Abstract
BACKGROUND:
Very preterm adolescents display persistent deficits in neuropsychological functions.
OBJECTIVE:
To compare cognitive and language outcomes at 16 years and cognitive and receptive vocabulary trajectories throughout school years between very preterm and term children and to determine child and family factors associated with better developmental trajectories.
DESIGN AND METHODS:
At 8, 12, and 16 years, 322 very preterm children with birth weights of 1250 g or less and 41 term children had cognitive and language testing. Hierarchical growth-curve modeling was used to delineate the differences in cognitive and receptive vocabulary development between participants. Cluster analyses allowed for the characterization of very preterm children with different patterns of cognitive and receptive vocabulary development.
RESULTS:
At 16 years, very preterm adolescents had deficits in general cognition and higher-order language skills (phonological awareness and phonemic decoding) compared with term peers. Although the between-group difference in cognitive scores remained stable from 8 to 16 years, very preterm children demonstrated catch-up gains in receptive vocabulary during the same period. Moreover, subgroups of very preterm children displayed developmental trajectories in cognition similar to term children (55% on the vocabulary and 46% on the block-design subtests). These children had lower rates of neurosensory impairment and mothers with higher education and were from an ethnic nonminority.
CONCLUSIONS:
Significant catch-up in receptive vocabulary is observed by the age of 16 years among very preterm children compared to term peers. The absence of neurosensory impairment and residing in a favorable socioeconomic milieu are associated with the most optimal developmental trajectories.
Keywords: very low birth weight, prematurity, cognitive development, language
WHAT'S KNOWN ON THIS SUBJECT:
Very preterm children display neuropsychological deficits that persist into adolescence.
WHAT THIS STUDY ADDS:
By adolescence, very preterm children show catch-up gains in receptive vocabulary. The absence of significant neurosensory impairment and a favorable socioeconomic milieu are associated with better cognitive developmental trajectories across school years.
Very preterm children are at higher risk of neuropsychological difficulties from early school age until adulthood, leading to lower school attainment.1–4 Studies have documented impairment in cognition among very preterm children,5 along with deficits in different language components, such as phonological processing and vocabulary6–10 and literacy skills.6,11–15 Comprehensive language assessment of more recent birth cohorts that have now reached adolescent years is scarce.
Moreover, the developmental trajectory of cognitive and language functions among very preterm children remains poorly defined because of few existing longitudinal studies using similar outcome measures over time. Long-term follow-up of very preterm children enrolled in the Multicenter Randomized Indomethacin Intraventricular Hemorrhage Prevention Trial has allowed for the identification of severe neonatal brain injury and socioeconomic disadvantage as predictors of slower development in receptive vocabulary from the age of 3 to 12 years.16 Taylor et al17 also have shown that children with birth weights less than 750 g generally display poorer cognitive progress during the school-aged years than their term counterparts, especially on tasks of visual-motor integration and executive function.
The aim of this study was to compare cognitive and language outcomes between very preterm and term adolescents at the age of 16 years. The second objective was to examine the trajectory of cognition and receptive vocabulary from the age of 8 to 16 years of very preterm children in comparison with term control children. Finally, child and family factors associated with better development among very preterm children were investigated. We hypothesized that (1) very preterm adolescents would have poorer language performance than term control children, especially with increasingly complex tasks, (2) although the gap in cognitive function between preterm and term children was expected to remain stable over time, slower gains in receptive vocabulary would be observed among those born very preterm, especially boys, given the increased vulnerability of the preterm male brain to sequelae in regions subserving language function,18,19 and (3) the absence of severe neonatal brain injury and favorable socioeconomic status would be associated with better developmental trajectories.
DESIGN AND METHODS
Population
The study population has been previously described and comes from a cohort of 505 infants with birth weight 1250 g or less admitted to 3 hospital centers in Rhode Island, Maine, and Connecticut, between September 1989 and August 1992.20,21 A total of 440 preterm children survived to 8 years of age and were followed until age 12 years. At 16 years, 437 survivors were available for follow-up.6,22 From the 8- to 16-year visits, 124 term control children were recruited from the local community or randomly selected from a telemarketing list and frequency matched on age, gender, race, and zip code. Informed consent was obtained from parents and children at each assessment. The institutional review boards of all participating institutions approved of the protocols.
Data Collection
Serial standardized neuropsychological tests were conducted by trained assessors. They were blinded to participants' perinatal history and to previous psychometric scores.
General intellectual ability was measured using the Wechsler Intelligence Scale for Children, Third Edition (WISC-III),23 from which the verbal, performance, and full-scale IQs were obtained. Raw scores on the vocabulary and block-design subtests, which are strongly correlated with general intelligence,24 were used for longitudinal analyses. Vocabulary subtest measures word knowledge and is computed in the verbal IQ, whereas block-design assesses visual-spatial problem-solving skills and composes the performance IQ.
Specific language skills were assessed with the Peabody Picture Vocabulary Test–Revised (PPVT-R)25 for receptive vocabulary development and the Comprehensive Test of Phonological Processing.26 The Comprehensive Test of Phonological Processing yields 3 composite scores: rapid naming (rapid digit naming and rapid letter-naming subtests) measures efficient retrieval of phonological information from memory; phonological awareness (blending and segmented nonwords subtests) assesses how well a person can reproduce and manipulate the sound structure (phonemes) of oral language; and phonological memory (nonword repetition subtest only) refers to coding information phonologically for short-term storage, which is important in decoding new words. Finally, reading abilities were evaluated with the Test of Word Reading Efficiency,27 in which participants were asked to read a list of real words (sight-word efficiency) and pronounceable nonwords (phonemic decoding) as rapidly as possible.
Data on neonatal, sociodemographic, and neurologic characteristics were retrieved from the study database. Bronchopulmonary dysplasia was defined as oxygen need at 28 days. Severe brain injury referred to grades 3–4 intraventricular hemorrhage, periventricular leukomalacia and grade 2 of higher ventriculomegaly on neonatal ultrasound. Neurosensory impairment (NSI) included the presence of abnormal neurologic examination, including cerebral palsy, ventriculo-peritoneal shunt, seizure disorder, hearing aids, or services for the blind.
Statistical Analysis
Because descriptive statistics on test scores at 8 and 12 years have been published previously,6,22 only the results of the cognitive and language assessment at the age of 16 years were compared between preterm and term children, with adjustment for potential confounders (gender, maternal education, minority status, and single parenthood). Between-group mean differences and odds ratios (ORs) with 95% confidence intervals (CIs) were computed by using regression analysis.
Trajectories of cognition and receptive vocabulary were delineated using a multilevel-model approach to individual growth modeling.28,29 Details on this technique were outlined in a previous article.16 Analyses were conducted on raw scores, which are more sensitive to change. Raw scores refer to the number of points achieved by the participant on a subtest. With increasing cognitive development, examinees succeed in passing more items on each subtest and therefore obtain higher raw scores with time. From raw scores, norm-referenced measures are obtained to facilitate comparison with an age-standardized population. Growth modeling analysis was performed on WISC-III vocabulary and block-design raw scores, because of their strong correlation with general intelligence, and on PPVT-R raw scores. Two main parameters are involved in growth modeling: the “intercept parameter,” which represents initial status at 8 years and the “slope parameter,” which describes the rate of growth per year in cognition and receptive language from the age of 8 to 16 years. The effect of very preterm birth on the intercept and slope parameters was examined first. Interaction between preterm status and gender also was assessed. Then, covariates thought to potentially influence developmental trajectories were entered in the model (maternal education, minority status, and household structure) for adjustment.
Finally, hierarchical agglomerative cluster analysis was performed on vocabulary, block-design, and PPVT-R raw scores to identify groups of very preterm children with similar developmental patterns.30 In this type of analysis, each individual initially forms its own cluster. Later, in successive steps, similar clusters are combined on the basis of Ward's method, which attempts to minimize the sum of squares of any 2 (hypothetical) clusters. Once a cluster is formed, it cannot be split. The mahalanobis distance was used to take into account correlation in the data.31
Only very preterm children with data at all 3 visits could be entered in the models for cluster analysis (vocabulary: n = 309; block design: n = 315; and PPVT-R: n = 302). Discriminant function analysis was used to estimate the percentage of children correctly classified to validate the model. Once a classification structure was retained that best represented the data, comparisons of child and family factors across the clusters were initially performed, using analysis of variance and χ2 tests. Then, multinomial logistic regression was performed to account for the different covariates in the logistic models. For all comparisons between clusters, P values of <.05 were considered statistically significant. All analyses were conducted by using SAS 9.2 (SAS Institute, Inc, Cary, NC).
RESULTS
Table 1 lists the characteristics of the preterm and term cohorts at 8, 12, and 16 years of age. Over the study period, 322 preterm and 41 term participants were seen at all 3 visits and had cognitive and language testing completed. They did not differ from the entire study population in terms of gestational age, birth weight, gender, social factors, and WISC full-scale IQ (data not shown).
TABLE 1.
Population Characteristics at the Ages of 8, 12, and 16 Years
| Preterm |
Term |
|||||
|---|---|---|---|---|---|---|
| 8 y | 12 y | 16 y | 8 y | 12 y | 16 y | |
| n seen/n eligible | 375/440 | 374/440 | 334/437 | 47/53 | 111/119 | 102/124 |
| Follow-up rate, % | 85 | 85 | 76 | 89 | 93 | 83 |
| Child characteristics | ||||||
| Gestational age, mean (SD), wk | 28 (2) | 28 (2) | 28 (2) | — | — | — |
| Birth weight, mean (SD), g | 961 (174) | 962 (174) | 960 (173) | — | — | — |
| Male gender, n (%) | 200 (53) | 202 (54) | 177 (53) | 22 (47) | 51 (46) | 49 (48) |
| Small for gestational age, n (%) | 89 (24) | 93 (25) | 77 (23) | |||
| Multiple births, n (%) | 74 (20) | 76 (20) | 69 (21) | |||
| Antenatal steroids, n (%) | 126 (34) | 129 (34) | 113 (34) | |||
| O2 need at 28 d | ||||||
| N | 374 | 373 | 333 | |||
| n (%) | 166 (44) | 171 (46) | 154 (46) | |||
| Severe brain injury | ||||||
| N | 371 | 370 | 331 | |||
| n (%)a | 35 (9) | 34 (9) | 31 (9) | |||
| Family factors | ||||||
| Maternal education (N = 368), n (%) | ||||||
| Less than high school | 48 (13) | 41(11) | 41 (12) | 7 (15) | 9 (8) | 5 (5) |
| High school | 132 (36) | 131 (35) | 109 (33) | 8 (17) | 27 (24) | 27 (26) |
| ≥1 y of college | 188 (51) | 201 (54) | 184 (55) | 32 (68) | 75 (68) | 70 (69) |
| N | 328 | 329 | 333 | 42 | 98 | |
| Race and ethnicity | ||||||
| Non-Hispanic white, n (%) | 225 (69) | 225 (68) | 226 (68) | 29 (69) | 69 (71) | 72 (70) |
| Black, n (%) | 57 (17) | 58 (18) | 60 (18) | 57(17) | 18 (18) | 19 (19) |
| Hispanic white, n (%) | 16 (5) | 17 (5) | 17 (5) | 1 (2) | 2 (2) | 2 (2) |
| Other, n (%) | 30 (9) | 29 (9) | 30 (9) | 5 (12) | 9 (9) | 9 (9) |
| Single-parent household | ||||||
| N | 369 | 369 | 44 | |||
| n (%) | 120 (33) | 125 (33) | 111 (33) | 11 (25) | 31 (28) | 25 (25) |
| Neurocognitive outcomes | ||||||
| Any NSI | ||||||
| N | 371 | 333 | 46 | 98 | ||
| n (%)b | 57 (15) | 56 (15) | 51 (15) | 0 (0) | 0 (0) | 0 (0) |
| WISC-III full-scale IQ | ||||||
| N | 373 | 366 | 326 | 99 | ||
| Mean (SD) | 91 (20) | 88 (18) | 87 (19) | 106 (16) | 104 (16) | 104 (16) |
The number of children (n) for whom data are available is mentioned only when there are missing data.
Severe brain injury includes grades 3 to 4 intraventricular hemorrhage, periventricular leukomalacia, and grade 2 and above ventriculomegaly on neonatal ultrasound.
NSI includes the presence of any of the following: abnormal neurologic examination, including cerebral palsy, ventriculo peritoneal shunt, seizure disorder, hearing aids, and services for the blind.
Outcomes at 16 Years
Differences in IQ scores between the very preterm and term cohorts remained significant at 16 years of age (Table 2).
TABLE 2.
Comparison of the Results Between Very Preterm Adolescents and Term Control Children on Tests of Cognition and Language
| Preterm |
Term |
Adjusted Mean Difference (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| n | Mean (SD) | Impairment <2 SDs, n (%) | n | Mean (SD) | Impairment <2 SDs, n (%) | ||
| WISC-III | |||||||
| Full-scale IQ | 326 | 87 (19) | 51 (16) | 99 | 104 (16) | 2 (2) | −13.3 (−19.0 to −7.5)a |
| Verbal IQ | 327 | 89 (19) | 39 (12) | 99 | 103 (15) | 3 (3) | −9.4 (−15.1 to −3.7)a |
| Performance IQ | 330 | 87 (19) | 52 (16) | 99 | 104 (17) | 0 | −15.5 (−21.6 to −9.4)a |
| Verbal comprehension | 326 | 91 (18) | 99 | 103 (15) | −8.0 (−13.3 to −2.6)a | ||
| Perceptual organization | 329 | 89 (19) | 99 | 104 (17) | −14.3 (−20.3 to −8.2)a | ||
| Freedom of distractibility | 325 | 89 (18) | 99 | 100 (15) | −8.6 (−14.3 to −2.9)a | ||
| Processing speed | 320 | 93 (21) | 97 | 107 (16) | −13.7 (−20.2 to −7.3)a | ||
| Vocabulary subtest raw score | 327 | 38 (12) | 99 | 43 (8) | −2.7 (−6.1 to 0.7) | ||
| Block-design subtest raw score | 330 | 44 (17) | 99 | 55 (10) | −8.9 (−14.1 to −3.7)a | ||
| PPVT-R | 330 | 95 (24) | 42 (13) | 101 | 106 (21) | 3 (3) | −5.5 (−12.3 to 1.3) |
| CTOPP composite scores | |||||||
| Rapid naming | 306 | 96 (23) | 37 (12) | 102 | 99 (14) | 3 (3) | −3.9 (−10.9 to 3.2) |
| Phonological awareness | 251 | 82 (16) | 46 (18) | 94 | 91 (13) | 2 (2) | −5.1 (−10.1 to −0.1)b |
| CTOPP subtest | |||||||
| Rapid digit naming | 309 | 9 (4) | 102 | 10 (2) | −0.5 (−1.6 to 0.7) | ||
| Rapid letter naming | 307 | 10 (4) | 102 | 10 (3) | −0.9 (−2.2 to 0.4) | ||
| Nonword repetition | 306 | 8 (3) | 102 | 9 (2) | −0.4 (−1.2 to 0.5) | ||
| Phoneme reversal | 252 | 7 (3) | 94 | 9 (3) | −0.7 (−1.7 to 0.3) | ||
| Blending nonwords | 251 | 8 (3) | 94 | 9 (2) | −0.5 (−1.5 to 0.5) | ||
| Segmented nonwords | 251 | 6 (3) | 94 | 8 (2) | −1.1 (−2.0 to −0.2)b | ||
| TOWRE | |||||||
| Sight word efficiency | 308 | 89 (15) | 25 (8) | 101 | 94 (10) | 2 (2) | −2.8 (−7.3 to 1.7) |
| Phonemic decoding | 308 | 88 (16) | 42 (14) | 101 | 94 (13) | 2 (2) | −5.6 (−10.5 to −0.7)b |
Adjustment for gender, maternal education, minority status, and single parent household. CTOPP indicates Comprehensive Test of Phonological Processing, TOWRE, Test of Word Reading Efficiency.
P < .005.
P < .05.
Although very preterm adolescents performed, on average, at a lower level than their term counterparts on language tasks, significant differences were not detected on tests of receptive vocabulary, rapid naming, and sight-word reading, after adjustment for gender and social factors (Table 2). Very preterm adolescents obtained significantly lower scores on more complex tasks of phonological awareness and phonemic decoding. Moreover, a higher proportion of very preterm adolescents scored in the impaired ranges (<70) on the PPVT-R (OR: 3.9 [95% CI: 1.1–13.1]), on rapid naming (OR: 4.3 [95% CI: 1.3–14.7]), on phonological awareness (OR: 9.1 [95% CI: 2.1–39.2]), and on phonemic decoding (OR: 6.5 [95% CI: 1.5–27.6]). On the sight word-efficiency scale, 8% of very preterm adolescents versus 2% of term control infants performed in the abnormal ranges. This difference, however, was not statistically significant (OR: 3.7 [95% CI: 0.9–16.3]).
Trajectories of Cognitive and Receptive Vocabulary Development
Table 3 shows the effect of preterm birth, male gender, and the interaction of these 2 factors on growth in cognitive and receptive vocabulary scores from the age of 8 through 16 years. The intercept indicates a mean raw score for the entire study population at the age of 8 years on WISC-III vocabulary, WISC-III block design, and PPVT-R. Preterm birth conferred a disadvantage in initial raw score at 8 years of age. Preterm participants had a 5.5-point gap behind term children on vocabulary, a 7.8-point negative difference on block design, and performed 14.4 points lower on the PPVT-R. A gender effect was observed on block design only among children born at term, with term boys performing better than term girls by 7.1 points.
TABLE 3.
Adjusted Individual Growth Models for Longitudinal Changes in WISC-III Vocabulary, WISC-III Block-Design, and PPVT-R Raw Scores and Effects of Preterm Birth and Gender on Growth From 8 Through 16 Years of Age
| Parameters and Growth Predictors | WISC-III Vocabulary Subtest, Estimate (SE) | WISC-III Block Design Subtest, Estimate (SE) | PPVT-R, Estimate (SE) |
|---|---|---|---|
| Fixed effects | |||
| Initial status | |||
| Intercept | 23.5 (1.5)a | 27.6 (2.6)a | 99.0 (5.3)a |
| Preterm birth | −5.5 (1.5)a | −7.8 (2.5)a | −14.4 (5.1)b |
| Male gender | 2.3 (2.0) | 7.1 (3.4)b | 3.1 (7.0) |
| Preterm birth × male | −2.9 (2.1) | −7.5 (3.6) | −4.2 (7.4) |
| Rate of change | 9.8 (0.8)a | 13.3 (1.2)a | 19.1 (1.7)a |
| Age, y | |||
| Age × preterm birth | 0.3 (0.8) | −0.9 (1.2) | 4.0 (1.8)b |
| Age × male | −1.7 (1.1) | −2.6 (1.7) | −0.3 (2.5) |
| Age × preterm birth × male | 1.9 (1.2) | 2.8 (1.8) | −0.9 (2.6) |
Models are adjusted for the level of maternal education, minority status, and single-parent household.
P < .001.
P < .05.
As expected, raw scores increased yearly by 9.8, 13.3, and 19.1 points on vocabulary, block design, and PPVT-R, respectively. Rates of change on WISC-III cognitive scores were similar between preterm and term children, regardless of gender, meaning that the overall difference in vocabulary and block-design scores observed between the 2 groups remained constant from the age of 8 through 16 years. Contrary to our hypothesis, increases in PPVT-R raw scores over time were higher among very preterm children compared with term control children, with an additional 4.0-point gain yearly. It is notable that gender was not associated with differential rate of change in PPVT-R raw scores.
Patterns of Cognitive and Receptive Vocabulary Development
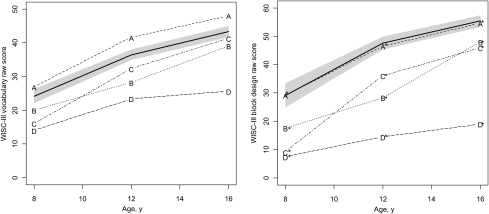
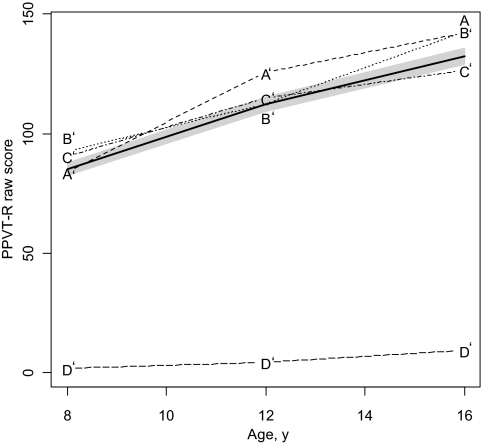
Cluster analyses revealed 4 patterns of development for WISC-III vocabulary and block-design subtests (Fig 1) and PPVT-R (Fig 2). Using this 4-cluster structure, discriminant analysis showed that 87% of the total preterm sample was correctly classified in the vocabulary model, 94% in block design, and 88% in PPVT-R.
FIGURE 1.
Patterns of cognitive development from the age of 8 to 16 years: A, WISC-III vocabulary raw score; B, WISC-III block-design raw score. Raw scores of term children are indicated with the bold line. The shadow represents CIs. Subgroups A and A* show similar patterns of cognitive development to the term cohort. Subgroup C also is catching up to the term group by the age of 16 years.
FIGURE 2.
Patterns of receptive language development from the age of 8 to 16 years. PPVT-R raw scores of term children are indicated with the bold line. The shadow represents CIs. By the age of 16 years, subgroups A' and B' are catching up to the term group.
In the vocabulary model, cluster A (17% of very preterm children) surpassed the term group at each assessment. Clusters B (21%) and C (38%) started with a lower score at 8 years of age compared with term control children. Scores improved over time, with cluster C catching up with term children by the age of 16 years. Finally, cluster D (25%) had significantly lower scores than all groups with an increasing gap from the age of 8 to 16 years.
On block design, cluster A* (46%) showed a similar pattern of visual-spatial cognitive development to the term group. Clusters B* (21%) and C* (13%) displayed lower scores compared with term control children from the age of 8 to 16 years, with cluster C* closing the gap with cluster B* by age 16 years. Cluster D* (20%) obtained very low scores with minimal gains over time.
Finally, regarding PPVT-R developmental trajectory, all clusters obtained lower scores at age 8 years. By age 12 years, cluster A' (28%) caught up to the term group and by age 16 years, cluster B' (33%) also displayed similar performance to control children.
Characteristics Differentiating Patterns of Development
Multinomial logistic regression allowed for the identification of factors that discriminated among the different clusters. All variables identified in Tables 4, 5 and 6 were entered except for gestational age, because of its high correlation with birth weight.
TABLE 4.
Characteristics of the 4 Clusters for Longitudinal Changes in WISC-III Vocabulary Raw Scores
| Cluster A | Cluster B | Cluster C | Cluster D | |
|---|---|---|---|---|
| n | 51 | 65 | 116 | 77 |
| Proportion of total preterm cohort, %, n = 309 | 17 | 21 | 38 | 25 |
| Child characteristics | ||||
| Gestational age, mean (SD), wk | 27.9 (1.9) | 28.0 (1.9) | 28.0 (1.9) | 27.9 (2.0) |
| Birth weight, mean (SD), g | 950 (173) | 969 (172) | 968 (157) | 905 (192) |
| Male gender, n (%) | 27 (53) | 38 (58) | 62 (53) | 41 (53) |
| Multiple, n (%) | 9 (18) | 12 (18) | 31 (27) | 16 (21) |
| Small for gestational age, n (%) | 11 (22) | 17 (26) | 29 (25) | 17 (22) |
| Maternal steroids, n (%) | 21 (41) | 23 (35) | 43 (37) | 23 (30) |
| Indomethacin, n (%) | 25 (49) | 31 (48) | 59 (52) | 39 (53) |
| Bronchopulmonary dysplasia, n (%) | 19 (38) | 32 (49) | 50 (43) | 39 (51) |
| Neonatal brain injury, n (%) | ||||
| None | 41 (80) | 48 (74) | 93 (80) | 49 (64) |
| Severe brain injurya | 6 (12) | 4 (6) | 3 (3) | 14 (18) |
| NSI, n (%)b | 6 (12) | 8 (12) | 7 (6) | 22 (29) |
| Family factors | ||||
| Maternal age at birth, mean (SD), y | 30.0 (6.0) | 27.2 (5.6) | 27.9 (5.9) | 27.0 (6.0) |
| Maternal education, n (%) | ||||
| High school or less | 7 (14) | 28 (43) | 39 (34) | 48 (62) |
| ≥1 year of college | 44 (86) | 37 (57) | 76 (66) | 29 (38) |
| Race and ethnicity, n (%) | ||||
| Non-Hispanic white | 44 (86) | 44 (68) | 78 (67) | 45 (59) |
| Others | 7 (14) | 21 (32) | 38 (33) | 31 (41) |
| Bilingual environment, n (%) | 6 (12) | 8 (12) | 12 (10) | 15 (20) |
| Single-parent household at the age of 16 y, n (%) | 12 (24) | 22 (34) | 32 (28) | 35 (45) |
Severe brain injury includes grades 3 to 4 intraventricular hemorrhage, periventricular leukomalacia, and grade 2 and above ventriculomegaly.
NSI includes the presence of any of the following: abnormal neurological examination including cerebral palsy, ventriculo peritoneal shunt, seizure disorder, hearing aids, services for the blind.
TABLE 5.
Characteristics of the 4 Clusters for Longitudinal Changes in WISC-III Block-Design Raw Scores
| Cluster A* | Cluster B* | Cluster C* | Cluster D* | |
|---|---|---|---|---|
| N | 145 | 67 | 41 | 62 |
| Proportion of total preterm cohort (N = 315), % | 46 | 21 | 13 | 20 |
| Child characteristics | ||||
| Gestational age, mean (SD), wk | 28.1 (1.9) | 27.8 (1.8) | 28.5 (2.0) | 27.3 (2.0) |
| Birth weight, mean (SD), g | 976 (168) | 953 (167) | 987 (180) | 913 (173) |
| Male gender, n (%) | 75 (52) | 33 (49) | 24 (58) | 37 (60) |
| Multiple, n (%) | 37 (26) | 10 (15) | 10 (24) | 11 (18) |
| Small for gestational age, n (%) | 41 (28) | 13 (19) | 11 (27) | 10 (16) |
| Maternal steroids, n (%) | 61 (42) | 24 (36) | 13 (32) | 12 (19) |
| Indomethacin, n (%) | 64 (45) | 39 (60) | 23 (56) | 31 (52) |
| Bronchopulmonary dysplasia, n (%) | 56 (39) | 33 (49) | 20 (49) | 32 (52) |
| Neonatal brain injury, n (%) | ||||
| None | 112 (77) | 53 (79) | 32 (78) | 39 (63) |
| Severe brain injurya | 6 (4) | 4 (6) | 3 (7) | 14 (23) |
| NSI, n (%) | 6 (4) | 8 (12) | 1 (2) | 28 (45) |
| Family factors | ||||
| Maternal age at birth, mean (SD), y | 28.1 (6.2) | 27.6 (5.8) | 27.1 (6.1) | 27.3 (5.9) |
| Maternal education, n (%) | ||||
| High school or less | 44 (30) | 30 (45) | 18 (44) | 34 (56) |
| ≥1 year of college | 101 (70) | 37 (55) | 23 (56) | 27 (44) |
| Race and ethnicity, n (%) | ||||
| Non-Hispanic white | 114 (79) | 45 (67) | 21 (52) | 33 (53) |
| Other | 31 (21) | 22 (33) | 19 (48) | 29 (47) |
| Single-parent household at the age of 16 y, n (%) | 12 (24) | 22 (34) | 32 (28) | 35 (45) |
Severe brain injury includes grades 3 to 4 intraventricular hemorrhage, periventricular leukomalacia, and grade 2 and above ventriculomegaly.
TABLE 6.
Characteristics of the 4 Clusters for Longitudinal Changes in PPVT-R Raw Scores
| Cluster A' | Cluster B' | Cluster C' | Cluster D' | |
|---|---|---|---|---|
| N | 84 | 101 | 111 | 6 |
| Proportion of total preterm cohort (N = 302), % | 28 | 33 | 37 | 2 |
| Child characteristics | ||||
| Gestational age, mean (SD), wk | 27.9 (2.2) | 27.9 (1.8) | 28.0 (1.9) | 28.0 (2.3) |
| Birth weight, mean (SD), g | 929 (186) | 973 (153) | 967 (184) | 884 (176) |
| Male gender, n (%) | 42 (50) | 59 (58) | 60 (54) | 5 (83) |
| Multiple, n (%) | 20 (24) | 27 (27) | 18 (16) | 0 (0) |
| Small for gestational age, n (%) | 19 (23) | 22 (22) | 31 (28) | 2 (33) |
| Maternal steroids, n (%) | 27 (32) | 41 (41) | 38 (34) | 1 (17) |
| Indomethacin, n (%) | 38 (46) | 48 (49) | 62 (56) | 3 (60) |
| Bronchopulmonary dysplasia, n (%) | 41 (49) | 47 (47) | 48 (43) | 5 (83) |
| Neonatal brain injury, n (%) | ||||
| None | 64 (76) | 78 (77) | 82 (74) | 2 (33) |
| Severe brain injurya | 5 (6) | 9 (9) | 7 (6) | 4 (67) |
| NSI, n (%) | 12 (14) | 9 (9) | 14 (13) | 6 (100) |
| Family factors | ||||
| Maternal age at birth, mean (SD), y | 28.9 (6.3) | 28.0 (5.9) | 27.2 (6.0) | 27.4 (6.5) |
| Maternal education, n (%) | ||||
| High school or less | 28 (33) | 41 (41) | 48 (44) | 3 (50) |
| ≥1 year of college | 56 (67) | 60 (59) | 62 (56) | 3 (50) |
| Race and ethnicity, n (%) | ||||
| Non-Hispanic white | 54 (64) | 79 (78) | 70 (64) | 5 (83) |
| Other | 30 (36) | 22 (22) | 40 (36) | 1 (17) |
| Bilingual environment, n (%) | 13 (15) | 12 (12) | 14 (13) | 2 (33) |
| Single-parent household at 16 y, n (%) | 20 (24) | 33 (33) | 44 (40) | 1 (17) |
Severe brain injury includes grades 3 to 4 intraventricular hemorrhage, periventricular leukomalacia, and grade 2 and above ventriculomegaly.
Table 4 shows child and family characteristics for each pattern of verbal cognitive development. Clusters did not differ on birth weight, antenatal steroids, gender, multiple birth, prophylactic indomethacin, being small for gestational age, bronchopulmonary dysplasia, maternal age, bilingual environment, and household structure. Mothers in cluster A, in which children obtained the best performance over time, had higher educational levels than mothers in clusters B, C, and D (detailed ORs available on request). Mothers in clusters B and C also were more educated than those in cluster D. Cluster A had a lower percentage of children from an ethnic minority than the other clusters. Finally, children in cluster D, who had the slowest cognitive gains over time, displayed the highest rate of NSI.
Table 5 outlines characteristics of the different clusters for longitudinal changes in block-design scores. Once again, clusters differed on maternal education, child's race and ethnicity, and presence of NSI. Children in cluster A* had mothers with higher education than in clusters B* and D*. There was a higher proportion of children from a nonminority group in cluster A* in comparison with clusters C* and D*, as well as in cluster B* compared with D*. Children in cluster D* differed from those in the other clusters by their higher rate of NSI. Children in cluster B* also were more likely to display NSI compared with those in cluster A*.
Finally, when exploring factors that allowed differentiation of the 4 patterns of receptive vocabulary development, analyses showed that children in cluster D' displayed higher rates of severe brain injury than those in the other clusters. Other child and family factors were not significant.
DISCUSSION
This study demonstrated continuing difficulties among very preterm adolescents in cognition and higher-order language tasks when compared with term peers. However, it also highlighted the potential for catch-up in cognitive skills among very preterm children during the school-aged years. As a whole, very preterm adolescents displayed lower IQs at the age of 16 years compared with term counterparts, although this gap remained constant throughout school years. Nonetheless, subgroups of very preterm children showed progress over time and even achieved performance similar or close to term peers. Moreover, the difference in receptive vocabulary development between very preterm and term children diminished over time.
To our knowledge, only Guarini et al15 showed that certain aspects of phonological awareness were affected during school years in Italian children born at 33 weeks' gestation or earlier. Our study also outlined persisting difficulties among very preterm adolescents on a composite measure of phonological awareness, an important skill for reading accuracy,32 especially for nonword deciphering.33 It comes as no surprise that preterm adolescents displayed lower scores compared with term peers on phonemic decoding. However, differences between preterm and term adolescents were not detected on rapid naming and sight word reading, which are correlated tasks that also involve visual processing (ie, orthographic decoding that relies on mental representation of letters or words to allow later automatic recognition), thus decreasing the demand on phonological processing.33,34 Microstructural-imaging studies of neural pathways subserving rapid naming suggest the emergence of compensatory mechanisms in very preterm adolescents with engagement of both arcuate fasciculi, which connect frontal cortices to temporoparietal regions,35 in contrast to left-dominant activation in typically developing individuals. Likewise, in a functional imaging study comparing normal to poor readers, a positive correlation was found between increased activation of both dorsal inferior frontal gyri and better skills on phonological awareness among poor readers.36
One encouraging finding is the improvement in receptive vocabulary over time in our very preterm cohort. Imaging studies show that very preterm children develop alternative neural connections for semantic processing compared with term control children, which could explain enhanced language skills.37 However, the constant gap across ages between preterm and term children on WISC-III vocabulary (which requires expressive language and conceptualization) and block design could either reflect maturational lag or a limit to cerebral plasticity in the recovery of certain higher-order cognitive functions in very preterm children as a group. Nonetheless, at an individual level, some children showed potential for cognitive catch-up.
From the age of 8 to 16 years, 55% of very preterm children (clusters A and C) displayed cognitive trajectories similar to term control children, as measured by the WISC-III Vocabulary subtest, whereas 46% (cluster A*) performed at the same level as their term counterparts on block design. Factors linked to favorable socioeconomic status in the United States, such as higher maternal education and membership to a nonminority group, were associated with better developmental trajectories. Children in clusters A or A*, who exhibited the best performance over time, had both favorable child and family factors, whereas those in clusters D or D*, who fared poorly, displayed higher rates of NSI and cumulated factors associated with lower socioeconomic status. The importance of social factors as major determinants of child physical and developmental health is well recognized regardless of prematurity status. Children evolving in families with lower socioeconomic status have poorer health38 as well as delayed motor and sociocognitive development.39,40 Moreover, the combined effects of preterm birth and social adversity expose the vulnerable child to a greater risk of slower development and lower educational attainment.41,42 This study provides additional evidence that aggregation of both significant medical morbidities and socioeconomic disadvantage lead to unfavorable developmental trajectories. However, it cannot provide an explanation for the underlying mechanism leading to better or poorer outcomes. Favorable family factors in this study could be proxy measures of a stronger genetic background for higher IQ, better nutrition, decreased exposure to stress, easier access to medical, rehabilitation, and educational resources or better neighborhood, all potential mediators in the pathway linking the social environment to cognition. Furthermore, this study did not look at other medical factors that could affect outcomes, such as white matter abnormalities43 or postnatal steroids,44 because this information was not collected during the neonatal period.
Our study draws its strength from its longitudinal nature and the use of similar measures across time, thus allowing the current statistical analyses. Despite complete sets of data on only 74% of our very preterm cohort, the 322 participants were representative of the entire group and constitute one of the largest recent preterm cohorts followed into adolescent years.
CONCLUSIONS
Although very preterm adolescents continue to display deficits in general cognition and higher-level language skills compared with term peers, significant catch up in receptive vocabulary is observed by the age of 16 years. Moreover, subgroups of very preterm children demonstrate remarkable progress with increasing age. Continued research is needed to identify perinatal interventions that prevent morbidities associated with significant NSI and educational programs that promote early developmental stimulation and parenting qualities in vulnerable families to improve long-term outcomes.45,46
ACKNOWLEDGMENTS
We thank Victoria Watson, Susan Delancy, Jill Maller-Kessleman, Marjorene Ainley, June Gagnon, Karol Katz, Marilyse Julien, and all participating families for their contributions to the study.
FINANCIAL DISCLOSURE: The authors have indicated they have no personal financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH) (NS 27116).
Abbreviations:
- WISC-III
- Wechsler Intelligence Scale for Children, Third Edition
- PPVT-R
- Peabody Picture Vocabulary Test–Revised
- NSI
- neurosensory impairment
- OR
- odds ratio
- CI
- confidence interval
REFERENCES
- 1. Aylward GP. Cognitive and neuropsychological outcomes: more than IQ scores. Ment Retard Dev Disabil Res Rev. 2002;8(4):234–240 [DOI] [PubMed] [Google Scholar]
- 2. Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–728 [DOI] [PubMed] [Google Scholar]
- 3. Bhutta AT, Cleves MA, Casey PH, Cradock MM, Anand KJ. Cognitive and behavioral outcomes of school-aged children who were born preterm: a meta-analysis. JAMA. 2002;288(6):728–737 [DOI] [PubMed] [Google Scholar]
- 4. Pritchard VE, Clark CA, Liberty K, Champion PR, Wilson K, Woodward LJ. Early school-based learning difficulties in children born very preterm. Early Hum Dev. 2009;85(4):215–224 [DOI] [PubMed] [Google Scholar]
- 5. Watts JL, Saigal S. Outcome of extreme prematurity: as information increases so do the dilemmas. Arch Dis Child Fetal Neonatal Ed. 2006;91(3):F221–F225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luu TM, Ment LR, Schneider KC, Katz KH, Allan WC, Vohr BR. Lasting effects of preterm birth and neonatal brain hemorrhage at 12 years of age. Pediatrics. 2009;123(3):1037–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Taylor HG, Minich N, Bangert B, Filipek PA, Hack M. Long-term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J Int Neuropsychol Soc. 2004;10(7):987–1004 [DOI] [PubMed] [Google Scholar]
- 8. Wolke D, Samara M, Bracewell MA, Marlow N. Specific language difficulties and school achievement in children born at 25 weeks of gestation or less. Pediatrics. 2008;152(2):256–262 [DOI] [PubMed] [Google Scholar]
- 9. Guarini A, Sansavini A, Fabbri C, Alessandroni R, Faldella G, Karmiloff-Smith A. Reconsidering the impact of preterm birth on language outcome. Early Hum Dev. 2009;85(10):639–645 [DOI] [PubMed] [Google Scholar]
- 10. Sansavini A, Guarini A, Justice LM, et al. Does preterm birth increase a child's risk for language impairment? Early Hum Dev. 2010;86(12):765–772 [DOI] [PubMed] [Google Scholar]
- 11. Saigal S, Hoult LA, Streiner DL, Stoskopf BL, Rosenbaum PL. School difficulties at adolescence in a regional cohort of children who were extremely low birth weight. Pediatrics. 2000;105(2):325–331 [DOI] [PubMed] [Google Scholar]
- 12. Samuelsson S, Finnstrom O, Flodmark O, Gaddlin PO, Leijon I, Wadsby M. A longitudinal study of reading skills among very-low-birthweight children: is there a catch-up? J Pediatr Psychol. 2006;31(9):967–977 [DOI] [PubMed] [Google Scholar]
- 13. Grunau RE, Whitfield MF, Fay TB. Psychosocial and academic characteristics of extremely low birth weight (≤800 g) adolescents who are free of major impairment compared with term-born control subjects. Pediatrics. 2004;114(6). Available at: www.pediatrics.org/cgi/content/full/114/6/e725 [DOI] [PubMed] [Google Scholar]
- 14. Taylor HG, Klein N, Drotar D, Schluchter M, Hack M. Consequences and risks of <1000-g birth weight for neuropsychological skills, achievement, and adaptive functioning. J Dev Behav Pediatr. 2006;27(6):459–469 [DOI] [PubMed] [Google Scholar]
- 15. Guarini A, Sansavini A, Fabbri C, et al. Long-term effects of preterm birth on language and literacy at eight years. J Child Lang. 2010;37(4):865–885 [DOI] [PubMed] [Google Scholar]
- 16. Luu TM, Vohr BR, Schneider KC, et al. Trajectories of receptive language development from 3 to 12 years of age for very preterm children. Pediatrics. 2009;124(1):333–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor HG, Minich NM, Klein N, Hack M. Longitudinal outcomes of very low birth weight: neuropsychological findings. J Int Neuropsychol Soc. 2004;10(2):149–163 [DOI] [PubMed] [Google Scholar]
- 18. Constable RT, Ment LR, Vohr BR, et al. Prematurely born children demonstrate white matter microstructural differences at 12 years of age, relative to term control subjects: an investigation of group and gender effects. Pediatrics. 2008;121(2):306–316 [DOI] [PubMed] [Google Scholar]
- 19. Kesler SR, Reiss AL, Vohr B, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152(4):513–520, 520e511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin and prevention of intraventricular hemorrhage: a multicenter randomized trial. Pediatrics. 1994;93(4):543–550 [PubMed] [Google Scholar]
- 21. Ment LR, Oh W, Ehrenkranz RA, et al. Low-dose indomethacin therapy and extension of intraventricular hemorrhage: a multicenter randomized trial. J Pediatr. 1994;124(6):951–955 [DOI] [PubMed] [Google Scholar]
- 22. Vohr BR, Allan WC, Westerveld M, et al. School-age outcomes of very low birth weight infants in the indomethacin intraventricular hemorrhage prevention trial. Pediatrics. 2003;111(4 pt 1). Available at: www.pediatrics.org/cgi/content/full/113/2/e340 [DOI] [PubMed] [Google Scholar]
- 23. Wechsler D. Wechsler Scale of Intelligence for Children. 3rd ed New York, NY: Psychological Corporation/Harcourt Brace; 1991 [Google Scholar]
- 24. Kaufman AS. Intelligent Testing With the WISC-III. Tampa, FL: University of South Florida/Wiley Interscience; 1994 [Google Scholar]
- 25. Dunn L, Dunn L. PPVT: Peabody Picture Vocabulary Test: Revised Form. Circle Pines, MN: American Guidance Service; 1981 [Google Scholar]
- 26. Wagner RK, Torgesen JK, Rashotte CA. Comprehensive Test of Phonological Processing. Austin, TX: PRO-ED; 1999 [Google Scholar]
- 27. Wagner RK, Torgesen JK, Rashotte CA. Test of Word Reading Efficiency. Austin, TX: PRO-ED; 1999 [Google Scholar]
- 28. Singer JD. Using SAS PROC MIXED to fit multilevel models, hierarchical models, and individual growth models. J Educ Behav Stat. 1998;24(4):33 [Google Scholar]
- 29. Singer JD, Willet JB. Applied Longitudinal Data Analysis: Modeling Change and Event Occurence. New York, NY: Oxford University Press; 2003 [Google Scholar]
- 30. Koller H, Lawson K, Rose SA, Wallace I, McCarton C. Patterns of cognitive development in very low birth weight children during the first six years of life. Pediatrics. 1997;99(3):383–389 [DOI] [PubMed] [Google Scholar]
- 31. Mahalanobis PC. On the generalised distance in statistics. Proc Natl Inst Sci India. 1936;2(1):49–55 [Google Scholar]
- 32. Wocadlo C, Rieger I. Phonology, rapid naming and academic achievement in very preterm children at eight years of age. Early Hum Dev. 2007;83(6):367–377 [DOI] [PubMed] [Google Scholar]
- 33. Grizzle KL. Developmental dyslexia. Pediatr Clin North Am. 2007;54(3):507–523, vi [DOI] [PubMed] [Google Scholar]
- 34. Schatschneider C, Torgesen JK. Using our current understanding of dyslexia to support early identification and intervention. J Child Neurol. 2004;19(10):759–765 [DOI] [PubMed] [Google Scholar]
- 35. Mullen KM, Vohr BR, Katz KH, et al. Preterm birth results in alterations in neural connectivity at age 16 years. Neuroimage. 2011;54(4):2563–2570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bach S, Brandeis D, Hofstetter C, Martin E, Richardson U, Brem S. Early emergence of deviant frontal fMRI activity for phonological processes in poor beginning readers. Neuroimage. 2010;53(2):682–693 [DOI] [PubMed] [Google Scholar]
- 37. Schafer RJ, Lacadie C, Vohr B, et al. Alterations in functional connectivity for language in prematurely born adolescents. Brain. 2009;132(pt 3):661–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen E, Martin AD, Matthews KA. Trajectories of socioeconomic status across children's lifetime predict health. Pediatrics. 2007;120(2). Available at: www.pediatrics.org/cgi/content/full/120/2/e297 [DOI] [PubMed] [Google Scholar]
- 39. To T, Guttmann A, Dick PT, et al. What factors are associated with poor developmental attainment in young Canadian children? Can J Public Health. 2004;95(4):258–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sameroff AJ. Environmental risk factors in infancy. Pediatrics. 1998;102(5 suppl E):1287–1292 [PubMed] [Google Scholar]
- 41. Escalona SK. Babies at double hazard: early development of infants at biologic and social risk. Pediatrics. 1982;70(5):670–676 [PubMed] [Google Scholar]
- 42. Msall ME. Optimizing early development and understanding trajectories of resiliency after extreme prematurity. Pediatrics. 2009;124(1):387–390 [DOI] [PubMed] [Google Scholar]
- 43. Woodward LJ, Anderson PJ, Austin NC, Howard K, Inder TE. Neonatal MRI to predict neurodevelopmental outcomes in preterm infants. N Engl J Med. 2006;355(7):685–694 [DOI] [PubMed] [Google Scholar]
- 44. Barrington KJ. The adverse neuro-developmental effects of postnatal steroids in the preterm infant: a systematic review of RCTs. BMC Pediatr. 2001;1:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Anderson LM, Shinn C, Fullilove MT, et al. The effectiveness of early childhood development programs: a systematic review. Am J Prev Med. 2003;24(3 suppl):32–46 [DOI] [PubMed] [Google Scholar]
- 46. McCormick MC, Brooks-Gunn J, Buka SL, et al. Early intervention in low birth weight premature infants: results at 18 years of age for the Infant Health and Development Program. Pediatrics. 2006;117(3):771–780 [DOI] [PubMed] [Google Scholar]