Abstract
Importance of the field
Novel approaches are needed for patients with small cell lung cancer (SCLC), as response after relapse is poor with standard therapies. p53 gene mutations often occur, resulting in tumoral protein overexpression and allowing for their recognition by p53-specific cytotoxic T cells.
Areas covered in this review
We describe the characteristics and manufacturing of INGN-225, a p53-modified adenovirus-tranduced dendritic cell vaccine, and review available data, to understand INGN-225’s role in SCLC treatment. We discuss our pre-clinical, early Phase I/II, and ongoing randomized Phase II studies.
What the reader will gain
INGN-225 was well tolerated (all toxicities ≤grade 2) in the Phase I/II trial (54 patients receiving at least 1 dose). Specific antip-53 immune response was positive in 18/43 (41.8%) patients, with overall post-INGN-225 response observed in 17/33 (51.5%) and immune response data available in 29 (14 positive, 15 negative). Post-INGN-225 response was observed in 11/14 (78.6%) and 5/15 (33%) patients with positive and negative immune responses, respectively.
Take home message
INGN-225 is safe, induces a significant immune response, and appears to sensitize SCLC to subsequent chemotherapy. Improvements in immune response induction and understanding the chemotherapy–immunotherapy synergism will determine INGN-225’s future role as an anticancer therapy.
Keywords: adenovirus, dendritic cells, INGN-225, p53 gene, small cell lung cancer
1. Introduction
1.1 Overview of small cell lung cancer
In the United States, 215,000 new cases of lung cancer were diagnosed and 161,840 patients with lung cancer died in 2008 [1], with small cell lung cancer (SCLC) accounting for 13 – 15% of them. SCLC typically disseminates early, with 70 – 80% of patients diagnosed with metastatic disease (extensive stage, ES-SCLC), and is initially also chemo-responsive, so that considerable survival improvements are achieved with (first-line) chemotherapy [2–4].
Etoposide–platinum remains the preferred first-line treatment and the standard against which new therapies are measured. By this standard, patients with ES-SCLC achieve overall response rates (ORR) of ≥ 70% and complete response rates of 20 – 30%, but rarely survive beyond 2 years (10 – 20%), with median survival times (MST) ranging from 7 to 10 months [2–4]. The ORR to second-line therapy is also very disappointing and dependent on the previous chemotherapy response. Patients with ‘platinum-sensitive’ disease (ORR = 20 – 25%) progress ≥ 90 days after initial chemotherapy, whereas those with ‘platinum-resistant’ disease (ORR ≤ 10%) progress sooner [5–7].
Clearly, new therapies are urgently needed, and evidence suggests that immunotherapy may have a potential role in SCLC treatment, particularly if used in conjunction with chemotherapy [8–12].
1.2 p53 as a target for cancer immunotherapy
The p53 tumor suppressor gene plays a central role in the control of cell growth and differentiation. Normally, p53 is a short-lived protein localized in the nuclei of cells. Approximately 50% of all human cancers and > 90% of patients with SCLC have altered p53 function, mostly as a result of single-point mutations or abnormalities in degradation of wild-type (wt) p53. This leads to accumulation of mutant (mu) or wt-p53 protein (whereas normal tissues have low to undetectable levels) and expression of p53-derived epitopes on the surface of tumor cells in the context of MHC class I [13]. Furthermore, since mutant forms of p53 can result in oncogenic gain of function [14,15], it is unlikely that they can escape anti-p53 cytotoxic T lymphocytes (CTLs) by restoring wt-p53 status (no antigen-loss variants). Thus, p53 has many characteristics of an ‘ideal’ tumor-associated antigen (TAA), which makes it a very attractive candidate target for immune recognition and anti-p53-based cancer immunotherapy.
1.3 Anti-p53 dendritic cell-based immunotherapy
Different approaches to p53-based cancer immunotherapy have been explored. The role of wt-p53 peptide sequences in the induction of anti-tumor CTL responses has been investigated in both human and animal in vitro studies, with encouraging results [13,16–20]. However, peptide-based approaches assume knowledge of precise HLA types and the peptides present on particular tumors, leading to several limitations [21,22], such as technical difficulties with generating custom mutant-specific peptides and clinical trial designs that adequately assess this approach.
These difficulties can be circumvented with the use of TAA proteins and dendritic cells (DCs). DCs are the most potent antigen presenting cells (APCs) and the most effective in inducing a primary CTL response. There are numerous methods of loading DCs with a variety of different TAAs, and animal models show that the approach of using viral vectors to introduce these TAAs into DCs is practical, safe, and effective [23–25]. Because cells with mu-p53 usually overexpress the protein and because there is a large human experience in melanoma with targeting overexpressed but not mutant proteins such as MAGE and MART, a much more practical approach would be to develop a strategy that targets the non-mutant portions of the p53 overexpressed in tumors.
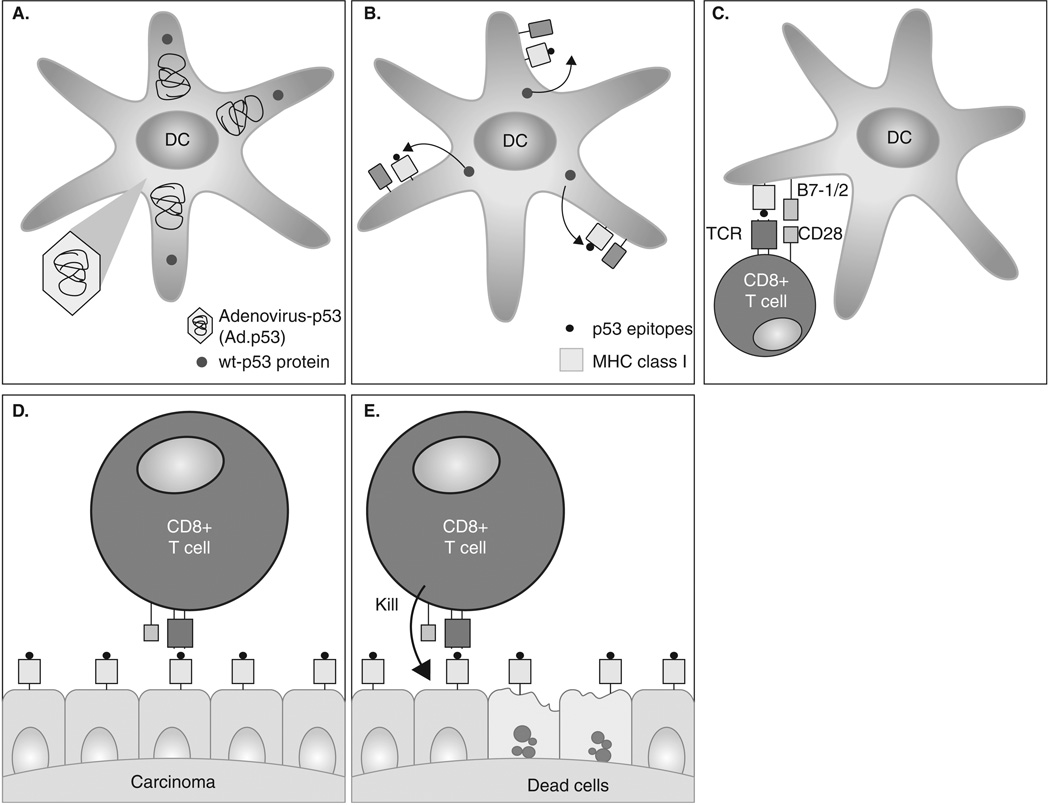
Therefore, we have focused on utilizing DCs transfected with the full-length p53 gene. The rationale for this approach is based on i) the assumption that multiple MHC class I and II matching p53-derived epitopes are present on the surface of DCs, eliminating the need for selecting matching patients as well as providing conditions for activation of CD4+ T cells; and ii) the fact that previous studies have demonstrated that each of the different minimal epitopes combined in a single fusion protein can be recognized by specific CTLs (Figure 1)[26].
Figure 1. Anti-p53 DC-based immunotherapy.
A. Replication null adenoviral particles containing the wild-type (wt)-p53 gene are transfected ex vivo into the patient autologous DCs (antigen presenting cells) to produce INGN-225 (Ad.p53-DC). B. p53 protein is synthesized and processed by the DCs. p53 peptides (epitopes) are expressed on the DC surface in the context of MHC I molecules. C. Naïve T cells recognize the p53 peptide on the surface of the antigen presenting cell (Ad.p53-DC/INGN-225) and are activated to proliferate and differentiate into effector T cells capable of recognizing and eliminating any p53 (over)expressing cell (malignant cells). D. Epithelial malignant cells (SCLC) with mutant (mu)-p53 gene and overexpressing p53 epitopes or TAAs are recognized and attacked by activated effector T cells. E. Cytokines (IL-2, INF-γ, granzymes) are produced, and malignant cells are destroyed.
2. INGN-225 (Ad.p53-DC)
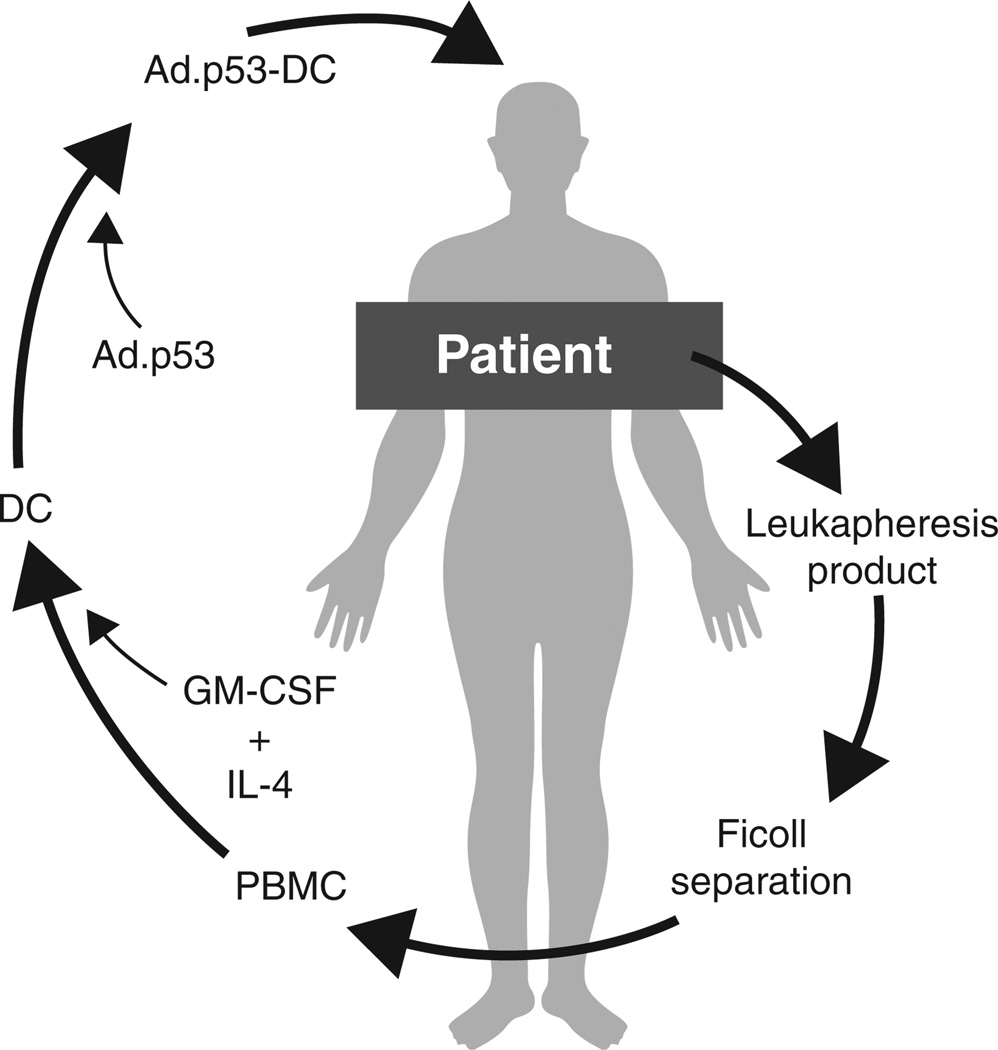
Adenovirus (Ad) provides a high-level transduction efficacy for many cell types, regardless of their mitotic status [27], and replication-defective Ad (deletions in the E1 region) has been safely injected into patients [28]. Successful transduction of DCs with model antigens has been reported, and transduced DCs have effectively presented the recombinant protein antigens [29–31]. In this case, DCs grown by culturing PBMC in the presence of GM-CSF and IL-4 were infected with an adenoviral construct containing wt-p53 (Ad.p53 vector, Advexin, Invitrogen Technologies; Contusugene Ladenovec) to generate INGN-225 (Box 1, Figure 2).
Box 1. Drug summary.
| Drug name | INGN-225 |
| Phase | Phase II |
| Indication | Extensive-stage small cell lung cancer |
| Pharmacology description/mechanism of action | Immunotherapy |
| Route of administration | Intradermal |
| Pivotal trial(s) | [37] |
Figure 2. INGN-225 (Ad.p53-DC) manufacture.
PBMCs are extracted from the patient through Ficoll separation of the leukapheresis product and cultured ex vivo with GM-CSF and IL-4 to produce autologous DCs. Replication null adenoviral particles containing the wild type p53 gene (Ad.p53) are transfected ex vivo into the patient DCs (antigen presenting cells) for the final product (Ad.p53-DC/INGN-225).
2.1 Manufacture of INGN-225
PBMCs are collected by leukapheresis, separated over a Ficoll density gradient, washed, suspended in Plasmalyte-A solution, supplemented with autologous plasma and DMSO, and cryostored in liquid nitrogen. After thawing, cells are washed, suspended in X-VIVO 15 culture medium, and transferred to tissue culture flasks for repetitive incubation with GM-CSF and IL-4. Preliminarily, non-adherent cells are removed; however, after full incubation, all non-adherent cells are harvested and tested by flow cytometry for the fraction of recovered DCs. DCs are centrifuged, resuspended in medium, and reincubated with adenovirus (Ad.p53). After viral incubation, cell product samples are taken to determine the quality of INGN-225 (Ad.p53-DC).
2.2 Preclinical animal studies with INGN-225
To determine the optimal dose of Ad.p53 vector that produces the highest level of human p53 expression with the least amount of toxicity to the DCs, mice were given a human p53-transduced DC vaccine [32,33]. We found that infecting murine DCs with the Ad.p53 vector at a multiplicity of infection (MOI) of 50 – 200 viral particles per cell (vp/cell) did not adversely affect DC viability. MOI in excess of 500 significantly reduced DC viability. However, transduction of DCs at an MOI of 100 produced very good transduction efficiency, with 40 – 45% of the DCs becoming positive for p53. Similarly, infecting human DCs with the Ad.p53 vector at a concentration of 10,000 – 20,000 vp/cell did not adversely affect DC viability and showed better transduction efficiency with the higher dose. At a concentration of 40,000 vp/cell, DC viability was adversely affected despite transduction rates similar to the lower doses. Thus, 20,000 vp/cell was the concentration chosen to manufacture INGN-225.
Additionally, T cells recovered from immunized mice contained significant numbers of CTLs that could specifically kill tumor cells previously modified to express the human p53 gene, and INGN-225-immunized mice developed a significant CTL response to murine p53, probably due to the significant homology between murine and human p53. INGN-225-induced anti-p53 CTL response resulted in protection of mice from challenges with tumor cells that overexpressed human or murine p53. Furthermore, tumors that were established in mice prior to immunization exhibited significantly slowed growth as a result of the immunization with INGN-225 [32,33].
2.3 Preclinical experiments using human T cells
To determine whether this response was sufficient to recognize and eliminate tumor cells in cancer patients, peripheral-blood-derived T cells and autologous DCs were obtained from three healthy volunteers and nine cancer patients, all of whom were HLA-A2-positive [34]. T cells were in vitro primed with INGN-225 and then tested for their ability to kill (HLA-A2-positive) target cells with normal to low or overexpressed levels of p53 protein.
Selective CTL killing of p53-overexpressing cells but not of cells with normal p53 expression levels was generated in blood from all three healthy donors and eight cancer patients. Furthermore, when cells that expressed normal to low levels of p53 were forced to overexpress p53 by gene transfection, they became sensitive to INGN-225-primed CTLs. Additionally, when an excess of NK cell-sensitive target cells or an anti-CD4 antibody was added to the cytotoxicity assays, very little effect or a small decrease in cytotoxicity activity was seen. Conversely, when anti-CD8 was added, we observed a very significant decrease in cytotoxicity, demonstrating that neither NK nor CD4 cells but CD8-positive CTLs are the relevant effector cells primed with INGN-225.
3. Phase I/II clinical trial of INGN-225 in SCLC
p53 gene mutations and p53 protein overexpression are present in ≥ 90% of SCLC cases [35,36]. With many characteristics of an ‘ideal’ TAA present, p53 is an attractive candidate for cancer immunotherapy. Therefore, an approach where an adenoviral vector carrying the intact human wt-p53 gene is used to overexpress the p53 protein in autologous DCs and that allows endogenous processing mechanisms to select and present the appropriate p53 peptides for each individual’s HLA type encourages the use of these transduced cells (INGN-225) as a vaccine.
3.1 Study design and patient characteristics
The trial was designed to assess the clinical and immune responses of SCLC to INGN-225 [37,38]. A total of 54 patients (24 male, 30 female) with extensive-stage disease (initial or recurrent) were enrolled. All had previously been treated with chemotherapy. Patients with stable disease or better underwent leukapheresis 8 weeks after the last dose of chemotherapy to manufacture INGN-225. Patients received three doses of INGN-225 intradermally every two weeks. If after reassessment, stable disease or better persisted, three more monthly doses of INGN-225 were given.
3.2 INGN-225 dose and safety
The number of p53+ DCs (INGN-225 dose) was evaluated using flow cytometry. The initial goal was to escalate the dose from 5 × 106 to 5 × 107 p53+ DC. However, generation of > 5 × 106 p53+ DC per dose was difficult to achieve (on average, 7.7 × 107 DCs and 8.6 × 106 p53+ DCs were generated per dose and ≥ 107 p53+ DCs were generated in < 10% of cases). Thus, to maintain consistency throughout the trial, the single doses of p53+ DC were not escalated beyond 5 × 106 cells. On average, each patient received 3.8 × 106 p53+ DCs per dose. However, five patients received < 106 p53+ DCs because of production difficulties.
INGN-225 toxicities were infrequent and mostly mild. Only two patients experienced grade 2 toxicities (one fatigue, one arthralgia), and INGN-225 was never withheld. The most frequent toxicities were grade 1 arthralgia/myalgia (nine patients), fatigue and injection site erythema (five patients each), and injection site pain (four patients). Occurrence of toxicities was independent of the number of INGN-225 doses received.
3.3 Immune response to INGN-225
The p53-specific immune response was evaluated by spot ELISA (ELISPOT) using autologous PBMCs infected with a canary pox virus (ALVAC) containing wt-p53 or empty vector as control. The number of IFN-γ-producing cells was evaluated using an automated ELISPOT reader [39]. In 12 HLA-A2-positive patients, immune responses were tested with p53-derived or control HLA-A2 matched peptides pulsed onto autologous PBMCs and tetramers. Response was considered significant if it was ≥ 2 sd higher than the response to ALVAC or peptide controls. Increase over baseline (pre-INGN-225) was considered significant if p53-specific responses (post-INGN-225) were ≥ 2 sd higher than p53-specific responses pre-INGN-225 and at least 2 sd higher than responses to ALVAC or peptides. Because the generation of a p53-specific T-cell response not only depends on the quality of antigen stimulation but also on the functional activity of T cells and DCs in the host, they were also tested.
Full immune response evaluation was possible in 43 patients. Overall, 18 patients (41.8%) had a statistically significant p53-specific response using ALVAC and 7 of 12 patients (58.3%) using p53-derived peptide. Three patients with a significant response to INGN-225 using the p53-derived peptide were not tested with ALVAC (technical reasons). The baseline p53-specific immunity level was similar in INGN-225-responsive and non-responsive patients. The level of the p53-specific immune response decreased 2 months after patients completed INGN-225, coinciding with the start of additional chemotherapy.
Both presence and functional activity of DCs were decreased, and the p53-specific immune response to INGN-225 did not correlate with T cell functional activity, presence of Tregs, or pre-existing levels of DC activity. Because myeloid-derived suppressive cells (MDSC) are implicated in the host’s immunosuppressive state [40,41], we examined patients for the presence of these cells. Pre-INGN-225 MDSC (Lin−HLA-DR−CD3+) levels were elevated compared with control donors (p = 0.01). Post-INGN-225 levels increased even further (p = 0.002). All patients with normal levels of MDSCs prior to INGN-225 developed a p53-specific immune response compared to only 33% of patients who had elevated levels of MDSC (p = 0.012).
3.4 Clinical efficacy of INGN-225 and enhanced effect on salvage chemotherapy
Using Response Evaluation Criteria In Solid Tumors (RECIST), two patients (3.7%) achieved a partial response, and 13 had stable disease with INGN-225. All but five patients developed progressive disease, and response data were available for 33 patients who received additional chemotherapy post-INGN-225 progression. The ORR for all 33 patients treated with ‘second’-line chemotherapy was 51.5 and 45.5% for the 22 platinum-resistant patients. The MST (from the date of the first INGN-225 dose) for platinum-resistant patients was 10.5 months (95% CI = 5.3 – 14.4) and 8.8 months (95% CI = 5.2 – 11.8) for all 54 patients.
We also evaluated the relation between immune response to INGN-225 and clinical response (RECIST) to second-line chemotherapy. Out of 14 patients with a positive immune response 11 (78.6%) experienced a clinical response to second-line chemotherapy compared with 5 of 15 patients (33.3%) with a negative immune response (p = 0.014). Patients with a positive immune response had a trend towards improved survival; however, the difference did not reach statistical significance (MST = 12.6 versus 8.2 months, p = 0.131) [38].
4. Current advances in INGN-225 immunotherapy strategies
4.1 Rationale for the use of ATRA in combination with INGN-225
Because all-trans-retinoic acid (ATRA) causes differentiation of acute promyelocytic leukemia cells and lineage features between MDSCs and myeloid acute promyelocytic leukemia cells are similar, the effect of ATRA on DC differentiation and function was tested in patients with metastatic renal cell carcinoma [42]. As expected, patients had a substantially increased proportion of MDSCs, decreased presence of DCs, and decreased MDSC:DC ratio. Treatment with ATRA significantly decreased the presence of MDSC and improved MDSC:DC ratio to control levels. Patients had profound defects in the ability to respond to tetanus toxoid and to stimulate allogeneic T cells and treatment with ATRA improved those defects as well, although not significantly.
Gr-1+ cells (analogous to human MDSC) inhibit antigen-specific T cell response and are present in excess in tumor-bearing mice. ATRA differentiates these cells in vitro and removes their immunosuppressive effect [43,44]. Furthermore, in vivo administration of ATRA to tumor-bearing mice dramatically reduces the presence of Gr-1+ cells and improves the effect of tumor vaccines [45]. Similarly, in vitro treatment of human MDSCs with ATRA results in their differentiation (two thirds become DC-like and one third myeloid) [46]. These data confirm the effect of ATRA on DCs and MDSCs and strongly suggest a valuable role in cancer immunotherapy.
4.2 Randomized Phase II clinical trial in SCLC
We are currently conducting a randomized Phase II study where untreated patients with ES-SCLC, after receiving initial chemotherapy, are randomized to i) observation (standard of care); ii) INGN-225; or iii) INGN-225 plus ATRA. Upon progression, all patients are treated with single-agent paclitaxel (salvage chemotherapy). Our hypotheses are i) salvage chemotherapy in combination with INGN-225 results in a substantial improvement in clinical outcomes; and ii) ATRA, by reversing the immunosuppressive influence of MDSCs, substantially improves the p53-specific immune response to INGN-225 and hence clinical outcomes.
Even though topotecan is the only second-line therapy approved for SCLC, paclitaxel has shown reasonable activity in this setting [47]. Furthermore, paclitaxel was chosen as salvage chemotherapy due to our previous observation of improved response post-INGN-225 therapy [37], consistency in the new trial design, and pre-clinical laboratory data suggesting paclitaxel-induced upregulation of DR5 levels [48] when loss of DR5 is a recognized mechanism of immune escape in SCLC [49,50].
5. Conclusions
Very little progress has been made in SCLC using conventional treatment modalities, and applying novel modalities represents a rational approach. Immunotherapies that target normal proteins overexpressed in tumor cells have shown selective killing of these tumor cells without killing of cells with normal protein expression levels. Due to mutations, p53 protein overexpression occurs in ≥ 90% of SCLC, and selective killing of p53-overexpressing tumor cells by anti-p53 CTLs has been demonstrated. Thus, given that DCs represent the most potent APCs and can be cultured from PBMCs of cancer patients and that wt-p53 is a good antigenic target that can be exploited to prime a tumor-specific T cell response, we developed and tested a DC vaccine transfected with the wt-p53 gene (INGN-225) in SCLC.
Our Phase I/II clinical trial demonstrated that INGN-225 is safe and results in substantial immunological responses (40 – 50%). Furthermore, the absence of an immune response to INGN-225 may be due to a close association with the accumulation of immunosuppressive MDSCs present in the blood of these patients. However, the most significant finding from the trial was the unusually high frequency of objective tumor regressions in patients treated with chemotherapy immediately after INGN-225, particularly if a positive immune response was observed. This observation was quite unexpected since the existing paradigm suggests that chemotherapy is detrimental to the maintenance of an immune response. The paramount implications and significance of this issue deserves further testing, currently underway.
6. Expert opinion
These trials represent the first steps toward defining the role that IGN-225 will play in the treatment of SCLC and other p53-overexpressing tumors. This role will occur in the context of additional therapies (sequential or concurrent), and two areas deserving of future investigation to better define these associations are i) the synergistic mechanism observed with immunotherapy and chemotherapy; and ii) combinations with drugs to enhance a specific immune response.
The immunotherapy–chemotherapy synergism that we observed has been described with other solid tumors [51–53], and proposed mechanisms are divided into local and systemic [54–58]. Local effects include i) disruption of tumor stroma, resulting in improved penetration of CTLs to the tumor site; ii) increased permeability of tumor cells to CTL-derived granzymes; iii) increased TAA expression that enhances targeting by CTLs; iv) upregulation of Fas-FasL on tumor cells and CTLs; and v) synergistic effects in caspase 3 activation between chemotherapeutics, granzymes, and Fas. Systemic effects include i) chemotherapy and CTLs inducing apoptosis through different mechanisms; ii) tumor debulking to reduce the immunosuppressed milieu; iii) elimination of immunosuppressive cells (MDSCs, Tregs); iv) non-specific activation of APCs; v) improved cross-presentation of TAAs; and vi) lymphopenia with resultant homeostatic T cell proliferation.
The second area of interest includes drugs with immunostimulatory properties, and we have previously demonstrated that, by reducing and differentiating MDSCs, ATRA removes their immunosuppressive effect, creating a positive immune stimulus. Many new agents, with different molecular structures, pharmacokinetic and pharmacodynamic properties, biologic activities, and mechanisms of action (sunitinib malate [59], 1-methyl-dl-tryptophan (1-MT) [60], and anti-CTLA-4 [61] or anti-CD40 [62] monoclonal antibodies) but similar immunostimulatory activity, have been developed and are under clinical investigation. Thus, further research using any of these drugs as immunostimulatory agents is warranted, and we and many other groups are currently pursuing trials with 1-MT, sunitinib, anti-CD40 and anti-CTLA-4.
Acknowledgments
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
This paper has been sponsored by NCI grant 5P50- CA- 119997.
Footnotes
Declaration of interest
D Gabrilovich had a licensing agreement with Introgen Technologies for INGN-225, which no longer exists.
Bibliography
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Johnson DH. Management of small cell lung cancer: current state of the art. Chest. 1999;116:525S–530S. doi: 10.1378/chest.116.suppl_3.525s. [DOI] [PubMed] [Google Scholar]
- 3.Johnson BE. Management of small cell lung cancer. Clin Chest Med. 2002;23:225–239. doi: 10.1016/s0272-5231(03)00070-4. [DOI] [PubMed] [Google Scholar]
- 4.Simon GR, Wagner H. Small cell lung cancer. Chest. 2003;123:259S–271S. doi: 10.1378/chest.123.1_suppl.259s. [DOI] [PubMed] [Google Scholar]
- 5.von Pawel J, Schiller JH, Shepherd FA, et al. Topotecan versus cyclophosphamide, doxorubicin, and vincristine for the treatment of recurrent small-cell lung cancer. J Clin Oncol. 1999;17:658–667. doi: 10.1200/JCO.1999.17.2.658. [DOI] [PubMed] [Google Scholar]
- 6.Ardizzoni A. Topotecan in the treatment of recurrent small cell lung cancer: an update. Oncologist. 2004;9 Suppl 6:4–13. doi: 10.1634/theoncologist.9-90006-4. [DOI] [PubMed] [Google Scholar]
- 7.Davies AM, Evans WK, Mackay JA, et al. Treatment of recurrent small cell lung cancer. Hematol Oncol Clin North Am. 2004;18:387–416. doi: 10.1016/j.hoc.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Mattson K, Niiranen A, Ruotsalainen T, et al. Interferon maintenance therapy for small cell lung cancer: improvement in long-term survival. J Interferon Cytokine Res. 1997;17:103–105. doi: 10.1089/jir.1997.17.103. [DOI] [PubMed] [Google Scholar]
- 9.Prior C, Oroszy S, Oberaigner W, et al. Adjunctive interferon-alpha-2c in stage IIIB/IV small-cell lung cancer: a phase III trial. Eur Respir J. 1997;10:392–396. doi: 10.1183/09031936.97.10020392. [DOI] [PubMed] [Google Scholar]
- 10.Zarogoulidis K, Ziogas E, Papagiannis A, et al. Interferon alpha-2a and combined chemotherapy as first line treatment in SCLC patients: a randomized trial. Lung Cancer. 1996;15:197–205. doi: 10.1016/0169-5002(95)00583-8. [DOI] [PubMed] [Google Scholar]
- 11.Clamon G, Herndon J, Perry MC, et al. Interleukin-2 activity in patients with extensive small-cell lung cancer: a Phase II trial of Cancer and Leukemia Group B. J Natl Cancer Inst. 1993;85:316–320. doi: 10.1093/jnci/85.4.316. [DOI] [PubMed] [Google Scholar]
- 12.Kelley MJ, Linnoila RI, Avis IL, et al. Antitumor activity of a monoclonal antibody directed against gastrin-releasing peptide in patients with small cell lung cancer. Chest. 1997;112:256–261. doi: 10.1378/chest.112.1.256. [DOI] [PubMed] [Google Scholar]
- 13.Chikamatsu K, Nakano K, Storkus WJ, et al. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clin Cancer Res. 1999;5:1281–1288. [PubMed] [Google Scholar]
- 14.Liu DP, Song H, Xu Y. A common gain of function of p53 cancer mutants in inducing genetic instability. Oncogene. 2009;29:949–956. doi: 10.1038/onc.2009.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Oijen MG, Slootweg PJ. Gain-of-function mutations in the tumor suppressor gene p53. Clin Cancer Res. 2000;6:2138–2145. [PubMed] [Google Scholar]
- 16.Vierboom MP, Nijman HW, Offringa R, et al. Tumor eradication by wild-type p53-specific cytotoxic T lymphocytes. J Exp Med. 1997;186:695–704. doi: 10.1084/jem.186.5.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayordomo JI, Loftus DJ, Sakamoto H, et al. Therapy of murine tumors with p53 wild-type and mutant sequence peptide-based vaccines. J Exp Med. 1996;183:1357–1365. doi: 10.1084/jem.183.4.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zwaveling S, Vierboom MP, Ferreira Mota SC, et al. Antitumor efficacy of wild-type p53-specific CD4+ T-helper cells. Cancer Res. 2002;62:6187–6193. [PubMed] [Google Scholar]
- 19.Parajuli P, Pisarev V, Sublet J, et al. Immunization with wild-type p53 gene sequences coadministered with Flt3 ligand induces an antigen-specific type 1 T-cell response. Cancer Res. 2001;61:8227–8234. [PubMed] [Google Scholar]
- 20.Eura M, Chikamatsu K, Katsura F, et al. A wild-type sequence p53 peptide presented by HLA-A24 induces cytotoxic T lymphocytes that recognize squamous cell carcinomas of the head and neck. Clin Cancer Res. 2000;6:979–986. [PubMed] [Google Scholar]
- 21.Theobald M, Ruppert T, Kuckelkorn U, et al. The sequence alteration associated with a mutational hotspot in p53 protects cells from lysis by cytotoxic T lymphocytes specific for a flanking peptide epitope. J Exp Med. 1998;188:1017–1028. doi: 10.1084/jem.188.6.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vierboom MP, Gabrilovich DI, Offringa R, et al. p53: a target for T cell mediated immunotherapy. In: Kast WM, editor. Peptide-based cancer vaccines. Georgetown, TX: Landes Bioscience; 2000. pp. 40–55. [Google Scholar]
- 23.Brossart P, Goldrath AW, Butz EA, et al. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 24.Wan Y, Bramson J, Carter R, et al. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 25.Specht JM, Wang G, Do MT, et al. Dendritic cells retrovirally transduced with a model antigen gene are therapeutically effective against established pulmonary metastases. J Exp Med. 1997;186:1213–1221. doi: 10.1084/jem.186.8.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thomson SA, Khanna R, Gardner J, et al. Minimal epitopes expressed in a recombinant polyepitope protein are processed and presented to CD8+ cytotoxic T cells: implications for vaccine design. Proc Natl Acad Sci USA. 1995;92:5845–5849. doi: 10.1073/pnas.92.13.5845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Becker TC, Noel RJ, Coats WS, et al. Use of recombinant adenovirus for metabolic engineering of mammalian cells. Methods Cell Biol. 1994;43:161–176. doi: 10.1016/s0091-679x(08)60603-2. [DOI] [PubMed] [Google Scholar]
- 28.Roth JA, Cristiano RJ. Gene therapy for cancer: what have we done and where are we going? J Natl Cancer Inst. 1997;89:21–39. doi: 10.1093/jnci/89.1.21. [DOI] [PubMed] [Google Scholar]
- 29.Brossart P, Goldrath AW, Butz EA, et al. Virus-mediated delivery of antigenic epitopes into dendritic cells as a means to induce CTL. J Immunol. 1997;158:3270–3276. [PubMed] [Google Scholar]
- 30.Dietz AB, Vuk-Pavlovic S. High efficiency adenovirus-mediated gene transfer to human dendritic cells. Blood. 1998;91:392–398. [PubMed] [Google Scholar]
- 31.Wan Y, Bramson J, Carter R, et al. Dendritic cells transduced with an adenoviral vector encoding a model tumor-associated antigen for tumor vaccination. Hum Gene Ther. 1997;8:1355–1363. doi: 10.1089/hum.1997.8.11-1355. [DOI] [PubMed] [Google Scholar]
- 32.Nikitina EY, Chada S, Muro-Cacho C, et al. An effective immunization and cancer treatment with activated dendritic cells transduced with full-length wild-type p53. Gene Ther. 2002;9:345–352. doi: 10.1038/sj.gt.3301670. [DOI] [PubMed] [Google Scholar]
- 33.Ishida T, Chada S, Stipanov M, et al. Dendritic cells transduced with wild-type p53 gene elicit potent anti-tumour immune responses. Clin Exp Immunol. 1999;117:244–251. doi: 10.1046/j.1365-2249.1999.00913.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nikitina EY, Clark JI, Van Beynen J, et al. Dendritic cells transduced with full-length wild-type p53 generate antitumor cytotoxic T lymphocytes from peripheral blood of cancer patients. Clin Cancer Res. 2001;7:127–135. [PubMed] [Google Scholar]
- 35.D’Amico D, Carbone D, Mitsudomi T, et al. High frequency of somatically acquired p53 mutations in small-cell lung cancer cell lines and tumors. Oncogene. 1992;7:339–346. [PubMed] [Google Scholar]
- 36.Bodner SM, Minna JD, Jensen SM, et al. Expression of mutant p53 proteins in lung cancer correlates with the class of p53 gene mutation. Oncogene. 1992;7:743–749. [PubMed] [Google Scholar]
- 37.Antonia SJ, Mirza N, Fricke I, et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin Cancer Res. 2006;12:878–887. doi: 10.1158/1078-0432.CCR-05-2013. [DOI] [PubMed] [Google Scholar]
- 38.Chiappori A, Sereno M, Gabrilovich D, et al. Phase II trial of patients with extensive stage small cell lung cancer immunized with p53-transduced dendritic cells: immune sensitization to chemotherapy. Proc Am Soc Clin Oncol. 2007;25 abstract 3012. [Google Scholar]
- 39.Pisarev V, Yu B, Salup R, et al. Full-length dominant-negative survivin for cancer immunotherapy. Clin Cancer Res. 2003;15:6523–6533. [PubMed] [Google Scholar]
- 40.Kusmartsev S, Gabrilovich DI. Immature myeloid cells and cancer-associated immune suppression. Cancer Immunol Immunother. 2002;51:293–298. doi: 10.1007/s00262-002-0280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gabrilovich D. The mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 42.Mirza N, Fishman M, Fricke I, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bronte V, Apolloni E, Cabrelle A, et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood. 2000;96:3838–3846. [PMC free article] [PubMed] [Google Scholar]
- 44.Gabrilovich DI, Velders MP, Sotomayor EM, et al. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 45.Kusmartsev S, Cheng F, Yu B, et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 46.Almand B, Clark JI, Nikitina E, et al. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 47.Smit EF, Fokkema E, Biesma B, et al. A phase II study of paclitaxel in heavily pretreated patients with small-cell lung cancer. Br J Cancer. 1998;77:347–351. doi: 10.1038/bjc.1998.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nimmanapalli R, Perkins CL, Orlando M, et al. Pretreatment with paclitaxel enhances apo-2 ligand/tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis of prostate cancer cells by inducing death receptors 4 and 5 protein levels. Cancer Res. 2001;61:759–763. [PubMed] [Google Scholar]
- 49.Hopkins-Donaldson S, Ziegler A, Kurtz S, et al. Silencing of death receptor and caspase-8 expression in small cell lung carcinoma cell lines and tumors by DNA methylation. Cell Death Differ. 2003;10:356–364. doi: 10.1038/sj.cdd.4401157. [DOI] [PubMed] [Google Scholar]
- 50.Ramakrishnan R, Antonia S, Gabrilovich DI. Combined modality immunotherapy and chemotherapy: a new perspective. Cancer Immunol Immunother. 2008;57:1523–1529. doi: 10.1007/s00262-008-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Radfar S, Wang Y, Khong HT. Activated CD4+ T cells dramatically enhance chemotherapeutic tumor responses in vitro and in vivo. J Immunol. 2009;183:6800–6807. doi: 10.4049/jimmunol.0901747. [DOI] [PubMed] [Google Scholar]
- 52.Wheeler CJ, Das A, Liu G, et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10:5316–5326. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 53.Gribben JG, Ryan DP, Boyajian R, et al. Unexpected association between induction of immunity to the universal tumor antigen CYP1B1 and response to next therapy. Clin Cancer Res. 2005;11:4430–4436. doi: 10.1158/1078-0432.CCR-04-2111. [DOI] [PubMed] [Google Scholar]
- 54.Arlen PM, Gulley JL, Parker C, et al. A randomized Phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin Cancer Res. 2006;12:1260–1269. doi: 10.1158/1078-0432.CCR-05-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arlen PM, Madan RA, Hodge JW, et al. Combining vaccines with conventional therapies for cancer. Update Cancer Ther. 2007;2:33–39. doi: 10.1016/j.uct.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schlom J, Arlen PM, Gulley JL. Cancer vaccines: moving beyond current paradigms. Clin Cancer Res. 2007;13:3776–3782. doi: 10.1158/1078-0432.CCR-07-0588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulley JL, Madan RA, Arlen PM. Enhancing efficacy of therapeutic vaccinations by combination with other modalities. Vaccine. 2007;25 Suppl 2:B89–B96. doi: 10.1016/j.vaccine.2007.04.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schlom J, Gulley JL, Arlen PM. Paradigm shifts in cancer vaccine therapy. Exp Biol Med (Maywood) 2008;233:522–534. doi: 10.3181/0708-MR-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ozao-Choy J, Ma G, Kao J, et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 2009;69:2514–2522. doi: 10.1158/0008-5472.CAN-08-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cady SG, Sono M. 1-Methyl-DL-tryptophan, beta-(3-benzofuranyl)-DL-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-DL-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Arch Biochem Biophys. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- 61.Ribas A, Hanson DC, Noe DA, et al. Tremelimumab (CP-675,206), a cytotoxic T lymphocyte associated antigen 4 blocking monoclonal antibody in clinical development for patients with cancer. Oncologist. 2007;12:873–883. doi: 10.1634/theoncologist.12-7-873. [DOI] [PubMed] [Google Scholar]
- 62.Vonderheide RH, Flaherty KT, Khalil M, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–883. doi: 10.1200/JCO.2006.08.3311. [DOI] [PubMed] [Google Scholar]