Abstract
OBJECTIVE:
Susceptibility to cigarette smoking in tobacco-naive youth is a strong predictor of smoking initiation. Identifying mechanisms that contribute to smoking susceptibility provide information about early targets for smoking prevention. This study investigated whether sensitivity to secondhand smoke exposure (SHSe) contributes to smoking susceptibility.
PARTICIPANTS AND METHODS:
Subjects were high-risk, ethnically diverse 8- to 13-year-old subjects who never smoked and who lived with at least 1 smoker and who participated in a longitudinal SHSe reduction intervention trial. Reactions (eg, feeling dizzy) to SHSe were assessed at baseline, and smoking susceptibility was assessed at baseline and 3 follow-up measurements over 12 months. We examined the SHSe reaction factor structure, association with demographic characteristics, and prediction of longitudinal smoking susceptibility status.
RESULTS:
Factor analysis identified “physically unpleasant” and “pleasant” reaction factors. Reported SHSe reactions did not differ across gender or family smoking history. More black preteens reported feeling relaxed and calm, and fewer reported feeling a head rush or buzz compared with non-Hispanic white and Hispanic white counterparts. Longitudinally, 8.5% of subjects tracked along the trajectory for high (versus low) smoking susceptibility. Reporting SHSe as “unpleasant or gross” predicted a 78% reduction in the probability of being assigned to the high–smoking susceptibility trajectory (odds ratio: 0.22 [95% confidence interval: 0.05–0.95]), after covariate adjustment.
CONCLUSIONS:
Assessment of SHSe sensitivity is a novel approach to the study of cigarette initiation etiology and informs prevention interventions.
Keywords: secondhand smoke, sensitivity, smoking susceptibility, trajectories, preteens
WHAT'S KNOWN ON THIS SUBJECT:
Passive exposure to cigarette smoke in children is toxic and associated with susceptibility to cigarette smoking. In turn, smoking susceptibility predicts smoking initiation. These relationships suggest that exposure to cigarette smoke in childhood contributes to risk for future cigarette smoking.
WHAT THIS STUDY ADDS:
Sensitivity to exposure to cigarette smoke may be a mechanism that helps explain the relationship between passive exposure and smoking susceptibility. Tobacco-naive preteens who report cigarette smoke as “unpleasant or gross” have substantially reduced susceptibility to smoking.
Childhood secondhand smoke exposure (SHSe) is associated with adverse health consequences1–5 and contributes to medical problems into adulthood.6–10 It also is associated with smoking susceptibility,11,12 which is an independent predictor of smoking initiation.13–15 SHSe is associated with symptoms of addiction in children who had never smoked16 and with progression to weekly smoking in adulthood,17 independent of family and peer smoking. One mechanism that could account for the relationship between SHSe and smoking behavior is genetics, because children who live with parents who smoke likely have an inherited predisposition to smoking.18–21 A second mechanism is individual differences in sensitivity to SHSe,22 which could be influenced by genetic predisposition to smoking, genetic predisposition in sensitivity to SHSe,23,24 by cumulative SHSe, by existing medical conditions, or a combination of these and other factors. A third mechanism is access to cigarettes and the modeling of smoking behavior to which exposed children are subject. Social reactions to the child's early imitations of their parents' smoking may strongly influence smoking initiation.
We posited that individual differences in sensitivity to SHSe may represent markers for vulnerability to smoking behavior. Studies have shown that subjective reactions (eg, relaxed, dizzy) to first-time smoking are related to escalation in smoking behavior and nicotine dependence.25–27
We previously reported the assessment of sensitivity to SHSe, presented psychometric findings of SHSe sensitivity, and predicted smoking susceptibility in cross-sectional analyses.28 The current investigation extends our previous findings by examining sensitivity to SHSe and smoking susceptibility longitudinally.
MATERIALS AND METHODS
All procedures were approved by the San Diego State University institutional review board.
Participants
Low-income families were recruited throughout San Diego County, California, with a total of 18 673 recruitment contacts made over 3 years. A total of 1836 interested families were contacted by telephone between 2004 and 2007, identifying 618 potential families on the basis of child age (8–13 years) and resident smoker status; 388 families qualified for an in-home baseline interview. Parents signed informed consent and preteens signed assents. Of 388 families, 211 families were eligible for the randomized clinical trial if reported SHSe in the home was 2 or more cigarettes per day or if the preteen's urine cotinine level (a biomarker of SHSe) was 2.0 ng/mL or higher. Of these, 9 families refused to continue, 1 family was lost, and 201 families were randomly assigned to either the intervention or control and were followed at 5, 9, and 12 months.
Analysis in this study was limited to the longitudinal subsample of 201 preteens (1 from each family) who had never smoked at baseline and throughout the 12-month follow-up (n = 182) and who had smoking susceptibility data on at least 2 of 4 assessments (n = 165).
Assessments
Sequential interviews were completed with the parent and preteen separately. The preteen interview included demographics; general health information; tobacco-use history; peer smoking behavior; SHSe in the home, school, church, and neighborhood; SHSe reactions; rules about smoking in the home; parenting and home environment; alcohol use; and popular-culture items. The parent interview included race/ethnicity information and detailed family smoking history. Urine was collected from the preteen at baseline and follow-up measures.
Variables
Reactions to SHSe
Assessment of SHSe sensitivity was adapted from measures used to assess sensitivity to the first smoked cigarette.29,30 Preteens were asked to respond “yes” or “no” to the questions “When you have breathed other people's smoke, did you ever feel any of the following?”: (1) “Did you feel dizzy?”; (2) “Did you feel like you wanted to throw-up?”; (3) “Did you think it was unpleasant or gross?”; (4) “Did your heart beat faster?”; (5) “Did you feel relaxed or calm?”; (6) “Did you feel a rush or buzz in your head?”; (7) “Did you think it was nice or pleasant?”; (8) “Did you like the smell?”; and (9) “Did you start coughing or choking?” SHSe sensitivity was assessed at the baseline interview only.
Smoking Susceptibility
To be classified as nonsusceptible, subjects who never smoked had to respond with “definitely not” to the following: “Do you think you will try a cigarette soon?”, “Do you think you will be smoking one year from now?”, and “If one of your best friends were to offer you a cigarette, would you smoke it?” Otherwise, preteens were classified as susceptible.31 Never smoking was defined as answering “no” to both “Have you ever smoked a cigarette?” and “Have you ever tried cigarette smoking, even a few puffs?” Smoking susceptibility was assessed at baseline and at each follow-up interview.
Covariates
The following were covariates, including variables shown to be associated with smoking susceptibility13,32: (1) gender; (2) age, categorized as ages 8 to 9, 10 to 11, and 12 to 13 years; (3) race/ethnicity, categorized as non-Hispanic white, Hispanic white, black, combined Native American, Asian, and Pacific Islander, and mixed. Nonwhite groups included both Hispanics and non-Hispanics; (4) parent education, categorized as less than high school, high school, and more than high school; (5) family smoking index, defined as the proportion of first- and second-degree relatives who ever smoked regularly relative to the total number of relatives (an expanded explanation can be found in ref 28 or requested from Dr Lessov-Schlaggar)33,34; (6) school grades in the past year (mostly As and Bs versus all others); (7) any friends who smoke (yes or no); (8) urine cotinine level (log transformed; analytic chemistry of urine cotinine has been described28,35); and (9) membership in the experimental or control arms of the study.
Data Analysis
Factor analysis was used to investigate the pattern of correlations among SHSe reactions and to examine whether reactions cluster into “pleasant” and “unpleasant” dimensions. Factor analysis with promax rotation (allowing for correlated factors) was performed using SAS software version 9.1.36 The choice of the final factor solution was based on (1) the common variance accounted for by each factor, (2) the scree plot, (3) at least 2 items with factor loadings 0.3 or higher, and (4) items with high loadings (≥0.3) on 1 factor had to have lower loadings on all remaining factors.37
Association of SHSe reactions and demographic characteristics was investigated in a series of regressions by using Stata 9,38 where the dependent variable was either each reaction (logistic regression) or a 3-category summary score (ordinal logistic regression). Pairwise comparisons between categories of the independent variables were computed using the Wald χ2 test. For the ordinal regression, the proportional odds assumption was tested using the Brant test. The assumption was not violated in any of the models.
Smoking susceptibility trajectories were estimated using a semiparametric, multinomial-mixture modeling approach that identifies the optimal number of trajectory growth curves in the population.34,39–41 The most parsimonious Bayesian information criterion42 was used for model selection. Model building proceeded using the general recommendation to add trajectory classes as long as the Bayesian information criterion continued to decrease and the model was meaningful.41
Trajectory models were fit to the values of the dichotomous smoking susceptibility variable across the 4 assessments using a logit model. Time since baseline was the independent variable. Baseline was indexed as 0, 5-month and 9-month follow-ups were indexed as 0.417 and 0.75 fractions of 1 year, and 1 represented the 1-year follow-up. Trajectory analyses were conducted on data from preteens who never smoked throughout and who had susceptibility data from at least 2 of 4 assessments (final n = 165).
Association of SHSe reactions and susceptibility trajectories was investigated by using logistic regression predicting trajectory class assignment from baseline SHSe reaction items and summary scores. Trajectory class assignment was based on computation of a posterior probability of preteen assignment to each trajectory class modeled. Thus, if 2 trajectories were modeled, each individual had 2 posterior probabilities of trajectory class assignment. A person was assigned to the trajectory for which they had the higher posterior probability.
RESULTS
Demographics and prevalence of baseline SHSe reactions are shown in Table 1. Factor analysis of SHSe reactions resulted in 2 factors (Table 2). A total of 5 of 9 items had loadings of 0.30 or higher on the first “physical/unpleasant” factor. The item “unpleasant or gross” did not have a factor loading 0.30 or higher; however, it was included in the computation of the factor 1 summary score because it had a sufficiently high loading in the factor analysis for the full sample (factor loading: 0.31) and was significantly related to smoking susceptibility.28 This 6-item factor had acceptable internal consistency (Cronbach α = 0.63). The factor accounted for 24.9% of the variance in these 6 items. None to all 6 of the “unpleasant/physical reactions” were endorsed with a median of 2 and mean of 2.11 (SD: 1.57). To increase cell size for this quasicontinuous measure, a discrete variable was defined capturing roughly the lower one-third (scores 0 and 1 [39.9%]), median (score 2 [24.2%]), and upper one-third (score ≥3 [36.0%]) of the distribution (Table 2).
TABLE 1.
Demographic Characteristics and Prevalence of Baseline Reactions to SHSe in the Longitudinal Sample
| Demographics | Sample (N = 165) |
|---|---|
| Age (SD; range) | 10.3 (1.6; 8–13) |
| Gender, % girls | 55.2 |
| Race/ethnicity, % | |
| Non-Hispanic white | 23.0 |
| Hispanic white | 26.7 |
| Black | 29.7 |
| Native | 8.5 |
| Alaskan/Asian/Pacific Islander | |
| Mixed | 12.1 |
| Parent education, % | |
| Less than high school | 25.5 |
| High school or equivalent | 29.7 |
| More than high school | 44.9 |
| Family smoking index (SD; range) | 71.6 (24.6) |
| Reactions, % | Sample (N = 154–165) |
| Dizzy | 26.1 |
| Wanted to throw up | 20.3 |
| Unpleasant or gross | 72.6 |
| Heart beat faster | 22.1 |
| Relaxed or calm | 24.4 |
| Head rush or buzz | 22.6 |
| Nice or pleasant | 2.4 |
| Liked the smell | 0.6 |
| Coughing or choking | 51.2 |
TABLE 2.
Factor Analysis of Baseline Reactions to SHSe in the Longitudinal Sample
| Reactions | Factor Loadings |
|
|---|---|---|
| F1 | F2 | |
| Dizzy | 0.52 | −0.02 |
| Wanted to throw up | 0.64 | 0.05 |
| Unpleasant or gross | 0.27a | 0.06 |
| Heart beat faster | 0.46 | 0.08 |
| Relaxed or calm | −0.03 | 0.53 |
| Head rush or buzz | 0.61 | −0.1 |
| Nice or pleasant | 0.25 | 0.39 |
| Liked the smell | −0.08 | 0.11 |
| Coughing or choking | 0.40 | −0.21 |
| Cronbach α | 0.64 | 0.44 |
| Interfactor correlation | 0.10 | |
| Summary score categories, % | ||
| F1 low (score 0 or 1)a | 39.9 | |
| F1 medium (score 2) | 24.2 | |
| F1 high (score ≥3) | 36.0 | |
| F2 low (score 0) | 75.6 | |
| F2 high (score 1 or 2) | 24.4 | |
F1 indicates factor 1 (unpleasant factor); F2, factor 2 (pleasant factor).
The unpleasant or gross item was included in the computation of the F1 summary score because it loaded significantly in the factor analysis in the full baseline sample.28
Feeling relaxed or calm and thinking that SHSe is nice or pleasant loaded on a second factor. The internal consistency of this “pleasant” factor was low (Cronbach α = 0.44), and it accounted for 21.7% of the variance in the 2 items. The summary score captured endorsement of neither of these 2 items (score: 0) or either or both of them (score: 1 or 2). Factors 1 and 2 were weakly correlated (r = 0.10).
Associations of baseline SHSe reactions and summary scores with demographic characteristics are shown in Table 3. Overall, more 8- to 9-year-old subjects, relative to older age-groups, reported wanting to throw up, feeling relaxed or calm, and coughing or choking, and more endorsed 3 or more “physical/unpleasant” reactions and any “pleasant” reactions. Relative to non-Hispanic white and Hispanic white preteens, significantly more black preteens reported feeling relaxed or calm and any “pleasant” reactions, and significantly fewer reported a rush or buzz. More preteens with parents who had less than a high school education reported wanting to throw up compared with preteens with more highly educated parents. There were no differences by gender or family smoking index (data not shown) across SHSe reactions or summary score categories.
TABLE 3.
Relationship of Baseline SHSe Reactions and Factor Score Categories With Baseline Demographic Characteristics in the Longitudinal Sample of Preteens Who Never Smoked (N = 165)
| Gender |
Age-Groups |
Race/Ethnicity |
Parent Education |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male (N = 67–74) | Female (N = 87–91) | 8–9 y (N = 52–53) | 10–11 y (N = 62–69) | 12–13 y (N = 40–43) | Non-Hispanic White (N = 37–38) | Hispanic White (N = 42–44) | Black (N = 44–49) | Native Alaskan, Asian, or Pacific Islander (N = 13–14) | Mixed (N = 18–20) | Less Than High School (N = 40–42) | High School or Equivalent (N = 49) | More Than High School (N = 65–74) | |
| Reactions, % endorsed | |||||||||||||
| Dizzy | 24.3 | 27.5 | 35.9 | 23.2 | 18.6 | 26.3 | 29.6 | 22.5 | 28.6 | 25.0 | 28.6 | 30.6 | 21.6 |
| Wanted to throw up | 18.9 | 21.4 | 32.71 | 14.72 | 14.02 | 21.6 | 25.0 | 14.6 | 21.4 | 20.0 | 31.01 | 20.4 | 13.92 |
| Unpleasant or gross | 68.9 | 75.6 | 69.8 | 72.1 | 76.7 | 76.3 | 70.5 | 72.9 | 78.6 | 65.0 | 71.4 | 69.4 | 75.3 |
| Heart beat faster | 22.4 | 21.8 | 28.9 | 19.4 | 17.5 | 24.3 | 14.3 | 20.5 | 30.8 | 33.3 | 25.0 | 24.5 | 18.5 |
| Relaxed or calm | 28.8 | 20.9 | 34.61 | 21.7 | 16.32 | 13.21 | 14.01 | 40.82 | 21.4 | 30.0 | 29.3 | 22.5 | 23.0 |
| Rush or buzz in head | 24.3 | 21.1 | 28.3 | 20.6 | 18.6 | 31.61 | 29.61 | 8.32 | 21.4 | 25.0 | 28.6 | 22.5 | 19.2 |
| Nice or pleasant | 2.7 | 2.2 | 7.6 | 0.0 | 0.0 | 0.0 | 0.0 | 4.1 | 7.1 | 5.0 | 0.0 | 2.0 | 4.1 |
| Liked smell | 1.4 | 0.0 | 1.9 | 0.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 | 0.0 | 0.0 | 2.0 | 0.0 |
| Coughing or choking | 54.1 | 48.9 | 62.31 | 48.5 | 41.92 | 62.2 | 43.2 | 51.0 | 57.1 | 45.0 | 46.3 | 53.1 | 52.7 |
| Factor score categories, n | 67–73 | 86–91 | 51–52 | 62–69 | 40–43 | 36 | 42 | 44 | 13 | 18 | 40 | 49 | 64 |
| F1 low (score 0 or 1), % | 41.7 | 38.3 | 27.5 | 46.8 | 45.0 | 36.1 | 42.9 | 38.6 | 30.8 | 50.0 | 30.0 | 38.8 | 46.9 |
| F1 medium (score 2), % | 16.4 | 30.2 | 23.5 | 21.0 | 30.0 | 19.4 | 19.1 | 36.4 | 38.5 | 5.6 | 25.0 | 24.5 | 23.4 |
| F1 high (score ≥3), % | 41.8 | 31.4 | 49.01 | 32.32 | 25.02 | 44.4 | 38.1 | 25.0 | 30.8 | 44.4 | 45.0 | 36.7 | 28.7 |
| F2 low (score 0), % | 71.2 | 79.1 | 65.4 | 78.3 | 83.7 | 86.8 | 86.1 | 59.2 | 78.6 | 70.0 | 70.7 | 77.5 | 77.0 |
| F2 high (score 1 or 2), % | 28.8 | 20.9 | 34.61 | 21.7 | 16.32 | 13.21 | 13.91 | 40.82 | 21.4 | 30.0 | 29.3 | 22.5 | 23.0 |
Different sample sizes are a result of a different number of missing values across SHSe reactions. Factor scores have a lower sample size in some cases compared with individual items because if an individual has missing data for just 1 of the items that are summed across to create the factor score, that individual will be dropped from the total factor score. Prevalence estimates with different superscripts are significantly different from each other at P < .05. F1 indicates factor 1 (unpleasant factor); F2, factor 2 (pleasant factor). Mixed respondents are those who endorsed 2 or more racial/ethnic categories.
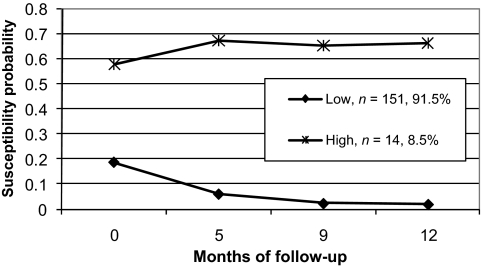
The Bayesian information criterion values of models estimating 1, 2, and 3 trajectories were −243.72, −224.54, and −228.90, respectively. The best-fitting susceptibility trajectory model by Bayesian information criterion was the 2-class solution (Fig 1), characterized by high (8.5%) and low (91.5%) susceptibility trajectories. In covariate-adjusted analyses, experiencing SHSe as “unpleasant or gross” predicted a 78% reduction in the probability of being assigned to the high smoking susceptibility trajectory compared with the low smoking susceptibility trajectory (Table 4) (odds ratio: 0.22 [95% confidence interval: 0.05–0.95]). There was a trend for reduced risk for assignment to the high susceptibility trajectory, given endorsement of 3 or more “physical/unpleasant” SHSe reactions. Approximately one-half of the preteens in each of the 2 trajectories had been assigned to the intervention arm of the SHSe reduction trial. As a consequence, group assignment to intervention or control was not significantly associated with trajectory class assignment. Preteens of Native American, Asian, or Pacific Islander background were more likely to be in the high susceptibility trajectory. Having grades of As and Bs in school was associated with a lower risk for assignment to the high susceptibility trajectory.
FIGURE 1.
Smoking susceptibility trajectories in 8- to 13-year-old preteens who have never smoked.
TABLE 4.
Relationship of SHSe Reactions and Summary Score Categories With Smoking Susceptibility Trajectories (Low Susceptibility Trajectory Is the Referent Category)
| Reactions by Trajectory Group |
Association With Trajectories |
Covariates | |||
|---|---|---|---|---|---|
| Low (N = 140–151) | High (N = 14) | Unadjusted Odds Ratio (95% Confidence Interval) | Adjusted Odds Ratio (95% Confidence Interval) | ||
| Reactions, % | |||||
| Dizzy | 25.8 | 28.6 | 1.15 (0.34–3.87) | 0.75 (0.16–3.45) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Wanted to throw up | 20.1 | 21.4 | 1.08 (0.28–4.12) | 0.63 (0.12–3.31) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Unpleasant or gross | 74.7 | 50.0 | 0.34 (0.11–1.03)a | 0.22 (0.05–0.95)b | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Heart beat faster | 22.1 | 21.4 | 0.96 (0.25–3.65) | 0.79 (0.13–4.80) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Relaxed or calm | 24.0 | 28.6 | 1.27 (0.37–4.28) | 2.28 (0.43–12.0) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Head rush or buzz | 23.3 | 14.3 | 0.55 (0.12–2.56) | 0.28 (0.05–1.74) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Nice or pleasant | 2.0 | 7.1 | 3.79 (0.37–39.1) | 2.73 (0.09–80.5) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Liked the smell | 0.7 | 0.0 | Native Alaskan/Asian/Pacific Islandera; School gradesb | ||
| Coughing or choking | 52.7 | 35.7 | 0.50 (0.16–1.56) | 0.34 (0.08–1.40) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| Summary score categories, n | 139–150 | 14 | |||
| F1 low (score 0 or 1), % | 38.3 | 57.4 | 1.00 | 1.00 | |
| F1 medium (score 2), % | 25.2 | 14.3 | 0.38 (0.08–1.89) | 0.26 (0.04–1.83) | |
| F1 high (score ≥3), % | 36.7 | 28.6 | 0.52 (0.15–1.83) | 0.20 (0.03–1.14)a | Native Alaskan/Asian/Pacific Islandera; School gradesb |
| F2 low (score 0), % | 76.0 | 71.4 | 1.00 | 1.00 | |
| F2 high (score 1 or 2), % | 24.0 | 28.6 | 1.27 (0.37–4.28) | 2.28 (0.43–12.0) | Native Alaskan/Asian/Pacific Islandera; School gradesb |
Adjusted for age, gender, race, parent education, family smoking history, school grades, smoking friends, urine cotinine levels, and intervention group. F1 indicates factor 1 (unpleasant factor); F2, factor 2 (pleasant factor); NA, not applicable.
P < .10.
P < .05.
DISCUSSION
This longitudinal study, together with our previous cross-sectional analysis, investigated a research question that has not been previously addressed. In particular, we examined whether reported reactivity to exposure to SHS in high-risk preteens predicts susceptibility to cigarette smoking. Subjective reactivity to the first smoked cigarette has been shown to predict risk for tobacco dependence26,43 and to be have some genetic basis.23,24 Both longitudinal and cross-sectional investigation showed that experiencing SHS as “unpleasant or gross” is protective against smoking susceptibility, suggesting that it may reflect a mechanism for targeted prevention efforts. In this longitudinal study, reactions to SHSe seemed to capture “physical/unpleasant” and “pleasant” dimensions, consistent with results for subjective reactions to the first cigarette.27 However, in the full baseline sample, reactions like feeling relaxed or calm, thinking SHSe was nice or pleasant, or liking the smell did not load on a second “pleasant” factor,28 which might reflect little or no “positive reinforcing” reactions to SHSe, at least in the way that such reactions were assessed in this study.
There were differences in endorsement rates of some SHSe reactions by age, race/ethnicity, or parent education but no differences by gender or family smoking history. Results suggest decreasing sensitivity to both unpleasant and pleasant reactions to SHSe as preteens get older. This decreased sensitivity cannot be explained by decreased SHSe because there was no significant relationship between urine cotinine levels (a biomarker of exposure) and age (r = 0.005). In addition, urine cotinine did not predict susceptibility trajectory assignment (odds ratio: 1.15 [95% confidence interval: 0.76–1.75]; P = .498). It could be that the interpretation of SHSe reactions is different across age-groups. Because reactions to SHSe were asked in relation to lifetime exposure (“When you have breathed other people's smoke, did you ever feel any of the following?”) and not specifically to recent exposure, it is possible that older preteens forgot reactions to exposures when they were younger or are simply recalling their more recent experiences rather than their lifetime experiences. Lower endorsement rates could also reflect changes in social-reinforcement contingencies and adaptations to cigarette smoke, where contingencies lead to more exposure, and overexposure episode adaptation takes place where one no longer reacts as negatively and/or downplays the negative effects of SHSe. It could also be that cumulative exposure is associated with decreasing sensitivity, perhaps through development of tolerance to SHSe over time. It is not possible to evaluate this possibility in this study, but because lifetime exposure would be difficult to assess, assessment of reported sensitivity to SHSe may be a practical alternative.
The prevalence of feeling relaxed or calm was 3 times higher in black preteens, and the prevalence of a head rush or buzz was 3 and one-half times lower compared with non-Hispanic white and Hispanic white groups. Approximately 75% of black smokers smoke mentholated cigarettes, compared with 23% to 30% of non-Hispanic white and white smokers.44,45 It is possible that black preteens are more commonly exposed to the smoke from mentholated cigarettes, compared with preteens of other racial/ethnic groups, and menthol-flavored smoke may be experienced as more pleasant (feeling more relaxed or calm and less of a head rush or buzz) compared with SHSe from nonmentholated cigarettes. There is evidence that cigarette smoke from mentholated cigarettes is associated with sensations of coolness in the mouth and throat of smokers.46 These results may have implications for Food and Drug Administration regulation of menthol cigarettes, which currently are exempt from regulation.47
Significantly more preteens with lower-educated parents reported wanting to throw up. It could be that preteens in such homes are exposed to greater amounts of SHS because lower educational achievement is associated with higher rates of cigarette smoking.48–51 Baseline urine cotinine levels did not systematically differ across parent education levels, although cotinine levels were significantly lower in preteens whose parents had less than a high school education compared with those whose parents had a high school or equivalent education (P = .047), suggesting that SHSe at the time of assessment could not explain prevalence differences.
Using longitudinal smoking susceptibility data, we identified low and high smoking susceptibility trajectories. The low-susceptibility trajectory comprised the highest proportion of the sample, suggesting that despite the high-risk sample, for this age range and ethnic diversity, preteen smoking susceptibility was low overall. The mean age of preteens in the high (mean age: 10.2, SD: 2.2, and range: 8–13) versus low (mean age: 10.3, SD: 1.6, and range: 8–13) trajectories were the same, suggesting that those at high susceptibility risk were not simply older. Experiencing SHSe as “unpleasant or gross” was the only reaction that predicted a significant reduction in risk for assignment to the high smoking susceptibility trajectory. In cross-sectional analyses,28 this item showed a trend for a significant 40% reduction in smoking susceptibility risk. The combined results suggest that this reaction may be a marker for mechanisms that protect against smoking susceptibility. Among the covariates, the strongest protective factor for smoking susceptibility was having grades of As and Bs in school. This finding may have important implications for future intervention strategies that focus on behavior change to encourage good school performance by preteens along with providing strategies for limiting or eliminating SHSe.
The results from this study may not be generalizable to children younger than 8 or older than 13 years of age or to children at lower smoking risk, such as those who do not live with smokers. Assessment of SHSe reactions needs to be refined to include items that are specific to secondhand smoke rather than reactions more related to the experience of the first smoked cigarettes. The small portion of item variance explained by the factor structure makes evident the need for refinement. Low endorsement rates of the “pleasant” reactions to SHSe precluded identification of statistically significant associations between these items and smoking susceptibility. Larger samples would be needed to detect the effects of low prevalence measures. Longitudinal follow-up through the age period of risk for smoking initiation would be very important and informative for understanding how sensitivity to SHSe relates to smoking initiation.
CONCLUSIONS
This investigation, together with our previous cross-sectional analysis, suggest that (1) experiencing SHSe as “unpleasant or gross” may be a phenotypic marker for mechanisms associated with protection against smoking susceptibility; (2) identifying subgroups of individuals with different smoking susceptibility profiles over time allows for better resolution of the relationship between SHSe reactions and smoking susceptibility; (3) even in this high-risk diverse sample of subjects aged 8 to 13 years who never smoked, smoking susceptibility was low, suggesting that this age range may represent an opportune window for targeted intervention to keep susceptibility low and to decrease smoking initiation risk; and (4) sensitivity to SHSe is a measure of importance in tobacco-use etiology, in terms of flagging early risk or protective factors for smoking susceptibility and, possibly, in turn, smoking initiation. This study provides empirical support for more expansive designs to determine the combination of factors that most reliably predict smoking susceptibility. Including measures of early exposure to smoke and sensitivity to exposure in language competent children (eg, ages 3–4 years) within the longitudinal National Children's Study may enable validation of these measures in relation to future smoking risk. This analysis should be replicated with youth representative of the general population. If results are replicated, intervention studies should test prevention of tobacco initiation with children who are “at risk of initiation” as well as those who are not to determine the utility of using the measures as bases for prevention programs.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health Grants HL066307 (to Dr Hovell), DA027046 (to Dr Lessov-Schlaggar), and DA018019 (to Dr Swan) and discretionary funds from the Center for Behavioral Epidemiology and Community Health (to Dr Hovell, director).
Dr Lessov-Schlaggar conducted all analyses and wrote the article, Mr Wahlgren, Ms Jones, and Drs Hughes and Hovell designed and conducted the study; Mr Wahlgren served as the measurement coordinator for the overall trial, designed the study measures, and oversaw data collection; Mr Liles conducted poststudy data management and consulted on study design and statistical issues; Dr Ji consulted on analytical approaches; Drs Winickoff and Swan consulted on the scientific direction and approach; and Dr Hovell supervised all aspects of the study, analyses, and manuscript preparation. All coauthors provided extensive, critical edits and feedback throughout the writing process.
FINANCIAL DISCLOSURE: The authors have indicated they have no personal financial relationships relevant to this article to disclose.
Funded by the National Institutes of Health (NIH).
Abbreviation:
- SHSe
- secondhand smoke exposure
REFERENCES
- 1. California Environmental Protection Agency Proposed Identification of Environmental Tobacco Smoke as a Toxic Air Contaminant. Sacramento, CA: California Environmental Protection Agency, Office of Environmental Health Hazard Assessment; 2005 [Google Scholar]
- 2. Landau LI. Parental smoking: asthma and wheezing illnesses in infants and children. Paediatr Respir Rev. 2001;2(3):202–206 [DOI] [PubMed] [Google Scholar]
- 3. Tager IB. The effects of second-hand and direct exposure to tobacco smoke on asthma and lung function in adolescence. Paediatr Respir Rev. 2008;9(1):29–37; quiz 37–28 [DOI] [PubMed] [Google Scholar]
- 4. US Department of Health and Human Services The Health Consequences of Involuntary Exposure to Tobacco Smoke: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health; 2006 [Google Scholar]
- 5. World Health Organization Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France: International Agency for Research on Cancer Press; 2004 [Google Scholar]
- 6. David GL, Koh WP, Lee HP, Yu MC, London SJ. Childhood exposure to environmental tobacco smoke and chronic respiratory symptoms in non-smoking adults: the Singapore Chinese Health Study. Thorax. 2005;60(12):1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lovasi GS, Diez Roux AV, Hoffman EA, Kawut SM, Jacobs DR, Jr, Barr RG. Association of environmental tobacco smoke exposure in childhood with early emphysema in adulthood among nonsmokers: the MESA-lung study. Am J Epidemiol. 2010;171(1):54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Olivo-Marston SE, Yang P, Mechanic LE, et al. Childhood exposure to secondhand smoke and functional mannose binding lectin polymorphisms are associated with increased lung cancer risk. Cancer Epidemiol Biomarkers Prev. 2009;18(12):3375–3383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Svanes C, Omenaas E, Jarvis D, Chinn S, Gulsvik A, Burney P. Parental smoking in childhood and adult obstructive lung disease: results from the European Community Respiratory Health Survey. Thorax. 2004;59(4):295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang FL, Love EJ, Liu N, Dai XD. Childhood and adolescent passive smoking and the risk of female lung cancer. Int J Epidemiol. 1994;23(2):223–230 [DOI] [PubMed] [Google Scholar]
- 11. Centers for Disease Control and Prevention Exposure to secondhand smoke among students aged 13–15 years: worldwide, 2000–2007. MMWR Morb Mortal Wkly Rep. 2007;56(20):497–500 [PubMed] [Google Scholar]
- 12. Seo DC, Torabi MR, Weaver AE. Factors influencing openness to future smoking among nonsmoking adolescents. J Sch Health. 2008;78(6):328–336; quiz 356–328 [DOI] [PubMed] [Google Scholar]
- 13. Gritz ER, Prokhorov AV, Hudmon KS, et al. Predictors of susceptibility to smoking and ever smoking: a longitudinal study in a triethnic sample of adolescents. Nicotine Tob Res. 2003;5(4):493–506 [DOI] [PubMed] [Google Scholar]
- 14. Jackson C. Cognitive susceptibility to smoking and initiation of smoking during childhood: a longitudinal study. Prev Med. 1998;27(1):129–134 [DOI] [PubMed] [Google Scholar]
- 15. Unger JB, Johnson CA, Stoddard JL, Nezami E, Chou CP. Identification of adolescents at risk for smoking initiation: validation of a measure of susceptibility. Addict Behav. 1997;22(1):81–91 [DOI] [PubMed] [Google Scholar]
- 16. Bélanger M, O'Loughlin J, Okoli CT, et al. Nicotine dependence symptoms among young never-smokers exposed to secondhand tobacco smoke. Addict Behav. 2008;33(12):1557–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Becklake MR, Ghezzo H, Ernst P. Childhood predictors of smoking in adolescence: a follow-up study of Montreal schoolchildren. CMAJ. 2005;173(4):377–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Boardman JD. State-level moderation of genetic tendencies to smoke. Am J Public Health. 2009;99(3):480–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Han C, McGue MK, Iacono WG. Lifetime tobacco, alcohol and other substance use in adolescent Minnesota twins: univariate and multivariate behavioral genetic analyses. Addiction. 1999;94(7):981–993 [DOI] [PubMed] [Google Scholar]
- 20. Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry. 2008;65(6):674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Maes HH, Woodard CE, Murrelle L, et al. Tobacco, alcohol and drug use in eight- to sixteen-year-old twins: the Virginia Twin Study of Adolescent Behavioral Development. J Stud Alcohol. 1999;60(3):293–305 [DOI] [PubMed] [Google Scholar]
- 22. Anthonisen N, Murray R. A new childhood pathway for transmission of an increased likelihood of smoking? CMAJ. 2005;173(4):382–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ehringer MA, Clegg HV, Collins AC, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am J Med Genet B Neuropsychiatr Genet. 2007;144B(5):596–604 [DOI] [PubMed] [Google Scholar]
- 24. Ehringer MA, McQueen MB, Hoft NR, et al. Association of CHRN genes with “dizziness” to tobacco. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(2):600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen X, Stacy A, Zheng H, et al. Sensations from initial exposure to nicotine predicting adolescent smoking in China: a potential measure of vulnerability to nicotine. Nicotine Tob Res. 2003;5(4):455–463 [DOI] [PubMed] [Google Scholar]
- 26. DiFranza JR, Savageau JA, Fletcher K, et al. Recollections and repercussions of the first inhaled cigarette. Addict Behav. 2004;29(2):261–272 [DOI] [PubMed] [Google Scholar]
- 27. Hu MC, Davies M, Kandel DB. Epidemiology and correlates of daily smoking and nicotine dependence among young adults in the United States. Am J Public Health. 2006;96(2):299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lessov-Schlaggar CN, Wahlgren DR, Liles S, et al. Sensitivity to secondhand smoke exposure predicts smoking susceptibility in 8 to 13 year-old never smokers. J Adolesc Health. 2011;48(3):234–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Meliska CJ, Gilbert DG. Hormonal and subjective effects of smoking the first five cigarettes of the day: a comparison in males and females. Pharmacol Biochem Behav. 1991;40(2):229–235 [DOI] [PubMed] [Google Scholar]
- 30. Pomerleau OF. Individual differences in sensitivity to nicotine: implications for genetic research on nicotine dependence. Behav Genet. 1995;25(2):161–177 [DOI] [PubMed] [Google Scholar]
- 31. Pierce JP, Choi WS, Gilpin EA, Farkas AJ, Merritt RK. Validation of susceptibility as a predictor of which adolescents take up smoking in the United States. Health Psychol. 1996;15(5):355–361 [DOI] [PubMed] [Google Scholar]
- 32. Kaufman NJ, Castrucci BC, Mowery PD, Gerlach KK, Emont S, Orleans CT. Predictors of change on the smoking uptake continuum among adolescents. Arch Pediatr Adolesc Med. 2002;156(6):581–587 [DOI] [PubMed] [Google Scholar]
- 33. Drobes DJ, Munafo MR, Leigh F, Saladin ME. A family smoking index to capture genetic influence in smoking: rationale and two validation studies. Nicotine Tob Res. 2005;7(1):41–46 [DOI] [PubMed] [Google Scholar]
- 34. Milne BJ, Moffitt TE, Crump R, et al. How should we construct psychiatric family history scores? A comparison of alternative approaches from the Dunedin Family Health History Study. Psychol Med. 2008;38(12):1793–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johnson-Kozlow M, Wahlgren DR, Hovell MF, et al. Adolescents validly report their exposure to secondhand smoke. J Clin Epidemiol. 2010;63(8):914–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. SAS Institute SAS/STAT User's Guide Version 9. Cary, NC: SAS Institute; 2004 [Google Scholar]
- 37. Tabachnick GB, Fidell LS. Using Multivariate Statistics. 5th ed Upper Saddle River, NJ: Pearson Education; 2007 [Google Scholar]
- 38. Stata Statistical Software [computer program]. Release 9.0 College Station, TX: Stata Corporation; 2005 [Google Scholar]
- 39. Jones BL, Nagin D, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29(3):374–393 [Google Scholar]
- 40. Nagin D. Analyzing developmental trajectories: semi-parametric, group-based approach. Psychol Methods. 1999;4(2):139–177 [DOI] [PubMed] [Google Scholar]
- 41. Nagin DS, Tremblay RE. Analyzing developmental trajectories of distinct but related behaviors: a group-based method. Psychol Methods. 2001;6(1):18–34 [DOI] [PubMed] [Google Scholar]
- 42. Schwarz G. Estimating the dimension of a model. Ann Statistics. 1978;6(2):461–464 [Google Scholar]
- 43. Kandel DB, Hu MC, Griesler PC, Schaffran C. On the development of nicotine dependence in adolescence. Drug Alcohol Depend. 2007;91(1):26–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Giovino GA, Sidney S, Gfroerer JC, et al. Epidemiology of menthol cigarette use. Nicotine Tob Res. 2004;6(suppl 1):S67–S81 [DOI] [PubMed] [Google Scholar]
- 45. Substance Abuse and Mental Health Services Administration Office of Applied Studies The NSDUH Report: Use of Menthol Cigarettes. Rockville, MD: Substance Abuse and Mental Health Services Administration; 2009 [Google Scholar]
- 46. Wiseman EJ, McMillan DE. Rationale for cigarette smoking and for mentholation preference in cocaine- and nicotine-dependent outpatients. Compr Psychiatry. 1998;39(6):358–363 [DOI] [PubMed] [Google Scholar]
- 47. Curfman GD, Morrissey S, Drazen JM. The FDA and tobacco regulation. N Engl J Med. 2008;359(10):1056–1057 [DOI] [PubMed] [Google Scholar]
- 48. Centers for Disease Control and Prevention Disparities in secondhand smoke exposure-United States, 1988–1994 and 1999–2004. MMWR Morb Mortal Wkly Rep. 2008;57(27):744–747 [PubMed] [Google Scholar]
- 49. Kandel DB, Griesler PC, Schaffran C. Educational attainment and smoking among women: risk factors and consequences for offspring. Drug Alcohol Depend. 2009;104(suppl 1):S24–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCaffery JM, Papandonatos GD, Lyons MJ, Niaura R. Educational attainment and the heritability of self-reported hypertension among male Vietnam-era twins. Psychosom Med. 2008;70(7):781–786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Siegel D, Faigeles B. Smoking and socioeconomic status in a population-based inner city sample of African-Americans, Latinos and whites. J Cardiovasc Risk. 1996;3(3):295–300 [PubMed] [Google Scholar]