Abstract
OBJECTIVE: To review surgical results of endoscopic transthoracic limited sympathotomy for palmar-plantar hyperhidrosis during the past decade.
PATIENTS AND METHODS: We retrospectively reviewed 155 consecutive patients who underwent surgery from June 30, 2000, through December 31, 2009, for medically refractory palmar-plantar hyperhidrosis using a technique of T1-T2 sympathotomy disconnection, designed for successful palmar response and minimization of complications.
RESULTS: Of the 155 patients, 44 (28.4%) were male, and 111 (71.6%) were female; operative times averaged 38 minutes. No patient experienced Horner syndrome, intercostal neuralgia, or pneumothorax. The only surgical complication was hemothorax in 2 patients (1.3%); in 1 patient, it occurred immediately postoperatively and in the other patient, 10 days postoperatively; treatment in both patients was successful. All 155 patients had successful (warm and dry) palmar responses at discharge. Long-term follow-up (>3 months; mean, 40.2 months) was obtained for 148 patients (95.5%) with the following responses to surgery: 96.6% of patients experienced successful control of palmar sweating; 69.2% of patients experienced decreased axillary sweating; and 39.8% of patients experienced decreased plantar sweating. At follow-up, 5 patients had palmar sweating (3 patients, <3 months; 1 patient, 10-12 months; 1 patient, 16-18 months). Compensatory hyperhidrosis did not occur in 47 patients (31.7%); it was mild in 92 patients (62.2%), moderate in 7 patients (4.7%), and severe in 2 patients (1.3%).
CONCLUSION: In this series, a small-diameter uniportal approach has eliminated intercostal neuralgia. Selecting a T1-T2 sympathotomy yields an excellent palmar response, with a very low severe compensatory hyperhidrosis complication rate. The low failure rate was noted during 18 months of follow-up and suggests that longer follow-up is necessary in these patients.
rH = relative humidity; TST = thermoregulatory sweat test
Sympathetic nervous system surgery for hyperhidrosis has been practiced by the Mayo Clinic Department of Neurosurgery for more than 75 years.1 The palmar component of palmar-plantar hyperhidrosis has always been the target of thoracic surgery, with less predictable beneficial results for the axillae and feet. Endoscopic transthoracic approaches were introduced by Kux2 in 1951, and with improved optics and light sources, this approach has been used in virtually every hyperhidrosis surgical series for the past 20 years. Uniportal approaches are possible with improved optics when performed by experienced surgeons.3 Although the transthoracic endoscopic approach is currently practiced at all medical centers, the operation devised to interrupt the sympathetic flow to the hands varies considerably: resection, ablation, clips, and cautery have all been applied to various regions of the upper sympathetic thoracic chain.4-9 Life-threatening complications are rare with the endoscopic transthoracic approach, regardless of how the sympathetic chain is operated. Because there is no uniformly agreed on approach for interruption of the sympathetic chain (the actual operation for this condition), rates of success and risks of complication vary. However, recent series strongly suggest what we have practiced for more than a decade: simple T2 sympathectomy yields excellent results for the hands and lessens the chances of severe postoperative compensatory hyperhidrosis, and the more aggressive resections of the sympathetic chain increase the risk of severe postoperative compensatory hyperhidrosis.10-16 The extremely disagreeable complication of severe compensatory hyperhidrosis (in which the patient regrets the operation because of resultant profound increased new sweating elsewhere) remains the most problematic because no satisfactory treatment is available if it occurs.
We designed our operative approach to render a high success rate in the treatment of severe palmar hyperhidrosis and minimize possible complications (which we previously described17,18) by initiating the following strategies.
Maximize Palmar Response to Surgery. We designed the surgical procedure to effectively eliminate any sympathetic output to the brachial plexus in the hand, except through the T1 ganglion (or C8 and T1 fusion, the stellate ganglion) by disconnecting all sympathetic connections below this level (T2 sympathotomy). Others have found excellent results using this technique as well.10,11 We added an intraoperative laser Doppler palmar blood flow monitoring machine (PeriFlux System 5000; Perimed AB, Järfäll, Sweden) and computer analysis. The system injects laser light into tissue through Doppler probes adhered to the palmar aspect of each thumb and measures wavelength alterations caused by flowing blood. This provides real-time feedback of completed loss of sympathetic tone to the hand, and this approach is markedly more effective than relying on fingertip temperature changes alone.19 The operation is anatomically completed when all visualized sympathetic branches between the T1 ganglion and the T2 ganglion have been severed across the second rib, and it is physiologically confirmed by an instantaneous increase in palmar blood flow when the loss of sympathetic tone is complete.
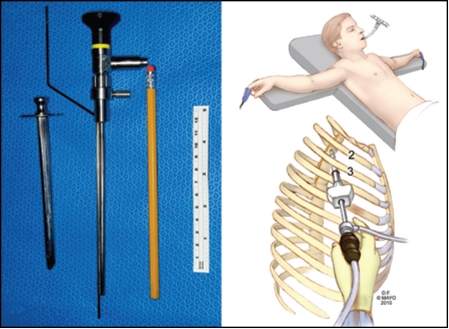
Minimize Intercostal Neuralgia. This painful thoracic nerve injury is due to instrumental pressure on the intercostal nerve in the narrow confines between the ribs; it occurs in 2% to 3% of endoscopic transthoracic procedures, and in severe cases, the injured intercostal nerve may emanate pain for months or even years.4-9,13,14 We designed a very small single-incision, uniportal trochar approach, using a straight lens 3-mm Gaab endoscope, engineered at Mayo Clinic, to couple with a sleeve-attached monopolar cautery matching the focal point of the lens, to minimize thoracic nerve irritation between the ribs by minimizing the whole operating instrument to smaller than the width of a pencil (Figure 1). The diameter of the device allows placement through the intercostal approach without compressing the intercostal nerve and does not compromise optical visualization.
Minimize Severe Compensatory Hyperhidrosis. Mild increased sweating occurs postoperatively in new compensatory areas in a large percentage of patients, regardless of how the procedure is performed. However, severe compensatory hyperhidrosis is generally an accepted term when a patient develops new areas of increased sweating postoperatively that are so severe the patient regrets having undergone the procedure. In most series in which sym path ec tomy (removal of the sympathetic chain) has been performed, the unpredictable complication of severe com pensatory hyperhidrosis occurs in 5% to 20% of patients.4-9,13,14 We designed our surgical approach to minimize this complication on the basis of evidence that the more destructive the procedure, the higher the risk of postoperative severe compensatory hyperhidrosis.9-15 Therefore, we perform only a limited, single-level sympathotomy and do not remove any of the sympathetic chain (sympathectomy). All branches and connections to the brachial plexus across the second rib are disconnected, isolating the T1 (or stellate) ganglion as the only input to the hand and ensuring that ganglion cells in T1 and T2 remain undisturbed (Figure 2). These ganglia may be important if injured because axons from the spinal cord to the ganglia would necessarily be injured as well, which could theoretically cause synaptic reorganization of spinal cord sympathetic reflexes and may increase the risk of severe compensatory hyperhidrosis. In essence, the procedure is the least invasive that can be performed to ensure success of the surgery and minimize complications.
FIGURE 1.
Left, Hollow trochar with obturator (first object) for perforating chest wall allows penetration of the chest wall through a small (<1 cm) incision, removal of the inner obturator, and passage of the Gaab straight lens endoscope with engineered attached monopolar cautery probe (second object). Pencil and tape measure provide perspectives of size. The Mayo engineered hollow trochar with blunt perforating obturator removed mates perfectly with the engineered endoscope/cautery unit. Right: upper, Position of patient at surgery; lower, Single small incision and uniportal access with endoscope and cautery combined.
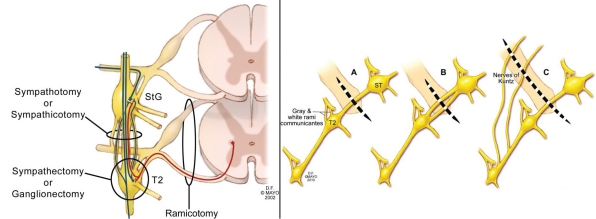
FIGURE 2.
Left, Schematic drawing of sympathectomy vs sympathotomy. Note that sympathectomy, with use of ganglionectomy by definition, must sever the primary axon from the neuron in the intermediolateral cell column of the spinal cord (red) before primary or collateral synapse in the T2 ganglion. This injures the neurons at this level of the spinal cord, some of which may die, and may predispose the patient to spinal cord neuronal synaptic reorganization and severe compensatory hyperhidrosis. Sympathotomy interrupts only axons after potential T2 ganglion synapses, a less injurious effect on the neuron, and is the least destructive procedure possible for successful treatment of palmar hyperhidrosis. StG = stellate ganglion. Right, All sympathetic connections across the second rib between the StG and the T2 ganglion are severed. (A) depicts a single trunk, (b) multiple trunks (6 patients), (C) trunk with lateral nerves of Kuntz (16 patients). Severing all sympathetic innervation across the second rib between the StG and T2 ganglion ensures that only the StG can provide sympathetic outflow to the hand through the brachial plexus, and this strategy minimizes axonal injury at the spinal cord level (left panel).
The aforementioned information serves as background. The purpose of this report was to review surgical results of endoscopic transthoracic limited sympathotomy for palmar-plantar hyperhidrosis during the past decade.
PATIENTS AND METHODS
We retrospectively reviewed 155 consecutive patients with medically refractory palmar-plantar hyperhidrosis who underwent surgery in the Mayo Clinic Department of Neurosurgery from June 30, 2000, through December 31, 2009. The Mayo Clinic Institutional Review Board approved the study, and no patient was contacted for follow-up against his or her wishes. Preoperatively, a variety of oral agents, topical agents, and in many cases tap water iontophoresis had failed in all patients, and all were judged to have severe refractory palmar-plantar hyperhidrosis. Some patients had received off-label use of botulinum toxin injections to the hands. Importantly, patients with hyperhidrosis that predominantly affected the axillae or patients with primarily a craniofacial hyperhidrosis were not deemed surgical candidates. All patients were preoperatively assessed outside the Department of Neurosurgery by the department of neurology, dermatology, or pediatrics and referred for surgical consideration after noted failure of medical strategies. All patients had a confirmatory history, had undergone a physical examination, and had undergone thermoregulatory sweat testing (TST) and were observed to have beading or drippage of sweat from palms during physical examination or in the thermoregulatory laboratory before heat exposure. The TST, using indicator powder, provided visual confirmation of the preheat, resting sweat distribution, and the heat-stimulus portion of the TST ruled out compensatory palmar sweating due to anhidrosis elsewhere. Later in the series, quantitative emotional sweat output was obtained (Figures 3 and 4). A ventilated capsule containing a calibrated humidity sensor was fashioned, and a recording was made simultaneously from both palms and the left forearm. Such data have been advocated to objectively quantitate the severity of a patient's hyperhidrosis and stratify treatment options on the basis of severity.20
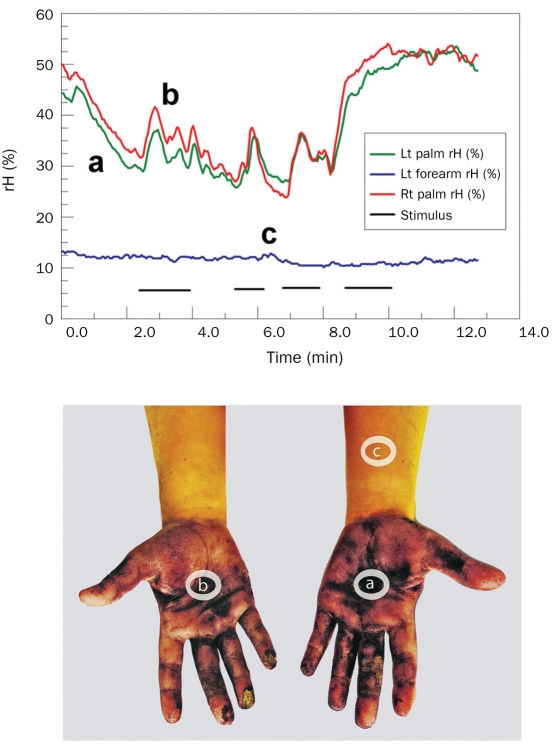
FIGURE 3.
Two ways of assessing palmar hyperhidrosis. Upper, ventilated capsule measurements capture the detailed synchronous pulsatile and localized palmar (a and b traces) sweat output with emotional stimuli. Lt = left; rH = relative humidity; Rt = right. Lower, In same patient, the resting sweat produces a purple discoloration of the alizarin red indicator powder in palms but not the forearm. Lettered ovals refer to capsule locations.
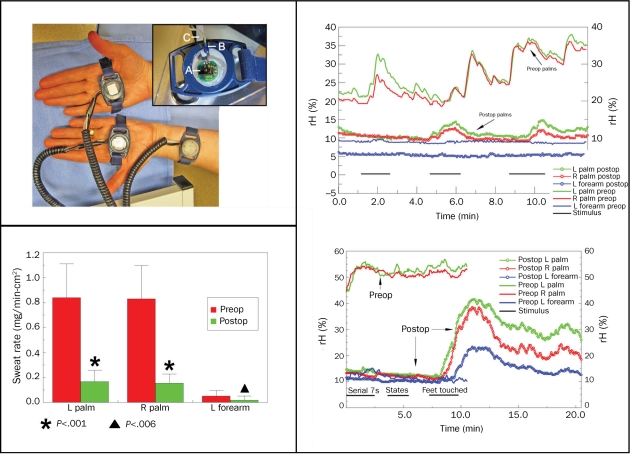
FIGURE 4.
Linear relationship between mean measured relative humidity (rH) and normalized sweat rate. Top left, ventilated capsule setup for recording quantitative palmar and left (L) forearm sweat rates. Inset: A, The HIH 3610 (humidity sensor; Honeywell Sensing and Control, Golden valley, MN); b, thermistor probe to measure skin temperature; and C, desiccated air tubing connecting to capsule. All 3 capsule sites are plotted. Clustering of responses below 10% rH relates to low sweat rates in the forearm site. Lower left, Quantitative sweat output in patients studied before and after upper thoracic endoscopic sympathotomy. Mann-Whitney u test reveals highly significant reductions in sweat rate for both palms and less robust reduction in forearm. Preop = preoperatively; Postop = postoperatively. Lower right, Surprising palmar and forearm emotional sweat outburst in patient postop. Marked reduction in resting sweat was noted postop with serial 7 and naming states stimuli (from 0-5 minutes); however, when examiner touched patient's feet (7.5-10 minutes), a remarkable sweating response was noted in palms and L forearm postop. R = right. Upper right, Mild emotional-induced sweating is often detected postop but is usually unnoticed by the patient.
Attempts were made to contact all patients postoperatively for follow-up by outpatient consultation, telephone interview, or questionnaire and written correspondence. The 155 patients consisted of 111 females (71.6%) and 44 males (28.4%). The median age for females was 22 years and for males, 23 years; 25 females (22.5%) and 15 males (34.0%) were 18 years of age or younger. Family history was positive in 84 patients (54.2%). Onset of symptoms occurred at the following ages: 0 to 3 years old in 42 patients (27.1%); 4 to 8 years old in 74 patients (47.7%); 9 to 12 years old in 29 patients (18.7%); and greater than12 years old in 10 patients (6.5%).
Operation
At arrival of the patient to the operating room, general anesthesia was induced in the supine position, followed by placement of a double-lumen endotracheal tube for intermittent one-lung ventilation. Then, both arms were abducted to 90°, and the back was elevated to 40°. After sterile skin preparation and draping, the chest wall was perforated through a 1-cm or less skin incision with a small blunt obturator and hollow trochar, engineered for perfect passage of the small endoscope and attached monopolar cautery unit. Gravity was used to descend the lung a few centimeters from the apex to perform the procedure because there is no pressurized gas insufflation with this technique (Figure 1). We performed the procedure (described subsequently) on the right side first; once the procedure was completed, a pediatric feeding tube was inserted to serve as a vent for retained air, and the right lung was ventilated. We then performed the identical procedure on the left side, again inserting a pediatric feeding tube and ventilating both lungs. The wounds were closed, and the intrapleural air was aspirated through the bilateral pediatric feeding tubes, which were then removed. Chest radiography confirmed postoperative obliteration or minimal intrapleural air remaining postoperatively. In general, for comfort measures, patients were discharged after a 23-hour observation period, although occasional patients were discharged the same day.
Sympathotomy was performed in all patients by cautery disconnection of the sympathetic trunk and all visible branches above the T2 ganglion and below the T1 ganglion (or stellate) across the second rib. No ganglia were violated anatomically on the basis of correct anatomic location and endoscopic visualization. A minimal (generally ≤1 cm) transaxillary incision was performed in 150 patients, and 5 patients underwent an anterior mid-clavicular approach between the second and third ribs by uniportal access (≤1-cm incision) because of the size of the chest cavity or body mass index limiting an axillary approach. The Gaab straight lens endoscope with Mayo engineered coapted insulated monopolar cautery probe (Figure 1) was used, and the laser Doppler palmar blood flow device was instituted to ensure physiologic response of the sympathotomy in all but the first 12 patients (143 patients). Blood flow elevation without fluctuations during further ipsilateral cautery or contralateral stimulation ensured physiologic removal of sympathetic tone to the vasculature of the operated hand. This provided a markedly improved real-time feedback during severance of sympathetic tone to the hand, over and above fingertip temperature probes alone.
The following describes the procedure, which is presented in the supplementary narrated video (see Supporting Online Material, a link to which is provided at the end of this article). The skin blood flow monitor is displayed as a “picture-in-picture” overlay on the surgical videoscope monitors. After the probes are secured to the palm on each hand of the patient simultaneously, a cutaneous temperature probe is also placed on both index fingertips. The operation is performed on the right side first. The right lung is nonventilated to allow visualization of the sympathetic chain. As the electrocautery probe moves across the chain overlying the second rib, marked increases in ipsilateral skin blood flow are nearly instantaneous. On the left palm, the skin blood flow is reduced because of the normal sympathetically mediated vasoconstrictor response to surgical stimulation. When the procedure is performed on the left side, separation of the left sympathetic chain evokes an increase in skin blood flow on the left side. Importantly, as the electrocautery probe courses along the left chain, profound vasodilation is present on both sides that is no longer reactive to any surgical or sympathetic stimulation. This confirms bilateral sympathotomy and predicts a more successful outcome in patients with hyperhidrosis than fingertip temperature alone.
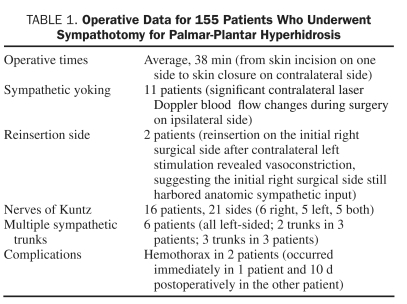
Operative times from skin incision on one side to skin closure on the contralateral side (completed surgery on both sides) averaged 38 minutes. In 2 patients, the operative site was reopened on the initial right side, and further cautery performed after surgery on the left side revealed laser blood flow changes on the right side, suggesting that sympathetic tone was still present. The sympathotomy was completed in these 2 patients by further cautery, which produced a marked rise in measured palmar blood flow concomitant with an absence of further reactivity in Doppler blood flow. Interestingly, 11 patients exhibited significant yoking of the sympathetic response, presumably through spinal cord reflexes, with dramatic increases in blood flow on the contralateral side after the initial right-sided sympathotomy, which further increased after completion of sympathotomy on the contralateral side. Nerves of Kuntz21,22 were identified in 16 patients (10%), on 21 sides (6 on the right, 5 on the left, and 5 on both sides). More than 1 visible trunk was identified in 6 patients, and all were on the left side (2 trunks in 3 patients; 3 trunks in 3 patients) (Figure 2). Operative information is summarized in Table 1.
TABLE 1.
Operative Data for 155 Patients Who Underwent Sympathotomy for Palmar-Plantar Hyperhidrosis
RESULTS
All 155 patients had dry hands immediately postoperatively and during hospitalization.
No patient experienced Horner syndrome, intercostal neuralgias perioperatively or in long-term follow-up, or pneumothorax. Two patients experienced hemothorax and required treatment. In the one patient, the hemothorax was immediate; a chest tube was inserted, atelectasis occurred subsequently, and the patient was hospitalized for 6 days but had an uneventful discharge. The other patient returned 10 days after surgery with increasing shortness of breath; chest radiography revealed a moderate left-sided hemothorax. The patient was hospitalized for 3 days and underwent chest tube placement, followed by successful thoracentesis and irrigation removal of a liquefied hematoma.
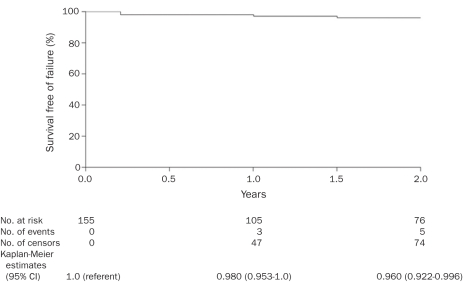
All patients were advised preoperatively that long-term follow-up was necessary. Patients were contacted and assessed by return evaluations, telephone interviews, or questionnaires. No follow-up was obtained beyond the day of discharge for 2 patients. In 5 patients, follow-up was less than 3 months (on the basis of short-term follow-up of these patients, the procedure was successful. It is highly probable that, if the procedure had failed in any of these 7 patients, the surgical team would have been contacted. Figure 5 summarizes the 2-year follow-up and all documented surgical failures, with Kaplan-Meier estimates for the total surgical group of 155 patients.
FIGURE 5.
Kaplan-Meier estimated survival free of surgical failure in 155 patients. Five of these patients had documented surgical failure, all within 2 years postoperatively. The median length of follow-up after surgery in the remaining 150 patients was 2.1 years; range, 1 day to 9.9 years. CI = confidence interval.
Excluding the 7 patients with short-term follow-up, follow-up of the remaining 148 patients (95%) ranged from 3 months to 120 months, with a median of 34 months and mean of 40.2 months. The surgical outcomes of patients with follow-up greater than 3 months are discussed subsequently.
The palmar response to sympathotomy was considered successful by the patient in 143 patients (96.6%); 128 patients (86.5%) considered their hands very dry or with no or minimal sweating, and 15 patients (10.1%) considered the operation highly successful, and their hands exhibited normal heat- or exercise-induced sweating, but with none of the anxiety- or emotional-induced sweating that was present before surgery. Of the 143 patients with successful palmar response to sympathotomy, 99 patients (69.2%) had dryer armpits, whereas 44 (30.8%) had no change; 57 patients (39.8%) had dryer feet, 84 (58.7%) had no change, and 2 (1.3%) had increased sweating of their feet. Results for the axillae and feet are patient responses to questions whether or not these areas had excessive hyperhidrosis preoperatively.
Fifty patients (32%) underwent preoperative quantitative measurement of palmar and left forearm sweating rate via a calibrated ventilated capsule technique. The capsule components and placement are shown in Figure 4, upper left. Capsules were ventilated with dried air at 0.2 L/min. After a 2-minute stabilization period, the percent baseline relative humidity (rH) was obtained. This was followed by a 12-minute observation period during which each patient was subjected to emotional stimuli, including performing serial 7 calculations, timed recitation of US state names, and touching of their feet by the examiner. Capsule percent rH was measured in real time and used to calculate the normalized sweat rate. A linear relationship between mean percent rH and the cumulative sweat rate in milligrams/minute per centimeter squared was found. Sweat rates of the forearm remained near baseline, whereas palmar sweat rates ranged from 0.25 to 0.50 for mild, 0.5 to 0.75 for moderate, and above 0.75 mg/min-cm2 for severe (beading or sweat drippage visible) palmar sweating. A patient who had severe sweating is shown in Figure 3.
Sixteen patients (10%) were studied at a mean of 4 months postoperatively, and the palmar and forearm sweat rates preoperatively and postoperatively are shown in Figure 4, lower left. For this smaller subset, the preoperative vs postoperative sweat rate at each site was compared using the Mann-Whitney U test. The postoperative sweat rate reduction was significant for all sites, especially for the palms (P<.001): preoperative palmar sweat rate for left (0.840±0.27 mg/min-cm2) and right (0.830±0.27 mg/min-cm2) compared with postoperative palmar sweat rates for left (0.166±0.09 mg/min-cm2) and right (0.153±0.07 mg/min-cm2). Preoperative left forearm sweat rate was 0.050±0.04 mg/min-cm2, and postoperative, 0.018±0.03 mg/min-cm2 (P<.006). Postoperative palmar sweat rates were still greater than preoperative forearm sweat rates in most patients, and occasionally, emotional palmar sweat outbursts occurred (Figure 4, lower right). The cause of this residual sweating is speculated to be the result of nerve regeneration through the stellate ganglia, increased neural traffic through existing pathways from the stellate, denervation supersensitivity of adrenergic or cholinergic receptors, or reorganization and up-regulation of the density of sweat glands.23 These speculative mechanisms may also explain the 5 bilateral palmar hyperhidrosis failures; 3 patients experienced failure within 3 months postoperatively; 1 patient between 10 months and 12 months; and 1 patient between 16 and 18 months postoperatively. Two patients had mild increased compensatory hyperhidrosis on the trunk and thighs but did not seek postoperative treatment; the 3 other patients in whom surgery failed had no increased sweating elsewhere. Additionally, these 5 patients did not have decreased sweating in their axillary and plantar components. Thus, all 5 patient failures, with recurrence of palmar hyperhidrosis, reported absent or only mild new compensatory sweating.
Compensatory hyperhidrosis was graded as none (patient develops no new sweating elsewhere); mild (patient develops new mild sweating in other areas, but it does not bother patient); moderate (patient develops new sweating in other areas to the degree that patient desires treatment); and severe (patient develops new sweating so severe that patient regrets undergoing the procedure). New areas of increased sweating were most commonly described on the low back, abdomen, buttocks, and thighs, and all long-term follow-up patients are included, whether the procedure was a success (143 patients) or a failure (5 patients). Our compensatory hyperhidrosis results are as follows: no increased sweating in 47 patients (31.7%); mild increased sweating in 92 patients (62.2%); moderate increased sweating in 7 patients (4.7%); and severe increased sweating in 2 patients (1.3%). Interestingly, the 2 patients with severe compensatory hyperhidrosis who regretted undergoing the procedure had very dry hands and no new increased sweating in a temperature-controlled environment, but they had a profoundly increased sweating response to environmental heat or exercise, which is why they regretted undergoing the procedure. Three patients had increased sweating in presurgical areas already involved with hyperhidrosis: 1 patient with a mild increase in gustatory sweating, and 2 patients with mild increased sweating in their feet.
The effect of sympathotomy on cardiovascular function has been previously published in a subset of patients from this series. Results suggest that the sympathotomy procedure used at Mayo Clinic causes detectable changes in cardiovascular control, but this likely has minimal clinical or quality-of-life consequences.
DISCUSSION
The sympathotomy operation performed at our institution divides all branches of the sympathetic chain (separate trunks and nerves of Kuntz) across the second rib between the T1 and T2 ganglia. The operation intentionally does not remove or injure ganglia of the chain or axons from spinal cord neurons innervating the ganglia. As a result, this approach decreases synaptic reorganization at the sympathetic chain level, as well as at the spinal cord level. The operation was designed to shorten operative time, to facilitate a high degree of success for the palmar component of this condition, and to minimize all possible complications, especially severe compensatory hyperhidrosis. The 0% rate of intercostal neuralgia and Horner syndrome is lower than that in reported series, and the 1.3% rate of chest tube for hemothorax is at the lower end of the reported complication range of 1.35% to 5%.5-7,10,11 The high success rate and very low severe compensatory hyperhidrosis rate demonstrated in this large series of patients prove the validity of this approach, suggesting that a more uniform technique could be considered by other surgeons as well. Also, by adopting a very small incision uniportal access and using a smaller endoscope, we have shortened total surgical time to an average of 38 minutes, and no intercostal neuralgia complications occurred.
The limitations of the current study are that it is a retrospective analysis and subject to the biases of patient responses. Despite every effort to stay in touch with these patients, most are young, mobile, and sometimes difficult to locate. Only 2 patients regretted undergoing the procedure, which is as low or lower than that reported in other series. We found no discernible difference in success or failure of the procedure with patient size or weight, age, sex, time of onset, or family history, nor did we find any preoperative predictors in the 7 patients who developed moderate compensatory hyperhidrosis or in the 2 patients who developed severe compensatory hyperhidrosis. However, a general trend was noted—if patients sweat excessively before surgery in areas other than their palms, axillae, and feet, at room temperature, they have an increased likelihood of experiencing some degree of compensatory hyperhidrosis in these same areas after surgery. Of note, only 1 patient, regardless of the degree of compensatory hyperhidrosis as a result of the surgery, considered sweating to be moderately disagreeable in a temperature-controlled environment. In all other patients who had increased sweating in compensatory areas (thermoregulatory-driven), the increased sweating was primarily caused or exacerbated by heat or exercise. One of the 2 patients who regretted undergoing the procedure worked in a petroleum industry around an oil well, so heavy, hot, nonventilated garments made working conditions unbearable. However, his postoperative sweat pattern, documented by TST, showed his hands to be dry and his sweating to be minimal or normal in a temperature-controlled environment. All 155 patients had palmar successes at hospital discharge. Of note, the 5 patients in whom the surgery failed experienced failure within 18 months, and 3 experienced failure within 3 months postoperatively. These data suggest that a minimum of 18 months follow-up postoperatively may be necessary to determine failed procedures and patients with compensatory hyperhidrosis.
CONCLUSION
Long-term follow-up of sympathotomy for palmar-plantar hyperhidrosis, as conceived and performed in the Department of Neurosurgery at Mayo Clinic by T1-T2 sympathetic disconnection, laser Doppler blood flow monitoring, small single-incision uniportal access, and minimization of the size of the endoscope and cautery to less than the width of a pencil, revealed the following: no Horner syndrome, no intercostal neuralgia, no treated pneumothorax; 2 cases of hemothorax that required treatment (1 immediate, 1 delayed); minimal clinically detectable cardiovascular changes; 96.6% of patients experienced long-term satisfaction, with markedly decreased or absent sweating of the hands; 69.2% of patients experienced decreased axillary sweating; 39.8% of patients experienced decreased plantar sweating; no compensatory hyperhidrosis in 31.7% of patients; mild compensatory hyperhidrosis (no bother to the patient) in 62.2% of patients; moderate compensatory hyperhidrosis (patient requesting treatment) in 4.7% of patients; and severe compensatory hyperhidrosis (patient regrets undergoing the procedure) in 1.3% of patients. Of note, all mild, moderate, and severe cases of compensatory hyperhidrosis except one case were primarily thermoregulatory-driven and precipitated most commonly by a hot ambient temperature and/or exercise.
Our results compare favorably with or exceed those of other large series of patients in which different techniques were used. Highly selective T1-T2 sympathotomy for palmar hyperhidrosis offers a very high long-term success rate and, as importantly, a very low complication rate compared with other surgical techniques for this condition.
Supplementary Material
Footnotes
For editorial comment, see page 717
Supporting Online Material
www.mayoclinicproceedings.com/content/86/8/721/suppl/DC1 Video
References
- 1. Adson AW, Craig WM, Brown GE. Essential hyperhidrosis cured by sympathetic ganglionectomy and trunk resection. Arch Surg. 1935;31(5):794-806 [Google Scholar]
- 2. Kux E. The endoscopic approach to the vegetative nervous system and its therapeutic possibilities: especially in duodenal ulcer, angina pectoris, hypertension and diabetes. Dis Chest. 1951;20(2):139-147 [DOI] [PubMed] [Google Scholar]
- 3. Vanaclocha V, Sáiz-Sapena N, Panta F. Uniportal endoscopic superior thoracic sympathectomy. Neurosurgery. 2000;46(4):924-928 [DOI] [PubMed] [Google Scholar]
- 4. Steiner Z, Kleiner O, Hershkovitz Y, Mogilner J, Cohen Z. Compensatory sweating after thoracoscopic sympathectomy: an acceptable trade-off. J Pediatr Surg. 2007;42(7):1238-1242 [DOI] [PubMed] [Google Scholar]
- 5. Chwajol M, Barrenechea IJ, Chakraborty S, Lesser JB, Connery CP, Perin NI. Impact of compensatory hyperhidrosis on patient satisfaction after endoscopic thoracic sympathectomy. Neurosurgery. 2009;64(3):511-518 [DOI] [PubMed] [Google Scholar]
- 6. Li X, Tu YR, Lin M, Lai FC, Chen JF, Miao HW. Minimizing endoscopic thoracic sympathectomy for primary palmar hyperhidrosis: guided by palmar skin temperature and laser Doppler blood flow. Ann Thorac Surg. 2009;87(2):427-431 [DOI] [PubMed] [Google Scholar]
- 7. Rodriguez PM, Freixinet JL, Hussein M, et al. Side effects, complications and outcome of thoracoscopic sympathectomy for palmar and axillary hyperhidrosis in 406 patients. Eur J Cardiothorac Surg. 2008;34(3):514-519 [DOI] [PubMed] [Google Scholar]
- 8. Katara AN, Domino JP, Cheah WK, So JB, Ning C, Lomanto D. Comparing T2 and T2-T3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: a randomized control trial. Surg Endosc. 2007;21(10):1768-1771 [DOI] [PubMed] [Google Scholar]
- 9. Baumgartner FJ. Surgical approaches and techniques in the management of severe hyperhidrosis. Thorac Surg Clin. 2008;18(2):167-181 [DOI] [PubMed] [Google Scholar]
- 10. Wait SD, Killory BD, Lekovic GP, Ponce FA, Kenny KJ, Dickman CA. Thoracoscopic sympathectomy for hyperhidrosis: analysis of 642 procedures with special attention to Horner's syndrome and compensatory hyperhidrosis. Neurosurgery. 2010;67(3):652-656 [DOI] [PubMed] [Google Scholar]
- 11. Miller DL, Bryant AS, Force SD, Miller JI., Jr Effect of sympathectomy level on the incidence of compensatory hyperhidrosis after sympathectomy for palmar hyperhidrosis. J Thorac Cardiovasc Surg. 2009;138(3):581-585 [DOI] [PubMed] [Google Scholar]
- 12. Katara AN, Domino JP, Cheah WK, So JB, Ning C, Lomanto D. Comparing T2 and T2-T3 ablation in thoracoscopic sympathectomy for palmar hyperhidrosis: a randomized control trial. Surg Endosc. 2007;21(10):1768-1771 [DOI] [PubMed] [Google Scholar]
- 13. Kopelman D, Hashmonai M. The correlation between the method of sympathetic ablation for palmar hyperhidrosis and the occurrence of compensatory hyperhidrosis: a review. World J Surg. 2008;32(11):2343-2356 [DOI] [PubMed] [Google Scholar]
- 14. O'Riordain DS, Maher M, Waldron DJ, O'Donovan B, Brady MP. Limiting the anatomic extent of upper thoracic sympathectomy for primary palmar hyperhidrosis. Surg Gynecol Obstet. 1993;176(2):151-154 [PubMed] [Google Scholar]
- 15. Weksler B, Blaine G, Souza ZB, Gavina R. Transection of more than one sympathetic chain ganglion for hyperhidrosis increases the severity of compensatory hyperhidrosis and decreases patient satisfaction. J Surg Res. 2009;156(1):110-115 [DOI] [PubMed] [Google Scholar]
- 16. Miller DL, Bryant AS, Force SD, Miller JI., Jr Effect of sympathectomy level on the incidence of compensatory hyperhidrosis after sympathectomy for palmar hyperhidrosis. J Thorac Cardiovasc Surg. 2009;138(3):581-585 [DOI] [PubMed] [Google Scholar]
- 17. Atkinson JLD, Fealey RD. Sympathotomy instead of sympathectomy for palmar hyperhidrosis: minimizing postoperative compensatory hyperhidrosis. Mayo Clin Proc. 2003;78(2):167-172 [DOI] [PubMed] [Google Scholar]
- 18. Eisenach JH, Atkinson JL, Fealey RD. Hyperhidrosis: evolving therapies for a well-established phenomenon [published correction appears in Mayo Clin Proc. 2005;80(6):828]. Mayo Clin Proc. 2005;80(5):657-666 [DOI] [PubMed] [Google Scholar]
- 19. Li X, Tu YR, Lin M, Lai FC, Chen JF, Miao HW. Minimizing endoscopic thoracic sympathectomy for primary palmar hyperhidrosis: guided by palmar skin temperature and laser doppler blood flow. Ann Thorac Surg. 2009;87(2):427-431 [DOI] [PubMed] [Google Scholar]
- 20. Tetteh HA, Groth SS, Kast T, et al. Primary palmoplantar hyperhidrosis and thoracoscopic sympathectomy: a new objective assessment method. Ann Thorac Surg. 2009;87(1):267-275 [DOI] [PubMed] [Google Scholar]
- 21. Kuntz A. Distribution of the sympathetic rami to the brachial plexus: its relation to sympathectomy affecting the upper extremity. Arch Surg. 1927; 15:871-877 [Google Scholar]
- 22. Kirgis HD, Kuntz A. Inconstant sympathetic neural pathways: their relation to sympathetic denervation of the upper extremity. Arch Surg. 1942;44:95-102 [Google Scholar]
- 23. Ramos R, Masuet C, Badia M, et al. Quantification of eccrine sweat glands with acetylcholine sweat-spot test and anatomical redistribution of sweating after T2-T3 thoracoscopic sympathicolysis. Surg Endosc. 2009; 23(2):321-326 [DOI] [PubMed] [Google Scholar]
- 24. Schmidt JE, Wehrwein EA, Gronbach LA, et al. Autonomic function following endoscopic thoracic sympathotomy for hyperhidrosis. Clin Auton Res. 2011;21(1):11-17 [DOI] [PubMed] [Google Scholar]
- 25. Wehrwein EA, Schmidt JE, Elvebak RL, et al. Hemodynamics following endoscopic thoracic sympathotomy for palmar hyperhidrosis. Clin Auton Res. 2011;21(1):3-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.