Abstract
Histone deacetylase inhibitors (HDACi), including trichostatin A (TSA) and valproic acid, can alter the acetylation of histones in chromatin and enhance gene transcription. Previously we demonstrated that HDACi-treated tumor cells are capable of presenting antigen via the MHC class II pathway. In this study, we show that treatment with HDACi enhances the expression of molecules (TAP1, TAP2, LMP2, LMP7, Tapasin and MHC class I) involved in antigen processing and presentation via the MHC class I pathway in melanoma cells. HDACi treatment of B16F10 cells also enhanced cell surface expression of class I and costimulatory molecules CD40 and CD86. Enhanced transcription of these genes is associated with a significant increase in direct presentation of whole protein antigen and MHC class I-restricted peptides by TSA-treated B16F10 cells. Our data indicate that epigenetic modification can convert a tumor cell to an antigen presenting cell capable of activating IFN-γ secreting T cells via the class I pathway. These findings suggest that the abnormalities, observed in some tumors in the expression of MHC class I antigen processing and presentation molecules, may result from epigenetic repression.
Electronic supplementary material
The online version of this article (doi:10.1007/s00262-007-0402-4) contains supplementary material, which is available to authorized users.
Keywords: Epigenetic regulation, Histone deacetylase inhibitor, Antigen presentation, MHC class I, Melanoma
Introduction
Defects in MHC class I surface expression on tumor cells may lead to the inability of the cytotoxic T cell (CTL) to directly destroy tumor cells. Loss or dysfunction of molecules involved in antigen processing and presentation (such as, TAP1, TAP2, LMP2, LMP7, Tapasin) via the class I pathway contributes to deficient class I expression in several tumor types [29, 37]. Mutations of several genes in the class I pathway have been shown to be responsible for these defects in certain tumor cells. Treatment with IFN-γ and TNF-α [12, 32] or transfection of specific genes including TAP1 and TAP2 [36] can restore MHC class I expression in certain tumor cells treated in vitro with these agents. In addition, restoration of TAP activity by transfection of tumor cells enhances class I mediated antigen presentation and induces susceptibility to CTL killing, both in vitro and in vivo [1, 23]. In contrast, IFN-γ and TNF-α treatment failed to elicit class I expression in some class I deficient tumors which are associated with a defective β2m gene [5] or DNA hypermethylation [40]. However, mutation of β2m is not a common mechanism of class I deficiency in tumors [9, 15]. In addition to mutations, epigenetic silencing of genes is potentially important in cancer [3, 7] and has recently been suggested to be etiologically related to immune gene and tumor associated antigen repression that facilitate tumor escape [41]. Several covalent modifications of histone proteins (acetylation, methylation, phosphorylation, ubiquitination and ADP ribosylation) are responsible for a “histone code” that epigenetically regulates chromatin and gene expression [14]. Deacetylation of histone has been found to contribute to repression of MHC class I and costimulatory genes in various mouse and human tumor cells [19, 25, 26]. As mentioned above, DNA methylation has also been identified as a mechanism of both class I and tumor associated antigen repression in several tumor types [10, 24, 40]. The above considerations raise the possibility that individual class I pathway components responsible for the generation and transport of peptide–class I complexes might also be epigenetically repressed in tumor cells. Thus, the reversal of epigenetically repressed class I pathway components and costimulatory molecules in tumor cells could potentially be an effective means of activating anti-tumor CTL responses.
Histone deacetylase inhibitors (HDACi), a new generation of chemical agents being used to develop therapy against cancer, can alter ∼5% of the genome depending on the cell type and the HDACi analyzed [16]. Treatment with HDACi, such as trichostatin A (TSA), promotes acetylation of histones by inhibiting HDACs and is generally associated with enhanced transcription. TSA has been reported to enhance expression of MHC class I, CD40 and B7-1/2 genes in several tumor cell lines, including adenocarcinoma, neuroblastoma and melanoma, [17, 18, 26]. Another HDACi valproic acid (VA), a well-known anti-epileptic drug, can also induce expression of several immune genes including class I and NKG2D ligands on tumor cells, and although generally less effective than TSA, treatment of tumor cells with VA enhances cell lysis by CTLs in vitro [2, 28]. Furthermore, it has been shown that TSA treatment can convert tumor cells to antigen presenting cells (APC) capable of presenting whole protein antigen via the class II pathway in vitro [6]. However, the activation of specific components of the class I antigen processing and presentation pathway in tumor cells by HDACi has not been previously defined.
Here, we show that the expression of the MHC class I antigen processing pathway components, which include class I, TAP1, TAP2, LMP2, LMP7 and Tapasin, in tumor cells can be upregulated by TSA. Treatment with TSA and VA can also enhance surface expression of class I and the costimulatory molecules, CD40 and B7-2, in the B16F10 melanoma cells. Additionally, tumor cells treated with TSA can present protein antigens via the class I pathway and stimulate antigen specific T cells that are capable of producing IFN-γ.
Materials and methods
Cells, mice and reagents
Mouse melanoma B16F0 and B16F10 (ATCC, Manassas, VA) as well as the adenocarcinoma Colon 26 [generously provided by Elizabeth A. Repasky, Roswell Park Cancer Institute (RPCI), Buffalo, NY] were maintained in Dulbecco’s modified Eagle medium (Invitrogen, Grand Island, NY) supplemented with 10% fetal bovine serum and penicillin/streptomycin. C57BL/6-Tg(OT-I)-RAG1tm1Mom [OT-I] (Taconic, Germantown, NY) mice were maintained in the Department of Laboratory Animal Resources at RPCI and 6–8 weeks-old mice were used for all experiments. Principles of laboratory animal care (NIH publication No. 85-23, revised 1986) were followed and all work was carried out under RPCI IACUC approval. TSA (Wako Biochemical, Richmond, VA) and VA (Sigma, St Louis, MO) were diluted in ethanol and water, respectively. Ovalbumin (ova) (Grade V, Sigma), MHC class I specific ova–peptide257–264 and mouse melanoma mgp10025–33 peptide (Invitrogen, Grand Island, NY) were diluted in water. Recombinant mouse IFN-γ (R&D Systems, Minneapolis, MN) was diluted in phosphate-buffered saline.
RT and quantitative real-time PCR
Total RNA was prepared from cells using an RNeasy kit (Qiagen, Valencia, CA), RNasin and RQ1 DNase I (Promega, Madison, WI) and two μg of RNA was used to synthesize cDNA using Superscript II™ RNase H− reverse transcriptase (Invitrogen, Carlsbad, CA). Amplification of cDNA samples was performed either with Taq DNA Polymerase (Invitrogen) or SYBR Green Master Mix (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocol. Different sets of primers for mouse MHC class I (H-2D), TAP1, TAP2, LMP2, LMP7, β2m and Tapasin (for sequences see Supplementary Table 1) were used for standard RT-PCR and quantitative real-time PCR. Both PCR techniques were carried out as previously described [20, 26]. SYBR Green quantitation was performed on an ABI7900HT (Applied Biosystems) cycling machine and data was analyzed using the ΔΔCT method [26].
Flow cytometry
Flow cytometric analysis was conducted by published methods on a FACScan (Becton Dickinson, San Jose, CA) as previously described [26]. R-phycoerythrin conjugated anti-mouse H-2Db, CD40, CD80, CD86 mAb and isotype controls matched to each antibody (Pharmingen, San Diego, CA) were used in these experiments. Forward scatter versus side scatter gating was set to include all non-aggregated cells.
Antigen presentation assays
Popliteal lymph node T cells were isolated from groups of OT-I mice 7 days after priming by footpad injection of ova in complete Freund’s adjuvant (DIFCO, Detroit, MI) and purified magnetically with microbead-labeled antibodies using a Pan T cell isolation kit (Miltenyi, Auburn, CA). The mouse IFN-γ ELISpot kit (BD Bioscience, San Diego, CA) was used to determine antigen specific IFN-γ secreting T cells as described by the manufacturer. Briefly, tumor cells were treated with TSA for 48 h and simultaneously pulsed with ova, ova–peptide257–264 or unrelated control peptide mgp10025–33 (10 μM) for the last 16 h in culture. Pulsed tumor cells were irradiated (2,000 Gy) before use in the ELISpot plate. T cells (2 × 105) from OT-I mice were incubated in triplicate wells with untreated controls or TSA-treated tumor cells (1 × 105) in IFN-γ coated ELISpot plates for 24 h. Splenocytes isolated from OT-I mice were used as controls after antigen stimulation and irradiation (30 Gy). A standard ELISpot assay protocol was followed to measure IFN-γ secreting T cell spots.
Statistical analysis
T cell reactivity as measured by the ELISpot assay was considered significant if the average number of spots in test wells was higher than that in control wells when using an unpaired student’s t test for p < 0.05.
Results
HDACi treatments enhance expression of the genes involved in antigen processing and presentation via MHC class I in tumor cells
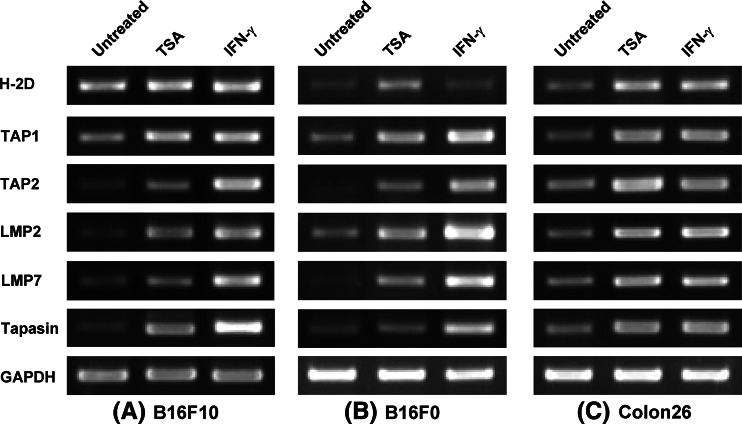
Previous studies have shown that the expression of IFN-γ-inducible immune genes including CIITA and MHC class I can be enhanced by treatment with HDACi [26] and defective expression of TAP1, TAP2, LMP2, and LMP7 can be reversed by treatment with IFN-γ in tumor cells [37]. To determine the role of histone deacetylation in the regulation of genes involved in antigen processing and presentation, TSA-treated (50 nM–1 μM, 12–48 h) adherent B16F10 cells were analyzed by RT-PCR and real-time quantitative RT-PCR for the expression of TAP1, TAP2, LMP2, LMP7, Tapasin, β2m and class I genes. The conditions inducing maximal expression of indicated genes are presented in Figs. 1 and 2. These figures demonstrate that TSA treatment (250 and 500 nM for 24 h) significantly enhanced the expression of TAP2, LMP2, LMP7 and Tapasin in B16F10 cells. The low-level expression of class I and TAP1 genes found in untreated B16F10 (Fig. 1a) was also substantially enhanced after TSA treatment as shown by real-time PCR (Fig. 2). In addition to TSA we used VA, which enhanced gene expression levels of TAP, LMP, Tapasin and class I in B16F10 cells but to a lower level than TSA (data not shown). Although the mechanisms responsible for the difference in gene expression by TSA and VA is uncertain, target specificity and drug potency may be involved, since TSA is highly reactive and inhibits more HDACs than VA [16, 41]. The constitutive expression of β2m in B16F10 cells was unaltered after TSA or VA treatment (data not shown). These results show that, similar to IFN-γ, HDACi treatment can enhance the expression of genes required for antigen processing and presentation via the MHC class I pathway in B16F10 cells. To determine whether HDACi can elicit class I antigen processing components in other tumor cells, we analyzed gene expression by RT-PCR in several tumor cells, including B16F0 and Colon 26. Figure 1b demonstrates that, similar to B16F10, TSA treatment (250 nM for 24 h) enhanced the expression of TAP2, LMP7 and class I in B16F0 cells. In addition, the low-level expression of TAP1 and LMP2 genes found in untreated B16F0 cells was enhanced after TSA treatment (Fig. 1b). The constitutive expression of TAP1, TAP2, LMP2, LMP7, Tapasin and class I genes found in untreated Colon 26 cells was also enhanced by TSA treatment (250 nM for 24 h) and gene expression levels were similar to those of IFN-γ-treated cells (Fig. 1c). IFN-γ treatment (100 U/ml for 24 h) enhanced high-level expression of all genes analyzed, with the notable exception of class I, in B16F0 (Fig. 1). These data demonstrate that HDACi can up-regulate class I antigen processing components in different tumor types. The difference in gene expression by TSA and IFN-γ treatment in B16F0 tumors suggests that, in certain tumors, enhanced TAP, LMP and Tapasin by IFN-γ may not be sufficient to restore class I and that epigenetic modification by HDACi is needed.
Fig. 1.
HDACi treatments enhance the genes involved in antigen processing and presentation via MHC class I pathway in tumor cells. a B16F10, b B16F0 and c Colon 26 cells were treated with TSA (250 nM) or IFN-γ (100 U/ml) and mRNA for H-2D, TAP1, TAP2, LMP2, LMP7 and Tapasin was amplified by RT-PCR at 24 h. The data presented represent more than three independent experiments
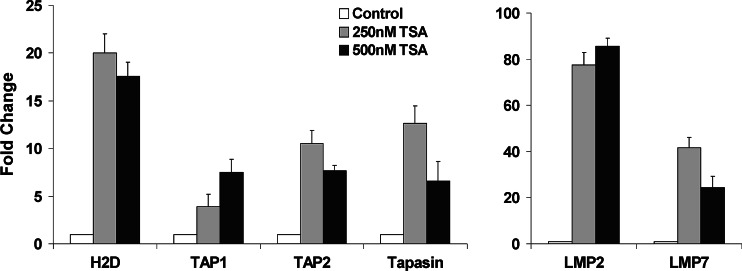
Fig. 2.
Quantitative analysis of MHC class I antigen processing gene expression in HDACi-treated melanoma cells. TSA treatment (24 h) induced approximately 5–20-fold increase in mRNA for TAP1, TAP2, Tapasin and H-2D in B16F10 cells. LMP2 and LMP7 mRNA was also increased approximately 75 and 30-fold respectively in B16F10 cells after TSA treatment compared to untreated control. These real-time PCR experiments were repeated three times with similar results
Enhanced cell surface expression of MHC class I, CD40 and CD86 in tumor cells after HDACi treatments
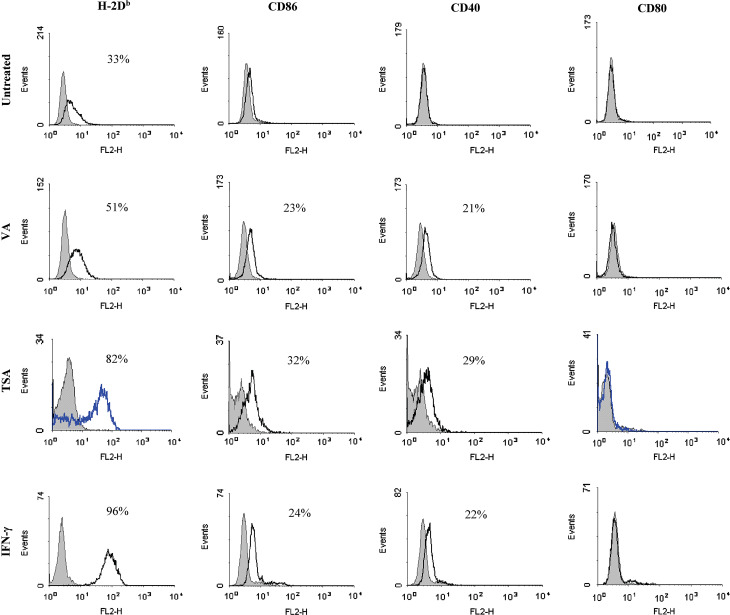
Since HDACi enhance surface expression of MHC class I and costimulatory molecules in various tumor cells, including B16F0 (A. N. H. Khan et al., in press) and B16/BL6 [18], we first investigated whether this also occurred with the metastatic B16F10 melanoma, which is known to constitutively express low-levels of class I [39]. B16F10 cells were treated with different concentrations of TSA (100 nM–1 μM) and VA (100 μM–1 mM) for multiple time periods (12, 24 and 48 h) and adherent cells were analyzed by flow cytometry for the expression of class I, CD40, CD80 and CD86. The conditions inducing maximal expression of class I and costimulatory molecules are presented in Fig. 3. This figure shows that expression of class I was significantly enhanced in B16F10 cells after treatment with 500 nM TSA for 48 h. TSA treatment also induced costimulatory molecule CD40 and CD86 expression, but not CD80, in B16F10 cells (Fig. 3). Similar to TSA, an optimal concentration of VA treatment (500 μM for 48 h) enhanced class I expression substantially, CD40 and CD86 expression minimally (Fig. 3) with no enhancement of CD80. IFN-γ treatment (100 U/ml for 24 h) was also performed as a known positive control to compare with HDACi. These results demonstrate that TSA and VA treatments can enhance expression of class I, CD40 and B7-2 in B16F10 cells and the effects of TSA are comparable to IFN-γ treatment. VA treatments gave generally lesser enhancement than TSA (Fig. 3). These findings suggest that HDAC mediated epigenetic mechanisms, established for other immune genes, may also be involved in the regulation of the class I pathway genes in tumor cells.
Fig. 3.
HDACi treatments enhance expression of MHC class I, CD40 and CD86 on melanoma cells. B16F10 cells were stained with specific mAb and isotype controls after treatment with TSA (500 nM for 48 h), VA (500 μM for 48 h) or IFN-γ (100 U/ml for 24 h) and analyzed by flow cytometry to assay expression of the indicated surface markers. Isotype controls are shown as shaded peaks and heavy lines represent expression determined by specific antibody staining. Values indicated in the histograms are the percent of cells positive for the respective mAb relative to the isotype staining. The data presented represent more than three independent experiments
TSA treatment enhances MHC class I specific antigen presentation by tumor cells
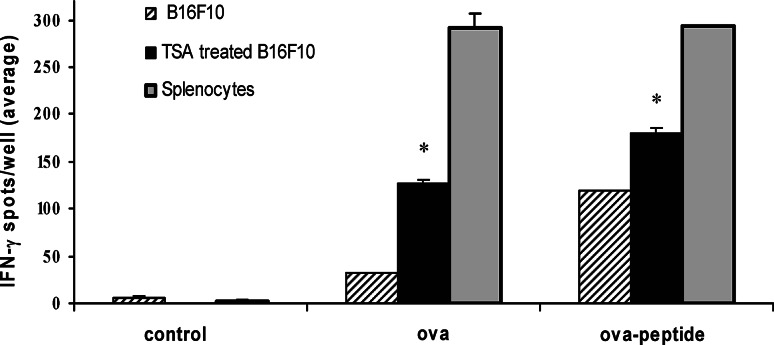
To determine the potential functional relevance of epigenetically induced expression of class I, costimulatory molecules and the genes involved in antigen processing, we studied the ability of TSA treated B16F10 cells to present antigen in the context of class I. TSA-treated (500 nM for 48 h) or untreated B16F10 cells were pulsed with ova, ova–peptide257–266 or control peptide. Antigen-pulsed cells were irradiated and incubated with purified CD8+ T cells for 24 h. Peripheral lymph node T cells isolated from OT-I transgenic mice that express a T cell receptor for ova–peptide257–266, shown to be specifically presented by MHC class I [4, 13], were used in these experiments to demonstrate class I mediated antigen presentation. Standard ELISpot assays were employed to measure IFN-γ production by CD8+ T cells. Figure 4 demonstrates that ova-pulsed TSA-treated B16F10 cells can stimulate significantly higher numbers of IFN-γ producing CD8+ T cells compared to untreated cells. Although ova–peptide257–264-pulsed untreated B16F10 cells are capable of stimulating antigen specific T cells, levels of activation by the pulsed TSA-treated cells are significantly higher than untreated cells. Activation of CD8+ T cells was antigen specific, since control antigen-pulsed TSA-treated or untreated B16F10 cells and splenocytes did not induce significant IFN-γ producing T cells. Ova or ova–peptide257–266-pulsed splenocytes of OT-I mice were used as a positive control. In addition, controls with CD8+ T cells and TSA-treated B16F10 or splenocytes without antigen pulsing did not elicit significant IFN-γ production (data not shown). These results demonstrate that treatment with TSA generates tumor cells that are efficient in presenting class I-restricted peptides and effective in processing and presenting whole protein antigens. Enhanced expression of TAP1, TAP2, LMP2, LMP7 and Tapasin after TSA treatment in B16F10 tumor cells, together with the demonstration of protein antigen presentation by TSA-treated tumor cells, suggests that epigenetic mechanisms may be involved in the regulation of antigen processing and presentation, via the class I pathway in melanoma tumor cells. Moreover, the data demonstrates that treatment with HDACi can functionally restore the class I pathway.
Fig. 4.
MHC class I restricted antigen presentation by epigenetically altered melanoma cells. CD8+ T cells, isolated from ova-primed OT-I mice and purified magnetically with microbead-labeled antibodies, were cultured in triplicate with TSA treated (500 nM for 48 h) or untreated B16F10 cells for 24 h. Splenocytes isolated from OT-I mice were used as positive control. Splenocytes and B16F10 cells (TSA-treated and untreated) were pulsed with ova, ova–peptide257–266 or the control peptide mgp100 and irradiated before use in the assay. Standard ELISpot assays were performed to measure IFN-γ secreting CD8+ T cells. Asterisks indicate number of IFN-γ spots detected with ova and ova–peptide257–266-pulsed TSA-treated B16F10 is statistically significant compared with untreated cells. Similar results were obtained from three independent experiments
Discussion
To our knowledge this is the first report demonstrating that HDACi treatment can activate the proteasomal components (LMP2, LMP7), transporters associated with antigen processing (TAP1, TAP2) and the TAP-associated glycoprotein (Tapasin) gene in tumor cells. The data presented here also confirm previous observations [18, 19] that treatment with HDACi can enhance mRNA and surface expression of MHC class I in B16F10 melanoma cells. Expression of these genes is regulated mainly at the transcriptional level. HDAC-mediated chromatin repression has been reported as a major mechanism for down regulation of class I transcription in tumor cells [21, 42]. Many laboratories have shown that TSA and other HDACi regulate chromatin structure and induce CIITA, MHC class II, B7-1/2 and other genes in tumor cells [25, 26, 30, 43 reviewed in 41]. We have recently demonstrated that HDACi also initiate methylation of histone in several mouse and human tumor cells, and defined the spatial distribution of histone acetylation and methylation markers along the MHC class II gene during different phases of the transcription process (S.-D. Chou et al., in press). These reports and the enhanced gene expression following TSA and VA treatment, showed in this study, suggest that HDAC-mediated chromatin regulation is involved in repression of class I antigen processing genes in tumor cells. In addition to histone acetylation, HDACi may utilize other mechanisms to enhance gene expression. For example, HDACi may inhibit DNA methylation and activate repressed immune genes in tumor cells [31]. Furthermore, HDACi may also enhance gene expression through acetylation of non-histone proteins, such as transcription factors, signal transduction mediators and molecular chaperones [22]. Moreover, recent data suggests that TSA may, depending on the concentration and tumor type, activate MAPK pathways which may directly phosphorylate histone H3 and alter chromatin [41; unpublished data]. These and other basic epigenetic mechanisms were not directly addressed in this study and remain to be elucidated in the class I pathway.
A coordinated suppression of LMP, TAP, Tapasin and MHC class I was observed in murine melanoma cells [38] and also in a human bladder carcinoma [33]. It has been shown that deficient expression of LMP and TAP alters the peptide repertoire presented in the context of MHC class I [11] and limits the presentation of antigens by tumor cells for CTL recognition [8]. Reduced Tapasin expression also affects MHC class I expression [35]. Various methods including cytokine treatment and transfection of TAP1/TAP2 have been employed to recover class I expression in tumor cells. However, TAP1/TAP2 transfection in a murine melanoma cell line [39] was not sufficient to induce class I expression suggesting that the mechanisms underlying the total or partial repression of class I in tumor cells are complex and may vary in different tumors. In our study, simultaneous enhancement of TAP, LMP, Tapasin genes and surface class I expression in B16F10 cells after treatment with optimal concentration of HDACi suggest that a coordinated epigenetic repression involving multiple genes may be responsible for reduced class I expression in this tumor. Importantly, this study also shows that HDACi treatments are associated with enhanced expression of class I antigen processing and presentation genes together with costimulatory molecules CD40 and B7-2 and the conversion of B16F10 melanoma cells to APCs. Whether additional unidentified effects of HDACi are involved in the restoration of class I antigen presentation is uncertain. Moreover, similar conversion of other tumor cells to APCs as shown here for B16 model remains to be determined.
The induction of antigen presentation by tumor cells could be an additional or alternative pathway to induce T cell mediated immune responses. Direct antigen presentation in the MHC class II pathway by HDACi treated tumor cells has been shown to activate anti-tumor T cell responses in vitro and in vivo (A. N. H. Khan et al., in press). MHC class I mediated direct priming of CTLs has also been observed in an engineered tumor model and is dependent on the density of MHC/peptide complexes and the expression of B7 costimulatory molecules on tumor cells [34]. Similarly, our study demonstrates that antigen presentation via the class I pathway by HDACi-treated B16F10 tumor cells can activate CD8+ T cells in vitro. Further investigation is required to determine whether in vivo activation of tumor specific CTL responses occur following systemic HDACi treatment. Effective induction of CTL responses through the class I pathway by HDACi could potentially improve anti-tumor immunity as suggested in other tumor models [17, 28].
In recent years, systemic use of several HDACi, including VA, SAHA and depsipeptide, have shown anti-tumor effects in clinical trials [27]. Although these drugs were used on the basis of their ability to induce differentiation, growth arrest and apoptosis of tumor cells, the precise molecular pathways involved in the anti-tumor effects have not been fully determined. Our findings suggest that systemic administration of deacetylase inhibitors could enhance the expression of immune genes, including class I, in the tumor cells making them more susceptible to CTL mediated killing. The various HDAC inhibitors may have substantially different quantitative activities in inducing immune genes and this will require further evaluation. In addition, the finding that TSA, but not IFN-γ, induces class I in the B16F0 melanoma suggests that HDACi might be combined with cytokines in the treatment of certain cancers. Other effects of systemic HDACi on host immunity may also contribute to systemic therapeutic treatments. It will be important, as a component of HDACi clinical trials, to evaluate the effects of epigenetic agents on MHC, costimulatory molecules, class I pathway components and other immune genes in tumor cells as well as in host immune cells. In vivo conversion of tumor cells to APCs, although currently problematic, could provide useful information in developing effective immunotherapeutic strategies against cancer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Supported by a National Institutes of Health grant HD 17013 and utilized core facilities of Roswell Park Cancer Institute’s NCI Cancer Center Support Grant CA16056.
Abbreviations
- CTL
Cytotoxic T lymphocyte
- HDACi
Histone deacetylase inhibitors
- TSA
Trichostatin A
- VA
Valproic acid
- APC
Antigen presenting cell
- ova
Ovalbumin
References
- 1.Agrawal S, Reemtsma K, Bagiella E, Oluwole SF, Braunstein NS. Role of TAP-1 and/or TAP-2 antigen presentation defects in tumorigenicity of mouse melanoma. Cell Immunol. 2004;228:130–137. doi: 10.1016/j.cellimm.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 2.Armeanu S, Bitzer M, Lauer UM, Venturelli S, Pathil A, Krusch M, Kaiser S, Jobst J, Smirnow I, Wagner A, Steinle A, Salih HR. Natural killer cell-mediated lysis of hepatoma cells via specific induction of NKG2D ligands by the histone deacetylase inhibitor sodium valproate. Cancer Res. 2005;65:6321–6329. doi: 10.1158/0008-5472.CAN-04-4252. [DOI] [PubMed] [Google Scholar]
- 3.Baylin SB, Ohm JE. Epigenetic gene silencing in cancer—a mechanism for early oncogenic pathway addiction? Nat Rev Cancer. 2006;6:107–116. doi: 10.1038/nrc1799. [DOI] [PubMed] [Google Scholar]
- 4.Bertholet S, Debrabant A, Afrin F, Caler E, Mendez S, Tabbara KS, Belkaid Y, Sacks DL. Antigen requirements for efficient priming of CD8+ T cells by Leishmania major-infected dendritic cells. Infect Immun. 2005;73:6620–6628. doi: 10.1128/IAI.73.10.6620-6628.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang C-C, Ogino T, Mullins DW, Oliver JL, Yamshchikov GV, Bandoh N, Slingluff CL, Jr, Ferrone S. Defective human leukocyte antigen class I-associated antigen presentation caused by a novel beta2-microglobulin loss-of-function in melanoma cells. J Biol Chem. 2006;281:18763–18773. doi: 10.1074/jbc.M511525200. [DOI] [PubMed] [Google Scholar]
- 6.Chou S-D, Khan ANH, Magner WJ, Tomasi TB. Histone acetylation regulates the cell type specific CIITA promoter, MHC class II expression and antigen presentation in tumor cells. Int Immunol. 2005;17:1483–1494. doi: 10.1093/intimm/dxh326. [DOI] [PubMed] [Google Scholar]
- 7.Ducasse M, Brown MA (2006) Epigenetic aberrations and cancer. Mol Cancer. http://www.molecular-cancer.com/content/5/1/60 [DOI] [PMC free article] [PubMed]
- 8.Evans M, Borysiewicz LK, Evans AS, Rowe M, Jones M, Uzi Gileadi U, Vincenzo Cerundolo V, Man S. Antigen processing defects in cervical carcinomas limit the presentation of a CTL epitope from human papillomavirus 16 E6. J Immunol. 2001;167:5420–5428. doi: 10.4049/jimmunol.167.9.5420. [DOI] [PubMed] [Google Scholar]
- 9.Feenstra M, Rozemuller E, Duran K, Stuy I, van den Tweel J, Slootweg P, de Weger R, Tilanus M. Mutation in the beta-2 m gene is not a frequent event in head and neck squamous cell carcinomas. Hum Immunol. 1999;8:697–706. doi: 10.1016/S0198-8859(99)00015-4. [DOI] [PubMed] [Google Scholar]
- 10.Fonsatti E, Sigalotti L, Coral S, Colizzi F, Altomonte M, Maio M. Methylation-regulated expression of HLA class I antigens in melanoma. Int J Cancer. 2003;105:430–431. doi: 10.1002/ijc.11077. [DOI] [PubMed] [Google Scholar]
- 11.Groettrup M, Soza A, Kuckelkorn U, Kloetzel PM. Peptide antigen production by the proteasome: complexity provides efficiency. Immunol Today. 1996;17:429–435. doi: 10.1016/0167-5699(96)10051-7. [DOI] [PubMed] [Google Scholar]
- 12.Hicklin DJ, Marincola FM, Ferrone S. HLA class I antigen downregulation in human cancers: T cell immunotherapy revives an old story. Mol Med Today. 1999;5:178–186. doi: 10.1016/S1357-4310(99)01451-3. [DOI] [PubMed] [Google Scholar]
- 13.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76:17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 14.Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- 15.Jimenez P, Canton J, Concha A, Cabrera T, Fernández M, Real LM, García A, Serrano A, Garrido F, Ruiz-Cabello F. Microsatellite instability analysis in tumor with different mechanisms for total loss of HLA expression. Cancer Immunol Immunother. 2000;48:684–90. doi: 10.1007/s002620050017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Johnstone RW, Licht JD. Histone deacetylase inhibitors in cancer therapy: is transcription the primary target? Cancer Cell. 2003;4:13–18. doi: 10.1016/S1535-6108(03)00165-X. [DOI] [PubMed] [Google Scholar]
- 17.Khan ANH, Magner WJ, Tomasi TB. An epigenetically altered tumor cell vaccine. Cancer Immunol Immunother. 2004;53:748–754. doi: 10.1007/s00262-004-0513-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komatsu Y, Hayashi H. Histone deacetylase inhibitors up-regulate the expression of cell surface MHC class I molecules in B16/BL6 cells. J Antibiot. 1998;51:89–91. doi: 10.7164/antibiotics.51.89. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu Y, Tomizaki K, Tsukamoto M, Kato T, Nishino N, Sato S, Yamori T, Tsuruo T, Furumai R, Yoshida M, Horinouchi S, Hayashi H. Cyclic hydroxamic-acid-containing peptide 31, a potent synthetic histone deacetylase inhibitor with antitumor activity. Cancer Res. 2001;61:4459–4466. [PubMed] [Google Scholar]
- 20.Lentschat A, Karahashi H, Michelsen KS, Thomas LS, Zhang W, Vogel SN, Arditi M. Mastoparan, a G protein agonist peptide, differentially modulates TLR4- and TLR2-mediated signaling in human endothelial cells and murine macrophages. J Immunol. 2005;174:4252–4261. doi: 10.4049/jimmunol.174.7.4252. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Ou X, Xiong J, Wang T. HPV16E7 mediates HDAC chromatin repression and downregulation of MHC class I genes in HPV16 tumorigenic cells through interaction with an MHC class I promoter. Biochem Biophys Res Commun. 2006;349:1315–1321. doi: 10.1016/j.bbrc.2006.08.182. [DOI] [PubMed] [Google Scholar]
- 22.Lin HY, Chen CS, Lin SP, Weng JR, Chen CS. Targeting histone deacetylase in cancer therapy. Med Res Rev. 2006;26:397–413. doi: 10.1002/med.20056. [DOI] [PubMed] [Google Scholar]
- 23.Lou Y, Vitalis TZ, Basha G, Cai B, Chen SS, Choi KB, Jeffries AP, Elliott WM, Atkins D, Seliger B, Jefferies WA. Restoration of the expression of transporters associated with antigen processing in lung carcinoma increases tumor-specific immune responses and survival. Cancer Res. 2005;65:7926–7933. doi: 10.1158/0008-5472.CAN-04-3977. [DOI] [PubMed] [Google Scholar]
- 24.Luo W, Wang X, Kageshita T, Wakasugi S, Karpf, Ferrone S. Regulation of high molecular weight-melanoma associated antigen (HMW-MAA) gene expression by promoter DNA methylation in human melanoma cells. Oncogene. 2006;25:2873–2884. doi: 10.1038/sj.onc.1209319. [DOI] [PubMed] [Google Scholar]
- 25.Maeda T, Towatari M, Kosugi H, Saito H. Up-regulation of costimulatory/adhesion molecules by histone deacetylase inhibitors in acute myeloid leukemia cells. Blood. 2000;96:3847–3856. [PubMed] [Google Scholar]
- 26.Magner WJ, Kazim AL, Stewart C, Romano MA, Catalano G, Grande C, Keiser N, Santaniello F, Tomasi TB. Activation of MHC class I, II, and CD40 gene expression by histone deacetylase inhibitors. J Immunol. 2000;165:7017–7024. doi: 10.4049/jimmunol.165.12.7017. [DOI] [PubMed] [Google Scholar]
- 27.Marks PA, Dokmanovic M. Histone deacetylase inhibitors: discovery and development as anticancer agents. Expert Opin Investig Drugs. 2005;14:1497–1511. doi: 10.1517/13543784.14.12.1497. [DOI] [PubMed] [Google Scholar]
- 28.Mora-García ML, Duenas-González A, Hernández-Montes J, Cruz-Hernández ED, Pérez-Cárdenas E, Weiss-Steider B, Santiago-Osorio E, Ortíz-Navarrete VF, Rosales VH, Cantú D, Lizano-Soberón M, Rojo-Aguilar MP, Monroy-García A. Up-regulation of HLA class-I antigen expression and antigen-specific CTL response in cervical cancer cells by the demethylating agent hydralazine and the histone deacetylase inhibitor valproic acid. J Transl Med. 2006;4:55. doi: 10.1186/1479-5876-4-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogino T, Shigyo H, Ishii H, Katayama A, Miyokawa N, Harabuchi Y, Ferrone S. HLA class I antigen down-regulation in primary laryngeal squamous cell carcinoma lesions as a poor prognostic marker. Cancer Res. 2006;66:9281–9289. doi: 10.1158/0008-5472.CAN-06-0488. [DOI] [PubMed] [Google Scholar]
- 30.Osborne A, Zhang H, Yang WM, Seto E, Blanck G. Histone deacetylase activity represses gamma interferon-inducible HLA-DR gene expression following the establishment of a DNase I-hypersensitive chromatin conformation. Mol Cell Biol. 2001;21:6495–6506. doi: 10.1128/MCB.21.19.6495-6506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ou J-N, Torrisani J, Unterberger A, Provençal N, Shikimi K, Karimi M, Ekström TJ, Szyf M. Histone deacetylase inhibitor trichostatin A induces global and gene-specific DNA demethylation in human cancer cell lines. Biochem Pharmacol. 2007;73:1297–1307. doi: 10.1016/j.bcp.2006.12.032. [DOI] [PubMed] [Google Scholar]
- 32.Raffaghello L, Prigione I, Bocca P, Morandi, Camoriano M, Gambini C, Wang X, Ferrone S, Pistoia V. Multiple defects of the antigen-processing machinery components in human neuroblastoma: immunotherapeutic implications. Oncogene. 2005;24:4634–4644. doi: 10.1038/sj.onc.1208594. [DOI] [PubMed] [Google Scholar]
- 33.Romero JM, Jimenez P, Cabrera T, Cózar JM, Pedrinaci S, Tallada M, Garrido F, Ruiz-Cabello F. Coordinated downregulation of the antigen presentation machinery and HLA class I/beta2-microglobulin complex is responsible for HLA-ABC loss in bladder cancer. Int J Cancer. 2005;113:605–610. doi: 10.1002/ijc.20499. [DOI] [PubMed] [Google Scholar]
- 34.Schoenberger SP, Jonges LE, Mooijaart RJ, Hartgers F, Toes RE, Kast WM, Melief CJ, Offringa R. Efficient direct priming of tumor specific cytotxic T lymphocyte in vivo by an engineered APC. Cancer Res. 1998;58:3094–3100. [PubMed] [Google Scholar]
- 35.Schoenhals GJ, Krishna RM, Grandea AG, 3rd, Spies T, Peterson PA, Yang Y, Früh K. Retention of empty MHC class I molecules by Tapasin is essential to reconstitute antigen presentation in invertebrate cells. EMBO J. 1999;18:743–753. doi: 10.1093/emboj/18.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seliger B, Harders C, Lohmann S, Momberg F, Urlinger S, Tampe R, Huber C. Down-regulation of the MHC class I antigen-processing machinery after oncogenic transformation of murine fibroblasts. Eur J Immunol. 1998;28:122–133. doi: 10.1002/(SICI)1521-4141(199801)28:01<122::AID-IMMU122>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 37.Seliger B, Maeurer MJ, Ferrone S. Antigen-processing machinery breakdown and tumor growth. Immunol Today. 2000;21:455–464. doi: 10.1016/S0167-5699(00)01692-3. [DOI] [PubMed] [Google Scholar]
- 38.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Coordinate downregulation of multiple MHC class I antigen processing genes in chemical-induced murine tumor cell lines of distinct origin. Tissue Antigens. 2000;56:327–336. doi: 10.1034/j.1399-0039.2000.560404.x. [DOI] [PubMed] [Google Scholar]
- 39.Seliger B, Wollscheid U, Momburg F, Blankenstein T, Huber C. Characterization of the major histocompatibility complex class I deficiencies in B16 melanoma cells. Cancer Res. 2001;61:1095–1099. [PubMed] [Google Scholar]
- 40.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F. Expression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer. 2001;94:243–251. doi: 10.1002/ijc.1452. [DOI] [PubMed] [Google Scholar]
- 41.Tomasi TB, Magner WJ, Khan ANH. Epigenetic regulation of immune escape genes in cancer. Cancer Immunol Immunother. 2006;55:1159–1184. doi: 10.1007/s00262-006-0164-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhao B, Hou S, Ricciardi RP. Chromatin repression by COUP-TFII and HDAC dominates activation by NF-kappaB in regulating major histocompatibility complex class I transcription in adenovirus tumorigenic cells. Virology. 2003;306:68–76. doi: 10.1016/S0042-6822(02)00079-X. [DOI] [PubMed] [Google Scholar]
- 43.Zika E, Greer SF, Zhu X-S, Ting JP. Histone deacetylase 1/mSin3A disrupts gamma interferon-induced CIITA function and major histocompatibility complex class II enhanceosome formation. Mol Cell Biol. 2003;23:3091–3102. doi: 10.1128/MCB.23.9.3091-3102.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.