Abstract
High-altitude studies offer insight into the evolutionary processes and physiological mechanisms affecting the early phases of the human lifespan. Chronic hypoxia slows fetal growth and reduces the pregnancy-associated rise in uterine artery (UA) blood flow. Multigenerational vs. shorter-term high-altitude residents are protected from the altitude-associated reductions in UA flow and fetal growth. Presently unknown is whether this fetal-growth protection is due to the greater delivery or metabolism of oxygen, glucose or other substrates or to other considerations such as mechanical factors protecting fragile fetal villi, the creation of a reserve protecting against ischemia/reperfusion injury, or improved placental O2 transfer as the result of narrowing the A-V O2 difference and raising uterine PvO2. Placental growth and development appear to be normal or modified at high altitude in ways likely to benefit diffusion. Much remains to be learned concerning the effects of chronic hypoxia on embryonic development. Further research is required for identifying the fetoplacental and maternal mechanisms responsible for transforming the maternal vasculature and regulating UA blood flow and fetal growth. Genomic as well as epigenetic studies are opening new avenues of investigation that can yield insights into the basic pathways and evolutionary processes involved.
Keywords: Adaptation, Andean, genetic, pregnancy, Tibetan, uteroplacental blood flow
1.0 Introduction
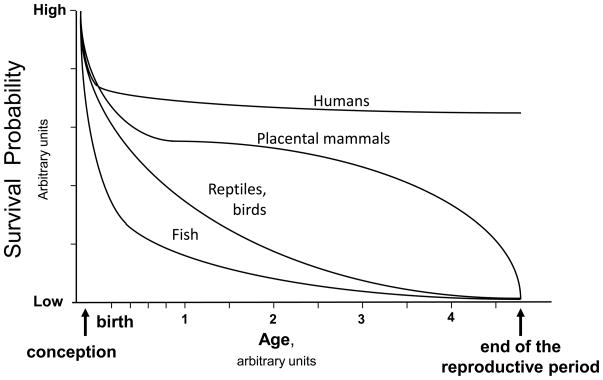
Studies at high altitude provide an important perspective for understanding energetics and oxygen transport in human beings. While much can be learned from whole animal, cell-based and other model systems as noted elsewhere in this issue, human studies are of special importance given the profound species differences in physiological and evolutionary processes. Exemplifying the former is the uniquely human susceptibility to the pregnancy disorder of preeclampsia and the latter is the variation between taxonomic groups in survival probabilities following conception. As shown in Figure 1, mammals compared to other vertebrates such as fish, reptiles, or birds have an improved chance of survival. This relates, in part, to variation in r- vs. k-selection strategies among these animals (Stearns, 1992), but these too are evolutionary processes. Survival probabilities are further enhanced in placental (eutherian) compared to egg-laying or marsupial (metatherian or prototherian) mammals given their longer intrauterine period. Social factors further benefit postnatal survival, with human beings and other primates surviving well past the end of the reproductive period. All these influences have the effect of concentrating the period of differential mortality prior to the end of the reproductive period to the perinatal period (i.e., the interval from conception through infancy), subjecting embryonic and fetal life to considerable selective pressure and making this period of special relevance for understanding evolutionary process.
Figure 1.
depicts a theoretical model for describing variation in the probability of survival from the time of conception through the end of the reproductive period in various taxonomic groups. The protection afforded by the shell of an egg as well as other factors increase survival probability in reptiles and birds relative to fish. Placental mammals have a further advantage commensurate with their longer period of intrauterine protection and improved survivorship during adult years. Since considerable differential mortality occurs soon after conception in all groups, the effects of natural selection are especially concentrated in the perinatal period, with this being particularly true in humans and other placental mammals.
We approach the evolutionary processes affecting embryonic and fetal development through the lens of pregnancy studies at high altitude given the importance of O2 tension as both a regulator and enabler of embryonic and fetal development, and the pervasive reduction in O2 tension in the inspired air at high altitude. Studies at high altitude have clinical and public health importance as well. There are 140 M persons living at above 2500 m (the conventional definition of high altitude as that where arterial O2 saturation (SaO2) measurably begins to fall), comprising the largest group of persons at risk of fetal growth restriction (Krampl, 2002). Additional large numbers of persons visit high altitude or experience intermittent hypoxia due to anemia, cardiovascular or pulmonary diseases. Recognition of the importance of prenatal development for the risk of cardiovascular or other disorders later in life (Barker, 1992) further underscores the importance of understanding the mechanisms by which hypoxia influences fetal and embryonic development.
We begin by reviewing the history of research on fetal growth and embryonic development at high altitude. The physiological processes governing fetal oxygen supply and their variation among human populations are then considered, first emphasizing those maternal attributes that determine the amount of oxygen and other nutrients that are transported to the uteroplacental circulation and then considering placental factors. The results of recent genome scans are also considered as these can provide new insights into the physiological pathways involved.
2.0 History of research concerning fetal growth and embryonic development at high altitude
Research on fetal growth at high altitude began before studies of embryonic development. Recognition of human variation in fetal growth at high altitude has further enlarged our appreciation of the physiological responses involved.
2.1 Fetal Growth
While difficulties posed by high altitude for human or nonhuman animal reproduction have been recognized for some time, systematic scientific studies aimed at investigating the mechanisms responsible began in the 1950s. At that time “premature” was used to describe any “baby [who] weighs 2500 gm or less regardless of the period of gestation” (Moore, 2001b). Since it was recognized that small-sized babies were more likely to die but it was considered impractical to record the length of gestation, birth weight was used as a single standard for identifying at-risk babies. Surprisingly this remained World Health Organization policy until 1975 when shortened gestation and fetal growth restriction were recognized as separable causes of low birth weight. Studies in Colorado were an important impetus for this change since it was there that fetal growth restriction was first demonstrated to lower birth weight independently of gestational age on a population scale.
In the 1950s Colorado had the highest “prematurity” rate in the country (Lichty et al., 1957). Dr. John Lichty was recruited from New York state to determine its cause (Moore, 2001b). Notably, one of his team’s first actions was to revise the 1949 Colorado birth certificate form to include pregnancy duration. Their state-wide review showed that the counties with the highest “prematurity” rates were located at high altitudes, the highest of which was Lake County whose population was centered in Leadville at 3100 m (10,200 ft). There the average birth weight was 2655 gm and 45% of newborns weighed less than 2500 gm (5.5 lbs). Comparison of the Lake County records with those from Denver (1600 m, 5280 ft) and Baltimore (sea level) revealed that the entire birth-weight distribution shifted leftward with increasing elevation, but no change occurred in gestational age (Lichty et al., 1957). Ethnicity, prepregnant body weight, pregnancy weight gain, week of onset of prenatal care, number of prenatal visits, maternal nutrition (by 24-hr dietary recall), delivery type, or levels of trace metals in the water supply could not explain the results observed. Reinforcing reduced O2 availability as the likely cause, Leadville newborns had higher cord hemoglobin levels and lower SaO2 values prior to the first breath than babies born in Denver. However, until recently the Colorado pediatric and obstetrical communities continued to think that factors such as ethnicity and inadequate medical care rather than altitude per se were primarily responsible (Moore, 2001b; Schwartz et al., in review).
Studies in the 1950s were also taking place in the Peruvian Andes by a distinguished group of scientists headed by Donald Barron from Yale University. Their work was aimed at discovering how fetal development was possible at an altitude of 4900 m where less than half as much oxygen was present in the inspired air compared to sea level. It was known from Joseph Barcroft’s pioneering observations that normal fetal development takes place in a low-oxygen environment, with placental intervillous O2 tensions being approximately 20 mm Hg at 7–10 weeks of gestation or one-third the values present on the maternal side of the placenta (Rodesch et al., 1992; Stave, 1970). Unless compensations occurred, halving maternal inspired O2 tensions would reduce fetal oxygenation below levels compatible with life, let alone those needed for fetal growth and development. Their studies in native sheep (which interestingly did not demonstrate lower birth weights) revealed that compensations occurred at each step in the oxygen transport chain, with the greatest change being a markedly higher uterine artery (UA) blood flow. These investigators speculated that if similar increases occurred in humans at high altitude, intervillous O2 tensions would only be slightly reduced compared to sea level (Metcalfe et al., 1967), an observation that was remarkably prescient given the reports to be published some 50 yrs later.
More recent studies show that birth weight falls, on average, 102 gm per 1000 m elevation gain and that approximately three times as many babies are born who are small for their gestational age and sex (SGA1) at high compared to low altitude (Jensen and Moore, 1997; Julian et al., 2007; Krampl, 2002). While the birth-weight reduction can be modeled as linear in large samples, finer-scale analyses reveal that it begins gradually and becomes marked at altitudes > 2500 m (8000 ft) (Mortola et al., 2000). The birth weight reduction can be observed after 29–31 wk gestation in babies born prematurely (Unger et al., 1988) or 25–29 wk by fetal biometry (Krampl et al., 2000). While poor nutrition, low socioeconomic status, primiparity, and limited health care contribute to birth-weight variation in any population, such factors cannot account for the altitude-associated fall (Giussani et al., 2001; Jensen and Moore, 1997; Keyes et al., 2003). Approximately half the fall can be attributed to a tripling of the incidence of preeclampsia2 (PE) (Jensen and Moore, 1997; Keyes et al., 2003; Palmer et al., 1999). However since only about half the babies born to PE women are growth restricted and SGA and PE are, increasingly, being recognized as having distinct etiologies (Rajakumar et al., 2007), chronic hypoxia appears to be the dominant factor that gives rise not only to more SGA babies but also more cases of PE.
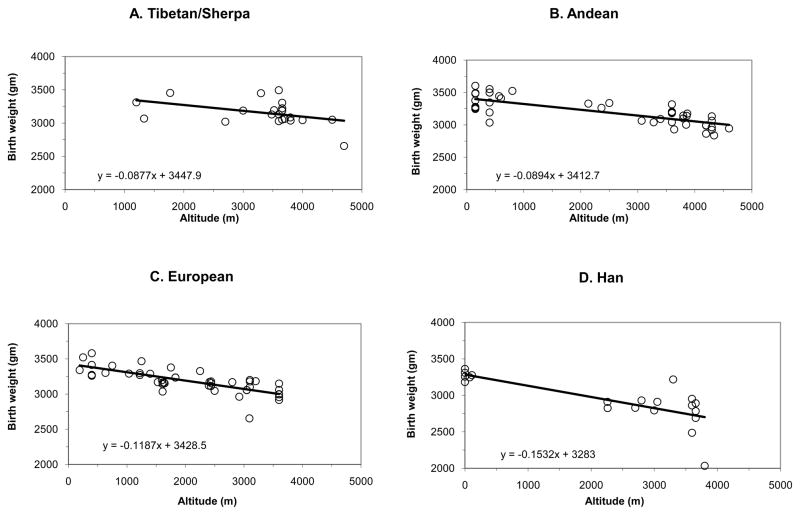
Although birth-weight reductions are seen in every population to date, the magnitude of the birth-weight fall varies among populations. Figure 2 summarizes all published studies from more than 4.6 million newborns in four major world populations – Andeans, Europeans, Han (“Chinese”), and Tibetans. Populations with 8000 or more years of high-altitude residence (Tibetans and Andeans) have less altitude-associated birth-weight decline than groups with < 400 yrs residence (Europeans and Han). In particular, Tibetan and Andean birth weights fall one-third as much as Europeans and half as much as Han. Similar findings were observed when comparisons were restricted to data acquired at low vs. high altitude within the same study so as to better control for other factors influencing fetal growth (Moore, 2010) and for variation in sample size (Moore, 2001a), reinforcing the view that multigenerational high-altitude residents are protected from altitude-associated fetal growth restriction. Interestingly, the sea-level birth weights (y-intercepts, Figure 2) are similar in all groups, contrasting with the view voiced by Zamudio and co-workers that “Andeans and Tibetans appear to have increased fetal growth, regardless of altitude” (Zamudio et al., 2007). As commented elsewhere (Moore, 2010), the heavier birth weights in that study likely resulted from the fact that subjects were required to be undergoing elective C-sections and therefore likely to be of above-average socioeconomic status, a factor known to raise birth weight at high altitude (Giussani et al., 2001; Unger et al., 1988). As shown in Figure 2, Tibetan and Andean newborns at 4000 m weigh ~300 gm less than their sea-level counterparts whereas European and Han infants are >400 and >700 gm lighter respectively. Comparing populations, Tibetans and Andeans weigh essentially the same at 4000 m but European newborns are >100 gm and Han >400 gm lighter. Thus whether comparisons are restricted to studies conducted by the same investigative team at high vs. low altitudes (Moore, 2010) or all studies are considered (Figure 2), the magnitude of altitude’s effect on birth weight is ordered such that it is least in those populations with the greatest number of generations of high-altitude residence (Tibetans and Andeans) and progressively greater in groups with shorter duration of residence (Europeans and Han). Unfortunately, the lack of complete vital statistics or prenatal care prevents reliable determination of whether population differences exist in the timing with which hypoxia influences fetal growth or the frequency of other pregnancy complications.
Figure 2.
Shown are the average birth weights and regression lines for all published studies from 1957 to the present for the geographic regions shown. A total of 4,690,093 newborns are represented, encompassing 12,129 Tibetans (including Sherpa, Panel A); 140,105 Andeans (Panel B); 4,409,917 Europeans (Panel C); and 127,942 Han (“Chinese”, Panel D) living at the altitudes specified. While birth weight declines with increasing altitude in each population, the decline is markedly less in Tibetans (−88 gm per 1000 m) or Andeans (−89 gm per 1000 m) than in Europeans (−119 gm per 1000 m), all of whom show less birth-weight decline than Han (−153 gm per 1000 m). Compared to the sea-level average (3393 gm), newborns at 4000 m weigh 296 gm less if Tibetan, 338 gm less if Andean, 439 gm less if European or 723 gm less if Han. Compared to Tibetans or Andeans at 4000 m, Europeans weigh 122 gm less and Han 406 gm less. See (Moore, 2010; Moore, 2001a) for original citations, with data added from (Gonzales et al., 2009).
2.2 Embryonic development
Studies on the effects of chronic hypoxia on embryonic development have identified the critical role played by physiologic hypoxia in orchestrating cellular differentiation and organogenesis. Shortly after implantation, trophoblast cells (i.e., the specialized epithelial cells forming the outer layer of the blastocyst) migrate into the uterine tissue and the lumen of maternal spiral arterioles. This serves to occlude the vessels and prevent direct exposure of placental tissue to oxidative damage. The trophoblast plugs loosen and initiate the intravillous circulation after ~12 wk, but by this time embryogenesis is complete. The resultant three-fold increase in placental O2 tensions is accompanied by increased expression of antioxidant enzymes that serves, in turn, to defend the placenta and the developing fetus against the progressive rise in O2 tensions (Jauniaux et al., 2000). Underscoring the importance of these developments, oxidative stress resulting from incomplete or shallow trophoblast invasion is considered to contribute to PE and miscarriage (Burton and Jauniaux, 2004).
While hypoxia plays an important role in normal embryogenesis, several studies indicate that the superimposed chronic hypoxia of residence at high altitude has deleterious effects. For example congenital anomalies, many of which stem from impaired embryonic development, are more common at high than low altitudes in Colorado (Jensen and Moore, 1997). Given the key role played by trophoblast-induced remodeling of maternal spiral arterioles in the etiology of PE, the increased frequency of PE at high vs. low altitude (Keyes et al., 2003; Palmer et al., 1999) and the accompanying reduction in pregnancy-associated trophoblast remodeling of placental vessels (Tissot van Patot et al., 2003) suggest that chronic hypoxia interferes with such processes. Experimental animal studies also indicate that maternal exposure to chronic hypoxia impairs pulmonary development. Rat pups whose mothers were exposed to a 10% inspired O2 concentration on the last day of gestation and for 1–2 hours after birth had a delayed increase in lung volume, impaired septation of gas exchange saccules, blunted expansion of gas-exchange surface area, and accelerated thinning of alveolar walls at 7 and 30 days after birth (Massaro et al., 1989). Perinatal hypoxia also affects the chemoreceptor pathway, delaying the onset and decreasing ventilatory sensitivity to hypoxia (Joseph et al., 2000; Okubo and Mortola, 1990). Further, babies born at low altitude who have experienced exaggerated perinatal hypoxia have a markedly greater rise in pulmonary arterial pressure than do age-matched controls when exposed to hypoxia during adulthood (Sartori et al., 1999), suggesting that changes in the pulmonary circulation are also involved.
The above studies indicate that embryonic and/or fetal hypoxia affect more than just fetal growth. As first proposed by David Barker, birth weight has a continuous, inverse relationship with the risk of obesity, hypertension, diabetes, heart as well as pulmonary disease and stroke later in life (Barker, 1992). A range of potential mechanisms might be involved. Fetal hypoxia can impair myocardial development and adult cardiac performance by acting through hypoxia-inducible factor (HIF)-1 and HIF-regulated genes important in heart formation (Patterson and Pouliot, 1987). Epigenetic modification of gene expression may also be involved by increasing the methylation of highly conserved CpG islands (i.e., genomic regions with a high frequency of sequential cytosine-guanine nucleotides). For example, hypermethylation of CpG islands located in the promoter region of the homeobox 1 transcription factor Pdx1 has been shown to decrease Pdx1 expression and lead to type-II diabetes in rats (Park et al., 2008). In short, insufficient fetal growth can initiate a cascade of epigenetic events that could silence critical genes and predispose persons for developing diabetes or other chronic diseases later in life.
3.0 Maternal factors influencing fetal growth
Given the nature of eutherian mammalian reproduction, maternal factors profoundly affect fetal growth. We consider here first the effects of pregnancy on ventilation and UA blood flow as these influence the amount of oxygen and other nutrients being transported to the uteroplacental circulation, and then turn to genetic factors. We address each of these by using the experiment of nature afforded by the existence of populations at high altitude with varying susceptibility to hypoxia-associated reductions in fetal growth.
3.1 Ventilation and arterial O2 content
Pregnancy raises maternal ventilation more than 25%, due principally to higher basal metabolic rate and the effects of progesterone and estrogen acting to increase hypoxic chemosensitivity. Whereas the ventilation rise has little effect on the already nearly-maximal SaO2 levels present at low altitude, maternal SaO2 increases appreciably at high altitude. Hemoglobin concentration falls during pregnancy due to the normal 40% rise in plasma volume and lack of commensurate increase in red cell mass. Since pre-pregnant hemoglobin concentration is higher at high than low altitude, values also tend to be higher during pregnancy. Combined with the ventilatory-induced rise in SaO2, arterial O2 content (CaO2) is maintained during pregnancy close to low-altitude values (see (Moore, 2010) for review).
Variation in the extent to which women raise their ventilation and defend CaO2 during pregnancy relates positively to their infant’s birth weight at high altitude (Moore et al., 1982; Moore et al., 2001). However, comparisons between groups at the same altitude indicate that Andeans or Tibetans do not have higher SaO2 or CaO2 levels than their European or Han counterparts (Julian et al., 2009; Moore et al., 2001; Vargas et al., 2007; Zamudio et al., 2007), making it unlikely that differences in arterial oxygenation underlie the protection from altitude-associated fetal growth reduction seen in long-resident groups.
3.2 Uterine artery (UA) blood flow
Pregnancy prompts profound circulatory changes, lowering systemic vascular resistance and raising cardiac output more than 25%. The greater fall in uteroplacental vascular resistance preferentially directs blood flow to this vascular bed, raising values from 20–50 ml/min in the nonpregnant state to 450–800 ml/min or more than one liter/min in twin pregnancies near term (Palmer et al., 1992). Humans are more reliant on lower vascular resistance in the uteroplacental than non-uteroplacental portions of the systemic circulation for sustaining high UA blood flow during the 3rd trimester than other mammalian species due to the fact that our bipedal posture and anatomical organization of the pelvic cavity results in uterine compression of the vena caval compression, reduction in venous return, and cessation of the continued rise in cardiac output. We have speculated elsewhere that this may be one factor contributing to human susceptibility to PE, a disorder which accounts for 10% of maternal mortality and considerable infant mortality and morbidity worldwide (Rockwell 2003).
More than 80% of total uterine blood flow is supplied by the two UA, with the remainder coming from a branch of the ovarian artery that anastomoses with the UA to form a dually-perfused cascade on each side of the uterus. Prior to week 20, the increase in UA blood flow is due primarily to an enlarged vessel diameter whereas faster blood flow velocity accounts for most of the late-pregnancy rise (Palmer et al., 1992). The main UA undergoes marked alterations to facilitate and accommodate this increased blood flow. At low altitude UA diameter doubles due to vascular growth and remodelling as well as to alterations in vasoreactivity and distensibility. These changes begin early with UA diameter doubling by week 6.5 (Burchell, 1967), suggesting that it is initiated by hormonal factors. Estradiol is likely a key player given its angiogenic properties and stimulatory effects on nitric-oxide mediated vasodilation (Kublickiene et al., 2000a). Once the placental circulation is established, uterine vascular resistance continues to decline due to trophoblast-induced remodelling of end-spiral arterioles (Burton et al., 2009). Hypertrophy and hyperplasia in all layers of the UA wall are evident by mid-gestation (Keyes et al., 1997). The mid-pregnant or near-term UA also shows greater distensibility and increased vasodilator response to pharmacologic substances and blood flow (Kublickiene et al., 1997; Mateev et al., 2003; White et al., 2000).
High altitude decreases the pregnancy-associated rise in UA blood flow (Julian et al., 2008). Consistent with the likelihood that alterations in UA vascular growth are involved, there is only half as much pregnancy-associated increase in UA DNA synthesis in chronically hypoxic vs. normoxic guinea pigs (Rockwell et al., 2006). The proliferative response to serum stimulation in cultured UA vascular smooth muscle cells is also diminished, suggesting that chronic hypoxia interferes with the ability of vascular smooth muscle cells to de-differentiate to a proliferative phenotype (Rockwell et al., 2006). The changes in growth may be due, in part, to less pregnancy-associated increase in nitric oxide (NO). NO synthase inhibition reduced the relaxation response to acetylcholine in UA isolated from chronically-hypoxic vs. normoxic guinea pigs, suggesting that a diminished stimulatory effect of pregnancy at high altitude (White et al., 2000). Isolated UA from chronically-hypoxic vs. normoxic pregnant animals also show less vasodilator response to flow (Mateev et al., 2003), similar to what has been observed in myometrial arteries from PE women (Kublickiene et al., 2000b). Also consistent with reduced NO production or activity, pregnant high- vs. low-altitude women in Colorado not only have smaller UA diameters but also lower levels of circulating NO metabolites (NOx) and higher endothelin-1 (ET1) to NOx ratios (Julian et al., 2008).
These adverse effects of high altitude on UA responses to pregnancy are absent or greatly diminished in long- vs. short-resident high-altitude populations. Pregnant Tibetan compared with Han women have higher UA flow velocity (Moore et al., 2001) and larger UA diameters (Chen et al., 2002), both of which would be expected to raise UA blood flow. Andean women undergo the normal doubling of UA diameter during pregnancy at high altitude whereas European women show only about half as much increase (Wilson et al., 2007). As a result, Andean women achieve much higher UA blood flows and O2 delivery than Europeans at high altitude. Values are the same, however, at low altitude indicating that the protective effects of Andean ancestry are only evident under circumstances of chronic hypoxia (Julian et al., 2009; Wilson et al., 2007; Zamudio et al., 2007). Our recent Bolivian investigations support the likelihood that a more favorable balance of angiogenic to anti-angiogenic factors plays a role in the Andeans’ larger UA diameters. Specifically, Andean compared with European women at high altitude had lower ratios of the anti-angiogenic substance sFlt-1 relative to the pro-angiogenic PlGF, which was due entirely to lower sFlt-1 levels (Davila et al., 2010).
Recently the functional significance of the pregnancy rise in UA blood flow and blood-flow differences between long- and short-residents groups has been questioned (Postigo et al., 2009; Zamudio et al., 2007). We consider that such differing interpretations are due to characteristics of study design and consideration of the ways in which UA blood flow can influence fetal growth.
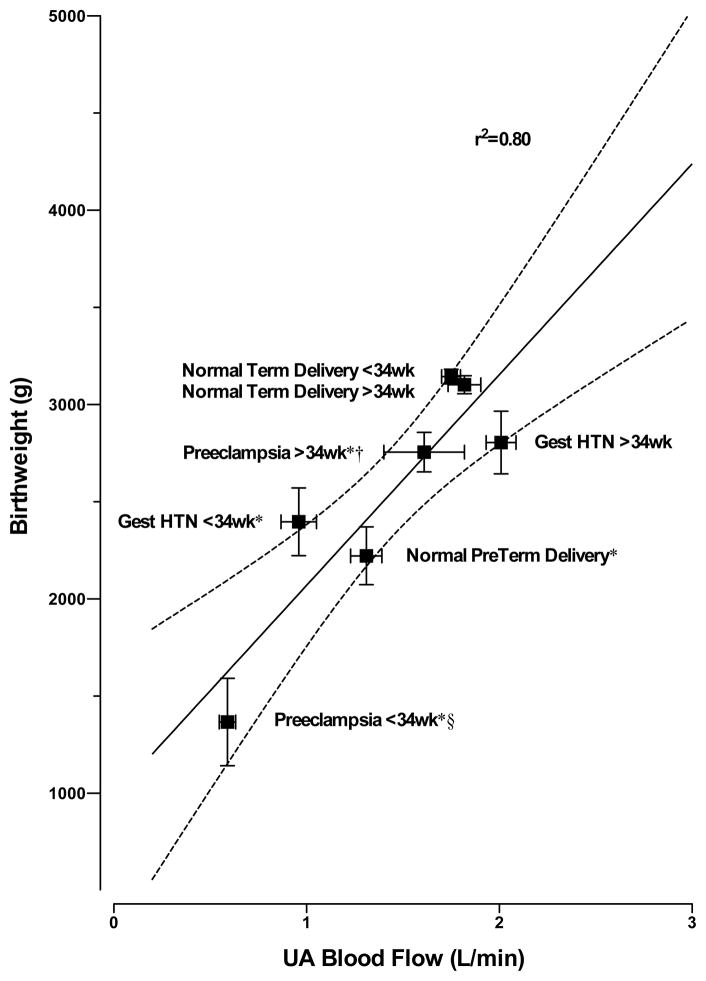
With respect to study design, the Zamudio study was conducted near term as it was designed to collect placentas and evaluate fetal oxygenation at delivery (Postigo et al., 2009; Zamudio et al., 2007). As we have pointed out, measurements at term cannot address the origin of differences in fetal growth (Julian et al., 2009; Moore, 2010). Our longitudinal studies have shown that ancestry-group differences in UA blood flow are present by week 20 (Julian et al., 2009; Wilson et al., 2007), which is well before altitude-associated reductions in fetal growth are apparent (Krampl, 2002; Unger et al., 1988). Another factor pertaining to study design is that there is a limited range of variation in UA blood flow or birth weight when only healthy high-altitude women are considered. As shown in Figure 3, when the full range of variation in birth weight is present by including women with hypertensive complications and preterm deliveries, 80% of the variation in birth weight can be attributed to UA blood flow (Browne et al., 2011).
Figure 3.
Third trimester uterine artery (UA) artery blood flows in 157 Andean residents of 3600–4100 m measured either before or after week 34 are strongly associated with their infant’s birth weight (R2 = 0.80, y = 1085 × + 985 where x is UA blood flow in liters/min and y is birth weight in grams, P<0.007). Normal women were without any pregnancy complications and delivered at term (n=119) or preterm (n=6). Twenty (20) were diagnosed with preeclampsia, and 12 had gestational hypertension (Gest HTN). The solid line represents the regression line with dotted 95% confidence intervals. *= p<0.05 compared with normal women who delivered at term; §= p<0.05 compared with Gest HTN; and †= p<0.05 compared with ≤34 wk. Figure adapted from (Browne et al., 2011) where study details may be found.
There are several considerations affecting the ways in which UA blood flow can influence fetal growth. One is the question of where the UA blood flow goes and, in particular, how much is distributed to the uterine, placental and fetal vascular beds. Anastomoses have recently been observed by ultrasound between arterial and venous vessels in the myometrial vasculature (Schaaps et al., 2005), which means that some of the UA flow does not reach the intervillous space. These shunts have been suggested to protect fragile fetal villi from mechanical damage due to high velocity or collapse from high intervillous pressure (Burton et al., 2009). Given that such a collapse would reduce umbilical vein blood flow, the lower umbilical vein blood flows seen in European than Andean pregnancies at high altitude (Postigo et al., 2009) are consistent with such mechanical considerations being important. Such shunts might also serve to increase the delivery of substrates to the myometrial (non-decidualized) portions of the uterine circulation and/or raise uterine PvO2. A related consideration pertains to how much O2 is consumed by the placenta. While it has been estimated that placental O2 consumption constitutes 40% of the fetoplacental total (Carter, 2000), this is derived from acute studies at low altitude and may not be applicable to the high-altitude case. To our knowledge, there are no direct measurements of placental O2 consumption at high altitude. Supporting the possibility of higher placental O2 consumption, a recent report shows a 71% increase in oxidative phosphorylation in placentas from native Tibetan compared with immigrant Han residents of 3600 m (Zhao, 2007). As noted below, placental morphology is remarkably well conserved at high vs. low altitude, which may also reflect higher placental O2 consumption. The recent report by Zamudio and co-workers of greater glucose extraction at high than low altitude is also consistent with increased placental metabolism (Zamudio et al., 2010).
A second possibility is that high UA flow constitutes a reserve that protects against intermittent hypoxia and ischemia/reperfusion (I/R) injury. Blood flow is likely to be highly variable during pregnancy (Jeffreys et al., 2006); the resultant intermittent hypoxia and I/R injury would be expected to stimulate the production of reactive oxygen species (ROS), damage the endothelium, reduce vasodilator production or activity, and decrease uterine vessel diameter and flow. The higher antioxidant activity seen in high-altitude Andean than European women during pregnancy might be one mechanism by which such oxidant damage is prevented and larger UA diameters and flow preserved (Julian et al., (in review)).
A final consideration stems from the nature of human placental exchange. It has been suggested that the human placenta operates like a venous equilibration exchanger for which a large uterine-umbilical venous PO2 gradient is required to draw O2 across the placental barrier (Wilkening and Meschia, 1992). As suggested long ago (Metcalfe et al., 1967) and predicted by the Fick equation, the benefit of high UA blood flow may therefore be to narrow the maternal A-V O2 content difference and thereby raise uterine PvO2 and fetal oxygenation. Such an effect would not be detected by measurements of maternal-fetal O2 gradients as reported by Zamudio (Postigo et al., 2009) since uterine PvO2data are required.
We conclude that a rise in UA blood flow is clearly important for sustaining fetal growth and maternal well-being during pregnancy. The lesser rise in UA blood flow seen during pregnancy in short- vs. long-resident high-altitude populations seems likely to be an important contributor to their relative protection from altitude-associated fetal growth restriction, but the precise mechanisms involved are presently unknown.
3.3 Genetic factors
The population differences in the altitude-related decline in birth weight (Figure 2) and in the maternal physiological responses to pregnancy described above raise the question as to whether genetic factors are involved. We have approached this question by medical records, gene marker, and single nucleotide polymorphism (SNP) studies.
Using medical records surnames to classify population ancestry, we showed that European newborns at high altitude were nearly five-times more likely than Andeans to be SGA whether or not variation in maternal hypertensive complications of pregnancy, parity, pregnancy weight gain, near-term body weight, week of onset and number of prenatal visits were taken into account (Julian et al., 2007). Finer-scale groupings using all surname combinations (Andean-Andean, Andean-Mestizo, Mestizo-Mestizo, Mestizo-European, European-European) revealed that altitude-associated reductions in birth weight were inversely related to the number of Andean surnames present (Bennett et al., 2008). Intriguingly, the baby’s birth weight was 74 g higher when classified by the father’s rather than the mother’s ancestry, or 81 g higher if the effects of gestational age were taken into account. Such findings are consistent with genetic imprinting in which maternally-transmitted genes restrict and paternally-transmitted ones enhance fetal growth (Moore and Haig, 1991) and with associations observed in Pima Indians between paternally-expressed genes on chromosome 11 and birth weight (Lindsay et al., 2002).
More direct assessment of the role of genetic factors is available from studies using ancestry-informative gene markers (AIMs). We found that the percent maternal indigenous American AIMs was positively correlated with UA blood flow, uteroplacental O2 delivery, fetal head circumference and infant birth weight at high altitude (Julian et al., 2009). No such relationships were present at low altitude, implying that the protective effect of indigenous American ancestry was confined to high altitude. These observations differ from those of Zamudio and co-workers insofar as they found that the percent fetal indigenous American AIMs was related to birth weight at low as well as high altitude (Zamudio et al., 2007); this, as we have suggested elsewhere, may be due to the influence of paternal factors on fetal growth (Julian et al., 2009).
Genome scans using SNP mapping arrays permit fuller evaluation of the role of genetic factors and in particular, HIF-regulatory or targeted genes (Moore et al., 2006). We have shown that nine HIF-related gene regions were distinctive in high-altitude Andeans when compared with low-altitude indigenous American, East Asian, European and West African populations using the Affymetrix 500k GeneChip® Mapping array (Bigham et al., 2009) (Table 1). Three of these gene regions were confirmed using the higher density Affymetrix 1M GeneChip® (Bigham et al., 2010). Each of these -- EGLN1, PRKAA1, and NOS2A -- plays a key role in relation to oxygen-sensing and maternal physiological response to pregnancy. EGLN1, also called PHD2, is involved in the degradation of HIF under normoxia or conversely its preservation in hypoxia. NOS2A, also called iNOS, is the inducible form of NO synthase and hence an important source of NO. PRKAA1, also called AMPK or AMPKa1, plays a key role in the regulation of protein synthesis by acting via inhibitory effects on the mTOR (mammalian target of rapamycin) pathway. AMPK functions as a cellular energy sensor, being activated by the accumulation of AMP and hence energy depletion, and has recently been implicated in the etiology of intrauterine growth restriction and PE (Burton et al., 2010). Of note, one of these, EGLN1, distinguished both Andeans and Tibetans from the low-altitude control populations considered. Three other studies employing genome scans appeared about the same time as our report (Table 1). All were conducted in Tibetans and implicated one of the same gene regions, EPAS (also called HIF2a) that we had seen (Bigham et al., 2010) although we found this gene region to be somewhat less distinctive, perhaps because of our broader set of control populations. In any event, the convergence of these three lines of evidence as well as confirmation by other investigators strongly implicates genetic factors in human adaptation to high altitude.
Table 1.
Genome scans implicating gene regions in long-term high-altitude adaptation.
| Study | Gene | Definition | Chromosome |
|---|---|---|---|
| (Bigham et al., 2009) 500k SNP genome scan in Andeans vs. low-altitude controls: | |||
| EDNRA | Endothelin receptor A | 4q31.22 | |
| EGLN1 | Egl nine homolog 1; also called HIF prolyl hydroxylase 2, HIF-PH2, HIF-prolyl hydroxylase 2, HPH-2, nine-like protein 1, hypoxia-inducible factor prolyl hydroxylase 2, prolyl hydroxylase domain-containing protein 2, zinc finger MYND domain-containing protein 6); | 1q42.1 | |
| NOS2A | Inducible or nitric oxide 2a | 17q11.2-q12 | |
| PRKAA1 | Protein kinase, AMP-activated, alpha 1 catalytic subunit; also called AMPK, AMPKa1 | 5p12 | |
| ELF2 | E74-like factor 2; also called ets domain transcription factor, EU32, NERF, NERF-1A, NERF-1B, NERF-1a, b, NERF-2 | 4q28 | |
| CDH | Cadherin 1; also called E-cadherin | 18q12.1 | |
| PIK3CA | Phosphoinositide-3-kinase, catalytic, alpha polypeptide; also called MGC142161, MGC142163, PI3K, p110-alpha | 3q26.3 | |
| TNC | Tenascin C | 9q33 | |
| VEGFA | Vascular endothelial growth factor; also called RP1-261G23.1, MGC70609, MVCD1, VEGF, VPF | 6p12 | |
| (Bigham et al., 2010) 1 M SNP genome scan in Andeans and Tibetans vs. low-altitude controls: | |||
| EGLN1 | See above | See above | |
| EPAS1 | Endothelial PAS domain protein 1; also called ECYT4, HIF2A, HLF, MOP2, PASD2, bHLHe73 | 2p21-p16 | |
| NOS2A | See above | See above | |
| PRKAA1 | See above | See above | |
| (Simonson et al., 2010) Genome-wide allelic differentiation scan of Tibetans with lowland Han: | |||
| EPAS1 | Endothelial PAS domain protein 1; also called ECYT4, HIF2A, HLF, MOP2, PASD2, bHLHe73 | 2p21-p16 | |
| (Yi et al., 2010) Sequencing 50 exomes in Tibetans, encompassing 92% of coding genes: | |||
| EPAS1 | Endothelial PAS domain protein 1; also called ECYT4, HIF2A, HLF, MOP2, PASD2, bHLHe73 | 2p21-p16 | |
4.0 Placental factors influencing fetal growth
The placenta plays a key role in implantation as well as maternal cardiovascular and metabolic responses to pregnancy. Here we summarize previous studies concerning placental morphology and its functional attributes in relation to fetal growth at low and high altitude.
4.1 Placental morphology
Placentas from growth-restricted babies at low altitude are reduced in size and show multiple vascular abnormalities, including poorly branched or capillarized villi and thickened exchange barriers (Kingdom et al., 2000). These characteristics are consistent with increased fetal vascular resistance as seen clinically by absent or reversed umbilical artery blood flow.
In contrast, reduced birth weights at high altitude are not accompanied by Doppler indices of augmented fetal vascular resistance (Julian et al., 2008; Julian et al., 2009; Krampl, 2002; Postigo et al., 2009; Wilson et al., 2007; Zamudio et al., 2007) and placental morphology appears well preserved. While there is considerable variation among high-altitude studies, placental size or weight at high altitude has generally been found to be similar to low-altitude values but larger in relation to fetal size since birth weights are reduced (Kruger and Arias-Stella, 1970; Tissot van Patot et al., 2009). Variation between studies may be due to differences in methodology (e.g., the use of wet vs. dry weight specimens since the former includes blood and other fluids that do not reflect placental tissue) or between the Colorado, Bolivia, Kirghizstan, and Saudi Arabian populations being considered (Ali et al., 1996; Jackson et al., 1987; Reshetnikova et al., 1994). Larger placental relative to fetal size would be expected to benefit diffusion, as would the thinning of the villous membrane as described in Bolivian materials (Jackson et al., 1985). The greater branching of fetal capillaries and shorter, more-closely spaced loops that were seen in the terminal villi in Saudi Arabian reports would also be expected to facilitate diffusion (Ali et al., 1996). The only population comparison at high altitude found similar placental weights but more trophoblast, more villous stroma and longer, thinner fetal capillaries in Andean vs. European/Mestizo placentas (Jackson et al., 1987). Thus existing data indicate that the high-altitude placenta is relatively protected from the abnormalities seen in SGA pregnancies at low altitude but well-controlled comparisons with validation of genetic ancestry are needed to establish whether population differences exist in placental morphology.
4.2
Functional attributes of the placenta reside in its role as an immunological barrier between the fetus and mother and in the nature of placental exchange. While studies of such characteristics at high altitude are only beginning, it is constructive to examine what is known as a means for directing future investigations.
4.2.1. Role as an immunological barrier
Following recognition of fetally-derived antigens, the maternal immune system reacts by initiating a range of protective mechanisms. Previously we found higher levels of the pro-inflammatory cytokines interleukin (IL)-6 and tumor necrosis factor relative to the anti-inflammatory IL-10 at 3100 than 1600 m in Colorado (Coussons-Read et al., 2002). To determine if differences in inflammatory response contributed to Andean protection from altitude-associated reductions in fetal growth, we compared pro- and anti-inflammatory cytokines in pregnant Andean and Bolivian women at low and high altitude (Davila et al., 2011). While both pregnancy and altitude altered cytokine levels, the only population difference was that high altitude lowered the pro-inflammatory IL-1β during pregnancy in Andean but not European women, suggesting that the Andeans had relatively less inflammatory response.
4.2.2. Placental exchange
The placenta’s primary exchange barriers are the two cell layers between the maternal and fetal blood vessels; namely the syncytiotrophoblast (i.e., the placenta’s transporting epithelium) and the fetal capillary endothelium. The syncytiotrophoblast consists of the microvillus plasma membrane (MVM) that is in contact with the maternal blood and the basal plasma membrane (BM), which faces the fetal circulation (Desforges and Sibley, 2010).
Diffusion accounts for most placental exchange (Desforges and Sibley, 2010). According to Fick’s Law, the net rate of diffusion is dependent on (a) the surface area available for diffusion per gm placental tissue; (b) the path length over which diffusion takes place or membrane thickness; (c) the diffusion coefficient for the substance, which is inversely related to the square root of the gas molecular weight; and (d) the maternal-fetal pressure gradient affecting the diffusion of gases in liquids. In growth-restricted fetuses at low altitude, smaller placental size and impaired vascularization reduce surface area and increase path length, thereby impairing placental exchange. However the larger placental relative to fetal size, membrane thinning and greater branching of fetal capillaries at high altitude would be expected to benefit diffusion by (a) increasing surface area and (b) reducing the path length for diffusion. Factor (c) is unlikely to be important since the molecular sizes of O2, glucose, water and the various ions necessary for fetal growth are unaffected by altitude. The primary determinant of factor (d) is the rate of blood flow through the uterine and umbilical circulations as well as the metabolic consumption of the intervening tissues.
A considerable number of studies at low altitude support a causal role for maternal uterine blood flow. For example, experimentally-induced reduction of uterine blood flow in animal models and low maternal UA blood flows observed clinically are clearly associated with reductions in placental weight that can be detected well before any attenuation in fetal growth (Hafner et al., 2003; Jansson et al., 1986). As reviewed above, it seems reasonable to suppose that the Andeans’ higher UA blood flow during pregnancy at high altitude improves placental exchange and therefore is one factor serving to protect placental morphology as well as fetal growth.
4.2.3. Transporters present
Flux across the MVM is the rate-limiting step in maternal-fetal transfer of glucose, amino acids and ions. Transporters are required for the movement of these less-readily diffusible substances. Reduced activity of placental amino acid transporters and decreased fetal plasma amino acid concentrations play important roles in fetal growth restriction at low altitude (Cetin and Antonazzo, 2009). To our knowledge no studies of amino acid transporters have been done at high altitude. With respect to glucose, the activity and expression of the glucose transporters GLUT 1 and GLUT 3 have been reported as unaltered or upregulated in IUGR fetuses at low altitude (Jansson et al., 2002). At high altitude, GLUT 1 on the BM was decreased at 3100 vs. 1600 m in Colorado (Zamudio et al., 2006) as well as at somewhat higher altitudes (3600 m) in Bolivia, but this was judged to be a consequence, not a cause, of reduced maternal-fetal transfer (Zamudio et al., 2010). Of note in our genome scan, none of the 64 SNPs in or near GLUT 1 gene regions was distinctive in Andean compared with low-altitude populations (Bigham, personal communication).
Recent data support the possibility that fetoplacental glucose metabolism is altered under conditions of high altitude. Confirming previous reports (Krampl et al., 2001), Zamudio and coworkers found lower maternal venous glucose concentrations at high vs. low altitude. These were not due to diet, since arterialized (and nonpregnant) levels were unaffected, but rather to a widened A-V gradient at high altitude. The authors interpreted this widened A-V gradient as indicating an increased placental glucose consumption that, in turn, spared oxygen but limited glucose for fetal growth (Zamudio et al., 2010). This study makes a valuable contribution by advancing our knowledge of how chronic hypoxia affects fetal-placental metabolism but we think underestimates the importance of UA blood flow. Abundant data support the likelihood that widened A-V gradients indicate that blood flow is insufficient to meet metabolic demands (Reeves et al., 1961). The authors’ own data underscore the importance of UA blood flow since it was the major determinant of uteroplacental glucose delivery and strongly (R2=0.43) associated with fetal glucose consumption (Zamudio et al., 2010). However, the authors interpreted the altitudinal differences in UA blood flow as not being the cause of the widened A-V gradient based on prior in vitro observations indicating that the magnitude of flow differences would only lower fetal glucose transfer half as much as observed (by ~12% vs. ~28%). Nonetheless, half is a considerable fraction and their data indicate that less glucose was transported to the uteroplacental circulation at high than low altitude (2.3 vs. 2.9 mmol/min), pointing to an important influence of UA blood flow on fetal glucose consumption and growth.
5.0 Summary and conclusions
Studies at high altitude have had a rich history, demonstrating the separable influences of fetal growth restriction and length of gestation on birth weight and improving our understanding of the compensatory responses enabling successful fetal growth and development. The chronic hypoxia of residence at high altitude influences maternal physiological responses to pregnancy in ways that serve to reduce the rise in UA blood flow and slow fetal growth. Multigenerational high-altitude populations are relatively protected from these adverse effects. The precise mechanisms by which their higher UA flow preserves fetal growth remain uncertain but likely involve increased delivery and/or metabolism of oxygen, glucose or other substrates; mechanical factors protecting fragile fetal villi; creation of a reserve protecting against I/R injury of the uteroplacental vasculature; and improved placental exchange. Placental growth and development appear remarkably well protected at high altitude, with the modifications observed likely to benefit placental exchange by increasing the surface area and/or reducing the path length over which diffusion occurs. Compared to the placental abnormalities seen in fetal growth restriction at low altitude, the relative protection of placental morphology at high altitude may be due to differences in the timing of the insults on placental vs. fetal growth, the more chronic nature of the growth restriction present, and perhaps also the portion(s) of the vasculature or other organ systems affected. The recent study of Browne and co-workers showing that Andean ancestry preserves UA enlargement but does not prevent the impaired trophoblast remodelling and invasion characteristic of PE (Browne et al., 2011) suggests that chronic hypoxia influences maternal and fetoplacental factors in fundamentally different ways. Perhaps the chronic hypoxia of residence at high altitude alters maternal but not fetoplacental vascular responses to pregnancy, unless the hypoxia is sufficiently severe to compromise placental function.
Much remains to be learned concerning the effects of chronic hypoxia on embryonic development and the early placental (intravillous) circulation. Important will be studies that determine the fetoplacental and maternal factors responsible for the transformation of the uterine vasculature from a high resistance, low flow to a low resistance, high flow vascular bed. Comparative studies of placental morphology, exchange, and metabolism in long- vs. short-resident high-altitude populations are also needed. Genomic as well as epigenetic studies have opened new avenues for investigation that are likely to yield insights into the basic pathways involved in regulating maternal and fetoplacental physiologic responses to pregnancy and chronic hypoxia. Continued use of the experiment of nature afforded by studying human populations that have resided at high altitudes for varying lengths of time promises to improve our understanding of the physiological mechanisms regulating maternal vascular responses to pregnancy, genes responsible for influencing susceptibility to pregnancy disorders, and the evolutionary processes by which high-altitude adaptation has been achieved. Such information, in turn, can lead to tests for predicting persons at risk and for identifying new treatments.
Acknowledgments
The authors express their gratitude to the subjects who participated in these studies and the many investigators who provided invaluable help in their conduct. Grant support from the NIH (HL060131, TW001188, HL079647, HL14985), the NSF (Graduate Research Fellowships to Megan Wilson and Abigail Bigham, Dissertation Improvement Grant to Colleen G. Julian, and BNS8919645 to Lorna G. Moore), and the AHA (predoctoral fellowship to Colleen G. Julian) is gratefully acknowledged.
Footnotes
Birth weights < 10th percentile of sea-level values for a given gestational age and sex (Williams et al., 1982).
Two or more resting blood pressures >140/90 mmHg accompanied by ≥1+ proteinuria (or ≥300 mg in 24 hours) after the 20th week of pregnancy in a previously normotensive women.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
8.0 References
- Ali KZ, Burton GJ, Morad N, Ali ME. Does hypercapillarization influence the branching pattern of terminal villi in the human placenta at high altitude? Placenta. 1996;17:677–682. doi: 10.1016/s0143-4004(96)80018-x. [DOI] [PubMed] [Google Scholar]
- Barker DJP, editor. The fetal and infant origins of adult disease. British Medical Journal Books; London: 1992. [Google Scholar]
- Bennett A, Sain SR, Vargas E, Moore LG. Evidence that parent-of-origin affects birth-weight reductions at high altitude. American Journal of Human Biology. 2008;20:592–597. doi: 10.1002/ajhb.20784. [DOI] [PubMed] [Google Scholar]
- Bigham A, Bauchet M, Pinto D, Mao X, Akey J, Mei R, Scherer S, Julian C, Wilson M, Lopez Herraez D, Brutsaert T, Parra E, Moore L, Shriver M. Identifying signatures of natural selection in Tibetan and Andean populations using dense genome scan data. PLoS Genet. 2010;6:1–14. doi: 10.1371/journal.pgen.1001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigham AW, Mao X, Mei R, Brutsaert T, Wilson MJ, Julian CG, Parra EJ, Akey JM, Moore LG, Shriver MD. Identifying positive selection candidate loci for high-altitude adaptation in Andean populations. Human Genomics. 2009;4:79–90. doi: 10.1186/1479-7364-4-2-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne V, Toledo-Jaldin L, Davila R, Lopez L, Yamashiro H, Cioffi-Ragan D, Wilson M, Bigham A, Shriver M, Honigman B, Vargas E, Roach RC, Moore L. High end-arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2011 Feb 16; doi: 10.1152/ajpregu.91046.2008. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchell RC. Arterial blood flow in the human intervillous space. American Journal of Obstetrics and Gynecology. 1967;98:303–311. doi: 10.1016/0002-9378(67)90149-4. [DOI] [PubMed] [Google Scholar]
- Burton G, Jauniaux E, Charnock-Jones DS. The influence of the intrauterine environment on human placental development. Int J Dev Biol. 2010;54:303–312. doi: 10.1387/ijdb.082764gb. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Jauniaux E. Placental oxidative stress: from miscarriage to preeclampsia. Journal of the Society for Gynecological Investigation. 2004;11:342–352. doi: 10.1016/j.jsgi.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Burton GJ, Woods AW, Jauniaux E, Kingdom JC. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta. 2009;30:473–482. doi: 10.1016/j.placenta.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AM. Placental oxygen consumption. Part I: in vivo studies--a review. Placenta. 2000;21(Suppl A):S31–37. doi: 10.1053/plac.1999.0513. [DOI] [PubMed] [Google Scholar]
- Cetin I, Antonazzo P. The role of the placenta in intrauterine growth restriction (IUGR) Z Geburtshilfe Neonatol. 2009;213:84–88. doi: 10.1055/s-0029-1224143. [DOI] [PubMed] [Google Scholar]
- Chen D, Zhou X, Zhu Y, Zhu T, Wang J. Comparison study on uterine and umbilical artery blood flow during pregnancy at high altitude and at low altitude. Zhonghua Fu Chan Ke Za Zhi. 2002;37:69–71. [PubMed] [Google Scholar]
- Coussons-Read ME, Mazzeo RS, Whitford MH, Schmitt M, Moore LG, Zamudio S. High altitude residence during pregnancy alters cytokine and catecholamine levels. American Journal of Reproductive Immunology. 2002;48:344–354. doi: 10.1034/j.1600-0897.2002.01078.x. [DOI] [PubMed] [Google Scholar]
- Davila RD, Julian CG, Wilson MJ, Browne VA, Rodriguez C, Bigham AW, Shriver MD, Vargas E, Moore LG. Do anti-angiogenic or angiogenic factors contribute to the protection of birth weight at high altitude afforded by Andean ancestry? Reproductive Science. 2010;17:861–870. doi: 10.1177/1933719110372418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila RD, Julian CG, Wilson MJ, Browne VA, Rodriguez C, Bigham AW, Shriver MD, Vargas E, Moore LG. Do cytokines contribute to the Andean-associated protection from reduced fetal growth at high altitude? Reproductive Science. 2011;18:79–87. doi: 10.1177/1933719110380061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desforges M, Sibley CP. Placental nutrient supply and fetal growth. International Journal of Developmental Biology. 2010;54:377–390. doi: 10.1387/ijdb.082765md. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips S, Anstee S, Barker DJP. Effects of altitude versus economic status on birth weight and body shape at birth. Pediatric Research. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Gonzales G, Steenland K, Tapia V. Maternal hemoglobin level and fetal outcome at low and high altitudes. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;297:R1477–1485. doi: 10.1152/ajpregu.00275.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E, Metzenbauer M, Hofinger D, Munkel M, Gassner R, Schuchter K, Dillinger-Paller B, Philipp K. Placental growth from the first to the second trimester of pregnancy in SGA-foetuses and pre-eclamptic pregnancies compared to normal foetuses. Placenta. 2003;24:336–342. doi: 10.1053/plac.2002.0918. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Joy CF, Mayhew TM, Haas JD. Stereological studies on the true thickness of the villous membrane in human term placentae: a study of placentae from high-altitude pregnancies. Placenta. 1985;6:249–258. doi: 10.1016/s0143-4004(85)80054-0. [DOI] [PubMed] [Google Scholar]
- Jackson MR, Mayhew TM, Haas JD. Morphometric studies on villi in human term placentae and the effects of altitude, ethnic grouping and sex of newborn. Placenta. 1987;8:487–495. doi: 10.1016/0143-4004(87)90077-4. [DOI] [PubMed] [Google Scholar]
- Jansson T, Ekstrand Y, Bjorn C, Wennergren M, Powell TL. Alterations in the activity of placental amino acid transporters in pregnancies complicated by diabetes. Diabetes. 2002;51:2214–2219. doi: 10.2337/diabetes.51.7.2214. [DOI] [PubMed] [Google Scholar]
- Jansson T, Thordstein M, Kjellmer I. Placental blood flow and fetal weight following uterine artery ligation: temporal aspects of intrauterine growth retardation in the guinea pig. Biology of the Neonate. 1986;49:172–180. doi: 10.1159/000242528. [DOI] [PubMed] [Google Scholar]
- Jauniaux E, Watson AL, Hempstock J, Bao YP, Skepper JN, Burton GJ. Onset of maternal arterial blood flow and placental oxidative stress: a possible factor in human early pregnancy failure. American Journal of Pathology. 2000;157:2111–2122. doi: 10.1016/S0002-9440(10)64849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffreys RM, Stepanchak W, Lopez B, Hardis J, Clapp JF., 3rd Uterine blood flow during supine rest and exercise after 28 weeks of gestation. British Journal of Obstetrics and Gynecology. 2006;113:1239–1247. doi: 10.1111/j.1471-0528.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- Jensen GM, Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? American Journal of Public Health. 1997;87:1003–1007. doi: 10.2105/ajph.87.6.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph V, Soliz J, Pequignot J, Sempore B, Cottet-Emard JM, Dalmaz Y, Favier R, Spielvogel H, Pequignot JM. Gender differentiation of the chemoreflex during growth at high altitude: functional and neurochemical studies. American Journal of Physiology Regulatory Integrative and Comparative Biology. 2000;278:R806–R816. doi: 10.1152/ajpregu.2000.278.4.R806. [DOI] [PubMed] [Google Scholar]
- Julian C, McCord J, Browne V, Rodriguez A, Wilson M, Rodriguez C, Yamashiro H, Hageman J, Davilá R, Bigham A, Vargas E, Moore L. Enhanced enzymatic antioxidant activity during pregnancy in Andean compared with European residents of high altitude in review. [Google Scholar]
- Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J, Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295:R906–915. doi: 10.1152/ajpregu.00164.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S, Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Archives of Disease in Childhood: Fetal and Neonatal Edition. 2007;92:F372–377. doi: 10.1136/adc.2006.109579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian CG, Wilson MJ, Lopez M, Yamashiro H, Tellez W, Rodriguez A, Bigham AW, Shriver MD, Rodriguez C, Vargas E, Moore LG. Augmented uterine artery blood flow and oxygen delivery protect Andeans from altitude-associated reductions in fetal growth. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2009;296:R1564–1575. doi: 10.1152/ajpregu.90945.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyes LE, Armaza JF, Niermeyer S, Vargas E, Young D, Villena M, Moore LG. Intrauterine growth restriction, preeclampsia and intrauterine mortality at high altitude in Bolivia. Pediatric Research. 2003;54(1):20–25. doi: 10.1203/01.PDR.0000069846.64389.DC. [DOI] [PubMed] [Google Scholar]
- Keyes LE, Majack R, Dempsey EC, Moore LG. Pregnancy stimulation of DNA synthesis and uterine blood flow in the guinea pig. Pediatric Research. 1997;41:708–715. doi: 10.1203/00006450-199705000-00017. [DOI] [PubMed] [Google Scholar]
- Kingdom J, Huppertz B, Seaward G, Kaufmann P. Development of the placental villous tree and its consequences for fetal growth. European Journal of Obstetrics & Gynecology and Reproductive Biology. 2000;92:35–43. doi: 10.1016/s0301-2115(00)00423-1. [DOI] [PubMed] [Google Scholar]
- Krampl E. Pregnancy at high altitude. Ultrasound in Obstetrics & Gynecology. 2002;19:535–539. doi: 10.1046/j.1469-0705.2002.00738.x. [DOI] [PubMed] [Google Scholar]
- Krampl E, Kametas NA, Cacho-Zegarra AM, Roden M, Nicolaides KH. Maternal plasma glucose at high altitude. British Journal of Obstetrics and Gynaecology. 2001;108:254–257. doi: 10.1111/j.1471-0528.2001.00072.x. [DOI] [PubMed] [Google Scholar]
- Krampl E, Lees C, Bland JM, Dorado JE, Gonzalo M, Campbell S. Fetal biometry at 4300 m compared to sea level in Peru. Ultrasound in Obstetrics and Gynecology. 2000;16:9–18. doi: 10.1046/j.1469-0705.2000.00156.x. [DOI] [PubMed] [Google Scholar]
- Kruger H, Arias-Stella J. The placenta and the newborn infant at high altitudes. American Journal of Obstetrics and Gynecology. 1970;106:586–591. doi: 10.1016/0002-9378(70)90045-1. [DOI] [PubMed] [Google Scholar]
- Kublickiene KR, Cockell AP, Nisell H, Poston L. Role of nitric oxide in the regulation of vascular tone in pressurized and perfused resistance myometrial arteries from term pregnant women. American Journal of Obstetrics and Gynecology. 1997;177:1263–1269. doi: 10.1016/s0002-9378(97)70048-6. [DOI] [PubMed] [Google Scholar]
- Kublickiene KR, Nisell H, Poston L. 17B-Estradiol upregulates flow-mediated nitric oxide-induced dilation in small sub-cutaneous arteries from postmenopausal women. Journal of the Society for Gynecologic Investigation. 2000a;7:186A. [Google Scholar]
- Kublickiene KR, Nisell H, Poston L, Kruger K, Lindblom B. Modulation of vascular tone by nitric oxide and endothelin 1 in myometrial resistance arteries from pregnant women at term. American Journal of Obstetrics and Gynecology. 2000b;182:87–93. doi: 10.1016/s0002-9378(00)70495-9. [DOI] [PubMed] [Google Scholar]
- Lichty JA, Ting RY, Bruns PD, Dyar E. Studies of babies born at high altitude. I. Relation of altitude to birth weight. American Medical Association Journal of Diseases of Children. 1957;93:666–669. doi: 10.1001/archpedi.1957.02060040668009. [DOI] [PubMed] [Google Scholar]
- Lindsay RS, Kobes S, Knowler WC, Hanson RL. Genome-wide linkage analysis assessing parent-of-origin effects in the inheritance of birth weight. Human Genetics. 2002;110:503–509. doi: 10.1007/s00439-002-0718-2. [DOI] [PubMed] [Google Scholar]
- Massaro GD, Olivier J, Massaro D. Short-term perinatal 10% O2 alters postnatal development of lung alveoli. American Journal of Physiology. 1989;257:L221–L225. doi: 10.1152/ajplung.1989.257.4.L221. [DOI] [PubMed] [Google Scholar]
- Mateev S, Sillau AH, Mouser R, McCullough RE, White MM, Young DA, Moore LG. Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. American Journal of Physiology: Heart and Circulatory Physiology. 2003;284:H820–H829. doi: 10.1152/ajpheart.00701.2002. [DOI] [PubMed] [Google Scholar]
- Metcalfe J, Novy MJ, Peterson EN. Reproduction at high altitude. In: Benirschke K, editor. Comparative Aspects of Reproductive Failure. Springer-Verlag; 1967. [Google Scholar]
- Moore L. Uterine blood flow as a determinant of feto-placental development. In: Burton G, Barker D, editors. Placenta and Fetal Programming. Univ Cambridge Press; Cambridge, UK: 2010. [Google Scholar]
- Moore LG. Human genetic adaptation to high altitude. High Altitude Medicine & Biology. 2001a;2:257–279. doi: 10.1089/152702901750265341. [DOI] [PubMed] [Google Scholar]
- Moore LG. Small babies and big mountains: John Lichty solves a Colorado mystery in Leadville. In: Reeves JT, Grover FT, editors. Attitudes on Altitude. Univ Colorado Press; Boulder: 2001b. pp. 137–159. [Google Scholar]
- Moore LG, Rounds SS, Jahnigen D, Grover RF, Reeves JT. Infant birth weight is related to maternal arterial oxygenation at high altitude. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1982;52:695–699. doi: 10.1152/jappl.1982.52.3.695. [DOI] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, Vargas E. An evolutionary model for identifying genetic adaptation to high altitude. Advances in Experimental Medicine and Biology. 2006;588:101–118. doi: 10.1007/978-0-387-34817-9_10. [DOI] [PubMed] [Google Scholar]
- Moore LG, Zamudio S, Zhuang J, Sun S, Droma T. Oxygen transport in Tibetan women during pregnancy at 3658 m. American Journal of Physical Anthropology. 2001;114:42–53. doi: 10.1002/1096-8644(200101)114:1<42::AID-AJPA1004>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Moore T, Haig D. Genomic imprinting in mammalian development: a parental tug-of-war. Trends in Genetics. 1991;7:2361–2366. doi: 10.1016/0168-9525(91)90230-N. [DOI] [PubMed] [Google Scholar]
- Mortola JP, Frappell PB, Aguero L, Armstrong K. Birth weight and altitude: a study in Peruvian communities. Journal of Pediatrics. 2000;136:324–329. doi: 10.1067/mpd.2000.103507. [DOI] [PubMed] [Google Scholar]
- Okubo S, Mortola JP. Control of ventilation in adult rats hypoxic in the neonatal period. Journal of Applied Physiology. 1990;259:R836–R841. doi: 10.1152/ajpregu.1990.259.4.R836. [DOI] [PubMed] [Google Scholar]
- Palmer SK, Moore LG, Young DA, Cregger B, Berman JC, Zamudio S. Altered blood pressure course during normal pregnancy and increased preeclampsia at high altitude (3100 meters) in Colorado. American Journal of Obstetrics and Gynecology. 1999;180:1161–1168. doi: 10.1016/s0002-9378(99)70611-3. [DOI] [PubMed] [Google Scholar]
- Palmer SK, Zamudio S, Coffin C, Parker S, Stamm E, Moore LG. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstetrics and Gynecology. 1992;80:1000–1006. [PubMed] [Google Scholar]
- Park J, Stoffers D, Nicholls R, Simmons R. Development of type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. Journal of Clinical Investigation. 2008;118:16–24. doi: 10.1172/JCI33655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RM, Pouliot MR. Neonatal morphometrics and perinatal outcome: who is growth retarded? American Journal of Obstetrics and Gynecology. 1987;157:691–693. doi: 10.1016/s0002-9378(87)80030-3. [DOI] [PubMed] [Google Scholar]
- Postigo L, Heredia G, Illsley NP, Torricos T, Dolan C, Echalar L, Tellez W, Maldonado I, Brimacombe M, Balanza E, Vargas E, Zamudio S. Where the O2 goes to: preservation of human fetal oxygen delivery and consumption at high altitude. Journal of Physiology. 2009;587:15. doi: 10.1113/jphysiol.2008.163634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajakumar A, Jeyabalan A, Markovic N, Ness RB, Gilmour C, Conrad K. Placental HIF-1alpha, HIF-2alpha, membrane and soluble VEGF receptor-1 proteins are not increased in normotensive pregnancies complicated by late-onset intrauterine growth restriction. American Journal of Physiology: Regulatiory, Integrative and Comparative Physiology. 2007;293:R766–774. doi: 10.1152/ajpregu.00097.2007. [DOI] [PubMed] [Google Scholar]
- Reeves JT, Grover RF, Blount SG, Jr, Filley GF. The cardiac output response to standing and treadmill walking. Journal of Applied Physiology. 1961;16:283–288. doi: 10.1152/jappl.1961.16.2.283. [DOI] [PubMed] [Google Scholar]
- Reshetnikova OS, Burton GJ, Milovanov AP. Effects of hypobaric hypoxia on the fetoplacental unit: the morphometric diffusing capacity of the villous membrane at high altitude. American Journal of Obstetrics and Gynecology. 1994;171:1560–1565. doi: 10.1016/0002-9378(94)90402-2. [DOI] [PubMed] [Google Scholar]
- Rockwell LC, ECD, Moore LG. Chronic hypoxia diminishes the proliferative response of guinea pig uterine artery vascular smooth muscle cells in vitro. High Altitude Medicine & Biology. 2006;6:237–244. doi: 10.1089/ham.2006.7.237. [DOI] [PubMed] [Google Scholar]
- Rodesch F, Simon P, Donner C, Jauniaux E. Oxygen measurements in endometrial and trophoblastic tissues during early pregnancy. Obstetrics and Gynecology. 1992;80:283–285. [PubMed] [Google Scholar]
- Sartori C, Allemann Y, Trueb L, Delabays A, Nicod P, Scherrer U. Augmented vasoreactivity in adult life associated with perinatal vascular insult. Lancet. 1999;353:2205–2207. doi: 10.1016/S0140-6736(98)08352-4. [DOI] [PubMed] [Google Scholar]
- Schaaps JP, Tsatsaris V, Goffin F, Brichant JF, Delbecque K, Tebache M, Collignon L, Retz MC, Foidart JM. Shunting the intervillous space: new concepts in human uteroplacental vascularization. American Journal of Obstetrics and Gynecology. 2005;192:323–332. doi: 10.1016/j.ajog.2004.06.066. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Cioffi-Ragan D, Beaty B, DeSilva W, Wilson M, Julian C, Moore L, Galan H. Altitude effects on fetal subcutaneous and lean tissues and on the fetal circulation in review. [Google Scholar]
- Simonson T, Yang Y, Huff C, Yun H, Qin G, Witherspoon D, Bai Z, Lorenzo F, Xing J, Jorde L, Prchal J, Ge R. Genetic evidence for high-altitude adaptation in Tibet. Science. 2010;329(5987):72–75. doi: 10.1126/science.1189406. [DOI] [PubMed] [Google Scholar]
- Stave U. Physiology of the Perinatal Period: Functional and Biochemical Development in Mammals. Appleton-Century-Crofts; New York: 1970. [Google Scholar]
- Stearns S. The Evolution of Life Histories. Oxford University Press; Oxford: 1992. [Google Scholar]
- Tissot van Patot M, Grilli A, Chapman P, Broad E, Tyson W, Heller DS, Zwerdlinger L, Zamudio S. Remodeling of uteroplacental arteries is decreased in high altitude placentae. Placenta. 2003;24:326–335. doi: 10.1053/plac.2002.0899. [DOI] [PubMed] [Google Scholar]
- Tissot van Patot MC, Valdez M, Becky V, Cindrova-Davies T, Johns J, Zwerdling L, Jauniaux E, Burton GJ. Impact of pregnancy at high altitude on placental morphology in non-native women with and without preeclampsia. Placenta. 2009;30:523–528. doi: 10.1016/j.placenta.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Unger C, Weiser JK, McCullough RE, Keefer S, Moore LG. Altitude, low birth weight, and infant mortality in Colorado. Journal of the American Medical Association. 1988;259:3427–3432. [PubMed] [Google Scholar]
- Vargas M, Vargas E, Julian C, Armaza J, Rodriguez A, Tellez W, Niermeyer N, Wilson M, Parra E, Shriver M, Moore L. Determinants of blood oxygenation during pregnancy in Andean and European residents of high altitude American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2007;293:R1303–1312. doi: 10.1152/ajpregu.00805.2006. [DOI] [PubMed] [Google Scholar]
- White MM, McCullough RE, Dyckes R, Robertson AD, Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. American Journal of Physiology: Heart and Circulatory Physiology. 2000;278:H2069–H2075. doi: 10.1152/ajpheart.2000.278.6.H2069. [DOI] [PubMed] [Google Scholar]
- Wilkening RB, Meschia G. Current topic: comparative physiology of placental oxygen transport. Placenta. 1992;13:1–15. doi: 10.1016/0143-4004(92)90002-b. [DOI] [PubMed] [Google Scholar]
- Williams RL, Creasy RK, Cunningham GC, Hawes WE, Norris FD, Tashiro M. Fetal growth and perinatal viability in California. Obstetrics and Gynecology. 1982;59:624–632. [PubMed] [Google Scholar]
- Wilson MJ, Lopez M, Vargas M, Julian C, Tellez W, Rodriguez A, Bigham A, Armaza JF, Niermeyer S, Shriver M, Vargas E, Moore LG. Greater uterine artery blood flow during pregnancy in multigenerational (Andean) than shorter-term (European) high-altitude residents. American Journal of Physiology: Regulatory, Integrative and Comparative Physiology. 2007;293:R1313–1324. doi: 10.1152/ajpregu.00806.2006. [DOI] [PubMed] [Google Scholar]
- Yi X, Liang Y, Huerta-Sanchez E, Jin X, Cuo Z, Pool J, Xu X, Jiang H, Vinckenbosch N, Korneliussen T, Zheng H, Liu T, He W, Li K, Luo R, Nie X, Wu H, Zhao M, Cao H, Zou J, Shan Y, Li S, Yang Q, Asan, Ni P, Tian G, Xu J, Liu X, Jiang T, Wu R, Zhou G, Tang M, Qin J, Wang T, Feng S, Li G, Huasang, Luosang J, Wang W, Chen F, Wang Y, Zheng X, Li Z, Bianba Z, Yang G, Wang X, Tang S, Gao G, Chen Y, Luo Z, Gusang L, Cao Z, Zhang Q, Ouyang W, Ren X, Liang H, Zheng H, Huang Y, Li J, Bolund L, Kristiansen K, Li Y, Zhang Y, Zhang X, Li R, Li S, Yang H, Nielsen R, Wang J, Wang Sequencing of 50 human exomes reveals adaptation to high altitude. Science. 2010;329:75–78. doi: 10.1126/science.1190371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Baumann MU, Illsley NP. Effects of chronic hypoxia in vivo on the expression of human placental glucose transporters. Placenta. 2006;27:49–55. doi: 10.1016/j.placenta.2004.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Armeller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. Journal of Physiology. 2007;582:12. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamudio S, Torricos T, Fik E, Oyala M, Echalar L, Pullockaran J, Tutino E, Martin B, Belliappa S, Balanza E, Illsley NP. Hypoglycemia and the origin of hypoxia-induced reduction in human fetal growth. PLoS One. 2010;5:e8551. doi: 10.1371/journal.pone.0008551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X. Placental mitochondrial respiratory function of native Tibetan at high altitude. Zhonghua Yi Xue Za Zhi. 2007 Apr 3;87(13):3. [PubMed] [Google Scholar]