Abstract
Objective
To determine associations between older adults’ baseline painful medical conditions and their 10-year drinking behavior, and whether personal and life context characteristics moderate these associations.
Method
At baseline, then 1, 4, and 10 years later, late-middle-aged community residents (M=61 years; n=1,291) were surveyed regarding their painful medical conditions, use of alcohol, and personal and life context characteristics. Latent growth modeling was used to determine concurrent and prospective relationships between painful medical conditions and 10-year drinking behavior, and moderating effects of personal and life context characteristics on these relationships.
Results
At baseline, individuals reporting more numerous painful medical conditions consumed alcohol less frequently, but had more frequent drinking problems, than did individuals with fewer such conditions. Being female and having more interpersonal social resources strengthened the association between painful medical conditions and less ethanol consumed. For men more so than women, more numerous painful medical conditions were associated with more frequent drinking problems. Baseline painful medical conditions alone had no prospective effect on 10-year change in drinking behavior, but being older and having more interpersonal social resources made it more likely that baseline painful medical conditions would predict decline over time in frequency of alcohol consumption and drinking problems.
Conclusions
Late-middle-aged individuals who have more numerous painful medical conditions reduce alcohol consumption but nonetheless remain at risk for more frequent drinking problems. Gender, age, and interpersonal social resources moderate the influence of painful medical conditions on late-life alcohol use. These results imply that older individuals with pain are at little immediate or long-term risk for increased alcohol consumption, but clinicians should remain alert to drinking problems among their older pain patients, especially men.
Keywords: pain, alcohol, drinking problems, older adults
Pain is a nearly ubiquitous experience for adults of all ages, and it increases in prevalence and salience as individuals grow older (1–7). Furthermore, most late-middle-aged adults continue to use alcohol and have potential to engage in risky or problem drinking as they grow older (8–15). However, almost no research has focused on associations between late-life pain and use of alcohol. In this study we extend earlier research on the pain-alcohol relationship by examining in a sample of older adults: (a) concurrent and prospective associations between baseline painful medical conditions and late-life drinking behavior (amount of ethanol consumed, frequency of drinking, and frequency of drinking problems), and (b) moderating effects of personal and life context characteristics on these associations.
Several hypotheses have been advanced to explain associations between more elevated, widespread, and chronic pain, and higher levels of alcohol consumption and more frequent negative consequences of alcohol use (drinking problems) (16), but there is very little empirical evidence to support or refute these hypotheses. For example, the “self medication hypothesis” suggests that individuals increase their alcohol consumption in order to combat pain and associated distress (16). The “stress-diathesis hypothesis” conceives of pain as a stressor with potential to overwhelm individuals’ adaptational resources, resulting in substance misuse (17). Finally, the “risky lifestyle hypothesis” posits that pain and alcohol use are associated because a lifestyle that includes substance misuse increases the risk of painful illness and injuries (18, 19).
To date, only the self-medication and risky lifestyle hypotheses have been examined empirically. With regard to the self-medication hypothesis, several studies of mixed-age samples have shown positive associations between various pain characteristics (e.g., location; chronicity) and larger amounts of alcohol consumed and more frequent drinking (20–23), as well as elevated self-reported use of alcohol to manage pain (24, 25). However, other studies have demonstrated a negative association (26, 27), or no association (28, 29), between various pain characteristics and alcohol consumption.
With respect to the risky lifestyle hypothesis, several studies substantiate a positive association between pain and substance use disorders (16, 30–32), and between pain characteristics and drinking behavior indicative of alcohol abuse or dependence (19, 24, 33–36). However, the positive association between pain and alcohol use disorders does not consistently hold (37). Furthermore, although the risky-lifestyle hypothesis implies that substance misuse temporally precedes pain-related illness and injury, the direction of causality in the relationship between substance misuse and pain has not yet been firmly established (cf. 32,33).
Almost all of these studies have focused on younger and mixed-age samples and have assessed alcohol use with single measures (e.g., number of drinks per week) which precludes consideration of effects of pain on distinct features of alcohol use, such as amount of ethanol consumed per drinking occasion, the frequency with which alcohol is consumed, and negative consequences of alcohol use (drinking problems). Perhaps more important, nearly all of these studies have cross-sectional designs. Thus, they cannot address direction of causality in the pain-drinking association, nor whether pain at baseline prospectively influences subsequent rate of change in use of alcohol. From a preventive health care perspective, it is important to know whether pain experienced by late-middle-aged individuals prospectively influences change in their subsequent use of alcohol. This research approach might provide findings that inform clinical treatment practices aimed at preventing adverse effects of pain on older adults’ drinking behavior.
Finally, studies thus far provide little information about which individuals, under what circumstances, might be more likely to respond to pain with change in alcohol consumption and drinking problems. Gender (38, 39), chronological age (2, 6), and race (40, 41) are each known or suspected to influence the experience of pain, as well as alcohol consumption and drinking problems (9, 42, 43). Thus, these personal characteristics should be considered potential moderators of the pain-drinking relationship.
Having a lifetime history of problem drinking, reliance on avoidance coping, and life context (stressors and social resources), may also moderate the pain-alcohol association. Having a history of drinking problems has enduring negative consequences for older drinkers (15); these may also include increased risk that pain will result in more negative consequences of alcohol use. Previous research has shown the detrimental effects of avoidance coping strategies on drinking outcomes (44), and that their use can strengthen the relationship between stressors and drinking problems (45–47). Finally, results from several studies imply that associations between pain and drinking behavior may be strengthened by encapsulation within more stressful life contexts, and within those low on interpersonal resources (48–52).
In sum, this study addresses three questions: (1) Among late-middle-aged individuals at baseline, is there a positive concurrent association between number of painful medical conditions and drinking behavior (amount of ethanol consumed, frequency of drinking, and frequency of drinking problems)? (2) Is there a positive, prospective relationship between number of painful medical conditions at baseline, and subsequent rate of change in older adults’ drinking behavior over a 10-year interval? and (3) Are concurrent and prospective relationships between baseline painful medical conditions and drinking behavior moderated by personal characteristics (gender, age, race, history of drinking problems, avoidance coping responses) and life context (interpersonal stressors and social resources)?
Method
Sample
Sample participants were recruited from a pool of community residents who had had contact with outpatient clinics, to obtain a variety of medical treatments, sometime within the past three years, and were between the ages of 55 and 65. Because of our interest in drinking behavior, lifetime abstainers and very infrequent drinkers were excluded from the sample.
Telephone contact was made successfully with 96% of eligible respondents, and 96% (n = 2,125) of these individuals agreed to participate in the first wave of data collection. Overall, 1,884 (89%) of the individuals agreeing to participate completed the baseline data collection (see 53,54 for additional recruitment details). Informed consent was obtained from all participants. This research was approved by the institutional review board of Stanford University.
The baseline sample was comparable to similarly-aged community samples with regard to such health characteristics as prevalence of chronic illness and hospitalization (53). Participants were followed 1 and 4 years after baseline; a 94% response rate was attained at each of these follow-ups. Ten years after baseline, 93% of all surviving participants (n = 1,291) completed another follow-up. There were no statistically significant differences between these surviving participants and surviving nonparticipants (n = 68) on any demographic characteristics or baseline drinking variables. Compared with the surviving participants (n = 1,291), participants who died or were too seriously impaired by illness (e.g., stroke) to participate further in the study (n = 525; 93% deceased, 7% too ill) were more likely to be male, unmarried, and non-white, and to have somewhat less education and lower income at baseline. However, there was no difference between these groups on baseline drinking variables.
At baseline, the mean age in the sample of 1,291 participants was 61 (SD = 3.2) years; the sample comprised 41% women (n=529) and 59% men (n = 762). Overall, 92% of the participants were White and 71% of them were married.
Measures
Data were collected through a combination of mailed surveys and telephone follow-ups.
Pain and Drinking Behavior
Painful medical conditions
Painful medical conditions were assessed with items from the Life Stressors and Resources Inventory (LISRES; 55). Medical conditions items in the LISRES health stressors subscale comprise a list of 13 serious medical conditions diagnosed by a physician; 5 of them indicate back pain, chest pain, headache, stomach pain, and joint pain. Participants were asked whether they had, in the past year, experienced (0=no, 1=yes) each of the LISRES medical conditions. Participants’ affirmative responses to the 5 painful medical conditions were summed to comprise a count of painful medical conditions at baseline.
We combined the pain items to comprise the global painful medical conditions measure for two reasons. First, preliminary data analyses, focusing separately on the relationships of pain in specific body locations (head, chest, stomach, back, joints) with drinking outcomes, showed similar pain-drinking relationships across specific body locations and the summed painful medical conditions measure. Second, frequency distributions showed that, although most (64%) participants had at least one painful medical condition, participants, on average, reported having only 1 or 2 painful medical conditions at baseline. Thus, the measure of painful medical conditions in essence indicated presence of pain in one or more body locations, occuring sometime in the year prior to baseline.
Drinking behavior
At baseline, then at the 1-year, 4-year, and 10-year follow-ups, three drinking indices were calculated using items from the Health and Daily Living Form (56). Amount of ethanol consumed was assessed by first determining the number of drinks within each of three beverage categories (wine, beer, and distilled spirits) participants had consumed, on usual drinking occasions, during the last month, then converting them to ounces of ethanol and summing ounces of ethanol across beverages. Frequency of drinking was calculated by summing responses to three questions, scored on a 5-point scale, that asked how often during the week (never to nearly every day) participants typically consumed wine, beer, and distilled spirits during the last month. Frequency of drinking problems was assessed with the Drinking Problem Index (DPI; 57,58) by summing frequency (0=never to 4=often) responses to 17 items tapping negative consequences of alcohol consumption, including physical problems (e.g., craving for alcohol), psychological difficulties (e.g., feeling confused after drinking), and social conflicts (e.g., family members’ complaints about participant’s use of alcohol) during the past year. The DPI has high internal consistency (α=.94), good construct validity (53, 57, 59), and acceptable sensitivity and specificity for identification of late-middle-aged and older adults who have problems with alcohol (60).
Moderating Variables
Personal characteristics
In addition to gender (0=female, 1= male), baseline age (range=55 to 65 years), and race (0=non-White, 1=White), personal characteristics included history of drinking problems and reliance on avoidance coping. Lifetime history of drinking problems was assessed with DPI items in the baseline survey and in a brief pre-baseline screening inventory of negative consequences of alcohol use (0=no current or past drinking problems; 1=current and/or past drinking problems). Coping was assessed with the Coping Responses Inventory (CRI; 61,62), which asks participants to identify their most important stressor of the past 12 months, then to rate the frequency with which they used specific avoidance and approach strategies to manage it. CRI subscales have internal consistencies ranging from .61 to .74 and are moderately positively intercorrelated (average r =.29; 55). To calculate reliance on avoidance coping we divided participants’ summed CRI avoidance coping subscale scores by their summed responses to the avoidance and approach coping subscales.
Life context
Participants’ interpersonal stressors and social resources were assessed with the Life Stressors and Social Resources Inventory (LISRES; 55). Four domains of interpersonal stressors (i.e., spouse/partner, child, extended family, and friends) were assessed using a set of five items (e.g., “Is he or she critical or disapproving of you”; “Do you have fights or arguments with him or her”) which participants rated on 5-point scales varying from 0=never to 4=often. These four indices were summed, then divided by the number of life stressor indices completed by the participant (e.g., participants without extended family completed only 3 of the 4 interpersonal life stressors indices) to create a weighted average measure of overall interpersonal stressors.
Interpersonal social resources were likewise assessed in the domains of spouse/partner, children, extended family, and friends. Within each domain, five items (e.g., “Can you count on him or her to help you when you need it”; “Does he or she really understand how you feel about things”) were rated on 5-point scales varying from 0=never to 4=often. These indices were summed, then divided by the number of indices completed by the participant, to create a weighted average measure of overall interpersonal resources. Internal consistencies of the individual interpersonal stressor subscales ranged from .77 to .86; those for individual interpersonal resources subscales ranged from .82 to .92. These subscales have good concurrent and predictive validity as indicated by their relation to measures of functioning such as depression (56).
Summary of Analyses
We used Mplus software (version 5.12; 64) to analyze the data. We first determined descriptive statistics at the group level for participants’ pain and drinking characteristics. Next, we conducted latent growth modeling (63;64) to describe participants’ unconditional 10-year drinking trajectories, to identify effects of baseline painful medical conditions on drinking trajectory growth characteristics, and to determine moderating effects of participants’ personal characteristics (gender, age, race, history of drinking problems, and avoidance coping responses) and life context (interpersonal stressors and social resources) on the relationships between baseline painful medical conditions and participants’ growth trajectory characteristics.
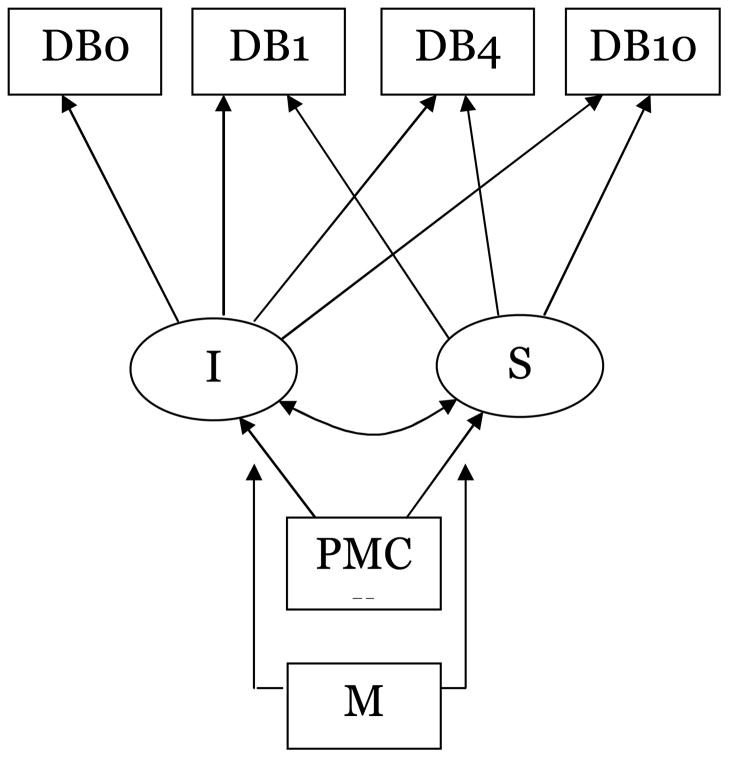
Figure 1 summarizes the latent growth model examined in this study. Participants’ drinking trajectories comprised measured information about their drinking behavior (i.e., amount of ethanol consumed, frequency of drinking, and drinking problem frequency) at four assessment points (DB0, DB1, DB4, DB10). This information was linked to latent growth parameters I (“intercept”, the estimated average initial level of drinking behavior), and S (“slope”, estimated average linear growth in drinking behavior) using the time metric of years since baseline assessment (0, 1, 4, and 10 years). Important but not shown in Figure 1, for simplicity, are variance in the I and S growth parameters. Measured variable PMC represents baseline painful medical conditions, hypothesized in this study to influence the initial level and growth in participants’ 10-year drinking trajectories. Measured variable M represents moderating variables (e.g., gender, age, race, history of drinking problems) hypothesized to influence the relationship between baseline painful medical conditions and growth parameters I and S.
Figure 1.
Latent growth model: Effect of baseline painful medical conditions on 10- drinking trajectories
Notes. DB0, DB1, DB4, DB10=measured drinking behavior (amount of ethanol consumed; frequency of drinking; frequency of drinking problems) variables assessed at baseline and 1, 4, and 10 years later; I=estimated average initial level of drinking behavior; S=estimated average linear growth rate; PMC= baseline painful medical conditions; M=moderating variable (gender, age, race, history of drinking problems, avoidance coping responses, interpersonal stressors, social resources) hypothesized to influence the relationship between baseline painful medical conditions and growth parameters I and S.
We first estimated unconditional linear growth models (i.e., models describing the drinking trajectory growth characteristics, without painful medical conditions as predictor) that describe participants’ drinking behavior over a 10-year interval. In Table 2, iM = mean initial status, and sM = mean linear growth rate, in drinking behavior.
Table 2.
Effect of Painful Medical Conditions on Growth in 10-year Ethanol Consumption, Frequency of Drinking, and Frequency of Drinking Problems
| Amount of Ethanol | Outcomes | Frequency of Drinking Problems | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency of Drinking | ||||||||||||||
| iM | iC | sM | sC | iM | iC | sM | sC | iM | iC | sM | sC | |||
| Unconditional linear growth models | 2.02** | --- | −0.04** | --- | 4.08** | --- | −.06** | --- | 2.85** | --- | −0.12** | --- | ||
| Predictor | Moderator | |||||||||||||
| #PMC | ----- | 2.13** | −0.05 | −0.06** | −0.01 | 3.74** | −0.27** | −0.10** | −0.00 | 3.38** | 0.29* | −0.20** | −0.01 | |
| #PMC | Gender: | Male | 2.38** | 0.03a | −0.06** | −0.01 | 3.99** | −0.16 | −0.09** | −0.01 | 4.02** | 0.62**b | −0.22** | −0.03 |
| Female | 1.65** | − 0.12**a |
−0.07** | 0.00 | 3.22** | −0.37** | −0.11** | 0.01 | 2.21** | −0.02b | −0.17** | 0.01 | ||
| #PMC | Age: | Younger | 2.22** | −0.03 | −0.07** | −0.01 | 3.69** | −0.35** | −0.09** | 0.01 | 3.98** | 0.29 | −0.24** | 0.01a |
| Older | 1.97** | −0.08 | 0.01** | −0.01 | 3.83** | −0.14 | −0.12** | −0.02 | 2.41** | 0.28 | −0.09* | −0.04*a | ||
| #PMC | Race: | Non-White | 2.11** | 0.11 | −0.07* | −0.05 | 3.24** | −0.06 | −0.14** | −0.04 | 3.30** | 0.42 | −0.20** | −0.07 |
| White | 2.00** | −0.07 | −0.03** | −0.00 | 4.15** | −0.29** | −0.06** | 0.00 | 2.71** | 0.24 | −0.10 | −0.01 | ||
| #PMC | History of Drinking Problems: | No | 1.51** | −0.05 | −0.01 | −0.01 | 3.72** | −0.13 | −0.08** | −0.00 | 0.52** | 0.01 | −0.02 | −0.00 |
| Yes | 2.37** | −0.07 | −0.08** | −0.01 | 3.75** | −0.32** | −0.10 | −0.00 | 4.55** | 0.24 | −0.27** | −0.01 | ||
| #PMC | Percentage Avoidance Coping: | Lower | 1.80** | −0.08 | −0.03** | −0.00 | 3.34** | −0.27* | −0.05* | −0.01 | 2.25** | 0.27 | −0.12** | −0.03 |
| Higher | 2.35** | −0.06 | −0.08** | −0.00 | 3.98** | −0.26* | −0.13** | 0.00 | 4.22** | 0.20 | −0.26 | −0.01 | ||
| #PMC | Spouse/partner Stressors: | Lower | 1.88** | −0.06 | −0.05** | −0.00 | 3.80** | −0.12 | −0.10** | −0.02 | 2.87** | 0.13 | −0.20** | −0.02 |
| Higher | 2.26** | 0.00 | −0.04** | −0.01 | 4.00** | −0.25* | −0.09** | 0.01 | 4.18** | 0.41 | −0.21** | 0.03 | ||
| #PMC | Family Stressors: | Lower | 2.02** | −0.07 | −0.04** | −0.00 | 3.79** | −0.21* | −0.10** | 0.00 | 2.97** | 0.13 | −0.17** | −0.00 |
| Higher | 2.28** | −0.05 | −0.08** | −0.01 | 3.76** | −0.28* | −0.09** | −0.00 | 3.96** | 0.38 | −0.24** | −0.03 | ||
| #PMC | Friend Stressors: | Lower | 2.07** | −0.04 | −0.07** | −.01* | 3.47** | −0.16 | −0.09** | −0.01 | 2.50** | 0.17 | −0.13** | −0.02 |
| Higher | 2.23** | −0.07 | −0.05** | 0.00 | 4.10** | −0.38** | −0.11** | 0.00 | 4.33** | 0.32 | −0.26** | −0.01 | ||
| #PMC | Overall Interpersonal Stressors: | Lower | 1.84** | −0.07 | −0.05** | −0.00 | 3.41** | −0.23 | −0.08 | −0.01 | 2.27** | −0.00 | −0.14** | −0.01 |
| Higher | 2.30** | −0.08 | −0.06** | −0.01 | 4.03** | −0.33 | −0.11** | 0.00 | 4.25** | 0.29 | −0.26** | −0.01 | ||
| #PMC | Spouse/partner Resources | Lower | 2.30** | 0.02 | −0.06** | −0.01 | 4.08** | −0.17 | −0.11** | 0.01a | 4.34** | 0.45* | −0.25** | −0.03 |
| Higher | 1.81** | −0.11 | −0.03* | −0.01 | 3.47** | −0.13 | −0.04 | −0.03*a | 1.65** | 0.11 | −0.07* | −0.03 | ||
| #PMC | Family Resources | Lower | 2.30** | −0.02 | −0.06** | −0.01 | 3.87** | −0.17 | −0.10** | −0.00 | 3.88** | 0.42* | −0.22** | −0.02 |
| Higher | 1.97** | −0.09 | −0.06** | −0.00 | 3.62** | −0.33** | −0.09** | 0.00 | 2.91** | 0.07 | −0.18** | −0.01 | ||
| #PMC | Friend Resources | Lower | 2.27** | 0.02a | −0.07** | −0.01 | 3.86** | −0.23* | −0.10** | 0.00 | 4.04** | 0.44* | −0.27** | −0.01 |
| Higher | 1.84** | −0.18**a | −0.03* | 0.00 | 3.45** | 0.32** | −0.08** | −0.01 | 2.23** | −0.02 | −0.09* | −0.01 | ||
| #PMC | Overall Interpersonal Resources | Lower | 2.27** | 0.01a | −0.07** | −0.01 | 3.84** | −0.19 | −0.11** | 0.00 | 4.26** | 0.37 | −0.26** | −0.01 |
| Higher | 1.82** | −0.16**a | −0.04** | −0.00 | 3.48** | −0.40** | −0.08** | −0.01 | 1.84** | 0.12 | −0.09** | −0.03 | ||
Notes:
p<.05;
p<.01.
Chronological age at baseline and race were statistically controlled in all of the growth models except those in which they were conceived to be moderating factors (e.g., in the model examining race as a moderator, only chronological age was statistically controlled).
PMC= number painful medical condition at baseline.
iM = mean initial status; ic = initial status regression coefficient; sM = mean linear growth rate; sc = linear growth regression coefficient.
significant χ2 difference test p<.05
significantχ2 difference test p<.01
We next estimated linear growth models that incorporated baseline painful medical conditions as a predictor of latent growth model parameters. In Table 2 ic= initial status regression coefficient, and sc= linear growth regression coefficient; these indicate, respectively, the estimated effect of baseline painful medical conditions on participants’ initial drinking behavior levels, and on linear growth in participants’ 10-year drinking trajectories.
To determine whether gender, age, race, history of drinking problems, avoidance coping responses, interpersonal stressors, and social resources had statistically significant moderating effects on the relationship between baseline painful medical conditions and drinking trajectory growth characteristics, we conducted chi-square difference tests to ascertain increment in fit of the latent growth models with and without invariance constraints placed on relevant parameter estimates (64, 65). In Table 2, parameters demonstrated by these tests to differ significantly share the letters a (χ2 difference test, significant at p< .05) or b (χ2 difference test, significant at at p< .01).
Chronological age at baseline and race were statistically controlled in all of the growth models except those in which age and race were conceived to be moderating factors. For example, in models examining race as a moderator, only chronological age was statistically controlled. In the predictive growth models, baseline number of painful medical conditions was centered about the group mean; in modeling to examine moderator effects, higher and lower levels of continuous moderating variables were calculated using the median split method.
Results
Baseline Pain and Drinking Characteristics
Most (64%) participants reported having one or more painful medical conditions at baseline assessment (Table 1). The most frequently reported painful medical conditions were joint/muscle pain, reported by 39% of the sample, and back pain, reported by 36% . About 35% of the sample had multiple painful medical conditions.
Table 1.
Painful Medical Conditions and Drinking Characteristics of Participants at Baseline
| Overall Sample (N=1291) | |
|---|---|
| Painful Medical Conditions (% yes) | |
| 1+ painful medical conditions | 63.8% |
| Headaches | 16.0% |
| Stomach pain | 11.2% |
| Chest pain | 17.0% |
| Joint/muscle pain | 38.6% |
| Back pain | 36.4% |
| 2+ painful medical conditions | 34.6% |
| Drinking Characteristics | |
| Amount of ethanol consumed (oz; usual drinking occasions, during last month) | |
| Baseline | 1.99 (SD = 1.88) |
| Year 1 | 2.02 (SD = 1.84) |
| Year 4 | 1.86 (SD = 1.67) |
| Year 10 | 1.64 (SD = 1.75) |
| Frequency of alcohol consumption (per week, during last month) | |
| Baseline | 4.25 (SD = 3.66) |
| Year 1 | 4.02 (SD = 3.53) |
| Year 4 | 3.60 (SD = 3.25) |
| Year 10 | 3.53 (SD = 3.39) |
| Frequency of drinking problems (range=0–68) | |
| Baseline | 3.28 (SD = 6.99) |
| Year 1 | 2.67 (SD = 6.08) |
| Year 4 | 2.19 (SD = 4.99) |
| Year 10 | 1.70 (SD = 4.51) |
At baseline participants consumed an average of almost 2 ounces of ethanol per drinking occasion, about three “standard drinks” of 0.6 fluid ounces each (66 ). Note that this is a liberal or maximum estimate of number of standard drinks, because calculation of this variable summed across all three beverage types consumed in the past month, and most individuals do not consume more than one type of beverage per drinking occasion. The frequency distribution of this variable, converted to standard drinks, indicates most participants were drinking at or below recommended drinking guidelines (66) at baseline assessment and subsequently. On average, participants consumed ethanol about 4 times per week.
At baseline, 37% of participants had one or more drinking problems (not shown in Table 1); in the sample overall, the average frequency of experiencing drinking problems was 3.28 (range=0–68; SD=6.99). Thus, whereas a noteworthy percentage of this sample experienced some negative consequences related to their drinking at baseline, negative drinking-related consequences for the group overall were relatively infrequent and mild. However, the standard deviation of drinking problem frequency indicates considerable variability in drinking problem frequency in this sample. Table 1 shows that, at the group level, amount of alcohol consumed, frequency of drinking, and frequency of drinking problems all declined moderately over the 10-year follow-up period.
Concurrent Associations Between Baseline Painful Medical Conditions and Alcohol Use
Table 2 shows unconditional linear growth models of amount of ethanol consumed, frequency of drinking, and frequency of drinking problems over the 10-year study interval. As expected, estimates of initial levels (IM) of these drinking behaviors approximated their baseline mean values (Table 1), indicating that, at baseline, participants were consuming about 2.02 ounces of ethanol, 4.08 times per week, and experienced drinking problems relatively infrequently (2.85). The unconditional linear growth models also show that, on average, participants declined moderately but significantly in drinking behavior over the 10-year interval, at a linear rate of- 0.04 for amount of ethanol consumed, −0.06 for drinking frequency, and −.12 for frequency of drinking problems.
Concurrent associations between baseline number of painful medical conditions and drinking behavior are indicated by iC coefficients, which show effects of number of painful medical conditions on initial levels of drinking behavior. These indicate that there is no statistically significant association between baseline painful medical conditions and baseline amount of ethanol consumed (ic= −.05, ns), but that there is a small negative concurrent association between number of painful medical conditions and frequency of drinking (ic=−.27, p<.01). That is, individuals who had painful medical conditions at baseline were, on average, consuming alcohol somewhat less frequently than were individuals without pain. Furthermore, Table 2 shows a small positive concurrent association between painful medical conditions and higher drinking problem frequency (ic= .29, p<.05).
Moderators of Concurrent Baseline Associations
As indicated by two group (e.g., men versus women) differences in the size of iC coefficients shown in Table 2, some personal and contextual characteristics moderated the concurrent association between number of painful medical conditions and drinking outcomes. However, only a few of these differences reached statistical significance. The moderating effect of gender is shown in Table 2 by the statistically significant difference in magnitude of ic (effect of pain on level of ethanol consumption) for men and for women. Whereas women with more baseline painful medical conditions consumed smaller amounts of ethanol at baseline than did women without pain (ic=−.12, p< .01), there was no association between painful medical conditions and ethanol consumption (ic=.03, ns) among men. Gender also moderated the effect of baseline painful medical conditions on frequency of drinking problems: for women, there was no association between these variables (ic=−.02, ns); for men, having more numerous painful medical conditions was associated with more frequent drinking problems (ic= .62, p<.01).
Race, history of drinking problems, percentage avoidance coping, and interpersonal stressors had no moderating effect on the concurrent relationship between baseline painful medical conditions and drinking behavior. However, social resources did have a moderating effect. Having higher levels of overall interpersonal resources at baseline facilitated a negative relationship between baseline painful medical conditions and amount of ethanol consumed (ic=−.16, p<.01), whereas there was no statistically significant relationship between painful medical conditions and amount of ethanol consumption among participants with lower levels of overall interpersonal resources (iC=.01, ns). This same moderating pattern was found for each of the individual social resource domains; however, the moderating effect reached statistical significance only for the friend resource domain, where painful medical conditions were associated with less ethanol consumption among individuals with higher levels of interpersonal resources from friends (ic=−.18, p<.01) but not among individuals with lower levels of interpersonal resources from this source (ic=.02, ns).
Prospective Relationship Between Baseline Painful Medical Conditions and 10-year Alcohol Use
In Table 2, SM coefficients indicate average rate of change in drinking behaviors, and SC coefficients represent the predictive effect of baseline painful medical conditions on 10-year rate of change in drinking behaviors. Baseline number of painful medical conditions alone had no effect on the 10-year pattern of decline in drinking problems, as indicated by the predictive linear growth coefficients SC= −.01 (ns) for amount of ethanol consumed, SC=.00 for frequency of drinking, and SC= −.01 (ns) for frequency of drinking problems.
Moderators of Prospective Relationships Between Painful Medical Conditions and Drinking Behavior
Only interpersonal resources and age showed statistically significant moderating effects on prospective relationships between painful medical conditions and drinking behavior. Specifically, interpersonal spouse/partner resources moderated the effect of painful medical conditions on 10-year change in drinking frequency, such that, among individuals with more spouse/partner resources, having more painful medical condition at baseline hastened decline in drinking frequency (SC= −.03, p<.05), whereas they had no effect on drinking frequency of individuals with fewer spouse/partner resources (SC= .01, ns). Age also moderated the relationship between baseline painful medical conditions and 10-year change in frequency of drinking problems, with painful conditions hastening the decline in drinking problem frequency in the slightly older (i.e., age 61–65 at baseline) participants (SC=−.04, p<.05), but having no effect (SC=.01) in the younger (age 55–60) participants.
Discussion
Our findings highlight the importance of distinguishing between the concepts of alcohol consumption and of negative consequences of alcohol consumption (drinking problems) in efforts to understand the relationship between late-life pain and drinking behavior. We found no association between having more numerous painful medical conditions at baseline and amount of ethanol consumed, but individuals with more numerous painful medical conditions consumed alcohol less frequently than did pain-free individuals. These findings do not support the alcohol self-medication hypothesis (16), which predicts a positive association between pain and both quantity and frequency of alcohol consumption. The negative concurrent association between pain and drinking frequency suggests new hypotheses about the relationship between late-life pain and alcohol consumption. For example, it is possible that late-middle-aged adults in pain self-regulate their drinking toward reduced frequency in order to avoid adverse medical consequences, such as painkiller-alcohol interactions. Another possibility is that older individuals’ pain symptoms and pain medication use prevent them from participating in social events and activities where alcohol is commonly served, thus accounting for the negative association between painful medical conditions and less frequent alcohol consumption.
In contrast, our results add to previous evidence of a positive association between pain and indicators of substance misuse (drinking problems; diagnosed alcohol use disorders) (16,19,24, 30–36). One explanation of this association is engendered by the risky-lifestyle hypothesis, which suggests that a lifestyle that includes substance misuse increases the risk of painful illness and injuries (18,19). However, the risky lifestyle hypothesis may be too narrow to fully explain the positive association between the presence of pain and drinking problem frequency found here. Future research should examine the possibility that pain exacerbates negative social and psychological consequences of drinking (e.g., drinking-related social friction; increased alcohol cravings), as well as negative physical consequences (e.g., more falls and injuries) associated with alcohol use. It should also more closely examine whether pain exacerbates drinking problems, or vice versa. We found no prospective association between baseline painful medical conditions and 10-year growth in drinking problem frequency, which detracts from the idea that it is pain that precedes drinking problems. However, because we found a baseline, positive concurrent association between number of painful medical conditions and drinking problems, the direction of causality between pain and drinking problems in this sample remains uncertain.
Our results extend previous research in this area by showing that gender and interpersonal social resources moderate the concurrent relationship between painful medical conditions and drinking behavior. The moderating effects of gender suggest that older women and men may differ in how they use alcohol when they have painful medical conditions. In line with research showing that women are more attentive and responsive to pain symptoms than are men (67), women in our sample may be more likely than men to respond to painful conditions by reducing alcohol consumption to accommodate increased use of pain medications and to avoid negative medication-alcohol interactions. The correspondence between more painful medical conditions and higher drinking problem frequency among men, but not women, is consistent with previous studies that have shown a stronger link between stressors and drinking problems among men than among women (45–47).
We also identified moderating effects that suggest interpersonal social resources may affect use of alcohol in response to pain. Having more painful medical conditions was associated with less alcohol consumption only among individuals with higher levels of friend and overall interpersonal resources. This is consistent with findings from previous research showing that interpersonal social resources can protect against the influence of stressors on poorer health outcomes (68). In this study, interpersonal social resources may have been protective because they provided sources of solace, advice, and assistance that steered participants away from alcohol consumption as a way of managing pain. More research is needed to determine how gender and interpersonal resources might support more adaptive use of alcohol in response to late-life pain.
Prospectively, painful medical conditions alone did not predict 10-year rate of change in drinking behavior. Previous longitudinal research (13, 69) has shown that over medium-term intervals most older adults’ use of alcohol declines moderately. Baseline painful medical conditions had almost no influence on this pattern of decline. On one hand, this is somewhat surprising, because having more numerous painful medical conditions might be assumed to be associated with lower levels of socializing, and more use of pain medication, both of which might herald declining 10-year alcohol consumption. On the other, a count of painful medical conditions, our only available measure of baseline pain, was limited; it did not encompass specific pain characteristics (e.g., recent severity, duration, chronicity) that may be more potent prospective influences upon 10-year rate of change in older adults’ alcohol use.
Although baseline painful medical conditions alone had no prospective influence on 10-year change in alcohol use, baseline chronological age and interpersonal social resources appeared to moderate this relationship. Our baseline age range was small (55 to 65 years) but individuals in the upper half of this range were more likely than those in the lower half to experience a decline in frequency of drinking problems in response to more numerous baseline painful medical conditions. The need to modify amount of alcohol consumed in relation to pain may be more pressing in older compared with younger cohorts. The possible protective influence of interpersonal social resources on concurrent pain-alcohol relationships appears to extend to include the prospective effect of baseline painful conditions on 10-year change in alcohol use. Individuals in pain who had more protective interpersonal influences on their drinking may have continued to benefit from these resources by reducing their frequency of alcohol use over the next 10 years.
Our findings may be of some use to clinicians who treat older patients with pain. They suggest that physicians and health care providers should have little concern that their late-middle-aged patients presenting with pain are at immediate or long-term risk for increased alcohol consumption. It appears, in fact, that many such patients will voluntarily, or with little prompting from physicians, reduce their frequency of alcohol consumption. However, clinicians should be alert to whether their late-middle-aged pain patients have drinking problems. Especially when experienced by late-middle-aged men, drinking problems may have potential to complicate pain prognoses and the course of effective pain treatment. Thus, it may be more important for clinicians to address at initial pain care evaluations, and during the course of pain treatment, older patients’ qualitative experiences involving alcohol use (i.e., incidents involving negative physical, psychological, or social consequences associated with alcohol use) than the quantitative features (i.e., amount and frequency) of their alcohol use.
This study has several important limitations. As mentioned, our measure of pain was very limited; it tapped, in essence, only the presence or absence of some physical pain at baseline assessment. Further research is needed to determine whether specific pain characteristics, such as recent pain severity, duration, chronicity, disruptiveness, and perceived controllability, are more apt than presence of pain per se to influence individuals’ use of alcohol. Similarly, our alcohol quantity and frequency measures did not assess risky alcohol consumption (e.g., drinking at levels known to cause organ damage; combined alcohol and prescription medication use); we thus cannot infer from our findings complete health-related implications of the pain-alcohol consumption relationship. Temporal design characteristics of this study, such as examining the data for prospective effects of pain over a 10-year interval, and long intervals between assessments, may have prevented detection of relationships between the presence of painful medical conditions and drinking behavior. Use of time-varying measures of pain, over shorter (e.g., daily) time intervals may be needed to more accurately describe the interplay between pain characteristics and individuals’ use of alcohol. Many statistical tests were conducted to identify moderating effects of personal and contextual characteristics on the pain-alcohol relationship, and our sample was comprised almost entirely of White drinkers. Finally, although our prospective design has the strength of temporal precedence in its favor, we cannot infer causality from the findings, and further research is needed to determine the clinical significance of the associations between baseline pain per se and late-life drinking trajectory characteristics described here.
Notwithstanding these limitations, this study adds to the literature by examining longitudinally the association between late-life pain and alcohol use, and by demonstrating that, among late-middle-aged and older adults, personal characteristics, such as gender and age, and life context, such as interpersonal social resources, moderate concurrent and prospective associations between painful medical conditions and drinking behavior. Overall, the findings suggest a need for additional research to explain how specific pain characteristics prospectively influence drinking behavior, and the direction of causality of the pain-drinking relationship. A research focus especially on the relationship between pain and drinking problems holds promise for generating clinically relevant information toward improving the course and outcomes of older adults’ pain treatment.
Acknowledgments
This research was supported by National Institute on Alcohol Abuse and Alcoholism Grants AA017477 and AA15685, and by Department of Veterans Affairs Health Services Research and Development Services research funds. The views expressed in this article are those of the authors and do not necessarily represent those of the Department of Veterans Affairs. We thank Eleanor Lewis and Alex Sox-Harris for their comments on an earlier version of this manuscript.
References
- 1.The management of persistent pain in older persons. J Am Geriatr Soc. 2002;50:S205–24. doi: 10.1046/j.1532-5415.50.6s.1.x. [DOI] [PubMed] [Google Scholar]
- 2.Blyth FM, March LM, Brnabic AJ, Jorm LR, Williamson M, Cousins MJ. Chronic pain in Australia: a prevalence study. Pain. 2001;89:127–34. doi: 10.1016/s0304-3959(00)00355-9. [DOI] [PubMed] [Google Scholar]
- 3.Ferrell B. Pain. In: Abrams WB, Beers MH, Berkow R, editors. Merk manual of geriatrics. Rahway, NJ: Merk Publishing; 2000. pp. 383–96. [Google Scholar]
- 4.Gloth FM., 3rd Pain management in older adults: prevention and treatment. J Am Geriatr Soc. 2001;49:188–99. doi: 10.1046/j.1532-5415.2001.49041.x. [DOI] [PubMed] [Google Scholar]
- 5.Mobily PR, Herr KA, Clark MK, Wallace RB. An epidemiologic analysis of pain in the elderly. J Aging Health. 1994;6:139–54. [Google Scholar]
- 6.Thomas E, Peat G, Harris L, Wilkie R, Croft PR. The prevalence of pain and pain interference in a general population of older adults: cross-sectional findings from the North Staffordshire Osteoarthritis Project (NorStOP) Pain. 2004;110:361–8. doi: 10.1016/j.pain.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Turk DC, Okifuji A, Scharff L. Assessment of older women with chronic pain. In: Roberto KA, editor. Older women with chronic pain. New York: Hawthorne Press; 1994. pp. 25–42. [Google Scholar]
- 8.Blow FC, Walton MA, Barry KL, Coyne JC, Mudd SA, Copeland LA. The relationship between alcohol problems and health functioning of older adults in primary care settings. J Am Geriatr Soc. 2000;48:769–74. doi: 10.1111/j.1532-5415.2000.tb04751.x. [DOI] [PubMed] [Google Scholar]
- 9.Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP. The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991-1992 and 2001-2002. Drug Alcohol Depend. 2004;74:223–34. doi: 10.1016/j.drugalcdep.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 10.Fink A, Morton SC, Beck JC, Hays RD, Spritzer K, Oishi S, et al. The alcohol-related problems survey: identifying hazardous and harmful drinking in older primary care patients. J Am Geriatr Soc. 2002;50:1717–22. doi: 10.1046/j.1532-5415.2002.50467.x. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 12.Kirchner JE, Zubritsky C, Cody M, Coakley E, Chen H, Ware JH, et al. Alcohol consumption among older adults in primary care. J Gen Intern Med. 2007;22:92–7. doi: 10.1007/s11606-006-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moos RH, Brennan PL, Schutte KK, Moos BS. High-risk alcohol consumption and late-life alcohol use problems. Am J Public Health. 2004;94:1985–91. doi: 10.2105/ajph.94.11.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pringle KE, Ahern FM, Heller DA, Gold CH, Brown TV. Potential for alcohol and prescription drug interactions in older people. J Am Geriatr Soc. 2005;53:1930–6. doi: 10.1111/j.1532-5415.2005.00474.x. [DOI] [PubMed] [Google Scholar]
- 15.Schutte KK, Nichols KA, Brennan PL, Moos RH. A ten-year follow-up of older former problem drinkers: risk of relapse and implications of successfully sustained remission. J Stud Alcohol. 2003;64:367–74. doi: 10.15288/jsa.2003.64.367. [DOI] [PubMed] [Google Scholar]
- 16.Aira M, Hartikainen S, Sulkava R. Drinking alcohol for medicinal purposes by people aged over 75: a community-based interview study. Fam Pract. 2008;25:445–49. doi: 10.1093/fampra/cmn065. [DOI] [PubMed] [Google Scholar]
- 17.Ilgen MA, Perron B, Czyz EK, McCammon RJ, Trafton JA. The timing of onset of pain and substanceuse disorders. Am J Addict. doi: 10.1111/j.1521-0391.2010.00065.x. in press. [DOI] [PubMed] [Google Scholar]
- 18.Gatchel RJ, Dersh J. Psychological disorders and chronic pain: Are there cause-and-effect relationships? In: Turk DC, Gatchel RJ, editors. Psychological approaches to pain management: a practitioner’s handbook. New York: Guilford Press; 2002. pp. 30–51. [Google Scholar]
- 19.Dersh J, Polatin PB, Gatchel RJ. Chronic pain and psychopathology: research findings and theoretical considerations. Psychosom Med. 2002;64:773–86. doi: 10.1097/01.psy.0000024232.11538.54. [DOI] [PubMed] [Google Scholar]
- 20.Armenian HK, Halabi SS, Khlat M. Epidemiology of primary health problems in Beirut. J Epidemiol Community Health. 1989;43:315–18. doi: 10.1136/jech.43.4.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Castillo RC, MacKenzie EJ, Wegener ST, Bosse MJ. Prevalence of chronic pain seven years following limb threatening lower extremity trauma. Pain. 2006;124:321–29. doi: 10.1016/j.pain.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 22.Strine TW, Hootman JM. US national prevalence and correlates of low back and neck pain among adults. Arthritis Rheum. 2007;57:656–65. doi: 10.1002/art.22684. [DOI] [PubMed] [Google Scholar]
- 23.Urquhart DM, Bell R, Cicuttini FM, Cui J, Forbes A, Davis SR. Low back pain and disability in community-based women: prevalence and associated factors. Menopause. 2009;16:24–29. doi: 10.1097/gme.0b013e31817e5ce0. [DOI] [PubMed] [Google Scholar]
- 24.Brennan PL, Schutte KK, Moos RH. Pain and use of alcohol to manage pain: prevalence and 3-year outcomes among older problem and non-problem drinkers. Addiction. 2005;100:777–86. doi: 10.1111/j.1360-0443.2005.01074.x. [DOI] [PubMed] [Google Scholar]
- 25.Riley JL, 3rd, King C. Self-report of alcohol use for pain in a multi-ethnic community sample. J Pain. 2009;10:944–52. doi: 10.1016/j.jpain.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ang DC, Peloso PM, Woolson RF, Kroenke K, Doebbeling BN. Predictors of incident chronic widespread pain among veterans following the first Gulf War. Clin J Pain. 2006;22:554–63. doi: 10.1097/01.ajp.0000208907.42506.21. [DOI] [PubMed] [Google Scholar]
- 27.Bergman S, Herrstrom P, Jacobsson LT, Petersson IF. Chronic widespread pain: a three year followup of pain distribution and risk factors. J Rheumatol. 2002;29:818–25. [PubMed] [Google Scholar]
- 28.Booker EA, Haig AJ, Geisser ME, Yamakawa K. Alcohol use self report in chronic back pain - relationships to psychosocial factors, function, performance, and medication use. Disabil Rehabil. 2003;25:1271–77. doi: 10.1080/09638280310001608609. [DOI] [PubMed] [Google Scholar]
- 29.Byles J, Young A, Furuya H, Parkinson L. A drink to healthy aging: The association between older women’s use of alcohol and their health-related quality of life. J Am Geriatr Soc. 2006;54:1341–47. doi: 10.1111/j.1532-5415.2006.00837.x. [DOI] [PubMed] [Google Scholar]
- 30.Dersh J, Gatchel RJ, Mayer T, Polatin P, Temple OR. Prevalence of psychiatric disorders in patients with chronic disabling occupational spinal disorders. Spine. 2006;31:1156–62. doi: 10.1097/01.brs.0000216441.83135.6f. [DOI] [PubMed] [Google Scholar]
- 31.Trafton JA, Oliva EM, Horst DA, Minkel JD, Humphreys K. Treatment needs associated with pain in substance use disorder patients: implications for concurrent treatment. Drug Alcohol Depend. 2004;73:23–31. doi: 10.1016/j.drugalcdep.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 32.Polatin PB, Kinney RK, Gatchel RJ, Lillo E, Mayer TG. Psychiatric illness and chronic low-back pain. The mind and the spine--which goes first? Spine. 1993;18:66–71. doi: 10.1097/00007632-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 33.Brown RL, Patterson JJ, Rounds LA, Papasouliotis O. Substance abuse among patients with chronic back pain. J Fam Pract. 1996;43:152–60. [PubMed] [Google Scholar]
- 34.Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, et al. Mental disorders among persons with chronic back or neck pain: results from the World Mental Health Surveys. Pain. 2007;129:332–42. doi: 10.1016/j.pain.2007.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Lawton J, Simpson J. Predictors of alcohol use among people experiencing chronic pain. Psychol Health Med. 2009;14:487–501. doi: 10.1080/13548500902923177. [DOI] [PubMed] [Google Scholar]
- 36.Von Korff M, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, et al. Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain. 2005;113:331–39. doi: 10.1016/j.pain.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Gureje O, Von Korff M, Kola L, Demyttenaere K, He Y, Posada-Villa J, et al. The relation between multiple pains and mental disorders: results from the World Mental Health Surveys. Pain. 2008;135:82–91. doi: 10.1016/j.pain.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 38.Giles BE, Walker JS. Gender differences in pain. Curr Opin Anaesthesiol. 1999;12:591–95. doi: 10.1097/00001503-199910000-00017. [DOI] [PubMed] [Google Scholar]
- 39.Unruh AM. Gender variations in clinical pain experience. Pain. 1996;65:123–67. doi: 10.1016/0304-3959(95)00214-6. [DOI] [PubMed] [Google Scholar]
- 40.Edwards RR, Doleys DM, Fillingim RB, Lowery D. Ethnic differences in pain tolerance: clinical implications in a chronic pain population. Psychosom Med. 2001;63:316–23. doi: 10.1097/00006842-200103000-00018. [DOI] [PubMed] [Google Scholar]
- 41.Green CR, Anderson KO, Baker TA, Campbell LC, Decker S, Fillingim RB, et al. The unequal burden of pain: confronting racial and ethnic disparities in pain. Pain Med. 2003;4:277–94. doi: 10.1046/j.1526-4637.2003.03034.x. [DOI] [PubMed] [Google Scholar]
- 42.Amoako EP, Richardson-Campbell L, Kennedy-Malone L. Self-medication with over-the-counter drugs among elderly adults. J Gerontol Nurs. 2003;29:10–5. doi: 10.3928/0098-9134-20030801-05. [DOI] [PubMed] [Google Scholar]
- 43.NIAAA. Alcohol use and alcohol use disorders in the United States: Main findings from the 2001-2002 National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) Data Reference Manual. 2006:1–247. [Google Scholar]
- 44.Timko C, Finney JW, Moos RH. The 8-year course of alcohol abuse: gender differences in social context and coping. Alcohol Clin Exp Res. 2005;29:612–21. doi: 10.1097/01.alc.0000158832.07705.22. [DOI] [PubMed] [Google Scholar]
- 45.Brennan PL, Moos RH. Late-life problem drinking: personal and environmental risk factors for 4-year functioning outcomes and treatment seeking. J Subst Abuse. 1996;8:167–80. doi: 10.1016/s0899-3289(96)90227-8. [DOI] [PubMed] [Google Scholar]
- 46.Cooper ML, Russell M, George WH. Coping, expectancies, and alcohol abuse: a test of social learning formulations. J Abnorm Psychol. 1988;97:218–30. doi: 10.1037//0021-843x.97.2.218. [DOI] [PubMed] [Google Scholar]
- 47.Cooper ML, Russell M, Skinner JB, Frone MR, Mudar P. Stress and alcohol use: moderating effects of gender, coping, and alcohol expectancies. J Abnorm Psychol. 1992;101:139–52. doi: 10.1037//0021-843x.101.1.139. [DOI] [PubMed] [Google Scholar]
- 48.Armeli S, Carney MA, Tennen H, Affleck G, O’Neil TP. Stress and alcohol use: a daily process examination of the stressor-vulnerability model. J Pers Soc Psychol. 2000;78:979–94. doi: 10.1037//0022-3514.78.5.979. [DOI] [PubMed] [Google Scholar]
- 49.Carney MA, Armeli S, Tennen H, Affleck G, O’Neil TP. Positive and negative daily events, perceived stress, and alcohol use: a diary study. J Consult Clin Psychol. 2000;68:788–98. [PubMed] [Google Scholar]
- 50.Holtzman S, Newth S, Delongis A. The role of social support in coping with daily pain among patients with rheumatoid arthritis. J Health Psychol. 2004;9:677–95. doi: 10.1177/1359105304045381. [DOI] [PubMed] [Google Scholar]
- 51.Lepore SJ, Evans GW. Coping with multiple stressors in the environment. In: Zeidner M, Endler NS, editors. Handbook of coping: Theory, research, applications. New York: John Wiley; 1996. pp. 350–77. [Google Scholar]
- 52.Peirce RS, Frone MR, Russell M, Cooper ML. Financial stress, social support, and alcohol involvement: a longitudinal test of the buffering hypothesis in a general population survey. Health Psychol. 1996;15:38–47. doi: 10.1037//0278-6133.15.1.38. [DOI] [PubMed] [Google Scholar]
- 53.Brennan PL, Moos RH. Life stressors, social resources, and late-life problem drinking. Psychol Aging. 1990;5:491–501. doi: 10.1037//0882-7974.5.4.491. [DOI] [PubMed] [Google Scholar]
- 54.Moos RH, Brennan PL, Fondacaro MR, Moos BS. Approach and avoidance coping responses among older problem and nonproblem drinkers. Psychol Aging. 1990;5:31–40. doi: 10.1037//0882-7974.5.1.31. [DOI] [PubMed] [Google Scholar]
- 55.Moos RH, Moos BS. Life Stressors and Social Resources Inventory: Adult Form manual. Odessa, FL: Psychological Assessment Resources; 1994. [Google Scholar]
- 56.Moos RH, Cronkite R, Finney J. Health and Daily Living Form manual. 2. Redwood City, CA: Mind Garden; 1992. [Google Scholar]
- 57.Finney JW, Moos RH, Brennan PL. The Drinking Problems Index: a measure to assess alcohol-related problems among older adults. J Subst Abuse. 1991;3:395–404. doi: 10.1016/s0899-3289(10)80021-5. [DOI] [PubMed] [Google Scholar]
- 58.Allen JP, Wilson VB. NIH Publication No 03-3745. 2. Bethesda, MD: US Department of Health and Human Services, NIAAA; 2003. Assessing alcohol problems: A guide for clinicians and researchers. [Google Scholar]
- 59.Kopera-Frye K, Wiscott R, Sterns H. Can the Drinking Problem Index provide valuable therapeutic information for recovering alcoholic adults? Aging Ment Health. 1999;3:246–56. [Google Scholar]
- 60.Bamberger PA, Sonnenstuhl WJ, Vashdi D. Screening older, blue-collar workers for drinking problems: an assessment of the efficacy of the drinking problems index. J Occup Health Psychol. 2006;11:119–34. doi: 10.1037/1076-8998.11.1.119. [DOI] [PubMed] [Google Scholar]
- 61.Moos RH. Coping Responses Inventory: Adult Form Manual. Odessa, FL: Psychological Assessment Resources; 1993. [Google Scholar]
- 62.Moos RH. The Coping Responses Inventory: An update on research applications and validity. Odessa, FL: Psychological Assessment Resources; 2004. [Google Scholar]
- 63.Muthén B, Khoo ST. Longitudinal studies of achievement growth using latent variable modeling. Learn Individ Differ, Special issue: latent growth curve analysis. 1998;10:73–101. [Google Scholar]
- 64.Muthén L, Muthén B. Mplus User’s Guide. Los Angeles, CA: Muthén & Muthén; 1998–2007. [Google Scholar]
- 65.Preacher KJ, Wichman AL, MacCallum RC, Briggs NE. Latent growth curve modeling. Thousand Oaks, CA: Sage Publications; 2008. [Google Scholar]
- 66.NIH Publication No 10-3770. Bethesda, MD: US Department of Health and Human Services, NIAAA; 2010. Rethinking drinking: Alcohol and your health. [Google Scholar]
- 67.Keefe FJ, Lefebre JC, Egert JR, Affleck G, Sullivan MJ, Caldwell DS. The relationship of gender to pain, pain behavior, and disability in osteoarthritis patients: the role of catastrophizing. Pain. 2000;87:325–334. doi: 10.1016/S0304-3959(00)00296-7. [DOI] [PubMed] [Google Scholar]
- 68.Cohen S. Social relationships and health. American Psychologist. 2004;59:676–684. doi: 10.1037/0003-066X.59.8.676. [DOI] [PubMed] [Google Scholar]
- 69.Brennan PL, Schutte KK, Moos RH. Patterns and predictors of late-life drinking trajectories: A 10-year longitudinal study. Psychol Addict Behav. 2010;24:254–264. doi: 10.1037/a0018592. [DOI] [PMC free article] [PubMed] [Google Scholar]