Abstract
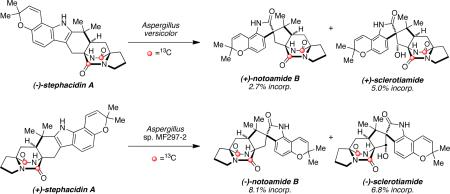
The advanced natural product stephacidin A is proposed as a biosynthetic precursor to notoamide B in various Aspergillus species. Doubly 13C-labeled racemic stephacidin A was synthesized and fed to cultures of the terrestrial-derived fungus, Aspergillus versicolor NRRL 35600, and the marine-derived fungus, Aspergillus sp. MF297-2. Analysis of the metabolites revealed enantiospecific incorporation of intact (–)-stephacidin A into (+)-notoamide B in Aspergillus versicolor, and (+)-stephacidin A into (–)-notoamide B in Aspergillus sp. MF297-2. 13C-Labeled sclerotiamide was also isolated from both fungal cultures.
Elucidation of the biosynthesis of prenylated indole alkaloids containing a unique bicyclo[2.2.2]diazaoctane ring system has been an area of intense research for several years.1 Mainly produced by fungi in the genera Aspergillus and Penicillium, the stephacidins,2 notoamides,3 paraherquamides,4 malbrancheamides,5 marcfortines,6 asperparalines,7 and brevianamide8 families of natural products are biosynthetically derived from three different building blocks, tryptophan, a cyclic amino acid, and one or two mevalonate-derived isoprene units.9 Early studies by Sammes10a and Birch,10b as well as by our laboratory,1 have suggested that the bicyclo[2.2.2]diazaoctane core found in these families of secondary metabolites is formed biosynthetically via an intramolecular hetero-Diels–Alder (IMDA) reaction of a 5-hydroxy-pyrazin-2(1H)-one. The proposed biosynthetic IMDA reaction has been extensively validated by its application to the biomimetic total synthesis of several members of this family of prenylated indole alkaloids including, brevianamide B,11 VM55599,12 marcfortine C,13 stephacidin A,14 malbrancheamide,15 and versicolamide B.3c,16 Nonetheless, the complete biosynthetic pathways of these secondary metabolites are yet to be fully elucidated.
In 2007, Tsukamoto and co-workers isolated the known metabolites stephacidin A and sclerotiamide from a marine-derived Aspergillus sp. MF297-2.3a Along with these metabolites, Tsukamoto reported the isolation of several new metabolites, some of which possess the same pyranoindole moiety and/or core bicyclo[2.2.2]diazaoctane ring system as stephacidin A and sclerotiamide, and named this new family of prenylated indole alkaloids the notoamides. One novel metabolite in particular, notoamide B, contains a similar structural core to both stephacidin A and sclerotiamide, which suggested that notoamide B might serve as a biosynthetic metabolite derived from stephacidin A. Thus, questions arose as to whether or not the stephacidins and notoamides share a common biogenesis, and if stephacidin A could be a direct precursor to notoamide B.
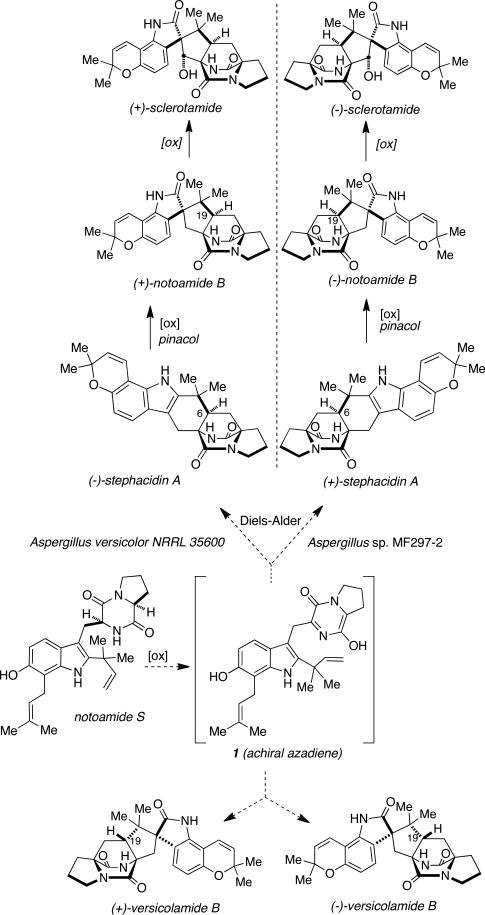
Elucidation of the biosynthesis of the stephacidins and notoamides garnered additional intrigue when Gloer et al. reported the isolation of the correspond ing enantiomers of stephacidin A and notoamide B from the closely related fungus, Aspergillus versicolor NRRL 35600.3c In addition, the individual (+)- and (–)-enantiomers of versicolamide B were isolated from Aspergillus versicolor NRRL 35600 (Gloer) and the marine-derived Aspergillus sp. MF297-2 (Tsukamoto). As depicted in Scheme 1, we recently proposed that oxidation and tautomerization of the proposed precursor notoamide S would yield the key achiral azadiene species (1).17 (Cycloaddition of the azadiene intermediate, followed by oxidative ring closure to the pyran would produce the (+)- and (–)-enantiomers of stephacidin A in the respective producing organisms. Face-selective oxidative rearrangement of the 2,3-disubstituted indole would then yield the corresponding spiro-oxindoles, (+)- and (–)-notoamide B, respectively. A second face-selective oxidation of notoamide B at the C-10 position (sclerotamide numbering) would give the respective (+)- and (–)-sclerotiamide enantiomers.
Scheme 1.
Possible biosynthetic relationship of some antipodal metabolites.
Our laboratory recently provided corroborating laboratory support for the proposed biosynthetic conversion of stephacidin A to notoamide B through the efficient biomimetic oxidation of stephacidin A to notoamide B by deploying an oxaziridine.14 However, it still remained to be demonstrated that this chemically feasible oxidative transformation was indeed valid in Nature. Furthermore, the biosynthetic conversion of stephacidin A to sclerotiamide (which presumably proceeds via notoamide B) was still unknown. To specifically investigate the biosynthetic formation of these metabolites in both Aspergillus fungal cultures, we relied on traditional isotopically-labeled substrate incorporation techniques. Herein we report the preparation of doubly 13C-labeled (±)-stephacidin A and the enantioselective biotransformation of this substrate to (+)-notoamide B and (+)-sclerotiamide in Aspergillus versicolor NRRL 36500, as well as (–)-notoamide B and (–)-sclerotiamide in Aspergillus) sp. MF297-2.
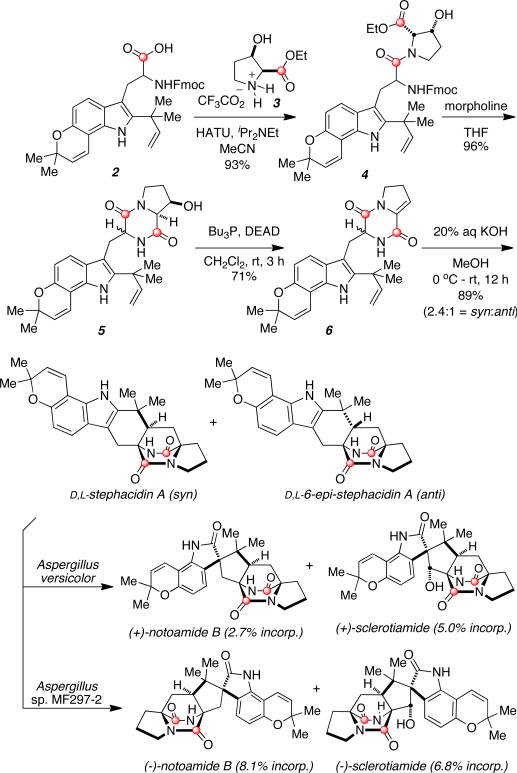
Following an unlabeled biomimetic strategy recently described by our laboratory,14 the synthesis of doubly 13C-labeled stephacidin A commenced with the amino acid coupling of the 13C1-labeled reverse-prenylated tryptophan derivative 214 with 13C1-labeled L-proline derivative 3 in the presence of HATU and iPr2NEt (Scheme 2). Deprotection and cyclization of amide 4 was achieved in one step with a solution of morpholine in THF to afford a mixture of diastereomers of dioxopiperazine 5. Both diastereomers were subjected together through the Mitsunobu-type elimination with PBu3 and DEAD to yield enamide 6. Treatment of 6 with aqueous KOH in MeOH effected the intramolecular hetero-Diels-Alder reaction to produce racemic cycloadducts D,L-stephacidin A and D,L-6-epi-stephacidin A as a 2.4:1 separable mixture of diastereomers favoring the desired syn stereochemistry as previously observed.
Scheme 2.
Doubly 13C-Labeled Stephacidin A and biotransformation to notoamide B.
With the desired 13C-labeled substrate in hand, D,L-stephacidin A was added to cultures of A. versicolor NRRL 35600 in a precursor incorporation experiment. Fungal extracts from this experiment were analyzed by LC-MS and 13C-NMR spectroscopy, and further analysis of the electrospray mass spectrum showed 2.7% intact incorporation of labeled (–)- stephacidin A into (+)-notoamide B.15b,18 The unreacted stereoisomer (+)-stephacidin A was also isolated from the fungal extract, which was anticipated since it possesses the opposite absolute configuration of the metabolite notoamide B produced by A. versicolor. The metabolite (+)-scleotiamide3a,19 was also found to contain intact double-13C-labels (5.0% incorporation).
In a similar precursor incorporation study with D,L-stephacidin A in the marine-derived Aspergillus sp. MF297-2, analysis of the fungal extract via ESI mass spectrometry revealed significant 13C-incorporation in to (–)-notoamide B (8.1%), as well as 6.8% intact incorporation into the known metabolite, (–)-sclerotiamide. In addition to the detection and isolation of (–)-notoamide B and (–)-sclerotiamide, the unreacted enantiomer, (–)-stephacidin A, was similarly recovered from the precursor incorporation study with Aspergillus sp. MF297-2.
In conclusion, these results provide experimental validation for the hypothesis that complementary, face-selective oxidative enzymes (currently presumed to be flavo-enzymes) are present in both Aspergillus versicolor NRRL 35600 and Aspergillus sp. MF297-2. Specifically in A. versicolor NRRL 35600, this oxidase is responsible for the biosynthetic conversion of (–)-stephacidin A into (+)-notoamide B, while a stereochemically complementary oxidase in the Tsukamoto marine-derived Aspergillus sp. MF297-2 converts (+)-stephacidin A into (–)-notoamide B. Furthermore, these results show that the known metabolite sclerotiamide is biosynthetically derived from stephacidin A in both Aspergillus fungal cultures in an enantioselective manner. While these results provide further insight into the antipodal biogenesis of the stephacidins and notoamides, several questions remain unanswered. In particular, mid-stage precursors between notoamide S and stephacidin A remain to be characterized. A combination of genome mining, proteomics and biotransformation experiments aimed at elucidating these questions remain the focus of ongoing studies in these laboratories and will be reported in due course.
Supplementary Material
Acknowledgment
This work was financially supported by the National Institutes of Health (CA70375 to RMW), by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (No. 23108518 to S.T.), and by a grant from the Nagase Science and Technology Foundation (to S.T.).
Footnotes
Supporting Information Available. Synthetic procedures, characterization, and calculation of percent incorporation. This material is free of charge via the Internet at http://pubs.acs.org.
References
- 1.a Williams RM. Chem. Pharm. Bull. 2002;50:711–740. doi: 10.1248/cpb.50.711. [DOI] [PubMed] [Google Scholar]; b Williams RM, Cox RJ. Acc. Chem. Res. 2003;36:127–139. doi: 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; c Stocking E, Williams RM. Angew. Chem. Int. Ed. Engl. 2003;42:3078–3115. doi: 10.1002/anie.200200534. [DOI] [PubMed] [Google Scholar]; d Miller KA, Williams RM. Chem. Soc. Rev. 2009;38:3160–3174. doi: 10.1039/b816705m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a Qian-Cutrone J, Huang S, Shu Y-Z, Vyas D, Fairchild C, Menendez A, Krampitz K, Dalterio R, Klohr SE, Gao Q. J. Am. Chem. Soc. 2002;124:14556–14557. doi: 10.1021/ja028538n. [DOI] [PubMed] [Google Scholar]; b Fenical W, Jensen PR, Cheng XC. 2000 U.S Patent 6,066,635.; c Sugie Y, Hirai H, Inagaki T, Ishiguro M, Kim Y-J, Kojima Y, Sakakibara T, Sakemi S, Sugiura A, Suzuki Y, Brennan L, Duignan J, Huang LH, Sutcliffe J, Kojima N. J. Antibiot. 2001;54:911–916. doi: 10.7164/antibiotics.54.911. [DOI] [PubMed] [Google Scholar]
- 3.a Kato H, Yoshida T, Tokue T, Nojiri Y, Hirota H, Ohta T, Williams RM, Tsukamoto S. Angew. Chem. Int. Ed. 2007;46:2254–2256. doi: 10.1002/anie.200604381. [DOI] [PubMed] [Google Scholar]; b Tsukamoto S, Kato H, Samizo M, Nojiri Y, Onuki H, Hirota H, Ohta T. J. Nat. Prod. 2008;71:2064–2067. doi: 10.1021/np800471y. [DOI] [PubMed] [Google Scholar]; c Greshock TJ, Grubbs AW, Jiao P, Wicklow DT, Gloer JB, Williams RM. Angew. Chem. Int. Ed. 2008;47:3573–3577. doi: 10.1002/anie.200800106. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tsukamoto S, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. J. Am. Chem. Soc. 2009;131:3834. doi: 10.1021/ja810029b. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Tsukamoto S, Kawabata T, Kato H, Greshock TJ, Hirota H, Ohta T, Williams RM. Org. Lett. 2009;11:1297–1300. doi: 10.1021/ol900071c. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Tsukamoto S, Umaoka H, Yoshikawa K, Ikeda T, Hirota H. J. Nat. Prod. 2010;73:1438–1440. doi: 10.1021/np1002498. [DOI] [PubMed] [Google Scholar]
- 4.a Yamazaki M, Okuyama E, Kobayashi M, Inoue H. Tetrahedron Lett. 1981;22:135–136. [Google Scholar]; b Ondeyka JG, Goegelman RT, Schaeffer JM, Kelemen L, Zitano L. J. Antibiot. 1990;43:1375–1379. doi: 10.7164/antibiotics.43.1375. [DOI] [PubMed] [Google Scholar]; c Liesch JM, Wichmann CF. J. Antibiot. 1990;43:1380–1386. doi: 10.7164/antibiotics.43.1380. [DOI] [PubMed] [Google Scholar]; d Blanchflower SE, Banks RM, Everet JR, Manger BR, Reading C. J. Antibiot. 1991;44:492–497. doi: 10.7164/antibiotics.44.492. [DOI] [PubMed] [Google Scholar]; e Blanchflower SE, Banks RM, Everet JR, Reading C. J. Antibiot. 1993;46:1355–1363. doi: 10.7164/antibiotics.46.1355. [DOI] [PubMed] [Google Scholar]; f Authrine C, Gloer JB. J. Nat. Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
- 5.a Martinez-Luis S, Rodriguez R, Acevedo L, Gonzalez MC, Lira-Rocha A, Mata R. Tetrahedron. 2006;62:1817. [Google Scholar]; b Figueroa M, Del Carmen González M, Mata R. Nat. Prod. Res. 2008;22:709–714. doi: 10.1080/14786410802012361. [DOI] [PubMed] [Google Scholar]
- 6.a Polonsky J, Merrien M-A, Prange T, Pascard C. J. Chem. Soc., Chem. Commun. 1980:601–602. [Google Scholar]; b Prange T, Billion M-A, Vuilhorgne M, Pascard C, Polonsky J. Tetrahedron Lett. 1981;22:1977–1980. [Google Scholar]
- 7.a Hayashi H, Nishimoto Y, Nozaki H. Tetrahedron Lett. 1997;38:5655–5658. [Google Scholar]; b Banks RM, Blanchflower SE, Everett JR, Manger BR, Reading C. J. Antibiot. 1997;50:840–846. doi: 10.7164/antibiotics.50.840. [DOI] [PubMed] [Google Scholar]
- 8.a Birch AJ, Wright JJ. Tetrahedron. 1970;26:2329–2344. doi: 10.1016/s0040-4020(01)92812-1. [DOI] [PubMed] [Google Scholar]; b Birch AJ, Wright JJS. J. Chem. Soc., Chem. Commun. 1969:644. [Google Scholar]; c Birch AJ, Russell RA. Tetrahedron. 1972;28:2999–3008. [Google Scholar]; d Steyn PS. Tetrahedron. 1973;29:107–120. [Google Scholar]
- 9.a Williams RM, Stocking EM, Sanz-Cervera JF. Top. Curr. Chem. 2000;209:97–173. [Google Scholar]; b Stocking EM, Sanz-Cervera JF, Unkefer CJ, Williams RM. Tetrahedron. 2001;57:5303–5320. [Google Scholar]
- 10.a Porter AEA, Sammes PG. J. Chem. Soc. Chem. Commun. 1970:1103. [Google Scholar]; b Baldas J, Birch AJ, Russell RA. J. Chem. Soc. Perkin Trans I. 1974:50–52. [Google Scholar]
- 11.a Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. J. Am. Chem. Soc. 1998;120:1090–1091. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; b Williams RM, Sanz-Cervera JF, Sancenón F, Marco JA, Halligan K. Bioorg. Med. Chem. 1998;6:1233–1241. doi: 10.1016/s0968-0896(98)00102-3. [DOI] [PubMed] [Google Scholar]; c Sanz-Cervera JF, Williams RM, Marco JA, López-Sánchez JM, González F, Martínex ME, Sancenón F. Tetrahedron. 2000;56:6345. [Google Scholar]; d Adams LA, Valente MWN, Williams RM. Tetrahedron. 2006;62:5195. [Google Scholar]
- 12.a Stocking EM, Sanz-Cervera JF, Williams RM. J. Am. Chem. Soc. 2000;122:1675–1683. [Google Scholar]; b Sanz-Cervera JF, Williams RM. J. Am. Chem. Soc. 2002;124:2556–2559. doi: 10.1021/ja017425l. [DOI] [PubMed] [Google Scholar]
- 13.Greshock TJ, Grubbs AW, Williams RM. Tetrahedron. 2007;63:6124–6130. doi: 10.1016/j.tet.2007.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.a Greshock TJ, Grubbs AW, Tsukamoto S, Williams RM. Angew. Chem. Int. Ed. 2007;46:2262–2265. doi: 10.1002/anie.200604378. [DOI] [PubMed] [Google Scholar]; b Greshock TJ, Williams RM. Org. Lett. 2007;9:4255–4258. doi: 10.1021/ol701845t. [DOI] [PubMed] [Google Scholar]
- 15.a Miller KA, Welch TR, Greshock TJ, Ding Y, Sherman DH, Williams RM. J. Org. Chem. 2008;73:3116–3119. doi: 10.1021/jo800116y. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Ding Y, Greshock TJ, Miller KA, Sherman DH, Williams RM. Org. Lett. 2008;10:4863–4866. doi: 10.1021/ol8019633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller KA, Tsukamoto S, Williams RM. Nature Chemistry. 2009;1:63–68. doi: 10.1038/nchem.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, de Wet JR, Cavalcoli J, Li S, Greshock TJ, Miller KA, Finefield JM, Sunderhaus JD, McAfoos T, Tsukamoto S, Williams RM, Sherman DH. J. Am. Chem. Soc. 2010;132:12733–12740. doi: 10.1021/ja1049302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Calculated according to the method outlined in: Lambert JB, Shurvell HB, Lightner DA, Cooks RG. Organic Structural Spectroscopy. Prentice Hall; Upper Saddle River, NJ: 1998. pp. 447–448.
- 19.Whyte AC, Gloer JB, Wicklow DT, Dowd PF. J. Nat. Prod. 1996;59:1093–1095. doi: 10.1021/np960607m. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.