Abstract
Objective: To determine the effect on mortality of the left atrial volume index (LAVI) and left ventricular (LV) geometry (normal, concentric remodeling, eccentric hypertrophy, and concentric hypertrophy).
PATIENTS AND METHODS: From January 1, 2004, through December 31, 2006, we evaluated 36,561 patients with preserved ejection fraction with an average follow-up of 1.7±1.0 years. The LAVI was categorized as normal (≤28 mL/m2) or increased (mild, 29-33 mL/m2; moderate, 34-39 mL/m2; severe, ≥40 mL/m2).
RESULTS: Progressive increases in LAVI and mortality were noted with abnormal LV geometry. Similarly, abnormal LV geometry and mortality were significantly higher in patients with increased LAVI. In patients who died vs surviving patients, the LAVI ± SD was significantly higher (33.0±14.8 vs 28.1±10.8 mL/m2; P<.001) and abnormal LV geometry was significantly more prevalent (62% vs 44%; P<.001). Compared with those with a normal LAVI, patients with a severe LAVI had a 42% increased risk of mortality. In patients with normal LV geometry or concentric remodeling, a severe LAVI was a significant independent predictor of mortality, with an increased risk of 28% and 46%, respectively. Similarly, in patients with eccentric hypertrophy and concentric hypertrophy, the mortality risk in patients with a severe LAVI was twice that of patients with a normal LAVI. Comparison of area under the curve (0.565 [without LAVI] vs 0.596 [with LAVI]; P<.001] and predictive models with and without LAVI for mortality prediction were significant, indicating increased mortality prediction by the addition of LAVI to other independent predictors.
CONCLUSION: The LAVI significantly predicts mortality risk, independent of LV geometry, and adds to the overall mortality prediction in a large cohort of patients with preserved systolic function.
AUC = area under the curve; BMI = body mass index; BP = blood pressure; Ch = concentric hypertrophy; CI = confidence interval; CR = concentric remodeling; CV = cardiovascular; EH = eccentric hypertrophy; HR = hazard ratio; LA = left atrial; LAE = LA enlargement; LAVI = LA volume index; LV = left ventricular; LVEF = LV ejection fraction; LVH = LV hypertrophy; LVMI = LV mass index; RWT = relative wall thickness
Left ventricular (LV) geometry, as an expression of the structural adaptation to an increased cardiovascular (CV) risk factor burden, is a well-known predictor of CV morbidity and mortality.1-5 These structural changes in the left ventricle also lead to functional impairment, notably impaired LV filling, thereby facilitating the development of diastolic dysfunction6 and ultimately leading to left atrial (LA) enlargement (LAE).7 The left atrium is directly exposed to LV pressure during diastole through the open mitral valve, and therefore any morphologic changes in the left atrium are largely determined by the same factors that influence diastolic LV filling.8 Recent studies have shown that LA volume increases with the severity of diastolic dysfunction and may express long-term exposure to abnormal LV filling pressures, providing a more sensitive and stable morphophysiologic expression of LV diastolic dysfunction.9
Current evidence strongly suggests that LA size, as assessed by echocardiography, is strongly associated with mortality.7,10-12 However, it is still unknown whether LAE predicts mortality independent of LV geometry and also whether it provides additional prognostic information to that of other predictors of mortality in a large cohort of patients with preserved systolic function.
To address this gap in the literature, we conducted a study of a large clinical cohort of 36,561 patients with preserved LV ejection fraction (LVEF) to determine how the LA volume index (LAVI) affected all-cause mortality and whether LAVI predicted mortality independent of LV geometry.
PATIENTS AND METHODS
We obtained clinical and echocardiographic data from a clinical echocardiographic report database (CVIS) of 86,147 studies that were recorded at Ochsner Clinic Foundation (New Orleans, LA) between January 1, 2004, and December 31, 2006. After excluding patients with missing clinical information (body mass index [BMI], n=10,682 [12.4%]; systolic blood pressure [BP], n=25,068 [29.1%]; diastolic BP, n=25,068 [29.1%]; heart rate, n=25,241 [29.3%]) or echocardiographic findings (LV mass, n= 20,158 [23.4%]; relative wall thickness [RWT], n=20,072 [23.3%]; LAVI, n=36,855 [42.8%]), a subgroup of 36,561 patients (42.4%) was selected for the study who had preserved systolic function (defined as LVEF ≥50% and absence of moderate or severe valvular heart disease). Survival status was obtained from the National Death Index for the entire cohort during a mean ± SD follow-up of 1.7±1.0 years. The end point was death due to all causes. This study was approved by the Institutional Review Board of the Ochsner Clinic Foundation.
General Examination
Height and weight were measured to calculate BMI (calculated as the weight in kilograms divided by height in meters squared). Single systolic and diastolic BP measurements as well as heart rate were obtained before echocardiographic examination. No other clinical information was available for the study.
Echocardiographic Methods
M-mode and 2-dimensional images were obtained with commercially available instruments that operated at 2.0 to 3.5 MHz. Two-dimensional imaging was performed in the standard fashion in parasternal long- and short-axis views and apical 4- and 2-chamber views. The LV dimensions and wall thickness were measured according to the guidelines of the American Society of Echocardiography.13 Intraobserver variability in our laboratory for quantitation of LV dimensions was less than 10%. End-diastolic LV dimensions (ie, interventricular septal dimension, LV internal dimension, and posterior wall thickness) were used to calculate LV mass by an anatomically validated formula, with good reproducibility.14 Left ventricular hypertrophy (LVH) was considered present when the LV mass index (LVMI) was greater than 116 g/m2 for men and greater than 96 g/m2 for women, as previously described.13 Relative wall thickness was calculated as twice the posterior wall thickness in diastole divided by the LV internal diameter. Increased RWT was present when this ratio was greater than 0.42.14 Normal geometry was present when the LVMI and RWT were normal. Increased RWT and normal LVMI were classified as concentric remodeling (CR); increased LVMI but normal RWT as eccentric LVH (eccentric hypertrophy [EH]); and increases in the 2 variables as concentric LVH (concentric hypertrophy [CH]).15
Left Atrial Volume Assessment
Left atrial volume was measured using the modified biplane area-length method and was corrected for body surface area.16,17 The LAVI was categorized as either normal (≤28 mL/m2) or increased (mild, 29-33 mL/m2; moderate, 34-39 mL/m2; severe, ≥40 mL/m2).13
Statistical Analyses
All statistical analyses were performed with SAS statistical software, version 9.1 (SAS Institute, Cary, NC). Two-tailed P<.05 was considered statistically significant. Clinical, echocardiographic, and mortality data were compared between groups with differing LV geometry, between patients who died and surviving patients, and between groups with differing LAVI categories. Mortality prevalence was compared by LAVI groups in the 4 LV geometric patterns and in the total cohort. Continuous variables are reported as mean ± SD, and the comparison was performed using the t test. Categorical variables are reported as percentages, and the comparison was performed using the χ2 test. Mortality prevalence was compared among multiple groups using Cox proportional hazards regression models. Comparison among multiple groups and trend assessment were performed using analysis of variance and Mantel-Haenszel statistics.
Univariate and multivariate associations of all-cause mortality with clinical (age, sex, BMI, systolic and diastolic BP, heart rate) and echocardiographic (LVEF, LVMI, RWT, LAVI) variables were assessed using Cox proportional hazards regression models in the total population. Independent predictability of mortality by LAVI was also assessed in different LV geometric groups using a Cox proportional hazards regression model that also included age, sex, BMI, systolic and diastolic BP, heart rate, and LVEF. Independent variables included in the multivariate regression analysis were selected using a stepwise method. Hazard ratios (HRs) and their associated 95% confidence intervals (CIs) were reported. A Kaplan-Meier survival curve was constructed to assess the survival over time for patients in different LAVI categories; comparisons were made using the log rank test. The incremental value of LAVI was assessed in 2 modeling steps. The first step consisted of fitting a multivariate model of age, BMI, systolic and diastolic BP, heart rate, LVEF, and LVMI. Then, LAVI was included in the model in a second step. The change in overall log likelihood ratio χ2 was used to assess the increase in predictive power after the addition of LAVI. For each of these 2 regression models, individual hazard functions were generated and compared using receiving operating characteristic curves, and areas under the curve (AUCs) were calculated and compared using z statistics, as described by DeLong et al.18 Detection of a significant difference between AUCs indicates a significant difference in predictive ability, with a larger area representing a better predictive model.
RESULTS
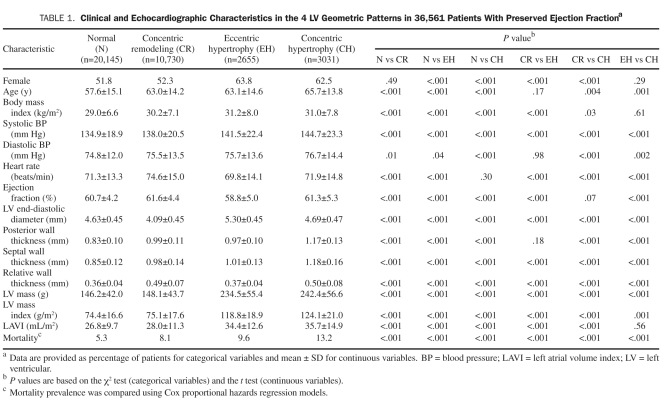
Clinical and echocardiographic characteristics of patients grouped by LV geometric patterns are shown in Table 1. Abnormal LV geometry was identified in 16,416 patients (45%) in the study cohort. Concentric remodeling, the most frequently observed abnormal LV geometric pattern, was identified in 10,730 patients (29%). Abnormal LV geometry was associated with older age, a higher BMI, a higher LAVI, and a greater prevalence of mortality.
TABLE 1.
Clinical and echocardiographic Characteristics in the 4 LV Geometric Patterns in 36,561 Patients With Preserved ejection Fractiona
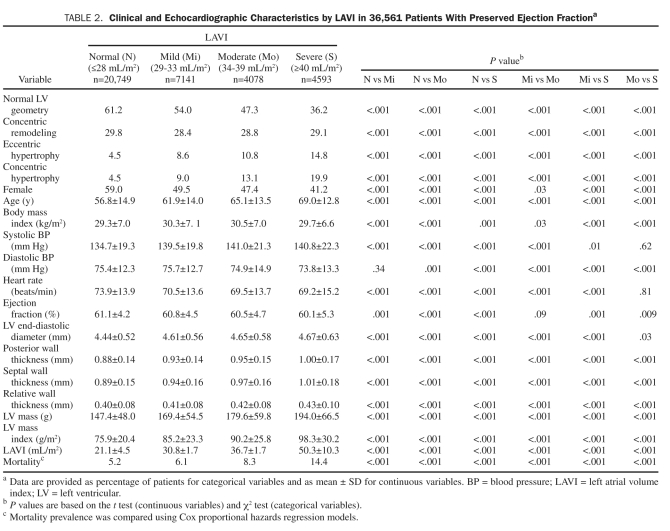
Patients were also divided into normal, mild, moderate, and severe LAVI groups, and their clinical and echocardiographic characteristics were compared as shown in Table 2. Abnormal LAVI was identified in 15,812 patients (43%). An increased prevalence of abnormal LV geometry was noted with increasing LAVI (39% [normal], 46% [mild], 53% [moderate], 64% [severe]). Importantly, with increasing LAVI, the prevalence of frank LVH significantly increased. Increasing LAVI was also associated with lower LVEF, older age, LV end-diastolic diameter, RWT, LVMI, and mortality.
TABLE 2.
Clinical and echocardiographic Characteristics by LAVI in 36,561 Patients With Preserved ejection Fractiona
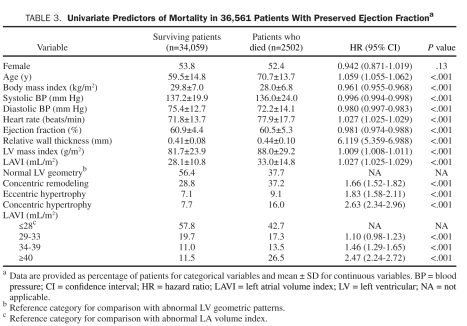
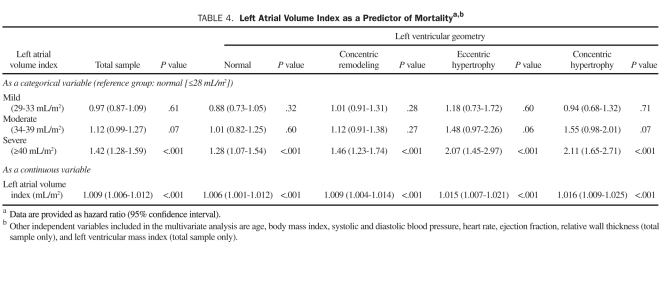
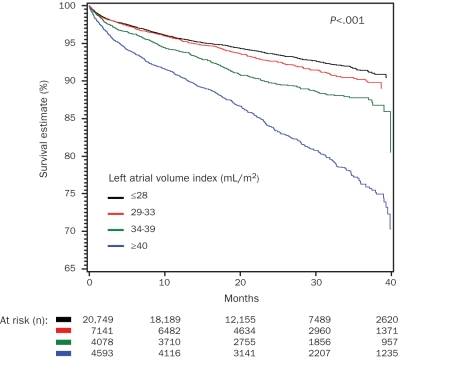
During the mean follow-up ± SD period of 1.7±1.0 years, 2502 patients (6.8%) died. As shown in Table 3, patients who died vs surviving patients had a significantly higher LAVI ± SD (33.0±14.8 vs 28.1±10.8; P<.001) and prevalence of abnormal LV geometry (62% vs 44%; P<.001). In patients who died, BMI, systolic and diastolic BP, and LVEF were lower, whereas age, heart rate, RWT, and LVMI were higher. Univariate predictors of all-cause mortality were identified (Table 3). In univariate analysis, all 3 patterns of abnormal LV geometry predicted mortality, with mortality risk increasing with changes from CR to EH and then to CH (CR: HR, 1.66; EH: HR, 1.83; CH: HR, 2.63; all P<.001]. Similarly, all categories of abnormal LAVI were significant predictors of mortality in comparison with normal LAVI category and also demonstrated an increasing mortality risk with a higher level of LAVI (mild: HR, 1.10; moderate: HR, 1.46; severe: HR, 2.47; all P<.001). Other parameters, including older age, lower BMI, lower systolic and diastolic BP, higher heart rate, lower LVEF, higher RWT, and higher LVMI, also independently predicted mortality. Because the current study lacks the information regarding antihypertensive medication use, the results regarding the association between BP and mortality are essentially uninterpretable. In multivariate analysis, the independent association of LAVI, both as a continuous and a categorical variable, was assessed in the total study cohort. As shown in Table 4, patients with a severe LAVI had a 42% increased mortality compared with patients with a normal LAVI. Similarly, with every milliliter per meter squared increase in LAVI, mortality risk independently increased by 0.9% (P<.001). Of note, this independent association between LAVI and mortality was significant even after adjusting for LVMI (HR, 1.006; 95% CI, 1.004-1.007; P<.001) and RWT (HR, 4.39; 95% CI, 2.88-6.70]; P<.001). Kaplan-Meier survival analysis in the study cohort by LAVI categories showed a statistically significant gradation, with worse survival for those with a severely elevated LAVI (Figure 1).
TABLE 3.
Univariate Predictors of Mortality in 36,561 Patients With Preserved ejection Fractiona
TABLE 4.
Left Atrial Volume Index as a Predictor of Mortalitya,b
FIGURE 1.
Kaplan-Meier survival analysis by left atrial volume index.
To remove the confounding effect of LV geometric changes on the association of LAVI and mortality, the study cohort was divided into the 4 LV geometric groups, and then the independent association of LAVI and abnormal LAVI categories in reference to normal LAVI was assessed within each LV geometric pattern. With every milliliter per meter squared increase in LAVI, mortality risk independently increased by 0.6% in the group with normal LAVI, by 0.9% in the group with CR, by 1.5% in the group with EH, and by 1.6% in the group with CH (all P<.001). Similarly, a severe LAVI was a significant independent predictor of mortality in all LV geometric patterns (normal: HR, 1.28; CR: HR, 1.46; EH: HR, 2.07; CH: HR, 2.11; all P<.001).
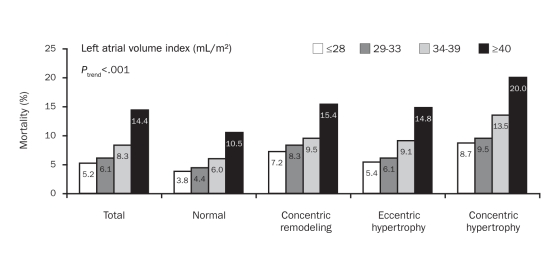
We also examined the prevalence of mortality by LAVI in the total study cohort as well as in groups divided by LV geometric patterns. As shown in Figure 2, significant increases in mortality with increasing LAVI were observed in the total cohort as well as in all 4 LV geometric groups. Mortality in patients with a severe LAVI was more than twice that in patients with a normal LAVI in all 4 geometric groups.
FIGURE 2.
Mortality prevalence by left atrial volume index in the total cohort and by 4 distinct left ventricular geometric patterns, including normal, concentric remodeling, eccentric hypertrophy, and concentric hypertrophy.
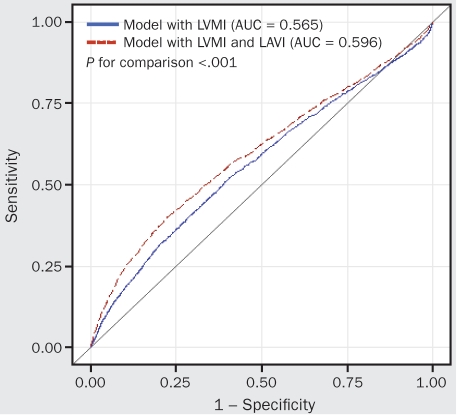
The comparison of 2 multivariate regression models, one with and one without the LAVI parameter (both included age, BMI, systolic and diastolic BP, heart rate, LVEF, and LVMI), using the likelihood ratio test, yielded significant results (χ2=34.4; P<.001). Wald statistics were examined to assess the relative weight of LVMI and LAVI within these Cox regression models. The model with LVMI had a LVMI Wald statistic of 117.7 (P<.001). With the addition of LAVI into the predictive model based on LVMI, the Wald statistic was 95.3 for LVMI (P<.001) and 36.2 for LAVI (P<.001). Furthermore, as shown in Figure 3, the AUC comparison between these 2 regression models was significant. Thus, although the addition of LAVI reduces the relative weight of LVMI in the model, it improves the overall accuracy for mortality prediction.
FIGURE 3.
Receiving operating characteristic curves and areas under the curves (Aucs) for cumulative hazard functions of left ventricular mass index (LVMI) with or without left atrial volume index (LAVI). Models are adjusted for age, body mass index, systolic and diastolic blood pressure, heart rate, and ejection fraction.
DISCUSSION
In our retrospective study of a very large clinical cohort of patients with preserved LV systolic function, LAVI was strongly associated with all-cause mortality, with mortality risk increasing with worsening LAVI. Further, LAVI predicted mortality independent of all LV geometric patterns and provided prognostic information incremental to other significant independent predictors of mortality.
The association of LAE and mortality, both CV and all-cause, has been reported in a few population-based studies6,9-11 as well as in several high-risk populations, such as patients with dilated cardiomyopathy,19 LV dysfunction,20 atrial arrhythmias,21 and acute myocardial infarction.22 Only a very few of these studies used LAVI, which is more accurate and reproducible, as a measure of LA size and were performed in a population with preserved LV systolic function as determined by LVEF. To further assess patients' risk, we evaluated the association between LA size and mortality by stratifying LAVI into normal, mild, moderate, and severely dilated LAVI categories as recommended by the American Society of Echocardiography. The progressive increase in mortality with increasing LA size was in line with other studies using different classification criteria.9-11
In patients without primary LA pathology or congenital heart or mitral valve disease, LAE mainly occurs as a result of pressure overload related to diastolic dysfunction and elevated LV filling pressures but can also result from volume overload related to factors such as obesity. The structural changes in the left ventricle expressed as different LV geometric forms are associated with abnormal diastolic properties, further increasing LV filling pressures and leading to LAE. Because LA size determinants, including LV geometry and diastolic dysfunction, are well-known predictors of mortality, the association between LAE and mortality could be explained in part by its strong association with abnormal LV geometry23 or diastolic dysfunction.9 This mechanism of association was further supported by the findings from a few population-based studies, in which an association between LAE and mortality was attenuated after adjusting for LV mass,10 LVH,12 or diastolic dysfunction.7 In the Framingham Heart Study, adjusting for LV mass negated the relationship between LA size and mortality.10 Similarly, in the Kuopio Ischaemic Heart Disease (KIHD) Risk Factor Study,12 no significant association between LA size and mortality was found after adjusting for LVH. Both of these studies suggested a strong association between LA size and LV mass or LVH as a partial explanation for this lack of association. The findings from our study support the notion that abnormal LV geometry is associated with LAVI and mortality; however, our results demonstrate substantial attenuation in mortality prediction by LAVI after adjusting for the presence of abnormal LV geometry. Further, the level and strength of this association were noticeably different in the 4 distinct LV geometric patterns.
The mortality risk associated with elevated LAVI was higher in patients with frank LVH compared with normal or CR groups. These findings raise the possibility of risk redistribution between LVMI and LAVI. We speculate that the burden of confounding risk factors (eg, obesity or hypertension) that influence both LV geometry24 and LAVI25 could be important, along with the presence and severity of diastolic dysfunction, in explaining the differences in the association between LAE and mortality in the different LV geometric groups.
Recently, De Simone et al26 reported that, in addition to LVMI, LA dilatation assessed by LA linear dimension was significantly associated with increased CV risk in hypertensive patients. However, in contrast to our study's findings, the effect of LA dimension, when considered together with LV mass, appeared to be incorporated into the information gained from LV mass. The population studied by De Simone et al had higher LV mass (189.2 vs 161.1 g) and higher prevalence of LVH (31.0% vs 15.6%) than our study population. In addition, the strength of the association between CV diseases and LA size has been reported to be stronger for LA volume than for LA linear dimension.27 These differences in population characteristics as well as the variability in the definitions used may explain at least in part the difference in the study results, but further investigation is certainly warranted.
Several other study limitations should be noted. First, because this study was a retrospective analysis of an echocardiographic report database, we do not have access to some important clinical data, including the reason for referral; any history of hypertension, diabetes, coronary artery disease, heart failure, clinical symptoms, or functional limitations; and use of medications. Second, our study lacks Doppler echocardiographic indices or tissue Doppler imaging assessment of mitral annulus motion to determine diastolic dysfunction, which may have in part explained our study findings. Third, data were unavailable on cause of death or cardiac events, although all-cause mortality is certainly an important end point. Fourth, the current retrospective study cannot address the issue of causality and underlying mechanisms governing the observed associations. Finally, because our cohort was not population-based but rather consisted of patients referred for echocardiography for routine clinical indications, selection bias is a possibility. Additionally, for various logistical reasons, many patients were excluded because of lack of LAVI data; although we do not think that these logistical issues significantly affected our major study findings, this possibility cannot be completely excluded.
We strongly consider, however, that our study findings have significant clinical importance because the patient sample is very large and reflective of the patient population commonly encountered by large, busy clinical CV practices.
CONCLUSION
Our data based on a retrospective analysis of a large clinical cohort demonstrate that LAE has important prognostic implications in terms of mortality prediction, independent of LV geometry, in patients with preserved LVEF and could improve clinical risk stratification for CV outcomes. Further studies are needed to confirm these findings as well as to expand the understanding of the natural history of LA remodeling and the effect of such changes on clinical outcomes.
Supplementary Material
Footnotes
Presented in part at the American heart Association Scientific Sessions; November 2008; New Orleans, LA.
References
- 1. Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561-1566 [DOI] [PubMed] [Google Scholar]
- 2. Milani RV, Lavie CJ, Mehra M, Ventura HO, Kurtz JD, Messerli FH. Left ventricular geometry and survival in patients with normal left ventricular ejection fraction. Am J Cardiol. 2006;97(7):959-963 [DOI] [PubMed] [Google Scholar]
- 3. Lavie CJ, Milani RV, Ventura HO, Messerli FH. Left ventricular geometry and mortality in patients >70 years of age with normal ejection fraction. Am J Cardiol. 2006;98(10):1396-1399 [DOI] [PubMed] [Google Scholar]
- 4. Lavie CJ, Milani RV, Cardenas GA, Mehra MR, Messerli FH. Disparate effects of left ventricular geometry and obesity on mortality in patients with preserved left ventricular ejection fraction. Am J Cardiol. 2007;100(9):1460-1464 [DOI] [PubMed] [Google Scholar]
- 5. Artham SM, Lavie CJ, Milani RV, Patel DA, Verma A, Ventura HO. Clinical impact of left ventricular hypertrophy and implications of regression. Prog Cardiovasc Dis. 2009;52(2):153-167 [DOI] [PubMed] [Google Scholar]
- 6. Grossman W. Diastolic dysfunction and congestive heart failure. Circulation. 1990;81(suppl III):III1-III7 [PubMed] [Google Scholar]
- 7. Pritchett AM, Mahoney DW, Jacobson SJ, Rodeheffer RJ, Karon BL, Redfield MM. Diastolic dysfunction and left atrial volume: a population based study. J Am Coll Cardiol. 2005;45(1):87-92 [DOI] [PubMed] [Google Scholar]
- 8. Greenberg B, Chatterjee K, Parmley WW, Werner JA, Holly AN. The influence of left ventricular filling pressure on atrial contribution to cardiac output. Am Heart J. 1979;98(6):742-751 [DOI] [PubMed] [Google Scholar]
- 9. Tsang TS, Barnes ME, Gersh BJ, Bailey KR, Seward JB. Left atrial volume as a morphophysiologic expression of left ventricular diastolic dysfunction and relation to cardiovascular risk burden. Am J Cardiol. 2002;90(12):1284-1289 [DOI] [PubMed] [Google Scholar]
- 10. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolfe PA, Levy D. Left atrial size and the risk of stroke and death: the Framingham Heart Study. Circulation. 1995;92(4):835-841 [DOI] [PubMed] [Google Scholar]
- 11. Gardin JM, McClelland R, Kitzman R, et al. M-mode echocardiographic predictors of six- to seven-year incidence of coronary heart disease, stroke, congestive heart failure, and mortality in an elderly cohort (the Cardiovascular Health Study). Am J Cardiol. 2001;87(9):1051-1057 [DOI] [PubMed] [Google Scholar]
- 12. Laukkanen JA, Kurl S, Eränen J, Huttunen M, Salonen JT. Left atrium size and the risk of cardiovascular death in middle-aged men. Arch Intern Med. 2005;165(15):1788-1793 [DOI] [PubMed] [Google Scholar]
- 13. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. Am J Soc Echocardiogr. 2005;18(12):1440-1463 [DOI] [PubMed] [Google Scholar]
- 14. Reichek N, Devereux RB. Reliable estimation of peak left ventricular systolic pressure by M-mode echographic-determined end-diastolic relative wall thickness: identification of severe valvular aortic stenosis in adult patients. Am Heart J. 1982;103(2):202-203 [DOI] [PubMed] [Google Scholar]
- 15. Ganau A, Devereux RB, Roman MJ, et al. Patterns of left ventricular hypertrophy and geometric remodeling in essential hypertension. J Am Coll Cardiol. 1992;19(7):1550-1558 [DOI] [PubMed] [Google Scholar]
- 16. Tsang TS, Barnes ME, Bailey KR, et al. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76(5):467-475 [DOI] [PubMed] [Google Scholar]
- 17. Fatema K, Bailey KR, Petty GW, et al. Increased left atrial volume index: potent biomarker for first-ever ischemic stroke. Mayo Clin Proc. 2008;83(10):1107-1115 [DOI] [PubMed] [Google Scholar]
- 18. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a non-parametric approach. Biometrics. 1988;44(3):837-845 [PubMed] [Google Scholar]
- 19. Rossi A, Cicoira M, Zanolla L, et al. Determinants and prognostic value of left atrial volume in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2002;40(8):1425-1430 [DOI] [PubMed] [Google Scholar]
- 20. Giannuzzi P, Temporelli PL, Bosimini E, et al. Independent and incremental prognostic value of Doppler-derived mitral deceleration time of early filling in both symptomatic and asymptomatic patients with left ventricular dysfunction. J Am Coll Cardiol. 1996;28(2):383-390 [DOI] [PubMed] [Google Scholar]
- 21. Cabin HS, Clubb KS, Hall C, Perlmutter RA, Feinstein AR. Risk for systemic embolization of atrial fibrillation without mitral stenosis. Am J Cardiol. 1990;65(16):1112-1116 [DOI] [PubMed] [Google Scholar]
- 22. Moller JE, Hillis GS, Oh JK, et al. Left atrial volume: a powerful predictor of survival after acute myocardial infarction. Circulation. 2003;107(17):2207-2212 [DOI] [PubMed] [Google Scholar]
- 23. Cioffi G, Mureddu GF, Stefenelli C, de Simone G. The relationship between left ventricular geometry and left atrial size and function in patients with systemic hypertension. J Hypertens. 2004;22(8):1589-1596 [DOI] [PubMed] [Google Scholar]
- 24. Gardin JM, Arnold A, Gottdiener JS, et al. Left ventricular mass in the elderly: the Cardiovascular Health Study. Hypertension. 1997;29(5):1095-1103 [DOI] [PubMed] [Google Scholar]
- 25. Stritzke J, Markus MRP, Duderstadt S, et al. The aging process of the heart: obesity is the main risk factor for left atrial enlargement during aging. J Am Coll Cardiol. 2009;54(21):1982-1989 [DOI] [PubMed] [Google Scholar]
- 26. De Simone G, Izzo R, Chinali M, et al. Does information on systolic and diastolic function improve prediction of a cardiovascular event by left ventricular hypertrophy in arterial hypertension? Hypertension. 2010;56(1):99-104 [DOI] [PubMed] [Google Scholar]
- 27. Tsang TS, Abhayaratna WP, Barnes ME, et al. Prediction of cardiovascular outcomes with left atrial size: is volume superior to area or diameter? Am J Coll Cardiol. 2006;47(5):1018-1023 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.