Summary
A dysregulated fear response is one of the hallmark clinical presentations of patients suffering from posttraumatic stress disorder (PTSD). These patients show overgeneralization of fear and in tandem an inability to inhibit fear responses in the presence of safety. Here, we summarize our recent findings using a conditional discrimination paradigm, which assesses safety signal processing (AX+/BX−) in combat and civilian PTSD populations. Overall, PTSD subjects demonstrate a lack of safety signal learning and an inability to modulate the fear responses with safety cues. We then review studies of the neurobiology of fear expression and inhibition in humans and non-humans, in order to provide a background for preliminary studies using reverse translation procedures in which the same AX+/BX− paradigm was used in rhesus macaques.
1. Clinical Phenomenology of Fear Inhibition to Safety Signals
Excessive fear and anxiety, along with an inability to overcome these emotions, are some of the defining characteristics of many anxiety disorders, such as phobias, panic disorder and posttraumatic stress disorder (PTSD). Several theorists (Amstedler et al., 2009; Friedman, 2010; Keane et al., 1985) have proposed that fear conditioning processes are involved in the etiology and maintenance of PTSD. According to the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; APA, 1994), the diagnosis of PTSD requires exposure to a traumatic event and a cluster of symptoms associated with that event (e.g., psychological and physiological reactions to trauma reminders, and avoidance of such reminders). Over-generalization of trauma-related stimuli or situations (i.e. an impaired ability to discriminate between danger and safety cues (Jovanovic et al., 2010a; Jovanovic et al., 2009) can lead to hyper-vigilance and exaggerated physiological responses that are part of the PTSD clinical presentation. For example, combat veterans with PTSD may experience uncontrollable fear in response to a previously learned fearful cue (e.g. helicopter sound), even when surrounded by many cues that should signal safety (e.g. the company of a loved one, far away from site of combat experience, etc.).
Conceptualizing PTSD within the framework of fear conditioning allows for the use laboratory paradigms, such as fear-potentiated startle, to better understand altered fear processing and to develop better treatments for this disorder. Although exposure to severe trauma is a defining criterion of PTSD, approximately 80% of individuals exposed to such trauma do not develop the disorder (Gillespie et al., 2009; Hoge et al., 2004), indicating that there are factors that increase vulnerability in some individuals. As noted above, most trauma victims show fear and related reactions after a traumatic event but these effects diminish over time in resilient individuals (Rothbaum and Davis, 2003). This resilience may reflect an intact ability to inhibit learned fear while the development of chronic PTSD in traumatized individuals that do not recover represents a failure of fear inhibition. The inability to suppress conditioned fear may be due to a complex gene x environment interaction between one‘s individual predisposition(s) and environmental factors, such as early life stress and the frequency, degree, and intensity of traumatic event(s).
1.1. Testing Safety Signal Learning in a Human Paradigm
Fear-potentiated startle is defined by the relative increase in the amplitude of the acoustic startle reflex when elicited in the presence of a conditioned stimulus (CS+) previously paired with an aversive stimulus (unconditioned stimulus, US) compared to startle amplitude elicited in the absence of that cue. Fear-potentiated startle can be demonstrated in animals and humans (Ameli et al., 2001; Davis et al., 1993; Grillon and Baas, 2003; Grillon and Davis, 1997). As a result, it provides an objective measure of the fear response and is an ideal paradigm for translational research. Fear inhibition involves learning of safety signals, i.e. the ability to discriminate between danger and safety cues and suppressing fear responses in the presence of safety cues. In the laboratory, fear inhibition is typically measured by pairing one cue with a fearful event and another that signals the absence of that event.
Myers and Davis (2004) developed a discrimination procedure in rats that allows for an independent evaluation of fear acquisition and inhibition of fear. The procedure, referred to as a conditional discrimination and abbreviated as AX+/BX−, is based on earlier learning theory experiments that were designed for other purposes (Wagner et al., 1968; Wagner and Rescorla, 1972). In this experiment, an aversive event is paired with a third stimulus, X, depending on the presence of either A or B. A evokes the fear response with training as the subject learns that A and X presented together predict the aversive unconditioned stimulus (US). B becomes inhibitory in that B presented with X predicts safety from the US. In a critical subsequent test trial, presentation of A and B together (AB) results in a reduced fear response compared to the response to A.
In translating this model to a human paradigm (Jovanovic et al., 2005), we only used compound stimuli in the experiment since we were concerned that people may see single cues as categorically different from two cues (i.e. a configural strategy - (Grillon and Ameli, 2001). To avoid such configural strategies, we presented A or B in compound with X designated by different shapes of different colors on a computer monitor with a “+” sign between the elements of the compound (see Figure 1 for description of the trials; Figure 2 shows a diagram of the entire experimental session used with our clinical populations).
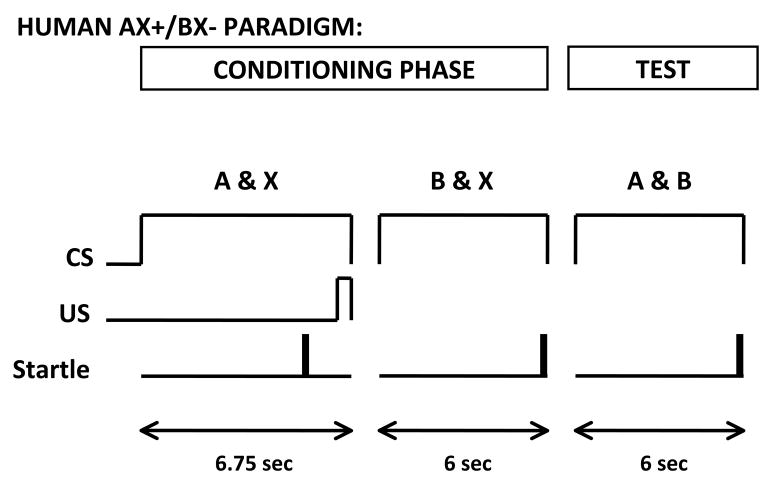
Figure 1.
Diagram of the trial design in the AX+/BX− human paradigm. CS=conditioned stimulus, US=unconditioned stimulus
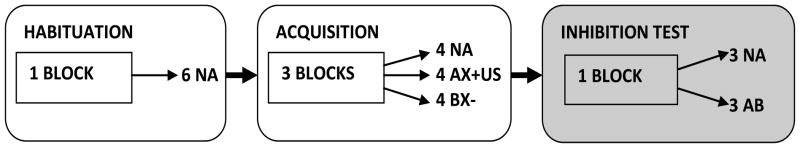
Figure 2.
Diagram of the AX+/BX− session in the human paradigm.
Furthermore, in order to assess awareness of experimental contingencies, we used a response keypad during training to assess expectation of the US on a trial-by-trial basis (Lovibond and Shanks, 2002). We used an aversive blast of air to the larynx as the US; this stimulus has produced robust fear conditioning in our studies without discouraging participation from patients (Jovanovic et al., 2009). We found that the aware subjects startled more in the presence of AX than to noise alone, and startled less in the presence of AB than AX, indicating they were both potentiating to the fear stimulus and inhibiting the fear response in the presence of safety signals (Jovanovic et al., 2005). To demonstrate that B was indeed a safety cue, we had to show that subjects could immediately transfer safety on a subsequent AB test trial, that is, that the decrement in startle to AB relative to AX was not an effect of learning that AB was non-reinforced. In order to ensure that this reduction is not due simply to the absence of X, we included a fourth trial type, AC, in which A was paired with a novel stimulus C (Jovanovic et al., 2005). In some studies, novelty has been found to reduce fear conditioned responses; this phenomenon is referred to as external inhibition (Pavlov, 1927). The novelty of combining AB could have reduced fear responses irrespective of the safety cue — therefore the AC trial controlled for this effect. We tested this by focusing on the first three presentations of AB and found that the subjects did indeed show immediate transfer on those trials and that this effect was not due to external inhibition, given that AB was significantly lower than AC (Jovanovic et al., 2005). Furthermore, the response pad data indicate that the subjects were transferring safety on a cognitive level as well: on the very first presentation of AB, the subjects immediately recognized B as a safety signal and dramatically reduced the level of danger expected with A when it was paired with B. Having validated the paradigm in healthy humans, we applied it to a clinical population of combat veterans with PTSD.
1.2. Safety Signal Learning and Transfer in Combat-Related PTSD
Very few studies in the literature have used Pavlovian fear conditioning procedures measured by potentiation of the startle in combat veterans. Studies that used instructed fear paradigms, in which the subjects are explicitly told which cues are associated with aversive stimuli, found increased potentiated startle responses in PTSD subjects compared to controls across the entire startle session (Grillon et al., 1998; Morgan et al., 1995). The authors argued that heightened contextual anxiety in the PTSD subjects, rather than more explicit cue fear, accounted for their overall increase in startle. Contextual conditioning occurs when a combination of various stimuli associated with the context results in a increased fear potentiated startle; in the human paradigm this was observed by a shift in the startle response to the noise alone trials in anticipation of the shock once the electrodes were attached to the subject‘s wrist (Ameli et al., 2001; Grillon et al., 1998). In cue conditioning, startle is increased in the presence of the CS+ relative to the noise alone trial; i.e. the difference between the CS+ and NA trials is the operation measure of fear of the specific CS+ (Davis et al., 1993). One study of Gulf war veterans (Grillon and Morgan, 1999) used a conditioning paradigm and found equivalent levels of fear potentiation to the CS+ in the PTSD and control groups. However, the PTSD subjects also potentiated to the CS−, whereas the controls did not. This result indicates that PTSD subjects may either have had learning deficits that precluded them from learning about which specific cue predicts the US, or they could not inhibit fear-potentiated startle to the context in the presence of a safety signal.
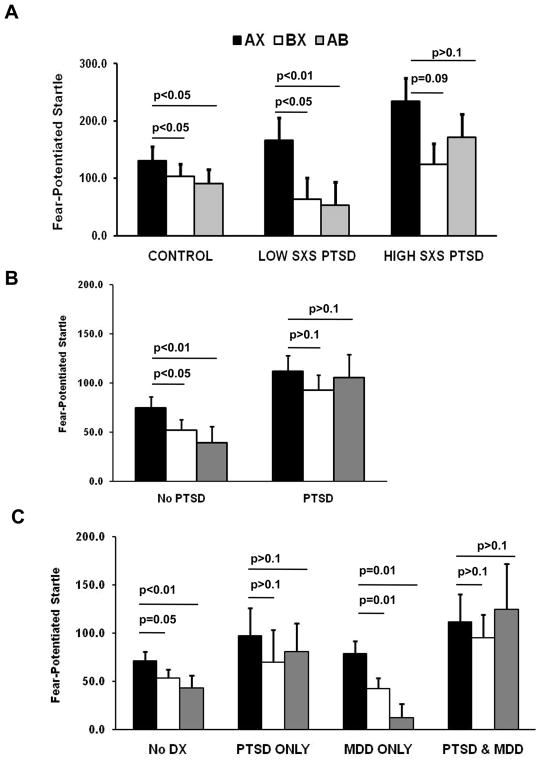
We examined inhibition of conditioned fear using startle as well as contingency awareness in order to differentiate between these two hypotheses. Subjects were instructed to respond on each CS trial by pressing one of three buttons: a button marked ‘+’ when they expected the US, a second button marked ‘-‘ when they did not expect the US, and a third button marked 0′ they were uncertain of the contingency. Our study of Vietnam veterans indicated that all subjects, regardless of PTSD status, showed significantly greater US expectancy to AX than to either BX or AB trials, indicating an intact ability to learn danger and safety signals on a cognitive level (Jovanovic et al., 2009). With the startle data, however, a current diagnosis of PTSD was related to impaired fear inhibition. Subjects with a history of PTSD, but with low current symptoms, responded similarly to healthy controls, showing both fear potentiation to the danger signal (AX+), discrimination between danger (AX+) and safety (BX−) trials, and transfer of safety on conditioned inhibition trials (AB). On the other hand, the PTSD patients with high symptoms showed strong fear potentiation to danger, but no significant difference in discrimination between danger and safety (AX+ vs. BX−), and did not transfer safety on the AB test trials (see Figure 3A), (Jovanovic et al., 2009). We replicated these findings in a sample of Croatian combat veterans with PTSD; in this population, which was culturally different and much younger than the Vietnam veteran cohort, PTSD patients also demonstrated a lack of fear inhibition on the AB transfer test on the startle measure but had normal safety signal contingency awareness (Jovanovic, et al., unpublished data).
Figure 3.
Mean Fear-Potentiated Startle on AX+, BX−, and AB trials across diagnostic groups from three studies. A. Fear-potentiated startle in Vietnam veterans with high symptoms of PTSD (n=13) and low symptoms of PTSD (n=14), and healthy age-matched controls (n=28). Figure adapted from (Jovanovic et al., 2009). B. Fear-potentiated startle in a traumatized civilian sample with PTSD (n=29) and without PTSD (n=61). Figure adapted from (Jovanovic et al., 2010b). C. Fear-potentiated startle in a traumatized civilian sample with comorbid PTSD and MDD (n=22), PTSD only (n=14), MDD only (n=17), and neither diagnosis (n=53). Figure adapted from (Jovanovic et al., 2010a). The Y-axis represents average percent startle potentiation for each trial type. This value was derived as follows: Percent Startle Potentiation = 100 x (startle magnitude during CS trials – NA startle)/(NA startle).
The discrepancy between the expectancy ratings and startle data was most apparent on the inhibition test trials. On the first AB test trial, all subjects indicated they did not expect to get an airblast: although this corresponded to the inhibition in startle observed in the low symptom patients, startle in the high symptom patients was still increased regardless of their expectancy. In our previous studies of fear-potentiated startle and US expectancy ratings, we found that startle and expectancy can be dissociated, especially with regard to safety cues (Jovanovic et al., 2006; Norrholm et al., 2006).
1.3. Safety Signal Learning and Transfer in Civilian-Related PTSD
Although the PTSD diagnosis was developed to better diagnose Vietnam veterans, PTSD is not limited to combat trauma. Studies of PTSD in civilian populations have demonstrated a wide range of traumatic events that lead to the development of the disorder, such as motor vehicle accidents (Jones et al., 2005), sexual assault (Rothbaum et al., 2001), including intimate-partner violence (Krause et al., 2006), and natural disasters, such as hurricanes (Galea et al., 2007). A growing number of studies (Breslau et al., 2004; Schwartz et al., 2005; Switzer et al., 1999) indicate that low income, African Americans living in urban environments are at especially high risk for both exposure to traumatic events and PTSD. We used the AX+/BX− paradigm in a highly traumatized civilian population from inner-city Atlanta (Jovanovic et al., 2010a; Jovanovic et al., 2010b). As was the case in our combat PTSD studies, individuals who met criteria for PTSD had higher potentiation of the startle response to safety signals than traumatized controls and, furthermore, neither did they demonstrate startle discrimination between danger (AX+) and safety signals (BX− ), nor did they transfer safety on the AB trial (Figure 3B) (Jovanovic et al., 2010b). Again we found that impaired safety signal learning was limited to the startle data; the contingency awareness data demonstrated that both groups learned to expect the US only on the reinforced trials (Jovanovic et al., 2010b). The discrepancy between the awareness data and the startle data further replicates that PTSD is associated with difficulty in appropriately responding to safety cues physiologically, even when there is cognitive awareness of safety. These laboratory data correspond to the clinical presentation of patients who respond to reminders with uncontrollable fear, even when they realize that they are no longer in danger.
It is important to note that, unlike the early studies by Grillon and colleagues (Morgan et al., 1995), we did not find group differences in baseline startle magnitudes to the noise probe in either the combat or civilian PTSD studies (Jovanovic et al., 2010a; Jovanovic et al., 2009). In our studies, baseline startle magnitude refers to the noise alone (NA) startle trials, i.e., those that are delivered in the session in the absence of CS presentation. Therefore, the effects of PTSD were only seen in cue specific fear-potentiated startle, i.e. the difference between startle in the presence of the CS and startle to the probe alone. Subtracting noise alone startle from the CS trials reduces the effect of individual differences in baseline startle response and focuses only on the effect of fear conditioning (as shown in Figure 3A, B, and C).
1.4. Safety Signal Learning in PTSD vs. MDD
PTSD and major depressive disorder (MDD) can develop independently, but are frequently comorbid in individuals who experience traumatic events. The National Comorbidity Survey of 1995 (Kessler et al., 1995) estimated that 48% of individuals who met criteria for PTSD also met criteria for MDD. Given the complexity of clinical symptoms, biomarkers of specific symptoms could provide a useful tool to discriminate between these disorders. Additionally, understanding physiological differences that may specifically underlie PTSD symptomatology is critical for elucidating differential neural circuitry in stress-related psychopathology.
Given the high comorbidity between PTSD and depression, we wanted to see whether safety signal learning deficits were specific to PTSD. In order to investigate whether the AX+/BX− paradigm can be used to differentiate between these disorders, we categorized our subjects into four groups: No diagnosis control, MDD only, PTSD only, and Comorbid PTSD and MDD (Jovanovic et al., 2010a). Patients with a PTSD diagnosis with or without comorbid depression did not demonstrate discrimination between danger and safety cues and did not inhibit fear on the safety transfer trials (Figure 3C); however, this finding may be secondary to the lack of discrimination. On the other hand, subjects with MDD without PTSD showed equivalent levels of discrimination and safety transfer as the no diagnosis controls. This study provides strong evidence that a reduced capacity for inhibition of fear-potentiated startle and impaired safety signal learning are specific to PTSD, as the MDD and control subjects did not show these deficits (see Table 1).
Table 1.
Summary of human clinical findings with the AX+/BX− paradigm.
| Fear Learning (AX+ > NA) | Safety Signal Learning (BX−< AX+) | Safety Signal Transfer (AB < AX+) | |
|---|---|---|---|
|
| |||
| Healthy volunteers | Yes | Yes | Yes |
| Combat PTSD | Yes | Yes/No | No |
| Civilian PTSD | Yes | No | No |
| Depression w PTSD | Yes | No | No |
| Depression w/o PTSD | Yes | Yes | Yes |
MDD in addition to PTSD appeared to exacerbate the impairment because this group had the greatest level of startle potentiation to AB (Figure 3C). However, this group also had much higher symptoms of PTSD than the PTSD only group. Because we controlled for level of trauma history by including it as a covariate in between-group analyses, the lack of safety signal expression may relate to the disorder rather than just trauma-related pathology (Breslau et al., 2000).
1.5. Discussion of Clinical Findings
The findings in our clinical populations strongly suggest that safety signal learning and transfer are specific markers of PTSD symptoms. These results support an increasing number of investigations that have found deficient safety signal processing in PTSD (Blechert et al., 2007; Bremner et al., 2005; Jovanovic et al., 2009; Lissek et al., 2009; Milad et al., 2008; Peri et al., 2000; Wessa and Flor, 2007). A small number of prospective studies suggest that impaired suppression of fear may be associated with vulnerability for PTSD (Guthrie and Bryant, 2006; Pole et al., 2009). A study of police academy cadets found that greater skin conductance responses to threatening stimuli and slower habituation prior to trauma exposure were predictive of PTSD symptom severity after trauma exposure (Pole et al., 2009).
A similar study with firefighters found that reduced extinction of fear conditioned responses examined before the index trauma explained almost a third of the symptoms in later traumatized individuals (Guthrie and Bryant, 2006). While fear acquisition refers to learning that something is dangerous, extinction is a mechanism by which an individual learns that something which was previously fear eliciting is no longer dangerous, i.e., that it is safe. In fear extinction paradigms, a stimulus that was previously paired with an aversive stimulus (the CS+) is then repeatedly presented without the US, so that it no longer elicits a fear response (Myers et al., 2006; Norrholm et al., 2006). A great deal of evidence indicates that, following extinction, fear to the CS is not erased, but instead the CS now also engages a parallel inhibitory process which competes with or suppresses fear elicited by that same CS (Myers and Davis, 2002). Because the CS now has both excitatory and inhibitory properties, it is difficult to tease apart whether a given experimental manipulation that affects extinction does so by affecting inhibition or excitation. Data from our recent study on extinction (Norrholm et al., 2010a) suggest that the early phase of extinction involves excitation, as is it predicted by the level of fear expression to the CS+ (i.e., the danger signal) at the end of acquisition. On the other hand, a high degree of fear remaining during late extinction is related to impaired inhibition, as it is best predicted by responses to the CS- (i.e. safety signal) at the end of acquisition (Norrholm et al., 2010a). Safety signal learning, such as can be measured using the AX+/BX− paradigm, appears to independently target inhibitory processes; however, extinction likely engages the same mechanisms.
Although extinction and safety signal learning (i.e. conditioned inhibition) are similar in many respects they also differ. Conditioned inhibition is defined by the fact that the safety cue will transfer inhibition to another excitatory cue (Rescorla, 1969). This is what we see in the AB transfer test after AX+/BX− training in rats, monkeys and humans. Thus the B cue (the safety signal) transfers inhibition to the A cue. In fear extinction paradigms, a stimulus that was previously paired with an aversive stimulus (the CS+) is then repeatedly presented without the US, so that it no longer elicits a fear response (Myers et al., 2006; Norrholm et al., 2006). Extinction is context specific because if the subject is tested in a context other than the context present during extinction training, the fear response returns (Bouton and Bolles, 1979). One might expect, therefore, that the ”extinction context” would act like a safety signal so that it would also inhibit fear to another excitatory cue. However, a direct test of this hypothesis found no evidence for such transfer (Bouton and King, 1983). Thus, although both conditioned inhibition and extinction in a particular context both involve inhibitory processes they behave differently vis-a-vis transfer of inhibition to another cue.
It is possible that a decreased ability to inhibit fear is a risk factor for developing the disorder and contributes to the maintenance of the disorder. In support of this hypothesis, a recent study demonstrated that genetic polymorphisms of the serotonin transporter were linked to a faster rate of fear-potentiated startle acquisition (Lonsdorf et al., 2009). On the other hand, our study, which examined current symptom severity in Vietnam veterans, suggests that fear inhibition may be a state-dependent rather than a trait phenomenon (Jovanovic et al., 2009). Another possibility is that some aspects of fear inhibition, such as the ability to learn safety cues, may be vulnerability traits (Guthrie and Bryant, 2006), whereas others, such as memory of safety cues, may be acquired as part of the disorder (Milad et al., 2008).
Conditioned inhibition focuses on active suppression of fear responses through learned safety signals; although fear itself may involve subcortical areas of the brain located primarily in the limbic circuitry, safety signals may require a cognitive, cortical component (Bremner et al., 2005; Weike et al., 2008). This premise is supported by data showing that awareness of the non-reinforcement contingency of the CS- is necessary for inhibiting fear responses on the AX+/BX− paradigm (Jovanovic et al., 2006), however Figures 2 and 3 clearly show that it is not sufficient. Furthermore, a recent study by Weike and colleagues examined the temporal domain of fear conditioning with a danger and safety signal and demonstrated that safety signal processing was slower than danger processing (Weike et al., 2008). The authors argued that top-down cognitive processes are involved in responses to safety signals, which accounts for the increased latency in response.
Impaired safety signal processing in PTSD is consistent with current neurocircuitry models of exaggerated amygdala activity and decreased prefrontal cortex activity in this disorder (Liberzon and Sripada, 2007; Rauch et al., 2006; Rauch et al., 2000; Shin et al., 2005). Recent studies have also indicated that inhibitory control from the dorsal anterior cingulate cortex is deficient in PTSD (Lanius et al., 2004; Shin et al., 2007); this brain area has also been found to be associated with fear expression in humans using fMRI (Milad et al., 2007a). Functional neuroimaging studies that have examined connectivity between prefrontal cortex and the amygdala have demonstrated impaired inhibition of the amygdala in PTSD (Lanius et al., 2004).
Although this review has focused on negative affect such as fear, impairments in processing positive affect may also be part of the PTSD syndrome. Appetitive reversal learning tasks and decision-making tasks such as the Iowa Gambling Task could be useful in evaluating patients with PTSD. It would be very interesting to know if the ventromedial prefrontal cortex is hypoactive in PTSD patients not only during aversive modulation/suppression of fear, but also under appetitive conditions, thus suggesting a general deficit in PFC function. Several studies have suggested that PTSD patients have impaired reward processing (Elman et al., 2009; Hopper et al., 2008; Sailer et al., 2008). In all of these studies, there appeared to be a “lack of motivation” element to the performance of PTSD patients, associated with alterations in the striatum. The Iowa Gambling Task might provide insights outside of just issues of motivation. In support of this, a recent fMRI study examining normal brain activity during the Iowa Gambling Task demonstrated activation in both the vmPFC as well as the ventral striatum (Li et al., 2010). In addition, Rogan et al., 2005 reported electrophysiological evidence in mice that a cue signaling the absence of footshock markedly activated field potentials in the caudate-putamen (Rogan et al., 2005).
A general deficit in inhibitory function is also supported by studies of altered response inhibition in PTSD. For example, in a simple Go/No Go task the subject is instructed to press a button when they see one character on the screen, but suppress the button-press under certain conditions (such as a change in background color) (Mueller et al., 2010). This task engages neural circuitry involved in cognitive control of action, such as the anterior cingulate cortex (ACC). Activation in the rostral part of the ACC has been found to be deficient during such cognitive control tasks in PTSD populations (Carrion et al., 2008; Falconer et al., 2008). Interestingly, a study of treatment responders and non-responders, found that PTSD patients who responded to CBT showed increases in volume of this area with structural MRI (Bryant et al., 2008). Interestingly, this same rostral area of the ACC in the vmPFC is activated during fear extinction recall (Milad et al., 2007b). Although a more general impairment in inhibitory processes mediated by the vmPFC may very well be an underlying abnormality associated with several psychiatric disorders, the deficits in inhibiting fear responses appear to be uniquely associated with re-experiencing and hyperarousal symptoms of PTSD (Norrholm and Jovanovic, 2010). Another benefit of specifically targeting inhibition of fear responses in PTSD research is that the groundwork in neurocircuitry for these systems has been greatly developed in animal models (Jovanovic and Ressler, 2010), and the field is well poised to discover novel approaches to prevention, intervention, and therapy of the disorder (Friedman, 2010; Norrholm et al., 2010b; Ressler et al., 2004; Rothbaum et al., 2008).
2. Reverse Translation of Fear Inhibition to Safety Signals
Fear conditioning offers a unique framework for translational studies, given that it can be modeled in animal experiments. Animal models of fear conditioning and fear inhibition provide useful tools for the study of these phenomena; therefore, it is essential to translate these models to human research. Moreover, the use of similar methods across different species allows for reverse translation, whereby a clinical phenomenon can be explored more rigorously in animal models. Because AX+/BX− lends itself to study in both human and non-human primates, we can examine the underlying neurobiology of safety signal learning by using targeted lesion studies in rhesus macaques and then testing them with this startle paradigm. We have begun to examine this hypothesis using rhesus macaque neonates that were tested in the AX+BX− paradigm as adults (manuscript in prep). These results have only appeared in abstract form (Kazama et al., 2010), thus only a summary will be provided here.
2.1. Testing Safety Signal Learning in a Monkey Model
The AX+/BX− paradigm was modified for non-human primates in several ways (Winslow et al., 2008). First, the startle training was conducted in three stages followed by the test session. In the first stage, the monkeys are fear conditioned to the A stimulus paired with the airblast US (danger cue). In the next stage, the safety cue (B) is added to the training session, so that the A cue is reinforced, and the B cue is never reinforced. In the third stage, X is added to the training session, so that A and X presented together are reinforced, and B and X presented together are never reinforced. The X cue was included for three reasons. First, Myers and Davis (2004) found that prior experience with compound cues reduced the probability that reduced fear on the critical AB transfer test would occur because the AB compound cue was novel. In other words, prior experience with compound cues reduced external inhibition. Secondly, because A and B are never presented prior to test, this reduces the probability that the AB compound will be viewed as a single cue. Third, because B is never put in compound with A prior to test, this reduces the amount of second order fear conditioning that can accrue to B, which would interfere learning that B is a safety signal. Another difference between the non-human primate and human paradigm, is the use of multimodal stimuli, including visual, auditory, and tactile stimuli.
In the final test, which occurred 48 hours after the last AX+/BX− training session, the monkeys were startled in the presence of each individual cue, as well as the AX and BX compounds, and the AB compound presented for the first time. As in the human paradigm, this procedure produces robust fear potentiation to AX, significant discrimination between AX and BX (safety signal learning), and significant reduction of fear on AB, i.e., safety signal transfer (Winslow et al., 2008).
2.2. Neural Underpinnings of Fear and Fear Inhibition
In humans, neuroimaging studies in normal subjects together with studies of patients suffering from PTSD have revealed several key brain areas involved in the emotional regulation of fear, with the primary focus being the hyper-excitation of the amygdala, for review see (Shin et al., 2006). Through its connections with hypothalamic and brainstem areas, the central nucleus of the amygdala has been repeatedly shown to mediate specific signs of fear and anxiety, including all aspects of the fight or flight response, such as increased heart-rate, cortisol, and an increase in acoustic startle response (Davis and Whalen, 2001).
Although activation of the amygdala is central to the fear response (Davis, 1992; LeDoux, 2000), other brain areas, including the hippocampus and prefrontal cortex, are thought to play a critical role in both learning safety signals and using those signals to functionally down-regulate the amygdala and reduce the fear response (Myers and Davis, 2007; Quirk and Beer, 2006; Sotres-Bayon and Quirk, 2010).
2.3. Role of the Amygdala in Safety Signal Learning
2.3.1. Human Amygdala
Many human neuroimaging studies have reported activation of the amygdala to be strongly correlated with various aspects of the fear response, including during fear acquisition (LaBar et al., 1998; Sehlmeyer et al., 2009) and extinction (Phelps et al., 2004), as well as the expression and recall of emotional memories (Hamann et al., 1999). Additionally, neuroimaging data suggest that hyperactivity in the amygdala is common for many anxiety disorders, including PTSD, social anxiety disorder, specific phobias and others (Lorberbaum et al., 2004; Phan et al., 2006; Rauch et al., 2000; Schienle et al., 2005; Shin et al., 2004; Stein et al., 2002; Straube et al., 2004; Tillfors et al., 2001; Veltman et al., 2004; Williams et al., 2006).
2.3.2. Non-Human Primate Amygdala
As is the case in humans, the monkey amygdala appears to be critical for processing emotional information; for review, see Kalin and Shelton (2003). Although several studies have shown that the monkey amygdala is critical for adaptively responding to threats (Aggleton and Passingham, 1981; Izquierdo and Murray, 2005; Kalin et al., 2001; Machado et al., 2009), until recently only one study had specifically looked at its role in the acquisition of learned fear (Antoniadis et al., 2007). Using a fear-potentiated startle paradigm adapted for monkeys, Antoniadis and colleagues (Antoniadis et al., 2009, 2007) found that selective ibotenic acid lesions of the amygdala blocked the acquisition, but not the expression of fear-potentiated startle, re-confirming the evidence seen in both humans and rats that the amygdala is critical for fear learning. Our own preliminary AX+/BX− data (manuscript in prep), supports the role of the amygdala in fear learning, however safety-signal learning and the flexible modulation of the fear response appear to be unaffected (see Table 2), suggesting that areas outside the amygdala may be more important for these abilities.
Table 2.
Summary of non-human primate findings with the AX+/BX− paradigm.
| Fear Learning (AX+ > NA) | Safety Signal Learning (BX−< AX+) | Safety Signal Transfer (AB < AX+) | |
|---|---|---|---|
|
| |||
| Control (sham lesions) | Yes | Yes | Yes |
| Amygdala lesions | No | Yes | Yes |
| OFC lesions | Yes | Yes | Yes |
| Hippocampus lesions | Yes | Yes | Yes |
| Hippocampus/striatum lesions | Yes/No | No | No |
2.4. Role of the Hippocampus in Safety Signal Learning
2.4.1. Human Hippocampus
As with the amygdala, several human neuroimaging studies have noted hippocampal activity during fear learning, as well as during the modulation of emotion (Sehlmeyer et al., 2009), and two studies reported activation during Pavlovian extinction (Knight et al., 2004; Milad et al., 2007b). Additionally, human studies examining the potential involvement of hippocampal dysfunction in PTSD have revealed several important findings. First, decreased hippocampal activity was found while PTSD patients experienced a symptomatic state (Bremner et al., 1997; Shin et al., 1999). Second, human patients with PTSD have shown decreased hippocampal volumes, compared to either trauma-exposed control subjects or trauma-unexposed healthy subjects (Bremner et al., 1995). However, in a volumetric study using identical twins in which one sibling developed PTSD and the other did not, Gilbertson and colleagues (Gilbertson et al., 2002) discovered that smaller hippocampal volumes were found in both twins, suggesting that small hippocampal volume may be a risk factor for developing PTSD. Third, fMRI studies have shown decreased hippocampal activation that correlated with PTSD symptom severity (Bremner et al., 1999; Shin et al., 1999). Conversely, Semple and colleagues (Semple et al., 2000) reported elevated hippocampal activation in PTSD patients during baseline conditions, without decreases in hippocampal activation during symptom provocation.
Additional studies, which have looked at cognitive deficits in PTSD patients, have found evidence of possible hippocampal dysfunction, even going as far as to posit that PTSD should be classified as a memory disorder; for review, see (Elzinga and Bremner, 2002). For instance, PTSD patients have been reported to have deficits in declarative memory, intrusive memories, fragmentation of memories, and trauma-related amnesia, all of which may indicate hippocampal dysfunction (Elzinga and Bremner, 2002).
2.4.2. Non-Human Primate Hippocampus
There have been very few monkey studies that have investigated the role of the hippocampus in the expression and regulation of emotion. First, studies investigating the role of the hippocampus in defensive behaviors have demonstrated that lesions of the hippocampus blunted emotional reactivity in response to threatening stimuli (Chudasama et al., 2008; Machado et al., 2009). However, the ability to acquire learned fear, measured with fear-potentiated startle, was not impacted by selective hippocampal damage (Antoniadis et al., 2007). We investigated the potential role of this structure in safety signal processing using adult non-human primates with early selective hippocampal damage tested on the AX+/BX− paradigm (Kazama et al., 2010). As seen in Table 2, with the exception of two animals that sustained inadvertent damage to both the dorsomedial and ventral striatum, the data showed that the hippocampus is not necessary for either fear or safety signal learning, or the down-regulation of the fear response during the safety transfer test.
2.5. Role of the Prefrontal Cortex in Safety Signal Learning
2.5.1. Human Prefrontal cortex
Recent reports from human studies suggest that the ventromedial and lateral aspects of the prefrontal cortex may play a role in down-regulating the amygdala (for review see (Davidson et al., 2002; Quirk and Beer, 2006), and that a dysfunction of the ventromedial prefrontal cortex may be responsible for some PTSD symptoms, particularly the inability to control the fear response long after the traumatic event has passed. Evidence supporting this conclusion includes: 1) Morphometric MRI studies showing decreased ventromedial prefrontal cortex volumes in PTSD patients (Fennema-Notestine et al., 2002; Rauch, 2003), 2) Decreased activation in ventromedial prefrontal cortex in PTSD patients during negative trauma-related narratives, combat pictures and/or sounds, fearful facial expressions, and performance of emotional Stroop interference tasks, as well as during a variety of symptom provocation paradigms (for review see (Shin et al., 2006). 3) Neuroimaging studies demonstrating that ventromedial prefrontal cortex activation is negatively correlated with PTSD symptom severity (Shin et al., 2004; Williams et al., 2006) and with the magnitude of the conditioned response during extinction in healthy subjects.
2.5.2. Non-Human Primate Prefrontal Cortex
It has long been known that damage to the ventral surface of the prefrontal cortex (orbital frontal cortex, OFC) causes striking deficits in social cognition (Butter et al., 1970). Some have hypothesized that these deficits are due in part to an inability to modulate emotion-related behaviors (Kalin et al., 2007). Monkeys with damage to the orbital frontal cortex show 1) abnormal aggressive behaviors (either increased or decreased) (Butter et al., 1970; Machado and Bachevalier, 2007, 2006); 2) loss of dominance status (Butter and Snyder, 1972); and 3) decreased threat-induced freezing and marginally decreased fearful responses to threatening stimuli (Kalin et al., 2007). Although these findings clearly show a deficit in emotion regulation, currently, no published studies have directly looked at the potential role of the orbital frontal cortex in fear learning or safety signal learning in non-human primates. We have begun to examine the potential role of various sub-regions of the OFC. Thus far, it appears that selective damage to Brodmann areas 11 & 13 of the OFC do not impact fear and safety-signal learning, or the flexible modulation of the fear response as measured by the AX+/BX− Paradigm (see Table 2, manuscript in prep). We are currently investigating other sub-regions of the OFC with the hopes of determining which areas are critical for these abilities.
3. General Discussion and Future Directions
Taken together, the clinical and animal model literature suggests several discreet but interconnected areas of the brain wherein a healthy fear response becomes uncontrollable. Furthermore, a reverse translational approach promises to shed further light on the complicated interaction between areas such as the amygdala, which appear to be important for fear learning, and that of other areas such as the hippocampus and prefrontal cortices, which may be important for regulating learned fears. Understanding how these areas contribute to healthy fear regulation will be critical for providing novel avenues of detection and intervention, some of which may be proactive, particularly in the case of combat-related PTSD.
3.1. Where Do We Go From Here With Clinical Research?
We suggest that one of the first studies to be conducted in clinical patients is to assess whether lack of safety signal learning represents a risk factor for PTSD. To examine this question, AX+/BX−, or similar experimental paradigms in which subjects discriminate between danger and safety cues, should be administered prior to trauma exposure. Such prospective studies can be done in populations at high risk for trauma exposure, such as soldiers, police, or firefighters. Such studies have been started with fear extinction paradigms (Guthrie and Bryant, 2006); however AX+/BX− is optimal for targeting safety signal learning. Alternatively, this paradigm might be administered in the immediate aftermath of trauma to predict who will develop PTSD after six months, when the response to trauma normalizes in most people. Although no conditioning studies of this nature have been done, a prospective study of traumatized women found that a heightened startle response is associated with later psychopathology (Griffin, 2008). Additionally, AX+/BX− can be tested before and after PTSD treatment to see whether an improvement in symptoms corresponds to improvements in safety signal learning. If this proves to be the case, than safety signal learning deficits may be useful both in identifying vulnerable individuals, and in tracking treatment progress.
Finally, functional neuroimaging studies such as fMRI can be conducted during the AX+/BX− paradigm to see which brain areas are active during safety signal learning as well as transfer. This may prove to be a challenging task, given that large numbers of trials are needed in order to capture the blood oxygen level dependent (BOLD) change. However, fear conditioning and fear extinction experiments have successfully been developed for use in fMRI (LaBar et al., 1998; Milad et al., 2009; Phelps et al., 2004; Rauch et al., 2006).
3.2. Where Do We Go From Here With Animal Models?
As the unpublished findings presented above are the first studies to examine the neural basis of safety-signal learning and conditioned inhibition using non-human primates, they should certainly be considered just a starting point for further research. First, while we have investigated the effects of early damage to the amygdala, hippocampus, and areas 11 and 13 of the orbital frontal cortex, there are several other areas of interest including the ventromedial prefrontal cortex, lateral prefrontal cortex, anterior cingulate cortex, and the striatum. Second, although the early lesion model is arguably more clinically relevant for examining neurodevelopmental psychopathology, the use of temporary inactivation would be more useful in answering questions while bypassing issues of re organization or recovery of function. Thus, the combined knowledge gained from both approaches would give us a more complete picture of the neural underpinnings of emotion regulation. Third, understanding how safety signal learning and the flexible modulation of the fear response develops in normal animals could be highly relevant for clinical work, and could be used to identify critical windows within development where treatment/intervention would be most effective. Currently, we are investigating other sub-regions of the orbital frontal cortex: ventromedial areas 14 and 25; lateral area 12; and middle areas 11, 13, and anterior insular cortex, using non-human primates with damage received in adulthood. Additionally, we will be investigating the normal development of these abilities using a modified version of the AX+/BX− fear-potentiated startle paradigm.
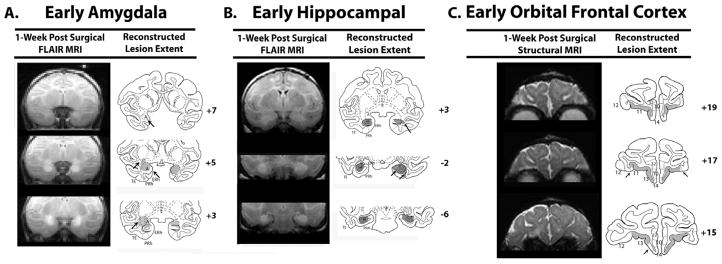
Figure 4.
Representative cases of each lesion group are represented by both the MR images showing either edema resulting from cell death after neurotoxic damage for (A) and (B), or loss of tissue due to aspiration for (C). Damage was then plotted onto an infant brain atlas as seen on the right hand columns for (A, B, & C). Arrows indicate either unintended damage or unintended sparing. Abbreviations: A=amygdala; ERh=entorhinal cortex; H=hippocampus; PRh=perirhinal cortex; TE, temporal cortical area and TH/TF=cytoarchitectonic fields of the parahippocampal gyrus as defined by von Bonin and Bailey (1947); Orbital Frontal Cortex Areas 10, 11, 12, 13, 14, & 25 as defined by Broadmann (1909).
Acknowledgments
The work described in this review has been supported by funding from the National Institutes of Mental Health, including the following grants: Kirschstein National Research Service Award Individual Fellowship 1F32 MH070129-01A2 (TJ), R37 MH47840 (MD), HD35471 and MH-58846 (JB) as well as grant MH086947 (JB and MD).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Passingham RE. Syndrome produce by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Ameli R, Ip C, Grillon C. Contextual fear-potentiated startle conditioning in humans: replication and extension. Psychophysiology. 2001;38:383–390. [PubMed] [Google Scholar]
- Amstedler AB, Nugent NR, Koenen KC. Genetics of PTSD: Fear conditioning as a model for future research. Psychiatric Annals. 2009;39:338–369. doi: 10.3928/00485713-20090526-01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. The nonhuman primate amygdala is necessary for the acquisition but not the retention of fear-potentiated startle. Biol Psychiatry. 2009;65:241–248. doi: 10.1016/j.biopsych.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: effects of chronic lesions in the rhesus monkey. J Neurosci. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blechert J, Michael T, Vriends N, Margraf J, Wilhelm FH. Fear conditioning in posttraumatic stress disorder: Evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behaviour Research and Therapy. 2007;45:2019–2033. doi: 10.1016/j.brat.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Contextual control of the extinction of conditioned fear. Learning and Motivation. 1979;10:455–466. [Google Scholar]
- Bouton ME, King DA. Contextual control of conditioned fear: tests for the associative value of the context. Journal of Experimental Psychology: Animal Behavior Processes. 1983;9:248–256. [PubMed] [Google Scholar]
- Bremner JD, Innis RB, Ng CK, Staib L, Duncan J, Bronen R, Zubal G, Rich D, Krystal JH, Dey H, Soufer R, Charney DS. PET measurement of cerebral metabolic correlates of yohimbine administration in posttraumatic stress disorder. Archives of General Psychiatry. 1997;54:246–256. doi: 10.1001/archpsyc.1997.01830150070011. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, Bronen RA, Seibyl JP, Southwick SM, Delaney RC, McCarthy G, Charney DS, Innis RB. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry. 1995;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Staib L, Kaloupek D, Southwick SM, Soufer R, Charney DS. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: A positron emission tomography study. Biological Psychiatry. 1999;45:806–816. doi: 10.1016/s0006-3223(98)00297-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS. Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychological Medicine. 2005;35:791–806. doi: 10.1017/s0033291704003290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N, Davis GC, Peterson EL, Schultz LR. A second look at comorbidity in victims of trauma: the posttraumatic stress disorder-major depression connection. Biological Psychiatry. 2000;48:902–909. doi: 10.1016/s0006-3223(00)00933-1. [DOI] [PubMed] [Google Scholar]
- Breslau N, Wilcox HC, Storr CL, Lucia VC, Anthony JC. Trauma exposure and posttraumatic stress disorder: a study of youths in urban America. Journal of Urban Health. 2004;81:530–544. doi: 10.1093/jurban/jth138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant RA, Felmingham K, Whitford TJ, Kemp A, Hughes G, Peduto A, Williams LM. Rostral anterior cingulate volume predicts treatment response to cognitive-behavioural therapy for posttraumatic stress disorder. Journal of Psychiatry & Neuroscience. 2008;33:142–146. [PMC free article] [PubMed] [Google Scholar]
- Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiol Exp (Wars) 1972;32:525–565. [PubMed] [Google Scholar]
- Butter CM, Snyder DR, McDonald JA. Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. J Comp Physiol Psychol. 1970;72:132–144. doi: 10.1037/h0029303. [DOI] [PubMed] [Google Scholar]
- Carrion VG, Garrett A, Menon V, Weems CF, Reiss AL. Posttraumatic stress symptoms and brain function during a response-inhibition task: an fMRI study in youth. Depression and Anxiety. 2008;25:514–526. doi: 10.1002/da.20346. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal Lesions in Rhesus Monkeys Disrupt Emotional Responses but Not Reinforcer Devaluation Effects. Biol Psychiatry. 2008 doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Lewis DA, Alloy LB, Amaral DG, Bush G, Cohen JD, Drevets WC, Farah MJ, Kagan J, McClelland JL, Nolen-Hoeksema S, Peterson BS. Neural and behavioral substrates of mood and mood regulation. Biological Psychiatry. 2002;52:478–502. doi: 10.1016/s0006-3223(02)01458-0. [DOI] [PubMed] [Google Scholar]
- Davis M. The role of the amygdala in fear and anxiety. Annual Review of Neuroscience. 1992;15:353–375. doi: 10.1146/annurev.ne.15.030192.002033. [DOI] [PubMed] [Google Scholar]
- Davis M, Falls WA, Campeau S, Kim M. Fear-potentiated startle: A neural and pharmacological analysis. Behavioral Brain Research. 1993;58:175–198. doi: 10.1016/0166-4328(93)90102-v. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Elman I, Lowen S, Frederick BB, Chi W, Becerra L, Pitman RK. Functional Neuroimaging of Reward Circuitry Responsivity to Monetary Gains and Losses in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66:1083–1090. doi: 10.1016/j.biopsych.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Bremner JD. Are the neural substrates of memory the final common pathway in PTSD? Journal of Affective Disorders. 2002;70:1–17. doi: 10.1016/s0165-0327(01)00351-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, Kemp AH, Gordon E, Peduto A, Olivieri G, Williams LM. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry & Neuroscience. 2008;33:413–422. [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry. 2002;52:1089–1101. doi: 10.1016/s0006-3223(02)01413-0. [DOI] [PubMed] [Google Scholar]
- Friedman MJ. Prevention of Psychiatric Problems among Military Personnel and Their Spouses. N Engl J Med. 2010;362:168–170. doi: 10.1056/NEJMe0911108. [DOI] [PubMed] [Google Scholar]
- Galea S, Brewin CR, Gruber M, Jones RT, King DW, King LA, McNally RJ, Ursano RJ, Petukhova M, Kessler RC. Exposure to Hurricane-Related Stressors and Mental Illness After Hurricane Katrina. Arch Gen Psychiatry. 2007;64:1427–1434. doi: 10.1001/archpsyc.64.12.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neuroscience. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie CF, Bradley RG, Mercer K, Smith AK, Conneely K, Gapen M, Weiss T, Schwartz AC, Cubells JF, Ressler KJ. Trauma Exposure and Stress-Related Disorders in Inner City Primary Care Patients. General Hospital Psychiatry. 2009;31:505–514. doi: 10.1016/j.genhosppsych.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin MG. A prospective assessment of auditory startle alterations in rape and physical assault survivors. Journal of Traumatic Stress. 2008;21:91–99. doi: 10.1002/jts.20300. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Conditioned inhibition of fear-potentiated startle and skin conductance in humans. Psychophysiology. 2001;38:807–815. [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–1579. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Effects of explicit and contextual cue conditioning following paired vs unpaired training. Psychophysiology. 1997;34:451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA., 3rd Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. Journal of Abnormal Psychology. 1999;108:134–142. doi: 10.1037//0021-843x.108.1.134. [DOI] [PubMed] [Google Scholar]
- Grillon C, Morgan CA, 3rd, Davis M, Southwick SM. Effects of experimental context and explicit threat cues on acoustic startle in Vietnam veterans with posttraumatic stress disorder. Biological Psychiatry. 1998;44:1027–1036. doi: 10.1016/s0006-3223(98)00034-1. [DOI] [PubMed] [Google Scholar]
- Guthrie RM, Bryant RA. Extinction learning before trauma and subsequent posttraumatic stress. Psychosomatic Medicine. 2006;68:307–311. doi: 10.1097/01.psy.0000208629.67653.cc. [DOI] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nature Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hoge CW, Castro CA, Messer SC, McGurk D, Cotting DI, Koffman RL. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. N Engl J Med. 2004;351:13–22. doi: 10.1056/NEJMoa040603. [DOI] [PubMed] [Google Scholar]
- Hopper JW, Pitman RK, Su Z, Heyman GM, Lasko NB, Macklin ML, Orr SP, Lukas SE, Elman I. Probing reward function in posttraumatic stress disorder: Expectancy and satisfaction with monetary gains and losses. Journal of Psychiatric Research. 2008;42:802–807. doi: 10.1016/j.jpsychires.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Opposing effects of amygdala and orbital prefrontal cortex lesions on the extinction of instrumental responding in macaque monkeys. Eur J Neurosci. 2005;22:2341–2346. doi: 10.1111/j.1460-9568.2005.04434.x. [DOI] [PubMed] [Google Scholar]
- Jones C, Harvey AG, Brewin CR. Traumatic brain injury, dissociation, and posttraumatic stress disorder in road traffic accident survivors. Journal of Traumatic Stress. 2005;18:181–191. doi: 10.1002/jts.20031. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Fear Potentiation and Fear Inhibition in a Human Fear-Potentiated Startle Paradigm. Biological Psychiatry. 2005;57:1559–1564. doi: 10.1016/j.biopsych.2005.02.025. [DOI] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010a;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Phifer JE, Weiss T, Davis M, Duncan E, Bradley B, Ressler K. Fear potentiation is associated with hypothalamic-pituitary-adrenal axis function in PTSD. Psychoneuroendocrinology. 2010b;35:846–857. doi: 10.1016/j.psyneuen.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, Davis M, Duncan EJ. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Res. 2009 doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Keyes M, Fiallos A, Jovanovic S, Myers KM, Davis M, Duncan EJ. Contingency awareness and fear inhibition in a human fear-potentiated startle paradigm. Behav Neurosci. 2006;120:995–1004. doi: 10.1037/0735-7044.120.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Ressler KJ. How the Neurocircuitry and Genetics of Fear Inhibition May Inform Our Understanding of PTSD. Am J Psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Ann NY Acad Sci. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biol Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama A, Heuer E, Davis M, Bachevalier J. Long-term effects of selective neonatal lesions of the amygdala, hippocampus, or areas 11 & 13 of the orbitofrontal cortex on fear regulation. Society for Neuroscience Meeting; San Diego, CA. 2010. [Google Scholar]
- Keane TM, Zimering RT, Caddell JM. A behavioral formulation of posttraumatic stress disorder in Vietnam veterans. The Behavior Therapist. 1985;8:9–12. [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Archives of General Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Knight DC, Smith CN, Cheng DT, Stein EA, Helmstetter FJ. Amygdala and hippocampal activity during acquisition and extinction of human fear conditioning. Cogn Affect Behav Neurosci. 2004;4:317–325. doi: 10.3758/cabn.4.3.317. [DOI] [PubMed] [Google Scholar]
- Krause ED, Kaltman S, Goodman L, Dutton MA. Role of distinct PTSD symptoms in intimate partner reabuse: A prospective study. Journal of Traumatic Stress. 2006;19:507–516. doi: 10.1002/jts.20136. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human Amygdala Activation during Conditioned Fear Acquisition and Extinction: a Mixed-Trial fMRI Study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Lanius RA, Williamson PC, Densmore M, Boksman K, Neufeld RW, Gati JS, Menon RS. The Nature of Traumatic Memories: A 4-T fMRI Functional Connectivity Analysis. Am J Psychiatry. 2004;161:36–44. doi: 10.1176/appi.ajp.161.1.36. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annual Review of Neuroscience. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Z-L, D’Argembeau A, Ng M, Bechara A. The Iowa Gambling Task in fMRI images. Human Brain Mapping. 2010;31:410–423. doi: 10.1002/hbm.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberzon I, Sripada CS. Progress in Brain Research. Elsevier; 2007. The functional neuroanatomy of PTSD: a critical review; pp. 151–169. [DOI] [PubMed] [Google Scholar]
- Lissek S, Rabin SJ, McDowell DJ, Dvir S, Bradford DE, Geraci M, Pine DS, Grillon C. Impaired discriminative fear-conditioning resulting from elevated fear responding to learned safety cues among individuals with panic disorder. Behaviour Research and Therapy. 2009;47:111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf TB, Weike AI, Nikamo P, Schalling M, Hamm AO, Öhman A. Genetic gating of human fear learning and extinction: Possible implications for gene-environment interaction in anxiety disorder. Psychological Science. 2009;20:198–206. doi: 10.1111/j.1467-9280.2009.02280.x. [DOI] [PubMed] [Google Scholar]
- Lorberbaum JP, Kose S, Johnson MR, Arana GW, Sullivan LK, Hamner MB, Ballenger JC, Lydiard RB, Brodrick PS, Bohning DE, George MS. Neural correlates of speech anticipatory anxiety in generalized social phobia. Neuroreport. 2004;15:2701–2705. [PubMed] [Google Scholar]
- Lovibond PF, Shanks DR. The role of awareness in Pavlovian conditioning: empirical evidence and theoretical implications. Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:3–26. [PubMed] [Google Scholar]
- Machado C, Kazama A, Bachevalier J. Impact of amygdala, orbital frontal or hippocampal lesions on threat avoidance and emotional reactivity in nonhuman primates. Emotion. 2009;9(2):147–163. doi: 10.1037/a0014539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. Eur J Neurosci. 2007;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: Results of a twin study. Journal of Psychiatric Research. 2008;42:515–520. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, Zeidan MA, Handwerger K, Orr SP, Rauch SL. Neurobiological Basis of Failure to Recall Extinction Memory in Posttraumatic Stress Disorder. Biological Psychiatry. 2009;66:1075–1082. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007a;62:1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007b;62:446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morgan CA, 3rd, Grillon C, Southwick SM, Davis M, Charney DS. Fear-potentiated startle in posttraumatic stress disorder. Biological Psychiatry. 1995;38:378–385. doi: 10.1016/0006-3223(94)00321-S. [DOI] [PubMed] [Google Scholar]
- Mueller SC, Maheu FS, Dozier M, Peloso E, Mandell D, Leibenluft E, Pine DS, Ernst M. Early-life stress is associated with impairment in cognitive control in adolescence: An fMRI study. Neuropsychologia. 2010;48:3037–3044. doi: 10.1016/j.neuropsychologia.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. AX+, BX− discrimination learning in the fear-potentiated startle paradigm: possible relevance to inhibitory fear learning in extinction. Learn Mem. 2004;11:464–475. doi: 10.1101/lm.74704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers KM, Davis M. Behavioral and Neural Analysis of Extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learning and Memory. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T. Tailoring therapeutic strategies for treating posttraumatic stress disorder symptom clusters. Neuropsychiatric Disease and Treatment. 2010;6:1–16. doi: 10.2147/NDT.S10951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, Ressler KJ. Fear Extinction in Traumatized Civilians with Posttraumatic Stress Disorder: Relation to Symptom Severity. Biological Psychiatry. 2010a doi: 10.1016/j.biopsych.2010.09.013. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrholm SD, Jovanovic T, Rothbaum BO, Davis M, Hasenkmap W, Crowe C, Duncan E, Leimbach L, Skelton K, Bradley B. The Opitimization of Research and Clinical Applications for Combat-related Posttraumatic Stress Disorder (PTSD): Progress Through Modern Translational Methodologies. Hauppage, NY: Nova Science; 2010b. [Google Scholar]
- Norrholm SD, Jovanovic T, Vervliet B, Myers KM, Davis M, Rothbaum BO, Duncan EJ. Conditioned fear extinction and reinstatement in a human fear-potentiated startle paradigm. Learning & Memory. 2006;13:681–685. doi: 10.1101/lm.393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned Reflexes. Oxford University Press; 1927. [Google Scholar]
- Peri T, Ben-Shakhar G, Orr SP, Shalev AY. Psychophysiologic assessment of aversive conditioning in posttraumatic stress disorder. Biological Psychiatry. 2000;47:512–519. doi: 10.1016/s0006-3223(99)00144-4. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Tancer ME. Association between amygdala hyperactivity to harsh faces and severity of social anxiety in generalized social phobia. Biological Psychiatry. 2006;59:424. doi: 10.1016/j.biopsych.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Pole N, Neylan TC, Otte C, Henn-Hasse C, Metzler TJ, Marmar CR. Prospective Prediction of Posttraumatic Stress Disorder Symptoms Using Fear Potentiated Auditory Startle Responses. Biological Psychiatry. 2009;65:235–240. doi: 10.1016/j.biopsych.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–727. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry Models of Posttraumatic Stress Disorder and Extinction: Human Neuroimaging Research-Past, Present, and Future. Biological Psychiatry. 2006;60:376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Segal E, Pitman RK, Carson MA, McMullin K, et al. Selectively reduced regional cortical volumes in post-traumatic stress disorder. Neuroreport. 2003;14:913–916. doi: 10.1097/01.wnr.0000071767.24455.10. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, Orr SP, Pitman RK. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, Hodges L, Davis M. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- Rogan MT, Leon KS, Perez DL, Kandel ER. Distinct Neural Signatures for Safety and Danger in the Amygdala and Striatum of the Mouse. Neuron. 2005;46:309–320. doi: 10.1016/j.neuron.2005.02.017. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Davis M. Applying learning principles to the treatment of post-trauma reactions. Annals of the New York Academy of Sciences. 2003;1008:112–121. doi: 10.1196/annals.1301.012. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Houry D, Heekin M, Leiner AS, Daugherty J, Smith LS, Gerardi M. A pilot study of an exposure-based intervention in the ED designed to prevent posttraumatic stress disorder. The American Journal of Emergency Medicine. 2008;26:326–330. doi: 10.1016/j.ajem.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Rothbaum BO, Kozak MJ, Foa EB, Whitaker DJ. Posttraumatic stress disorder in rape victims: autonomic habituation to auditory stimuli. Journal of Traumatic Stress. 2001;14:283–293. doi: 10.1023/A:1011160800958. [DOI] [PubMed] [Google Scholar]
- Sailer U, Robinson S, Fischmeister FPS, König D, Oppenauer C, Lueger-Schuster B, Moser E, Kryspin-Exner I, Bauer H. Altered reward processing in the nucleus accumbens and mesial prefrontal cortex of patients with posttraumatic stress disorder. Neuropsychologia. 2008;46:2836–2844. doi: 10.1016/j.neuropsychologia.2008.05.022. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neurosci Lett. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schwartz AC, Bradley RL, Sexton M, Sherry A, Ressler KJ. Posttraumatic Stress Disorder Among African Americans in an Inner City Mental Health Clinic. Psychiatr Serv. 2005;56:212–215. doi: 10.1176/appi.ps.56.2.212. [DOI] [PubMed] [Google Scholar]
- Sehlmeyer C, Schoning S, Zwitserlood P, Pfleiderer B, Kircher T, Arolt V, Konrad C. Human fear conditioning and extinction in neuroimaging: a systematic review. PLoS One. 2009;4:e5865. doi: 10.1371/journal.pone.0005865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semple WE, Goyer P, McCormick R, Donovan B, Muzic RF, Rugle L, McCutcheon K, Lewis C, Liebling D, Kowaliw S, Vapenik K, Semple MA, Flener CR, Schulz SC. Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared to controls. Psychiatry. 2000;63:65–74. doi: 10.1080/00332747.2000.11024895. [DOI] [PubMed] [Google Scholar]
- Shin LH, McNally RJ, Kosslyn SM, Thompson WL, Rauch SL, Alpert NM, Metzger LJ, Lasko NB, Orr SP, Pitman RK. Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related PTSD: A PET investigation. American Journal of Psychiatry. 1999;156:575–584. doi: 10.1176/ajp.156.4.575. [DOI] [PubMed] [Google Scholar]
- Shin LM, Bush G, Whalen PJ, Handwerger K, Cannistraro PA, Wright CI, Martis B, Macklin ML, Lasko NB, Orr SP, Pitman RK, Rauch SL. Dorsal anterior cingulate function in posttraumatic stress disorder. Journal of Traumatic Stress. 2007;20:701–712. doi: 10.1002/jts.20231. [DOI] [PubMed] [Google Scholar]
- Shin LM, Orr SP, Carson MA, Rauch SL, Macklin ML, Lasko NB, Peters PM, Metzger LJ, Dougherty DD, Cannistraro PA, Alpert NM, Fischman AJ, Pitman RK. Regional Cerebral Blood Flow in the Amygdala and Medial Prefrontal Cortex During Traumatic Imagery in Male and Female Vietnam Veterans With PTSD. Arch Gen Psychiatry. 2004;61:168–176. doi: 10.1001/archpsyc.61.2.168. [DOI] [PubMed] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, Medial Prefrontal Cortex, and Hippocampal Function in PTSD. Ann NY Acad Sci. 2006;1071:67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, Macklin ML, Lasko NB, Cavanagh SR, Krangel TS, Orr SP, Pitman RK, Whalen PJ, Rauch SL. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Quirk GJ. Prefrontal control of fear: more than just extinction. Curr Opin Neurobiol. 2010;20:231–235. doi: 10.1016/j.conb.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein MB, Goldin PR, Sareen J, Zorilla LTE, Brown GG. Increased amygdala activation to angry and contemptuous faces in generalized social phobia. Archives of General Psychiatry. 2002;59:1027–1034. doi: 10.1001/archpsyc.59.11.1027. [DOI] [PubMed] [Google Scholar]
- Straube T, Kolassa IT, Glauer M, Mentzel HJ, Miltner WH. Effect of task conditions on brain responses to threatening faces in social phobics: an event-related functional magnetic resonance imaging study. Biol Psychiatry. 2004;56:921–930. doi: 10.1016/j.biopsych.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Switzer GE, Dew MA, Thompson K, Goycoolea JM, Derricott T, Mullins SD. Posttraumatic Stress Disorder and Service Utilization Among Urban Mental Health Center Clients. Journal of Traumatic Stress. 1999;12:25–39. doi: 10.1023/A:1024738114428. [DOI] [PubMed] [Google Scholar]
- Tillfors M, Furmark T, Marteinsdottir I, Fischer H, Pissiota A, Langstrom B, Fredrikson M. Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry. 2001;158:1220–1226. doi: 10.1176/appi.ajp.158.8.1220. [DOI] [PubMed] [Google Scholar]
- Veltman DJ, Tuinebreijer WE, Winkelman D, Lammertsma AA, Witter MP, Dolan RJ, Emmelkamp PM. Neurophysiological correlates of habituation during exposure in spider phobia. Psychiatry Res. 2004;132:149–158. doi: 10.1016/j.pscychresns.2004.09.001. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Logan FA, Haberlandt K, Price T. Stimulus selection in animal discrimination learning. Journal of Experimental Psychology. 1968;76:171–180. doi: 10.1037/h0025414. [DOI] [PubMed] [Google Scholar]
- Wagner AR, Rescorla RA. Inhibition in Pavlovian conditioning: application of a theory. In: Boakes RA, Halliday MS, editors. Inhibition and Learning. London: Academic Press; 1972. pp. 301–336. [Google Scholar]
- Weike AI, Schupp HT, Hamm AO. In dubio pro defensio: Initial activation of conditioned fear is not cue specific. Behavioral Neuroscience. 2008;122:685–696. doi: 10.1037/0735-7044.122.3.685. [DOI] [PubMed] [Google Scholar]
- Wessa M, Flor H. Failure of Extinction of Fear Responses in Posttraumatic Stress Disorder: Evidence From Second-Order Conditioning. Am J Psychiatry. 2007;164:1684–1692. doi: 10.1176/appi.ajp.2007.07030525. [DOI] [PubMed] [Google Scholar]
- Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A, Gordon E, Bryant RA. Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. NeuroImage. 2006;29:347–357. doi: 10.1016/j.neuroimage.2005.03.047. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Noble PL, Davis M. AX+/BX− discrimination learning in the fear-potentiated startle paradigm in monkeys. Learn Mem. 2008;15:63–66. doi: 10.1101/lm.843308. [DOI] [PubMed] [Google Scholar]