clinical condition and of systemic inflammation were monitored as an indirect assessment of whether there were any adverse effects attributable to immunization. A slight decrease in hemoglobin and hematocrit levels was observed in the SIV-infected cohort following SIV-inoculation prior to immunization, reflective of slight anemia in these animals (data not shown). However, these values recovered to pre-SIV levels by week 12 post-immunization, and remained virtually stable for the remainder of the time points measured. Other measures of systemic inflammation and overall clinical condition (e.g. white blood cell count, platelet counts) remained relatively stable throughout the course of the experiment (data not shown). The one distinct outlier was macaque Mm503-02. In this animal, at the 9 week time point, liver enzymes (lactate dehydrogenase and aspartate amino transferase) were elevated, as were bilirubin and urea levels, along with decreased sodium and chloride levels cumulatively indicative of impaired liver and renal function (data not shown). These observations were consistent with the poor clinical status of this animal, which had exhibited high initial viral titres and significant wasting by week 9. Apart from this, there was little overall difference before and after immunization or between the 2 groups, indicating that the ΔleuD ΔpanCD did not provoke systemic toxicity or clinical deterioration in either group.
3.3 Immune responses of SIV-positive and SIV-negative non-human primates to ΔleuD ΔpanCD
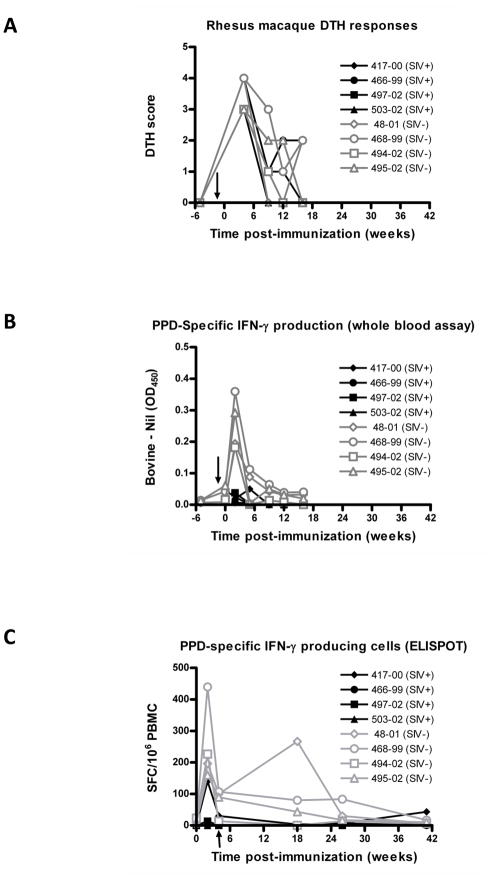
It is well known that protection against M. tuberculosis challenge is difficult and not consistently achieved in Rhesus macaques [19, 33, 34]. Consequently we assessed whether the highly attenuated ΔleuD ΔpanCD strain could elicit a mycobacterial antigen-specific cell-mediated immune response in these animals, by using 3 different approaches. First, we measured palpebral DTH responses to mammalian old tuberculin (MOT). At 4 weeks post-immunization, positive responses to MOT (DTH score ≥ 3; Fig. 5A) were elicited in all eight macaques, both SIV-negative and SIV-positive animals. We also used a commercial whole blood IFN-γ production assay (Primagam) to evaluate responses to Bovine PPD (Fig. 5B). In this assay, OD450 - Nil values above 0.1 are considered indicative of sensitization to mycobacterial antigens. Notably, for all 4 SIV-negative animals, stimulation of whole peripheral blood with M. bovis PPD elicited measureable IFN-γ production. No positive Primagam responses were observed in any SIV-infected macaques, generally confirming their immunodeficient state. Finally, we enumerated the number of IFN-γ producing PBMCs following PPD stimulation using an ELISPOT assay (Fig. 5C). With this method, we observed IFN-γ production above background levels for all 4 SIV-negative animals. Interestingly, positive responses were also observed for one SIV-positive macaque (Mm417-00) at 2, 4 and even 41 weeks post-immunization. In addition to these assays, we also assessed several other parameters, including the measurement of TNF-α, IL-12, IL-10 and IL-4 by multiplex cytokine analysis and quantification of cells positive for CD3, CD4, CD8, CCR5 and CXCR3 surface markers by surface staining and flow cytometry (data not shown). However, these did not reveal any significant differences between the 2 groups.
FIG. 5. Mycobacterial antigen-specific immune responses in ΔleuD ΔpanCD-immunized SIV-positive and SIV-negative Rhesus macaques.
Immune responses to mycobacterial antigens were measured in SIVmac251-positive (filled black symbols) and SIVmac251-negative (open grey symbols) immunized with ΔleuD ΔpanCD. (A) Delayed type hypersensitivity (DTH) responses were scored at 24 h following injection of Old Mammalian Tuberculin (MOT) into the eyelid. (B) IFN-γ production in response to stimulation of whole blood with bovine PPD was measured using the Primagam ELISA- based kit. Y-axis values are expressed as OD450 readings for Bovine-PPD stimulated vs nil antigen control stimulated wells, with values above 0.1 considered to be positive. (C) Numbers of IFN-γ-producing cells in response to stimulation of PBMCs with PPD were measured using the ELISPOT. Arrows on X-axes indicate time of SIV administration.
4. Discussion
The introduction of a safe and effective new vaccine into TB control has the potential to restrict the TB epidemic, with eventual elimination of TB as a public health threat as the ultimate goal [35]. However, for this to become a reality, extensive pre-clinical testing of any new vaccine candidate is an essential step. Several new live attenuated vaccine candidates based on M. tuberculosis are currently undergoing preclinical development [12]. These include candidates with attenuating mutations in essential biosynthetic pathways [17, 18], as well as in virulence genes [17, 36–38]. We previously reported on the development and pre-clinical testing of a recombinant auxotrophic strain of M. tuberculosis deleted for leuD and panCD, unable to make an essential amino acid and vitamin [18]. In accordance with recommendations in the first and second Geneva consensus documents on live mycobacterial vaccine development [39, 40], we demonstrated that these mutations were non-reverting and that the recombinant strain was fully sensitive to all frontline anti-tuberculosis drugs [18]. As further recommended, we demonstrated that the ΔleuD ΔpanCD auxotroph is fully attenuated even in severely immunodeficient SCID mice and its protective effectiveness was equivalent to BCG in short term experiments in mice (unpublished data) and guinea pigs [18]. In the present study, we provide additional efficacy data, and demonstrate, for the first time, long-term protective efficacy of the auxotrophic Mtb vaccine in the sensitive guinea pig aerosol challenge model. In addition, we provide further evidence supporting the safety of this auxotrophic strain in non-human primates. Most critically, we find that immunization of SIV-infected Rhesus macaques with the ΔleuD ΔpanCD Mtb vaccine results in no vaccine-attributable adverse effects.
Part of the underlying rationale for the development of live attenuated vaccines based on M. tuberculosis is that these will express a broader antigenic repertoire and thereby elicit a qualitatively superior immune response against Mtb than BCG. BCG vaccines lack more than a hundred genes that are present in the M. tuberculosis genome [7]. These include several critical antigens, as well as genes encoding the ESX-1 secretion system, suggesting that the immune response elicited by BCG will be restricted in comparison to M. tuberculosis-induced immunity. In the present study, we show that immunization with ΔleuD ΔpanCD elicits responses to the ESX-1 secreted antigen ESAT-6, a major Mtb antigen recognized by human T cells [22], which is not produced by BCG [7]. As expected, BCG-immunized guinea pigs produced negative DTH responses to ESAT-6. Therefore, despite the fact that the ΔleuD ΔpanCD strain does not replicate and is ultimately cleared in vivo, it nonetheless is able to stimulate the expansion of T cell populations responding to the secreted ESAT-6-antigen as well as to cell-associated antigens in PPD. Admittedly, our investigation focused on a single secreted antigen, nonetheless, these data support the notion that the ΔleuD ΔpanCD strain could elicit a broader immune response than BCG.
Consistent with previous findings [18] immunization with ΔleuD ΔpanCD resulted in a modest, but statistically significant reduction in lung bacterial burden post M. tuberculosis aerosol challenge in guinea pigs comparable to the protection afforded by BCG immunization. Herein we extended previous work, by assessing long-term survival post-challenge in guinea pigs, demonstrating that the ΔleuD ΔpanCD performed as well as BCG, conferring statistically significant protection for as long as 1 year.
Non-human primates most closely resemble humans in the range and nature of their physiological response to infection with M. tuberculosis, and therefore represent a valuable model for pre-clinical testing of TB vaccine candidates. In accordance with recommendations for the development of live mycobacterial vaccines [39], we went on to evaluate the safety of the ΔleuD ΔpanCD strain in a non-human primate model, the Rhesus macaque, which is naturally susceptible to M. tuberculosis infection to an even greater extent than the more commonly used Cynomologous macaques [34]. Since protection is very difficult to achieve in the Rhesus macaque model, the sole purpose of these experiments was to examine the safety of the auxotrophic Mtb vaccine in primates. Immunocompetent Rhesus macaques were immunized with ΔleuD ΔpanCD, and a range of clinical parameters were monitored for over 1 year following immunization. Previous reports have shown that Rhesus macaques infected via aerosol with a low dose (40–60 CFU) of M. tuberculosis succumb to disease with a mean survival time of approximately 3 months [33]. However, clinical observation of the animals in our study revealed no signs of adverse effects, and weight profiles remained stable or increased. Erythrocyte sedimentation rate, (a parameter which correlates with mycobacterial disease progression in non-human primates [41]), remained stable following immunization. Repeated blood cultures were negative for mycobacterial growth. Together, these findings strongly support the safety profile of the ΔleuD ΔpanCD strain.
An important characteristic of the non-human primate is that the animals can be infected with SIV to produce a model of human HIV/AIDS. The opportunity was presented to use these animals to model the safety of auxotrophic Mtb vaccination in HIV-infected populations. Because of the potential for disseminated disease development, BCG immunization is contra-indicated in HIV-positive individuals, a population with a very high risk of developing active TB [2], and therefore most in need of an effective new vaccine. Several new TB vaccine candidates have been evaluated in non-human primates [17, 19, 33, 42–45], but to our knowledge, none have previously exploited SIV co-infection as an additional measure of safety. To minimize the number of macaques used for humane and financial reasons, these experiments did not include an SIV-infected, non-vaccinated cohort, but instead relied on historical data for comparison [20, 27, Supporting Information]. Previous studies have linked BCG immunization with increased SIV replication [30, 31, 46], which could potentially have a detrimental effect on clinical status. Importantly, we observed no immunization-associated viral replication burst. One of the 4 SIV-infected animals presented with a viral titer approximately 10-fold higher than the other members of this group, and maintained a higher viral set point. This was not related to the immunization with ΔleuD ΔpanCD, as the initial viral titer was measured on samples drawn prior to administration of the auxotrophic vaccine. Previous reports have demonstrated that rapid early viral replication correlates with a high viral setpoint, which in turn correlates with a poor clinical prognosis [23]. In accordance with the latter, this animal had a downhill course, consistent with a rapid progressor phenotype [25, Supporting Information]. A full necropsy revealed no signs of mycobacterial disease, and no acid-fast bacteria were detected by culture or staining of any of the tissues tested. Such was also the case for a second SIV-infected, ΔleuD ΔpanCD-immunized animal that was euthanized prior to the end of the study. This animal exhibited an approximately 10-fold lower viral set point and was clinically healthy for almost a year following immunization, but ultimately developed SIV disease and was euthanized at 52 weeks for humane reasons. Once again, no signs of mycobacterial disease were detected, and the survival time for this animal was within the normal range of what has been observed by ourselves and others for SIV-infected Rhesus macaques [25–27, Supporting Information]. The 2 remaining SIV-infected animals remained clinically healthy for the duration of the study. Survival and clinical course of the cohort following vaccination was typical of what is historically observed with experimental SIVmac infection in Indian-origin rhesus macaques [24, 28, Supporting Information]. This indicates that the clinical decline of the SIV infected animals was unrelated to the immunization, and certainly not due to disseminated mycobacterial infection (as has been reported for BCG/SIV-co-infected macaques) [29–32].
A second goal of the primate study was to establish whether immunization with ΔleuD ΔpanCD elicited M. tuberculosis-specific immunity in this model system. We monitored three different measures of immunogenicity, all of which demonstrated that immunization with the double auxotroph elicited mycobacteria-specific immune responses. Importantly, this finding extended to the SIV-co-infected group, with all animals in this group demonstrating positive DTH responses following immunization. Mansoor et al recently showed that HIV-1 infection severely restricted the immunogenicity of BCG in an infant population [3], highlighting the need for new TB vaccine candidates that are safe as well as immunogenic in this vulnerable population. Our findings support the further exploration of ΔleuD ΔpanCD as a starting point for a viable new TB vaccine candidate in immune-compromised populations.
In summary, the present results demonstrate that the ΔleuD ΔpanCD auxotrophic Mtb strain engenders protective efficacy equivalent to BCG in a long-term guinea pig protection model. The double auxotroph was found to be safe when administered to both immune-competent and SIV-positive Rhesus macaques and engendered mycobacteria-specific immune responses in both groups that persisted for 1 year after immunization. We believe these findings further support development of ΔleuD ΔpanCD as a credible TB vaccine candidate. Additional preclinical development will require the manufacture and safety and toxicology testing of GMP (good manufacturing practice) seed and clinical lots before moving onto Phase I studies [40]. We envision that the ΔleuD ΔpanCD strain could potentially be used to safely replace BCG in a prime-boost strategy, with one of the new subunit antigen-plus-adjuvant candidates being considered as a booster. This approach could be employed in conjunction with alternative formulations for ΔleuD ΔpanCD, such as dry-powder preparations deliverable via aerosol which may improve both stability and immunogenicity as it has done with BCG immunization of mice and guinea pigs [47, 48]. A comparable formulation and delivery of ΔleuD ΔpanCD might similarly enhance protection without imposing the inherent risks associated with BCG vaccination.
Supplementary Material
Acknowledgments
We thank Sara Blakesley, Ilona Breiterene, Meredith Tatum and Kimberly Goldbach for technical assistance and Mike Piatak for advice and technical help with viral load determination. This study was supported by the Aeras Global TB Vaccine Foundation and the National Institute of Health (AI023545).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dye C, Williams BG. The Population Dynamics and Control of Tuberculosis. Science. 2010;328(5980):856–61. doi: 10.1126/science.1185449. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Tuberculosis Control: A Short Update To The 2009 Report. World Health Organization; Geneva: 2009. [Google Scholar]
- 3.Mansoor N, Scriba Thomas J, de Kock M, Tameris M, Abel B, Keyser A, et al. HIV-1 Infection in Infants Severely Impairs the Immune Response Induced by Bacille Calmette Guérin Vaccine. The Journal of Infectious Diseases. 2009;199(7):982–90. doi: 10.1086/597304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hesseling AC, Johnson LF, Jaspan H, Cotton MF, Whitelaw A, Schaaf HS, et al. Disseminated bacille Calmette-Guerin disease in HIV-infected South African infants. Bulletin of the World Health Organization. 2009;87(7):505–11. doi: 10.2471/BLT.08.055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trunz BB, Fine P, Dye C. Effect of BCG vaccination on childhood tuberculous meningitis and miliary tuberculosis worldwide: a meta-analysis and assessment of cost-effectiveness. Lancet. 2006 Apr 8;367(9517):1173–80. doi: 10.1016/S0140-6736(06)68507-3. [DOI] [PubMed] [Google Scholar]
- 6.Baily GV. Tuberculosis prevention Trial, Madras. Indian J Med Res. 1980;72(Suppl):1–74. [PubMed] [Google Scholar]
- 7.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, Rane S, et al. Comparative Genomics of BCG Vaccines by Whole-Genome DNA Microarray. Science. 1999;284:1520–3. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 8.Sirisanthana V. Complication of Bacillus Calmette-Guerin (BCG) Vaccine in HIV-infected Children. Journal of Infectious Disease and Antimicrobial Agents. 1995;2(2):63–7. [Google Scholar]
- 9.Rezai MS, Khotaei G, Mamishi S, Kheirkhah M, Parvaneh N. Disseminated Bacillus Calmette-Guerin Infection after BCG Vaccination. Journal of Tropical Pediatrics. 2008;54(6):413–6. doi: 10.1093/tropej/fmn053. [DOI] [PubMed] [Google Scholar]
- 10.Global Advisory Committee on Vaccine Safety. Wkly Epidemiol Rec. Vol. 82. 2007. Revised BCG vaccination guidelines for infants at risk for HIV infection; pp. 181–96. [PubMed] [Google Scholar]
- 11.World Health Organization, WHO. Recommendations on the Diagnosis of HIV infections in Adults and Children. World Health Organization; 2010. [Google Scholar]
- 12.Kaufmann SH. Future vaccination strategies against tuberculosis: thinking outside the box. Immunity. 2010 Oct 29;33(4):567–77. doi: 10.1016/j.immuni.2010.09.015. [DOI] [PubMed] [Google Scholar]
- 13.Kaufmann SHE, Hussey G, Lambert P-H. New vaccines for tuberculosis. Lancet. 2010;375:2110–9. doi: 10.1016/S0140-6736(10)60393-5. [DOI] [PubMed] [Google Scholar]
- 14.Grode L, Seiler P, Baumann S, Hess J, Brinkmann V, Nasser Eddine A, et al. Increased vaccine efficacy against tuberculosis of recombinant Mycobacterium bovis bacille Calmette-Guerin mutants that secrete listeriolysin. Journal of Clinical Investigation. 2005;115(9):2472–9. doi: 10.1172/JCI24617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoft DF, Blazevic A, Abate G, Hanekom WA, Kaplan G, Soler JH, et al. A New Recombinant Bacille Calmette Guérin Vaccine Safely Induces Significantly Enhanced Tuberculosis-Specific Immunity in Human Volunteers. The Journal of Infectious Diseases. 2008;198(10):1491–501. doi: 10.1086/592450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stop TB Partnership Working Group on New TB Vaccines. 2009 http://www.stoptb.org/wg/new_vaccines/documents.asp.
- 17.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, et al. Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27(34):4709–17. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sampson SL, Dascher CC, Sambandamurthy VK, Russell RG, Jacobs WR, Bloom BR, et al. Protection Elicited by a Double Leucine and Pantothenate Auxotroph of Mycobacterium tuberculosis in Guinea Pigs. Infection and Immunity. 2004;72(5):3031–7. doi: 10.1128/IAI.72.5.3031-3037.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verreck FAW, Vervenne RAW, Kondova I, Kralingen KWv, Remarque EJ, Braskamp G, et al. MVA.85A Boosting of BCG and an Attenuated, phoP Deficient M. tuberculosis Vaccine Both Show Protective Efficacy Against Tuberculosis in Rhesus Macaques. PLoS One. 2009;4(4):e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mansfield KG, Veazey RS, Hancock A, Carville A, Elliott M, Lin K-C, et al. Induction of Disseminated Mycobacterium avium in Simian AIDS Is Dependent upon Simian Immunodeficiency Virus Strain and Defective Granuloma Formation. American Journal of Pathology. 2001;159(2):693–702. doi: 10.1016/S0002-9440(10)61740-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cline A, Bess J, MP, Lifson J. Highly sensitive SIV plasma viral load assay: practical considerations, realistic performance expectations, and application to reverse engineering of vaccines for AIDS. J Med Primatol. 2005;34:303–12. doi: 10.1111/j.1600-0684.2005.00128.x. [DOI] [PubMed] [Google Scholar]
- 22.Ravn P, Demissie A, Eguale T, Wondwosson H, Lein D, Amoudy HA, et al. Human T cell responses to the ESAT-6 antigen from Mycobacterium tuberculosis. J Infect Dis. 1999 Mar;179(3):637–45. doi: 10.1086/314640. [DOI] [PubMed] [Google Scholar]
- 23.Lifson J, Nowak M, Goldstein S, Rossio J, Kinter A, Vasquez G, et al. The Extent of Early Viral Replication Is a Critical Determinant of the Natural History of Simian Immunodeficiency Virus Infection. Journal of Virology. 1997;71(12):9508–14. doi: 10.1128/jvi.71.12.9508-9514.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freeman LM, Mansfield KG, Goldin B, Woods M, Gualtieri L, Li W, et al. Body-composition changes in the simian immunodeficiency virus-infected juvenile rhesus macaque. J Infect Dis. 2004 Jun 1;189(11):2010–5. doi: 10.1086/386290. [DOI] [PubMed] [Google Scholar]
- 25.Brown CR, Czapiga M, Kabat J, Dang Q, Ourmanov I, Nishimura Y, et al. Unique Pathology in Simian Immunodeficiency Virus-Infected Rapid Progressor Macaques Is Consistent with a Pathogenesis Distinct from That of Classical AIDS. Journal of Virology. 2007;81(11):5594–606. doi: 10.1128/JVI.00202-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Letvin N, Mascola J, Sun Y, Gorgone D, Buzby A, Xu L, et al. Preserved CD4+ Central Memory T Cells and Survival in Vaccinated SIV-Challenged Monkeys. Science. 2006;9(312):5779–1533. doi: 10.1126/science.1124226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu J, O’Brien KL, Lynch DM, Simmons NL, La Porte A, Riggs AM, et al. Immune control of an SIV challenge by a T-cell-based vaccine in rhesus monkeys. Nature. 2008;457(7225):87–91. doi: 10.1038/nature07469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Freeman LM, Mansfield KG, Lackner AA, Naumova EN, Gorbach SL. Survival and failure to thrive in the SIV-infected juvenile rhesus monkey. J Acquir Immune Defic Syndr. 1999 Oct 1;22(2):119–23. doi: 10.1097/00126334-199910010-00002. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZW, Shen Y, Zhou D, Simon M, Kou Z, Lee-Parritz D, et al. In vivo T-lymphocyte activation and transient reduction of viral replication in macaques infected with simian immunodeficiency virus. J Virol. 2001 May;75(10):4713–20. doi: 10.1128/JVI.75.10.4713-4720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Croix D, Capuano S, Simpson L, Falert B, Fuller C, Klein E, et al. Effect of Mycobacterial Infection on Virus Loads and Disease Progression in Simian Immunodeficiency Virus-Infected Rhesus Monkeys. AIDS Research and Human Retroviruses. 2000;16(17):1895–908. doi: 10.1089/08892220050195856. [DOI] [PubMed] [Google Scholar]
- 31.Zhou D, Shen Y, Chalifoux L, Lee-Parritz D, Simon M, Sehgal P, et al. Mycobacterium bovis Bacille Calmette-Guerin Enhances Pathogenicity of Simian Immunodeficiency Virus Infection and Accelerates Progression to AIDS in Macaques: A Role of Persistent T cell Activation in AIDS Pathogenesis. Journal of Immunology. 1999;162:2204–16. [PubMed] [Google Scholar]
- 32.Shen Y, Shen L, Sehgal P, Zhou D, Simon M, Miller M, et al. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in Macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J Virol. 2001 Sep;75(18):8690–6. doi: 10.1128/JVI.75.18.8690-8696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, et al. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques, and an evaluation of endpoints for vaccine testing. Clinical and Vaccine Immunology. 2010;17(8):1170–82. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Langermans JAM, Andersen P, van Soolingen D, Vervenne RA, Frost PA, van der Laan T, et al. Divergent effect of bacillus Calmette-Guerin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: Implications for primate models in tuberculosis vaccine research. Proceedings of the National Academy of Sciences. 2001;98(20):11497–502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abu-Raddad LJ, Sabatelli L, Achterberg JT, Sugimoto JD, Longini IM, Dye C, et al. Epidemiological benefits of more-effective tuberculosis vaccines, drugs, and diagnostics. Proceedings of the National Academy of Sciences. 2009;106(33):13980–5. doi: 10.1073/pnas.0901720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cardona PJ, Asensio JG, Arbues A, Otal I, Lafoz C, Gil O, et al. Extended safety studies of the attenuated live tuberculosis vaccine SO2 based on phoP mutant. Vaccine. 2009 Apr 21;27(18):2499–505. doi: 10.1016/j.vaccine.2009.02.060. [DOI] [PubMed] [Google Scholar]
- 37.Martin C, Williams A, Hernandez-Pando R, Cardona PJ, Gormley E, Bordat Y, et al. The live Mycobacterium tuberculosis phoP mutant strain is more attenuated than BCG and confers protective immunity against tuberculosis in mice and guinea pigs. Vaccine. 2006 Apr 24;24(17):3408–19. doi: 10.1016/j.vaccine.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Sambandamurthy VK, Derrick SC, Hsu T, Chen B, Larsen MH, Jalapathy KV, et al. Mycobacterium tuberculosis DeltaRD1 DeltapanCD: a safe and limited replicating mutant strain that protects immunocompetent and immunocompromised mice against experimental tuberculosis. Vaccine. 2006 Sep 11;24(37–39):6309–20. doi: 10.1016/j.vaccine.2006.05.097. [DOI] [PubMed] [Google Scholar]
- 39.Kamath A, Fruth U, Brennan M, Dobbelaer R, Hubrechts P, Ho M, et al. New live mycobacterial vaccines: the Geneva consensus on essential steps towards clinical development. Vaccine. 2005;23(29):3753–61. doi: 10.1016/j.vaccine.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 40.Walker KB, Brennan MJ, Ho MM, Eskola J, Thiry G, Sadoff J, et al. The second Geneva Consensus: Recommendations for novel live TB vaccines☆. Vaccine. 2010;28(11):2259–70. doi: 10.1016/j.vaccine.2009.12.083. [DOI] [PubMed] [Google Scholar]
- 41.Capuano SV, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, et al. Experimental Mycobacterium tuberculosis Infection of Cynomolgus Macaques Closely Resembles the Various Manifestations of Human M. tuberculosis. Infection Infection and Immunity. 2003;71(10):5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kita Y, Tanaka T, Yoshida S, Ohara N, Kaneda Y, Kuwayama S, et al. Novel recombinant BCG and DNA-vaccination against tuberculosis in a cynomolgus monkey model. Vaccine. 2005;23(17–18):2132–5. doi: 10.1016/j.vaccine.2005.01.057. [DOI] [PubMed] [Google Scholar]
- 43.Langermans J, Doherty T, Vervenne R, Laan T, Lyashchenko K, Greenwald R, et al. Protection of macaques against infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23(21):2740–50. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 44.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, et al. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proceedings of the National Academy of Sciences. 2009;106(7):2301–6. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sugawara I, Sun L, Mizuno S, Taniyama T. Protective efficacy of recombinant BCG Tokyo (Ag85A) in rhesus monkeys (Macaca mulatta) infected intratracheally with H37Rv Mycobacterium tuberculosis. Tuberculosis. 2009;89(1):62–7. doi: 10.1016/j.tube.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 46.Cheynier R, Gratton S, Halloran M, Stahmer I, Letvin N, Wain-Hobson S. Antigenic stimulation by BCG vaccine as an in vivo driving force for SIV replication and dissemination. Nature Medicine. 1998;4(4):421–7. doi: 10.1038/nm0498-421. [DOI] [PubMed] [Google Scholar]
- 47.Garcia-Contreras L, Wong YL, Muttil P, Padilla D, Sadoff J, DeRousse J, et al. Immunization by a bacterial aerosol. Proceedings of the National Academy of Sciences. 2008;105(12):4656–60. doi: 10.1073/pnas.0800043105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wong YL, Sampson S, Germishuizen WA, Goonesekera S, Caponetti G, Sadoff J, et al. Drying a tuberculosis vaccine without freezing. Proc Natl Acad Sci U S A. 2007 Feb 20;104(8):2591–5. doi: 10.1073/pnas.0611430104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.