Abstract
BACKGROUND
Blast-related traumatic brain injuries have been common in the Iraq and Afghanistan wars, but fundamental questions about the nature of these injuries remain unanswered.
METHODS
We tested the hypothesis that blast-related traumatic brain injury causes traumatic axonal injury, using diffusion tensor imaging (DTI), an advanced form of magnetic resonance imaging that is sensitive to axonal injury. The subjects were 63 U.S. military personnel who had a clinical diagnosis of mild, uncomplicated traumatic brain injury. They were evacuated from the field to the Landstuhl Regional Medical Center in Landstuhl, Germany, where they underwent DTI scanning within 90 days after the injury. All the subjects had primary blast exposure plus another, blast-related mechanism of injury (e.g., being struck by a blunt object or injured in a fall or motor vehicle crash). Controls consisted of 21 military personnel who had blast exposure and other injuries but no clinical diagnosis of traumatic brain injury.
RESULTS
Abnormalities revealed on DTI were consistent with traumatic axonal injury in many of the subjects with traumatic brain injury. None had detectible intracranial injury on computed tomography. As compared with DTI scans in controls, the scans in the subjects with traumatic brain injury showed marked abnormalities in the middle cerebellar peduncles (P<0.001), in cingulum bundles (P = 0.002), and in the right orbitofrontal white matter (P = 0.007). In 18 of the 63 subjects with traumatic brain injury, a significantly greater number of abnormalities were found on DTI than would be expected by chance (P<0.001). Follow-up DTI scans in 47 subjects with traumatic brain injury 6 to 12 months after enrollment showed persistent abnormalities that were consistent with evolving injuries.
CONCLUSIONS
DTI findings in U.S. military personnel support the hypothesis that blast-related mild traumatic brain injury can involve axonal injury. However, the contribution of primary blast exposure as compared with that of other types of injury could not be determined directly, since none of the subjects with traumatic brain injury had isolated primary blast injury. Furthermore, many of these subjects did not have abnormalities on DTI. Thus, traumatic brain injury remains a clinical diagnosis. (Funded by the Congressionally Directed Medical Research Program and the National Institutes of Health; ClinicalTrials.gov number, NCT00785304.)
In the current wars in iraq and afghanistan, the number of blast-related traumatic brain injuries may be as high as 320,000.1 Most of these injuries are categorized as uncomplicated “mild” or “concussive” traumatic brain injury on the basis of clinical criteria and the absence of intracranial abnormalities on computed tomography (CT) or conventional magnetic resonance imaging (MRI).2 However, little is known about the nature of these “mild” injuries, and the relationship between traumatic brain injury and outcomes remains controversial.3,4 No human autopsy studies conducted with the use of current immunohistochemical methods5,6 have been published.7,8 Computer simulations of the effects of blast-induced pressure waves on the brain suggest that coup and contrecoup regions may be subject to high stresses.9,10 Simulations also suggest that the orbitofrontal regions and the posterior fossa (cerebellum and brain stem) may sustain intense stresses independently of the subject’s head orientation relative to the blast.10 Findings that are consistent with this view include a positron-emission tomographic study showing reduced cerebellar basal glucose metabolism11 and a case report documenting a lesion in cerebellar white matter on MRI after blast injury.12 In a swine model of experimental blast injury, traumatic axonal injury in several regions, including cerebellar tracts, was detected.13
We therefore hypothesized that traumatic axonal injury is a primary feature of human blast-related traumatic brain injury. To test this hypothesis noninvasively, we used an advanced MRI method called diffusion tensor imaging (DTI), which can be performed quickly on most clinical scanners.14 DTI involves the measurement of water diffusion in multiple directions. In the white matter of the brain, water diffuses faster along the predominant fiber direction and more slowly in perpendicular directions (see Fig. S1 and S2 in the Supplementary Appendix, available with the full text of this article at NEJM.org). The resulting anisotropy (directional asymmetry) of water diffusion is high in intact axons and reduced after axonal injury.15–17 The use of reduced anisotropy on DTI as a marker of traumatic axonal injury has been directly validated by means of comparison with immunohistochemical indicators of axonal injury in an animal model of traumatic brain injury, even when the findings on conventional MRI are normal.16,17 We explicitly assessed major orbitofrontal and posterior fossa white-matter tracts, along with other regions commonly affected by traumatic brain injury.
METHODS
SUBJECTS
U.S. military personnel with positive results on screening for traumatic brain injury, performed at the Landstuhl Regional Medical Center (LRMC), were eligible for inclusion in the study. Screening was based on U.S. military clinical criteria for traumatic brain injury18: loss of consciousness, amnesia for the event, or another change in neurologic status, such as feeling “dazed” or “confused” or “seeing stars” immediately after the trauma. Additional criteria for inclusion in the study were injury from a blast, defined as primary injury from blast exposure with or without additional mechanisms of injury, within 90 days before study enrollment; membership in the U.S. military; the ability to provide informed consent in person; no contraindications to MRI, such as retained metallic fragments; no history of major traumatic brain injury or psychiatric disorder; and agreement to communicate by telephone or e-mail monthly for 6 to 12 months after enrollment and to travel to Washington University in St. Louis for follow-up. Inclusion criteria for controls were the same except that negative results of screening for traumatic brain injury were required. All subjects provided written informed consent before enrollment.
DTI AND CONVENTIONAL MRI ASSESSMENTS
The initial MRI scans obtained at the LRMC were acquired with the use of a 1.5-tesla MRI scanner (Magnetom Avanto, Siemens), without the administration of sedation or medication beyond that being administered as part of routine clinical care. The DTI protocol involved the acquisition of two scans at a resolution of 2.5 mm by 2.5 mm by 2.5 mm with 23 diffusion directions. The conventional MRI scans obtained included T1-weighted and T2-weighted sequences, fluid-attenuated inversion recovery (FLAIR) sequences, and T2*-weighted sequences. Performance of the protocol required 21 minutes per subject (Table S1 in the Supplementary Appendix). (Further information on the image processing can be found in the Methods section in the Supplementary Appendix.) Subjects traveled to Washington University 6 to 12 months after enrollment for follow-up scans and in-person clinical assessments. The follow-up scans were obtained on another 1.5-tesla MRI scanner (Magnetom Avanto) at Washington University in accordance with the same protocol.
REGION-OF-INTEREST ANALYSIS
Analysts who were unaware of the clinical assessments of the subjects manually traced 17 regions of interest on each scan. Each region of interest consisted of multiple brain slices fully covering three-dimensional anatomical structures (Fig. 1). The anatomical structures were defined in accordance with definitions provided in a standard DTI atlas.19 Analyze software, version 6.1 (Mayo Foundation), was used to extract quantitative DTI parameters, including relative anisotropy, axial diffusivity, radial diffusivity, and mean diffusivity for each region of interest. (The definitions of these parameters are provided in the Supplementary Appendix.) Intrarater reliability (two analyses were performed, 2 weeks apart) was 96% or higher, and interrater reliability was 90% or higher. Therefore, each region of interest was traced by a single analyst to optimize consistency.
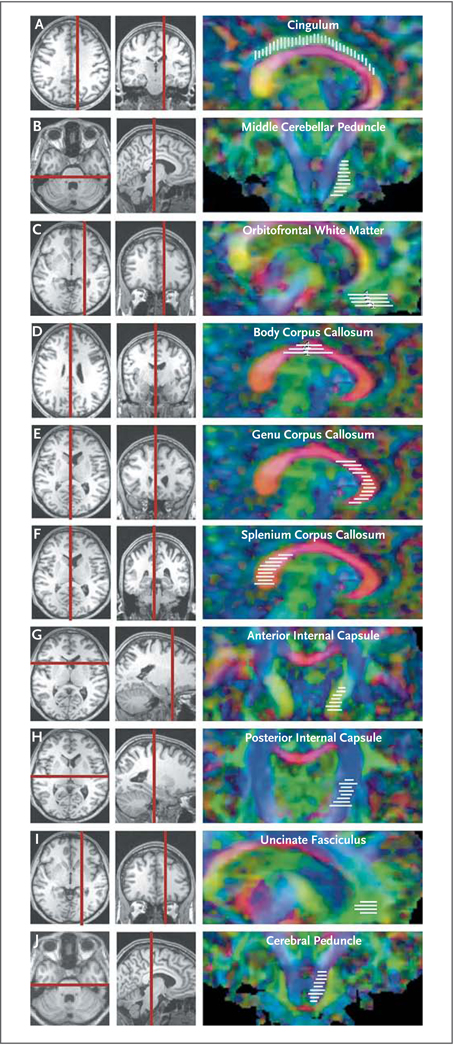
Figure 1. Brain Regions of Interest for Diffusion Tensor Imaging.
The scans in the left and center columns were obtained with conventional (T1-weighted) MRI and are shown for the purpose of anatomical localization. The scans in the right column are relative anisotropy maps obtained with diffusion tensor imaging (DTI). The vertical and horizontal red bars indicate the anatomical localization of the images in the right column. The white bars indicate the locations and orientation of the slices analyzed for multislice regions of interest. Red, green, and blue indicate the principal directions of diffusion, with red denoting right to left, green anterior to posterior, and blue dorsal to ventral. Panel A shows a sagittal section through the cingulum bundle, anterior– posterior, dorsal to the corpus callosum, and Panel B shows a coronal section through the middle cerebellar peduncle, anterior–posterior, in the dorsal brain stem and cerebellum. Panels C through F are sagittal sections, with Panel C showing orbitofrontal white matter, anterior–posterior, in the ventral frontal lobe, Panel D showing the body of the corpus callosum, right–left, between the lateral ventricles, Panel E showing the genu of the corpus callosum, right–left, anterior to the lateral ventricles, and Panel F showing the splenium of the corpus callosum, right–left, posterior to the lateral ventricles. Panels G and H are coronal sections, with Panel G showing the anterior limb of the internal capsule, anterior–posterior and right–left, between the caudate and putamen, and Panel H showing the posterior limb of the internal capsule, dorsal–ventral and right–left, between the putamen and thalamus. Panel I shows a sagittal section of the uncinate fasciculus, anterior–posterior, in the anterior frontal lobe, and dorsal and anterior to the orbitofrontal white-matter region of interest. Panel J shows a coronal section through the cerebral peduncle, dorsal–ventral in the midbrain and pons, medial to the middle cerebellar peduncle.
STATISTICAL ANALYSES
All data were analyzed with the use of Statistica software, version 6.0 (StatSoft). The relationship between measures of relative anisotropy in the regions of interest was assessed by examining scatter plots and performing correlation analyses of relative anisotropy in the 21 controls. When significant positive correlations were detected between relative anisotropy values in pairs of regions, the correlated regions of interest were combined. The combined regions of interest included the genu and splenium of the corpus callosum, the right and left middle cerebellar peduncles, the right and left cerebral peduncles, the right and left uncinate fasciculi, and the right and left cingulum bundles. This approach reduced the number of DTI regions of interest from 17 to 12. There were no significant correlations between relative anisotropy values among these 12 regions. We grouped these regions into two prespecified categories: 4 posterior fossa and orbitofrontal regions predicted to be vulnerable to primary blast injury, and 8 other regions commonly affected by traumatic brain injury.
The normal distribution of each continuous variable was assessed with the use of the Shapiro– Wilk test. All DTI data sets were found to be normally distributed. Hotelling’s T2-tests were used to assess overall differences in groups across the 12 regions of interest. Unpaired Student’s t-tests were then used to assess individual variables. For age, the only non-normally distributed continuous variable, the Mann–Whitney U test was used. Chi-square analyses were used to assess the relationships between categorical variables. One-sided tests were used when hypotheses were prespecified, and two-sided tests were used otherwise. Reported P values have not been corrected for multiple comparisons, but a P value of less than 0.05 was considered to be significant only after Bonferroni’s correction for multiple comparisons (e.g., P<0.0125 [0.05÷4] for each of the four prespecified orbitofrontal and posterior fossa DTI regions of interest and P<0.00625 [0.05 ÷ 8] for each of the eight other regions of interest).
For DTI assessments in individual subjects, the abnormalities consistent with traumatic axonal injury were defined as values for relative anisotropy that were more than 2 SD below the mean of the values for controls. To estimate the number of DTI abnormalities expected to occur by chance in each subject, a binomial distribution was used, with p = 0.02275 (the probability of each abnormality arising by chance) for 12 regions of interest in each subject. (In this instance, p denotes the parameter in the binomial distribution that indicates the probability of each event.) This estimate is based on the assumption that the regions of interest were statistically independent (see the Additional Statistical Methods section in the Supplementary Appendix).
RESULTS
CHARACTERISTICS OF THE SUBJECTS
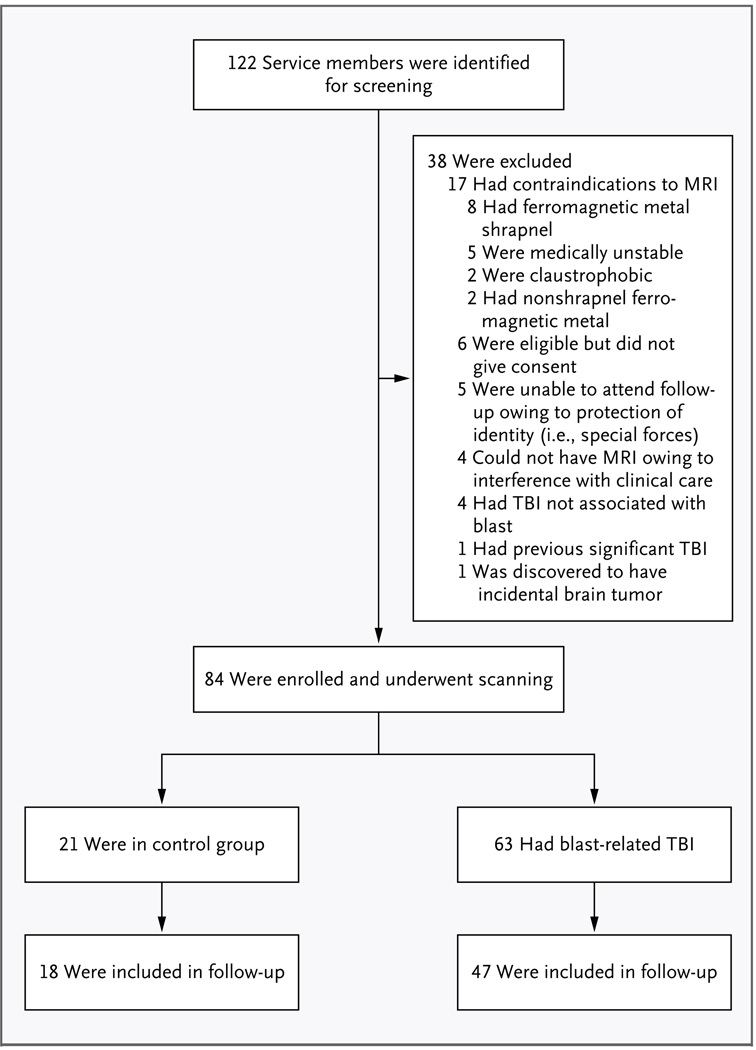
We enrolled 63 subjects with traumatic brain injury and 21 controls over the course of 5 noncontiguous months between November 2008 and October 2009 (Table 1 and Fig. 2). The median time from injury to enrollment was 14 days (range, 1 to 90). All available clinical histories for the subjects with traumatic brain injury indicated primary blast exposure plus another mechanism of head injury, such as being injured in a fall or motor-vehicle crash or being struck by a blunt object. None of the subjects had isolated primary blast injury.
Table 1.
Characteristics of the Study Participants.
| Characteristic | Controls (N = 21) |
Subjects with TBI* (N = 63) |
P Value† |
|---|---|---|---|
| Age — yr | 0.03‡ | ||
| Median | 31 | 24 | |
| Range | 19–49 | 19–58 | |
| Male sex — no. (%) | 21 (100) | 63 (100) | 1.0 |
| Race — no. (%)§ | 0.87 | ||
| White | 17 (81) | 48 (76) | |
| Other | 4 (19) | 18 (29) | |
| Branch of service — no. (%) | 0.92 | ||
| Army | 18 (86) | 56 (89) | |
| Air Force | 2 (10) | 0 | |
| Marine Corps | 1 (5) | 7 (11) | |
| Navy | 0 | 0 | |
| Rank — no. (%) | 0.46 | ||
| Officer | 2 (10) | 3 (5) | |
| Enlisted | 19 (90) | 60 (95) | |
| Theater of operation — no. (%) | 0.01 | ||
| Iraq | 15 (71) | 25 (40) | |
| Afghanistan | 6 (29) | 38 (60) |
TBI denotes traumatic brain injury.
P values were calculated with the use of the chi-square test unless noted otherwise.
The P value was calculated with the use of a two-tailed Mann–Whitney U test.
Race was self-reported, and subjects could select more than one category.
Figure 2. Screening and Enrollment of Study Subjects.
TBI denotes traumatic brain injury.
Inclusion in the group of subjects with traumatic brain injury was typically based on self-report of blast exposure, with immediate alteration of neurologic function meeting the standard criteria for traumatic brain injury used at the LRMC.18 All clinical histories were reviewed by study personnel, who also performed additional history taking and examined medical records. Medical documentation from the theater of operations regarding the duration of loss of consciousness and post-traumatic amnesia was often not available or not reliable. All available clinical histories indicated a change in level of consciousness or loss of consciousness for a few minutes and post-traumatic amnesia for less than 24 hours. Although the study had no restriction on the severity of injury, the requirement for in-person informed consent typically made patients with moderate-to-severe traumatic brain injury ineligible, and such patients were not enrolled. No intracranial abnormalities were detected on CT of the head without the administration of contrast material. Thus, all subjects with traumatic brain injury met the criteria from the Department of Defense for mild, uncomplicated traumatic brain injury.2 (These criteria are provided in the Methods section in the Supplementary Appendix.)
All controls had been exposed to blasts, but none had sustained traumatic brain injury according to the results of clinical screening.18 Specifically, most controls were evacuated to the LRMC for orthopedic or soft-tissue injuries to the arms or legs. Some controls also had gastrointestinal conditions. Many of these injuries occurred independently of blast exposure. None of the subjects in either group had other conditions that are known to or could reasonably be expected to affect DTI signal characteristics. Specifically, no subject in either group was known to have cerebro-vascular disease, hypoxic or ischemic brain injury, central nervous system infection, sepsis, infection with the human immunodeficiency virus, severe electrolyte disturbance, liver failure, renal failure, heart failure, a history of alcohol abuse, or a long-standing psychiatric condition.
FINDINGS ON DTI AND CONVENTIONAL MRI
Initial DTI scanning performed at LRMC revealed abnormalities that were consistent with traumatic axonal injury. Reductions in relative anisotropy were apparent in several brain regions (Fig. 3). The results of conventional MRI were normal even when abnormalities were present on DTI (Fig. S3 in the Supplementary Appendix). An abnormality related to traumatic brain injury was detected with the use of conventional MRI in only one subject; the review was performed by a board-certified neuroradiologist, who found a small occipital contusion.
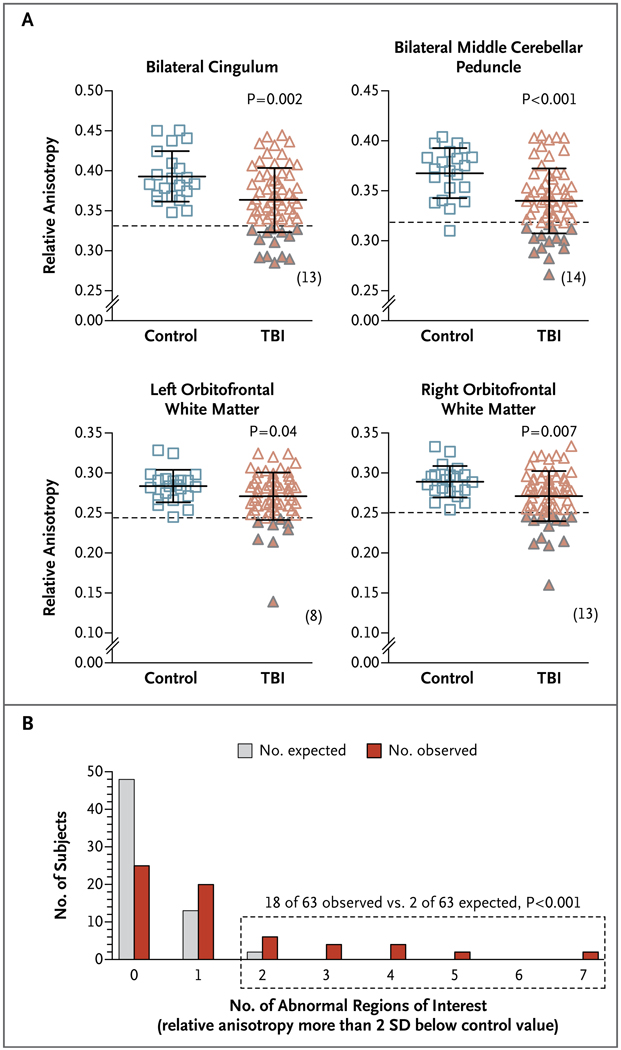
Figure 3. Abnormalities Detected on Diffusion Tensor Imaging in Subjects with Blast-Related Traumatic Brain Injury.
Panel A shows scatter plots of relative anisotropy in four regions of interest. P values were calculated with the use of one-sided Student’s t-tests, since the prespecified hypothesis was that relative anisotropy would be lower in subjects with traumatic brain injury (TBI) than in controls. The solid horizontal lines indicate means, and the I bars indicate standard deviations; the dashed horizontal lines are positioned 2 SD below the mean for the control group (solid triangles represent values in subjects with TBIs that are 2 SD below this level); the numbers in parentheses indicate the number of subjects with TBI for whom relative anisotropy was below this cut-off point. The formula for calculating relative anisotropy is available in Figure S1 in the Supplementary Appendix. Panel B shows the number of abnormalities detected on DTI as compared with the number that would be expected by chance in the 63 subjects with TBI. The dotted box indicates the group of subjects with two or more abnormal regions of interest. The P value was calculated with the use of the chi-square test.
Quantitative analyses indicated significant reductions in relative anisotropy in the group of subjects with traumatic brain injury as compared with the control group (P<0.02 according to Hotelling’s T2-test). Among the brain regions commonly affected in civilian cases of mild traumatic brain injury,15,20–26 abnormalities were most frequently found in the cingulum bundle (Fig. 3A), uncinate fasciculus, and anterior limb of the internal capsule (Fig. S4 in the Supplementary Appendix). However, there were few abnormalities in the corpus callosum or the posterior limb of the internal capsule; notably, abnormalities were more frequent in the middle cerebellar peduncles and orbitofrontal white matter (Fig. 3A), both of which are among the regions predicted to sustain the most intense stresses and therefore predicted to be vulnerable to primary blast injury.10
At an individual level, 18 of the 63 subjects with traumatic brain injury (29%) had abnormalities on DTI that were consistent with multifocal traumatic axonal injury. Specifically, relative anisotropy was reduced in two or more brain regions in each of these 18 subjects (Fig. 3B). Abnormalities detected on DTI were defined as relative anisotropy reductions of at least 2 SD below the mean for the 21 controls. On the basis of chance alone, no more than 2 of 63 healthy subjects would be expected to have two or more such abnormalities in 12 statistically independent regions of the brain (P<0.001 by chi-square analysis). An additional 20 subjects (32%) with traumatic brain injury had one abnormality detected on DTI and 25 (40%) had no abnormalities according to the aforementioned definitions.
There were imbalances in age and theater of operation between the subjects with traumatic brain injury and the controls (Table 1), but these differences were unlikely to account for the primary results. Specifically, there were no correlations between age and relative anisotropy in this cohort (Fig. S5 in the Supplementary Appendix). Likewise, there were no significant differences between controls or subjects with traumatic brain injury who were injured in Iraq and those injured in Afghanistan (Fig. S6 in the Supplementary Appendix). The differences between subjects with traumatic brain injury and controls were robust after adjustments for propensity score (Tables S2 and S3 in the Supplementary Appendix). Post hoc subgroup analyses indicated that these differences were unlikely to have resulted from effects restricted to any specific subgroup of subjects (Table S4 in the Supplementary Appendix).
EVOLUTION OF DTI SIGNAL ABNORMALITIES
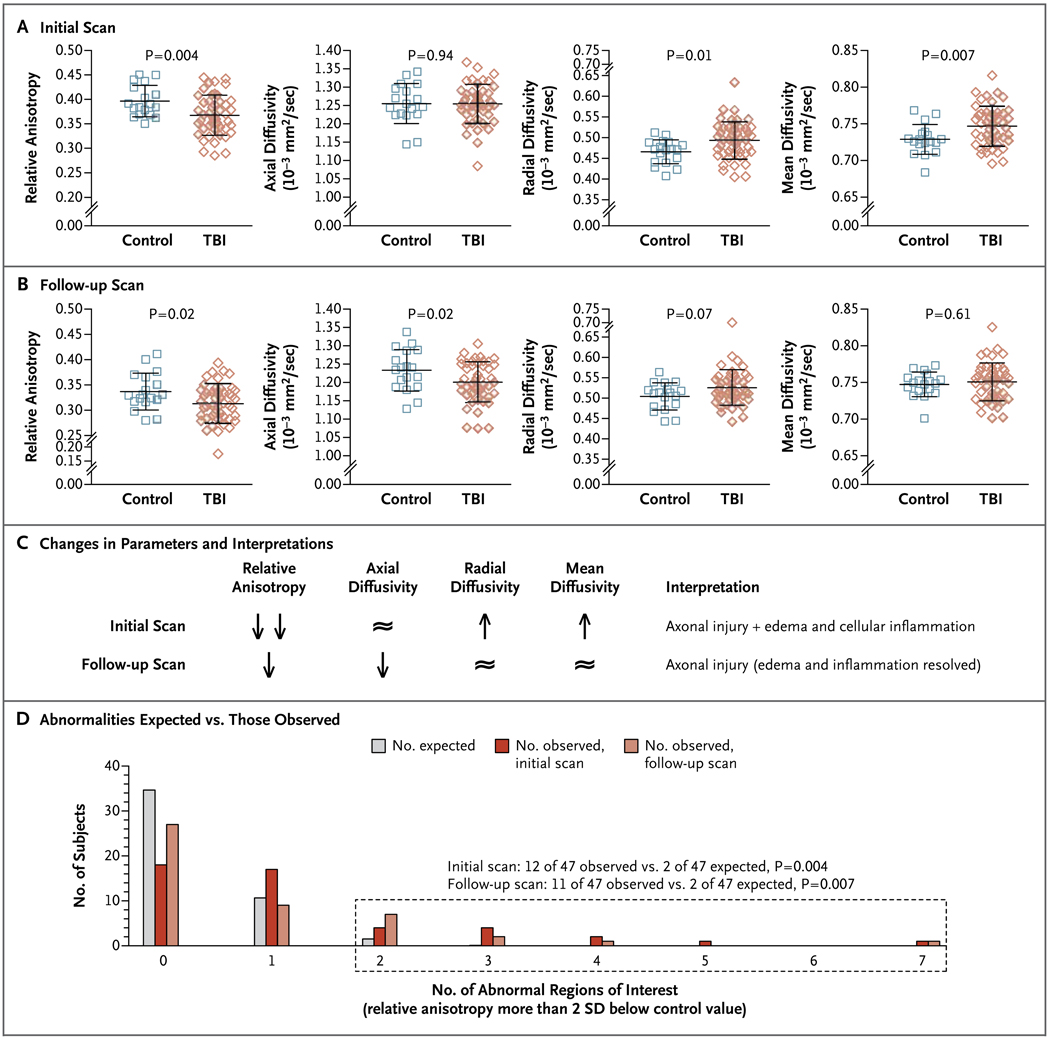
Relative anisotropy, the DTI parameter assessed in previous analyses, has been shown to be persistently reduced at several time points after traumatic brain injury in an animal model; however, other DTI parameters have been shown to change over time as the injuries evolve.16 We therefore analyzed these other DTI parameters and found clear evidence of changes in the DTI signal abnormalities over time in this cohort. Specifically, mean diffusivity and radial diffusivity were higher in subjects with traumatic brain injury than in controls on the initial scans (Fig. 4A) but normalized on follow-up scans (Fig. 4B). Axial diffusivity did not differ significantly between groups on the initial scans (Fig. 4A) but was lower in the subjects with traumatic brain injury than in controls on follow-up scans (Fig. 4B). These findings are consistent with an evolution of injury (Fig. 4C).
Figure 4. Evolution of Abnormalities over Time as Assessed with Diffusion Tensor Imaging.
All data in Panels A through D are from the 18 controls and 47 subjects with traumatic brain injury (TBI) who underwent both initial and follow-up diffusion tensor imaging (DTI). The formulas for calculating relative anisotropy, axial diffusivity, radial diffusivity, and mean diffusivity are available in Figure S1 and S8 in the Supplementary Appendix. In Panels A and B, the longer horizontal lines indicate the means and the I bars indicate standard deviations. Panel A shows the results of the initial scans (obtained within 90 days after injury) in the cingulum bundles, with reduced relative anisotropy, increased radial diffusivity, and increased mean diffusivity in the subjects with TBI as compared with the controls. Panel B shows the follow-up scans (obtained 6 to 12 months after study enrollment) in the cingulum bundles, with reduced relative anisotropy and reduced axial diffusivity. Panel C shows the changes in DTI parameters between initial and follow-up scanning in subjects with TBI as compared with controls and the interpretation of these changes (see also Fig. S4 in the Supplementary Appendix). The double arrows indicate more extensive reduction in relative anisotropy; the ≈ symbol indicates that there was no significant difference between subjects with TBI and controls. Panel D shows differences in observed versus expected DTI abnormalities on initial and follow-up scans in the 47 subjects with TBI. The dotted box indicates the group of subjects with two or more abnormal regions of interest.
On the basis of the results of analyses in individual subjects, the sensitivity of DTI did not decline substantially over time. Of the 47 subjects with traumatic brain injury who underwent scanning twice, 12 (26%) had two or more abnormal regions of interest on the initial scans and 11 (23%) had two or more abnormal regions of interest on follow-up scans (Fig. 4D). These proportions were both greater than would be expected by chance (P = 0.004 and P = 0.007, respectively, by chi-square analysis). There were no significant differences in initial relative anisotropy between the 47 subjects with traumatic brain injury who underwent follow-up scanning and the 16 who did not (Fig. S7 in the Supplementary Appendix). This finding indicated that subjects available for follow-up DTI scanning were representative of the entire cohort.
DISCUSSION
With the use of DTI, we found abnormalities consistent with traumatic axonal injury in U.S. military personnel with blast-related mild traumatic brain injury. Substantial numbers of abnormalities were found in regions of the brain not known to be commonly injured in civilian cases of mild traumatic brain injury but predicted to be vulnerable to blast on the basis of computational simulations.10 Abnormalities were also found in some brain regions that are commonly affected in civilian cases of mild traumatic brain injury.15,20–26 Other regions, such as the corpus callosum,5,15,20,23,25–27 were generally spared. Overall, the distribution of abnormalities can best be accounted for as a combination of traumatic axonal injuries in brain regions vulnerable to primary blast and in regions of the brain vulnerable to other mechanisms of injury. This explanation fits well with the clinical descriptions of the injuries, which in all cases included both primary blast exposure and another mechanism of injury, such as a fall, a motor-vehicle crash, or a blow to the head by a blunt object. However, it is also possible that injuries to the orbitofrontal white matter and cerebellar peduncles are more common in civilian cases of mild traumatic brain injury than currently recognized. Certainly, these and adjacent regions can be affected in more severe instances of civilian traumatic brain injury.28–31 Likewise, primary blast injury could sensitize these regions to subsequent insults. Thus, the exact contributions of primary blast exposure and other types of injury cannot be determined with certainty.
The characteristics of the abnormal DTI signals changed between initial scanning and follow-up scanning in a fashion that was consistent with the evolution of relatively acute injuries. The pattern of abnormalities on the initial scans was most consistent with axonal injury plus a cellular inflammatory response and edema (Fig. 4C, and Fig. S8 in the Supplementary Appendix). Axial diffusivity has been shown to be decreased with axonal injury but concomitantly increased with edema and cellular inflammation. Thus, axial diffusivity can be pseudonormalized in complex injuries.16 On the follow-up scans, the pattern of abnormalities was most consistent with persistent axonal injury plus resolution of the edema and cellular inflammation (Fig. 4C, and Fig. S8 in the Supplementary Appendix). This evolution over time also confirms that the DTI abnormalities were unlikely to have been preexisting.
The limitations of this study include a moderate sample size, an all-male study population, a finite number of prespecified regions of interest for DTI analysis, and the lack of a direct comparison with identically assessed subjects who had traumatic brain injury that was not blast-related. Another limitation, despite our best efforts at circumvention, is the possibility that some uncharacterized differences between the subjects and the controls, in addition to that of brain injury, affected the DTI signals in such as way as to produce the observed results. Additional research with independent cohorts will be required to validate these findings.
We have not been able to address questions regarding isolated primary blast-related traumatic brain injury. All our subjects had primary blast exposure plus another blast-related mechanism of injury, indicating that the incidence of isolated primary blast-related traumatic brain injury may be low (see the Discussion section in the Supplementary Appendix).
Our cohort consisted of active-duty U.S. military personnel with injuries or medical conditions severe enough to prompt commanding officers and medical personnel to at least temporarily remove them from duty. It is not known whether these subjects are representative of all U.S. military personnel with mild traumatic brain injury sustained in Iraq or Afghanistan. Military personnel were brought to the LRMC for a variety of reasons, the most common of which was to obtain specific types of medical care that were not available in Iraq or Afghanistan. Examples include consultations with specialists, certain surgical procedures, and radiologic studies such as MRI. It is possible that many of the subjects with the most mild injuries were returned to duty without being sent to the LRMC.32 Thus, there is a possibility of selection bias toward more seriously injured patients in our cohort. The LRMC serves as a central triage point for the wars in Iraq and Afghanistan; it is not yet possible to perform MRI-based studies in Iraq and Afghanistan because functioning scanners are not currently available to the U.S. military medical system in those countries.
Because DTI can be performed relatively quickly on the MRI scanners at U.S. military facilities and civilian hospitals, DTI-based assessments may be useful in diagnosis, triage, and treatment planning in clinical practice. The analytic methods used here allowed assessment of individual patients with traumatic brain injury, just as it would in a clinical setting. However, it must be emphasized that only 18 of the 63 subjects with traumatic brain injury had definitively abnormal scans when the scans were analyzed individually. For now, mild traumatic brain injury remains primarily a clinical diagnosis. Normal findings on a DTI scan do not rule out traumatic brain injury, nor are DTI findings in isolation sufficient to make this diagnosis with certainty (see the Discussion section in the Supplementary Appendix).
The relationship between DTI abnormalities and clinical outcomes in U.S. military personnel has yet to be determined. A great deal of research along these lines has been conducted in civilians with traumatic brain injury.20,21,31,33–38 However, unique aspects of traumatic brain injury sustained by military personnel include blast injuries and the high rate of post-traumatic stress disorder.3,39–43 The relationships among blast-related traumatic brain injury, axonal injury, and outcomes that include post-traumatic stress disorder are topics of active research. DTI and other advanced MRI techniques are tools that may be useful in probing these relationships.
Supplementary Material
Acknowledgments
Supported by a grant from the Congressionally Directed Medical Research Program (W81XWH-08-2-0061, to Dr. Brody) and the National Institutes of Health (F32NS062529, to Dr. Mac Donald; 5K23HD053212, to Dr. Shimony; P30NS048056, to Dr. Snyder; P50NS06833, to Drs. Raichle and Snyder; and 5K08NS49237, to Dr. Brody.)
We thank the participants, their families, the commanding officers, and the clinical care providers for making this study possible; the staff at the LRMC MRI clinic, including Don Albrant, Kenny Caywood, Kelly McKay, Tim McKay, Tim Roberts, Kris Robertson, Carl Russell, Stephen Sauter, M.D., Antoinette Sherman, and Ludwig Williams; the TBI Screening Team at the LRMC, including Marcel Flores, Shawn Nelson, Pamela Nyman, Shawna Scully, M.D., Karen Williams, and Janna Welch; the staff at the LRMC Trauma Program, including Daniel Lovasz, Kathleen Martin, Caroline Tuman, and Linda Wierzechowski; the Washington University assessment team, including Vera Bonsi, Justin Hampton, Leslie Schart, Ph.D., Eric Shumaker, and Elaine Tamez; and Gina D’Angelo, Ph.D., Washington University Statistical Consulting Service.
Footnotes
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of the Army, Department of the Air Force, Department of Defense, or federal government.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
Dedicated to the memory of John Witherow, who died in July 2010.
References
- 1.Tanielian TL, Jaycox LH, editors. Invisible wounds of war: psychological and cognitive injuries, their consequences, and services to assist recovery. Santa Monica, CA: RAND; 2008. [Google Scholar]
- 2.Casscells SW. Traumatic brain injury: definition and reporting. Washington, DC: Department of Defense; 2007. Oct, (memorandum). ( http://mhs.osd.mil/Content/docs/pdfs/policies/2007/07-030.pdf.) [Google Scholar]
- 3.Hoge CW, McGurk D, Thomas JL, Cox AL, Engel CC, Castro CA. Mild traumatic brain injury in U.S. soldiers returning from Iraq. N Engl J Med. 2008;358:453–463. doi: 10.1056/NEJMoa072972. [DOI] [PubMed] [Google Scholar]
- 4.Jones E, Fear NT, Wessely S. Shell shock and mild traumatic brain injury: a historical review. Am J Psychiatry. 2007;164:1641–1645. doi: 10.1176/appi.ajp.2007.07071180. [DOI] [PubMed] [Google Scholar]
- 5.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Staining of amyloid precursor protein to study axonal damage in mild head injury. Lancet. 1994;344:1055–1056. doi: 10.1016/s0140-6736(94)91712-4. [DOI] [PubMed] [Google Scholar]
- 6.Blumbergs PC, Scott G, Manavis J, Wainwright H, Simpson DA, McLean AJ. Topography of axonal injury as defined by amyloid precursor protein and the sector scoring method in mild and severe closed head injury. J Neurotrauma. 1995;12:565–572. doi: 10.1089/neu.1995.12.565. [DOI] [PubMed] [Google Scholar]
- 7.Mott FW. The microscopic examination of the brains of two men dead of commotio cerebri (shell shock) without visible external injury. J R Army Med Corps. 1917;29:662–677. doi: 10.1136/bmj.2.2967.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benzinger TL, Brody D, Cardin S, et al. Blast-related brain injury: imaging for clinical and research applications: report of the 2008 St. Louis workshop. J Neurotrauma. 2009;26:2127–2144. doi: 10.1089/neu.2009.0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chafi MS, Karami G, Ziejewski M. Biomechanical assessment of brain dynamic responses due to blast pressure waves. Ann Biomed Eng. 2010;38:490–504. doi: 10.1007/s10439-009-9813-z. [DOI] [PubMed] [Google Scholar]
- 10.Taylor PA, Ford CC. Simulation of blast-induced early-time intracranial wave physics leading to traumatic brain injury. J Biomech Eng. 2009;131:061007. doi: 10.1115/1.3118765. [DOI] [PubMed] [Google Scholar]
- 11.Peskind ER, Petrie EC, Cross DJ, et al. Cerebrocerebellar hypometabolism associated with repetitive blast exposure mild traumatic brain injury in 12 Iraq War veterans with persistent post-concussive symptoms. Neuroimage. 2011;54 Suppl 1:S76–S82. doi: 10.1016/j.neuroimage.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Warden DL, French LM, Shupenko L, et al. Case report of a soldier with primary blast brain injury. Neuroimage. 2009;47 Suppl 2:T152–T153. doi: 10.1016/j.neuroimage.2009.01.060. [DOI] [PubMed] [Google Scholar]
- 13.Bauman RA, Ling G, Tong L, et al. An introductory characterization of a combat-casualty-care relevant swine model of closed head injury resulting from exposure to explosive blast. J Neurotrauma. 2009;26:841–860. doi: 10.1089/neu.2008.0898. [DOI] [PubMed] [Google Scholar]
- 14.Pierpaoli C, Jezzard P, Basser PJ, Barnett A, Di Chiro G. Diffusion tensor MR imaging of the human brain. Radiology. 1996;201:637–648. doi: 10.1148/radiology.201.3.8939209. [DOI] [PubMed] [Google Scholar]
- 15.Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. AJNR Am J Neuroradiol. 2002;23:794–802. [PMC free article] [PubMed] [Google Scholar]
- 16.Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007;27:11869–11876. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007;205:116–131. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dempsey KE, Dorlac WC, Martin K, et al. Landstuhl Regional Medical Center: traumatic brain injury screening program. J Trauma Nurs. 2009;16:6–12. doi: 10.1097/01.JTN.0000348063.41099.a7. [DOI] [PubMed] [Google Scholar]
- 19.Mori S, Wakana S, Nagae-Poetscher LM, van Zijl PCM. MRI atlas of human white matter. London: Elsevier; 2005. [DOI] [PubMed] [Google Scholar]
- 20.Niogi SN, Mukherjee P, Ghajar J, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–973. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu TC, Wilde EA, Bigler ED, et al. Evaluating the relationship between memory functioning and cingulum bundles in acute mild traumatic brain injury using diffusion tensor imaging. J Neurotrauma. 2010;27:303–307. doi: 10.1089/neu.2009.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilde EA, Ramos MA, Yallampalli R, et al. Diffusion tensor imaging of the cingulum bundle in children after traumatic brain injury. Dev Neuropsychol. 2010;35:333–351. doi: 10.1080/87565641003696940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh M, Jeong J, Hwang D, Sungkarat W, Gruen P. Novel diffusion tensor imaging methodology to detect and quantify injured regions and affected brain pathways in traumatic brain injury. Magn Reson Imaging. 2010;28:22–40. doi: 10.1016/j.mri.2009.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geary EK, Kraus MF, Pliskin NH, Little DM. Verbal learning differences in chronic mild traumatic brain injury. J Int Neuropsychol Soc. 2010;16:506–516. doi: 10.1017/S135561771000010X. [DOI] [PubMed] [Google Scholar]
- 25.Lipton ML, Gellella E, Lo C, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: a voxel-wise analysis of diffusion tensor imaging. J Neurotrauma. 2008;25:1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- 26.Inglese M, Makani S, Johnson G, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- 27.Blumbergs PC, Jones NR, North JB. Diffuse axonal injury in head trauma. J Neurol Neurosurg Psychiatry. 1989;52:838–841. doi: 10.1136/jnnp.52.7.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strich SJ. Diffuse degeneration of the cerebral white matter in severe dementia following head injury. J Neurol Neurosurg Psychiatry. 1956;19:163–185. doi: 10.1136/jnnp.19.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adams JH, Doyle D, Ford I, Gennarelli TA, Graham DI, McLellan DR. Diffuse axonal injury in head injury: definition, diagnosis and grading. Histopathology. 1989;15:49–59. doi: 10.1111/j.1365-2559.1989.tb03040.x. [DOI] [PubMed] [Google Scholar]
- 30.Gurdjian ES, Gurdjian ES. Cerebral contusions: re-evaluation of the mechanism of their development. J Trauma. 1976;16:35–51. [PubMed] [Google Scholar]
- 31.Sidaros A, Engberg AW, Sidaros K, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- 32.Luethcke CA, Bryan CJ, Morrow CE, Isler WC. Comparison of concussive symptoms, cognitive performance, and psychological symptoms between acute blast-versus nonblast-induced mild traumatic brain injury. J Int Neuropsychol Soc. 2011;17:36–45. doi: 10.1017/S1355617710001207. [DOI] [PubMed] [Google Scholar]
- 33.Huisman TA, Schwamm LH, Schaefer PW, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. AJNR Am J Neuroradiol. 2004;25:370–376. [PMC free article] [PubMed] [Google Scholar]
- 34.Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 35.Niogi SN, Mukherjee P, Ghajar J, et al. Structural dissociation of attentional control and memory in adults with and without mild traumatic brain injury. Brain. 2008;131:3209–3221. doi: 10.1093/brain/awn247. [DOI] [PubMed] [Google Scholar]
- 36.Perlbarg V, Puybasset L, Tollard E, Lehéricy S, Benali H, Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: a diffusion tensor imaging study using voxel-based approaches. Hum Brain Mapp. 2009;30:3924–3933. doi: 10.1002/hbm.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang JY, Bakhadirov K, Devous MD, et al. Diffusion tensor tractography of traumatic diffuse axonal injury. Arch Neurol. 2008;65:619–626. doi: 10.1001/archneur.65.5.619. [DOI] [PubMed] [Google Scholar]
- 38.Lipton ML, Gulko E, Zimmerman ME, et al. Diffusion-tensor imaging implicates prefrontal axonal injury in executive function impairment following very mild traumatic brain injury. Radiology. 2009;252:816–824. doi: 10.1148/radiol.2523081584. [DOI] [PubMed] [Google Scholar]
- 39.Carlson KF, Kehle SM, Meis LA, et al. Prevalence, assessment, and treatment of mild traumatic brain injury and posttraumatic stress disorder: a systematic review of the evidence. J Head Trauma Rehabil. 2011;26:103–115. doi: 10.1097/HTR.0b013e3181e50ef1. [DOI] [PubMed] [Google Scholar]
- 40.Carlson KF, Nelson D, Orazem RJ, Nugent S, Cifu DX, Sayer NA. Psychiatric diagnoses among Iraq and Afghanistan war veterans screened for deployment-related traumatic brain injury. J Trauma Stress. 2010;23:17–24. doi: 10.1002/jts.20483. [DOI] [PubMed] [Google Scholar]
- 41.Chemtob CM, Muraoka MY, Wu-Holt P, Fairbank JA, Hamada RS, Keane TM. Head injury and combat-related posttraumatic stress disorder. J Nerv Ment Dis. 1998;186:701–708. doi: 10.1097/00005053-199811000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Vasterling JJ, Constans JI, Hanna-Pladdy B. Head injury as a predictor of psychological outcome in combat veterans. J Trauma Stress. 2000;13:441–451. doi: 10.1023/A:1007781107513. [DOI] [PubMed] [Google Scholar]
- 43.Levin HS, Wilde E, Troyanskaya M, et al. Diffusion tensor imaging of mild to moderate blast-related traumatic brain injury and its sequelae. J Neurotrauma. 2010;27:683–694. doi: 10.1089/neu.2009.1073. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.