Abstract
OBJECTIVE: To further understand the characteristics and behavior of malignant fibrous histiocytoma (MFH) in the clinical setting of chronic lymphocytic leukemia (CLL) or non-Hodgkin lymphoma (NHL).
PATIENTS AND METHODS: The patient database of MHF diagnosed at our institution from January 1, 1976, to December 31, 2008, was reviewed. For each MFH case with prior NHL or CLL, 3 matched controls from the same cohort without prior CLL or NHL were randomly selected. A retrospective chart review collected patient data, including sex; age; pathologic confirmation of MFH; tumor grade, size, and location; time since MFH diagnosis; history of chemotherapy or irradiation; treatment of MFH; recurrence; metastasis; and death. The Kaplan-Meier method was used to estimate overall survival, survival free of recurrence, and survival free of metastasis. Cox proportional hazards models were fit to evaluate associations between case status and outcomes.
RESULTS: Fifteen MFH cases with prior NHL or CLL were identified. Cases had frequent bone (n=7 [47%]) and cutaneous (n=5 [33%]) involvement. Five cases (33%) had previous irradiation, 6 (40%) had metastases, and 4 (27%) had recurrence. Overall survival and survival free of recurrence were not significantly different between the cases and controls (5-year overall survival, 49.9% and 58.7%; 12-month survival free of recurrence, 79.4% and 90.3%). However, cases were significantly more likely than controls to have metastasis (hazard ratio, 3.79; 95% confidence interval, 1.22-11.79; P=.02). In addition, survival free of metastasis at 12 months was 65.2% and 90.7%, respectively.
CONCLUSION: In the presence of CLL or NHL, MFH seems to behave more aggressively, suggested by the increased likelihood of metastases compared with controls without prior CLL or NHL. However, overall survival does not appear to be worse in cases of MFH and CLL or NHL than in MFH alone.
CI = confidence interval; CLL = chronic lymphocytic leukemia; HR = hazard ratio; MFH = malignant fibrous histiocytoma; NHL = non-Hodgkin lymphoma
Reports have suggested an increased incidence of solid tumors in persons with lymphoproliferative disorders during the past 30 years. Non-Hodgkin lymphoma (NHL) is among the top 10 most commonly diagnosed cancers in the United States and consists of a heterogeneous group of lymphoproliferative cancers.1 Chronic lymphocytic leukemia (CLL) is in the NHL family and is the most common leukemia occurring in the United States and other Western nations, representing 30% of all leukemias.2 The incidence of NHL has increased more than 100% in the past 50 years, in both Western Europe and the United States.1
Chronic lymphocytic leukemia consists of a marked accumulation of monoclonal CD5+ B lymphocytes in blood, lymphoid tissue, and bone marrow.2 Typically, CLL is seen more frequently in men and is usually diagnosed in patients older than 50 years.3,4
Malignant fibrous histiocytoma (MFH) is a high-grade pleomorphic soft tissue sarcoma that most frequently arises in the soft tissue of the extremities and retroperitoneum.5-9 Usually, MFH affects white men and makes up 20% to 25% of all soft tissue sarcomas.7 The prognosis of MFH differs widely and depends on the size, grade, and location of the primary tumor, as well as the presence of immunosuppression.9-11 Studies have shown an increased risk of many different forms of secondary cancers in patients with lymphoproliferative disorders such as CLL and NHL.12,13 Specifically, some studies have shown an association between lymphoproliferative disorders and the development of MFH.11,14
Patients with CLL have an increased risk of secondary cancers, including lung carcinoma, brain cancer, melanoma of the eye, and Hodgkin lymphoma, as well as malignant melanoma and nonmelanoma skin cancer.12,13,15 In fact, the risk of a cancer developing in a patient with CLL is 3-fold higher than that in corresponding control subjects; more specifically, the risk of skin cancer is 8-fold higher in a patient with CLL.13,15 Although the association of CLL and certain secondary cancers has been studied, no research has been performed on the effects of CLL and NHL on MFH.
PATIENTS AND METHODS
The Mayo Clinic Institutional Review Board approved this study. A data retrieval specialist performed an electronic search of the Mayo Clinic medical diagnosis index to identify patients with MFH diagnosed between January 1, 1976, and December 31, 2008. The search also identified a subset of the MFH patients with lymphoma (potential cases). A retrospective chart review was conducted to ensure that these cases fulfilled the study inclusion criteria of CLL or NHL diagnosed before MFH. Cases meeting criteria were matched 1:3 to patients with MFH from the same cohort without prior CLL or NHL (controls) on the basis of sex, birth date (±5 years), and MFH diagnosis date (±5 years).
The information extracted from the medical records included date of birth, sex, race, date of last follow-up, date and cause of death, secondary cancers, and history of irradiation. In regard to CLL and NHL, information was extracted involving date of diagnosis (based on histology) and treatment. The MFH information obtained included date and location; grade (all cases diagnosed by a Mayo Clinic pathologist); size; treatment methods; bone, muscle, or skin involvement; location and date of recurrence; location and date of metastases; and method of metastasis diagnosis.
Analysis was performed with the SAS version 9.2 software package (SAS Institute Inc, Cary, NC). Duration of follow-up was calculated from the date of MFH diagnosis to the date of death or last follow-up. Overall survival, survival free of recurrence, and survival free of metastasis were estimated with the Kaplan-Meier method. The association between case/control status and each of these outcomes was summarized with the hazard ratio (HR) and corresponding 95% confidence interval (CI) obtained through fitting separate Cox proportional hazards models. The assumption of proportional hazards was assessed by introducing a time-dependent coefficient in the Cox models. Calculated P values were 2-sided, and P<.05 was considered statistically significant.
Recently, the nomenclature of soft tissue sarcomas has shifted, with the diagnosis of MFH being frequently replaced with undifferentiated pleomorphic sarcoma or high-grade undifferentiated sarcoma.10,16-18 However, because malignant fibrous histiocytoma was the dominant nomenclature used throughout the study period and the most common term in the literature, we use this term herein.
RESULTS
We identified 72 patients who had MFH and lymphoma, 45 of whom had CLL. Fifteen patients fulfilled the study inclusion criteria of CLL or NHL diagnosed before the diagnosis of MFH. Of these 15 patients, 5 (33%) had CLL, and 10 (67%) had NHL. Nine (60%) of these 15 patients were men, and all study patients were white except for 2 whose ethnicity was unknown. The average age at diagnosis of lymphoma was 64 years (SD, 18.1 years; range, 28-88 years); the average age at diagnosis of MFH was 73 years (SD, 13.7 years; range, 36-93 years). Five (33%) of the 15 patients had other secondary cancers, including prostate cancer, NHL, squamous cell carcinoma, and basal cell carcinoma that occurred before MFH diagnosis. Interestingly, 1 (7%) of the 15 patients had 2 separate pathologically confirmed diagnoses of NHL or CLL that had developed before MFH diagnosis; 2 other patients (13%) had a secondary NHL after the diagnosis of MFH.
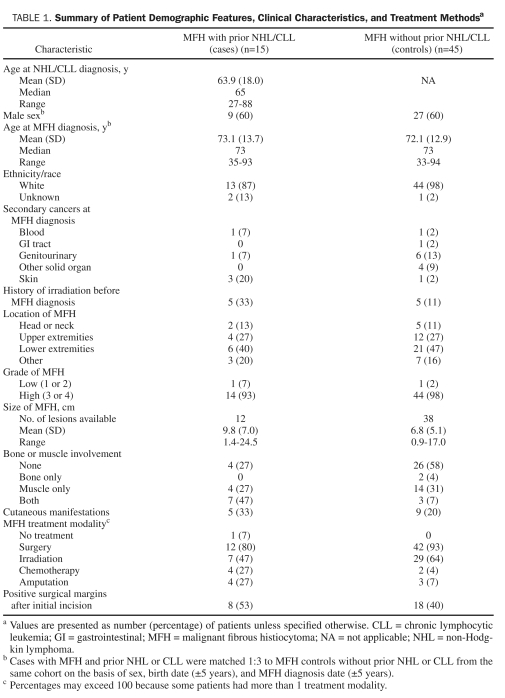
Patient demographics, clinical characteristics, and treatment of the 15 MFH cases with prior NHL or CLL and the 45 matched MFH controls without prior NHL or CLL are summarized in Table 1. All but 2 tumors (1 in each group) were high grade. Five (33%) of the 15 cases had cutaneous manifestations of MFH, such as fungating or ulcerated lesions. However, only 9 (20%) of the controls had cutaneous manifestations.
TABLE 1.
summary of Patient Demographic Features, Clinical Characteristics, and Treatment Methodsa
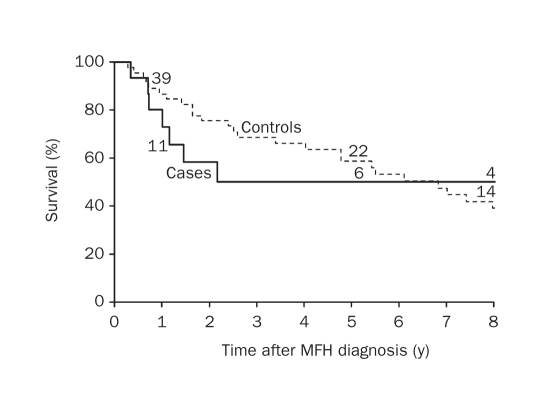
Among the 10 cases with MFH and prior NHL or CLL who were deceased at the time of data abstraction, the median time to death after the MFH diagnosis was 1.3 years (mean, 4.0 years; range, 0.3-14.0 years). Causes of death in this group were unknown (n=5), metastatic MFH (n=3), lymphoma (n=1), and organ failure (n=1). Among the 5 cases alive at last follow-up, the median duration of follow-up was 6.2 years (range, 0.8-10.6 years). On the basis of the Kaplan-Meier method, the overall survival at 1 and 5 years after the MFH diagnosis was 80.0% and 49.9%, respectively (Figure 1).
FIGURE 1.
Overall survival in cases of malignant fibrous histiocytoma (MFh) with prior non-hodgkin lymphoma (Nhl) or chronic lymphocytic leukemia (cll) and in MFh controls without prior Nhl or cll. Numbers above graph lines represent number of patients still at risk.
By comparison, 35 of the 45 matched controls were deceased at the time of the study. The median time to death after the diagnosis of MFH was 4.8 years (mean, 6.1; range, 0.3-21.2 years). Causes of death in this group included unknown (n=12), metastatic or recurrent MFH (n=8), other malignancy (n=5), cardiac or pulmonary (n=6), and infection or organ failure (n=4). Among the 10 controls alive at last follow-up, the median duration of follow-up was 5.0 years (range, 1.4-22.9 years). On the basis of the Kaplan-Meier method, the overall survival at 1 and 5 years after the MFH diagnosis was 86.7% and 58.7%, respectively (Figure 1). Cases were 1.3 times more likely to die than controls; however, this association was not statistically significant (HR, 1.30; 95% CI, 0.64-2.66; P=.47).
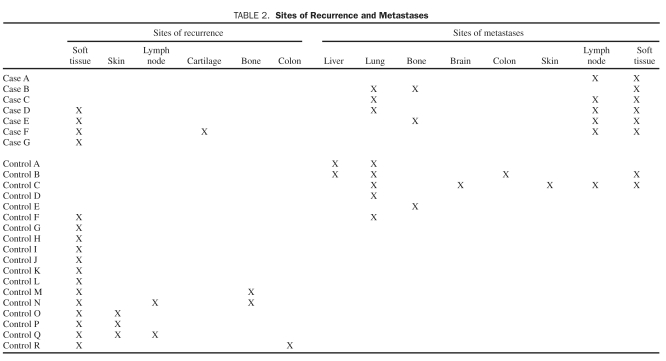
Sites of recurrence and metastasis for the cases and controls are summarized in Table 2. Among the 15 cases, 3 had metastases only, 1 had a local recurrence only, and 3 had both metastases and local recurrence. In 1 of these cases (case C), deep lung and para-aortic lymph node metastasis was confirmed with imaging, but in all other cases, metastasis was confirmed histologically. Among the 45 controls, 5 had metastases only, 12 had a local recurrence only, and 1 had both a metastasis and local recurrence. In the controls, recurrence was confirmed by reexcision and biopsy in instances of superficial lymph node, skin, and soft tissue. Recurrence in the site of the original MFH frequently invaded adjacent tissues and organs. In 1 control (control R) in whom the colon was involved, the original MFH had been found adjacent to the colon in the retroperitoneum and was confirmed histologically; this patient had no other malignancy. In another control (control Q), lymph node recurrence was found histologically, because the patient also had prior breast and uterine malignant neoplasms. With MFH metastases in the control group, deep organ sites were not frequently biopsied, but most of these patients had metastases to other more superficial sites in addition to deep locations that were confirmed histologically, as in controls B and C. For control F, who had a history of prostate cancer, MFH metastases were found in the lung during surgical excision. Control D also had prostate cancer and Paget disease, but lung metastases were not confirmed histologically; this patient died shortly thereafter with metastatic MFH. Control A had liver and lung metastases on imaging; however, this patient had no other malignancy and died within a year because of metastatic MFH. Soft tissue, lung, and lymph nodes were the most common sites of metastases.
TABLE 2.
sites of Recurrence and Metastases
The median time to recurrence after MFH diagnosis was 4.5 and 18 months, respectively, for the cases and controls. The survival free of recurrence at 6 and 12 months was 79.4% and 79.4% for the cases and 97.8% and 90.3% for the controls. Cases were 1.3 times more likely to have a recurrence; however, this association was not statistically significant (HR, 1.28; 95% CI, 0.42-3.95; P=.67).
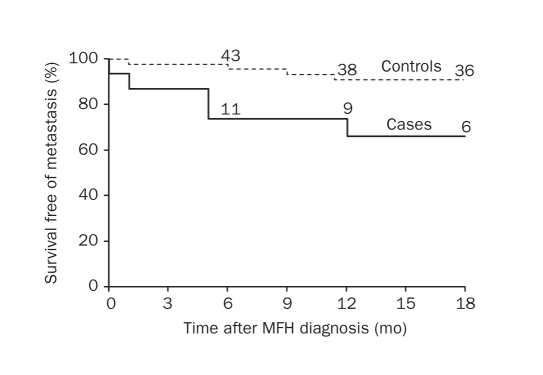
The median time to metastasis after MFH diagnosis was 5 and 10 months, respectively, for the cases and controls. Survival free of metastasis at 6 and 12 months was 73.3% and 65.2% for the cases and 95.5% and 90.7% for the controls. Cases were significantly more likely to have a metastasis (HR, 3.79; 95% CI, 1.22-11.79; P=.02; Figure 2).
FIGURE 2.
survival free of metastasis in cases of malignant fibrous histiocytoma (MFh) with prior non-hodgkin lymphoma (Nhl) or chronic lymphocytic leukemia (cll) and in MFh controls without prior Nhl or cll. Numbers above graph lines represent number of patients still at risk.
DISCUSSION
Our study group represented a similar proportion of males and females (3:2) as in previous studies of MFH and CLL or NHL.4,11 Locations of the tumors seen in our cohort followed national trends: most tumors were located on the extremities, with fewer on the trunk or retroperitoneum and in the head and neck region.8,9,17,19 The median age at diagnosis of CLL or NHL in our study (median, 65 years; mean [SD], 64 [18.0] years; range, 27-88 years) was comparable to the Surveillance Epidemiology and End Results median of 67 years for NHL.20 Also, when looking at the subset of 5 patients with CLL, the median age at time of CLL diagnosis was 79 years, which is higher than the Surveillance Epidemiology and End Results median of 72 years between the years of 2003 and 2007.20 Likewise, the 15 MFH cases with prior CLL or NHL had a median age at MFH diagnosis of 73 years, which is greater than the median age (56-64 years) reported in the literature.6,19 In both MFH and CLL or NHL, incidence increases with age.4,9 A diagnosis of CLL or NHL was a prerequisite in the current study; hence, it is understandable that the median age at MFH diagnosis in these cases is elevated compared with medians in other studies. Furthermore, this also could be a contributing influence in death and outcomes, since prior studies have pointed to increased age being associated with poorer prognosis in patients with MFH.17
A large proportion (n=5 [33%]) of our case group had numerous other malignant neoplasms besides MFH and CLL or NHL. Cases with CLL or NHL who had such an aggressive, rare soft tissue sarcoma as MFH may represent a subgroup of patients who have a more severe immunosuppressed state or a greater genetic predisposition to development of secondary malignancy. The concept of an underlying genetic association to an increased risk of secondary malignancy in patients with lymphoma has been studied, and evidence shows that patients with lymphoma, particularly those with CLL or with 17p, 6q, and 11q deletions or trisomy 12, may be at increased risk of additional malignant neoplasms.21
The impaired immune system of patients with CLL can influence the pathogenesis and outcomes of certain cancers.4,22,23 Patients with CLL have multiple factors that cause immunosuppression, including factors from CLL B cells, a defective antigen-presenting ability, and an anergic T-cell state.4,23,24 Other factors implicated in the development of CLL-related immunosuppression include hypogammaglobulinemia, altered leukemic cell expression of class II major histocompatibility complex antigens, impaired granulocyte function, low levels of complement, and altered expression of T-cell receptor variable region genes.4 In addition, therapy with nucleoside analogues and other chemotherapeutic treatments may exacerbate this already weakened immune response in patients with CLL.15,23
Reported survival for patients with MFH ranges from a 5-year rate of 50% to 70% to a 10-year rate of 38% to 60%.6,17,19 The 5-year survival rate in our cohort of cases was 49.9%. Although the 5-year survival rate is similar to previous reports documenting an overall survival rate of 50%,9 we were unable to estimate a rate at 10 years because only 3 of the 15 cases were still at risk at 10 years. Additionally, when we compared them with controls, we were unable to detect a statistically significant difference in overall survival.
Compared with controls with no prior CLL or NHL, cases were more likely to have recurrence, but recurrence was not statistically significant. However, the risk of metastasis in cases was significantly increased compared with controls, which could indicate more aggressive behavior of MFH in cases with CLL or NHL. Ultimately, 6 cases (40%) had metastasis, and 4 cases (27%) had MFH recurrence. This metastatic rate appears slightly higher than that reported in the literature (range, 25%-34%), whereas the recurrence rate is similar (range, 26%-28%).6,8,9,19 In the literature, lung was the most common site for distant metastases,9 with bone, soft tissue, and lymph nodes next in prominence.11,25
Reports of MFH reflect rare osseous involvement17; however, our cohort of cases with MFH and prior CLL or NHL showed 47% (n=7) with bone involvement at MFH diagnosis compared with 11% (n=5) in the control group. Bone involvement was verified histologically during biopsy or excision of MFH. Of the 7 cases with bone involvement, 3 died of metastatic MFH, and none of the 7 survived 10 years. Five (33%) of the 15 cases presented with cutaneous manifestations, including fungating or ulcerated tumors. Despite the fact that these tumors presented on the skin, some had aggressive deep tissue involvement, with 2 tumors (40%) extending to bone. By comparison, only 20% (n=9) of controls had a cutaneous presentation. Although formal statistical comparisons were not performed on these controls, bone and skin involvement seemed to occur more frequently in the cases and could indicate a unique presentation and behavior of MFH in the clinical setting of CLL and NHL.
The increased risk of sarcomas after irradiation has been reported previously, with MFH being one of the most frequent sarcomas after radiation therapy.5,8,26,27 Our study reiterated this point, with 5 cases (33%) having had a history of irradiation before MFH developed. In these cases, MFH developed in the same region of the radiation field26-28; none survived to 10 years. The increased risk of neoplasia in the treatment context of previous irradiation, in conjunction with the immunosuppression of NHL or CLL, makes patients with the combination of lymphoma and irradiation history potentially at high risk for the subsequent development of aggressive sarcomas, including MFH.
The predominant treatment method for MFH in our cases was surgery (n=12; 80%), with 27% (n=4) undergoing amputation. Those cases who did not undergo surgery had a poor prognosis, and none survived to 10 years. Chemotherapy was also used in 2 cases (27%) as an adjuvant therapy or as a therapy for metastatic disease.17 In 3 cases (75%), chemotherapy was given as an adjuvant with surgery or irradiation, or both, to treat metastatic MFH. For example, one of these cases underwent a chemotherapeutic regimen of carboplatin, mesna, and ifosfamide as a phase 1 experimental trial. Another case was treated with adriamycin, as a dual agent for MFH and high-grade follicular lymphoma. In another case, who was originally misdiagnosed as having a diffuse histiocytic lymphoma, ProMACE-CytaBOM (cyclophosphamide, doxorubicin, etoposide, cytarabine, bleomycin, vincristine, methotrexate, prednisone, and folinic acid) chemotherapy was used as the sole therapy for palliation; this case died 4 months after the diagnosis of metastatic MFH. The most common combined therapy was surgery and adjuvant irradiation; 6 cases (40%) underwent this combination approach.
In the controls, similar trends were found, with surgery (n=42 [93%]) as the most common treatment. However, a smaller proportion of controls had amputation (n=3 [7%]) than in the cases (n=4 [27%]). Also, a smaller group of controls (n=2 [4%]) underwent chemotherapy than in the cases (n=4 [27%]) with both MFH and CLL or NHL; chemotherapeutic regimens used in the control group included mitomycin, adriamycin, cisplatin, and ifosfamide.
In the 15 cases, NHL or CLL treatment included chemotherapy (n=9 [60%]), irradiation (n=6 [40%]), surgical excision of lymph nodes (n=3 [20%]), and observation. In fact, 3 cases (20%) were not treated at all, which reflects the indolent nature of some NHLs and points at the heterogeneity that exists in this group of malignancy. Chemotherapies in this group were varied, especially since treatment spanned many decades; some regimens included chlorambucil, adriamycin, COPA—either alone or with the addition of bleomycin or rituximab—and COP. Interestingly, of the 5 MFH cases in which there was a history of irradiation, 4 had MFH in the very radiation field that previously was used to treat NHL. This finding again shows the complexity of these cases, in which it is difficult to determine whether the NHL itself or the NHL treatments could have an influence on MFH development and behavior.
Because MFH is rare, the number of patients in our study was limited; nonetheless, we were able to see interesting trends when comparing the cases to the controls without NHL or CLL. In such patients with multiple malignant neoplasms, it is important to consider the complexity of factors that influence carcinogenesis, such as history of prior irradiation, chemotherapy, and the possibility of genetic alterations predisposing to multiple malignant neoplasms and worse outcomes. Additional study is required in larger cohorts with MFH and CLL or NHL to further solidify the effect that lymphoma may have on this rare and aggressive soft tissue sarcoma.
CONCLUSION
Although overall survival did not seem to differ between cases with MFH and a prior history of CLL or NHL and MFH controls, the metastatic rate was increased in MFH cases with prior CLL or NHL. In this context of a prior hematopoietic malignancy such as NHL or CLL, MFH may present in atypical ways, such as bone or skin involvement. Therefore, patients with NHL or CLL may necessitate a lower threshold for biopsy, and those with MFH may warrant closer follow-up and a multidisciplinary approach.
Footnotes
An abstract of this article was presented at the 69th Annual Meeting of the American Academy of dermatology, February 4-6, 2010, New Orleans, LA.
Dr brewer is a recipient of the dermatology Foundation career development Award for the study of lymphoma-associated skin cancer.
References
- 1. Hu S, Federman DG, Ma F, Kirsner RS. Skin cancer and non-Hodgkin's lymphoma: examining the link. Dermatol Surg. 2005;31(1):76-82 [DOI] [PubMed] [Google Scholar]
- 2. Montserrat E, Moreno C. Chronic lymphocytic leukaemia: a short overview. Ann Oncol. 2008;19(suppl 7):vii320-vii325 [DOI] [PubMed] [Google Scholar]
- 3. Dighiero G, Hamblin TJ. Chronic lymphocytic leukaemia. Lancet. 2008;371(9617):1017-1029 [DOI] [PubMed] [Google Scholar]
- 4. Kipps TJ. Chronic lymphocytic leukemia. Curr Opin Hematol. 2000;7(4):223-234 [DOI] [PubMed] [Google Scholar]
- 5. Kumar RV, Mukherjee G, Bhargava MK. Malignant fibrous histiocytoma of bone. J Surg Oncol. 1990;44(3):166-170 [DOI] [PubMed] [Google Scholar]
- 6. Rooser B, Willen H, Gustafson P, Alvegard TA, Rydholm A. Malignant fibrous histiocytoma of soft tissue: a population-based epidemiologic and prognostic study of 137 patients. Cancer. 1991;67(2):499-505 [DOI] [PubMed] [Google Scholar]
- 7. Stein A, Hackert I, Sebastian G, Meurer M. Cutaneous malignant fibrous histiocytoma of the scalp in a renal transplant recipient. Br J Dermatol. 2006;154(1):183-185 [DOI] [PubMed] [Google Scholar]
- 8. Shinjo K. Analysis of prognostic factors and chemotherapy of malignant fibrous histiocytoma of soft tissue: a preliminary report. Jpn J Clin Oncol. 1994;24(3):154-159 [PubMed] [Google Scholar]
- 9. Pezzi CM, Rawlings MS, Jr, Esgro JJ, Pollock RE, Romsdahl MM. Prognostic factors in 227 patients with malignant fibrous histiocytoma. Cancer. 1992;69(8):2098-2103 [DOI] [PubMed] [Google Scholar]
- 10. Skubitz KM, D'Adamo DR. Sarcoma. Mayo Clin Proc. 2007;82(11):1409-1432 [DOI] [PubMed] [Google Scholar]
- 11. Weiss SW, Enzinger FM. Malignant fibrous histiocytoma: an analysis of 200 cases. Cancer. 1978;41(6):2250-2266 [DOI] [PubMed] [Google Scholar]
- 12. Mellemgaard A, Geisler CH, Storm HH. Risk of kidney cancer and other second solid malignancies in patients with chronic lymphocytic leukemia. Eur J Haematol. 1994;53(4):218-222 [DOI] [PubMed] [Google Scholar]
- 13. Manusow D, Weinerman BH. Subsequent neoplasia in chronic lymphocytic leukemia. JAMA. 1975;232(3):267-269 [PubMed] [Google Scholar]
- 14. Oddou S, Vey N, Viens P, et al. Second neoplasms following high-dose chemotherapy and autologous stem cell transplantation for malignant lymphomas: a report of six cases in a cohort of 171 patients from a single institution. Leuk Lymphoma. 1998;31(1-2):187-194 [DOI] [PubMed] [Google Scholar]
- 15. Wiernik PH. Second neoplasms in patients with chronic lymphocytic leukemia. Curr Treat Options Oncol. 2004;5(3):215-223 [DOI] [PubMed] [Google Scholar]
- 16. Al-Agha OM, Igbokwe AA. Malignant fibrous histiocytoma: between the past and the present. Arch Pathol Lab Med. 2008;132(6):1030-1035 [DOI] [PubMed] [Google Scholar]
- 17. Nascimento AF, Raut CP. Diagnosis and management of pleomorphic sarcomas (so-called ``MFH'') in adults. J Surg Oncol. 2008;97(4):330-339 [DOI] [PubMed] [Google Scholar]
- 18. Rosenberg AE. Malignant fibrous histiocytoma: past, present, and future. Skeletal Radiol. 2003;32(11):613-618 [DOI] [PubMed] [Google Scholar]
- 19. Zagars GK, Mullen JR, Pollack A. Malignant fibrous histiocytoma: outcome and prognostic factors following conservation surgery and radiotherapy. Int J Radiat Oncol Biol Phys. 1996;34(5):983-994 [DOI] [PubMed] [Google Scholar]
- 20. Altekruse SF, Kosary CL, Krapcho M, et al., eds. SEER Cancer Statistics Review, 1975-2007. Bethesda, MD: National Cancer Institute; 2010. http://seer.cancer.gov/csr/1975_2007/ Accessed April 18, 2011 [Google Scholar]
- 21. Tsimberidou AM, Wen S, McLaughlin P, et al. Other malignancies in chronic lymphocytic leukemia/small lymphocytic lymphoma. J Clin Oncol. 2009;27(6):904-910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Levine PH, Hoover R. The emerging epidemic of non-Hodgkin's lymphoma: current knowledge regarding etiological factors. Cancer Epidemiol Biomarkers Prev. 1992;1(6):515-517 [PubMed] [Google Scholar]
- 23. Molica S. Second neoplasms in chronic lymphocytic leukemia: incidence and pathogenesis with emphasis on the role of different therapies. Leuk Lymphoma. 2005;46(1):49-54 [DOI] [PubMed] [Google Scholar]
- 24. Aslakson CJ, Lee G, Boomer JS, Gilman-Sachs A, Kucuk O, Beaman KD. Expression of regeneration and tolerance factor on B cell chronic lymphocytic leukemias: a possible mechanism for escaping immune surveillance. Am J Hematol. 1999;61(1):46-52 [DOI] [PubMed] [Google Scholar]
- 25. Spanier SS, Enneking WF, Enriquez P. Primary malignant fibrous histiocytoma of bone. Cancer. 1975;36(6):2084-2098 [DOI] [PubMed] [Google Scholar]
- 26. Fangman WL, Cook JL. Postradiation sarcoma: case report and review of the potential complications of therapeutic ionizing radiation. Dermatol Surg. 2005;31(8, pt 1):966-972 [PubMed] [Google Scholar]
- 27. Patel SR. Radiation-induced sarcoma. Curr Treat Options Oncol. 2000;1(3):258-261 [DOI] [PubMed] [Google Scholar]
- 28. Nonaka M, Kadokura M, Ohkubo F, et al. Post radiation inflammatory malignant fibrous histiocytoma arising from the chest wall. Ann Thorac Cardiovasc Surg. 2001;7(6):371-374 [PubMed] [Google Scholar]