Abstract
The majority of different cell types in the human body have a cilium, a thin rod-like structure of uniquely arranged microtubules that are encapsulated by the surface plasma membrane. The cilium originates from a basal body, a mature centriole that has migrated and docked to the cell surface. The non-motile cilia are microtubule-based organelles that are generally considered sensory structures. The purpose of this review is to discuss the practicality of the ciliary hypothesis as a unifying concept for polycystic kidney disease and to review current literature in the field of cilium biology, as it relates to mechanosensation and planar cell polarity. The polycystins and fibrocystin localization at the cilium and other subcellular localizations are discussed, followed by a hypothetical model for the cilium’s role in mechanosensing, planar cell polarity, and cystogenesis.
Keywords: Cilium, Polycystic kidney disease, Polarization, Review
2. INTRODUCTION
Polycystic Kidney Disease (PKD) is a group of renal cystic diseases in which many forms are inherited in a Mendelian fashion. The autosomal dominant form of PKD is the most common type, characterized by isolated, large symmetrical bi-lateral cysts. The mechanism of cyst formation is still unknown; however, indirect evidence showing many PKD gene products localize to the primary cilium led to a ciliary hypothesis for PKD (1). The primary cilium found in many cell types (2) is considered to be a fluid-flow sensor for the epithelial cells lining the nephron (3, 4). In essence, the ciliary hypothesis states that dysfunctions in proteins localized to the primary cilium cause PKD.
The inference from many studies investigating the primary cilium in association with PKD is that altered mechanosensation is a key etiological factor in the cellular pathogenesis of PKD, i.e., ciliary dysfunction leads to cyst formation. While this hypothesis is undoubtedly novel and intriguing, recent claims have further garnished this model by suggesting its potential to provide a "unifying pathogenic concept" for PKD (5). A unifying pathogenic concept should require the integration of many well-described cellular pathologies of PKD into a single testable experiment with the end result demonstrating strong evidence for a direct mechanistic link between cilium function and cyst formation.
This reductionist-like approach and its conclusions based on a cellular organelle with unknown functions in the human kidney is risky, because it fails to integrate the data from other studies showing the other cellular localizations and functions for polycystins, protein products of PKD genes (6–15), as well as other cystic disease proteins (16–26). Furthermore, functional data for proteins in the ciliary plasma membrane is limited. Balancing the published data on the pathogenic mechanisms for PKD is necessary to understand this complex disease. The balance begins by placing a weighted importance on these cellular organelles and subcellular structures to their contribution of cellular phenotypes observed in PKD that are based on functional studies with experimental reproducibility.
Dysfunction of the cilium and centrosome as a cause of renal cystic diseases is, so far, one of "guilty by association.” The ciliary hypothesis was developed from observations that most of the cystic disease proteins localize to the cilium. Localization does not directly imply importance; otherwise, the endoplasmic reticulum and Golgi apparatus, which certainly contain all of these proteins, should be equally as “guilty”. However, proteins associated with the human cystic diseases of the kidney (such as fibrocystin, nephronophthisis-1 to 5, polycystin-1 and -2) also localize to the lateral junctions with many of these proteins having other subcellular localizations and function (6, 14, 27). Moreover, proteins such as Kif3A/B, polaris, and cystin exclusively localize to the cilium-basal body-centriole axis, and when mutated in mice cause PKD. These proteins are cilium maintenance proteins, yet none have a known association with human disease (28–32).
A notable exception is the BBS proteins that localize only to the cilium-basal body-centriole axis where mutations of this protein group have been associated with Bardet-Biedl syndrome (33). Thus, the two major pieces of evidence implicating the primary cilium, i.e. ciliary localization and assembly, are circumstantial, given that there are no ciliary length defects in human cystic kidney disease. Furthermore, no human disease is associated with a protein that localizes exclusively to the cilium. Until we observe such differences, we must consider ciliary dysfunction in PKD as a contributing factor rather than a primary cause of PKD. Weighing the importance of a particular cellular compartment or localization better affords us the opportunity to design treatments that will likely have the most efficacies in treating PKD. Thus, we aim to carefully review the data on the subcellular localization of cystoproteins in renal tubular epithelial cells and the functional significance at these locales in PKD.
Conversely, the functional roles of the primary cilium are thought to be important in many cellular processes, with emerging data suggesting that cilium dysfunction as a primary cause in cystic diseases. Moreover, the "ciliary hypothesis" can explain the extra-renal phenotypes in many of these cases (34, 35). Despite this fact, researchers should not overextend themselves by focusing primarily on the cilium. Rather, a continuous effort should be made to integrate the cilium's role into basic cellular functions and then objectively assign the contribution of this organelle to the disease processes of PKD while giving careful consideration to the non-cilium localization and functions of the cystic proteins. Otherwise, we will fail to see the forest because we are too focused on the trees.
3. INHERITED POLYCYSTIC KIDNEY DISEASES
PKD is a leading cause of end stage renal disease (ESRD). Most forms of PKD are hereditary although it may be acquired in patients who have had acute renal failure and subsequent dialysis (34, 36–39). Autosomal dominant polycystic kidney disease (ADPKD) is a frequently-occurring genetic disease of the kidney affecting one in every 500 to 1,000 individuals. The principle genes mutated in ADPKD are PKD1 (Polycystic Kidney Disease 1) and PKD2 (Polycystic Kidney Disease 2), genes that encode for polycystin-1 (40, 41) and polycystin-2 (42), respectively. More than 85% of ADPKD cases occur as a result of mutations in PKD1, and mutations in PKD2 account for only 10%.
Autosomal recessive polycystic kidney disease (ARPKD) is caused by mutations in PKHD1 (Polycystic Kidney and Hepatic Disease 1) resulting in either a severe form, caused by two different truncating mutations, or a moderate form, due to missense mutations or a missense and truncating mutation in the encoded protein fibrocystin (43–45). The prevalence of ARPKD is one in 20,000 live births, characterized with renal failure and hepatic fibrosis as the primary cause of death in those infants.
While large focal cysts arising from the rapidly dividing tubular epithelial cells are the hallmark of ADPKD, elongated cysts due to collecting duct dilatations are the key feature of ARPKD. An important difference between the two is that cysts become isolated in ADPKD while in ARPKD the cysts remain in contact with their tubular origin (46). The mechanism for cyst formation in PKD is unclear. If or how cyst formation is a direct result of cilium dysfunction remains the pivotal question. Studies have shown that polycystin-1, polycystin-2, and fibrocystin localize to the primary cilium. These three proteins appear to be in the same complex (47, 48). Furthermore, these proteins have been proposed to have a role in mediating flow dependent mechanosensation (47, 49–54), suggesting a common pathway in cyst development.
While it appears that the ciliary hypothesis explains the major forms of PKD, it cannot explain other forms of renal cystic diseases found in humans. For example, mutations in MCKD2 result in medullary cystic kidney disease (MCKD); however, Tamm-Horsfall protein, the MCKD2 gene product, does not localize to the cilium or connecting substructures (55, 56). Other renal cystic diseases, such as Bardet-Biedl syndrome, have a direct link with a protein that is localized to the centrosome or basal body of primary cilia (57). Distinguishing between the cilium, basal body, and the centrosome is a very important task on both a morphological and functional level. Thus, it is imperative for us to weigh in the relevance of “cysto” proteins to their localizations at the cilium.
For a more detailed description of human cystic diseases, please see the reviews by Wilson (46), Igarashi (58) and others. For more detailed information on animal models of PKD, please refer to reviews by Guay-Woodford (59) and Torres (60).
4. LOCALIZATION OF POLYCYSTINS AND FIBROCYSTIN
4.1. Tissue distribution
Immunohistochemical detection of polycystin-1 in human renal tissue indicates polycystin-1 is expressed in most nephron segments, although the precise spatial pattern remains unclear (7, 61–63). This may be due to variations in the temporal and spatial expression pattern for polycystin-1 between species confounded by changing cellular localizations during development (63, 64). This is further complicated by individual studies using different antibodies to various regions of polycystin-1, introducing an uncertainty in the detection of full-length polycystin-1, and detection of protein products from homologous genes. Moreover, antigen detection in tissue is difficult, often masked from fixation and the documented results regarding the degradation of polycystin-1 (63).
Developmental studies show polycystin-1 localizes to the distal portion of the uteric bud (15) as well as the portion of the uteric bud deep within the nephrogenic zone (63). However, murine polycystin-1 localization studies have also demonstrated low levels of polycystin-1 at the uteric bud (64) along with no detectable PKD1 mRNA in the uteric bud of human embryos at 10 weeks, challenging the role of polycystin-1 in nephrogenic induction (65). Maturing cortical tubules and collecting ducts also express polycystin-1 at moderately high levels during embryonic development (7, 64, 66). Other localization studies show evidence for polycystin-1 expression within the glomerulus (66); either at the glomerular tuft or parietal cells of Bowman’s capsule (7, 67). Polycystin-1 expression has also been documented in vascular endothelial cells and smooth muscle cells (68–70). In general, polycystin-1 expression declines after birth and is present at low levels in adults with expression confined to the cortical and medullary collecting duct. Polycystin-1 expression is high in fetal tissues with tubular expression much lower in the collecting duct during adulthood (7, 61). This developmental expression pattern along the nephron has led to the hypothesis that polycystin-1 is involved in tubular differentiation during nephrogenesis and tubular maintenance in the adult kidney.
Initial distribution studies showed identical polycystin-1 and polycystin-2 expression patterns in human kidney tissue sections (71). This result was in conflict with data that suggested distinct polycystin-1 and polycystin-2 distribution patterns within the mouse kidneys (72). Subsequent localization studies revealed a temporal expression pattern for polycystin-1 and polycystin-2, thereby reconciling the apparent discrepancy (64). The current literature suggests that polycystin-2 expression occurs later in development than polycystin-1 and maintains a greater level of expression in the adult (73). Interestingly, PKD2 transcript and protein is detectable in human embryos earlier than PKD1 (72, 73). The temporal and spatial differences between these two proteins suggest that they each have two different roles, one of dependence and another of independence. The dependent role prompted the hypothesis that the proteins form a functional complex in vivo that serves as a convenient explanation for the similar phenotypes in ADPKD. This is further supported by the evidence of the two polycystins’ interaction at their cytosolic COOH termini (74, 75) to form functional complex in vivo (8, 53).
The Pkhd1 transcript has a complex pattern of splice variants (76, 77), and fibrocystin itself undergoes a complicated pattern of proteolytic processing (78, 79). Fibrocystin has been shown to express in a tissue-specific manner, which varies with the stage of tissue development and cellular differentiation (80, 81). During kidney development, fibrocystin is expressed in ureteric bud branches (80–82). Fibrocystin is also expressed in cortical and medullary collecting tubular epithelia (47, 80, 83) and thick ascending limbs of Henle (80, 81). Expressions of fibrocystin in other tissues, such as liver and pancreas, have also been reported (80, 82). The significance of fibrocystin in the tissue distribution during development remains to be elucidated; its subcellular localization and functional contributions in PKD will be discussed in the next sections.
4.2. Subcellular distribution
In vivo immunofluorescence and electron microscopy studies revealed that polycystin-1 localizes to the plasma membrane and cytoplasm (7, 62, 84, 85). In cultured cells, polycystin-1 localizes to numerous membrane and cytosolic compartments including apico-basolateral plasma membranes (62, 86–90), cytosolic vesicles (91), and endoplasmic reticulum (92) with cleavage products detected in the nucleus (49, 50). The trafficking of polycystin-1 to the lateral membranes may be due, in part, to a dependence on tuberin (88, 93). Furthermore, polycystin-1 trafficking to the plasma membrane is dependent upon the cytoskeleton as the COOH terminal-tail directly interacts with intermediate filaments (94). Polycystin-1 also has the capacity to bind to the extracellular matrix components such as collagen I, laminin, fibronectin, and integrin (85, 95, 96). The localization of polycystin-1 to multiple subcellular localizations suggests that it has multiple functions ranging from flow sensing to cellular adhesion and differentiation.
Tissue distribution studies have revealed the developmental regulation pattern for polycystin-1 during nephrogenesis, and cell culture studies seem to indicate a temporal and spatial pattern for polycystin-1 dependent upon the differentiation status. Polycystin-1 localizes to the desmosomes at the lateral membranes soon after establishment of cell-cell junctions (14, 91, 97). When cell polarity is achieved and cellular differentiation begins, polycystin-1 appears to interact with the tight junctional proteins E-cadherin and the catenins (98). Establishment of adhesion and cell polarity allows the cell to enter a more differentiated state (G0) where polycystin-1 can reside on the primary cilium (52, 99). Interestingly, these three sites are essential in mechanotransduction, and the unique structure of polycystin-1 lends itself to carrying out the diverse mechanosensing abilities required at multiple cellular sites. Thus, as polycystin-1 spatial expression in tissue is indicatives of its role in tubular differentiation, one would predict that cellular differentiation also results in a spatial distribution for polycystin-1 expression.
Polycystin-2 resides within the endoplasmic reticulum (9, 100), plasma membrane (101–103), primary cilium (52, 99, 104), and mitotic spindles (105). The details of how polycystin-2 traffics to the cilium are limited, but current data suggests that there is a specific ciliary signal sequence, which allows polycystin-2 to exit the endoplasmic reticulum and enter the ciliary plasma membrane (106, 107). Interestingly, the localization of the polycystins appears to be partially dependent on one another. For instance, in cells where polycystin-2 is absent, polycystin-1 localizes to the plasma membrane and the endoplasmic reticulum (92). In cultured epithelial cells with mutation in PKD1, localization of polycystin-2 to cilia is not that apparent (53, 54). In polycystin-1 null cells, however, polycystin-2 can still travel to the cilium, indicating that its localization is independent of polycystin-1 (106). Thus, polycystin-2 localization to cilia appears to partially depend on polycystin-1. Furthermore, polycystin-2 trafficking to the cilium is thought to be independent from its phosphorylation status in the endoplasmic reticulum, but requires the presence of IFT88 (108, 109). The role polycystin-1 and polycystin-2 have on each other as it relates to ciliary trafficking requires further investigation.
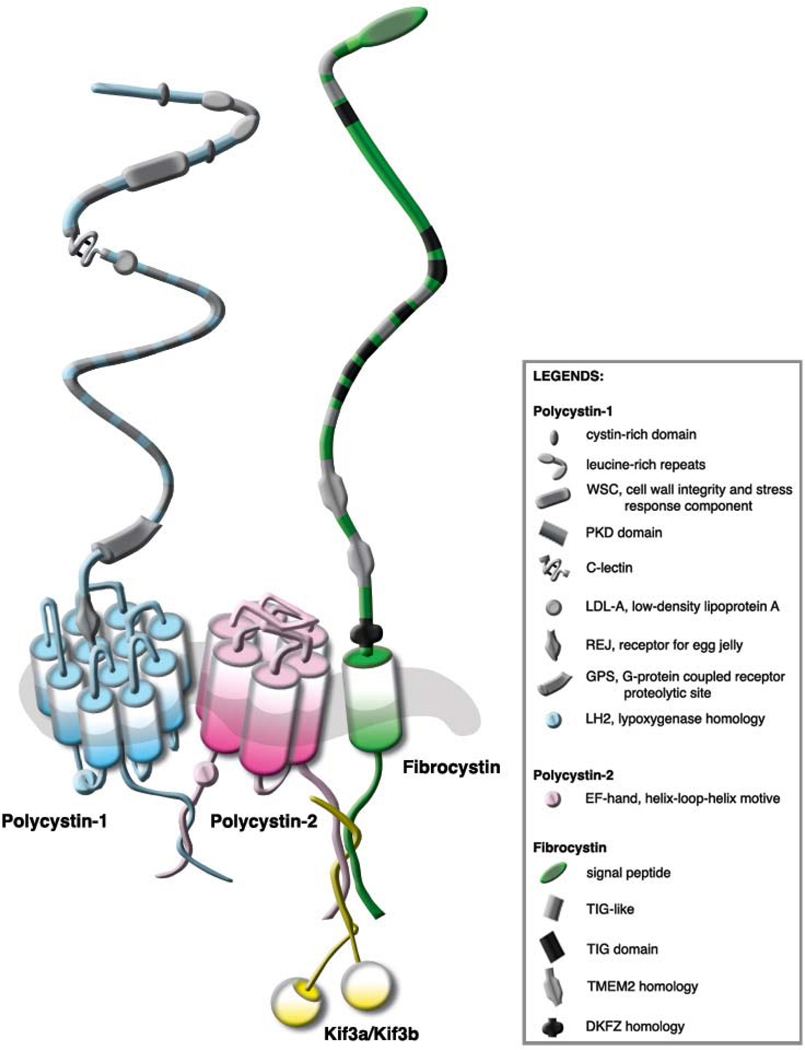
Immunohistohemical analysis demonstrates that fibrocystin, like other cysto proteins, is localized in centrosomes, basal bodies or primary apical cilia in renal epithelial cells. Fibrocystin is also found at other subcellular localizations, such as cytosol, basal lateral membrane and nucleus (78–83, 110). Recent studies show that fibrocystin indirectly interacts with polycystin-2 at the cilium (47, 48) (Figure 1). Furthermore, it has been shown that this indirect interaction between fibrocystin with polycystin-2 is necessary in regulating polycystin-2 channel activity (48). This suggests that a similar mechanism of cystogenesis may exist between ARPKD and ADPKD. The subcellular localization of fibrocystin being unaltered in Pkd1 cells (110) suggests that the localization of fibrocystin is independent of polycystin-1. Wang et al. have also shown in stable Pkhd1 knockdown cells, both polycystin-1 and -2 expressions and localizations are not altered (47).
Figure 1.
Mechanosensory protein complex. Among other subcellular localizations, it is thought that polycystin-1, polycystin-2 and fibrocystin form a mechanosensory complex protein in the cilium to sense fluid-shear stress. Polycystin-1 and polycystin-2 interact with each other at their COOH termini forming a polycystin complex. It is predicted that fibrocystin interacts with this complex through polycystin-2 via Kif as a possible adaptor protein.
5. PRIMARY CILIUM AND PKD
An increasingly greater link between the mammalian primary cilium and human diseases has put this organelle at the center of attention for many cell biologists. However, an absent mechanistic link between cilium and cyst formation has made the functional significance of this organelle elusive. The primary cilium is potentially a flow sensor for the luminal fluid of the nephron, where cilium bending results in increasing cytosolic calcium. This paradigm was established mainly from in vitro studies where cultured kidney cells were challenged with a fluid-flow shear stress that bent the cilium, thereby activating intracellular calcium stores and a subsequent rise in free intracellular calcium concentration (52, 111). Subsequent studies then demonstrated that polycystins localize to the primary cilium of renal tubular epithelial cells, and when absent from the cilium or if inactivated, the fluid shear-induced calcium response was abolished (52–54). However, the reproduction of these results using an in vivo model has not been forthcoming. Ex vivo experiments using isolated gastrulation stage node and perfused tubules have produced results confirming a mechanosensory function for the primary cilium (51, 112). Future studies are still necessary to confirm that the ciliary polycystins mediate the fluid-flow response in human adult nephron segments.
The cilium’s involvement in a broad range of cellular events such as cell cycle (3, 113) and cell polarity (114, 115), along with differentiation and establishment of planar cell polarity has made the task of determining its primary function in vivo difficult to establish (79, 116). How these functions are interconnected, if indeed they are, is an important question, as it may lead to insights into the pathogenesis of PKD. Furthermore, the quantitative properties of cilium sensitivity to shear stress will afford researchers the opportunity to relate cilium mechanosensory function to the luminal fluid-flow properties allowing for more accurate predictions about cilium involvement in the mature nephron (117). Two possibilities are that either the primary cilium is a low flow sensor that functions primarily during development to facilitate planar cell polarity for establishing luminal diameter, or the primary cilium may act as a maintenance sensor that allows the tubular cells to dedifferentiate and elongate along the tubule when the nephron has been challenged with an insult. Thus, one possible scenario is that during PKD development, the nephron is unable to repair itself correctly, because the maintenance signaling from the cilium is absent or altered, forcing the tubular cells into an unregulated repair mechanism.
5.1. Biology of a primary cilium
The primary cilium is a membrane-covered tubular-shaped extension filled with a highly organized array of microtubules. The ciliary microtubules elongate from the preexisting ones, which originated and grew from a basal body, a mature centriole that has migrated within the cytosol to the apical surface. The basal body is integrated into the cytoskeleton through its projections of transitional fibers, basal foot processes, and bundles of thin filaments connecting it to the other centriole. Together, the basal body and centriole form a centrosome, which serve as the cell’s main microtubule organizing center (118, 119).
The mammalian cilium is a diverse structure with multiple known functions, yet the main function for primary cilium of renal cells is still not well understood. The ultrastructure of the mammalian cilium was once thought to accurately predict its motility, e.g., 9+2 cilia were presumed to always be motile and 9+0 considered immotile. The consensus was that the 9+0 arrangement in many mammalian cilia was indicative of an inability to beat, since the inner doublet was important for the beating pattern exemplified by lung epithelial cilia. This is now known to be incorrect, as evidenced by the 9+0 motile cilium found in nodal cells (120, 121). Moreover, in the rabbit and mouse node, there are multiple cilium types ranging from 9+0, 9+2 and the newly discovered 9+4 (122).
The general distribution of the 9+0 or non-motile cilium occurs in tubular-based tissues such as the nephron, bile duct, vascular system and pancreas. The 9+0 cilium is likely to function as a flow sensor to monitor shear stress across the cell’s surface and subsequently relaying this information back to the cytosolic compartment. The flow sensing function appears critical in cystic disease, but whether or not the cilium has an important role in the adult animal remains to be seen. Furthermore, the flow sensing capacity of the primary cilium has been largely demonstrated in cultured cells. More in vivo data is warranted to support ciliary role as an actual flow sensor.
The motile cilium’s role in vivo is supported by strong data suggesting its primary function is to facilitate fluid movement in such places as the lung. Interestingly, under certain pathological conditions, a 9+2 cilium has been detected along the mammalian nephron (123, 124). This observation is intriguing and may occur to facilitate fluid movement along the nephron. In particular, it has been reported that primary cilium in the zebrafish nephron is motile (125). Future research will likely investigate such observations more closely, since current research indicates the 9+0 or 9+2 microtubule arrangement is not an absolute indicator of cilium motility (3).
Interestingly, the cilium appears to be integrated into the cell’s cytoskeletal and integrin signaling pathways; it forms an apparent communication system that begins at the ciliary membrane, diverges through the cell via the centrosome and microtubule organizing center, and ultimately ends at the basal cellular adhesion sites. The basics of this communication system were established by experiments where each component was systematically disrupted while using ciliary calcium flow response as the functional readout. Thus, when actin, tubulin, microfilaments, or integrins are not fully functional, the cell is unable to initiate a flow response (126).
For a more detailed description of cilia structure and function, please see the works by Praetorius (4), Satir (127), and others (3, 128, 129).
5.2. Ciliary mechanosensation proteins
Polycystin-1 (52, 99), polycystin-2 (52, 99, 104), and fibrocystins (82) are localized to the primary cilium in renal epithelial cells, and their contribution to mechanosensation is not fully understood. In vitro perfusion data strongly suggests that the polycystins (51– 54), and presumably fibrocystin (47), are involved in the ciliary mediated response to flow by initiating intracellular calcium release. This flow response is a result of cilium activation, apparently from a low fluid-shear that causes the cilium to bend, with subsequent activation of the polycystins and other interacting proteins in this complex along with the downstream signaling components. The rise in intracellular calcium is likely one of a multitude of flow-induced ciliary responses that may or may not arise directly from the cilium. This is an area of much needed investigation. For instance, polycystin-1 binds to various heterotrimeric G proteins (130–133), and the signal transduction arising from their activation may contribute to the overall cellular response to flow. The non-calcium mediated signaling pathways may possibly bridge ciliary information to the polycystins at other subcellular localizations (134).
Polycystin-1, polycystin-2, fibrocystin, and possibly other cysto proteins such as Kif3a/b (Figure 1), can form a multi-protein complex at the primary cilium with mechanosensing functions in cultured kidney lines (47, 48). Interestingly, the recent finding that a fibrocystin at the ciliary plasma membrane has a cleavage product released into the lumen indicates multiple functions for fibrocystin. The authors of this study went on to speculate that the cleavage product may function in a paracrine fashion, possibly maintaining cell polarity of the downstream tubular epithelium (79). Moreover, it is possible that fibrocystin cleavage is an extracellular signaling event in response to mechanosensation, similar to polycystin-1 in response to mechanical stress (49, 50). If these three proteins are in a signaling complex, and polycystin-1 and polycystin-2 mutants give rise to a similar phenotype with many large focal cysts, then it is interesting as to why the fibrocystin mutant results in a phenotype of tubular dilatation.
Fibrocystin has also been suggested to play important role in ciliary structure and morphology. Although there is conflicting evidence on the role of fibrocystin in regulating length of a cilium (47), a previous study shows that 90% of Pkhd1 knockdown cells fail to generate cilia (135). Furthermore, a more recent study shows that the primary cilia in the bile ducts of Pkhd1 mice are structurally shorter than those of wild-type animals (136). Using PCK rat as an animal model of ARPKD, Masyuk et. al. show that cilia are shorter and dysmorphic (137). They further show that cholangiocytes treated with Pkhd1 siRNA and lacking fibrocystin have shorter cilia. Further characterization of the PCK liver phenotype confirmed these findings, demonstrating that cilia from PCK rats are significantly shorter and malformed with bulbous extensions of the ciliary tip (138).
The Orpk murine model of ARPKD, which is genetically different from human ARPKD, has a stunted cilium (112, 139). Siroky et al. show that cells from the murine model of ARPKD have abnormal apical flow sensing (140). There is also evidence that fibrocystin may play a role in regulating intracellular calcium through ciliary mechanosensing (47). Another study suggests that fibrocystin interacts with calcium modulating cyclophilin ligand (CAML), a protein involved in calcium signaling (83). Since CAML is a calcium signaling-related protein, the authors suggest that fibrocystin may also contribute to the modulation of intracellular calcium in a manner similar to polycystins.
Although there is sufficient evidence to demonstrate that polycystins and fibrocystin are localized to cilium, there certainly are many more questions that we could not answer about these mechanosensory proteins. For example, as mentioned earlier in the review, the expression levels of polycystin-1 change substantially during development where polycystin-1 levels actually decrease towards adulthood. This raises the question of how important the role of polycystin-1 is at the cilium in the matured adult tissue. The most recent data shows that inactivation of Pkd1 in mice before postnatal day 13 results in severely cystic kidneys within 3 weeks, whereas inactivation at day 14 results in cysts after 5 months (141). This indicates that polycystin-1 functions are still important in adulthood, albeit more critical during development.
Generally speaking, studying the detailed nature of polycystin/fibrocystin signaling at the cilium is a daunting task especially when one considers the large size of the proteins and the technical difficulties associated with large glycoproteins and/or multiple proteolytic isoforms.
6. PLANAR CELL POLARITY
Kidney tubules are constructed from sheets of polarized epithelial cells in a single plane, which form a lumen for the transport of urinary fluid. Tubular formation is thought to occur by processes such as planar cell polarity (PCP) as most epithelial tissues develop in a polarized fashion identifiable by the microvilli and cilia extending from the cell surface. The processes behind lumen formation are unknown, but tubular elongation occurs by orienting cell division in the proliferative phase of tubular development.
One mechanism likely to play an important role in tubular development is PCP, a set of processes that govern the polarization of epithelial cells and allow for the transfer of such information to the newly developing cells. PCP involves a complex set of genes to establish a 3-dimensional orientation of organ development. PCP involvement in tubulogenesis appears to utilize another complex network of genes referred to as Wnt signaling (142). Polycystin-1 has been recently shown to interact with the morphogens of the Wnt pathway (143); however, it is not clear if this is directly related to PCP.
Wnt signaling pathways are grouped as canonical or non-canonical (144). Canonical Wnt utilizes Frizzled (Fz) and LDL-related protein (LRP) co-receptors. In general, Wnt binds to Fz receptors, inducing phosphorylation of disheveled (DSH). DSH activation inhibits the APC-Axin-glycogen synthase kinase-3, allowing β-catenin to escape proteasomal degradation. β-catenin levels in the nucleus presumably increase thereby activating Tcf family members and Wnt target genes. Cannonical Wnt pathway is a process that guides the proliferation of renal epithelial cells and aids the formation of apical-basolateral polarity.
Non-canonical Wnt signaling occurs without β-catenin involvement and appears to have some overlap with PCP in that these processes are principally concerned with the development of tissues along a particular axis other than the apical-basolateral axis. Defects in the development of tissue polarity in nephrogenesis are now being considered an essential part of the disease processes associated with PKD. Orientated cell division (Figure 2) is necessary for the elongation of the developing nephron. Recent work by Fischer et.al. demonstrates that abnormalities in the PCP process are present in PKD as indicated by differences in the programmed patterns of cell division in renal tubules elongating in normal and in PKD animal models (116). In these studies, the PCK rat was found to have mitotic spindles orientated outside of the tubular axis. In this disease model, as with the human disease, cysts arise in the later stages of the animal’s life, which allowed the researchers to conclude that mitotic spindle misalignment is not a result of cyst formation, but a likely contributing factor due to the aberrant function of fibrocystin.
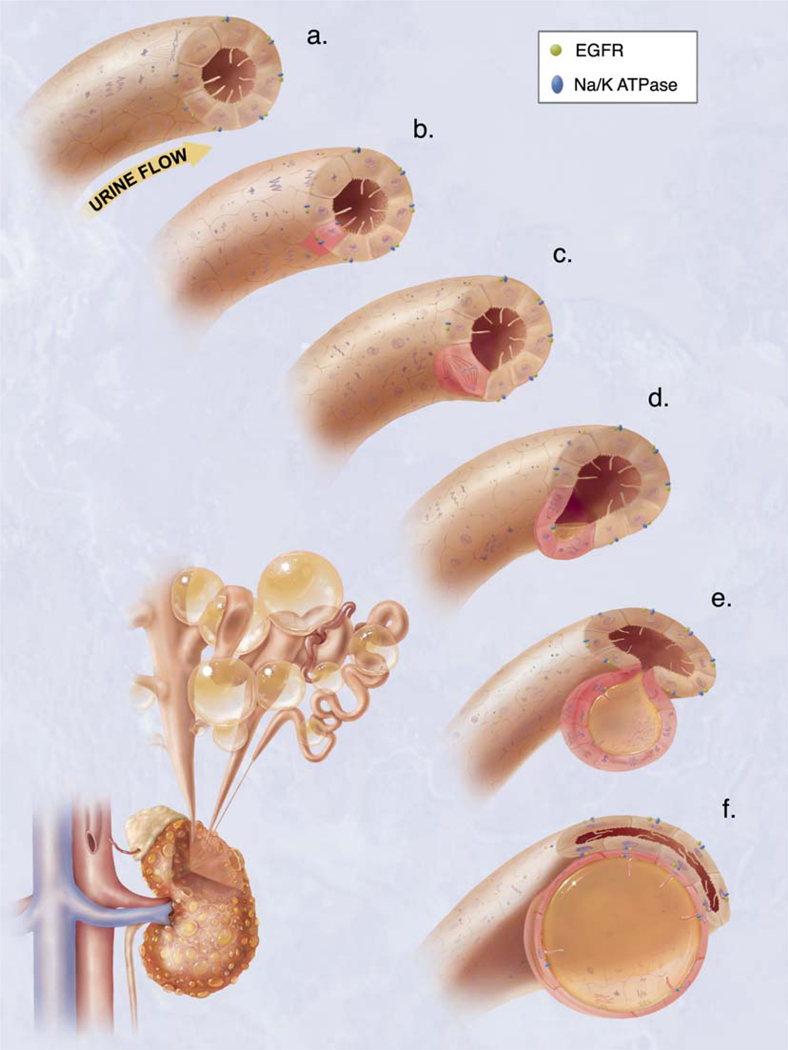
Figure 2.
Hypothetical model of cystogenesis. The illustration depicts mechanosensory function of renal tubular epithelial cilium. a. Each cilium plays an important role to transmit extracellular information, such as urine flow, into the cell. This message may provide critical signals to the cell regarding the direction of cell division along the tubule. b. Insults, such as genetic disorder or random mutation, will result in abnormal ciliary function to sense fluid movement. c. The functional abnormality in ciliary sensing may result in loss of planar cell polarity. d. Direction of cell division becomes randomized, resulting in increasing tubular diameter rather than tubular elongation. e. Budding of a cyst from the renal tubule and abnormal localizations of epidermal growth factor receptor (EGFR) and Na+/K+ ATPase pump are typical characteristics of the autosomal dominant polycystic kidney. f. The cyst is eventually enlarged and isolated. Multiple cysts from the neighboring nephrons are illustrated on the bottom left corner.
As mentioned earlier in this review, fibrocystin is in a complex with polycystin-1 and -2 at the cilium (47, 48), and polycystin-1 interacts with the morphogens of the Wnt pathway (143). It is interesting to speculate that the flow sensing capabilities of the cilium allow the cell to incorporate the multitude of cellular signaling pathways based on fluid-flow stimulation to activate the appropriate cell signaling pathway necessary for cellular differentiation. For instance, during development, urinary flow rate and directions would be sensed by the deflecting cilium allowing the cell to utilize the appropriate Wnt signaling pathway for tubular elongation and establishment of PCP pathways allowing for maintenance in the tubular architecture. Disruption of this complex abrogates normal flow sensing processes, which triggers unregulated Wnt signaling and PCP pathways, leading to cell division out of the tubular axis and promoting cyst formation.
Polycystin-1 has been shown to undergo cleavage in conditions where there is no fluid-flow stimulation. Under these static conditions, the COOH-tail translocates to the nucleus activating STAT6/P100 dependent genes; while under flow conditions, polycystin-1 is bound to STAT6/P100 effectively preventing nuclear translocation (50). The COOH-tail of polycystin-1 also reportedly acts to stabilize β-catenin, a well-characterized readout for canonical Wnt signaling (98, 143). These two pieces of data suggest that the polycystin-1 COOH-tail functions to stabilize proteins, which determine cell-fate specification. For example, fluid-flow stimulation provides a signal to the cell, via polycystin-1 COOH-tail stabilization, to modulate gene expression, which directs the cell towards a differentiated state. It is therefore easy to envision how under diseased states, where polycystin-1 COOH-tail is unable to function as a mediator of cellular differentiation by its inability to sequester proteins involved in proliferation, both Wnt and PCP signaling pathways could be disturbed to alter cellular proliferation and cellular dedifferentiation. Much work is still needed to determine the details of how the polycystins and other ciliary proteins are involved in the canonical and non-canonical Wnt pathways, and whether or not they play a significant role the initial events of cyst formation.
Currently, the literature suggests that β-catenin plays a pivotal role in the aberrant signaling processes of PKD. Experiments where β-catenin is constitutively activated in transgenic mice result in PKD in each segment of the nephron (21). Whether or not this directly relates to mechanosensation is not clear. However, in one study in which a cilia specific protein, Kif3A, was genetically altered in the mouse resulted in increased β-catenin levels and displayed the cystic phenotype (30). Such studies strongly suggest that the polycystin signaling complex at the cilium is important in Wnt signaling and maintenance of normal tubular architecture.
The early stages of nephron development are the key period for understanding the mechanisms behind the PKD disease process. This process may involve crucial events in establishing cell polarity, differentiation and tubulogenesis that involves Wnt signaling and PCP events. Because cyst development occurs late in human adult life and the molecular events behind cyst formation are those most active in development, this suggests that the developmental process remain active in adulthood (141). This further indicates that a transformation from development into maintenance process may occur in adult life.
7. PERSPECTIVES
Understanding how developmental events become critical in the adult kidney in PKD patients is an important concept requiring further investigation. For example, we need to further understand why in adult PKD cyst, the subcellular localizations of epidermal growth factor receptor (EGFR) and Na+/K+ ATPase pump are transformed into patterns seen in developing kidneys (145–148). At present, the molecular components mediating this switch are thought to reside at the primary cilium. The current view suggests that mechanosensation is important in maintaining tubular architecture and provides the cell with the ability to use fluid-flow stimulation as a means for monitoring tubular differentiation (Figure 2). The cilium is a fascinating organelle, which appears to be tightly integrated into many different cellular processes ranging from cell cycle to cellular differentiation. Using the polycystins and other cysto proteins as a mechanosensing complex, the cilium can rapidly respond to changes in urinary fluid-flow by activating specific signaling pathways unique to each of the individual proteins of this complex. Understanding the specific events connected to each of the mechanosensing protein’s components at the cilium will help researchers better understand both the functions of the primary cilium and more importantly, the events which trigger cyst formation in PKD.
8. ACKNOWLEDGEMENT
Work in Nauli’s laboratory is supported by the NIH (HL084451), AHA (0630257N), and deArce Memorial Endowment Fund offered by the University of Toledo. Due to the space limitation, we apologize to those whose work is not described in this review manuscript. Authors are thankful to two anonymous reviewers for critical comments on this manuscript. Authors would also like to thank T. Floyd-Bradstock for her illustration assistance, M. Takahashi for her secretarial help, B. Mell and C. Hodge for editing of the manuscript.
REFERENCES
- 1.Pazour GJ. Intraflagellar transport and cilia-dependent renal disease: the ciliary hypothesis of polycystic kidney disease. J Am Soc Nephrol. 2004;15:2528–2536. doi: 10.1097/01.ASN.0000141055.57643.E0. [DOI] [PubMed] [Google Scholar]
- 2.Wheatley DN, Wang AM, Strugnell GE. Expression of primary cilia in mammalian cells. Cell Biol Int. 1996;20:73–81. doi: 10.1006/cbir.1996.0011. [DOI] [PubMed] [Google Scholar]
- 3.Nauli SM, Zhou J. Polycystins and mechanosensation in renal and nodal cilia. Bioessays. 2004;26:844–856. doi: 10.1002/bies.20069. [DOI] [PubMed] [Google Scholar]
- 4.Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–529. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- 5.Hildebrandt F, Otto E. Cilia and centrosomes: a unifying pathogenic concept for cystic kidney disease? Nat Rev Genet. 2005;6:928–940. doi: 10.1038/nrg1727. [DOI] [PubMed] [Google Scholar]
- 6.Charron AJ, Nakamura S, Bacallao R, Wandinger-Ness A. Compromised cytoarchitecture and polarized trafficking in autosomal dominant polycystic kidney disease cells. J Cell Biol. 2000;149:111–124. doi: 10.1083/jcb.149.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Geng L, Segal Y, Peissel B, Deng N, Pei Y, Carone F, Rennke HG, Glucksmann-Kuis AM, Schneider MC, Ericsson M, Reeders ST, Zhou J. Identification and localization of polycystin, the PKD1 gene product. J Clin Invest. 1996;98:2674–2682. doi: 10.1172/JCI119090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanaoka K, Qian F, Boletta A, Bhunia AK, Piontek K, Tsiokas L, Sukhatme VP, Guggino WB, Germino GG. Co-assembly of polycystin-1 and -2 produces unique cation-permeable currents. Nature. 2000;408:990–994. doi: 10.1038/35050128. [DOI] [PubMed] [Google Scholar]
- 9.Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S. Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol. 2002;4:191–197. doi: 10.1038/ncb754. [DOI] [PubMed] [Google Scholar]
- 10.Kugoh H, Kleymenova E, Walker CL. Retention of membrane-localized beta-catenin in cells lacking functional polycystin-1 and tuberin. Mol Carcinog. 2002;33:131–136. doi: 10.1002/mc.10034. [DOI] [PubMed] [Google Scholar]
- 11.Markoff A, Bogdanova N, Knop M, Ruffer C, Kenis H, Lux P, Reutelingsperger C, Todorov V, Dworniczak B, Horst J, Gerke V. Annexin A5 interacts with polycystin-1 and interferes with the polycystin-1 stimulated recruitment of E-cadherin into adherens junctions. J Mol Biol. 2007;369:954–966. doi: 10.1016/j.jmb.2007.03.070. [DOI] [PubMed] [Google Scholar]
- 12.Roitbak T, Surviladze Z, Tikkanen R, Wandinger-Ness A. A polycystin multiprotein complex constitutes a cholesterol-containing signalling microdomain in human kidney epithelia. Biochem J. 2005;392:29–38. doi: 10.1042/BJ20050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheffers MS, van der Bent P, van de Wal A, van Eendenburg J, Breuning MH, de Heer E, Peters DJ. Altered distribution and co-localization of polycystin-2 with polycystin-1 in MDCK cells after wounding stress. Exp Cell Res. 2004;292:219–230. doi: 10.1016/j.yexcr.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 14.Silberberg M, Charron AJ, Bacallao R, Wandinger-Ness A. Mispolarization of desmosomal proteins and altered intercellular adhesion in autosomal dominant polycystic kidney disease. Am J Physiol Renal Physiol. 2005;288:F1153–F1163. doi: 10.1152/ajprenal.00008.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward CJ, Turley H, Ong AC, Comley M, Biddolph S, Chetty R, Ratcliffe PJ, Gattner K, Harris PC. Polycystin, the polycystic kidney disease 1 protein, is expressed by epithelial cells in fetal, adult, and polycystic kidney. Proc Natl Acad Sci U S A. 1996;93:1524–1528. doi: 10.1073/pnas.93.4.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bouillet P, Cory S, Zhang LC, Strasser A, Adams JM. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell. 2001;1:645–653. doi: 10.1016/s1534-5807(01)00083-1. [DOI] [PubMed] [Google Scholar]
- 17.Gilbert E, Morel A, Tulliez M, Maunoury R, Terzi F, Miquerol L, Kahn A. In vivo effects of activated H-ras oncogene expressed in the liver and in urogenital tissues. Int J Cancer. 1997;73:749–756. doi: 10.1002/(sici)1097-0215(19971127)73:5<749::aid-ijc23>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Morham SG, Langenbach R, Loftin CD, Tiano HF, Vouloumanos N, Jennette JC, Mahler JF, Kluckman KD, Ledford A, Lee CA, Smithies O. Prostaglandin synthase 2 gene disruption causes severe renal pathology in the mouse. Cell. 1995;83:473–482. doi: 10.1016/0092-8674(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 19.Nakayama K, Nakayama K, Negishi I, Kuida K, Sawa H, Loh DY. Targeted disruption of Bcl-2 alpha beta in mice: occurrence of gray hair, polycystic kidney disease, and lymphocytopenia. Proc Natl Acad Sci U S A. 1994;91:3700–3704. doi: 10.1073/pnas.91.9.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nechiporuk T, Fernandez TE, Vasioukhin V. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5−/− mice. Dev Cell. 2007;13:338–350. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C. Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene. 2001;20:5972–5981. doi: 10.1038/sj.onc.1204825. [DOI] [PubMed] [Google Scholar]
- 22.Schaffner DL, Barrios R, Massey C, Banez EI, Ou CN, Rajagopalan S, Aguilar-Cordova E, Lebovitz RM, Overbeek PA, Lieberman MW. Targeting of the rasT24 oncogene to the proximal convoluted tubules in transgenic mice results in hyperplasia and polycystic kidneys. Am J Pathol. 1993;142:1051–1060. [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon MB, Patton BL, Harvey SJ, Miner JH. A hypomorphic mutation in the mouse laminin alpha5 gene causes polycystic kidney disease. J Am Soc Nephrol. 2006;17:1913–1922. doi: 10.1681/ASN.2005121298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Togawa A, Miyoshi J, Ishizaki H, Tanaka M, Takakura A, Nishioka H, Yoshida H, Doi T, Mizoguchi A, Matsuura N, Niho Y, Nishimune Y, Nishikawa S, Takai Y. Progressive impairment of kidneys and reproductive organs in mice lacking Rho GDIalpha. Oncogene. 1999;18:5373–5380. doi: 10.1038/sj.onc.1202921. [DOI] [PubMed] [Google Scholar]
- 25.Trudel M, D'Agati V, Costantini F. C-myc as an inducer of polycystic kidney disease in transgenic mice. Kidney Int. 1991;39:665–671. doi: 10.1038/ki.1991.80. [DOI] [PubMed] [Google Scholar]
- 26.Veis DJ, Sorenson CM, Shutter JR, Korsmeyer SJ. Bcl-2-deficient mice demonstrate fulminant lymphoid apoptosis, polycystic kidneys, and hypopigmented hair. Cell. 1993;75:229–240. doi: 10.1016/0092-8674(93)80065-m. [DOI] [PubMed] [Google Scholar]
- 27.Wilson PD. Epithelial cell polarity and disease. Am J Physiol. 1997;272:F434–F442. doi: 10.1152/ajprenal.1997.272.4.F434. [DOI] [PubMed] [Google Scholar]
- 28.Brown NE, Murcia NS. Delayed cystogenesis and increased ciliogenesis associated with the re-expression of polaris in Tg737 mutant mice. Kidney Int. 2003;63:1220–1229. doi: 10.1046/j.1523-1755.2003.00863.x. [DOI] [PubMed] [Google Scholar]
- 29.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D'Eustachio P, Beier DR, Guay-Woodford LM. Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest. 2002;109:533–540. doi: 10.1172/JCI14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci U S A. 2003;100:5286–5291. doi: 10.1073/pnas.0836980100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taulman PD, Haycraft CJ, Balkovetz DF, Yoder BK. Polaris, a protein involved in left-right axis patterning, localizes to basal bodies and cilia. Mol Biol Cell. 2001;12:589–599. doi: 10.1091/mbc.12.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yoder BK, Tousson A, Millican L, Wu JH, Bugg CE, Jr., Schafer JA, Balkovetz DF. Polaris, a protein disrupted in orpk mutant mice, is required for assembly of renal cilium. Am J Physiol Renal Physiol. 2002;282:F541–F552. doi: 10.1152/ajprenal.00273.2001. [DOI] [PubMed] [Google Scholar]
- 33.Mykytyn K, Sheffield VC. Establishing a connection between cilia and Bardet-Biedl Syndrome. Trends Mol Med. 2004;10:106–109. doi: 10.1016/j.molmed.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 34.Badano JL, Mitsuma N, Beales PL, Katsanis N. The Ciliopathies: An Emerging Class of Human Genetic Disorders. Annu Rev Genomics Hum Genet. 2006;7:125–148. doi: 10.1146/annurev.genom.7.080505.115610. [DOI] [PubMed] [Google Scholar]
- 35.Hildebrandt F, Zhou W. Nephronophthisis-associated ciliopathies. J Am Soc Nephrol. 2007;18:1855–1871. doi: 10.1681/ASN.2006121344. [DOI] [PubMed] [Google Scholar]
- 36.Choyke PL. Inherited cystic diseases of the kidney. Radiol Clin North Am. 1996;34:925–946. [PubMed] [Google Scholar]
- 37.Choyke PL. Acquired cystic kidney disease. Eur Radiol. 2000;10:1716–1721. doi: 10.1007/s003300000601. [DOI] [PubMed] [Google Scholar]
- 38.Fick GM, Gabow PA. Hereditary and acquired cystic disease of the kidney. Kidney Int. 1994;46:951–964. doi: 10.1038/ki.1994.354. [DOI] [PubMed] [Google Scholar]
- 39.Rizk D, Chapman AB. Cystic and inherited kidney diseases. Am J Kidney Dis. 2003;42:1305–1317. doi: 10.1053/j.ajkd.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 40.The polycystic kidney disease 1 gene encodes a 14 kb transcript and lies within a duplicated region on chromosome 16. The European Polycystic Kidney Disease Consortium. Cell. 1994;77:881–894. doi: 10.1016/0092-8674(94)90137-6. [DOI] [PubMed] [Google Scholar]
- 41.Polycystic kidney disease: the complete structure of the PKD1 gene and its protein. The International Polycystic Kidney Disease Consortium. Cell. 1995;81:289–298. doi: 10.1016/0092-8674(95)90339-9. [DOI] [PubMed] [Google Scholar]
- 42.Mochizuki T, Wu G, Hayashi T, Xenophontos SL, Veldhuisen B, Saris JJ, Reynolds DM, Cai Y, Gabow PA, Pierides A, Kimberling WJ, Breuning MH, Deltas CC, Peters DJ, Somlo S. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272:1339–1342. doi: 10.1126/science.272.5266.1339. [DOI] [PubMed] [Google Scholar]
- 43.Bergmann C, Senderek J, Sedlacek B, Pegiazoglou I, Puglia P, Eggermann T, Rudnik-Schoneborn S, Furu L, Onuchic LF, De Baca M, Germino GG, Guay-Woodford L, Somlo S, Moser M, Buttner R, Zerres K. Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1) J Am Soc Nephrol. 2003;14:76–89. doi: 10.1097/01.asn.0000039578.55705.6e. [DOI] [PubMed] [Google Scholar]
- 44.Furu L, Onuchic LF, Gharavi A, Hou X, Esquivel EL, Nagasawa Y, Bergmann C, Senderek J, Avner E, Zerres K, Germino GG, Guay-Woodford LM, Somlo S. Milder presentation of recessive polycystic kidney disease requires presence of amino acid substitution mutations. J Am Soc Nephrol. 2003;14:2004–2014. doi: 10.1097/01.asn.0000078805.87038.05. [DOI] [PubMed] [Google Scholar]
- 45.Ward CJ, Hogan MC, Rossetti S, Walker D, Sneddon T, Wang X, Kubly V, Cunningham JM, Bacallao R, Ishibashi M, Milliner DS, Torres VE, Harris PC. The gene mutated in autosomal recessive polycystic kidney disease encodes a large, receptor-like protein. Nat Genet. 2002;30:259–269. doi: 10.1038/ng833. [DOI] [PubMed] [Google Scholar]
- 46.Wilson PD. Polycystic kidney disease. N Engl J Med. 2004;350:151–164. doi: 10.1056/NEJMra022161. [DOI] [PubMed] [Google Scholar]
- 47.Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J. Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol. 2007;27:3241–3252. doi: 10.1128/MCB.00072-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Y, Dai XQ, Li Q, Chen CX, Mai W, Hussain Z, Long W, Montalbetti N, Li G, Glynne R, Wang S, Cantiello HF, Wu G, Chen XZ. Kinesin-2 mediates physical and functional interactions between polycystin-2 and fibrocystin. Hum Mol Genet. 2006;15:3280–3292. doi: 10.1093/hmg/ddl404. [DOI] [PubMed] [Google Scholar]
- 49.Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S, Caplan MJ. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest. 2004;114:1433–1443. doi: 10.1172/JCI21753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T. Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell. 2006;10:57–69. doi: 10.1016/j.devcel.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 51.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/s0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 52.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 53.Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J. Loss of polycystin-1 in human cystlining epithelia leads to ciliary dysfunction. J Am Soc Nephrol. 2006;17:1015–1025. doi: 10.1681/ASN.2005080830. [DOI] [PubMed] [Google Scholar]
- 54.Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL. Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+ signaling. Am J Physiol Renal Physiol. 2007;292:F930–F945. doi: 10.1152/ajprenal.00285.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bernascone I, Vavassori S, Di Pentima A, Santambrogio S, Lamorte G, Amoroso A, Scolari F, Ghiggeri GM, Casari G, Polishchuk R, Rampoldi L. Defective intracellular trafficking of uromodulin mutant isoforms. Traffic. 2006;7:1567–1579. doi: 10.1111/j.1600-0854.2006.00481.x. [DOI] [PubMed] [Google Scholar]
- 56.Hart TC, Gorry MC, Hart PS, Woodard AS, Shihabi Z, Sandhu J, Shirts B, Xu L, Zhu H, Barmada MM, Bleyer AJ. Mutations of the UMOD gene are responsible for medullary cystic kidney disease 2 and familial juvenile hyperuricaemic nephropathy. J Med Genet. 2002;39:882–892. doi: 10.1136/jmg.39.12.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM, Mah AK, Johnsen RC, Cavender JC, Lewis RA, Leroux MR, Beales PL, Katsanis N. Basal body dysfunction is a likely cause of pleiotropic Bardet-Biedl syndrome. Nature. 2003;425:628–633. doi: 10.1038/nature02030. [DOI] [PubMed] [Google Scholar]
- 58.Igarashi P, Somlo S. Genetics and pathogenesis of polycystic kidney disease. J Am Soc Nephrol. 2002;13:2384–2398. doi: 10.1097/01.asn.0000028643.17901.42. [DOI] [PubMed] [Google Scholar]
- 59.Guay-Woodford LM. Murine models of polycystic kidney disease: molecular and therapeutic insights. Am J Physiol Renal Physiol. 2003;285:F1034–F1049. doi: 10.1152/ajprenal.00195.2003. [DOI] [PubMed] [Google Scholar]
- 60.Torres VE, Harris PC. Polycystic kidney disease: genes, proteins, animal models, disease mechanisms and therapeutic opportunities. J Intern Med. 2007;261:17–31. doi: 10.1111/j.1365-2796.2006.01743.x. [DOI] [PubMed] [Google Scholar]
- 61.Harris PC, Germino G, Klinger K, Landes G, van Adelsberg J. The PKD1 gene product. Nat Med. 1995;1:493. doi: 10.1038/nm0695-493a. [DOI] [PubMed] [Google Scholar]
- 62.Ibraghimov-Beskrovnaya O, Dackowski WR, Foggensteiner L, Coleman N, Thiru S, Petry LR, Burn TC, Connors TD, Van Raay T, Bradley J, Qian F, Onuchic LF, Watnick TJ, Piontek K, Hakim RM, Landes GM, Germino GG, Sandford R, Klinger KW. Polycystin: in vitro synthesis, in vivo tissue expression, and subcellular localization identifies a large membrane-associated protein. Proc Natl Acad Sci U S A. 1997;94:6397–6402. doi: 10.1073/pnas.94.12.6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Adelsberg J, Chamberlain S, D'Agati V. Polycystin expression is temporally and spatially regulated during renal development. Am J Physiol. 1997;272:F602–F609. doi: 10.1152/ajprenal.1997.272.5.F602. [DOI] [PubMed] [Google Scholar]
- 64.Geng L, Segal Y, Pavlova A, Barros EJ, Lohning C, Lu W, Nigam SK, Frischauf AM, Reeders ST, Zhou J. Distribution and developmentally regulated expression of murine polycystin. Am J Physiol. 1997;272:F451–F459. doi: 10.1152/ajprenal.1997.272.4.F451. [DOI] [PubMed] [Google Scholar]
- 65.Ekblom P, Miettinen A, Saxen L. Induction of brush border antigens of the proximal tubule in the developing kidney. Dev Biol. 1980;74:263–274. doi: 10.1016/0012-1606(80)90429-7. [DOI] [PubMed] [Google Scholar]
- 66.Palsson R, Sharma CP, Kim K, McLaughlin M, Brown D, Arnaout MA. Characterization and cell distribution of polycystin, the product of autosomal dominant polycystic kidney disease gene 1. Mol Med. 1996;2:702–711. [PMC free article] [PubMed] [Google Scholar]
- 67.Guillaume R, Trudel M. Distinct and common developmental expression patterns of the murine Pkd2 and Pkd1 genes. Mech Dev. 2000;93:179–183. doi: 10.1016/s0925-4773(00)00257-4. [DOI] [PubMed] [Google Scholar]
- 68.Griffin MD, Torres VE, Grande JP, Kumar R. Vascular expression of polycystin. J Am Soc Nephrol. 1997;8:616–626. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- 69.Kim K, Drummond I, Ibraghimov-Beskrovnaya O, Klinger K, Arnaout MA. Polycystin 1 is required for the structural integrity of blood vessels. Proc Natl Acad Sci U S A. 2000;97:1731–1736. doi: 10.1073/pnas.040550097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Qian Q, Li M, Cai Y, Ward CJ, Somlo S, Harris PC, Torres VE. Analysis of the polycystins in aortic vascular smooth muscle cells. J Am Soc Nephrol. 2003;14:2280–2287. doi: 10.1097/01.asn.0000080185.38113.a3. [DOI] [PubMed] [Google Scholar]
- 71.Ong AC, Ward CJ, Butler RJ, Biddolph S, Bowker C, Torra R, Pei Y, Harris PC. Coordinate expression of the autosomal dominant polycystic kidney disease proteins, polycystin-2 and polycystin-1, in normal and cystic tissue. Am J Pathol. 1999;154:1721–1729. doi: 10.1016/S0002-9440(10)65428-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foggensteiner L, Bevan AP, Thomas R, Coleman N, Boulter C, Bradley J, Ibraghimov-Beskrovnaya O, Klinger K, Sandford R. Cellular and subcellular distribution of polycystin-2, the protein product of the PKD2 gene. J Am Soc Nephrol. 2000;11:814–827. doi: 10.1681/ASN.V115814. [DOI] [PubMed] [Google Scholar]
- 73.Chauvet V, Qian F, Boute N, Cai Y, Phakdeekitacharoen B, Onuchic LF, Attie-Bitach T, Guicharnaud L, Devuyst O, Germino GG, Gubler MC. Expression of PKD1 and PKD2 transcripts and proteins in human embryo and during normal kidney development. Am J Pathol. 2002;160:973–983. doi: 10.1016/S0002-9440(10)64919-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qian F, Germino FJ, Cai Y, Zhang X, Somlo S, Germino GG. PKD1 interacts with PKD2 through a probable coiled-coil domain. Nat Genet. 1997;16:179–183. doi: 10.1038/ng0697-179. [DOI] [PubMed] [Google Scholar]
- 75.Tsiokas L, Kim E, Arnould T, Sukhatme VP, Walz G. Homo- and heterodimeric interactions between the gene products of PKD1 and PKD2. Proc Natl Acad Sci U S A. 1997;94:6965–6970. doi: 10.1073/pnas.94.13.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nagasawa Y, Matthiesen S, Onuchic LF, Hou X, Bergmann C, Esquivel E, Senderek J, Ren Z, Zeltner R, Furu L, Avner E, Moser M, Somlo S, Guay-Woodford L, Buttner R, Zerres K, Germino GG. Identification and characterization of Pkhd1, the mouse orthologue of the human ARPKD gene. J Am Soc Nephrol. 2002;13:2246–2258. doi: 10.1097/01.asn.0000030392.19694.9d. [DOI] [PubMed] [Google Scholar]
- 77.Onuchic LF, Furu L, Nagasawa Y, Hou X, Eggermann T, Ren Z, Bergmann C, Senderek J, Esquivel E, Zeltner R, Rudnik-Schoneborn S, Mrug M, Sweeney W, Avner ED, Zerres K, Guay-Woodford LM, Somlo S, Germino GG. PKHD1, the polycystic kidney and hepatic disease 1 gene, encodes a novel large protein containing multiple immunoglobulin-like plexin-transcription-factor domains and parallel beta-helix 1 repeats. Am J Hum Genet. 2002;70:1305–1317. doi: 10.1086/340448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hiesberger T, Gourley E, Erickson A, Koulen P, Ward CJ, Masyuk TV, Larusso NF, Harris PC, Igarashi P. Proteolytic cleavage and nuclear translocation of fibrocystin is regulated by intracellular Ca2+ and activation of protein kinase C. J Biol Chem. 2006;281:34357–34364. doi: 10.1074/jbc.M606740200. [DOI] [PubMed] [Google Scholar]
- 79.Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG. Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet. 2007;16:942–956. doi: 10.1093/hmg/ddm039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Menezes LF, Cai Y, Nagasawa Y, Silva AM, Watkins ML, Da Silva AM, Somlo S, Guay-Woodford LM, Germino GG, Onuchic LF. Polyductin, the PKHD1 gene product, comprises isoforms expressed in plasma membrane, primary cilium, and cytoplasm. Kidney Int. 2004;66:1345–1355. doi: 10.1111/j.1523-1755.2004.00844.x. [DOI] [PubMed] [Google Scholar]
- 81.Zhang MZ, Mai W, Li C, Cho SY, Hao C, Moeckel G, Zhao R, Kim I, Wang J, Xiong H, Wang H, Sato Y, Wu Y, Nakanuma Y, Lilova M, Pei Y, Harris RC, Li S, Coffey RJ, Sun L, Wu D, Chen XZ, Breyer MD, Zhao ZJ, McKanna JA, Wu G. PKHD1 protein encoded by the gene for autosomal recessive polycystic kidney disease associates with basal bodies and primary cilia in renal epithelial cells. Proc Natl Acad Sci U S A. 2004;101:2311–2316. doi: 10.1073/pnas.0400073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ward CJ, Yuan D, Masyuk TV, Wang X, Punyashthiti R, Whelan S, Bacallao R, Torra R, LaRusso NF, Torres VE, Harris PC. Cellular and subcellular localization of the ARPKD protein; fibrocystin is expressed on primary cilia. Hum Mol Genet. 2003;12:2703–2710. doi: 10.1093/hmg/ddg274. [DOI] [PubMed] [Google Scholar]
- 83.Nagano J, Kitamura K, Hujer KM, Ward CJ, Bram RJ, Hopfer U, Tomita K, Huang C, Miller RT. Fibrocystin interacts with CAML, a protein involved in Ca2+ signaling. Biochem Biophys Res Commun. 2005;338:880–889. doi: 10.1016/j.bbrc.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 84.Peters DJ, van de Wal A, Spruit L, Saris JJ, Breuning MH, Bruijn JA, de Heer E. Cellular localization and tissue distribution of polycystin-1. J Pathol. 1999;188:439–446. doi: 10.1002/(SICI)1096-9896(199908)188:4<439::AID-PATH367>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 85.Wilson PD, Geng L, Li X, Burrow CR. The PKD1 gene product, "polycystin-1," is a tyrosine-phosphorylated protein that colocalizes with alpha2beta1-integrin in focal clusters in adherent renal epithelia. Lab Invest. 1999;79:1311–1323. [PubMed] [Google Scholar]
- 86.Boletta A, Qian F, Onuchic LF, Bragonzi A, Cortese M, Deen PM, Courtoy PJ, Soria MR, Devuyst O, Monaco L, Germino GG. Biochemical characterization of bona fide polycystin-1 in vitro and in vivo. Am J Kidney Dis. 2001;38:1421–1429. doi: 10.1053/ajkd.2001.29282. [DOI] [PubMed] [Google Scholar]
- 87.Griffin MD, Torres VE, Grande JP, Kumar R. Immunolocalization of polycystin in human tissues and cultured cells. Proc Assoc Am Physicians. 1996;108:185–197. [PubMed] [Google Scholar]
- 88.Kleymenova E, Ibraghimov-Beskrovnaya O, Kugoh H, Everitt J, Xu H, Kiguchi K, Landes G, Harris P, Walker C. Tuberin-dependent membrane localization of polycystin-1: a functional link between polycystic kidney disease and the TSC2 tumor suppressor gene. Mol Cell. 2001;7:823–832. doi: 10.1016/s1097-2765(01)00226-x. [DOI] [PubMed] [Google Scholar]
- 89.Roitbak T, Ward CJ, Harris PC, Bacallao R, Ness SA, Wandinger-Ness A. A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells. Mol Biol Cell. 2004;15:1334–1346. doi: 10.1091/mbc.E03-05-0296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Streets AJ, Newby LJ, O'Hare MJ, Bukanov NO, Ibraghimov-Beskrovnaya O, Ong AC. Functional analysis of PKD1 transgenic lines reveals a direct role for polycystin-1 in mediating cell-cell adhesion. J Am Soc Nephrol. 2003;14:1804–1815. doi: 10.1097/01.asn.0000076075.49819.9b. [DOI] [PubMed] [Google Scholar]
- 91.Scheffers MS, van der Bent P, Prins F, Spruit L, Breuning MH, Litvinov SV, de Heer E, Peters DJ. Polycystin-1, the product of the polycystic kidney disease 1 gene, co-localizes with desmosomes in MDCK cells. Hum Mol Genet. 2000;9:2743–2750. doi: 10.1093/hmg/9.18.2743. [DOI] [PubMed] [Google Scholar]
- 92.Grimm DH, Cai Y, Chauvet V, Rajendran V, Zeltner R, Geng L, Avner ED, Sweeney W, Somlo S, Caplan MJ. Polycystin-1 distribution is modulated by polycystin-2 expression in mammalian cells. J Biol Chem. 2003;278:36786–36793. doi: 10.1074/jbc.M306536200. [DOI] [PubMed] [Google Scholar]
- 93.Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A, Walz G, Piontek KB, Germino GG, Weimbs T. The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci U S A. 2006;103:5466–5471. doi: 10.1073/pnas.0509694103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu GM, Sikaneta T, Sullivan BM, Zhang Q, Andreucci M, Stehle T, Drummond I, Arnaout MA. Polycystin-1 interacts with intermediate filaments. J Biol Chem. 2001;276:46544–46552. doi: 10.1074/jbc.M107828200. [DOI] [PubMed] [Google Scholar]
- 95.Malhas AN, Abuknesha RA, Price RG. Interaction of the leucine-rich repeats of polycystin-1 with extracellular matrix proteins: possible role in cell proliferation. J Am Soc Nephrol. 2002;13:19–26. doi: 10.1681/ASN.V13119. [DOI] [PubMed] [Google Scholar]
- 96.Weston BS, Bagneris C, Price RG, Stirling JL. The polycystin-1 C-type lectin domain binds carbohydrate in a calcium-dependent manner, and interacts with extracellular matrix proteins in vitro. Biochim Biophys Acta. 2001;1536:161–176. doi: 10.1016/s0925-4439(01)00046-1. [DOI] [PubMed] [Google Scholar]
- 97.N OB, Husson H, Dackowski WR, Lawrence BD, Clow PA, Roberts BL, Klinger KW, Ibraghimov-Beskrovnaya O. Functional polycystin-1 expression is developmentally regulated during epithelial morphogenesis in vitro: downregulation and loss of membrane localization during cystogenesis. Hum Mol Genet. 2002;11:923–936. doi: 10.1093/hmg/11.8.923. [DOI] [PubMed] [Google Scholar]
- 98.Huan Y, van Adelsberg J. Polycystin-1, the PKD1 gene product, is in a complex containing E-cadherin and the catenins. J Clin Invest. 1999;104:1459–1468. doi: 10.1172/JCI5111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yoder BK, Hou X, Guay-Woodford LM. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol. 2002;13:2508–2516. doi: 10.1097/01.asn.0000029587.47950.25. [DOI] [PubMed] [Google Scholar]
- 100.Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S. Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem. 1999;274:28557–28565. doi: 10.1074/jbc.274.40.28557. [DOI] [PubMed] [Google Scholar]
- 101.Luo Y, Vassilev PM, Li X, Kawanabe Y, Zhou J. Native polycystin 2 functions as a plasma membrane Ca2+-permeable cation channel in renal epithelia. Mol Cell Biol. 2003;23:2600–2607. doi: 10.1128/MCB.23.7.2600-2607.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Newby LJ, Streets AJ, Zhao Y, Harris PC, Ward CJ, Ong AC. Identification, characterization, and localization of a novel kidney polycystin-1-polycystin-2 complex. J Biol Chem. 2002;277:20763–20773. doi: 10.1074/jbc.M107788200. [DOI] [PubMed] [Google Scholar]
- 103.Scheffers MS, Le H, van der Bent P, Leonhard W, Prins F, Spruit L, Breuning MH, de Heer E, Peters DJ. Distinct subcellular expression of endogenous polycystin-2 in the plasma membrane and Golgi apparatus of MDCK cells. Hum Mol Genet. 2002;11:59–67. doi: 10.1093/hmg/11.1.59. [DOI] [PubMed] [Google Scholar]
- 104.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol. 2002;12:R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 105.Rundle DR, Gorbsky G, Tsiokas L. PKD2 interacts and co-localizes with mDia1 to mitotic spindles of dividing cells: role of mDia1 IN PKD2 localization to mitotic spindles. J Biol Chem. 2004;279:29728–29739. doi: 10.1074/jbc.M400544200. [DOI] [PubMed] [Google Scholar]
- 106.Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci. 2006;119:1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 107.Hidaka S, Konecke V, Osten L, Witzgall R. PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem. 2004;279:35009–35016. doi: 10.1074/jbc.M314206200. [DOI] [PubMed] [Google Scholar]
- 108.Cano DA, Murcia NS, Pazour GJ, Hebrok M. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development. 2004;131:3457–3467. doi: 10.1242/dev.01189. [DOI] [PubMed] [Google Scholar]
- 109.Streets AJ, Moon DJ, Kane ME, Obara T, Ong AC. Identification of an N-terminal glycogen synthase kinase 3 phosphorylation site which regulates the functional localization of polycystin-2 in vivo and in vitro. Hum Mol Genet. 2006;15:1465–1473. doi: 10.1093/hmg/ddl070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wang S, Luo Y, Wilson PD, Witman GB, Zhou J. The autosomal recessive polycystic kidney disease protein is localized to primary cilia, with concentration in the basal body area. J Am Soc Nephrol. 2004;15:592–602. doi: 10.1097/01.asn.0000113793.12558.1d. [DOI] [PubMed] [Google Scholar]
- 111.Praetorius HA, Spring KR. Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol. 2003;191:69–76. doi: 10.1007/s00232-002-1042-4. [DOI] [PubMed] [Google Scholar]
- 112.Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol. 2005;289:F978–F988. doi: 10.1152/ajprenal.00260.2004. [DOI] [PubMed] [Google Scholar]
- 113.Quarmby LM, Parker JD. Cilia and the cell cycle? J Cell Biol. 2005;169:707–710. doi: 10.1083/jcb.200503053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kolb RJ, Woost PG, Hopfer U. Membrane trafficking of angiotensin receptor type-1 and mechanochemical signal transduction in proximal tubule cells. Hypertension. 2004;44:352–359. doi: 10.1161/01.HYP.0000136645.90116.1a. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y, Wada J, Yasuhara A, Iseda I, Eguchi J, Fukui K, Yang Q, Yamagata K, Hiesberger T, Igarashi P, Zhang H, Wang H, Akagi S, Kanwar YS, Makino H. The role for HNF-1beta-targeted collectrin in maintenance of primary cilia and cell polarity in collecting duct cells. PLoS ONE. 2007;2 doi: 10.1371/journal.pone.0000414. e414. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 116.Fischer E, Legue E, Doyen A, Nato F, Nicolas JF, Torres V, Yaniv M, Pontoglio M. Defective planar cell polarity in polycystic kidney disease. Nat Genet. 2006;38:21–23. doi: 10.1038/ng1701. [DOI] [PubMed] [Google Scholar]
- 117.Resnick A, Hopfer U. Force-response considerations in ciliary mechanosensation. Biophys J. 2007;93:1380–1390. doi: 10.1529/biophysj.107.105007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Dirksen ER. Centriole and basal body formation during ciliogenesis revisited. Biol Cell. 1991;72:31–38. doi: 10.1016/0248-4900(91)90075-x. [DOI] [PubMed] [Google Scholar]
- 119.Lin W, Fung B, Shyamala M, Kasamatsu H. Identification of antigenically related polypeptides at centrioles and basal bodies. Proc Natl Acad Sci U S A. 1981;78:2373–2377. doi: 10.1073/pnas.78.4.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–837. doi: 10.1016/s0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 121.Okada Y, Takeda S, Tanaka Y, Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–644. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 122.Feistel K, Blum M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn. 2006;235:3348–3358. doi: 10.1002/dvdy.20986. [DOI] [PubMed] [Google Scholar]
- 123.Katz SM, Morgan JJ. Cilia in the human kidney. Ultrastruct Pathol. 1984;6:285–294. doi: 10.3109/01913128409018587. [DOI] [PubMed] [Google Scholar]
- 124.Lungarella G, de Santi MM, Tosi P. Ultrastructural study of the ciliated cells from renal tubular epithelium in acute progressive glomerulonephritis. Ultrastruct Pathol. 1984;6:1–7. doi: 10.3109/01913128409016659. [DOI] [PubMed] [Google Scholar]
- 125.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer's vesicle is required for normal organogenesis. Development. 2005;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 126.Alenghat FJ, Nauli SM, Kolb R, Zhou J, Ingber DE. Global cytoskeletal control of mechanotransduction in kidney epithelial cells. Exp Cell Res. 2004;301:23–30. doi: 10.1016/j.yexcr.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 127.Satir P, Christensen ST. Overview of structure and function of mammalian cilia. Annu Rev Physiol. 2007;69:377–400. doi: 10.1146/annurev.physiol.69.040705.141236. [DOI] [PubMed] [Google Scholar]
- 128.Cohen E, Meininger V. Ultrastructural analysis of primary cilium in the embryonic nervous tissue of mouse. Int J Dev Neurosci. 1987;5:43–51. doi: 10.1016/0736-5748(87)90047-5. [DOI] [PubMed] [Google Scholar]
- 129.Odor DL, Blandau RJ. Observations on the solitary cilium of rabbit oviductal epithelium: its motility and ultrastructure. Am J Anat. 1985;174:437–453. doi: 10.1002/aja.1001740407. [DOI] [PubMed] [Google Scholar]
- 130.Delmas P, Nauli SM, Li X, Coste B, Osorio N, Crest M, Brown DA, Zhou J. Gating of the polycystin ion channel signaling complex in neurons and kidney cells. Faseb J. 2004;18:740–742. doi: 10.1096/fj.03-0319fje. [DOI] [PubMed] [Google Scholar]
- 131.Kim E, Arnould T, Sellin L, Benzing T, Comella N, Kocher O, Tsiokas L, Sukhatme VP, Walz G. Interaction between RGS7 and polycystin. Proc Natl Acad Sci U S A. 1999;96:6371–6376. doi: 10.1073/pnas.96.11.6371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Parnell SC, Magenheimer BS, Maser RL, Rankin CA, Smine A, Okamoto T, Calvet JP. The polycystic kidney disease-1 protein, polycystin-1, binds and activates heterotrimeric G-proteins in vitro. Biochem Biophys Res Commun. 1998;251:625–631. doi: 10.1006/bbrc.1998.9514. [DOI] [PubMed] [Google Scholar]
- 133.Parnell SC, Magenheimer BS, Maser RL, Zien CA, Frischauf AM, Calvet JP. Polycystin-1 activation of c-Jun N-terminal kinase and AP-1 is mediated by heterotrimeric G proteins. J Biol Chem. 2002;277:19566–19572. doi: 10.1074/jbc.M201875200. [DOI] [PubMed] [Google Scholar]
- 134.Malone AM, Anderson CT, Tummala P, Kwon RY, Johnston TR, Stearns T, Jacobs CR. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc Natl Acad Sci U S A. 2007;104:13325–13330. doi: 10.1073/pnas.0700636104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mai W, Chen D, Ding T, Kim I, Park S, Cho SY, Chu JS, Liang D, Wang N, Wu D, Li S, Zhao P, Zent R, Wu G. Inhibition of Pkhd1 impairs tubulomorphogenesis of cultured IMCD cells. Mol Biol Cell. 2005;16:4398–4409. doi: 10.1091/mbc.E04-11-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH, LaRusso NF, Harris PC, Ward CJ. A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int. 2007;72:328–336. doi: 10.1038/sj.ki.5002294. [DOI] [PubMed] [Google Scholar]
- 137.Masyuk TV, Huang BQ, Ward CJ, Masyuk AI, Yuan D, Splinter PL, Punyashthiti R, Ritman EL, Torres VE, Harris PC, LaRusso NF. Defects in cholangiocyte fibrocystin expression and ciliary structure in the PCK rat. Gastroenterology. 2003;125:1303–1310. doi: 10.1016/j.gastro.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 138.Masyuk TV, Huang BQ, Masyuk AI, Ritman EL, Torres VE, Wang X, Harris PC, Larusso NF. Biliary dysgenesis in the PCK rat, an orthologous model of autosomal recessive polycystic kidney disease. Am J Pathol. 2004;165:1719–1730. doi: 10.1016/S0002-9440(10)63427-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–718. doi: 10.1083/jcb.151.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD. Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol. 2006;290:F1320–F1328. doi: 10.1152/ajprenal.00463.2005. [DOI] [PubMed] [Google Scholar]
- 141.Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG. A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med. 2007 doi: 10.1038/nm1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Stark K, Vainio S, Vassileva G, McMahon AP. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature. 1994;372:679–683. doi: 10.1038/372679a0. [DOI] [PubMed] [Google Scholar]
- 143.Kim E, Arnould T, Sellin LK, Benzing T, Fan MJ, Gruning W, Sokol SY, Drummond I, Walz G. The polycystic kidney disease 1 gene product modulates Wnt signaling. J Biol Chem. 1999;274:4947–4953. doi: 10.1074/jbc.274.8.4947. [DOI] [PubMed] [Google Scholar]
- 144.Widelitz R. Wnt signaling through canonical and non-canonical pathways: recent progress. Growth Factors. 2005;23:111–116. doi: 10.1080/08977190500125746. [DOI] [PubMed] [Google Scholar]
- 145.Du J, Wilson PD. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am J Physiol. 1995;269:C487–C495. doi: 10.1152/ajpcell.1995.269.2.C487. [DOI] [PubMed] [Google Scholar]
- 146.Orellana SA, Sweeney WE, Neff CD, Avner ED. Epidermal growth factor receptor expression is abnormal in murine polycystic kidney. Kidney Int. 1995;47:490–499. doi: 10.1038/ki.1995.62. [DOI] [PubMed] [Google Scholar]
- 147.Wilson PD, Sherwood AC, Palla K, Du J, Watson R, Norman JT. Reversed polarity of Na (+) -K (+)-ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am J Physiol. 1991;260:F420–F430. doi: 10.1152/ajprenal.1991.260.3.F420. [DOI] [PubMed] [Google Scholar]
- 148.Wilson SJ, Amsler K, Hyink DP, Li X, Lu W, Zhou J, Burrow CR, Wilson PD. Inhibition of HER-2 (neu/ErbB2) restores normal function and structure to polycystic kidney disease (PKD) epithelia. Biochim Biophys Acta. 2006;1762:647–655. doi: 10.1016/j.bbadis.2006.04.006. [DOI] [PubMed] [Google Scholar]