Abstract
The perioperative management of patients with inflammatory bowel disease is challenging given the altered immune system that results from a variety of biologic and immunomodulator therapies. Clinicians are often faced with challenges and complicated equations when deciding on the type and dose of medication. To understand the effect of these medications and review the evidence regarding the management of these medications in the perioperative setting, a PubMed-based literature search (January 1, 1960, through April 1, 2011) was conducted using the following search terms: perioperative management, risk, outcome, inflammatory bowel disease, ulcerative colitis, Crohn's disease, aminosalicylates, glucocorticoids, purine analogues, cyclosporine, methotrexate, biologic therapy, infliximab, and thromboembolism. The 414 articles identified were manually sorted to exclude those that did not address perioperative risk, outcomes, and medications in the abstracts, yielding 84 articles for review. Additional references were obtained from the citations within the retrieved articles. This review surveys the findings of the selected articles and presents guidelines and resources for perioperative medication management for patients with inflammatory bowel disease undergoing surgery.
ACTH = adrenocorticotropic hormone; 5-ASA = 5-aminosalicylic acid; CD = Crohn disease; CI = confidence interval; DVT = deep venous thrombosis; GI = gastrointestinal; HPA = hypo thalamic-pituitary-adrenal; IBD = inflammatory bowel disease; LMWH = low-molecular-weight heparin; OR = odds ratio; PE = pulmonary embolism; TNF = tumor necrosis factor; TPC = total proctocolectomy; UC = ulcerative colitis; VTE = venous thromboembolism
The perioperative management of patients with Crohn disease (CD) and ulcerative colitis (UC) requires a multifaceted approach involving both traditional and disease-specific considerations. As is true of any surgical patient, a thorough preoperative evaluation is crucial. Of note, the proportion of older patients among those with inflammatory bowel disease (IBD) is increasing, with a consequent increase in the complexity of comorbid conditions that would require perioperative care. Recent data have shown worse outcomes among elderly patients treated with certain immunosuppressant agents such as anti–tumor necrosis factor (TNF) compared with a younger population.
Substantial morbidity is associated with IBD, especially among malnourished patients, those aged 60 to 80 years, those requiring emergency surgery, those with fistulizing disease, and those treated at low-volume surgical centers.
The perioperative morbidity is associated as well with the nature of the surgical procedure: a total proctocolectomy (TPC) with J pouch may result in higher postoperative infection rates than TPC with an ileostomy; a strictureplasty or stoma revision may have a decreased rate of postoperative cardiopulmonary or infectious complications compared with a complicated resection for fistulizing disease. Laparoscopic surgery also has been consistently associated with decreased postoperative length of stay and complication rate.1,2
One of the factors with the greatest effect on medical and surgical outcomes is the long-term immunosuppressant therapy that makes patients susceptible to infections and poor wound healing.3
The advent of immunomodulators and biological agents poses new challenges for practicing physicians, who are increasingly faced with making clinical recommendations with little evidence to support their decision. This review focuses on recommendations for perioperative management of immunosuppressant and immunomodulator agents in patients with IBD.
TIMING AND TYPE OF SURGERY
Patients undergoing urgent IBD-related surgery are at additional risk of cardiac complications. Various factors limit optimization of preoperative management of cardiovascular risk in such patients. Mangano4 showed that cardiac complications were 2 to 5 times more likely to occur with emergent IBD and non–IBD-related surgical procedures than elective operations. Such increased risk might be expected, given the fact that these patients had chronically debilitating disease conditions.
The perioperative medication management in patients with IBD will vary depending on the clinical situation of the patient (UC vs CD; elective vs emergent surgery).
Article Highlights
Aminosalicylates (sulfasalazine and mesalamine) should be discontinued 1 day before surgery with resumption 3 days after surgery, especially in patients with susceptibility for decreased glomerular filtration
For patients receiving glucocorticoids, the most relevant issue is to ensure adequate stress glucocorticoid supplementation if required
Purine analogues (6-mercaptopurine/azathioprine) should be withheld on the day of surgery and resumed in the first 3 postoperative days when oral medications are resumed, if renal function remains normal
Cyclosporine should be continued in the preoperative and immediate postoperative period, given the existing evidence
Methotrexate should be discontinued 1 week before surgery until at least 1 week after surgery in patients with a history of infectious complications and resumed after successful wound healing
Immunomodulator therapy with anti–tumor necrosis factor agents should be continued in the perioperative setting
In patients with UC, gastrointestinal (GI) surgery, most commonly elective TPC with ileoanal anastomosis, would be the recommended approach. However, emergency surgery can be required, especially in the setting of fulminant colitis with no opportunity for preoperative optimization. Patients with UC who have previously undergone proctocolectomy and require surgery will no longer require immunosuppressant therapy, allowing for more straightforward perioperative medication management. Similarly, for patients with CD, clinical decision making will depend on whether procedures are elective (eg, strictureplasty) or emergent (eg, alleviation of small bowel obstruction).
Perioperative morbidity is also associated with the nature of the surgical procedure: a TPC with J pouch may result in higher rates of postoperative infection than a TPC with an ileostomy; a strictureplasty or stoma revision may have a decreased rate of postoperative cardiopulmonary or infectious complications compared with a complicated resection for fistulizing disease.5 Laparoscopic surgery has been consistently associated with decreased postoperative length of stay and complication rate.1,2 Staged procedures also result in decreased short-term complications.6,7
The perioperative management of medication will not differ between patients with IBD undergoing GI vs non-GI surgeries (eg, orthopedic procedures) because, regardless of the type of procedure, the adequate control of IBD is paramount given its systemic implications.
Most of these patients who undergo surgery understand that it carries a perioperative cardiac risk but have reconciled themselves to the fact that the anticipated morbidity and mortality from not operating overrides that risk. Because most surgical procedures in these patients are intraperitoneal and are traditionally classified into the intermediate-risk category, case-by-case evaluation of risk factors should determine the use of perioperative β-blockers.8-10
Given the morbidity associated with the underlying disease, these patients may require emergency surgery, which poses the additional risk of postoperative complications and blood loss.11
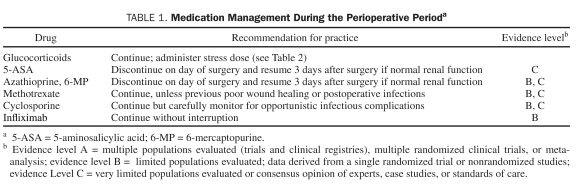
One of the most important aspects of the perioperative care of patients with IBD, the correct and efficient management of medications, is summarized in Table 1 and discussed in detail in the section that follows.
TABLE 1.
Medication Management During the Perioperative Perioda
PERIOPERATIVE MANAGEMENT OF MEDICATIONS USED IN IBD
Aminosalicylates
5-Aminosalicylic acid (5-ASA) agents remain the mainstay of therapy for the induction of remission in mild to moderately active UC12 and for the maintenance of remission in UC and possible CD.13 Sulfasalazine, the prototype aminosalicylate, was developed to deliver both an antibacterial agent (sulfapyridine) and an anti-inflammatory agent (5-ASA, mesalamine, or mesalazine); the 5-ASA component of sulfasalazine is primarily responsible for the therapeutic benefit. It is poorly absorbed in the colon and partially absorbed in the small intestine. Several sulfa-free aminosalicylates have been developed in recent years14 to target specific GI sites based on the assumption that the effects of 5-ASA are topical and not systemic. Sulfasalazine and mesalamine have multiple anti-inflammatory effects, including the inhibition of the arachidonic acid pathway along the cyclooxygenase, lipoxygenase, and platelet aggregation factor systems. These drugs are primarily eliminated by the kidneys. Important adverse effects include hypersensitivity reactions, bone marrow suppression, pneumonitis, pancreatitis, and hemolytic anemia. These compounds have a short half-life (6-10 hours) and are extensively metabolized. There is a paucity of clinical data for perioperative use of these medications.
In patients in whom decreased glomerular filtration is more likely (age, >65 years; American Society of Anesthesiologists physical status score, IV or V; revised cardiac risk index score, >2; chronic heart disease), a reasonable approach in the perioperative phase is to discontinue sulfasalazine and mesalamine a day before surgery with resumption 3 days after surgery.15
Patients undergoing proctocolectomy will not require postoperative resumption of these agents.
Glucocorticoids
Glucocorticoid use is common in IBD. The most relevant approach in the perioperative period is to ensure that adequate stress glucocorticoid supplementation is given. In patients with IBD, especially patients with active Crohn disease, glucocorticoids are highly effective in inducing clinical remission. However, the therapeutic role of glucocorticoids in the treatment of IBD is primarily to decrease the intensity of inflammation because they are ineffective in maintaining remission or healing mucosal lesions. Long-term use of glucocorticoids is associated with dependency as well as clinical relapses. In addition, long-term glucocorticoid use is associated with osteopenia and osteoporosis, glucose intolerance and diabetes mellitus, increased intraocular pressure and glaucoma, and severe infections.16
Adequate glucose control must be established in these patients in the perioperative setting because uncontrolled hyperglycemia is associated with worse outcome, including poor wound healing and increased infections.
The increased likelihood of infectious complications was demonstrated in the TREAT (The Crohn's Therapy, Resource, Evaluation, and Assessment Tool) registry, in which glucocorticoid use was an independent factor associated with serious infections (odds ratio [OR], 2.21; 95% confidence interval [CI], 1.46-3.34; P<.001).17 In a series of 100 patients with IBD who developed opportunistic infections, Toruner et al18 found that glucocorticoid use was significantly associated with the development of opportunistic infections (OR, 3.4; 95% CI, 1.8-6.2). In this series, in multivariate analysis, the risk for opportunistic infection increased substantially with the use of a single immunosuppressant (OR, 2.9; 95% CI, 1.5-5.3) vs a combination of 2 or 3 immunosuppressants (OR, 14.5; 95% CI, 4.9-43.0).
In an epidemiologic study in Olmsted County, Minnesota, glucocorticoid dependence at 1 year was found in 28% of patients with CD and 22% of patients with UC.19
The strategy to minimize adverse effects related to glucocorticoids in patients with IBD is to use the lowest effective dose to induce remission in patients with moderately to severely active CD and acute severe colitis,16,20 along with the early use of other immunosuppressant glucocorticoid-sparing agents and biologic therapy.
In a 1952 article, Fraser et al21 first reported iatrogenic adrenal insufficiency due to preoperative glucocorticoid withdrawal in a surgical patient. The publication of similar findings in a case report the following year led to recommendations for high-dose or “stress-dose” glucocorticoids in the perioperative period.22 Since then, the overall practice has evolved, and currently the doses of glucocorticoid replacement are lower than initially espoused because of concerns about the adverse effects of glucocorticoids, including impaired wound healing, elevated risk of infections, GI bleeding, and hyperglycemia.23 The entire practice of “stress-dose” glucocorticoid replacement, even in its current form, has been questioned by some investigators.22-25 In a recent study, Bruewer et al26 found that patients receiving high-dose and prolonged preoperative systemic glucocorticoid therapy who underwent bowel resection for CD experienced no more postoperative complications than did control patients.
In a series from the University of Tokyo, which analyzed data on all patients with UC from 1963 to 1994, those receiving high-dose glucocorticoids were more likely to undergo colectomy because they were more likely to have a refractory disease and to experience postoperative complications.27
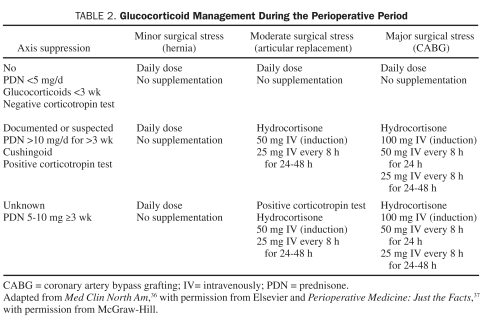
Surgical stress is a potent stimulant of the hypothalamic-pituitary-adrenal (HPA) axis. Stress acts by stimulating the release of corticotropin-releasing hormone and arginine vasopressin that in turn causes adrenocorticotropic hormone (ACTH) release. In surgical patients, the highest ACTH levels are noted during the immediate postoperative recovery period and are likely triggered by trauma and pain. Elevated levels of ACTH are also seen during extubation and reversal of anesthesia and at the time of surgical incision.27-29 Epidural or local anesthesia does not appear to stimulate the HPA axis.30 In addition, plasma ACTH response during surgery is attenuated by opiate drugs.28 Substantial variation exists in the degree of endogenous glucocorticoid release in response to surgical stress. Important factors that may influence this variation include concomitant medication use, antecedent illnesses, and age. In general, patients receiving 5 mg or less of prednisone each day, alternate-day glucocorticoids, or any dose of glucocorticoids for less than 3 weeks are not considered to have a suppressed HPA axis and do not require “stress-dose” glucocorticoids.31-34 In contrast, patients receiving less than 20 mg/d of prednisone (or its equivalent) for more than 3 weeks or with features of Cushing syndrome should be assumed to have a suppressed HPA axis and considered for “stress-dose” glucocorticoid supplementation perioperatively. For patients receiving between 5 and 10 mg of prednisone or its equivalent for more than 3 weeks, a clinical prediction rule cannot be implemented because, in this subset of patients, the HPA axis may or may not be suppressed. Generally speaking, rather than subjecting these patients to a corticotropin stimulation test, which may not reliably predict HPA axis suppression, it may be prudent to provide glucocorticoid supplementation in these patients. However, the administration of glucocorticoids in any patient who has been receiving prednisone doses of more than 5 mg/d for more than 1 week in the 6 to 12 months before surgery appears unnecessary.
The physician performing the preoperative assessment should bear in mind that patients receiving long-term high-dose inhaled or topical glucocorticoids for various conditions may have a suppressed HPA axis and may be candidates for “stress-dose” glucocorticoids. Although a healthy person is estimated to secrete between 20 and 30 mg of cortisol a day,35 the requirement for patients undergoing a surgical procedure, while varying according to the degree of expected stress, rarely exceeds 200 mg of cortisol secretion in 24 hours.22 Currently, expert consensus favors optimizing the glucocorticoid replacement dose to the magnitude of stress posed by the surgery.
Patients receiving glucocorticoid therapy should receive their daily requirement throughout the perioperative period, along with supplementation as outlined in Table 2.36,37
TABLE 2.
Glucocorticoid Management During the Perioperative Period
Purine Analogues (6-mercaptopurine/Azathioprine)
These agents have been widely used as glucocorticoid-sparing agents for maintenance of remission. Both agents are oxidized or methylated in erythrocytes or the liver. The perioperative use of immunomodulators such as purine analogues does not affect surgical outcomes or morbidity. Early complications after proctocolectomy with ileal pouch-anal anastomosis were not found in patients using azathioprine/6-mercaptopurine but were observed in patients receiving high-dose glucocorticoids in a study by Mahadevan et al.38 However, potential concerns related to purine analogues are pancreatitis, leukopenia, hepatitis, and bone marrow suppression.
Azathioprine is known to be antagonistic to neuromuscular blocking agents. Dretchen et al39 described a reversal of neuromuscular blockage produced by d-tubocurarine but an increase of the neuromuscular blockade produced by succinylcholine. The effects of azathioprine on neuromuscular transmission are considered to be secondary to inhibition of phosphodiesterase in the motor nerve terminal. Gramstad40 suggested that the initial dose of neuromuscular blocking drugs in renal transplant patients should be increased in the presence of azathioprine (atracurium by 37%, vecuronium by 20%, and pancuronium by 45%). In this study, the atracurium dose was unaffected by renal function, whereas dose requirements for vecuronium and pancuronium were reduced by 23.2% and 61.5%, respectively, compared with patients not taking azathioprine. These findings suggest a transient antagonism of neuromuscular blockade in the presence of azathioprine.
Aberra et al41 performed a retrospective cohort study in 159 patients with IBD who underwent elective bowel surgery, including 56 patients receiving glucocorticoids alone, 52 receiving 6-mercaptopurine/azathioprine with or without glucocorticoids, and 51 patients receiving neither glucocorticoids nor 6-mercaptopurine/azathioprine. The rate of postoperative infectious complications was not found to be significantly higher in the group receiving glucocorticoids or a combination of glucocorticoids and purine analogues. In an animal study conducted by Brokowski et al,42 the regeneration of a ureter and renal pelvis from a transected and anastomosed strip of the ureteral wall was not associated with any adverse effect on wound healing in dogs receiving prednisone and azathioprine.
In a study by Colombel et al,43 the rate of postoperative complications was not increased in 207 patients with CD who underwent intra-abdominal surgery while receiving glucocorticoids or immunosuppressive therapy with azathioprine, 6-mercaptopurine, methotrexate, or infliximab.
Myrelid et al44 reported an 8% risk of postoperative intra-abdominal septic complications in 343 consecutive patients undergoing surgery for CD. Overall, thiopurine therapy was associated with an increased risk of intra-abdominal septic complications (16% vs 6% without therapy; P=.044). The use of thiopurines was associated with a 24% risk of septic complications in patients who had known risk factors (both preoperative intra-abdominal sepsis and use of colo-colonic anastomosis), 13% in patients with only 1 of these risk factors, and only 4% in patients with none of these risk factors (P<.001).
A potential association between the use of thiopurines and myelotoxicity has been reported.45 It has been recommended, on the basis of weak evidence and physiologic considerations, that thiopurines be stopped on the day of surgery and resumed 3 days afterward because of their renal elimination and potential for toxic metabolite accumulation.46 However, the incidence rate of severe myelotoxicity is less than 1% per patient and year of treatment, and the mortality risk is less than 0.1%47; therefore, although a theoretical risk for perioperative myelotoxicity exists, it is almost negligible. Therefore, our recommendation is to withhold thiopurines on the day of surgery and, if renal function remains normal, to resume within the first 3 postoperative days when oral medications are resumed.
Cyclosporine
This potent immunosuppressive agent has been used in patients with glucocorticoid-refractory UC as rescue therapy before colectomy and occasionally in patients with CD. Cyclosporine, a potent inhibitor of T cells that is metabolized in the liver by CYP3A, is primarily excreted in bile; however, 6% of the drug is eliminated unchanged in urine. Major adverse effects include nephrotoxicity, seizures, and opportunistic infections. The mortality rate with opportunistic infections can be as high as 3.5%48; hence, patients receiving cyclosporine and glucocorticoid therapy should be carefully monitored for any signs of infection. These patients should also receive due consideration for Pneumocystis jiroveci prophylaxis with trimethoprim-sulfamethoxazole.
Preoperative cyclosporine has not been shown to have any detrimental effects during or after surgery. In a small case series of 25 patients, Pinna-Pintor et al49 found no increased postoperative complications in patients treated with intravenous or oral cyclosporine. These findings were consistent with previous studies.50,51
We recommend careful observation of patients receiving cyclosporine for deterioration in renal function and opportunistic infections. At the same time, cyclosporine levels ought to be carefully monitored. Current clinical data are inadequate to support the discontinuation of this drug before and immediately after surgery.
Methotrexate
Methotrexate competitively inhibits the enzyme dihydrofolate reductase, impairing DNA synthesis and therefore cellular replication. Evidence to support its use in UC is minimal.52 Methotrexate is excreted by the kidneys, and patients with renal impairment require dose adjustment. Major adverse effects include thrombocytopenia (up to 10%), pneumonitis, and hepatotoxicity. Perioperative considerations include, but are not limited to, an increase in infectious complications; in the setting of renal impairment, a toxic buildup of its metabolites can lead to bone marrow suppression.53,54 In a 1997 study of a population of patients undergoing elective orthopedic surgery for rheumatoid arthritis, Bridges and Moreland55 found increased perioperative complications in a small number of patients. Multiple subsequent studies in patients undergoing orthopedic surgery have suggested an increased risk of postoperative complications, consisting mainly of infections.56-58 Grennan et al59 published a retrospective study of 388 patients with rheumatoid arthritis who underwent surgery while receiving methotrexate and concluded that continuation of methotrexate does not increase the risk of either infections or surgical complications in patients within one year of elective orthopedic surgery. Most studies have not specifically addressed the effect of methotrexate in the perioperative period on renal function.
Concern has been raised about the potential interaction between nitrous oxide used for anesthesia and methotrexate. A substantial interaction between nitrous oxide–based anesthesia and methotrexate in cancer patients undergoing surgery has been demonstrated by in vivo studies. According to these studies, patients who receive methotrexate during the immediate postoperative period (within 6 hours) after nitrous oxide–based anesthesia often develop severe bone marrow depression and mucositis.60 However, no quantitative data for dose-effect interactions are available regarding the combined toxic effects of methotrexate and nitrous oxide.
Most published trials have included patients receiving low dosages of methotrexate (5-10 mg weekly). Only a paucity of data supports the currently recommended dosages of 15 mg weekly with escalation to 20 to 30 mg weekly depending on clinical response and tolerability.61,62
Existing data do not suggest a significant increase in the risk of perioperative infections or impaired wound healing. Given the lack of data, in patients with a history of previous or severe septic complications, it may be reasonable to discontinue methotrexate 1 week before surgery and resume it no sooner than 1 week after or when the wound has successfully healed. The risks vs the benefits of discontinuation should be discussed with the patient, and the potential for flare of the disease should be weighed against the potential of poor wound healing or infectious complications.63
Biologic Therapy
Treatment for IBD has entered an era of biologic response modifiers. One such agent, infliximab, a chimeric monoclonal antibody targeting TNF-α, is the first drug approved by the US Food and Drug Administration for the treatment of CD. Its role in the treatment of refractory CD is well established,64,65 and current data support its use in patients with moderately to severely active UC who have had an inadequate response to conventional therapy.66 Infliximab has a long half-life of 8.0 to 9.5 days and has its own unique adverse effect profile. Several adverse effects have been reported for TNF-α–blocking agents, including reactivation of tuberculosis; an increased risk of sepsis, pneumonia, and fatal and opportunistic infections (eg, invasive fungal infections, listeriosis, Pneumocystis infections); reactivation of chronic hepatitis B in carriers; worsened chronic heart failure; optic neuritis; demyelination reactions; bone-marrow toxicity infusion reactions; acute and delayed hypersensitivity reactions; and formation of anti–double-stranded DNA.67-70
Despite its potent immunosuppressive effects, preoperative use of infliximab does not seem to increase postoperative complications in patients with UC or CD. In a cohort of 314 patients with CD, 40 of whom received 1 or more infusions of infliximab before intestinal resection, Marchal et al71 found no increase in postoperative infections or prolongation of hospital stay after infliximab infusion. In a study by Jarnerot et al72 using infliximab as rescue therapy in patients with moderately severe UC, 7 patients who received infliximab underwent colectomy without any increase in the postoperative complication rate. On the basis of their retrospective study of 277 patients with CD who received infliximab within 8 weeks of surgery and 4 weeks after surgery in addition to other immunomodulators, Colombel et al43 concluded that infliximab is safe in the perioperative setting. A 30-day postoperative follow-up showed no increase in septic and nonseptic complications.
In a cohort study by Kunitake et al73 of 413 patients with IBD who underwent abdominal surgery, the rate of postoperative complications was similar in the 100 patients who had received infliximab 12 weeks or less before surgery vs those who had not.
Bordeianou et al74 compared 44 patients with UC and symptoms of unremitting disease who were taking infliximab before TPC or a subtotal colectomy with 127 patients who were not using infliximab. The outcomes in both groups were similar: rate of emergent surgery (4.5% vs 0.4%; P=.98), rate of subtotal colectomy (19.2% vs 18.0%; P=.99), or rate of ileoanal J pouch reconstruction (53.8% vs 62.0%; P=.98). The authors concluded that infliximab contributed no increased surgical morbidity in patients with UC.
Kraemer et al75 found that 16 of 19 patients who received 5 mg/kg of infliximab perioperatively during scheduled anal reconstructive surgery for complicated fistulizing anal CD had a favorable outcome, findings similar to those of other studies in the same population. In the TREAT registry study evaluating 6290 patients, Lichtenstein et al76 found that infliximab was not independently associated with increased risk after adjustment for corticosteroid use and disease severity; however, both corticosteroids and disease severity were associated with adverse outcomes. In a recent study by Gainsbury et al,77 infliximab was not associated with increased risk of short-term postoperative complications after proctocolectomy and ileoanal anastomosis.
A retrospective study of 389 patients with CD who underwent ileocolonic resection at the Cleveland Clinic, 60 of whom received infliximab within 3 months before surgery, found an increased rate of postoperative sepsis, abscess, and readmissions in the patients who received infliximab; the authors of this study suggested that these complications might have been prevented by a diverting stoma.78 Of these patients, those using infliximab had an increased rate of early complications (OR, 3.54; 95% CI, 1.51-8.31; P=.004) or sepsis (OR, 13.8; 95% CI, 1.8-105.0; P=.011) and an increased need for a 3-stage procedure (OR, 2.07; 95% CI, 1.18-3.63; P=.011), leading Mor et al79 to conclude that infliximab use has changed the surgical approach to UC by increasing the number of operations. However, in response to an invited editorial comment, they acknowledged that 3-stage procedures are safe in these patients.
In a multivariate analysis of 301 patients with UC who underwent ileal pouch anal anastomosis, 47 of whom received infliximab preoperatively, Selvasekar et al80 reported that infliximab was the only factor independently associated with infectious complications in this group of patients.
Toruner et al18 described a substantial increase in the risk for opportunistic infection in patients taking a combination of 2 or 3 immunosuppressants (OR, 14.5; 95% CI, 4.9-43) vs those taking a single immunosuppressant (OR, 2.9; 95% CI, 1.5-5.3).
A recent study by Cottone et al81 demonstrated a higher risk of severe infections (11%), neoplasms (3%), and mortality (10%) in patients older than 65 years who received TNF inhibitor therapy than in younger patients or in patients of the same age who did not receive such treatment.
Although new anti-TNFα drugs (eg, adalimumab and certolizumab pegol) are available for the treatment of CD, no data regarding their use in the perioperative setting have been reported. The safety profile of these medications appears to be similar to that of infliximab, especially for fistulizing CD.82-88
Another recently introduced agent that has been approved for the management of moderate to severe CD is natalizumab, a humanized monoclonal antibody against the α4 integrin subunit that inhibits leukocyte adhesion and migration to areas of inflammation. However, safety concerns regarding its association with progressive multifocal leukoencephalopathy have limited its use. Data regarding its use in the perioperative setting are unavailable.
Most evidence suggests that infliximab can be used safely in the perioperative period. Divergent data may reflect the higher burden of comorbidity (concomitant immunosuppressant use, increased severity of disease) in patients with adverse outcomes.
Practices being increasingly recommended to improve outcome include staged surgeries with temporary diverting stomas and the selection of 3-rather than 2-stage ileal pouch anal anastomosis.7,89
In a recent review, Beddy et al5 questioned whether it was justifiable to delay surgery in patients who have recently been administered infliximab or to create a proximal diverting stoma purely to deliver biologic medications. Currently, we do not recommend the discontinuation of immunomodulator therapy with anti-TNF agents in the perioperative setting. However, the clinician should be aware of all possible complications, including serious infections, in surgical patients receiving these agents.
Perioperative medication and Thromboembolic Events in IBD
Patients with IBD have long been known to be at increased risk of thromboembolism. In 1936, Bargen and Barker90 at Mayo Clinic reported that 18 of 1500 patients with UC had evidence of extensive arterial and venous thrombosis. Since then, the association of these complications with IBD has been increasingly recognized. Thromboembolic complications in the cerebral and retinal vasculature,91 the portal vein, and peripheral arteries92 have been reported. In a case series of IBD-related thromboembolism from Mayo Clinic, the activity of disease and extent of colonic involvement in patients with UC were found to be associated with increased risk; however, 87% of patients in this study had another risk factor for venous thrombosis, such as hospitalization, immobilization, malignancy, or recent surgery.93 These risk factors are common in patients who are undergoing surgery, and aggressive antivenous thrombosis prophylaxis should be considered in these patients. However, no guidelines for venous thrombosis prophylaxis specifically in IBD patients have been published. In a study by O'Connor et al,94 the event rate of clinical thrombosis after major abdominal and pelvic surgery for patients with UC was noted to be similar to that in patients without UC undergoing similar surgery. The authors of that study concluded that standard prophylaxis in a patient with UC is acceptable to reduce the occurrence of thrombotic events in the perioperative period.
Inherited risk factors for thrombosis, such as the factor V Leiden mutation, the G20210A mutation in the prothrombin gene, and the homozygous C677T mutation in the methylenetetrahydrofolate reductase gene, have not been attributed to increased thrombosis in patients with IBD.95 However, a thorough investigation of the coagulation profile and genetic testing is advisable in younger IBD patients with a first idiopathic thrombotic event.96
Hyperhomocysteinemia, which is considered a risk factor for arterial as well as venous thrombosis, has been found to be more prevalent in patients with IBD97 but has not been found to be a major contributory factor in the development of venous or arterial thrombosis in patients with IBD.98
Using a large outpatient database (Manitoba Health database), Bernstein et al99 demonstrated a 3-fold increased risk of developing deep venous thrombosis (DVT) or pulmonary embolism (PE) in patients with IBD, incidence rates of 31.4 per 10,000 person-years for DVT and 10.3 per 10,000 person-years for PE in patients with CD, and incidence rates of 30.0 per 10,000 person-years for DVT and 19.8 per 10,000 person-years for PE in patients with UC.99
In a more recent study among 13,756 patients with IBD and 71,672 matched controls, Grainge et al100 found that 139 patients and 165 controls developed venous thromboembolism (VTE); patients with IBD had a higher risk of VTE compared with controls (hazard ratio, 3.4; 95% CI, 2.7-4.3; P<.001; absolute risk, 2.6 per 1000 person-years).
In the inpatient setting, Nguyen and Sam101 evaluated a large national database of 522,704 discharges of non-IBD patients compared with 73,197 discharges of patients with CD and 43,645 discharges of patients with UC; the risk of VTE was 1.5- to 1.8-fold higher among patients with vs without IBD (P<.001). In patients with IBD, VTE risk was higher among patients with UC or fistulizing CD.101
The Joint Commission National Quality Core measures specifications include the appropriate use in patients undergoing general surgery of pharmacological VTE prophylaxis, including unfractionated heparin, low-molecular-weight heparin (LMWH), factor Xa inhibitor (fondaparinux), and any pharmacological choice combined with a physical measure (stockings or intermittent pneumatic compression devices). Nonpharmacological prophylaxis is used in patients with a high risk of bleeding.102
No studies have specifically evaluated the potential benefit of VTE prophylaxis in hospitalized or ambulatory patients with IBD. Studies to date do not support an increased bleeding risk with moderate doses of anticoagulant medications in patients with active IBD.103
During the perioperative period, we recommend that patients with IBD receive prophylaxis based on American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition).104 However, a recent study by Scarpa et al105 demonstrated that standard prophylactic LMWH may be insufficient for VTE prophylaxis in patients with IBD. In a series of patients undergoing major colorectal surgery from 1999 until 2006, they demonstrated an elevated risk of postoperative DVT in patients with UC colitis (OR, 7.4; 95% CI, 1.4-44.4; P=.017) despite prophylactic anticoagulation with 4000 IU/d of LMWH. The rate of DVT in patients with UC was higher than in patients with colorectal cancer (P=.009).
For patients undergoing open surgery, we recommend prophylaxis with 5000 U of subcutaneous heparin 3 times daily, 40 mg of subcutaneous enoxaparin once daily, or 2.5 to 5.0 mg of subcutaneous fondaparinux daily.
CONCLUSION
Perioperative physicians play a critical role in controlling and standardizing the management of surgical patients receiving immunomodulator or immunosuppressant therapy. However, sound research in this field has been limited, especially in the subgroup of patients with IBD.
Over the years, the management of patients receiving glucocorticoids perioperatively has become fairly standardized. Guidelines for the management of patients receiving methotrexate have been based largely on findings from orthopedic studies. Although the evidence on which these recommendations are based is admittedly less than robust, we recommend a conservative approach in patients older than 65 years and in patients with renal impairment.
Azathioprine and 6-mercaptopurine use should be carefully monitored in patients at risk for toxicity. Cyclosporine use poses a substantial risk of postoperative opportunistic infections. Patients receiving cyclosporine therapy tend to be much sicker and prone to an increased rate of opportunistic infections.
With the exciting advent of biologic agents have come new challenges. The experience in the perioperative use of most of these agents is certainly not sufficient to make any accurate predictions regarding their efficacy and safety; however, the current level of evidence, although limited, has been reassuring for the use of infliximab in the perioperative period.
Supplementary Material
References
- 1. Ananthakrishnan AN, McGinley EL, Saeian K, Binion DG. Laparoscopic resection for inflammatory bowel disease: outcomes from a nationwide sample. J Gastrointest Surg. 2010;14:58-65 [DOI] [PubMed] [Google Scholar]
- 2. Kaplan GG, Mccarthy EP, Ayanian JZ, Korzenik J, Hodin R, Sands BE. Impact of hospital volume on postoperative morbidity and mortality following a colectomy for ulcerative colitis. Gastroenterology. 2008;134(3):680-687 [DOI] [PubMed] [Google Scholar]
- 3. Sholter DE, Armstrong PW. Adverse effects of corticosteroids on the cardiovascular system. Can J Cardiol. 2000;16(4):505-511 [PubMed] [Google Scholar]
- 4. Mangano DT. Preoperative assessment of the patient with cardiac disease. Curr Opin Cardiol. 1995;10(5):530-542 [DOI] [PubMed] [Google Scholar]
- 5. Beddy D, Dozois EJ, Pemberton JH. Perioperative complications in inflammatory bowel disease [published online ahead of print October 25, 2010]. Inflamm Bowel Dis. Doi: 10.1002/ibd.21504 [DOI] [PubMed]
- 6. Pandey S, Luther G, Umanskiy K, et al. Minimally invasive pouch surgery for ulcerative colitis: is there a benefit in staging? Dis Colon Rectum. 2011;54(3):306-310 [DOI] [PubMed] [Google Scholar]
- 7. Holubar SD, Cima RR, Pemberton JH. Does infliximab increase complications after surgery for inflammatory bowel disease? F1000 Med Rep. 2009;1:10 doi: 10.3410/M1-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lindenauer PK, Pekow P, Wang K, et al. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005; 353(4):349-361 [DOI] [PubMed] [Google Scholar]
- 9. Wallace A, Layug B, Tateo I, et al. ; McSPI Research Group Prophylactic atenolol reduces postoperative myocardial ischemia. Anesthesiology. 1998;88(1):7-17 [DOI] [PubMed] [Google Scholar]
- 10. Mangano DT, Layug EL, Wallace A, Tateo I; Multicenter Study of Perioperative Ischemia Research Group Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1996; 335(23):1713-1720 [DOI] [PubMed] [Google Scholar]
- 11. Indar AA, Young-Fadok TM, Heppell J, Efron JE. Effect of perioperative immunosuppressive medication on early outcome in Crohn's disease patients. World J Surg. 2009;33(5):1049-1052 [DOI] [PubMed] [Google Scholar]
- 12. Kornbluth A, Sachar DB. Ulcerative Colitis Practice Guidelines in Adults: American College of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105(3):501-523 [DOI] [PubMed] [Google Scholar]
- 13. Lichtenstein GR, Hanauer SB, Sandborn WJ. Management of Crohn's disease in adults. Am J Gastroenterol. 2009;104:465-483 [DOI] [PubMed] [Google Scholar]
- 14. Wikberg M, Ulmius J, Ragnarsson G. Review article: targeted drug delivery in treatment of intestinal diseases. Aliment Pharmacol Ther. 1997;11(suppl 3):109-115 [DOI] [PubMed] [Google Scholar]
- 15. Borthwick E, Ferguson A. Perioperative acute kidney injury: risk factors, recognition, management, and outcomes. BMJ. 2010;341:c3365 doi: 10.1136/bmj.c3365 [DOI] [PubMed] [Google Scholar]
- 16. Rutgeerts PJ. Review article: the limitations of corticosteroid therapy in Crohn's disease. Aliment Pharmacol Ther. 2001;15(10):1515-1525 [DOI] [PubMed] [Google Scholar]
- 17. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621-630 [DOI] [PubMed] [Google Scholar]
- 18. Toruner M, Loftus EV, Jr, Harmsen WS, et al. Risk factors for opportunistic infections in patients with inflammatory bowel disease. Gastroenterology. 2008;134(4):929-936 [DOI] [PubMed] [Google Scholar]
- 19. Faubion WA, Jr, Loftus EV, Jr, Harmsen WS, et al. The natural history of corticosteroid therapy for inflammatory bowel disease: a population-based study. Gastroenterology. 2001;121(2):255-260 [DOI] [PubMed] [Google Scholar]
- 20. Van Assche G, Vermeire S, Rutgeerts P. Management of acute severe ulcerative colitis. Gut. 2011;60(1):130-133 [DOI] [PubMed] [Google Scholar]
- 21. Fraser CG, Preuss FS, Bigford WD. Adrenal atrophy and irreversible shock associated with cortisone therapy. J Am Med Assoc. 1952;149(17): 1542-1543 [DOI] [PubMed] [Google Scholar]
- 22. Salem M, Tainsh RE, Jr, Bromberg J, et al. Perioperative glucocorticoid coverage: a reassessment 42 years after emergence of a problem. Ann Surg. 1994;219(4):416-425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stuck AE, Minder CE, Frey FJ. Risk of infectious complications in patients taking glucocorticosteroids. Rev Infect Dis. 1989;11(6):954-963 [DOI] [PubMed] [Google Scholar]
- 24. Bromberg JS, Alfrey EJ, Barker CF, et al. Adrenal suppression and steroid supplementation in renal transplant recipients. Transplantation. 1991; 51(2):385-390 [DOI] [PubMed] [Google Scholar]
- 25. Glowniak JV, Loriaux DL. A double-blind study of perioperative steroid requirements in secondary adrenal insufficiency. Surgery. 1997;121(2):123-129 [DOI] [PubMed] [Google Scholar]
- 26. Bruewer M, Utech M, Rijcken EJ, et al. Preoperative steroid administration: effect on morbidity among patients undergoing intestinal bowel resection for Crohns disease. World J Surg. 2003;27(12):1306-1310 [DOI] [PubMed] [Google Scholar]
- 27. Shinozaki M, Suzuki K, Sawada T, et al. Steroid complications and surgery in intractable ulcerative colitis. J Gastroenterol. 1998;33(2):196-200 [DOI] [PubMed] [Google Scholar]
- 28. Raff H, Norton AJ, Flemma RJ, Findling JW. Inhibition of the adrenocorticotropin response to surgery in humans: interaction between dexamethasone and fentanyl. J Clin Endocrinol Metab. 1987;65(2):295-298 [DOI] [PubMed] [Google Scholar]
- 29. Udelsman R, Norton JA, Jelenich SE, et al. Responses of the hypothalamic-pituitary-adrenal and renin-angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J Clin Endocrinol Metab. 1987;64(5):986-994 [DOI] [PubMed] [Google Scholar]
- 30. Brandt M, Kehlet H, Binder C, et al. Effect of epidural analgesia on the glycoregulatory endocrine response to surgery. Clin Endocrinol (Oxf). 1976; 5(2):107-114 [DOI] [PubMed] [Google Scholar]
- 31. Ackerman GL, Nolsn CM. Adrenocortical responsiveness after alternate-day corticosteroid therapy. N Engl J Med. 1968;278(8):405-409 [DOI] [PubMed] [Google Scholar]
- 32. Fauci AS. Alternate-day corticosteroid therapy. Am J Med. 1978;64(5): 729-731 [DOI] [PubMed] [Google Scholar]
- 33. Harter JG, Reddy WJ, Thorn GW. Studies on an intermittent corticosteroid dosage regimen. N Engl J Med. 1963;269:591-596 [DOI] [PubMed] [Google Scholar]
- 34. Shaw M. When is perioperative `steroid coverage' necessary? Cleve Clin J Med. 2002;69(1):9-11 [DOI] [PubMed] [Google Scholar]
- 35. Coursin DB, Wood KE. Corticosteroid supplementation for adrenal insufficiency. JAMA. 2002;287(2):236-240 [DOI] [PubMed] [Google Scholar]
- 36. Schiff RL, Welsh GA. Perioperative evaluation and management of the patient with endocrine dysfunction. Med Clin North Am. 2003;87(1):175-192 [DOI] [PubMed] [Google Scholar]
- 37. Seyman GB, Halle DA. Adrenal insufficiency. In: Cohn SL, Smetana GW, Weed HG, eds. Perioperative Medicine: Just the Facts. Chapter 28. New York, NY: McGraw Hill; 2006:158-163 [Google Scholar]
- 38. Mahadevan U, Loftus EV, Jr, Tremaine WJ, et al. Azathioprine or 6-mercaptopurine before colectomy for ulcerative colitis is not associated with increased postoperative complications. Inflamm Bowel Dis. 2002;8(5):311-316 [DOI] [PubMed] [Google Scholar]
- 39. Dretchen KL, Morgenroth VH, III, Standaert FG, Walts LF. Azathioprine: effects on neuromuscular transmission. Anesthesiology. 1976;45(6):604-609 [PubMed] [Google Scholar]
- 40. Gramstad L. Atracurium, vecuronium and pancuronium in end-stage renal failure: dose-response properties and interactions with azathioprine. Br J Anaesth. 1987;59(8):995-1003 [DOI] [PubMed] [Google Scholar]
- 41. Aberra FN, Lewis JD, Hass D, et al. Corticosteroids and immunomodulators: postoperative infectious complication risk in inflammatory bowel disease patients. Gastroenterology. 2003;125(2):320-327 [DOI] [PubMed] [Google Scholar]
- 42. Brokowski A, Piechna K, Wyhowski J, Plata Z. Regeneration of the ureter and the renal pelvis in dogs from transversely transected and anastomosed strips of the ureteral wall: influence of long administration of azathioprine and prednisone on the newly formed wall; I: experimental. Eur Urol. 1979;5(6):352-358 [DOI] [PubMed] [Google Scholar]
- 43. Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. Early postoperative complications are not increased in patients with Crohn's disease treated perioperatively with infliximab or immunosuppressive therapy. Am J Gastroenterol. 2004;99(5):878-883 [DOI] [PubMed] [Google Scholar]
- 44. Myrelid P, Olaison G, Sjödahl R, Nyström PO, Almer S, Andersson P. Thiopurine therapy is associated with postoperative intra-abdominal septic complications in abdominal surgery for Crohn's disease. Dis Colon Rectum. 2009;52(8):1387-1394 [DOI] [PubMed] [Google Scholar]
- 45. Connell WR, Kamm MA, Ritchie JK, Lennard-Jones JE. Bone marrow toxicity caused by azathioprine in inflammatory bowel disease: 27 years of experience. Gut. 1993;34(8):1081-1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rosandich PA, Kelley JT, III, Conn DL. Perioperative management of patients with rheumatoid arthritis in the era of biologic response modifiers. Curr Opin Rheumatol. 2004;16(3):192-198 [DOI] [PubMed] [Google Scholar]
- 47. Gisbert JP, Gomollón F. Thiopurine-induced myelotoxicity in patients with inflammatory bowel disease: a review. Am J Gastroenterol. 2008;103(7): 1783-1800 [DOI] [PubMed] [Google Scholar]
- 48. Arts J, D'Haens G, Zeegers M, et al. Long-term outcome of treatment with intravenous cyclosporin in patients with severe ulcerative colitis. Inflamm Bowel Dis. 2004;10(2):73-78 [DOI] [PubMed] [Google Scholar]
- 49. Pinna-Pintor M, Arese P, Bona R, et al. Severe steroid-unresponsive ulcerative colitis: outcomes of restorative proctocolectomy in patients undergoing cyclosporin treatment. Dis Colon Rectum. 2000;43(5):609-613 [DOI] [PubMed] [Google Scholar]
- 50. Fleshner PR, Michelassi F, Rubin M, et al. Morbidity of subtotal colectomy in patients with severe ulcerative colitis unresponsive to cyclosporin. Dis Colon Rectum. 1995;38(12):1241-1245 [DOI] [PubMed] [Google Scholar]
- 51. Hyde GM, Jewell DP, Kettlewell MG, Mortensen NJ. Cyclosporin for severe ulcerative colitis does not increase the rate of perioperative complications. Dis Colon Rectum. 2001;44(10):1436-1440 [DOI] [PubMed] [Google Scholar]
- 52. Feagan BG, Fedorak RN, Irvine EJ, et al. ; North American Crohn's Study Group Investigators A comparison of methotrexate with placebo for the maintenance of remission in Crohn's disease. N Engl J Med. 2000; 342(22):1627-1632 [DOI] [PubMed] [Google Scholar]
- 53. Buchbinder R, Hall S, Ryan PF, et al. Severe bone marrow failure due to low dose oral methotrexate. J Rheumatol. 1988;15(10):1586-1588 [PubMed] [Google Scholar]
- 54. Gutierrez-Urena S, Molina JF, Garcia CO, et al. Pancytopenia secondary to methotrexate therapy in rheumatoid arthritis. Arthritis Rheum. 1996;39(2):272-276 [DOI] [PubMed] [Google Scholar]
- 55. Bridges SL, Jr, Moreland LW. Perioperative use of methotrexate in patients with rheumatoid arthritis undergoing orthopedic surgery. Rheum Dis Clin North Am. 1997;23(4):981-993 [DOI] [PubMed] [Google Scholar]
- 56. Perhala RS, Wilke WS, Clough JD, Segal AM. Local infectious complications following large joint replacement in rheumatoid arthritis patients treated with methotrexate versus those not treated with methotrexate. Arthritis Rheum. 1991;34(2):146-152 [DOI] [PubMed] [Google Scholar]
- 57. Sany J, Anaya JM, Canovas F, et al. Influence of methotrexate on the frequency of postoperative infectious complications in patients with rheumatoid arthritis. J Rheumatol. 1993;20(7):1129-1132 [PubMed] [Google Scholar]
- 58. Carpenter MT, West SG, Vogelgesang SA, Casey Jones DE. Postoperative joint infections in rheumatoid arthritis patients on methotrexate therapy. Orthopedics. 1996;19(3):207-210 [DOI] [PubMed] [Google Scholar]
- 59. Grennan DM, Gray J, Loudon J, Fear S. Methotrexate and early postoperative complications in patients with rheumatoid arthritis undergoing elective orthopaedic surgery. Ann Rheum Dis. 2001;60(3):214-217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Goldhirsch A, Gelber RD, Tattersall MN, et al. Methotrexate/nitrousoxide toxic interaction in perioperative chemotherapy for early breast cancer [letter]. Lancet. 1987;2(8551):151 [DOI] [PubMed] [Google Scholar]
- 61. Visser K, Katchamart W, Loza E, et al. Multinational evidence-based recommendations for the use of methotrexate in rheumatic disorders with a focus on rheumatoid arthritis: integrating systematic literature research and expert opinion of a broad international panel of rheumatologists in the 3E Initiative. Ann Rheum Dis. 2009;68:1086-1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Loza E, Martinez-Lopez JA, Carmona L. A systematic review on the optimum management of the use of methotrexate in rheumatoid arthritis patients in the perioperative period to minimize perioperative morbidity and maintain disease control. Clin Exp Rheumatol. 2009;27:856-862 [PubMed] [Google Scholar]
- 63. Heldman F, Braun J. Perioperative use of methotrexate. Clin Exp Rheumatol. 2010;28(5, suppl 61):S110-S113 [PubMed] [Google Scholar]
- 64. Present DH, Rutgeerts P, Targan S, et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N Engl J Med. 1999;340(18): 1398-1405 [DOI] [PubMed] [Google Scholar]
- 65. Targan SR, Hanauer SB, Van Deventer SJ, et al. ; Crohn's Disease cA2 Study Group A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med. 1997;337(15): 1029-1035 [DOI] [PubMed] [Google Scholar]
- 66. Sands BE, Tremaine WJ, Sandborn WJ, et al. Infliximab in the treatment of severe, steroid-refractory ulcerative colitis: a pilot study. Inflamm Bowel Dis. 2001;7(2):83-88 [DOI] [PubMed] [Google Scholar]
- 67. Rutgeerts P, D'Haens G, Targan S, et al. Efficacy and safety of retreatment with anti-tumor necrosis factor antibody (infliximab) to maintain remission in Crohn's disease. Gastroenterology. 1999;117(4):761-769 [DOI] [PubMed] [Google Scholar]
- 68. Ljung T, Karlen P, Schmidt D, et al. Infliximab in inflammatory bowel disease: clinical outcome in a population based cohort from Stockholm County. Gut. 2004;53(6):849-853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor α-neutralizing agent. N Engl J Med. 2001; 345(15):1098-1104 [DOI] [PubMed] [Google Scholar]
- 70. Colombel JF, Loftus EV, Jr, Tremaine WJ, et al. The safety profile of infliximab in patients with Crohn's disease: the Mayo Clinic experience in 500 patients. Gastroenterology. 2004;126(1):19-31 [DOI] [PubMed] [Google Scholar]
- 71. Marchal L, D'Haens G, Van AG, et al. The risk of post-operative complications associated with infliximab therapy for Crohn's disease: a controlled cohort study. Aliment Pharmacol Ther. 2004;19(7):749-754 [DOI] [PubMed] [Google Scholar]
- 72. Jarnerot G, Hertervig E, Friis-Liby I, et al. Infliximab as rescue therapy in severe to moderately severe ulcerative colitis: a randomized, placebo-controlled study. Gastroenterology. 2005;128(7):1805-1811 [DOI] [PubMed] [Google Scholar]
- 73. Kunitake H, Hodin R, Shellito PC, et al. Perioperative treatment with infliximab in patients with Crohn's disease and ulcerative colitis is not associated with an increased rate of postoperative complications. J Gastrointest Surg. 2008;12(10):1730-1736 [DOI] [PubMed] [Google Scholar]
- 74. Bordeianou L, Kunitake H, Shellito P, Hodin R. Preoperative infliximab treatment in patients with ulcerative and indeterminate colitis does not increase rate of conversion to emergent and multistep abdominal surgery. Int J Colorectal Dis. 2010;25(3):401-404 [DOI] [PubMed] [Google Scholar]
- 75. Kraemer M, Kirschmeier A, Marth T. Perioperative adjuvant therapy with infliximab in complicated anal Crohn's disease. Int J Colorectal Dis. 2008;23(10):965-969 [DOI] [PubMed] [Google Scholar]
- 76. Lichtenstein GR, Feagan BG, Cohen RD, et al. Serious infections and mortality in association with therapies for Crohn's disease: TREAT registry. Clin Gastroenterol Hepatol. 2006;4(5):621-630 [DOI] [PubMed] [Google Scholar]
- 77. Gainsbury ML, Chu DI, Howard LA, et al. Preoperative infliximab is not associated with an increased risk of short-term postoperative complications after restorative proctocolectomy and ileal pouch-anal anastomosis. J Gastrointest Surg. 2011;15(3):397-403 [DOI] [PubMed] [Google Scholar]
- 78. Appau KA, Fazio VW, Shen B, et al. Use of infliximab within 3 months of ileocolonic resection is associated with adverse postoperative outcomes in Crohn's patients. J Gastrointest Surg. 2008;12(10):1738-1744 [DOI] [PubMed] [Google Scholar]
- 79. Mor IJ, Vogel JD, da Luz Moreira A, et al. Infliximab in ulcerative colitis is associated with an increased risk of postoperative complications after restorative proctocolectomy. Dis Colon Rectum. 2008;51(8):1202-1207 [DOI] [PubMed] [Google Scholar]
- 80. Selvasekar CR, Cima RR, Larson DW, et al. Effect of infliximab on short-term complications in patients undergoing operation for chronic ulcerative colitis. J Am Coll Surg. 2007;204(5):956-962 [DOI] [PubMed] [Google Scholar]
- 81. Cottone M, Kohn A, Daperno M. Advanced age is an independent risk factor for severe infections and mortality in patients given anti–tumor necrosis factor therapy for inflammatory bowel disease. Clin Gastroenterol Hepatol. 2011;9:30-35 [DOI] [PubMed] [Google Scholar]
- 82. Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369(9573):1641-1657 [DOI] [PubMed] [Google Scholar]
- 83. Behm BW, Bickston SJ. Tumor necrosis factor-alpha antibody for maintenance of remission in Crohn's disease. Cochrane Database Syst Rev. 2008;(1):CD006893 [DOI] [PubMed] [Google Scholar]
- 84. Devlin SM, Panaccione R. Adalimumab for the treatment of Crohn's disease. Expert Opin Biol Ther. 2008;8(7):1011-1019 [DOI] [PubMed] [Google Scholar]
- 85. Juillerat P, Pittet V, Felley C, et al. Drug safety in Crohn's disease therapy. Digestion. 2007;76(2):161-168 [DOI] [PubMed] [Google Scholar]
- 86. Panes J, Gomollon F, Taxonera C, et al. Crohn's disease: a review of current treatment with a focus on biologics. Drugs. 2007;67(17):2511-2537 [DOI] [PubMed] [Google Scholar]
- 87. Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med. 2007;357(3):228-238 [DOI] [PubMed] [Google Scholar]
- 88. Fiorino G, Szabò H, Walter F, et al. Adalimumab in Crohn's disease: tips and tricks after 5 years of clinical experience. Curr Med Chem. 2011;18(8):1230-1238 [DOI] [PubMed] [Google Scholar]
- 89. Pandey S, Luther G, Umanskiy K, et al. Minimally invasive pouch surgery for ulcerative colitis: is there a benefit in staging? Dis Colon Rectum. 2011;54(3):306-310 [DOI] [PubMed] [Google Scholar]
- 90. Bargen JA, Barker NW. Extensive arterial and venous thrombosis complicating chronic ulcerative colitis. Arch Intern Med. 1936;58(1):17-31 [Google Scholar]
- 91. Schneiderman JH, Sharpe JA, Sutton DM. Cerebral and retinal vascular complications of inflammatory bowel disease. Ann Neurol. 1979;5(4): 331-337 [DOI] [PubMed] [Google Scholar]
- 92. Braverman D, Bogoch A. Arterial thrombosis in ulcerative colitis. Am J Dig Dis. 1978;23(12):1148-1150 [DOI] [PubMed] [Google Scholar]
- 93. Solem CA, Loftus EV, Tremaine WJ, Sandborn WJ. Venous thromboembolism in inflammatory bowel disease. Am J Gastroenterol. 2004;99(1): 97-101 [DOI] [PubMed] [Google Scholar]
- 94. O'Connor OJ, Cahill RA, Kirwan WO, Redmond HP. The incidence of postoperative venous thrombosis among patients with ulcerative colitis. Ir J Med Sci. 2005;174(3):20-22 [DOI] [PubMed] [Google Scholar]
- 95. Papa A, Danese S, Grillo A, et al. Review article: inherited thrombophilia in inflammatory bowel disease. Am J Gastroenterol. 2003;98(6):1247-1251 [DOI] [PubMed] [Google Scholar]
- 96. Seligsohn U, Lubetsky A. Genetic susceptibility to venous thrombosis. N Engl J Med. 2001;344(16):1222-1231 [DOI] [PubMed] [Google Scholar]
- 97. Cattaneo M, Vecchi M, Zighetti ML, et al. High prevalence of hyperchomocysteinemia in patients with inflammatory bowel disease: a pathogenic link with thromboembolic complications? Thromb Haemost. 1998;80(4): 542-545 [PubMed] [Google Scholar]
- 98. Oldenburg B, Fijnheer R, van der Griend R, vanBerge-Henegouwen GP, Koningsberger JC. Homocysteine in inflammatory bowel disease: a risk factor for thromboembolic complications? Am J Gastroenterol. 2000;95(10): 2825-2830 [DOI] [PubMed] [Google Scholar]
- 99. Bernstein CN, Blanchard JF, Houston DS, Wajda A. The incidence of deep venous thrombosis and pulmonary embolism among patients with inflammatory bowel disease: a population-based cohort study. Thromb Haemost. 2001;85:430-434 [PubMed] [Google Scholar]
- 100. Grainge MJ, West J, Card TR. Venous thromboembolism during active disease and remission in inflammatory bowel disease: a cohort study. Lancet. 2010;375:657-663 [DOI] [PubMed] [Google Scholar]
- 101. Nguyen GC, Sam J. Rising prevalence of venous thromboembolism and its impact on mortality among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(9):2272-2280 [DOI] [PubMed] [Google Scholar]
- 102. Joint Commission Specifications manual for Joint Commission Na tion al Quality Core Measures (2010B): VTE prophylaxis options for surgery. http://manual.jointcommission.org/releases/Archive/TJC2010B1/VTEProphylaxisOptionsforSurgery.html Accessed June 2, 2011
- 103. Murthy SK, Nguyen GC. Venous thromboembolism in inflammatory bowel disease: an epidemiological review [published online ahead of print March 15, 2011]. Am J Gastroenterol. doi:10.1038/ajg.2011.53 [DOI] [PubMed]
- 104. Douketis JD, Berger PB, Dunn AS, et al. The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133(6) (suppl):299S-339S [DOI] [PubMed] [Google Scholar]
- 105. Scarpa M, Pilon F, Pengo V, et al. Deep venous thrombosis after surgery for inflammatory bowel disease: is standard dose low molecular weight heparin prophylaxis enough? World J Surg. 2010;34(7):1629-1636 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.