Abstract
Inefficiency in systemic drug delivery and tumor residence as well microenvironmental selection pressures contribute to the development of multidrug resistance (MDR) in cancer. Characteristics of MDR include abnormal vasculature, regions of hypoxia, up-regulation of ABC-transporters, aerobic glycolysis, and an elevated apoptotic threshold. Nano-sized delivery vehicles are ideal for treating MDR cancer as they can improve the therapeutic index of drugs and they can be engineered to achieve multifunctional parameters. The multifunctional ability of nanocarriers makes them more adept at treating heterogeneous tumor mass than traditional chemotherapy. Nanocarriers also have preferential tumor accumulation via the EPR effect; this accumulation can be further enhanced by actively targeting the biological profile of MDR cells. Perhaps the most significant benefit of using nanocarrier drug delivery to treat MDR cancer is that nanocarrier delivery diverts the effects of ABC-transporter mediated drug efflux; which is the primary mechanism of MDR. This review discusses the capabilities, applications, and examples of multifunctional nanocarriers for the treatment of MDR. This review emphasizes multifunctional nanocarriers that enhance drug delivery efficiency, the application of RNAi, modulation of the tumor apoptotic threshold, and physical approaches to overcome MDR.
Keywords: Tumor hypoxia, Warburg’s effect, multidrug resistance, cancer-initiating (stem) cells, multifunctional nanoparticles
Introduction
Since the discovery of the first chemotherapeutic agent (nitrogen mustard) in 1946, chemotherapy has become tailored to treat the different types of cancer according to tissue-specific criteria. Yet, in recent years our understanding of this collection of diseases has revealed patient-to-patient variability within each type of cancer (sub-types; i.e. different types of breast cancer) and even intra-patient variability (heterogeneous cancer within one patient; i.e. a patient with multi-form breast cancer). Perhaps the most significant variation is the development of multi-drug resistant (MDR) cancer. The development of MDR cancer is largely responsible for aggressive, untreatable disease [1-6]. MDR is classically defined as a state of resilience against structurally and/or functionally unrelated drugs [1]. There are two types of MDR; MDR that is inherent (intrinsic) and MDR that is acquired after exposure to chemotherapeutic agents [1]. Selection pressures within the tumor microenvironment favour the development of intrinsic MDR while the common dose and schedule adjustments that accompany traditional chemotherapy cultivate acquired MDR.
The tumor microenvironment, cancer initiating (stem) cells, and drug resistance
The microenvironment of a tumor contributes to the development of MDR and determines how each cell will respond to chemotherapy. The microenvironmental selection pressures that contribute to the development of MDR include abnormal tumor vasculature, hypoxia, decreased pH, increased interstitial fluid pressure, and alterations in the expression of tumor suppressors and oncogenes [7]. Abnormal tumor vasculature is the most defining characteristic of the tumor microenvironment. The tumor/vasculature interface undergoes a perpetual battle; as the tumor initiates, angiogenesis recruits oxygen and nutrients but as the tumor expands many of these vessels are destroyed and new vessels must be created to fuel the growing tumor. Furthermore, the vasculature of a tumor is highly disorganized and inefficient relative to normal vasculature. These fluctuating states of vascularisation lead to regions of acute and chronic hypoxia.
Cancer cells undergo a complex phenotype transformation under hypoxic conditions; a transformation that is necessary for cell survival under such conditions. This survival cascade is initiated when the alpha subunit of hypoxia inducible factor (HIF) translocates from the cytoplasm to the nucleus where it then complexes with the beta subunit of HIF, forming an active transcription factor [8-10]. The HIF complex binds to hypoxia responsive elements (HRE’s) on target genes, inducing transcription [9, 10]. The vast array of HIF targets include genes involved in invasion, proliferation, metabolism, and drug resistance [9-17]. With less oxygen available for energy acquisition through oxidative phosphorylation, these hypoxic cancer cells revert to aerobic metabolism for the production of ATP (the Warburg effect) [18]. This increased incidence of glycolysis is exploited in clinical tumor imaging with the use of 18F-fluorodeoxyglucose positron emission tomography (FDG PET), which has been used since the 1970’s. FDG PET monitors the first step of glycolysis, the phosphorylation of 2-DG to 2-DG-6-phosphate. The enzymes of the glycolytic pathway are also HIF targets with HRE’s.
The key characteristics of the tumor microenvironment are listed in Table 1. The most pivotal characteristics that are common to all solid tumors are (1) abnormal tumor vasculature (leaky, unorganized) and (2) the occurrence of hypoxic regions (transient and/or chronic). Other hallmarks of the tumor microenvironment include up-regulation of oncogenes and DNA repair mechanisms and down-regulation of tumor suppressors and cell cycle regulation. Growth factor receptors and nutrient importers may also be increased to make the cells hyper-sensitive to growth stimulation. The tumor microenvironment is defined by the interplay of these traits.
Table 1.
Characteristics of the Tumor Microenvironment and the Selection Pressures that Contribute to MDR
| Increased | Decreased |
|---|---|
|
|
The response to selection pressures determine if the cancer cell will undergo apoptosis, undergo necrosis, become quiescent, propagate, or develop MDR. Cell responses that result in a combination of the traits listed in Table 1 often result in the development of MDR. For example, MDR cells often have increased DNA repair mechanisms, up-regulation of ABC transporters, and a decreased apoptotic response (increased threshold for cell death) [19]. Alternatively, hypoxia alone is often enough to induce MDR through the transcriptional activity of HIF, which activates MDR proteins and mechanisms [4, 9, 14, 17, 20-24]. The most characterized mechanism of MDR is up-regulation of ATP-Binding Cassette (ABC) transporters and the most studied ABC transporter is P-glycoprotein (P-gp, MDR1, ABCB1) [25, 26]. P-gp effluxes a wide range of drugs and efflux requires two molecules of ATP [19]. MDR is often initiated with P-gp overexpression but other ABC transporteres such as multi-drug resistance protein 1 (MRP-1, ABCC1) and breast cancer resistance protein (BCRP, ABCG2) also contribute to the development of MDR [6, 25, 27-29]. Most commonly, it is a dynamic and transient combination of the characteristics listed in Table 1 that result in MDR.
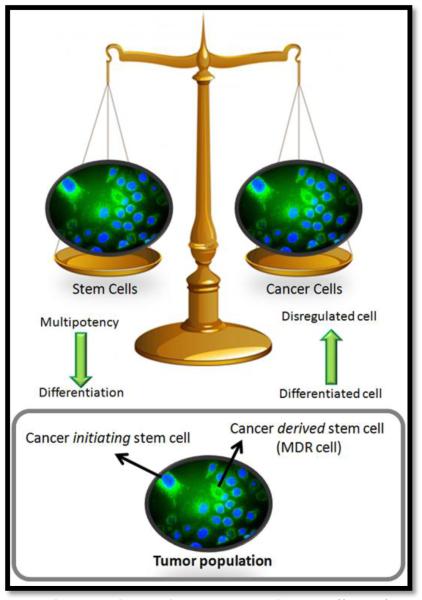
Although the concept of cancer stem cells has been explored since the late 1930’s, these concepts have solidified and attracted much attention in recent years. There seem to be two main schools of thought concerning cancer stem cells, (1) cancer stem cells are regular stem cells that have gone awry and cause cancer and (2) cancer stem cells arise from a subpopulation of cancer cells. It is most likely that both of these concepts are correct and both contribute to the microenvironment of a tumor. It is likely that some cancer is caused from the disregulation of stem cells and arises from cancer initiating stem cells. It is also likely that a subpopulation of cancer cells develops stem-like properties; and it is likely that this subpopulation of cancer stem cells is indeed MDR cells. Although there is still much debate over the nomenclature of cancer stem cell theories, it is well accepted that these cells represent a challenge for effective treatment of the disease. Figure 1 demonstrates the relationship between stem cells and cancer cells, which are on opposite ends of the biological scale in the sense that stem cells begin as multipotent cells that become differentiated whereas cancer cells are differentiated cells that lose their defining characteristics. Figure 1 also demonstrates that the two concepts of cancer stem cells co-exist; there are cancer initiating stem cells that originate as stem cells, but transform into cancer causing cells and secondly, there are cancer derived stem cells which are cancer cells that develop stem-like properties, these cells are better known as MDR cells [30]. In line with the concept that MDR cells can develop stem-like properties and be identified as cancer stem cells, different studies have shown that cell stressors such as hypoxia, which are efficient in inducing cancer aggression and MDR phenotypes, also induce stem-like properties in cancer cells such as the expression of stem cell factor (SCF) [9-17, 31]. Although cancer stem cells may be a challenge for therapy, as our understanding of these cells develops, new molecular targets will undoubtedly unfold. As such, inhibiting stem cell factor in MDR cells may increase the effectiveness of treatment by reducing the apoptotic threshold of these cells.
Figure 1. Cancer Stem Cell Theory:
Stem cells and cancer cells are on opposite ends of the biological scale; stem cells begin as multipotent cells that become differentiated whereas cancer cells originate as differentiated cells that become disregulated and lose their defining characteristics. It is most likely that two distinct types of cancer stem cells exist; cancer initiating stem cells that originate as stem cells, but transform into cancer causing cells and a distinct subpopulation of cancer derived stem cells which are cancer cells that develop stem-like properties, this subpopulation of cells are also known as MDR cells.
Although all of the characteristics listed in Table 1 synergistically define the microenvironment of a tumor, it is important to note that tumors consist of heterogeneous cells that can have very different traits. It is a likely scenario that a subpopulation of a tumor mass may have increased expression of certain proteins such as epidermal growth factor receptor (EGFR) and P-gp while neighbouring cells may be EGFR negative with minimal P-gp. These dynamic expression profiles of tumors require equally dynamic treatments that prove effective for different cancer phenotypes.
Drug delivery challenges
There are drug delivery challenges and biological barriers at every level of biological organization; at the level of the entire organism/body level, at the level of tissue/organs, at the cellular level, and at the sub-cellular or molecular level. The simple objective of drug delivery is to localize a therapeutic agent at its site of action for maximal effect (be it at the tissue, cellular, or molecular level) without resulting in a toxic distribution of the agent at non-target sites. Although this is a simple intent, achieving this result can be an expensive and time consuming process, without guaranteed success. This is evident from the large budgets and extended timelines that are required to bring a drug candidate into the market. In designing a drug delivery system, the appropriate and most effective route of administration must be selected, the pharmacokinetic properties of the system must be optimized to increase drug bioavailability and decrease residual toxicity, and the system must be replicable using Good Manufacturing Practices guidelines for regulatory approval.
The common challenge of drug delivery is to achieve a good therapeutic index, which is the ratio of the lethal dose for 50% of the population to the minimum effective dose for 50% of the population (LD50/ED50). Nanocarriers are capable of increasing the specificity of drugs for target cells, and thus are able to improve the therapeutic index of a drug. MDR cancer presents a unique set of delivery challenges. The most significant challenge in treating MDR is actually reaching these cells. MDR cells are often hypoxic cells that are either distant or transiently cut off from a local blood supply; acquiring oxygen and nutrients as well as therapeutic agents is a challenge. Nanocarriers can reduce this burden by exploiting the EPR effect. Nanocarriers also overcome the barrier of drug efflux pump extrusion by localizing drugs away from these cell-surface transporters. Nanocarriers offer a platform for drug delivery that can be optimized to overcome biological barriers and customized to achieve diverse treatment strategies that address the complexities of MDR cancer.
Multifunctional Nanoparticles
Rationale for using nanoparticle formulations in cancer therapy
The advantages of nanotechnology based therapeutics for cancer treatment are being explored in a plethora research projects and clinical trials. There are two main appeals for nanotechnology based cancer therapy (1) using nanocarriers can dramatically improve the therapeutic index of an agent and (2) nanocarriers can increase the capability of a therapeutic strategy by accomplishing two or more objectives (multifunctional). By increasing the therapeutic index of an agent, nanocarriers can reduce the toxicity associated with a drug, increase the bioavailability, and convert an agent with a low therapeutic potential into a drug candidate. By engineering multifunctional nanocarriers, or smart particles, cancer therapy becomes more effective as treatments achieve more than one task.
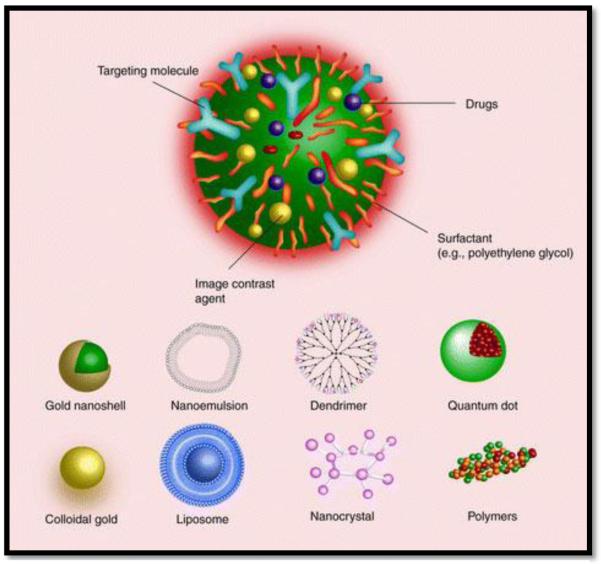
As demonstrated in Figure 2, a variety of organic and inorganic platforms are used to engineer nanoparticles. Common nanoparticle platforms include liposomes, micelles, nanoemulsions, polymers, quantum dots, gold, iron-oxide, and dendrimers. The nanoscale behaviour of the platform material is very different from the properties of the bulk platform material due to the large surface area to volume ratio that characterizes nanoparticles [32-35]. This large surface area to volume ratio allows the particles to be held in suspension, enables high drug encapsulation, and extensive surface absorption [32-35]. The primary intent of using multifunctional nanoparticles is to accomplish more than one objective using one therapeutic system. Nanocarrier platforms are versatile and customizable; they can be tuned to achieve a multitude of specifications and parameters including optimized pharmacokinetic properties. For nanocarrier systems that rely on drug encapsulation (not surface absorption), the platform material determines the pharmacokinetic profile of the system [36, 37]. These nanoparticle platforms can be engineered to have a tailored particle size, tailored shape, extensive surface modification, multiple therapeutic payloads, sustained drug release, and stimuli-triggered drug release. Most often, multifunctional nanoparticles are engineered to achieve two or more of the following objectives; drug delivery, RNAi/DNA delivery, active targeting, decreased clearance, imaging/tracking, and stimuli-responsive capabilities. Multiple diagnostic (optical, radioisotopic or magnetic) modalities can be incorporated in multifunctional nanocarriers to visualize details beyond the detection limit of conventional methods.
Figure 2. Multifunctional Nanoparticles:
The distinguishing benefit of nanocarriers is that they can be engineered to achieve more than one task such as drug delivery, gene/RNAi delivery, targeting, imaging, and reporting. It is easy to functionalize the surface of nanocarriers and to encapsulate multiple agents. Various platforms can be used as nanocarriers. Common nanocarrier platforms are illustrated [125]. *Reproduced from Nanomedicine (2007) 2(6), 789-803 with permission of Future Medicine Ltd.
Unlike most therapeutic agents, the surface of nanocarriers are easy to modify; as such, it is popular to incorporate poly(ethylene glycol) (PEG) and PEG-like molecules on the surface of nanocarriers to decrease clearance by the reticuloendothelial (RES) system [38, 39]. This increases the bioavailability of the nanocarrier system and improves the pharmacokinetics. Accumulation of nanocarriers at the site of a tumor is actually enhanced relative to normal tissue due to the enhanced permeability and retention (EPR) effect [40]. The EPR effect results in a higher accumulation of nanocarriers at the site of a tumor due to the leaky vasculature that allows passage of nanocarriers into the tumor matrix. Once in the tumor matrix, the nanocarriers are retained for a longer duration as tumors have poor lympathic drainage relative to normal tissue. This is a form of passive tumor targeting. Active targeting is also being extensively explored in experimental and clinical research. The objective of active targeting is to selectively increase the accumulation of a nanocarrier system at the site of a tumor by engaging a biological target that is over-expressed in cancer cells. Most often, the surface of nanocarriers are modified with a ligand or antibody for receptor targeting, antigen targeting, or carbohydrate targeting [38, 39]. Some multifunctional nanoparticles actually employ two different distinct targeting mechanisms to increase tumor specificity.
A key benefit of using nanocarriers for the treatment of MDR is that nanoparticles have been demonstrated to bypass efflux by ABC transporters as they are internalized via non-specific endocytosis (or facilitated uptake for targeted nanocarriers) and result in higher intracellular accumulation [41]. Free drugs are often internalized by diffusion across the cell membrane, making them spatially vulnerable to P-gp capture and efflux; on the other hand, nanocarriers are taken up by non-specific endocytosis, forming endosomes that internalize and release drugs near the peri-nuclear region, away from membrane bound P-glycoproteins [42, 43].
As our knowledge of cancer progresses, the high degree and relevance of tumor heterogeneity becomes more evident. Most of the current treatment strategies, as well as diagnostic techniques and prognostic evaluations do not account for the occurrence and significance of this heterogeneity (both intra- and inter-patient heterogeneity). Collectively, the capabilities of nanocarriers are far beyond what is achievable with traditional medicine. Carefully combining multifunctional features to address heterogeneous disease, side effects can be minimized while enhancing drug efficacy to provide an effective treatment for drug resistance disease.
Illustrative examples of multifunctional nano-delivery systems
Currently used anti-cancer agents do not greatly differentiate between cancer cells and normal cells and thus result in systemic toxicity and adverse effects. An appealing strategy for treating MDR is to co-administer a chemotherapeutic agent and a P-gp inhibitor. A representative nanocarrier system that utilizes this strategy employs doxorubicin as the chemotherapeutic agent, verapamil as the P-gp inhibitor, and liposomes as the delivery platform [44]. The specificity of this system for cancer cells was increased by attaching transferrin to the surface of the liposomes (for transferrin receptor targeting of cancer) [44]. This system was evaluated in a MDR leukemia cell line and was shown to significantly increase the cytotoxicity of doxorubicin; the IC50 of free doxorubicin was 23.4 μM which dropped to 4.18 μM for the transferrin-targeted doxorubicin and verapamil loaded liposomes [44]. Due to their enhanced binding, uptake and release properties, lipid based cationic nanoparticles are a promising option for tumor therapy [45]. A combination of cationic and neutral lipids forms the core to maintain charge and control particle size. In this context, Tekmira and a number of other companies have developed a series of cationic, lipid nanoparticles (LNPs) for tumor therapy [45]. Cationic lipids are carefully selected (pKa and redox properties), so that the particles survive the lower pH of the tumor microenvironment and successfully accumulate in tumor cells.
The active targeting approach is based on specific interactions such as that between ligands and receptors and between antibodies and antigens. Certain receptors and antigens are over expressed in many human cancer cells and can be exploited by active targeting strategies to achieve efficient drug uptake via receptor mediated endocytosis [46]. Active targeting also provides an alternative route for overcoming multi drug resistance. Folate, EGFR-2 (or HER2), and transferrin are some of the commonly used ligands. Folate receptors present in healthy cells are shown to be largely inaccessible to circulating ligands due to their location on apical membranes but they are over-expressed on the surface of many cancers such as ovary, brain, kidney, breast, and lung malignancies [47, 48]. Folate modified polymeric nanoparticles have been shown to have enhanced localization and internalization in target breast cancer cells [49]. Folate conjugated PEG-modified nanoparticles have also been demonstrated to have a ten-fold higher affinity for folate receptors compared to free folate affinity for the receptor [50]. Many of the folate targeted cancer therapies and diagnostic strategies under development have a strong clinical potential [46].
Similarly, attaching anti-HER2 to nanoparticle surfaces also improved the cellular internalization of gelatin/albumin and gold nanoparticles [51]. Transferrin, an iron binding glycoprotein, is a well studied ligand for tumor targeting due to the up regulation of these receptors in numerous types of cancer. One such system is a cyclodextrin polymer delivery system with a transferrin targeting moiety [52]. One of the components of this system is a cyclodextrin containing polycation for nucleic acid condensation; the other component is a transferrin linked PEG adamantane to stabilize the particle and to target the cell surface receptors [52]. This system has demonstrated efficient delivery in pre-clinical models [52].
In addition to being used as delivery vectors for therapeutic drugs, nanocarriers are also being loaded with imaging contrast agents and used for diagnostics [51]. The inherent properties of iron oxide, gold, gadolinium, and quantum dots make them ideal platforms for nanocarrier based hyperthermia, radiation, and photodynamic therapy [51]. Gold is the most common metallic nanocarrier platform. Iron oxide is a useful platform for nanocarrier therapies that utilize MRI as iron oxide functions as a contrast agent. Although MRI is a very useful technique for the detection of solid tumors, it is insensitive to small-scale cancer imaging such as detecting lymph node metastasis and evaluating the therapeutic efficacy of cancer treatment. Iron nanoparticles improve the sensitivity of MRI; using iron nanoparticles as MRI contrast agents over 90% of lymph node metastasis are detectable as opposed to 35% detection by conventional MRI in prostate cancer patients [53]. Combining this detection system with drug delivery could improve the treatment of metastatic prostate cancer.
Nanosystems to Enhance Drug Delivery Efficiency
Solubilization of hydrophobic drugs and protection of labile payloads
There are many cytotoxic compounds available for the reversal of MDR in cancer cells including P-gp inhibitors such as verapamil, cyclosporin A, quinidine, and tamoxifen that directly associate with P-gp to block drug efflux [43]. However these drugs are very hydrophobic and poorly soluble which results in low bioavailability. For a therapeutically relevant dose of these drugs to reach the site of a tumor, a high systemic dose must be administered which is accompanied by high systemic toxicity. Therefore, residual toxicity limits the application of these drugs. Using nanocarriers to deliver P-gp inhibitors can reduce the off-target effects and increase the therapeutic index of these agents. Poly(epsilon caprolactone) (PCL) nanoparticles surface-modified with poly(ethylene glycol) (PEO) have proven to be efficient delivery systems for hydrophobic drugs [43, 54-57]. A combination therapy of paclitaxel and tamoxifen loaded in PEO-PCL nanoparticles resulted in a higher accumulation of paclitaxel in the tumor and lowered the therapeutic dose of paclitaxel required to treat an established MDR cancer cell line, SKOV3TR cells [54].
Doxorubicin, which is used for the treatment of various types of cancer, is very hydrophobic and as a result has high plasma protein binding. A dose-limiting side effect of doxorubicin is cardio-toxicity. As is the case with doxorubicin, nanocarriers can improve the efficacy of hydrophobic drugs. Doxorubicin in long circulating liposomes (Doxil®) is an FDA approved preparation that was first approved for the treatment of AIDS-related Kaposi’s sarcoma in 1995 and has subsequently been approved for other oncology indications. [58]. Doxorubicin is encapsulated within the hydrophilic core of the liposome. This liposomal system aids the selective uptake of the drug into the tumor tissue and reduces non-target uptake, thus reducing the side effects of doxorubicin. In MDR cells, doxorubicin encapsulated in liposomes, have resulted in a 2-fold increase in the levels of intracellular doxorubicin compared to free doxorubicin accumulation [59].
In addition to improving the delivery of hydrophobic drugs, nanocarriers are also useful for protecting labile therapeutics such as DNA and RNAi. These agents have poor stability in systemic circulation and are prone to extracellular and intracellular degradation; it is the protection and adequate delivery of gene therapy and RNAi that limits the clinical application of these agents. Various nanocarrier platforms such as polymeric nanoparticles, cationic liposomes, and lipoplexes, have been shown to increase the stability of labile therapeutics. PEG- modified type B gelatin and thiolated gelatin nanoparticles have also been shown to increase the stability of therapeutic payloads. Remarkable, the stability of plasmid DNA encapsulated in gelatin nanoparticles can be maintained even in the presence of DNAse1 [60]. GFP plasmids are common reporter genes used to assess the transfection efficiency of different gene therapy strategies. Gelatin nanoparticles used to deliver a GFP plasmid have demonstrated transfection within 6 hrs of incubation, and the GFP plasmid expression was sustained until 96 hrs after transfection, indicating enhanced stability of the encapsulated plasmid compared to the naked plasmid [60]. PEO-PCL nanoparticles have also been used to simultaneously deliver paclitaxel and MDR-1 (P-gp) siRNA [61]. As P-gp (MDR-1) is responsible for the resistance phenotype of many MDR cells and is known to actively efflux paclitaxel, this strategy aimed to block paclitaxel efflux by silencing P-gp while increasing paclitaxel intracellular delivery using the nanocarrier system [61]. Nanoparticle delivery increased the intracellular concentration of paclitaxel relative to free drug administration. When administered to SKOV3TR cells (established MDR ovarian cancer cells), the concentration of paclitaxel was 8.2 nM/mg of total cellular protein for cells treated with paclitaxel loaded PEO-PCL nanoparticles (no siRNA), this concentration increased to 11.5 nM/mg of total protein for cells treated with PEO-PCL nanoparticles loaded with both paclitaxel and P-gp siRNA [61]. This enhanced accumulation of paclitaxel due to P-gp down-regulation was confirmed with RT-PCR [61]. This strategy of combination drug delivery and P-gp silencing could be applied to other P-gp substrates to enhance efficacy.
Enhancing tumor accumulation - passive and active targeting
Drugs loaded in nanoparticles selectively accumulate in tumor masses through an exploitation of tumor physiology. As previously mentioned, solid tumors have leaky vasculature with cell junction gaps ranging between 100 nm to 780 nm [62]. This leaky vasculature coupled with poor lymphatic drainage result in the EPR effect, which is a means of passive tumor targeting. To exploit the EPR effect the particles must be able to escape systemic recognition by the immune system; PEG modification of nanocarriers increases the circulation time of the nanocarrier by preventing opsonisation and clearance by the reticulo endothelial (RES) system [63]. The shielding effect of the PEG chains is due to the formation of a tightly bound network of water. This hydrophilic surface prevents recognition and interaction required for the uptake and clearance of the nanoparticles by macrophages. This increases the circulation time of the nanoparticles and results in an increase in tumor accumulation by 10 to 100 fold as compared to non-PEG-modified nanoparticles [58, 62]. PEG modification also prevents particle aggregation. Studies have demonstrated that PEG modification increases the residence half life of gelatin nanoparticles from 19 hours (no PEG) to 121 hours (with PEG) when administered systemically to C57/BL6J mice with Lewis Lung Carcinomas [58, 62, 63]. The increased half life of the PEG-modified gelatin nanoparticles was also accompanied by a 3-fold increase in reporter gene expression [58, 62, 63].
Active targeting is achieved through surface modification of the nanocarriers with targeting moieties, in addition to PEG modification. Tumor targeting can be achieved by various mechanisms; Table 2 illustrates some of the targeting strategies to overcome MDR. The anti-P-gp antibodies, MRK2 and MH162, directly bind and inhibit P-gp’s that are over-expressed on the surface of MDR cells [64]. The anti-Cripto antibody and anti-CD19 antibody, block Cripto and CD19 respectively, which are important proteins required for the complete functionalization of P-gp [12, 13, 14]. Binding of these antibodies sensitizes P-gp to inhibitors such as tamoxifen and cyclosporine A [12, 13, 14]. Certain targets are over-expressed by tumor blood vessels, such as the integrin receptor αvβ3, which binds tri-peptide ligands including arginine-glycine-aspartic acid (RGD) peptides [65]. HER2 is over expressed by many breast cancer cells; surface modification by the HER2 specific antibody, Herceptin®, results in rapid uptake of the drug conjugate by these breast cancer cells [62]. Other growth factor receptors and folate receptors are also common targets for nanocarrier therapeutics.
Table 2.
MDR Targeting Strategies
| Targeting Moiety | Mode of Action | Reference |
|---|---|---|
| MRK2 anti- Pgp monoclonal antibody and its F(ab)2 |
Direct binding and inhibition of P-gp |
[66] |
| Anti-CD-19 antibody, HD37 | Blocking of CD-19 causes e P-gp to migrate out of lipid rafts |
[67, 68] |
| Anti- Cripto antibody | Cripto is co-expressed with MDR-1/Pgp. Results in inhibition of Akt and activation of apoptosis |
[69] |
| Low density Lipoproteins (LDL) |
Targets lipid rafts where P- gp resides |
[70, 71] |
| MH162 chimeric anti-P-gp antibody |
Direct binding and inhibition of Pgp |
[64] |
| Anti-FAS antibody | Targets FAS-antigens expressed on the surface of MDR cells |
[72] |
| Arg-Gly-Asp (RGD) peptide | Has a strong affinity for αvβ3 integrin that is over- expressed in the vasculature of aggressive tumors |
[58] |
Prolonged tumor residence - alterations in drug pharmacokinetic profile
Since the pharmacokinetic profile of nanocarrier-encapsulated drugs is dictated by the properties of the nanoparticle systems, this approach provides a convenient strategy for altering the drug biodistribution and enhances delivery to target site. Drugs encapsulated in nanocarriers do not contribute to the pharmacokinetic profile; it is the nanomaterial and the physical and chemical properties of the nanocarriers that determine the pharmacokinetics. It is easy to customize these properties to achieve a desired pharmacokinetic profile for a carrier system; for example the size, charge, density, and surface modification of a nanocarrier system can be manipulated. Naked sFlt-1 plasmid has been shown to have a higher transfection efficiency in the liver compared to tumor mass; using a nanocarrier system to deliver the sFlt-1 plasmid results in significantly higher plasmid accumulation and expression in the tumor mass [61].
Although the EPR effect helps to retain nanoparticles in the tumor environment once they have reached the tumor, nanocarriers must remain in circulation long enough to reach the site of a tumor. As previously mentioned, the easiest and most common technique that is used to alter the pharmacokinetics of a nanoparticle formulation is PEG modification. Surface modification with PEG helps evade opsonisation and clearance by RES organs such as the kidney, liver, spleen, and lymph nodes. PEG surface modification has been shown to increase tumor drug accumulation by as much as 100-fold [1]. PEO-modified PCL nanoparticles loaded with paclitaxel have been shown to have prolonged circulation with paclitaxel drug clearance 8 times lower than the clearance of free paclitaxel [57, 61].
As with conventional delivery systems, the pharmacokinetic profile of PEG-modified nanocarrier systems is greatly affected by the route of administration. For example, the bioavailability of superoxide dismutase conjugated to PEG decreases to 71% when administered as an intraperitoneal injection, 54% for an intramuscular injection, and 29% for a subcutaneous injection (relative to 100% bioavailability for an intravenous injection) [73]. PEG-modified superoxide dismutase had a 100-fold higher bioavailability compared to non-PEG-modified superoxide dismutase [73].
Another consideration is selecting the appropriate molecular weight PEG for a given nanocarrier system. Interestingly, studies have shown that as the molecular weight of PEG increases, the local residence time of the system at the site of administration also increases [74, 75]. PEG modification can also be used to mask the charge of a carrier system. Cationic carrier systems such as cationic liposomes are removed by the kidneys faster than anionic and neutral systems. PEG modification can help mask the charge of cationic systems, reducing uptake by the kidneys.
PEG modification increases the circulation time of a drug without increasing the administered dose. The systemic half-life of a nanoparticle system increases as the molecular mass of the PEG chains increase. It has been calculated that the half-life increases by about 972 minutes when the molecular mass of the PEG chains increases from 6 to 50 kDa [75]. The volume of distribution of PEG-modified TNF-α dropped down to 8-12, compared to 31-73 for TNF-α alone and similarly the half-life of the TNF-α was found to increase from 2.3 hours to 50 hours when it was conjugated with PEG [76]. By developing nanocarrier systems for drug delivery it is possible to modify the pharmacokinetic profile of a drug. The pharmacokinetic profile of the nanocarrier can be further improved through PEG modification and by customizing the physical and chemical properties of the system.
Increasing intracellular availability and subcellular localization
As important it is for therapeutics to reach the site of a tumor, it is equally important for therapeutics to reach their intracellular site of action. For this to occur, the therapeutics must escape the endosomal pathway and subsequent lysosomal degradation. Many strategies have evolved to ensure endosomal escape; a popular strategy is to modify particles with CPP’s (cell penetrating peptides) which enable cell entry while evading lysosomal degradation [58, 71]. One such peptide that has been isolated to maximize cell entry is the transactivating transcriptional activator (TAT) peptide derived from HIV-1 [58, 71]. Surface coating of nanocarriers with TAT peptides will allow these particles to escape the lysosomal pathway; this is particularly important for MDR cells, since lysosomal degradation will result in release of the drug into the cytoplasm proximal to the cellular membrane, making the drug available for P-gp efflux [2,24]. Another method for endosomal escape is to use pH sensitive nanocarriers such as PEG-modified dioleoyl phosphatidylethanolamine (DOPE) pH sensitive liposomes [71]. The intra-tumor pH destabilizes the liposomes, causing them to fuse with the endosomal membrane and subsequently release the cargo in the cytoplasm. Another mechanism is endosomal burst, which relies on disruption of the endosomal membrane for release of therapeutics into the cytoplasm. Immuno-liposomes are also used to localize nanocarriers to specific sites.
These examples illustrate an important consideration; when designing a nanocarrier system it is important to consider the desired mechanism and kinetics of particle uptake. Even without an active targeting ligand, PEG-modified particles are internalized via non-specific endocytosis with appreciable uptake after one hour and eventual saturation. Nanocarriers targeted to a particular receptor will be internalized via the characteristic mechanism for the receptor; for example, nanoparticles targeting the EGFR receptor are rapidly internalized via a flip-flop mechanism within fifteen minutes of receptor-ligand engagement. Rapid saturation through EGFR binding is less likely as receptors are quickly recycled to the cell membrane. The same principles of cell-surface and extra-cellular targeting apply to intra-cellular targeting. As such, surface modification of nanocarriers can also be tailored to achieve sub-cellular localization. For example, mitochondrial targeting is often achieved using mitochondrial leader sequences or by exploiting the negative membrane potential of mitochondria through the use of “mitochondriotropics”, molecules that have delocalized positive charges such as triphenylphosphonium.
RNA Interference Therapy to Overcome MDR
Silencing strategies
RNA interference is an endogenous, well conserved mechanism that uses small non-coding RNA’s to silence gene expression. Two types of small RNA molecules, small interfering RNA (siRNA) and microRNA (miRNA), are central to RNA interference. When exogenous small double stranded siRNA is introduced into cells, it binds to the endogenous RNAi machinery to disrupt the expression of mRNA’s containing sequences with high specificity [77]. This pathway is initiated by the enzyme Dicer, which cleaves long double stranded RNA (dsRNA) into short fragments of ~20 nucleotides. One of the strands called the guide strand, is then incorporated into the RNA-induced silencing complex (RISC). The anti-sense RNA strand then guides RISC to homologous sequences on target mRNA and base-pairs with a complementary sequence of messenger RNA, inducing cleavage by Argonaute, the catalytic component of the RISC complex [77]. The cleaved target mRNA is no longer capable of producing functional protein and therefore the mRNA is “silenced”.
Endogenous microRNAs are originally processed from hairpin precursors and are known to regulate proteins involved in cell death, differentiation and development [78]. Although mature miRNAs are structurally similar to siRNAs, before reaching maturity, the miRNAs first undergo extensive post transcriptional modifications. Once they are mature, they can then be integrated into RISC and follow the same machinery as siRNA, leading to silencing [79]. This robust silencing effect of RNAi makes it a valuable research tool both in cell culture and in living organisms, although introducing siRNA into cells in vivo remains a significant obstacle. Silencing of P-gp and down regulation of multidrug resistance proteins could reverse the multidrug resistance phenotype, re-sensitizing MDR cells to cytotoxic agents.
Illustrative examples of RNAi in overcoming tumor MDR
Most often, in clinical scenarios of MDR tumors consist of a mixed population of malignant cells, some of which are drug-sensitive while others are drug-resistant. As P-gp and related ABC-transporters play a pivotal role in MDR, disruption of P-gp mediated drug extrusion is an attractive therapeutic approach; yet the use of small molecule drugs that inhibit P-gp has not resulted in much clinical success [80]. As such, therapeutic strategies using RNA interference technology to overcome multidrug resistance are actively being explored [80-82]. There are two main strategies for using RNAi to overcome P-gp associated MDR; transient RNAi mediated silencing can be attained through the application of siRNA, or stable RNAi-mediated gene silencing can be achieved through transfection with short hairpin RNA (shRNA) [80-82].
The first studies that demonstrated that the activation of the RNAi pathway could reverse the MDR phenotype of human cancer cells by knocking down the MDR1/P-gp encoding mRNA were reported in 2003 [80, 83]. In two simultaneous publications, chemically synthesized siRNA was used to transiently down regulate MDR1/P-gp mRNA and protein expression as well as MDR modulation [80, 83]. Following these pioneering investigations, similar studies of RNAi-dependent down-regulation of MDR1/P-gp and alternative ABC-transporters involved in MDR were conducted, demonstrating a transient MDR reversal of nearly 90% [80]. To further increase the efficacy of silencing, several investigators have designed stable anti-ABC transporter shRNA expression vectors to overcome cancer MDR [82]. Plasmid vectors used for shRNA expression impressively demonstrated the high potential of RNAi for treating MDR through silencing of ABC-transporter-specific transcripts [82]. Adenoviruses expressing well-characterized shRNA were also constructed and resulted in complete knockdown of P-gp encoding mRNA in multidrug resistant cell lines [80]. In these examples, the total knockdown of mRNA and protein was accompanied by a complete inhibition of the drug efflux activity of P-gp and reversal of the drug-resistant phenotype. Following these in vitro investigations, investigators initiated several in vivo studies for overcoming MDR with RNAi platforms. In initial studies, MDR cancer cells were stably transfected with anti-MDR shRNA expression plasmids, then these cells were grown as xenografts in nude mice [84]. Treatment with vincristine inhibited tumor growth of the shRNA expressing tumors by 42%; this study demonstrated proof-of- principle that RNAi can modulate MDR in an in vivo model [82]. Following this, a number of different studies have shown similar out comes [82]. Cells transfected with anti-MDR1/P-gp shRNA expressing retroviruses that were implanted into nude mice, produced 80-fold less tumor growth compared to the controls [82]. Although a number of studies have demonstrated RNAi modulation of cancer MDR in vivo, the drawback is the lack of an efficient delivery strategy for administering shRNA to cancer patients. Various strategies have been explored but with no great success. In one such study, a model of nude mice tumor xenografts was established and treated with anti-MDR1/P-gp siRNA with a hyperdermic syringe [82]. A non-viral jet-injection technology was also used to deliver shRNA [85]. This injection system uses high velocity jets of naked plasmid DNA that pass through the skin and penetrate deep into the underlying tissues. By injecting anti-P-gp shRNA expression vectors into mice with xenografts of human MDR cancer, P-gp encoding mRNA was reduced by more than 90% [85]. After 2 jet injections of vectors into tumors combined with two intravenous administration of doxorubicin, complete reversal of drug resistant phenotype was demonstrated [85]. Although it was very effective in vivo, a more simple method of delivery would be necessary in the clinical setting. These studies illustrate that the clinical application of RNAi requires a more efficient mode of delivery. Nanocarriers are a promising platform for the efficient delivery of RNAi.
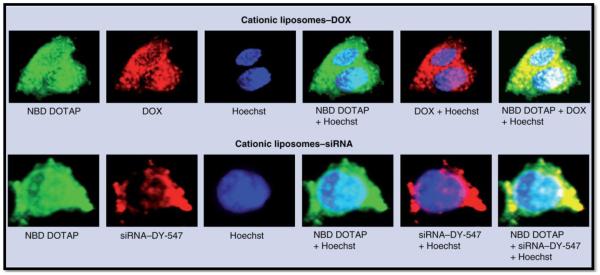
An intricate nanocarrier system designed to treat MDR small-cell lung carcinoma is a promising platform for the combination of small molecule drugs and RNAi therapy [86]. This system uses cationic liposomes to encapsulate and deliver doxorubicin, while siRNA targeted to MRP1 and BCL2 mRNA are complexed with the liposomal surface [86]. The therapeutic strategy is to increase the efficacy of doxorubicin by silencing MRP1 expression, preventing drug efflux through this ABC-transporter while also silencing anti-apoptotic BCL2 – and to achieve this using one nanocarrier system [86]. As demonstrated in Figure 3, this nanocarrier system was capable of localizing doxorubicin and siRNA in MDR cells. MDR human H69AR lung cancer cells were incubated with the liposomes; the top panel demonstrates doxorubicin localization while the bottom panel illustrates siRNA localization. The first image in each panel represents the localization of fluorescently (NBD) labeled DOTAP (labeled liposomes); the second image represents the distribution of doxorubicin (inherent fluorescence) and labeled siRNA, respectively; the third image in each panel demonstates nuclear staining; the forth image in each panel is an overlay of the liposomal distribution and nuclear staining; the fifth image in each panel is an overlay of nuclear staining and doxorubicin or labeled siRNA, respectively; and finally, the sixth image is an overlay of the liposome distribution, the nuclear staining and the doxorubicin or siRNA distribution [86]. The yellow regions in the last images represent co-localization of doxorubicin and siRNA with cytoplasmic liposomes while the white regions represent co-localization of doxorubicin and siRNA with the nucleus [86]. Not only was this system effective in delivering both doxorubicin and siRNA, this system dramatically improved the cytotoxicity of doxorubicin in MDR cells [86]. The IC50 for the combination nanocarrier therapy was approximately 80% lower than the IC50 of doxorubicin alone [86].
Figure 3. Nanocarrier Delivery of Doxorubicin, MRP1 and Bcl-2 Gene Silencing siRNA in MDR Cells:
MDR H69AR human lung cancer cells incubated with cationic liposomes containing doxorubicin (DOX) or siRNA. Liposomes were prepared using NBD DOTAP (green fluorescence), siRNA was labeled with DY-547 (red fluorescence), while DOX possesses an intrinsic red fluorescence, and cell nuclei were stained with Hoechst 33258 (blue fluorescence). The images were overlaid to determine regions of co-localization [79]. *Reproduced from Nanomedicine (2008) 3(6), 761-776 with permission of Future Medicine Ltd.
Modulation of Tumor Apoptotic Threshold
For a cell to undergo apoptosis it must overcome a minimum cellular threshold. In cancer cells this threshold is elevated to the extent that extra-cellular and intra-cellular insults which would be sufficient in inducing apoptosis in normal cells, have no effect. On the contrary, these insults become selection pressures to cancer cells, which adapt and transform with some adopting MDR character. One strategy for treating MDR cancer is to lower the apoptotic threshold required to initiate the process of cell death. MDR cells have developed various mechanisms for increasing their apoptotic threshold. Decreased ceramide levels and the Warburg effect are two representative mechanisms that MDR cells utilize to increase their apoptotic threshold.
Ceramide combination therapy
Ceramide is an endogenous constituent of the lipid bilayer that also functions as a second messenger in various signally pathways including the immune response and apoptosis [87, 88]. In response to cellular insults such as radiation and exposure to chemotherapeutic agents, ceramide activates the apoptotic pathway [88-90]. Ceramide is also involved in membrane clustering of the death receptor (CD95) [91]. In addition to its location in the cell membrane, ceramide also incorporates in the mitochondrial outer membrane, forming permeable channels that allow the release of pro-apoptotic factors such as cytochrome c [92-94]. MDR cells have adopted a mechanism to decrease intracellular ceramide levels, eliminating its signalling potential, and subsequently increasing the apoptotic threshold. This mechanism relies on the over-expression of glucosylceramide synthase, an enzyme that converts active ceramide into an inactive glcosylceramide [95-97].
Our lab has developed a nanocarrier system for the delivery of ceramide and paclitaxel combination therapy to treat MDR cancer. This system has been extensively evaluated in vitro and in vivo [98, 99]. The nanocarrier system consisted of two polymers; poly(beta-amino ester) (PbAE) which is a pH responsive polymer and poly(D,L-lactide-co-glycolide) (PLGA) [99]. Paclitaxel was associated with the PbAE to achieve rapid release upon exposure to the low pH of the tumor environment while ceramide was associated with the PLGA to achieve sustained release [99]. Combination treatment with ceramide and paclitaxel reduced the viability of MDR cells in vitro and this nanocarrier system was more effective at treating MDR tumor xenografts in nu/nu mice [98, 99]. Other strategies have been pursued to increase the intracellular level of ceramide. One such study demonstrated complete reversal of the MDR phenotype in human breast cancer cells after treatment with glucosylceramide synthase antisense cDNA [100]. Another strategy to increase intracellular ceramide is to use siRNA to silence glucosylceramide synthase; this approach actually decreased the expression of P-gp in MDR cells, validating the significance of ceramide in apoptotic modulation [101].
The Warburg effect and apoptotic modulation
A second mechanism that many MDR and drug-sensitive cancer cells adopt to increase the apoptotic threshold is the Warburg effect. The Warburg effect is an elevated level of glycolysis, even in the presence of oxygen (aerobic glycolysis) [102-106]. This phenomenon was first discovered by Otto Warburg in 1930, who noted at the time that even though some cancer cells revert to glycolysis for energy production, the majority of ATP is still acquired through oxidative phosphorylation [102-106]. The primary motive for elevated aerobic glycolysis is to provide a cancer cell with a survival advantage; less reliance on OXPHOS means that oxygen is not a rate limiting factor for ATP acquisition and less OXPHOS also means less ROS accumulation and damage, additionally, glycolysis is a more rapid process than OXPHOS [104, 107].
Glycolytic enzymes are actually targets of HIF transcription factors while some of these glycolytic enzymes also induce HIF activation; as such, the Warburg effect represents a positive feedback loop between hypoxia, cell stress, and glycolysis [108-111]. Hexokinase catalyzes the first step pf glycolysis, conversion of glucose and ATP to glucose-6-phosphate and ADP. The hexokinase 2 isoform is directly associated with mitochondria to enable immediate capture of ATP as it is released from the voltage dependent anion channel on the mitochondrial outer membrane. Hexokinase 2 association with the VDAC also has an anti-apoptotic effect as pro-apoptotic Bcl-2 family members are prevented from associating with the mitochondrial permeability transition pore complex [112-114].
Our lab has developed a strategy to treat MDR using a nanocarrier system that targets the Warburg effect in cancer cells. This system uses EGFR-targeted PCL-PLGA blend nanoparticles to simultaneously deliver paclitaxel and lonidamine. Lonidamine is a hexokinase 2 inhibitor that prevents association of hexokinase 2 with mitochondria, thus inhibiting aerobic glycolysis and enabling pro-apoptotic Bcl-2 proteins to bind to mitochondria [115-117]. Londiamine is actually an ideal drug candidate for nanoparticle delivery as its poor bioavailability limits its clinical use as a free drug. We have evaluated this nanocarrier combination therapy in vitro and are currently completing a pre-clinical evaluation of the system (unpublished work).
Physical Approaches to Overcome MDR
Combination drug and thermal therapies
Hyperthermia is the term used when any organ is heated to temperatures between 41°C to 46°C. Hyperthermia results in reversible cell damage, however when used as an adjunct treatment it can help increase the efficacy of chemotherapy and can enhance radiation induced tumor damage [118]. Hyperthermia has been used to change the morphology of a tumor to enhance the delivery of polymeric and liposomal nanoparticles by increasing the blood flow to the tumor [119]. Many groups have studied the use of super paramagnetic iron oxide particles to induce therapeutic hyperthermia; liposomes are often used to deliver the magnetic particles as this ensures higher intra-tumoral accumulation. Once the particles are administered, a magnetic field of 100-120 kHz is applied for 30 minutes to achieve temperatures between 40°C and 45°C. Using nanocarriers to simultaneously deliver magnetic particles and therapeutic agents could improve the efficacy and expand the application of hyperthermia combination therapy. Hyperthermia has been successfully combined with doxorubicin loaded liposomes that target the folate receptor [120]. This system was evaluated in MDR cervical carcinoma cells and hyperthermia (one hour of incubation at 42°C) was shown to decrease the IC50 of the drug loaded, targeted liposomes [120].
Temperature-sensitive nanocarriers are also being explored for cancer therapy. Temperature sensitive polymers are soluble in aqueous systems below a particular temperature and insoluble when the temperature of the system is raised. This temperature is termed the lower critical solution temperature (LCST). This polymer property can be exploited to synthesize self-assembling nanoparticles. A popular temperature-sensitive polymer is poly(N-isopropylacrylamide) (pNIPAAm) which has a LCST of 32°C; pNIPAAm is used as the hydrophilic segment of a diblock co-polymer micellar system along with a hydrophobic segment of cholesterol or cholic acid [119]. Variation to the structure of pNIPAAm can vary the LCST, for example the addition of an amine tail can help increase the LCST [58]. These temperature-sensitive systems allow control over nanocarrier solubility, as they can be designed to release drug payloads in the presence of specific temperature triggers.
Combination drug and ultrasound therapies
Ultrasound is another physical technique that is used to enhance drug delivery. Clinical improvements to ultrasound focusing are being developed to improve the control and precise targeting of ultrasonic waves [121]. Combining localized ultrasound with nanocarrier therapies could have a dramatic effect on reducing the residual toxicity associated with chemotherapy. A correlation between drug release and ultrasound frequency was demonstrated in a study using micelles loaded with doxorubicin [122]. This correlation was apparent in both wild-type and MDR ovarian carcinoma cells [122]. A similar study using Pluronic® P 105 micelles loaded with doxorubicin demonstrated that ultrasound combined with the nanocarriers increased the efficacy of the system in both MDR and drug sensitive cells [121]. Although these are in vitro studies, using ultrasound focusing to control drug release from nanocarriers is a promising strategy that may become a future treatment for patients with MDR disease.
Photodynamic therapy
Photodynamic therapy (PDT) is a form of cancer treatment that involves the use of photosensitizers as therapeutic agents. In the presence of light photosensitizers enter a triplet state of excitation. This triplet state of energy is readily transferred to oxygen molecules, which are subsequently converted into reactive oxygen species that are capable of causing cell damage [123-125]. This method of treatment has high selectivity, since only the cells which are exposed to both light and the photosensitizer are affected. Photofrin 2, which is a derivative of hematophorphyrin is the only PDT drug that is approved for clinical use in Canada, the Netherlands, and Japan, for the treatment of bladder, lung and esophageal cancer, respectively [123]. However the direct use of the photosensitizers is associated with many drawbacks such as poor tissue selectivity and porphyrin complex formation. Because of their poor extinction coefficient, photosensitizers have to be administered in large doses to achieve a therapeutic effect. These challenges have lead to the development of various nanocarriers for PDT [123]. Unilamellar dipalmytoylphosphotidyl-choline(DPPC) liposomes have been used to deliver hematoporphyrin and when evaluated in a MS-2 fibrosarcoma mouse model, the liposomal delivery of PDT resulted in a higher intra-tumoral accumulation of the photosensitizer relative to free drug administration [123]. Advances in PDT delivery systems have lead to the development of a “dynamic nanoplatform” (DNP) made from polyacrylamide nanoparticles [125]. This system originated as a delivery system for the photosensitizer, methylene blue, but adapted into a system for the direct delivery of oxygen to cancer cells [125]. Combining oxygen delivery with drug delivery could prove to be a very effective strategy for treating MDR cancer.
Conclusions
Cancer is a heterogeneous disease. As such, successful cancer treatment needs to address multiple phenotypes, including MDR phenotypes. Nanocarriers are ideal vectors for the treatment of MDR cancer as (1) nanocarriers can increase the therapeutic index of a drug, (2) nanocarriers can be engineered to achieve multifunctional parameters, (3) nanocarriers preferentially accumulate in the tumor due to the EPR effect, and (4) nanocarriers divert ABC-transporter mediated drug efflux which is a significant challenge in the treatment of MDR. It is undoubtedly only a matter of time until new multifunctional nanocarriers emerge from clinical trials and are approved for the treatment of MDR cancer.
Acknowledgements
Our work on multifunctional nanocarrier systems for overcoming tumor drug resistance has been supported by the National Cancer Institute’s Alliance for Nanotechnology in Cancer through the Platform Partnership grants (R01-CA119617 and U01-CA151452) and the ARRA Administrative Supplement (R01-CA119617S1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Harris AL, Hochhauser D. Mechanisms of multidrug resistance in cancer treatment. Acta oncologica (Stockholm, Sweden) 1992;31(2):205–213. doi: 10.3109/02841869209088904. [DOI] [PubMed] [Google Scholar]
- [2].Jamroziak K, Robak T. Pharmacogenomics of MDR1/ABCB1 gene: the influence on risk and clinical outcome of haematological malignancies. Hematology (Amsterdam, Netherlands) 2004;9(2):91–105. doi: 10.1080/10245330310001638974. [DOI] [PubMed] [Google Scholar]
- [3].Leighton JC, Jr., Goldstein LJ. P-glycoprotein in adult solid tumors. Expression and prognostic significance. Hematology/oncology clinics of North America. 1995;9(2):251–273. [PubMed] [Google Scholar]
- [4].Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. Journal of the National Cancer Institute. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- [5].Tsuruo T, Naito M, Tomida A, Fujita N, Mashima T, Sakamoto H, Haga N. Molecular targeting therapy of cancer: drug resistance, apoptosis and survival signal. Cancer science. 2003;94(1):15–21. doi: 10.1111/j.1349-7006.2003.tb01345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yague E, Arance A, Kubitza L, O’Hare M, Jat P, Ogilvie CM, Hart IR, Higgins CF, Raguz S. Ability to acquire drug resistance arises early during the tumorigenesis process. Cancer research. 2007;67(3):1130–1137. doi: 10.1158/0008-5472.CAN-06-2574. [DOI] [PubMed] [Google Scholar]
- [7].Nelson DA, Tan TT, Rabson AB, Anderson D, Degenhardt K, White E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes & development. 2004;18(17):2095–2107. doi: 10.1101/gad.1204904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Depping R, Steinhoff A, Schindler SG, Friedrich B, Fagerlund R, Metzen E, Hartmann E, Kohler M. Nuclear translocation of hypoxia-inducible factors (HIFs): involvement of the classical importin alpha/beta pathway. Biochimica et biophysica acta. 2008;1783(3):394–404. doi: 10.1016/j.bbamcr.2007.12.006. [DOI] [PubMed] [Google Scholar]
- [9].Harris AL. Hypoxia--a key regulatory factor in tumour growth. Nature reviews. 2002;2(1):38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- [10].Semenza GL. Targeting HIF-1 for cancer therapy. Nature reviews. 2003;3(10):721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- [11].Brahimi-Horn MC, Chiche J, Pouyssegur J. Hypoxia and cancer. Journal of molecular medicine (Berlin, Germany) 2007;85(12):1301–1307. doi: 10.1007/s00109-007-0281-3. [DOI] [PubMed] [Google Scholar]
- [12].Cosse JP, Michiels C. Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression. Anti-cancer agents in medicinal chemistry. 2008;8(7):790–797. doi: 10.2174/187152008785914798. [DOI] [PubMed] [Google Scholar]
- [13].Jewell UR, Kvietikova I, Scheid A, Bauer C, Wenger RH, Gassmann M. Induction of HIF-1alpha in response to hypoxia is instantaneous. Faseb J. 2001;15(7):1312–1314. [PubMed] [Google Scholar]
- [14].Kizaka-Kondoh S, Inoue M, Harada H, Hiraoka M. Tumor hypoxia: a target for selective cancer therapy. Cancer science. 2003;94(12):1021–1028. doi: 10.1111/j.1349-7006.2003.tb01395.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nanduri J, Yuan G, Kumar GK, Semenza GL, Prabhakar NR. Transcriptional responses to intermittent hypoxia. Respiratory physiology & neurobiology. 2008;164(1-2):277–281. doi: 10.1016/j.resp.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Semenza GL. Hypoxia-inducible factor 1 and cancer pathogenesis. IUBMB life. 2008;60(9):591–597. doi: 10.1002/iub.93. [DOI] [PubMed] [Google Scholar]
- [17].Shannon AM, Bouchier-Hayes DJ, Condron CM, Toomey D. Tumour hypoxia, chemotherapeutic resistance and hypoxia-related therapies. Cancer treatment reviews. 2003;29(4):297–307. doi: 10.1016/s0305-7372(03)00003-3. [DOI] [PubMed] [Google Scholar]
- [18].Guppy M. The hypoxic core: a possible answer to the cancer paradox. Biochemical and biophysical research communications. 2002;299(4):676–680. doi: 10.1016/s0006-291x(02)02710-9. [DOI] [PubMed] [Google Scholar]
- [19].Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nature reviews. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- [20].Brizel DM, Scully SP, Harrelson JM, Layfield LJ, Bean JM, Prosnitz LR, Dewhirst MW. Tumor oxygenation predicts for the likelihood of distant metastases in human soft tissue sarcoma. Cancer research. 1996;56(5):941–943. [PubMed] [Google Scholar]
- [21].Brown JM. Tumor hypoxia, drug resistance, and metastases. Journal of the National Cancer Institute. 1990;82(5):338–339. doi: 10.1093/jnci/82.5.338. [DOI] [PubMed] [Google Scholar]
- [22].Hockel M, Schlenger K, Hockel S, Vaupel P. Hypoxic cervical cancers with low apoptotic index are highly aggressive. Cancer research. 1999;59(18):4525–4528. [PubMed] [Google Scholar]
- [23].Nordsmark M, Overgaard M, Overgaard J. Pretreatment oxygenation predicts radiation response in advanced squamous cell carcinoma of the head and neck. Radiother Oncol. 1996;41(1):31–39. doi: 10.1016/s0167-8140(96)91811-3. [DOI] [PubMed] [Google Scholar]
- [24].Young SD, Hill RP. Effects of reoxygenation on cells from hypoxic regions of solid tumors: anticancer drug sensitivity and metastatic potential. Journal of the National Cancer Institute. 1990;82(5):371–380. doi: 10.1093/jnci/82.5.371. [DOI] [PubMed] [Google Scholar]
- [25].Gillet JP, Efferth T, Remacle J. Chemotherapy-induced resistance by ATP-binding cassette transporter genes. Biochimica et biophysica acta. 2007;1775(2):237–262. doi: 10.1016/j.bbcan.2007.05.002. [DOI] [PubMed] [Google Scholar]
- [26].Chinn LW, Kroetz DL. ABCB1 pharmacogenetics: progress, pitfalls, and promise. Clinical pharmacology and therapeutics. 2007;81(2):265–269. doi: 10.1038/sj.clpt.6100052. [DOI] [PubMed] [Google Scholar]
- [27].Buys TP, Chari R, Lee EH, Zhang M, MacAulay C, Lam S, Lam WL, Ling V. Genetic changes in the evolution of multidrug resistance for cultured human ovarian cancer cells. Genes, chromosomes & cancer. 2007;46(12):1069–1079. doi: 10.1002/gcc.20492. [DOI] [PubMed] [Google Scholar]
- [28].Kimura Y, Morita S, Matsuo M, Ueda K. Mechanism of multidrug recognition by MDR1/ABCB1. Cancer science. 2007;98(9):1303–1310. doi: 10.1111/j.1349-7006.2007.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Lemos C, Jansen G, Peters GJ. Drug transporters: recent advances concerning BCRP and tyrosine kinase inhibitors. British journal of cancer. 2008;98(5):857–862. doi: 10.1038/sj.bjc.6604213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Donnenberg VS, Donnenberg AD. Multiple drug resistance in cancer revisited: the cancer stem cell hypothesis. J Clin Pharmacol. 2005;45(8):872–877. doi: 10.1177/0091270005276905. [DOI] [PubMed] [Google Scholar]
- [31].Han ZB, Ren H, Zhao H, Chi Y, Chen K, Zhou B, Liu YJ, Zhang L, Xu B, Liu B, Yang R, Han ZC. Hypoxia-inducible factor (HIF)-1 alpha directly enhances the transcriptional activity of stem cell factor (SCF) in response to hypoxia and epidermal growth factor (EGF) Carcinogenesis. 2008;29(10):1853–1861. doi: 10.1093/carcin/bgn066. [DOI] [PubMed] [Google Scholar]
- [32].Hede S, Huilgol N. “Nano”: the new nemesis of cancer. Journal of cancer research and therapeutics. 2006;2(4):186–195. doi: 10.4103/0973-1482.29829. [DOI] [PubMed] [Google Scholar]
- [33].Moghimi SM, Hunter AC, Murray JC. Nanomedicine: current status and future prospects. Faseb J. 2005;19(3):311–330. doi: 10.1096/fj.04-2747rev. [DOI] [PubMed] [Google Scholar]
- [34].Thornton G. Materials science. Watching nanoparticles grow. Science (New York, N.Y. 2003;300(5624):1378–1379. doi: 10.1126/science.1085838. [DOI] [PubMed] [Google Scholar]
- [35].Pokropivny a.V.V.S. V.V. New dimensionality classifications of nanostructures. Physica E. 2008 [Google Scholar]
- [36].Hahn WC, Weinberg RA. Modelling the molecular circuitry of cancer. Nat Rev Cancer. 2002;2(5):331–341. doi: 10.1038/nrc795. [DOI] [PubMed] [Google Scholar]
- [37].Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [38].Moghimi SM, Hunter AC, Murray JC. Long-circulating and target-specific nanoparticles: theory to practice. Pharmacological reviews. 2001;53(2):283–318. [PubMed] [Google Scholar]
- [39].Sinha R, Kim GJ, Nie S, Shin DM. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Molecular cancer therapeutics. 2006;5(8):1909–1917. doi: 10.1158/1535-7163.MCT-06-0141. [DOI] [PubMed] [Google Scholar]
- [40].Maeda H, Fang J, Inutsuka T, Kitamoto Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. International immunopharmacology. 2003;3(3):319–328. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- [41].Davis ME, Chen ZG, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7(9):771–782. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- [42].Kope ek J, Kope kov· P, Minko T, Lu Z, Peterson C. Water soluble polymers in tumor targeted delivery. Journal of Controlled Release. 2001;74(1-3):147–158. doi: 10.1016/s0168-3659(01)00330-3. [DOI] [PubMed] [Google Scholar]
- [43].Shen F, Chu S, Bence A, Bailey B, Xue X, Erickson P, Montrose M, Beck W, Erickson L. Quantitation of doxorubicin uptake, efflux, and modulation of multidrug resistance (MDR) in MDR human cancer cells. Journal of Pharmacology and Experimental Therapeutics. 2008;324(1):95. doi: 10.1124/jpet.107.127704. [DOI] [PubMed] [Google Scholar]
- [44].Wu J, Lu Y, Lee A, Pan X, Yang X, Zhao X, Lee RJ. Reversal of multidrug resistance by transferrin-conjugated liposomes co-encapsulating doxorubicin and verapamil. J Pharm Pharm Sci. 2007;10(3):350–357. [PubMed] [Google Scholar]
- [45].Judge AD, Robbins M, Tavakoli I, Levi J, Hu L, Fronda A, Ambegia E, McClintock K, MacLachlan I. Confirming the RNAi-mediated mechanism of action of siRNA-based cancer therapeutics in mice. J Clin Invest. 2009;119(3):661–673. doi: 10.1172/JCI37515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Nie S, Xing Y, Kim GJ, Simons JW. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288. doi: 10.1146/annurev.bioeng.9.060906.152025. [DOI] [PubMed] [Google Scholar]
- [47].Leamon CP, Low PS. Folate-mediated targeting: from diagnostics to drug and gene delivery. Drug Discov Today. 2001;6(1):44–51. doi: 10.1016/s1359-6446(00)01594-4. [DOI] [PubMed] [Google Scholar]
- [48].Leamon CP, Reddy JA. Folate-targeted chemotherapy. Adv Drug Deliv Rev. 2004;56(8):1127–1141. doi: 10.1016/j.addr.2004.01.008. [DOI] [PubMed] [Google Scholar]
- [49].Reddy JA, Low PS. Folate-mediated targeting of therapeutic and imaging agents to cancers. Crit Rev Ther Drug Carrier Syst. 1998;15(6):587–627. [PubMed] [Google Scholar]
- [50].Stella B, Arpicco S, Peracchia MT, Desmaele D, Hoebeke J, Renoir M, D’Angelo J, Cattel L, Couvreur P. Design of folic acid-conjugated nanoparticles for drug targeting. J Pharm Sci. 2000;89(11):1452–1464. doi: 10.1002/1520-6017(200011)89:11<1452::aid-jps8>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- [51].van Vlerken LE, Amiji MM. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin Drug Deliv. 2006;3(2):205–216. doi: 10.1517/17425247.3.2.205. [DOI] [PubMed] [Google Scholar]
- [52].Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6(3):659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- [53].Harisinghani MG, Barentsz J, Hahn PF, Deserno WM, Tabatabaei S, van de Kaa CH, de la Rosette J, Weissleder R. Noninvasive detection of clinically occult lymph-node metastases in prostate cancer. N Engl J Med. 2003;348(25):2491–2499. doi: 10.1056/NEJMoa022749. [DOI] [PubMed] [Google Scholar]
- [54].Devalapally H, Duan Z, Seiden M, Amiji M. Modulation of drug resistance in ovarian adenocarcinoma by enhancing intracellular ceramide using tamoxifen-loaded biodegradable polymeric nanoparticles. Clinical Cancer Research. 2008;14(10):3193. doi: 10.1158/1078-0432.CCR-07-4973. [DOI] [PubMed] [Google Scholar]
- [55].Jabr-Milane L, van Vlerken L, Yadav S, Amiji M. Multi-functional nanocarriers to overcome tumor drug resistance. Cancer Treatment Reviews. 2008;34(7):592–602. doi: 10.1016/j.ctrv.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Vlerken L, Duan Z, Seiden M, Amiji M. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer research. 2007;67(10):4843. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- [57].Shenoy D, Amiji M. Poly (ethylene oxide)-modified poly ( -caprolactone) nanoparticles for targeted delivery of tamoxifen in breast cancer. International journal of pharmaceutics. 2005;293(1-2):261–270. doi: 10.1016/j.ijpharm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- [58].Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126(3):187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- [59].Thierry A, Vige D, Coughlin S, Belli J, Dritschilo A, Rahman A. Modulation of doxorubicin resistance in multidrug-resistant cells by liposomes. The FASEB Journal. 1993;7(6):572. doi: 10.1096/fasebj.7.6.8097173. [DOI] [PubMed] [Google Scholar]
- [60].Kommareddy S, Amiji M. Poly(ethylene glycol)-modified thiolated gelatin nanoparticles for glutathione-responsive intracellular DNA delivery. Nanomedicine. 2007;3(1):32–42. doi: 10.1016/j.nano.2006.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Yadav S, van Vlerken L, Little S, Amiji M. Evaluations of combination MDR-1 gene silencing and paclitaxel administration in biodegradable polymeric nanoparticle formulations to overcome multidrug resistance in cancer cells. Cancer Chemotherapy and Pharmacology. 2009;63(4):711–722. doi: 10.1007/s00280-008-0790-y. [DOI] [PubMed] [Google Scholar]
- [62].Kommareddy S, Tiwari SB, Amiji MM. Long-circulating polymeric nanovectors for tumor-selective gene delivery. Technol Cancer Res Treat. 2005;4(6):615–625. doi: 10.1177/153303460500400605. [DOI] [PubMed] [Google Scholar]
- [63].Vlerken L.E.v., Vyas TK, Amiji MM. Poly(ethylene glycol)-modified Nanocarriers for Tumor-targeted and Intracellular Delivery Pharmaceutical Research. 2007;24(8):1405–1414. doi: 10.1007/s11095-007-9284-6. [DOI] [PubMed] [Google Scholar]
- [64].Hamada H, Miura K, Ariyoshi K, Heike Y, Sato S, Kameyama K, Kurosawa Y, Tsuruo T. Mouse-human chimeric antibody against the multidrug transporter P-glycoprotein. Cancer research. 1990;50(11):3167. [PubMed] [Google Scholar]
- [65].Marcucci F, Lefoulon F. Active targeting with particulate drug carriers in tumor therapy: fundamentals and recent progress. Drug Discov Today. 2004;9(5):219–228. doi: 10.1016/S1359-6446(03)02988-X. [DOI] [PubMed] [Google Scholar]
- [66].Iwahashi T, Okochi E, Ariyoshi K, Watabe H, Amann E, Mori S, Tsuruo T, Ono K. Specific targeting and killing activities of anti-P-glycoprotein monoclonal antibody MRK16 directed against intrinsically multidrug-resistant human colorectal carcinoma cell lines in the nude mouse model. Cancer research. 1993;53(22):5475. [PubMed] [Google Scholar]
- [67].De Menezes L, Daniel E, Pilarski L, Allen T. In vitro and in vivo targeting of immunoliposomal doxorubicin to human B-cell lymphoma. Cancer research. 1998;58(15):3320. [PubMed] [Google Scholar]
- [68].Ghetie M, Marches R, Kufert S, Vitetta E. An anti-CD19 antibody inhibits the interaction between P-glycoprotein (P-gp) and CD19, causes P-gp to translocate out of lipid rafts, and chemosensitizes a multidrug-resistant (MDR) lymphoma cell line. Blood. 2004;104(1):178. doi: 10.1182/blood-2003-12-4255. [DOI] [PubMed] [Google Scholar]
- [69].Hu X, Li J, Yang E, Vandervalk S, Xing P. Anti-Cripto Mab inhibit tumour growth and overcome MDR in a human leukaemia MDR cell line by inhibition of Akt and activation of JNK/SAPK and bad death pathways. British journal of cancer. 2007;96(6):918. doi: 10.1038/sj.bjc.6603641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Rajendran L, Kn^lker H, Simons K. Subcellular targeting strategies for drug design and delivery. Nature Reviews Drug Discovery. 2010;9(1):29–42. doi: 10.1038/nrd2897. [DOI] [PubMed] [Google Scholar]
- [71].Torchilin VP. Recent approaches to intracellular delivery of drugs and DNA and organelle targeting. Annu Rev Biomed Eng. 2006;8:343–375. doi: 10.1146/annurev.bioeng.8.061505.095735. [DOI] [PubMed] [Google Scholar]
- [72].Morimoto H, Yonehara S, Bonavida B. Overcoming tumor necrosis factor and drug resistance of human tumor cell lines by combination treatment with anti-Fas antibody and drugs or toxins. Cancer research. 1993;53(11):2591. [PubMed] [Google Scholar]
- [73].Hamidi M, Azadi A, Rafiei P. Hydrogel nanoparticles in drug delivery. Adv Drug Deliv Rev. 2008;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- [74].Hamidi M, Azadi A, Rafiei P. Pharmacokinetic consequences of pegylation. Drug Delivery. 2006;13(6):399–409. doi: 10.1080/10717540600814402. [DOI] [PubMed] [Google Scholar]
- [75].Hamidi M, Rafiei P, Azadi A. Designing PEGylated therapeutic molecules: advantages in ADMET properties. Expert Opin. Drug Discov. 2008;3(11):1293–1307. doi: 10.1517/17460441.3.11.1293. [DOI] [PubMed] [Google Scholar]
- [76].Xu Z, Hoffman J, Patel I, Joubert P. Single-dose safety/tolerability and pharmacokinetic/pharmacodynamics (PK/PD) following administration of ascending subcutaneous doses of pegylated-interferon (PEG-IFN) and interferon -2a (IFN -2a) to healthy subjects. Hepatology. 1998;28(part 2):702A. [Google Scholar]
- [77].Dykxhoorn DM, Lieberman J. Running interference: prospects and obstacles to using small interfering RNAs as small molecule drugs. Annu Rev Biomed Eng. 2006;8:377–402. doi: 10.1146/annurev.bioeng.8.061505.095848. [DOI] [PubMed] [Google Scholar]
- [78].Zhao Y, Srivastava D. A developmental view of microRNA function. Trends Biochem Sci. 2007;32(4):189–197. doi: 10.1016/j.tibs.2007.02.006. [DOI] [PubMed] [Google Scholar]
- [79].Gregory RI, Chendrimada TP, Shiekhattar R. MicroRNA biogenesis: isolation and characterization of the microprocessor complex. Methods Mol Biol. 2006;342:33–47. doi: 10.1385/1-59745-123-1:33. [DOI] [PubMed] [Google Scholar]
- [80].Nieth C, Priebsch A, Stege A, Lage H. Modulation of the classical multidrug resistance (MDR) phenotype by RNA interference (RNAi) FEBS Lett. 2003;545(2-3):144–150. doi: 10.1016/s0014-5793(03)00523-4. [DOI] [PubMed] [Google Scholar]
- [81].Lage H. MDR1/P-glycoprotein (ABCB1) as target for RNA interference-mediated reversal of multidrug resistance. Curr Drug Targets. 2006;7(7):813–821. doi: 10.2174/138945006777709566. [DOI] [PubMed] [Google Scholar]
- [82].Lage H. Therapeutic potential of RNA interference in drug-resistant cancers. Future Oncol. 2009;5(2):169–185. doi: 10.2217/14796694.5.2.169. [DOI] [PubMed] [Google Scholar]
- [83].Wu H, Hait WN, Yang JM. Small interfering RNA-induced suppression of MDR1 (P-glycoprotein) restores sensitivity to multidrug-resistant cancer cells. Cancer Res. 2003;63(7):1515–1519. [PubMed] [Google Scholar]
- [84].Xiao H, Wu Z, Shen H, Luo AL, Yang YF, Li XB, Zhu DY. In vivo reversal of P-glycoprotein-mediated multidrug resistance by efficient delivery of stealth RNAi. Basic Clin Pharmacol Toxicol. 2008;103(4):342–348. doi: 10.1111/j.1742-7843.2008.00296.x. [DOI] [PubMed] [Google Scholar]
- [85].Stein U, Walther W, Stege A, Kaszubiak A, Fichtner I, Lage H. Complete in vivo reversal of the multidrug resistance phenotype by jet-injection of anti-MDR1 short hairpin RNA-encoding plasmid DNA. Mol Ther. 2008;16(1):178–186. doi: 10.1038/sj.mt.6300304. [DOI] [PubMed] [Google Scholar]
- [86].Saad M, Garbuzenko OB, Minko T. Co-delivery of siRNA and an anticancer drug for treatment of multidrug-resistant cancer. Nanomedicine (Lond) 2008;3(6):761–776. doi: 10.2217/17435889.3.6.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Kolesnick RN, Kronke M. Regulation of ceramide production and apoptosis. Annual review of physiology. 1998;60:643–665. doi: 10.1146/annurev.physiol.60.1.643. [DOI] [PubMed] [Google Scholar]
- [88].Struckhoff AP, Bittman R, Burow ME, Clejan S, Elliott S, Hammond T, Tang Y, Beckman BS. Novel ceramide analogs as potential chemotherapeutic agents in breast cancer. The Journal of pharmacology and experimental therapeutics. 2004;309(2):523–532. doi: 10.1124/jpet.103.062760. [DOI] [PubMed] [Google Scholar]
- [89].Schenck M, Carpinteiro A, Grassme H, Lang F, Gulbins E. Ceramide: physiological and pathophysiological aspects. Archives of biochemistry and biophysics. 2007;462(2):171–175. doi: 10.1016/j.abb.2007.03.031. [DOI] [PubMed] [Google Scholar]
- [90].Pettus BJ, Chalfant CE, Hannun YA. Ceramide in apoptosis: an overview and current perspectives. Biochimica et biophysica acta. 2002;1585(2-3):114–125. doi: 10.1016/s1388-1981(02)00331-1. [DOI] [PubMed] [Google Scholar]
- [91].Gulbins E, Grassme H. Ceramide and cell death receptor clustering. Biochimica et biophysica acta. 2002;1585(2-3):139–145. doi: 10.1016/s1388-1981(02)00334-7. [DOI] [PubMed] [Google Scholar]
- [92].Elrick MJ, Fluss S, Colombini M. Sphingosine, a product of ceramide hydrolysis, influences the formation of ceramide channels. Biophysical journal. 2006;91(5):1749–1756. doi: 10.1529/biophysj.106.088443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Siskind LJ. Mitochondrial ceramide and the induction of apoptosis. Journal of bioenergetics and biomembranes. 2005;37(3):143–153. doi: 10.1007/s10863-005-6567-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Siskind LJ, Kolesnick RN, Colombini M. Ceramide forms channels in mitochondrial outer membranes at physiologically relevant concentrations. Mitochondrion. 2006;6(3):118–125. doi: 10.1016/j.mito.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Itoh M, Kitano T, Watanabe M, Kondo T, Yabu T, Taguchi Y, Iwai K, Tashima M, Uchiyama T, Okazaki T. Possible role of ceramide as an indicator of chemoresistance: decrease of the ceramide content via activation of glucosylceramide synthase and sphingomyelin synthase in chemoresistant leukemia. Clin Cancer Res. 2003;9(1):415–423. [PubMed] [Google Scholar]
- [96].Morjani H, Aouali N, Belhoussine R, Veldman RJ, Levade T, Manfait M. Elevation of glucosylceramide in multidrug-resistant cancer cells and accumulation in cytoplasmic droplets. International journal of cancer. 2001;94(2):157–165. doi: 10.1002/ijc.1449. [DOI] [PubMed] [Google Scholar]
- [97].Senchenkov A, Litvak DA, Cabot MC. Targeting ceramide metabolism--a strategy for overcoming drug resistance. Journal of the National Cancer Institute. 2001;93(5):347–357. doi: 10.1093/jnci/93.5.347. [DOI] [PubMed] [Google Scholar]
- [98].van Vlerken LE, Duan Z, Seiden MV, Amiji MM. Modulation of intracellular ceramide using polymeric nanoparticles to overcome multidrug resistance in cancer. Cancer Res. 2007;67(10):4843–4850. doi: 10.1158/0008-5472.CAN-06-1648. [DOI] [PubMed] [Google Scholar]
- [99].van Vlerken LE, Duan Z, Little SR, Seiden MV, Amiji MM. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Mol Pharm. 2008;5(4):516–526. doi: 10.1021/mp800030k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Liu YY, Han TY, Giuliano AE, Cabot MC. Ceramide glycosylation potentiates cellular multidrug resistance. Faseb J. 2001;15(3):719–730. doi: 10.1096/fj.00-0223com. [DOI] [PubMed] [Google Scholar]
- [101].Gouaze V, Liu YY, Prickett CS, Yu JY, Giuliano AE, Cabot MC. Glucosylceramide synthase blockade down-regulates P-glycoprotein and resensitizes multidrug-resistant breast cancer cells to anticancer drugs. Cancer research. 2005;65(9):3861–3867. doi: 10.1158/0008-5472.CAN-04-2329. [DOI] [PubMed] [Google Scholar]
- [102].Altenberg B, Greulich KO. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84(6):1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- [103].Hsu PP, Sabatini DM. Cancer cell metabolism: Warburg and beyond. Cell. 2008;134(5):703–707. doi: 10.1016/j.cell.2008.08.021. [DOI] [PubMed] [Google Scholar]
- [104].Lopez-Lazaro M. The warburg effect: why and how do cancer cells activate glycolysis in the presence of oxygen? Anti-cancer agents in medicinal chemistry. 2008;8(3):305–312. doi: 10.2174/187152008783961932. [DOI] [PubMed] [Google Scholar]
- [105].Warburg O. On respiratory impairment in cancer cells. Science (New York, N.Y. 1956;124(3215):269–270. [PubMed] [Google Scholar]
- [106].Young CD, Anderson SM. Sugar and fat - that’s where it’s at: metabolic changes in tumors. Breast Cancer Res. 2008;10(1):202. doi: 10.1186/bcr1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Schumacker PT. Reactive oxygen species in cancer cells: live by the sword, die by the sword. Cancer cell. 2006;10(3):175–176. doi: 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [108].Liu H, Savaraj N, Priebe W, Lampidis TJ. Hypoxia increases tumor cell sensitivity to glycolytic inhibitors: a strategy for solid tumor therapy (Model C) Biochem Pharmacol. 2002;64(12):1745–1751. doi: 10.1016/s0006-2952(02)01456-9. [DOI] [PubMed] [Google Scholar]
- [109].Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. doi: 10.1074/jbc.M202487200. [DOI] [PubMed] [Google Scholar]
- [110].Lum JJ, Bui T, Gruber M, Gordan JD, DeBerardinis RJ, Covello KL, Simon MC, Thompson CB. The transcription factor HIF-1alpha plays a critical role in the growth factor-dependent regulation of both aerobic and anaerobic glycolysis. Genes Dev. 2007;21(9):1037–1049. doi: 10.1101/gad.1529107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Robey IF, Lien AD, Welsh SJ, Baggett BK, Gillies RJ. Hypoxia-inducible factor-1alpha and the glycolytic phenotype in tumors. Neoplasia. 2005;7(4):324–330. doi: 10.1593/neo.04430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25(34):4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Pedersen PL. Warburg, me and Hexokinase 2: Multiple discoveries of key molecular events underlying one of cancers’ most common phenotypes, the “Warburg Effect”, i.e., elevated glycolysis in the presence of oxygen. Journal of bioenergetics and biomembranes. 2007;39(3):211–222. doi: 10.1007/s10863-007-9094-x. [DOI] [PubMed] [Google Scholar]
- [114].Pedersen PL. Voltage dependent anion channels (VDACs): a brief introduction with a focus on the outer mitochondrial compartment’s roles together with hexokinase-2 in the “Warburg effect” in cancer. Journal of bioenergetics and biomembranes. 2008;40(3):123–126. doi: 10.1007/s10863-008-9165-7. [DOI] [PubMed] [Google Scholar]
- [115].Floridi A, Paggi MG, D’Atri S, De Martino C, Marcante ML, Silvestrini B, Caputo A. Effect of lonidamine on the energy metabolism of Ehrlich ascites tumor cells. Cancer research. 1981;41(11 Pt 1):4661–4666. [PubMed] [Google Scholar]
- [116].Giorgioni G, Ruggieri S, Di Stefano A, Sozio P, Cinque B, Di Marzio L, Santoni G, Claudi F. Glycosyl and polyalcoholic prodrugs of lonidamine. Bioorganic & medicinal chemistry letters. 2008;18(7):2445–2450. doi: 10.1016/j.bmcl.2008.02.046. [DOI] [PubMed] [Google Scholar]
- [117].Ravagnan L, Marzo I, Costantini P, Susin SA, Zamzami N, Petit PX, Hirsch F, Goulbern M, Poupon MF, Miccoli L, Xie Z, Reed JC, Kroemer G. Lonidamine triggers apoptosis via a direct, Bcl-2-inhibited effect on the mitochondrial permeability transition pore. Oncogene. 1999;18(16):2537–2546. doi: 10.1038/sj.onc.1202625. [DOI] [PubMed] [Google Scholar]
- [118].Jordan A, Scholz R, Wust P, FaKhling H, Felix R. Magnetic #uid hyperthermia (MFH): Cancer treatment with AC magnetic “eld induced excitation of biocompatible superparamagnetic nanoparticles. Journal of Magnetism and Magnetic Materials. 1999;201(1):413–419. [Google Scholar]
- [119].Kong G, Braun RD, Dewhirst MW. Hyperthermia enables tumor-specific nanoparticle delivery: effect of particle size. Cancer Res. 2000;60(16):4440–4445. [PubMed] [Google Scholar]
- [120].Gaber MH. Modulation of doxorubicin resistance in multidrug-resistance cells by targeted liposomes combined with hyperthermia. J Biochem Mol Biol Biophys. 2002;6(5):309–314. doi: 10.1080/10258140290033066. [DOI] [PubMed] [Google Scholar]
- [121].Rapoport N. Combined cancer therapy by micellar-encapsulated drug and ultrasound. International journal of pharmaceutics. 2004;277(1-2):155–162. doi: 10.1016/j.ijpharm.2003.09.048. [DOI] [PubMed] [Google Scholar]
- [122].Marin A, Sun H, Husseini GA, Pitt WG, Christensen DA, Rapoport NY. Drug delivery in pluronic micelles: effect of high-frequency ultrasound on drug release from micelles and intracellular uptake. J Control Release. 2002;84(1-2):39–47. doi: 10.1016/s0168-3659(02)00262-6. [DOI] [PubMed] [Google Scholar]
- [123].Konan YN, Gurny R, Allemann E. State of art in the delivery of photosensitizers for photodynamic therapy. Photochemistry and Photobiology. 2001;66(2):89–106. doi: 10.1016/s1011-1344(01)00267-6. [DOI] [PubMed] [Google Scholar]
- [124].Samia AC, Chen X, Burda C. Semiconductor quantum dots for photodynamic therapy. J Am Chem Soc. 2003;125(51):15736–15737. doi: 10.1021/ja0386905. [DOI] [PubMed] [Google Scholar]
- [125].Tang W, Xu H, Kopelman R, Philbert M. Photodynamic Characterisation and invitro application of Methylene Blue containing nanoparticle platform. Photochemistry and Photobiology. 2004;81(2):242–249. doi: 10.1562/2004-05-24-RA-176.1. [DOI] [PubMed] [Google Scholar]