Abstract
Statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) represent the cornerstone of drug therapy to reduce low-density lipoprotein (LDL) cholesterol and cardiovascular risk. However, even optimal statin management of LDL cholesterol leaves many patients with residual cardiovascular risk, in part because statins are more effective in reducing LDL cholesterol than apolipoprotein B (Apo B). Apo B may be a better marker of atherogenic risk than LDL cholesterol because Apo B measures the total number of all atherogenic particles (total atherosclerotic burden), including LDL, very low-density lipoprotein, intermediate-density lipoprotein, remnant lipoproteins, and lipoprotein(a). To determine whether Apo B is a better indicator of baseline cardiovascular risk and residual risk after lipid therapy compared with LDL cholesterol, a MEDLINE search of the literature published in English from January 1, 1975, through December 1, 2010, was conducted. On the basis of data from most population studies, elevated Apo B was more strongly associated with incident coronary heart disease than similarly elevated LDL cholesterol. Apo B was also a superior benchmark (vs LDL cholesterol) of statins' cardioprotective efficacy in both primary-prevention and secondary-prevention trials. To minimize cardiovascular risk among persons with hypercholesterolemia or dyslipidemia, the best available evidence suggests that intensive therapy with statins should be initiated to achieve the lowest possible Apo B level (with adequate drug toleration) and then other therapies (eg, niacin, bile acid resins, ezetimibe) added to potentiate these Apo B–lowering effects. In future consensus lipid-lowering treatment guidelines, Apo B should be considered as an index of residual risk, a potential parameter of treatment efficacy, and a treatment target to minimize risk of coronary heart disease.
ACCORD = Action to Control Cardiovascular Risk in Diabetes; Apo = apolipoprotein; ATP = Adult Treatment Panel; CHD = coronary heart disease; CI = confidence interval; DM = diabetes mellitus; FATS = Familial Atherosclerosis Treatment Study; FIELD = Fenofibrate Intervention and Event Lowering in Diabetes; HDL = high-density lipoprotein; HR = hazard ratio; IDL = intermediate-density lipoprotein; LDL = low-density lipoprotein; MI = myocardial infarction; P-OM3 = prescription omega-3-fatty acid ethyl ester; RCT = randomized controlled trial; TG = triglyceride; VA-HIT = Veterans Affairs High-Density Lipoprotein Intervention Trial; VLDL = very low-density lipoprotein
Elevated low-density lipoprotein (LDL) cholesterol is a pivotal lipid risk factor and target of lipid therapies to prevent cardiovascular events.1-15 Although statins (3-hydroxy-3-methylglutaryl-coenzyme A reductase inhibitors) are clinical mainstays of treatment, many statin recipients experience residual cardiovascular risk. Evidence supporting residual risk derives from clinical and observational studies,16-18 which have spawned efforts to seek other modifiable lipid/lipoprotein targets, including lipid components of diabetic, atherogenic, or mixed dyslipidemia. Many patients with these conditions have elevated apolipoprotein (Apo) B–containing lipoproteins, including very low-density lipoprotein (VLDL) and intermediate-density lipoprotein (IDL); LDL cholesterol alone does not capture the overall atherosclerotic burden as accurately as do levels of Apo B.
This review assesses the potential utility of elevations in Apo B levels as indicators of cardiovascular risk and as treatment targets to maximize the cardioprotective effects of lipid therapies.
METHODS
An English-language MEDLINE search of literature published from January 1, 1975, through December 1, 2010, was conducted. The title term *apo* was joined (using the Boolean operator “AND”) with terms including atheroscl*, cardiovasc*, *cholest*, dyslipidemia*,*trig*, *lip*, metabolism, and risk factors. The same title terms were also joined (using “AND”) with the title terms bile acid resin* (sequestrant*), docosahexaenoic acid, eicosapentaenoic acid, ezet*, *fibr*, hydroxymethylglutaryl-CoA reductase inhibitors (*statin*), niacin, nico*, and omega-3. Other reviews, consensus guidelines, and meta-analyses were included and their bibliographies searched to identify additional articles of interest.
DEFINITIONS AND PATHOBIOLOGY
Apo B represents the total number of circulating atherogenic particles (ie, total atherogenic burden).19 Each VLDL, IDL, LDL, and lipoprotein(a) particle contains 1 molecule of Apo B100, and each chylomicron or chylomicron remnant contains 1 molecule of Apo B48.19,20 Subendothelial trapping of Apo B is a key atherogenic trigger.21,22
Article Highlights
Low-density lipoprotein (LDL) cholesterol measures may not adequately evaluate cardiovascular risk when other apolipoprotein B–containing atherogenic particles predominate, such as in patients with the metabolic syndrome, diabetes mellitus, or coronary heart disease
Each LDL, intermediate-density lipoprotein, or very low-density lipoprotein particle has a single Apo B molecule. However, the amount of cholesterol in each LDL particle is more heterogeneous and can vary substantially in individual patients
Convergent evidence from major epidemiologic studies (eg, INTERHEART, ISIS, AMORIS) and clinical trials (eg, AFCAPS/TexCAPS, CARDS, IDEAL) strongly supports the use of apolipoproteins, particularly apolipoprotein B, to inform both population- and patient-based assessments and decision making in cardiovascular prevention
A potential exists for “treatment mismatch” and residual risk when only elevated LDL cholesterol levels are targeted for reduction, in part because 3-hydroxy-3-methylglutaryl-coen zyme A reductase inhibitors (statins) inhibit LDL cholesterol biosynthesis to a greater extent than they lower apolipoprotein B levels
Clinical trials show that monitoring levels of apolipoprotein B might be the optimal strategy to assess the adequacy of lipid-lowering therapy, particularly with statins
To maximize the cardioprotective benefits of lipid pharmacotherapy in many patients with hypercholesterolemia, hyperlipidemia, or dyslipidemia (or who are at otherwise elevated cardiovascular risk), the clinician should consider targeting treatment to the lowest achievable Apo B level with maximally tolerated statin doses, followed by further Apo B lowering using niacin, bile acid resins (sequestrants), or ezetimibe if necessary
The lipid triad (increased levels of triglycerides [TGs], low levels of high-density lipoprotein [HDL] cholesterol, and increased small, dense LDL particles) results from hepatic uptake of free fatty acids from adipose tissues, with hepatic overproduction of VLDL Apo B. Triglycerides in VLDL are then exchanged for cholesteryl esters in LDL and HDL by cholesteryl ester transfer protein, and TGs in the core of these lipoproteins are hydrolyzed by lipoprotein lipase. These processes result in smaller, denser LDL particles, which have greater propensity to elicit oxidative arterial-wall injury.19,23 In patients with increased numbers of small, dense LDL particles, the total number of LDL particles may be higher than the LDL cholesterol level, because LDL particles differ in their cholesterol contents. Conversely, there is a 1:1 relationship between Apo B levels and the total number of atherogenic particles. Apo A-1 is the core structural component of HDL.20
EPIDEMIOLOGIC EVIDENCE
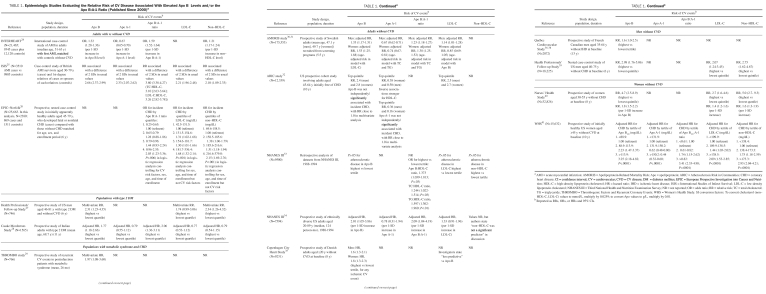
Many population studies have demonstrated the superior prognostic value of Apo B over LDL cholesterol and/or non-HDL cholesterol in predicting cardiovascular events (Table 1).24-41 In the 52-country INTERHEART study, the Apo B:A-1 ratio accounted for 54% of the population-attributable risk of acute myocardial infarction (MI). Apo B was also superior to LDL cholesterol and non-HDL cholesterol in predicting coronary heart disease (CHD) (Table 1).24 Further, the Health Professionals' Follow-up Study,39 involving 18,225 men without cardiovascular disease at baseline, showed that the relative risk of CHD in the top quintile was 3.01 (95% confidence interval [CI], 1.81-5.00) for Apo B, 2.76 (1.66-4.58) for non-HDL cholesterol, and 1.81 (1.12-2.93) for LDL cholesterol.
TABLE 1.
Epidemiologic Studies Evaluating the Relative Risk of CV Disease Associated With Elevated Apo B Levels and/or the Apo B:A-1 Ratio (Published Since 2000)a
In contrast, the ARIC (Atherosclerosis Risk in Communities) study suggested that Apo B was not independently predictive of incident CHD in persons without elevated LDL cholesterol and that the predictive value of Apo B was abolished after adjusting for elevated TG levels.32
Finally, the Emerging Risk Factors Collaborators42 demonstrated equivalent hazard ratios (HRs) for CHD for both Apo B and non-HDL cholesterol, and also for both Apo A-1 and HDL cholesterol. Both Apo B and non-HDL cholesterol were superior to directly measured LDL cholesterol as risk predictors. However, this meta-analysis did not include the INTERHEART study, one of the largest studies showing a positive relationship between apolipoproteins and cardiovascular risk.24
EFFECTS OF LIPID THERAPIES ON APO B AND POTENTIAL CLINICAL IMPLICATIONS
Statin-Containing Treatments
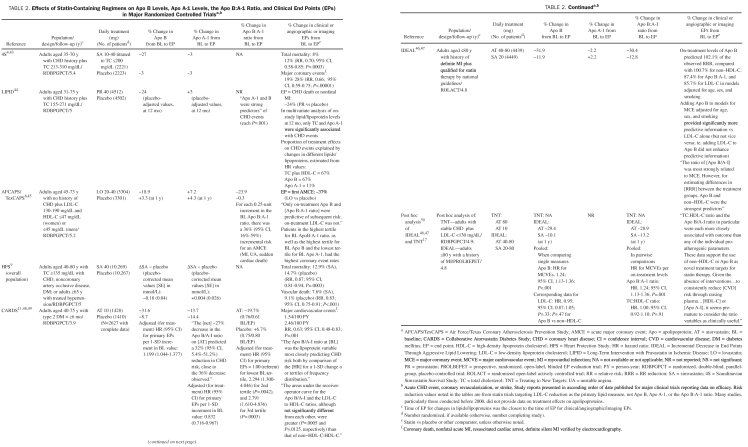
Effects on Apo B Levels and Clinical/Angiographic/Imaging End Points. Statins are the most effective available medications to reduce Apo B levels. Statin monotherapy lowered Apo B levels in major randomized controlled trials (RCTs) by 19% to 42% (Table 2).4,8,9,11,43-47 On-treatment elevations of Apo B levels could serve as indices of statin efficacy and as treatment targets to maximize the cardioprotective benefits of statins.
TABLE 2.
Effects of Statin-Containing Regimens on Apo B Levels, Apo A-1 Levels, the Apo B:A-1 Ratio, and Clinical End Points (EPs) in Major Randomized Controlled Trialsa,b
In the AFCAPS/TexCAPS (Air Force/Texas Coronary Atherosclerosis Prevention Study), lovastatin significantly reduced the relative risk of a first acute major coronary event by 37% (P<.001 vs placebo). Daily treatment with 20 to 40 mg of lovastatin for approximately 5 years reduced Apo B levels by 19% from baseline.45 Hence, for each 1% decline in Apo B levels, CHD risk was reduced by approximately 2%. Only Apo B—not LDL cholesterol—was significantly predictive of future cardiovascular events in patients receiving lovastatin. Apo B was the single most consistent, significant predictor of future coronary events, both at baseline and on treatment.
Similarly, in CARDS (Collaborative Atorvastatin Diabetes Study), which involved patients with type 2 diabetes mellitus (DM) and an LDL cholesterol level of 160 mg/dL (to convert to mmol/L, multiply by 0.0259) or greater but without CHD, 10 mg/d of atorvastatin for 1 year lowered Apo B levels by approximately 24% from baseline (P<.001 vs placebo).11,48,49 These changes in Apo B levels were associated with a 37% decline in the relative risk of major cardiovascular events. Hence, relative cardiovascular risk was reduced by approximately 1.5% for each 1% decrease in Apo B levels. The investigators concluded that “both Apo B and [non-HDL cholesterol] provide more consistent goals than [LDL cholesterol] for assessing the LDL particle response to statin therapy.”48
Finally, a pooled-data analysis of patients with a CHD history—stable CHD in TNT (Treating to New Targets)17 and an MI history in IDEAL (Incremental Decrease in End Points through Aggressive Lipid Lowering)46—showed that on-treatment (statin) Apo B and non-HDL cholesterol levels were more robustly predictive of cardiovascular outcomes than LDL cholesterol.50 The investigators suggested that Apo B and non-HDL cholesterol levels were more accurate predictors of cardiovascular disease even in patients with largely normal levels of TGs.
In addition to potential disparities in absolute CHD risk for given levels of Apo B and non-HDL cholesterol, a “mismatch” potentially exists between statins' effects in reducing LDL cholesterol compared with Apo B.51,52 Statins inhibit the rate-limiting enzyme in endogenous cholesterol (not Apo B) biosynthesis, and the cholesterol content of LDL is heterogeneous, whereas its Apo B content is fixed. Further, hepatocyte LDL receptors have a higher affinity for larger, more buoyant, cholesterol-rich IDL and LDL particles than for smaller, cholesterol-depleted particles. The mismatch in Apo B vs LDL cholesterol lowering may also be attributed to statin-associated reductions in the transfer of cholesteryl ester to VLDLs.53,54 More intensive lipid-lowering therapies and new treatments to lower Apo B levels may be needed to reduce Apo B levels to goal.55-58
Effects on Lipid End Points. The STELLAR (Statin Therapies for Elevated Lipid Levels compared Across doses to Rosuvastatin) trial provided further evidence that statin-containing regimens are the most effective single class of medications to lower Apo B levels.59 Treatment for 6 weeks reduced Apo B levels by 37% to 45% with 10 to 40 mg of rosuvastatin; 29% to 43% with 10 to 80 mg of atorvastatin, and 22% to 35% with 10 to 80 mg of simvastatin.
Niacin-Containing Regimens
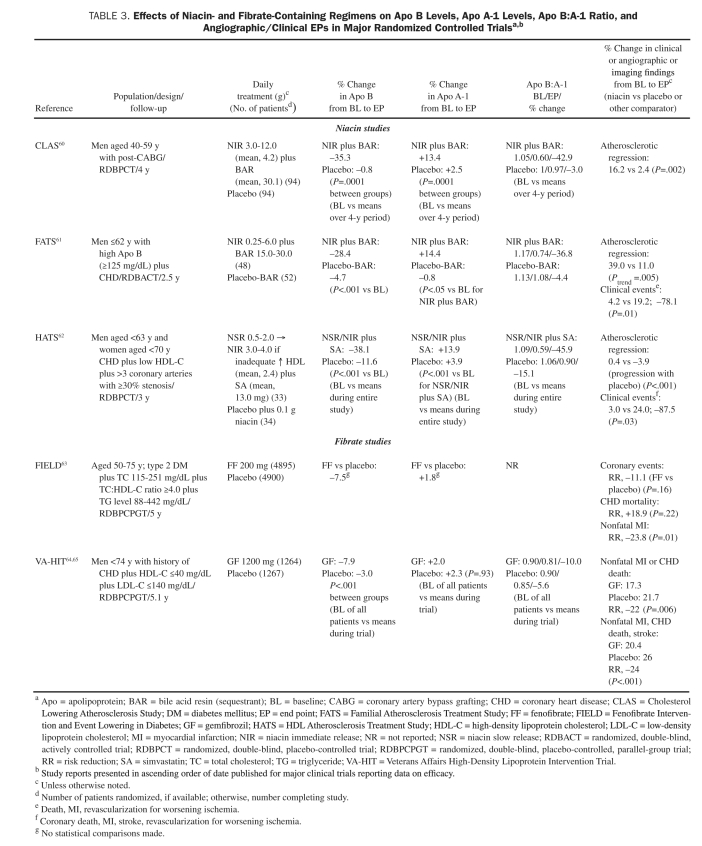
Effects on Apo B Levels and Clinical/Angiographic/Imaging End Points. Three major angiographic RCTs reported significant declines in atherosclerotic progression and/or increases in regression: CLAS II (Cholesterol-Lowering Atherosclerosis Study II), HATS (HDL Atherosclerosis Treatment Study), and FATS (Familial Atherosclerosis Treatment Study) (Table 3; niacin and fibrate studies).60-65 In FATS, reductions in Apo B levels more effectively predicted atherosclerotic lesion regression than did LDL cholesterol (r=0.38; P<.001), which was noted in 39% of patients treated with niacin plus a bile acid resin compared with 11% of controls (P=.005).61 Both FATS and HATS also reported significant relative risk reductions of 80% to 90% in favor of niacin-containing regimens (vs controls) in incidences of cardiovascular events. Given that niacin-containing therapies reduced Apo B levels by approximately 28% to 38%, the ratio between percent lowering in Apo B levels and in cardiovascular risk reduction was approximately 1:2 to 1:3.61,62 The largest RCT involving niacin was the Coronary Drug Project (N=1119), which did not measure Apo levels but demonstrated a reduction in relative risk of 14% in both coronary death and fatal MI and of 27% in nonfatal MI.66
TABLE 3.
Effects of Niacin- and Fibrate-Containing Regimens on Apo B Levels, Apo A-1 Levels, Apo B:A-1 Ratio, and Angiographic/Clinical EPs in Major Randomized Controlled Trialsa,b
Effects on Lipid End Points. In smaller clinical studies, treatment with niacin-containing regimens reduced Apo B levels by 10% to 20% and raised Apo A-1 levels by 5% to 10%.67-69
Fibrate-Containing Regimens
Effects on Apo B and Clinical/Angiographic/Imaging End Points. Certain RCTs and pooled-data analyses, including VA-HIT (Veterans Affairs High-Density Lipoprotein Intervention Trial), suggested that patients with diabetic dyslipidemia especially benefited from fibrate treatment.64,65,70,71 Baseline levels of Apo B were significantly predictive of recurrent CHD events in the VA-HIT, whereas levels of LDL cholesterol were not; for each increment of 10 mg/dL (to convert to g/L, multiply by 0.01) in Apo B levels (but not in LDL cholesterol levels) in VA-HIT, the relative risk of CHD increased by 6% (relative risk, 1.06; P=.01).65
In the FIELD (Fenofibrate Intervention and Event Lowering in Diabetes) study, treatment with 200 mg/d of fenofibrate for 5 years reduced CHD death or nonfatal MI by a statistically nonsignificant 11% (P=.16) in participants aged 50 to 75 years with type 2 DM.63 Fenofibrate reduced the relative risk of nonfatal MI by 24% (P=.01 vs placebo) and the relative risk of coronary revascularization by 21% (P=.003). Although fenofibrate treatment significantly lowered Apo B levels (by 6.9% vs placebo; P<.001), increments in total cardiovascular risk (HRs for cardiovascular events) were similar for each 1-SD increase in baseline Apo B and non-HDL cholesterol levels in both the placebo and active-treatment groups.72
The ACCORD (Action to Control Cardiovascular Risk in Diabetes) trial demonstrated that treatment with fenofibrate and simvastatin did not significantly lower major cardiovascular risk (vs simvastatin alone) in patients with type 2 DM.71 In a patient subset with TG levels of 204 mg/dL or higher (to convert to mmol/L, multiply by 0.0113) and HDL cholesterol levels of 34 mg/dL or lower (to convert to mmol/L, multiply by 0.0259), the risk for the primary end point was significantly reduced by 31% (nominal P=.032).73
Similar risk reductions have been observed in subgroups with low levels of HDL cholesterol and elevated levels of TGs in other major fibrate trials (Helsinki Heart Study, Bezafibrate Infarction Prevention trial, FIELD trial). In a recent meta-analysis, treatment with derivatives of fibric acid significantly reduced cardiovascular relative risk, by 30% (P<.001) in persons with TG levels higher than 200 mg/dL and HDL cholesterol levels lower than 35 mg/dL, and by 28% (P<.001) in those with TG levels higher than 200 mg/dL, but not significantly in those without atherogenic dyslipidemia (6% relative-risk reduction; P=.13).74 One possible reason for the statistically nonsignificant effect in the overall ACCORD study population might be that when fibrates are added to moderate- or high-dose statin regimens, they do not result in significant decreases in Apo B levels over statin therapy alone. Although Apo B levels were not measured in ACCORD, Apo B levels likely were low because all patients were already using statins.71
Effects on Lipid End Points. Reductions in Apo B levels from baseline with fibrate monotherapy range from approximately 12% to 18% in different populations.75-82 However, most trials of fibrates combined with medium- to high-dose statins have failed to show marked additional reductions in Apo B levels compared with statin monotherapy.
Ezetimibe-Containing Regimens
Effects on Apo B Levels and Clinical/Angiographic/Imaging End Points. Treatment with ezetimibe-simvastatin significantly reduced major atherosclerotic risk by 17% (P=.002 vs placebo) among 9438 patients with chronic kidney disease in SHARP (the Study of Heart and Renal Protection). Over the course of 2.5 years, the combination reduced Apo B levels by 27% (P<.001 vs placebo), for a 1.6:1.0 relationship between percent reduction in Apo B levels and in CHD risk.83
In contrast, patients with mild to moderate aortic stenosis but without overt ischemic heart disease who received ezetimibe-simvastatin for 4.4 years in the SEAS (Simvastatin and Ezetimibe Aortic Stenosis) trial did not experience a significantly lower incidence of a composite primary end point compared with placebo.84 However, the combination ezetimibe-simvastatin regimen significantly reduced (by 22%) the risk of first ischemic cardiovascular events (HR, 0.78; 95% CI, 0.63-0.97; P=.02 vs placebo). Given a placebo-adjusted decrease of 43% in Apo B levels at treatment year 1, the ratio between percent lowering in Apo B levels and in cardiovascular risk was 2:1.85
Effects on Lipid End Points. The greatest declines in Apo B levels (56%) have been reported in patients receiving 10/40 mg/d of ezetimibe-rosuvastatin for 6 weeks (P<.001 vs 40 mg of rosuvastatin alone) in the EXPLORER (Ezetimibe versus Rosuvastatin Alone) study.86 In another study, Apo B levels were reduced by about 48% with ezetimibe-simvastatin-niacin, 40% with ezetimibe-simvastatin, and 19% with niacin for 6 months (P<.001 for each treatment comparison).68
Bile Acid Resins (Sequestrants)
Treatment with sequestrants can reduce Apo B and LDL cholesterol levels by 12% to 15%, can potentiate the Apo B–lowering effects of other agents (eg, statins, niacin), and can reduce glycated hemoglobin (HbA1c) levels in diabetic patients. They have also been associated with significant declines in clinical events and atherosclerotic progression (Table 3).60,61,87,88 In FATS, treatment with either colestipollovastatin or colestipol-niacin decelerated atherosclerotic progression and significantly reduced cardiovascular risk in patients with CHD. On-treatment reductions in Apo B levels (r=0.38) were more predictive of improvements in percent stenosis than declines in LDL cholesterol levels (r=0.27). Thus, sequestrants can play an important role in potentiating the Apo B–reducing effects of other lipid therapies, but their use is limited in persons with very high levels of TGs.
Prescription Omega-3-Fatty Acid Ethyl Ester–Containing Regimens
These agents have emerged as possible treatment options to lower elevated levels of TGs and reduce CHD death in patients with previous MI.89,90 A systematic review suggested that treatment with non–lipid-lowering doses of prescription omega-3-fatty acid ethyl esters (P-OM3s) from fish oils reduced the incidence of all-cause mortality and sudden death but not nonfatal MI.91,92 However, the effects of P-OM3s on Apo B levels are small.93-95 Benefits of P-OM3 treatment on cardiovascular morbidity and mortality might be ascribed largely to nonlipid benefits.
CURRENT GUIDELINES AND EXPERT PANEL RECOMMENDATIONS
Mounting evidence has led expert panels to recommend consideration of Apo levels, particularly in patients with either increased cardiometabolic risk, diabetic dyslipidemia, or CHD.55,96 They have also recommended assessment of the adequacy of therapy for those already receiving treatment. A consensus panel of the American Diabetes Association/American College of Cardiology Foundation recommended a goal Apo B level of less than 80 mg/dL for patients at highest risk and less than 90 mg/dL for patients at high risk.55 The 2009 Canadian Cardiovascular Society/Canadian guidelines recommended an Apo B goal of less than 80 mg/dL, an LDL cholesterol level of less than 77 mg/dL, or a 50% decline in LDL cholesterol levels from baseline in patients at high and moderate risk.96 On the basis of recent (2000-2006) Third National Health and Nutrition Examination Survey population data, the Apo B treatment target should be less than 70 mg/dL for patients at high risk (ie, those with CHD) and less than 90 mg/dL for those at moderately high risk.97
EMERGING ISSUES AND THE WAY FORWARD
Potential Pathways to Improved Outcomes
Increasingly aggressive statin and other cholesterol-lowering therapies have led to significant incremental reductions in absolute cardiovascular risk in RCTs.15,98 Still, many clinicians justifiably contemplate which other atherogenic parameter needs to be altered to minimize atherosclerotic progression and cardiovascular events (Table 4).8,23,24,26-31,35,44,47,51,54,99-108
TABLE 4.
Potential Advantages and Disadvantages of Apolipoproteins as Risk Indices and Treatment Targets, and Potential Clinical Settings in Which These Measures Add Information to Traditional Lipid/Lipoprotein Test Resultsa,b
Levels of Apo B or non-HDL cholesterol are in general superior to levels of LDL cholesterol in stratifying patient populations according to baseline (pretreatment) cardiovascular risk. With their ease of computation, their improvement in risk prediction over LDL cholesterol levels, their consistency with the current “cholesterol” paradigm familiar to practitioners, and their cost-effectiveness, non-HDL cholesterol levels seem to represent an attractive route to more informed decision making.109 However, the unit of interest for clinicians is the individual patient. Health care professionals need to know which lipid or lipoprotein parameter(s) to target, and to what levels, in each patient. In other words, clinicians need to determine which parameter(s) will be the most effective measure of ongoing cardiovascular risk or benchmark of the adequacy of lipid therapies (ie, residual risk). The question currently under debate is whether the potential benefits of measuring Apo B levels in individual patients offset the additional costs in clinical practices.
From a pathophysiologic and clinical perspective, the current review argues in the affirmative: the benefits of Apo B measurement clearly outweigh the costs. From the pathophysiologic perspective, arterial infiltration with Apo B is a key atherogenic trigger, and the overall burden of atherosclerosis is most effectively estimated by the total exposure to atherogenic lipoprotein particles over time.22 Atherogenic Apo B–containing lipoprotein particles mediate arterial injury. Further, the cholesterol content of atherogenic lipoproteins is heterogeneous over time, and the measurement of LDL cholesterol may not reflect the full atherogenic burden. With Apo B, a more reliable 1:1 relationship exists between Apo B and each atherogenic lipoprotein particle. On a mechanistic basis, statins tend to reduce cholesterol biosynthesis to a greater extent than Apo B assembly and output. From the epidemiologic perspective, a recent meta-analysis demonstrated that treating individuals with Apo B or non-HDL cholesterol levels above the 70th percentile of the US adult population for 10 years would avert 500,000 or 300,000 more cardiovascular events, respectively, than treating those with LDL cholesterol levels above the 70th percentile.110 However, because of the exploratory nature of meta-analysis and metaregression analyses, such results need to be corroborated by RCTs.
The case for adding assays of Apo A-1, the chief pro tein constituent of the HDL particle, to routine lipid screening is inconclusive. Select epidemiologic and clinical studies have suggested that either Apo A-1 levels or the Apo B:A-1 ratio would be superior to traditional cholesterol values or ratios (eg, total cholesterol:HDL cholesterol) in predicting cardiovascular events.8,11,24,25,30,31,35,45,48,104-106,111,112 However, at this time, the potential benefits of introducing Apo A-1 to routine lipid/lipoprotein panels do not seem to outweigh their incremental costs. In the Emerging Risk Factors Collaboration, the largest pooled epidemiologic study to date, no advantage in risk prediction was found for Apo A-1 over HDL cholesterol.42 A recent metaregression analysis did not support the premise that raising HDL cholesterol levels (via diverse therapies) alone significantly reduced coronary death, coronary events, or all-cause mortality.113 Although HDL cholesterol and Apo A-1 levels are effective markers of risk, setting treatment targets for them would be premature.113
Evidence-Based Adjunctive Therapies to Further Reduce Cardiovascular Risk After Maximum-Tolerated Statin Therapy
One remaining issue is how physicians can maximize cardiovascular risk reductions for their patients. A first step would be to lower LDL cholesterol and non-HDL cholesterol to the newly revised Adult Treatment Panel (ATP) III LDL cholesterol goals using maximally tolerated statin doses.2,3 Achieving non-HDL cholesterol treatment targets is often more difficult than attaining only LDL cholesterol goals and may require additional therapies over and above the administration of the maximum-tolerated doses of statins.2,114 Residual elevations in Apo B levels will help to identify whether further therapy is justified after LDL cholesterol and non-HDL cholesterol treatment targets have been obtained. Given that statins lower LDL cholesterol and non-HDL cholesterol levels to a greater extent than Apo B, levels of the latter may represent more relevant targets of lipid therapies to maximize CHD risk reduction.
Although the primary-prevention JUPITER (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin) study was largely touted as providing a rationale for screening and treating patients with elevated high-sensitivity C-reactive protein, increased levels of this inflammatory biomarker helped to identify a population with even more marked Apo B levels, further justifying statin therapy to reduce cardiovascular risk. The marked reduction in the incidence of cardiovascular events (43%) in patients receiving intensive statin therapy in this trial was not surprising given that pretreatment Apo B levels were in the 65th percentile (vs the 25th percentile for LDL cholesterol and the 30th percentile for non-HDL cholesterol). Despite having relatively “normal” LDL cholesterol and non-HDL cholesterol levels at baseline, this population, which had a significant elevation in Apo B levels, still significantly benefited from statins.102,103
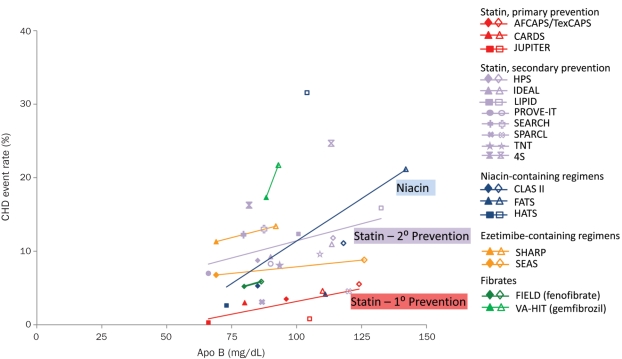
The Figure depicts relationships between Apo B levels in patients receiving treatment and CHD event rates in landmark trials. Irrespective of treatment and setting (primary vs secondary prevention), CHD event rates declined in an approximately linear fashion as a function of decreasing on-treatment Apo B levels, particularly with statins. To date, statins have the largest and most consistent database demonstrating cardioprotective benefits of Apo B lowering in almost all populations studied. The Figure suggests that, in primary prevention with statins, the CHD event rate approaches 0 as Apo B levels approach approximately 55 mg/dL. To further reduce cardiovascular risk, adjunctive therapies can be added to statins.
FIGURE.
The incidence of coronary heart disease (CHD) events as a function of apolipoprotein (Apo) B levels in randomized controlled trials involving regimens, including statins (red, purple), niacin (nicotinic acid; blue), fibrates (fibric-acid derivatives; green), or ezetimibe (orange). Separate regression lines are shown for trials of statins in primary and secondary prevention and niacin. Other lines represent individual trials for fibrates and ezetimibe. Individual studies for each treatment are detailed in the legend (filled symbol = treated group; empty symbol = control or placebo group). The event rate is predicted to approach 0 at an Apo B level of 54.6 mg/dL (statin, primary prevention population). Studies differ in methodology, CHD end point definitions, and comparator groups (eg, active treatment vs placebo, high dose vs low dose, combination therapy vs placebo). AFCAPS/TexCAPS = Air Force/Texas Coronary Atherosclerosis Prevention Study; CARDS = Collaborative Atorvastatin Diabetes Study; CLAS II = Cholesterol Lowering Atherosclerosis Study; FATS = Familial Atherosclerosis Treatment Study; FIELD = Fenofibrate Intervention and Event Lowering in Diabetes; HATS = HDL Atherosclerosis Treatment Study; HPS = Heart Protection Study; IDEAL = Incremental Decrease in End Points Through Aggressive Lipid Lowering; JUPITER = Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin; LIPID = Long-Term Intervention With Pravastatin in Ischaemic Disease; PROVE-IT = Pravastatin or Atorvastatin Evaluation and Infection, high-dose atorvastatin group; 4S = Scandinavian Simvastatin Survival Study, simvastatin group; SEARCH = Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine; SEAS = Simvastatin and Ezetimibe Aortic Stenosis, simvastatin-ezetimibe group; SHARP= Study of Heart and Renal Protection; SPARCL = Stroke Prevention by Aggressive Reduction in Cholesterol Levels; TNT = Treating to New Targets; VA-HIT = Veterans Affairs High-Density Lipoprotein Intervention Trial.
To answer which additional therapies to use, elevated Apo B levels may be more informative than increased levels of non-HDL cholesterol. In patients with elevated levels of non-HDL cholesterol, which reflect an excess of TG-rich lipoproteins, it is unclear whether the preferred additional agent would be a TG-lowering medication or an LDL cholesterol–lowering agent. However, if the goal is to decrease elevated Apo B levels, then it becomes clearer which adjunctive therapy to add to statins: medications with the most potent Apo B–lowering effects, including niacin, sequestrants, and ezetimibe. A discussion of these and other potential treatment adjuncts to potentiate statin-induced reductions in Apo B levels and cardiovascular risk follows; they are discussed in descending order of the strength of evidence based on the current review of the literature.
Niacin. The regression line for niacin (vs statins or ezetimibe) in the Figure suggests that nicotinic acid may have potential benefits beyond the reduction of Apo B levels, including niacin's ability to lower lipoprotein(a) levels; reduce LDL particle number; decrease small, dense LDL number; or raise HDL and Apo A-1 levels. However, of note, the niacin trials depicted in the Figure represent very small numbers of patients (<200 per study) and involve combination therapies with other agents. A recent meta-analysis that pooled data from 11 RCTs suggested that niacin treatment resulted in substantial risk reduction in major coronary events of 25% (95% CI, 13%-35%).115 The hypothesis that statin-niacin regimens further reduce the incidence of cardiovascular events is being evaluated in the AIM HIGH (Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides and Impact on Global Health Outcomes; NCT00120289 [*see note at end of article]) and HPS2-THRIVE (Treatment of High-density lipoprotein to Reduce the Incidence of Vascular Events; NCT00461630) trials.
Bile Acid Resins (Sequestrants). Sequestrant-containing regimens were associated with significant declines in clinical events and atherosclerotic progression in earlier studies (Table 3).60-62 Using colesevelam in patients with type 2 DM, clinicians can expect approximately 10% to 13% reductions in Apo B levels, and a 0.5-percentage-point decline in HbA1c levels.116 However, the presence of TG levels greater than 300 mg/dL may contraindicate use of these agents because of potential rebound hypertriglyceridemia.
Ezetimibe. Although the recent SHARP trial demonstrated significant benefits of ezetimibe-statin therapy over placebo in reducing cardiovascular risk in patients with chronic kidney disease, it did not determine whether ezetimibe-statin therapy conferred incremental cardioprotective benefits over statin monotherapy. This hypothesis is being evaluated in IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial; NCT00202878). Until this trial has been completed, maximizing the statin dose to its tolerable limits is still the preferred strategy, before adding ezetimibe.
Fibrates. Fibrate-containing regimens may be particularly useful in patients with DM, CHD, or insulin-resistance syndromes who have HDL cholesterol levels less than 40 mg/dL and/or TG levels greater than 200 mg/dL.117 However, the use of adjunctive treatment with fibrates to minimize residual risk remains controversial. In the Figure, reductions in CHD events were over a very narrow range of Apo B levels and occurred mainly in the VA-HIT with gemfibrozil, but not in the FIELD trial with fenofibrate.
Other potential drawbacks of fibrates are the following: (1) they do not significantly lower Apo B levels as adjuncts to statins; (2) they have never been demonstrated to significantly reduce all-cause mortality in major RCTs; (3) only treatment with gemfibrozil has been shown to significantly improve cardiovascular outcomes; and (4) concerns have been raised about myotoxicity/rhabdomyolysis with gemfibrozil-statin regimens (but not with fenofibrate-statin regimens).96,118,119
In summary, once maximally tolerated statin therapy has reduced Apo B to the lowest achievable levels, the clinician should consider adding niacin or a sequestrant to potentiate the Apo B– and cardiovascular risk–lowering effects of statins. Results from ongoing trials are eagerly awaited to evaluate the relative merits of combination treatment approaches.
“ATP IV-Cast”: Possible Changes in Upcoming Guidelines
The ATP IV should help specify the clinical circumstances in which Apo vs cholesterol levels would be the best and most informative choice in cardiovascular disease risk prediction (Table 4). In this context, Sniderman et al102 have commented that “if our objective is the best care for each patient, the challenge is to learn how to combine the information from cholesterol and apolipoproteins to enlarge our diagnostic accuracy rather than...to pit one against the other...our objective should be to add information, not eliminate it.”
One might justifiably ask which is the chief “biological culprit” and most worthy treatment target in atherosclerosis: LDL cholesterol, non-HDL cholesterol, or Apo B? Although non-HDL cholesterol includes the cholesterol content of all atherogenic lipoproteins, Apo B includes the number of atherogenic particles in all lipoproteins.2,55 As the ATP IV weighs the potential advantages and pitfalls of using traditional cholesterol measures or Apo measures to target and optimize treatment, the current review would advocate a “both/and” (not “either/or”) posture, in which physicians embrace the use of both Apo B and non-HDL cholesterol levels according to how risk-informative each is in a particular patient or population, as well as which helps to maximize cardioprotective benefits. Apo B should not supplant non-HDL cholesterol because the latter was part of the most recent ATP III guidelines, its measurement is free, and it is still superior to LDL cholesterol.109 Non-HDL cholesterol will most likely remain prominent in ATP IV and may even be placed alongside LDL cholesterol as a primary therapy target.
Apolipoproteins can be measured accurately in the postprandial state. Apolipoprotein assays are also standardized by the World Health Organization, are suitable for automation, and ultimately, with increased volume, can be performed at low cost.120,121 Apo B has advantages as an excellent measure of residual risk (in patients either at baseline or receiving statin treatment) and also as a rational means of helping to inform decisions about which other therapies to add to statins to prevent cardiovascular disease.
CONCLUSION
Many patients have residual cardiovascular risk despite optimal LDL cholesterol management, in part because statins are more effective in reducing cholesterol in LDL, which is heterogeneous, than in reducing Apo B, which is present in each atherogenic particle. Apo B elevations provide a sound measure of residual cardiovascular risk on treatment, act as a parameter of treatment efficacy, and are a rational target for adding other lipid therapies. To minimize CHD risk, clinicians should contemplate the use of lipid therapies to reduce Apo B levels to less than 70 mg/dL in patients at high risk and to less than 90 mg/dL in those at moderately high risk. Statins are the most effective medications to lower Apo B levels. After patients have achieved the lowest possible Apo B value with the highest dose of statins that they can tolerate, clinicians should contemplate adding other lipid-lowering agents (eg, niacin, sequestrants) to potentiate statins' Apo B–lowering effects and to maximize overall cardiovascular risk reduction.
Note Added in Proof
The AIM HIGH study was terminated prematurely for futility, because of a lack of incremental benefit for simvastatin-niacin extended-release combination therapy over simvastatin alone, in these secondary prevention patients with very well-controlled LDL.
Acknowledgments
Assistance in manuscript preparation was provided by Stephen W. Gutkin, BA, Angela Cimmino, PharmD, and Sara Glickstein, PhD, Rete Biomedical Communications Corp (Wyckoff, NJ), with funding from Abbott.
Footnotes
Research support was provided by Abbott Laboratories (Abbott Park, IL). Abbott had the opportunity to review and comment on the publication content; however, all decisions regarding content were made by the author.
Dr Jacobson received no compensation from Abbott. Dr Jacobson discloses that he has served as a consultant for Abbott, Amarin, AstraZeneca, GlaxoSmithKline, Kowa, and Merck.
References
- 1. NCEP Expert Panel Report of the National Cholesterol Education Program Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults: the Expert Panel. Arch Intern Med. 1988;148(1):36-69 [PubMed] [Google Scholar]
- 2. Adult Treatment Panel Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143-3421 [PubMed] [Google Scholar]
- 3. Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227-239 [DOI] [PubMed] [Google Scholar]
- 4. Scandinavian Simvastatin Survival Study Group Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383-1389 [PubMed] [Google Scholar]
- 5. Shepherd J, Cobbe SM, Ford I, et al. ; West of Scotland Coronary Prevention Study Group Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. N Engl J Med. 1995;333(20):1301-1307 [DOI] [PubMed] [Google Scholar]
- 6. Sacks FM, Pfeffer MA, Moye LA, et al. ; Cholesterol and Recurrent Events Trial Investigators The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med. 1996;335(14):1001-1009 [DOI] [PubMed] [Google Scholar]
- 7. Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339(19):1349-1357 [DOI] [PubMed] [Google Scholar]
- 8. Downs JR, Clearfield M, Weis S, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA. 1998;279(20):1615-1622 [DOI] [PubMed] [Google Scholar]
- 9. Heart Protection Study Collaborative Group MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360(9326):7-22 [DOI] [PubMed] [Google Scholar]
- 10. Collins R, Armitage J, Parish S, Sleigh P, Peto R. MRC/BHF Heart Protection Study of cholesterol-lowering with simvastatin in 5963 people with diabetes: a randomised placebo-controlled trial. Lancet. 2003;361(9374):2005-2016 [DOI] [PubMed] [Google Scholar]
- 11. Colhoun HM, Betteridge DJ, Durrington PN, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685-696 [DOI] [PubMed] [Google Scholar]
- 12. Sever PS, Dahlof B, Poulter NR, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial–Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149-1158 [DOI] [PubMed] [Google Scholar]
- 13. Baigent C, Keech A, Kearney PM, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267-1278 [DOI] [PubMed] [Google Scholar]
- 14. Kearney PM, Blackwell L, Collins R, et al. Efficacy of cholesterol-lowering therapy in 18,686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371(9607):117-125 [DOI] [PubMed] [Google Scholar]
- 15. Baigent C, Blackwell L, Emberson J, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barter P, Gotto AM, LaRosa JC, et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N Engl J Med. 2007; 357(13):1301-1310 [DOI] [PubMed] [Google Scholar]
- 17. LaRosa JC, Grundy SM, Waters DD, et al. Intensive lipid lowering with atorvastatin in patients with stable coronary disease. N Engl J Med. 2005; 352(14):1425-1435 [DOI] [PubMed] [Google Scholar]
- 18. Sazonov V, Beetsch J, Phatak H, Wentworth C, Evans M. Association between dyslipidemia and vascular events in patients treated with statins: report from the UK General Practice Research Database. Atherosclerosis. 2010; 208(1):210-216 [DOI] [PubMed] [Google Scholar]
- 19. Barter P. Managing diabetic dyslipidaemia—beyond LDL-C:HDL-C and triglycerides. Atheroscler Suppl. 2006;7(4):17-21 [DOI] [PubMed] [Google Scholar]
- 20. Genest J, Libby P, Gotto AM., Jr Lipoprotein disorders and cardiovascular disease. In: Zipes DP, Libby P, Bonow RO, Braunwald E, eds. Braunwald's Heart Disease: A Textbook of Cardiovascular Medicine. 7th ed. Philadelphia, PA: Elsevier Saunders; 2005:1013-1034 [Google Scholar]
- 21. Rosenson RS. Lp-PLA(2) and risk of atherosclerotic vascular disease. Lancet. 2010;375(9725):1498-1500 [DOI] [PubMed] [Google Scholar]
- 22. Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116(16):1832-1844 [DOI] [PubMed] [Google Scholar]
- 23. Barter PJ, Ballantyne CM, Carmena R, et al. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259(3):247-258 [DOI] [PubMed] [Google Scholar]
- 24. McQueen MJ, Hawken S, Wang X, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372(9634):224-233 [DOI] [PubMed] [Google Scholar]
- 25. Parish S, Peto R, Palmer A, et al. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30(17):2137-2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van der Steeg WA, Boekholdt SM, Stein EA, et al. Role of the apolipoprotein B-apolipoprotein A-I ratio in cardiovascular risk assessment: a case-control analysis in EPIC-Norfolk. Ann Intern Med. 2007;146(9):640-648 [DOI] [PubMed] [Google Scholar]
- 27. Jiang R, Schulze MB, Li T, et al. Non-HDL cholesterol and apolipoprotein B predict cardiovascular disease events among men with type 2 diabetes. Diabetes Care. 2004;27(8):1991-1997 [DOI] [PubMed] [Google Scholar]
- 28. Bruno G, Merletti F, Biggeri A, et al. Effect of age on the association of non-high-density-lipoprotein cholesterol and apolipoprotein B with cardiovascular mortality in a Mediterranean population with type 2 diabetes: the Casale Monferrato study. Diabetologia. 2006;49(5):937-944 [DOI] [PubMed] [Google Scholar]
- 29. Corsetti JP, Zareba W, Moss AJ, Sparks CE. Apolipoprotein B determines risk for recurrent coronary events in postinfarction patients with metabolic syndrome. Atherosclerosis. 2004;177(2):367-373 [DOI] [PubMed] [Google Scholar]
- 30. Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358(9298):2026-2033 [DOI] [PubMed] [Google Scholar]
- 31. Sniderman AD, Jungner I, Holme I, Aastveit A, Walldius G. Errors that result from using the TC/HDL C ratio rather than the apoB/apoA-I ratio to identify the lipoprotein-related risk of vascular disease. J Intern Med. 2006;259(5):455-461 [DOI] [PubMed] [Google Scholar]
- 32. Sharrett AR, Ballantyne CM, Coady SA, et al. Coronary heart disease prediction from lipoprotein cholesterol levels, triglycerides, lipoprotein(a), apolipoproteins A-I and B, and HDL density subfractions: the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. 2001;104(10):1108-1113 [DOI] [PubMed] [Google Scholar]
- 33. Hsia SH, Pan D, Berookim P, Lee ML. A population-based, cross-sectional comparison of lipid-related indexes for symptoms of atherosclerotic disease. Am J Cardiol. 2006;98(8):1047-1052 [DOI] [PubMed] [Google Scholar]
- 34. Sierra-Johnson J, Fisher RM, Romero-Corral A, et al. Concentration of apolipoprotein B is comparable with the apolipoprotein B/apolipoprotein A-I ratio and better than routine clinical lipid measurements in predicting coronary heart disease mortality: findings from a multi-ethnic US population. Eur Heart J. 2009;30(6):710-717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Benn M, Nordestgaard BG, Jensen GB, Tybjaerg-Hansen A. Improving prediction of ischemic cardiovascular disease in the general population using apolipoprotein B: the Copenhagen City Heart Study. Arterioscler Thromb Vasc Biol. 2007;27(3):661-670 [DOI] [PubMed] [Google Scholar]
- 36. St-Pierre AC, Cantin B, Bergeron J, et al. Inflammatory markers and long-term risk of ischemic heart disease in men: a 13-year follow-up of the Quebec Cardiovascular Study. Atherosclerosis. 2005;182(2):315-321 [DOI] [PubMed] [Google Scholar]
- 37. St-Pierre AC, Cantin B, Dagenais GR, et al. Low-density lipoprotein subfractions and the long-term risk of ischemic heart disease in men: 13-year follow-up data from the Quebec Cardiovascular Study. Arterioscler Thromb Vasc Biol. 2005;25(3):553-559 [DOI] [PubMed] [Google Scholar]
- 38. St-Pierre AC, Cantin B, Dagenais GR, Despres JP, Lamarche B. Apolipoprotein-B, low-density lipoprotein cholesterol, and the long-term risk of coronary heart disease in men. Am J Cardiol. 2006;97(7):997-1001 [DOI] [PubMed] [Google Scholar]
- 39. Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non-high-density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112(22):3375-3383 [DOI] [PubMed] [Google Scholar]
- 40. Shai I, Rimm EB, Hankinson SE, et al. Multivariate assessment of lipid parameters as predictors of coronary heart disease among postmenopausal women: potential implications for clinical guidelines. Circulation. 2004;110(18):2824-2830 [DOI] [PubMed] [Google Scholar]
- 41. Everett BM, Kurth T, Buring JE, Ridker PM. The relative strength of C-reactive protein and lipid levels as determinants of ischemic stroke compared with coronary heart disease in women. J Am Coll Cardiol. 2006;48(11): 2235-2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993-2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Pedersen TR, Olsson AG, Faergeman O, et al. Lipoprotein changes and reduction in the incidence of major coronary heart disease events in the Scandinavian Simvastatin Survival Study (4S). Circulation. 1998;97(15):1453-1460 [DOI] [PubMed] [Google Scholar]
- 44. Simes RJ, Marschner IC, Hunt D, et al. Relationship between lipid levels and clinical outcomes in the Long-term Intervention with Pravastatin in Ischemic Disease (LIPID) Trial: to what extent is the reduction in coronary events with pravastatin explained by on-study lipid levels? Circulation. 2002;105(10):1162-1169 [DOI] [PubMed] [Google Scholar]
- 45. Gotto AM Jr, Whitney E, Stein EA, et al. Relation between baseline and on-treatment lipid parameters and first acute major coronary events in the Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TexCAPS). Circulation. 2000;101(5):477-484 [DOI] [PubMed] [Google Scholar]
- 46. Pedersen TR, Faergeman O, Kastelein JJ, et al. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294(19): 2437-2445 [DOI] [PubMed] [Google Scholar]
- 47. Holme I, Cater NB, Faergeman O, et al. Lipoprotein predictors of cardiovascular events in statin-treated patients with coronary heart disease: insights from the Incremental Decrease In End-points Through Aggressive Lipid-lowering Trial (IDEAL). Ann Med. 2008;40(6):456-464 [DOI] [PubMed] [Google Scholar]
- 48. Charlton-Menys V, Betteridge DJ, Colhoun H, et al. Targets of statin therapy: LDL cholesterol, non-HDL cholesterol, and apolipoprotein B in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS). Clin Chem. 2009;55(3):473-480 [DOI] [PubMed] [Google Scholar]
- 49. Charlton-Menys V, Betteridge DJ, Colhoun H, et al. Apolipoproteins, cardiovascular risk and statin response in type 2 diabetes: the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia. 2009;52(2):218-225 [DOI] [PubMed] [Google Scholar]
- 50. Kastelein JJ, van der Steeg WA, Holme I, et al. ; Ideal Study Group Lipids, apolipoproteins, and their ratios in relation to cardiovascular events with statin treatment. Circulation. 2008;117(23):3002-3009 [DOI] [PubMed] [Google Scholar]
- 51. Harper CR, Jacobson TA. Using apolipoprotein B to manage dyslipidemic patients: time for a change? Mayo Clin Proc. 2010;85(5):440-445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gaw A, Packard CJ, Murray EF, et al. Effects of simvastatin on apoB metabolism and LDL subfraction distribution. Arterioscler Thromb. 1993; 13(2):170-189 [DOI] [PubMed] [Google Scholar]
- 53. Caslake MJ, Stewart G, Day SP, et al. Phenotype-dependent and -independent actions of rosuvastatin on atherogenic lipoprotein subfractions in hyperlipidaemia. Atherosclerosis. 2003;171(2):245-253 [DOI] [PubMed] [Google Scholar]
- 54. Ballantyne CM, Raichlen JS, Cain VA. Statin therapy alters the relationship between apolipoprotein B and low-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol targets in high-risk patients: the MERCURY II (Measuring Effective Reductions in Cholesterol Using Rosuvastatin) trial. J Am Coll Cardiol. 2008;52(8):626-632 [DOI] [PubMed] [Google Scholar]
- 55. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus statement from the American Diabetes Association and the American College of Cardiology Foundation. Diabetes Care. 2008;31(4):811-822 [DOI] [PubMed] [Google Scholar]
- 56. Wierzbicki AS, Hardman T, Prince WT. Future challenges for microsomal transport protein inhibitors. Curr Vasc Pharmacol. 2009;7(3):277-286 [DOI] [PubMed] [Google Scholar]
- 57. Akdim F, Stroes ES, Sijbrands EJ, et al. Efficacy and safety of mipomersen, an antisense inhibitor of apolipoprotein B, in hypercholesterolemic subjects receiving stable statin therapy. J Am Coll Cardiol. 2010;55(15):1611-1618 [DOI] [PubMed] [Google Scholar]
- 58. Cuchel M, Bloedon LT, Szapary PO, et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007; 356(2):148-156 [DOI] [PubMed] [Google Scholar]
- 59. Jones PH, Hunninghake DB, Ferdinand KC, et al. Effects of rosuvastatin versus atorvastatin, simvastatin, and pravastatin on non-high-density lipoprotein cholesterol, apolipoproteins, and lipid ratios in patients with hypercholesterolemia: additional results from the STELLAR trial. Clin Ther. 2004;26(9):1388-1399 [DOI] [PubMed] [Google Scholar]
- 60. Blankenhorn DH, Selzer RH, Crawford DW, et al. Beneficial effects of colestipol-niacin therapy on the common carotid artery: two- and four-year reduction of intima-media thickness measured by ultrasound. Circulation. 1993;88(1):20-28 [DOI] [PubMed] [Google Scholar]
- 61. Brown G, Albers JJ, Fisher LD, et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N Engl J Med. 1990;323(19):1289-1298 [DOI] [PubMed] [Google Scholar]
- 62. Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med. 2001;345(22):1583-1592 [DOI] [PubMed] [Google Scholar]
- 63. Keech A, Simes RJ, Barter P, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005; 366(9500):1849-1861 [DOI] [PubMed] [Google Scholar]
- 64. Rubins HB, Robins SJ, Collins D, et al. ; Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. N Engl J Med. 1999;341(6):410-418 [DOI] [PubMed] [Google Scholar]
- 65. Robins SJ, Collins D, Wittes JT, et al. Relation of gemfibrozil treatment and lipid levels with major coronary events: VA-HIT: a randomized controlled trial. JAMA. 2001;285(12):1585-1591 [DOI] [PubMed] [Google Scholar]
- 66. Coronary Drug Project Research Group Clofibrate and niacin in coronary heart disease. JAMA. 1975;231(4):360-381 [PubMed] [Google Scholar]
- 67. Ballantyne CM, Davidson MH, McKenney J, Keller LH, Bajorunas DR, Karas RH. Comparison of the safety and efficacy of a combination tablet of niacin extended release and simvastatin vs simvastatin monotherapy in patients with increased non-HDL cholesterol (from the SEACOAST I study). Am J Cardiol. 2008;101(10):1428-1436 [DOI] [PubMed] [Google Scholar]
- 68. Guyton JR, Brown BG, Fazio S, Polis A, Tomassini JE, Tershakovec AM. Lipid-altering efficacy and safety of ezetimibe/simvastatin coadministered with extended-release niacin in patients with type IIa or type IIb hyperlipidemia. J Am Coll Cardiol. 2008;51(16):1564-1572 [DOI] [PubMed] [Google Scholar]
- 69. McKenney JM, Jones PH, Bays HE, et al. Comparative effects on lipid levels of combination therapy with a statin and extended-release niacin or ezetimibe versus a statin alone (the COMPELL study). Atherosclerosis. 2007;192(2):432-437 [DOI] [PubMed] [Google Scholar]
- 70. Robins SJ, Rubins HB, Faas FH, et al. Insulin resistance and cardiovascular events with low HDL cholesterol: the Veterans Affairs HDL Intervention Trial (VA-HIT). Diabetes Care. 2003;26(5):1513-1517 [DOI] [PubMed] [Google Scholar]
- 71. Ginsberg HN, Elam MB, Lovato LC, et al. ; Action to Control Cardiovascular Risk in Diabetes (ACCORD) Study Group Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563-1574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Taskinen MR, Barter PJ, Ehnholm C, et al. Ability of traditional lipid ratios and apolipoprotein ratios to predict cardiovascular risk in people with type 2 diabetes. Diabetologia. 2010;53(9):1846-1855 [DOI] [PubMed] [Google Scholar]
- 73. Elam M, Lovato L, Ginsberg H. The ACCORD-Lipid study: implications for treatment of dyslipidemia in type 2 diabetes mellitus. Clin Lipidol. 2011;6(1):9-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bruckert E, Labreuche J, Deplanque D, Touboul PJ, Amarenco P. Fibrates effect on cardiovascular risk is greater in patients with high triglyceride levels or atherogenic dyslipidemia profile: a systematic review and metaanalysis. J Cardiovasc Pharmacol. 2010;57(2):267-272 [DOI] [PubMed] [Google Scholar]
- 75. Roth EM, McKenney JM, Kelly MT, et al. Efficacy and safety of rosuvastatin and fenofibric acid combination therapy versus simvastatin monotherapy in patients with hypercholesterolemia and hypertriglyceridemia: a randomized, double-blind study. Am J Cardiovasc Drugs. 2010;10(3):175-186 [DOI] [PubMed] [Google Scholar]
- 76. Goldberg AC, Bays HE, Ballantyne CM, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with atorvastatin in patients with mixed dyslipidemia. Am J Cardiol. 2009;103(4):515-522 [DOI] [PubMed] [Google Scholar]
- 77. Jones PH, Davidson MH, Kashyap ML, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: a phase 3 study. Atherosclerosis. 2009;204(1):208-215 [DOI] [PubMed] [Google Scholar]
- 78. Roth EM, Rosenson RS, Carlson DM, et al. Efficacy and safety of rosuvastatin 5 mg in combination with fenofibric acid 135 mg in patients with mixed dyslipidemia: a phase 3 study. Cardiovasc Drugs Ther. 2010;24(5-6):421-428 [DOI] [PubMed] [Google Scholar]
- 79. Mohiuddin SM, Pepine CJ, Kelly MT, et al. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: a phase 3, randomized, controlled study. Am Heart J. 2009;157(1):195-203 [DOI] [PubMed] [Google Scholar]
- 80. Grundy SM, Vega GL, Tomassini JE, Tershakovec AM. Correlation of non-high-density lipoprotein cholesterol and low-density lipoprotein cholesterol with apolipoprotein B during simvastatin + fenofibrate therapy in patients with combined hyperlipidemia (a subanalysis of the SAFARI trial). Am J Cardiol. 2009;104(4):548-553 [DOI] [PubMed] [Google Scholar]
- 81. Davidson MH, Rooney MW, Drucker J, Eugene GH, Oosman S, Beckert M. Efficacy and tolerability of atorvastatin/fenofibrate fixed-dose combination tablet compared with atorvastatin and fenofibrate monotherapies in patients with dyslipidemia: a 12-week, multicenter, double-blind, randomized, parallel-group study. Clin Ther. 2009;31(12):2824-2838 [DOI] [PubMed] [Google Scholar]
- 82. Grundy SM, Vega GL, Yuan Z, Battisti WP, Brady WE, Palmisano J. Effectiveness and tolerability of simvastatin plus fenofibrate for combined hyperlipidemia (the SAFARI trial). Am J Cardiol. 2005;95(4):462-468 [DOI] [PubMed] [Google Scholar]
- 83. SHARP Collaborative Group Study of Heart and Renal Protection (SHARP): randomized trial to assess the effects of lowering low-density lipoprotein cholesterol among 9,438 patients with chronic kidney disease. Am Heart J. 2010;160(5):785-794 [DOI] [PubMed] [Google Scholar]
- 84. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008; 359(13):1343-1356 [DOI] [PubMed] [Google Scholar]
- 85. Holme I, Boman K, Brudi P, et al. Observed and predicted reduction of ischemic cardiovascular events in the Simvastatin and Ezetimibe in Aortic Stenosis trial. Am J Cardiol. 2010;105(12):1802-1808 [DOI] [PubMed] [Google Scholar]
- 86. Ballantyne CM, Weiss R, Moccetti T, et al. Efficacy and safety of rosuvastatin 40 mg alone or in combination with ezetimibe in patients at high risk of cardiovascular disease (results from the EXPLORER study). Am J Cardiol. 2007;99(5):673-680 [DOI] [PubMed] [Google Scholar]
- 87. Kane JP, Malloy MJ, Ports TA, Phillips NR, Diehl JC, Havel RJ. Regression of coronary atherosclerosis during treatment of familial hypercholesterolemia with combined drug regimens. JAMA. 1990;264(23):3007-3012 [PubMed] [Google Scholar]
- 88. Blankenhorn DH, Nessim SA, Johnson RL, Sanmarco ME, Azen SP, Cashin-Hemphill L. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA. 1987;257(23):3233-3240 [PubMed] [Google Scholar]
- 89. Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet. 2007;369(9567):1090-1098 [DOI] [PubMed] [Google Scholar]
- 90. GISSI Study Group (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto miocardico) Dietary supplementation with n-3 polyunsaturated fatty acids and vitamin E after myocardial infarction: results of the GISSI-Prevenzione trial [published corrections appear in Lancet. 2007;369(9556):106 and 2001;357(9256):642]. Lancet. 1999;354(9177):447-455 [PubMed] [Google Scholar]
- 91. Jacobson TA. Beyond lipids: the role of omega-3 fatty acids from fish oil in the prevention of coronary heart disease. Curr Atheroscler Rep. 2007;9(2):145-153 [DOI] [PubMed] [Google Scholar]
- 92. Jacobson TA, Miller M, Schaefer EJ. Hypertriglyceridemia and cardiovascular risk reduction. Clin Ther. 2007;29(5):763-777 [DOI] [PubMed] [Google Scholar]
- 93. Maki KC, McKenney JM, Reeves MS, Lubin BC, Dicklin MR. Effects of adding prescription omega-3 acid ethyl esters to simvastatin (20 mg/day) on lipids and lipoprotein particles in men and women with mixed dyslipidemia. Am J Cardiol. 2008;102(4):429-433 [DOI] [PubMed] [Google Scholar]
- 94. Bays HE, McKenney J, Maki KC, Doyle RT, Carter RN, Stein E. Effects of prescription omega-3-acid ethyl esters on non–high-density lipoprotein cholesterol when coadministered with escalating doses of atorvastatin. Mayo Clin Proc. 2010;85(2):122-128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kromhout D, Giltay EJ, Geleijnse JM. n-3 fatty acids and cardiovascular events after myocardial infarction. N Engl J Med. 2010;363(21):2015-2026 [DOI] [PubMed] [Google Scholar]
- 96. Genest J, McPherson R, Frohlich J, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult—2009 recommendations. Can J Cardiol. 2009;25(10):567-579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. NHANES Investigators National Health and Nutrition Examination Survey (NHANES) 2005-2006 documentation, codebook, and frequencies triglyceride, LDL-cholesterol and Apoliprotein (ApoB) (TRIGLY_D). http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/TRIGLY_D.htm#Component_Description Accessed May 5, 2011
- 98. O'Keefe JH Jr, Cordain L, Harris WH, Moe RM, Vogel R. Optimal low-density lipoprotein is 50 to 70 mg/dl: lower is better and physiologically normal. J Am Coll Cardiol. 2004;43(11):2142-2146 [DOI] [PubMed] [Google Scholar]
- 99. Sniderman A, Williams K, Cobbaert C. ApoB versus non-HDL-C: what to do when they disagree. Curr Atheroscler Rep. 2009;11(5):358-363 [DOI] [PubMed] [Google Scholar]
- 100. Brunzell JD, Davidson M, Furberg CD, et al. Lipoprotein management in patients with cardiometabolic risk: consensus conference report from the American Diabetes Association and the American College of Cardiology Foundation. J Am Coll Cardiol. 2008;51(15):1512-1524 [DOI] [PubMed] [Google Scholar]
- 101. Sniderman AD. Differential response of cholesterol and particle measures of atherogenic lipoproteins to LDL-lowering therapy: implications for clinical practice. J Clin Lipidol. 2008;2(1):36-42 [DOI] [PubMed] [Google Scholar]
- 102. Sniderman AD, Williams K, McQueen MJ, Furberg CD. When is equal not equal? J Clin Lipidol. 2010;4(2):83-88 [DOI] [PubMed] [Google Scholar]
- 103. Ridker PM, Danielson E, Fonseca FA, et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359(21):2195-2207 [DOI] [PubMed] [Google Scholar]
- 104. Sniderman AD, Furberg CD, Keech A, et al. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin treatment. Lancet. 2003;361(9359):777-780 [DOI] [PubMed] [Google Scholar]
- 105. Walldius G, Jungner I. The apoB/apoA-I ratio: a strong, new risk factor for cardiovascular disease and a target for lipid-lowering therapy—a review of the evidence. J Intern Med. 2006;259(5):493-519 [DOI] [PubMed] [Google Scholar]
- 106. Liem AH, van de Woestijne AP, Roeters van Lennep HW, Zwinderman AH, van der Steeg WA, Jukema JW. ApoB/A1 and LDL-C/HDL-C and the prediction of cardiovascular risk in statin-treated patients. Curr Med Res Opin. 2008;24(2):359-364 [DOI] [PubMed] [Google Scholar]
- 107. Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42(12):1355-1363 [DOI] [PubMed] [Google Scholar]
- 108. Lavie CJ, Milani RV, O'Keefe JH. To B or not to B: is non-high-density lipoprotein cholesterol an adequate surrogate for apolipoprotein B? Mayo Clin Proc. 2010;85(5):446-450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Ramjee V, Sperling LS, Jacobson TA. Non-HDL versus ApoB in cardiovascular risk stratification: do the math. J Am Coll Cardiol. In press [DOI] [PubMed] [Google Scholar]
- 110. Sniderman AD, Williams K, Contois JH, et al. A meta-analysis of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B as markers of cardiovascular risk. Circ Cardiovasc Qual Outcomes. 2011;4(3):337-345 [DOI] [PubMed] [Google Scholar]
- 111. Luc G, Bard JM, Ferrieres J, et al. ; PRIME Study Group Value of HDL cholesterol, apolipoprotein A-I, lipoprotein A-I, and lipoprotein A-I/A-II in prediction of coronary heart disease: the PRIME Study. Arterioscler Thromb Vasc Biol. 2002;22(7):1155-1161 [DOI] [PubMed] [Google Scholar]
- 112. Meisinger C, Loewel H, Mraz W, Koenig W. Prognostic value of apolipoprotein B and A-I in the prediction of myocardial infarction in middle-aged men and women: results from the MONICA/KORA Augsburg cohort study. Eur Heart J. 2005;26(3):271-278 [DOI] [PubMed] [Google Scholar]
- 113. Briel M, Ferreira-Gonzalez, You JJ, et al. Association between change in high density lipoprotein cholesterol and cardiovascular disease morbidity and mortality: systematic review and meta-regression analysis. BMJ. 2009;338:b92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Davidson MH, Maki KC, Pearson TA, et al. Results of the National Cholesterol Education (NCEP) Program Evaluation ProjecT Utilizing Novel E-Technology (NEPTUNE) II survey and implications for treatment under the recent NCEP Writing Group recommendations. Am J Cardiol. 2005;96(4):556-563 [DOI] [PubMed] [Google Scholar]
- 115. Bruckert E, Labreuche J, Amarenco P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis. 2010;210(2):353-361 [DOI] [PubMed] [Google Scholar]
- 116. Jialal I, Abby SL, Misir S, Nagendran S. Concomitant reduction in low-density lipoprotein cholesterol and glycated hemoglobin with colesevelam hydrochloride in patients with type 2 diabetes: a pooled analysis. Metab Syndr Relat Disord. 2009;7(3):255-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet. 2010;375(9729):1875-1884 [DOI] [PubMed] [Google Scholar]
- 118. Jacobson TA. Myopathy with statin-fibrate combination therapy: clinical considerations. Nat Rev Endocrinol. 2009;5(9):507-518 [DOI] [PubMed] [Google Scholar]
- 119. Jacobson TA. Toward ``pain-free'' statin prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc. 2008;83(6): 687-700 [DOI] [PubMed] [Google Scholar]
- 120. Hlatky MA, Greenland P, Arnett DK, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119(17):2408-2416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jacobson TA, Harper CR. Using apolipoprotein B to manage dyslipidemia [letter reply]. Mayo Clin Proc. 2010;85(8):769-771 [DOI] [PMC free article] [PubMed] [Google Scholar]