Abstract
E. coli UvrD is a superfamily 1 (SF1) DNA helicase and single stranded (ss) DNA translocase that functions in DNA repair, plasmid replication and as an anti-recombinase by removing RecA protein from ssDNA. UvrD couples ATP binding and hydrolysis to unwind double-stranded DNA (dsDNA) and translocate along ssDNA with 3′ to 5′ directionality. Although a UvrD monomer is able to translocate along ssDNA rapidly and processively, DNA helicase activity in vitro requires a minimum of a UvrD dimer. Previous crystal structures of UvrD bound to a ss-duplex DNA junction show that its 2B sub-domain exists in a “closed” state and interacts with the duplex DNA. Here we report a crystal structure of an apo form of UvrD in which the 2B sub-domain is in an “open” state that differs by a ~160° rotation of the 2B sub-domain. To study the rotational conformational states of the 2B sub-domain in various ligation states, a series of double cysteine UvrD mutants were constructed and labeled with fluorophores such that rotation of the 2B sub-domain results in changes in fluorescence resonance energy transfer (FRET). These studies show that the open and closed forms can interconvert in solution with low salt favoring the closed conformation and high salt favoring the open conformation in the absence of DNA. Binding of UvrD to DNA as well as ATP binding and hydrolysis also affect the rotational conformational state of the 2B sub-domain suggesting that 2B sub-domain rotation is coupled to the function of this nucleic acid motor enzyme.
Keywords: DNA repair, fluorescence, FRET, crystal structure, allostery
Introduction
DNA helicases are nucleoside triphosphate hydrolyzing motor proteins that function in all aspects of DNA replication, recombination and repair that require formation of single stranded (ss) DNA intermediates.1; 2 Structurally, these enzymes can generally be grouped as either hexameric3 or non-hexameric,4 with the latter class having examples of functional monomers, dimers or filamentous oligomers.2; 5 These enzymes are also classified in different families or superfamilies based on conserved regions of primary structure,6 with the superfamily 1 and 2 (SF1 and SF2) classes being the largest.
E. coli UvrD is a non-hexameric SF1 helicase and ss-DNA translocase that functions in methyl-directed mismatch repair7 and nucleotide excision repair8 of DNA, reversal of replication forks9; 10 and replication of some plasmids.11 UvrD also functions to remove proteins from DNA12; 13 and as an anti-recombinase by displacing RecA filaments from ss-DNA intermediates, thus preventing homologous recombination.14; 15 In fact, these enzymes generally display multiple functions, including unwinding and strand separation of duplex (ds) DNA, translocation along ss-DNA, as well as protein displacement from DNA.16; 17 Thus it is likely that the helicase activity of these enzymes may not be their only function in vivo. In many cases, the different activities of these enzymes require different forms of the enzyme. For example, the monomeric forms of the SF1 enzymes, E. coli Rep, E. coli UvrD and B. stearothermophilus PcrA, are all capable of rapid, highly processive and directional (3′ to 5′) translocation along ssDNA,18; 19; 20 yet the monomeric forms are unable to unwind duplex DNA by themselves in vitro.21; 22; 23; 24; 25 Either some self-assembly or interaction with an accessory factor is required to activate its helicase activity.4; 26
The three SF1 helicases, B. stearothermophilus PcrA,27; 28 E. coli Rep,29 and E. coli UvrD30 are structurally similar (see Figure 1), possessing a two domain structure with each domain (1 and 2) being composed of two sub-domains (1A, 1B, 2A and 2B). ATP analogs bind between the 1A and 2A sub-domains and ssDNA binds at the junction above the 1A and 2A sub-domains in the orientation shown in Figure 1. Interestingly, two conformations of the Rep monomer (“closed” and “open” forms) were observed in the asymmetric unit of the Rep-ssDNA crystals29. The major difference between the two forms is the rotational configuration of the 2B sub-domain which can rotate by ~130 degrees about a hinge region connecting the 2B and 2A sub-domains. The apo27 and 3′-ss-ds DNA junction bound28 forms of the PcrA monomer also showed a large rotation of the 2B sub-domain by ~160 degrees, with the apo enzyme having an open conformation and the DNA bound form being in a closed conformation with the 2B sub-domain contacting the duplex DNA. Similarly, structures of a UvrD monomer bound to a 3′-ss-ds DNA junction also show a closed orientation with the 2B sub-domain contacting the duplex region.30 Single molecule fluorescence resonance energy transfer (FRET) studies have shown that the 2B sub-domain of a Rep monomer is primarily in a closed conformation when bound to a 3′-ss-ds DNA junction.31
Figure 1. Crystal structures of apo UvrD and a UvrD-DNA complex show that the 2B sub-domain can rotate to either an “open” or “closed” conformation.
Ribbon diagram representations of crystal structures of (a) apo UvrD (this report, PDB# 3LFU) showing the 2B sub-domain in an “open” conformation, and (b) UvrD bound to a 3′-ssDNA-duplex junction34 (PDB# 2IS1) showing the 2B sub-domain in a “closed” conformation. Sub-domains 1A, 1B, 2A, and 2B are colored yellow, green, red, and blue, respectively. DNA in panel (b) is shown as a tubular model. The DNA strand with the 3′-single stranded DNA extension is colored light-pink and the partner strand cyan. ATP analog AMPPNP is shown in sticks and colored as magenta. (c) The sub-domains 1A, 1B, and 2A of the two structures in panels (a) and (b) are superimposed showing the difference in the rotational conformational states of the 2B sub-domains. Sub-domains 1A, 1B, and 2A in UvrD structures are colored grey and sub-domain 2B is colored green in the closed conformation and blue in the open conformation, respectively.
The function of the 2B sub-domain is the subject of some debate. Based on the crystal structures of monomers of UvrD and PcrA bound to the 3′-ss-ds-DNA junctions,28; 30 it was suggested that the monomeric forms of these enzymes have processive helicase activity and that the interactions of the 2B sub-domain with the duplex region of the DNA junction is essential for helicase activity. However, there is substantial evidence that the monomeric forms of PcrA, UvrD and Rep are not processive helicases.19; 23; 25; 32 In fact, there is no evidence that the monomeric form of Rep can even initiate partial DNA unwinding.21 Furthermore, removal of the Rep 2B sub-domain to form RepΔ2B monomer activates the helicase activity of the monomer indicating that the 2B sub-domain in Rep is auto-inhibitory for monomeric helicase activity.19 This suggests that the extensive 2B sub-domain-duplex DNA contacts inferred from the crystal structures of PcrA and UvrD monomers may not be important for their helicase activities. Rather, the 2B sub-domain may play a role in regulating the various activities of these enzymes through self-assembly or interactions with accessory proteins.4 In fact, the rotational conformation of the 2B sub-domain may play a role in regulating its auto-inhibitory and other functions.
Here we report a crystal structure of the apo form of UvrD that has its 2B sub-domain in an open conformation indicating that the 2B sub-domain of UvrD, just as for Rep and PcrA, is capable of undergoing a similar large rotational conformational change. Using ensemble fluorescence resonance energy transfer (FRET) studies, we show that rotational conformational state of the 2B sub-domain of UvrD can be influenced by binding of ligands (e.g., nucleotides, DNA) and changes in solution conditions. The fact that rotational motion of the 2B sub-domain is coupled to the binding of nucleotides and DNA suggests that it is likely to be functionally important for some activities of this enzyme and/or their regulation.
Results
Apo UvrD structure
Crystal structures of a monomer of E. coli UvrDΔ40, UvrD with the last 40 amino acids deleted from its C-terminus, bound to a series of short 3′-ss-ds-DNA junctions have been reported by Lee and Yang.30 In all of these structures, one of which is depicted in Figure 1b, the 2B sub-domain (blue) of UvrD is in a “closed” orientation, relative to the 1B sub-domain (green). In this orientation, the 2B sub-domain contacts the 1B sub-domain, and these two sub-domains bind the duplex region of the DNA junction. This orientation is similar to the closed orientation observed in one of the structures of E. coli Rep bound to ssDNA ((dT)16).29 In the “open” structure of Rep also bound to (dT)16, the 2B sub-domain has rotated by ~130° about a hinge region connected to the 2A sub-domain and there is no interaction between the 1B and 2B sub-domains.29
We have determined a crystal structure to 1.8 Å of an apo form of a UvrDΔ73 monomer (residues 1 to 647) in which 73 amino acids have been deleted from its C-terminus. This UvrD mutant was used because this region of the C-terminus is not visible in the crystal structures of Rep and thus is likely unstructured and/or flexible. Deletion of these 73 amino acids also increased its solubility significantly. This mutant retains ATPase activity, monomeric ss-DNA translocation activity (158 ± 8 nucleotides/s compared to wild type monomer (191 ± 3 nucleotides/s20) as well as helicase activity (~30% of wild type with UvrDΔ73 in large molar excess over DNA).
In the UvrDΔ73 apo structure, the conformations of the 1A and 2A sub-domains are nearly identical to those in the open form of PcrA (1PJR), the open Rep-ssDNA, and the UvrD-DNA complexes (2IS1), with an rmsd of 1.3 Å among the 360 Cα atoms of the 1A and 2A sub-domains of the open and closed UvrD forms (Figure 1c). There is a small rotation of the 1B sub-domain when comparing the open and closed UvrD structures resulting in movement of the apical part (residue 360) by 8 Å.
The 2B sub-domain in the apo UvrD structure is in an “open” conformation, relative to the other three sub-domains (1A, 2A and 1B) (Figures 1a). It rotates by nearly 160 degrees, moving from one side of the 1B sub-domain in the open apo form to the opposite interface of the 1B sub-domain in the closed DNA-bound form (Figures 1c). The magnitude of this 2B swiveling is very similar to that observed upon comparing the apo PcrA structure27 and its DNA complexes.28 Importantly, the ssDNA- and dsDNA-binding sites are partially blocked in the UvrD and PcrA open forms (see Discussion). A large swiveling of the 2B sub-domain of an SF1 helicase was first observed in the crystal structure of two monomers of Rep bound to ssDNA ((dT)16);29 however, the magnitude of the Rep 2B sub-domain swiveling is smaller (~130°) and the 2B sub-domain does not block ssDNA binding to the 2A sub-domain, as is the case in the UvrD and PcrA open conformations. Overall, three different rotational conformations of the 2B sub-domain have been observed in various crystal structures of Rep, UvrD and PcrA. These include the open and closed forms of UvrD and PcrA which are stabilized by extensive contacts with the 1B sub-domain and thus may represent stable conformations. The third is the open ssDNA-bound Rep form in which the ssDNA binding site is fully accessible. To examine the ttransitions among these conformational states further, we wished to probe the conformational changes of the 2B sub-domain in solution and upon binding different ligands.
Double UvrD mutant construction and fluorophore labeling
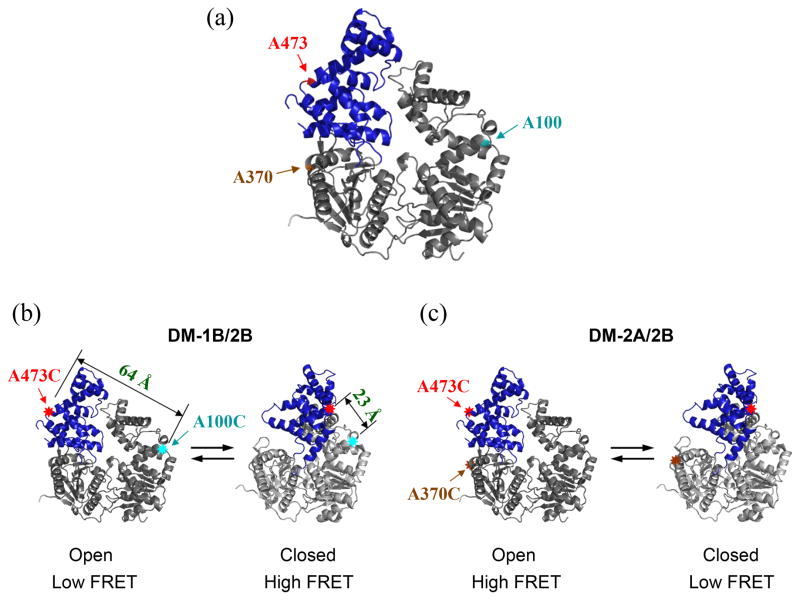
We used fluorescence resonance energy transfer (FRET) to study 2B sub-domain rotation in solution. For this, we mutated three different amino acids (two at a time) in UvrD to Cys for subsequent labeling with donor and acceptor fluorophores. One mutated position (A473C) is within the 2B sub-domain while the other positions are located in either the 1B sub-domain (A100C) or the 2A sub-domain (A370C) (Figure 2a). Each position is exposed on the surface of UvrD, enabling convenient fluorescent labeling. Two different double Cys mutants were constructed, UvrDΔCys[A100C, A473C] (referred to as DM-1B/2B) (Figure 2b) and UvrDΔCys[A370C, A473C] (referred to as DM-2A/2B) (Figure 2c). These mutations were made within an otherwise Cys-less UvrD protein, UvrDΔCys (see Materials and Methods). These positions are highlighted in the closed and open conformations of the UvrD structure in Figure 2. DM-1B/2B was designed to yield a high FRET signal when 2B is in its closed conformation and a low FRET signal when 2B is in its open conformation (Figure 2b), whereas DM-2A/2B was designed to observe a low FRET signal with 2B in its closed conformation and a high FRET signal with 2B in its open conformation (Figure 2c).
Figure 2. Design and fluorescent labeling of the UvrD mutants used to monitor the 2B sub-domain rotation by FRET.
Two different double Cys UvrD mutants, UvrDΔCys[A100C, A473C] (DM-1B/2B) and UvrDΔCys[A370C, A473C] (DM-2A/2B) were made by the substitution of alanine with cysteine at the indicated positions. (a) The positions of the three Ala to Cys mutations are indicated in the apo UvrD structure. (b) UvrD(DM-1B/2B) labeled with a mixture of Cy3 and Cy5 should show high FRET in the closed form and low FRET in the open form. The distances between A100C and A473C in the two conformations are also indicated. (c) UvrD(DM-2A/2B) labeled with a mixture of Cy3 and Cy5 should show low FRET in the closed form and high FRET in the open form.
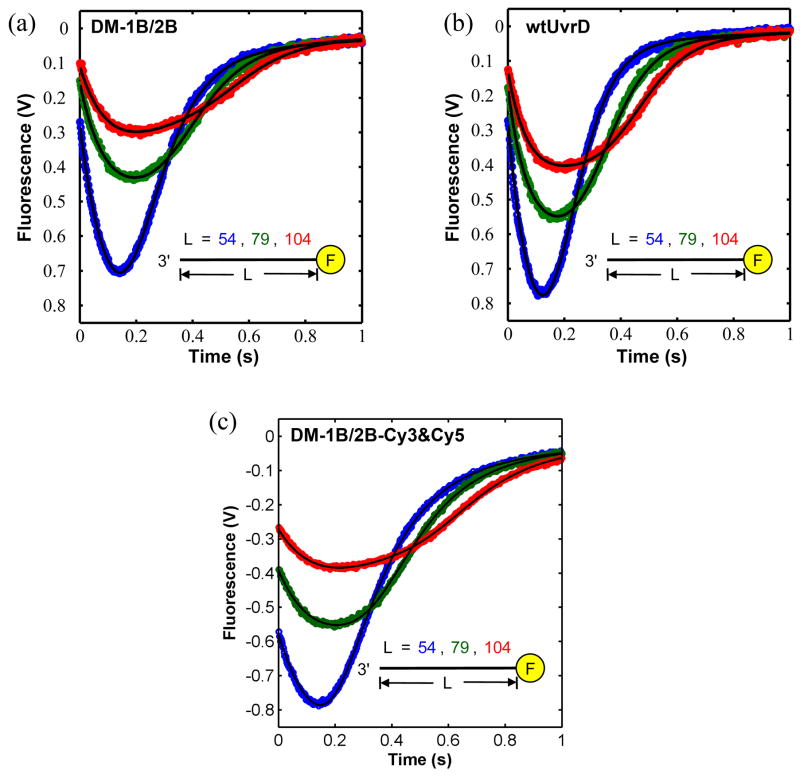
UvrD(DM-1B/2B) and UvrD(DM-2A/2B) were labeled stochastically with an equimolar mixture of Cy3 (donor) and Cy5 (acceptor) maleimides (see supplemental materials). The average labeling efficiencies per protein were 85% for Cy3 and 92% for Cy5, respectively. The ssDNA-stimulated steady-state ATPase activity was reduced by about 25% for the labeled UvrD mutants compared to wtUvrD (data not shown). In addition, DNA unwinding activities of the labeled mutants were within 22% of wtUvrD (data not shown). The kinetics of ss-DNA translocation of the monomeric UvrD double mutants were similar to those of wtUvrD monomers. Figure 3a, 3b and 3c show the time courses of ssDNA translocation for unlabeled UvrD(DM-1B/2B), wtUvrD, and double-labeled UvrD(DM-1B/2B) respectively, for a series of different ssDNA lengths ((dT)54, (dT)79, and (dT)104) labeled with fluorescein at the 5′ end. These single round experiments monitor quenching of fluorescein fluorescence upon arrival of a translocating UvrD monomer at the 5′ end followed by UvrD dissociation from DNA and trapping by heparin.20; 33 The macroscopic translocation rate determined from a global non-linear least squares (NLLS) analysis of the unlabeled UvrD (DM-1B/2B) monomer time courses is 182 ± 8 nucleotides/s, which is the same within error as the rate for the wt UvrD monomer (191 ± 3 nucleotides/s) under the same conditions.20 The translocation rate for the double-labeled UvrD (DM-1B/2B) monomer is slightly slower (155 ± 10 nucleotides/s).
Figure 3. ssDNA translocation kinetics of UvrD(DM-1B/2B) and wtUvrD monomers.
Kinetics of UvrD monomer translocation along (dT)54 (blue), (dT)79 (green) and (dT)104 (red), were examined by monitoring the decrease in fluorescein fluorescence accompanying the arrival of UvrD at the 5′-end of fluorescein labeled ssDNA, followed by an increase in fluorescence upon dissociation of the UvrD translocase. ss-DNA translocation kinetics of (a)- UvrD(DM-1B/2B) monomer (not labeled with Cy3 and Cy5), (b)- wtUvrD monomer, and (c)- UvrD(DM-1B/2B) monomer (labeled with Cy3 and Cy5). The continuous lines are simulated time courses using the best-fit parameters from a global NLLS analysis of the data as described.20 UvrD (25 nM, postmixing) was preincubated with 5′-F-(dT)L (50 nM, postmixing) in buffer T20 and translocation was initiated with the addition of ATP (0.5 mM), MgCl2 (2 mM), and heparin (4 mg/ml) (all final concentration) at 25 °C.
The 2B sub-domain rotational conformation is sensitive to salt concentration and type
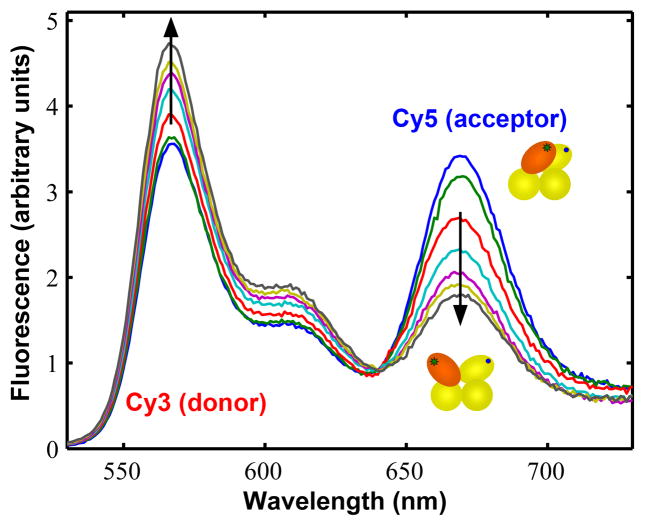
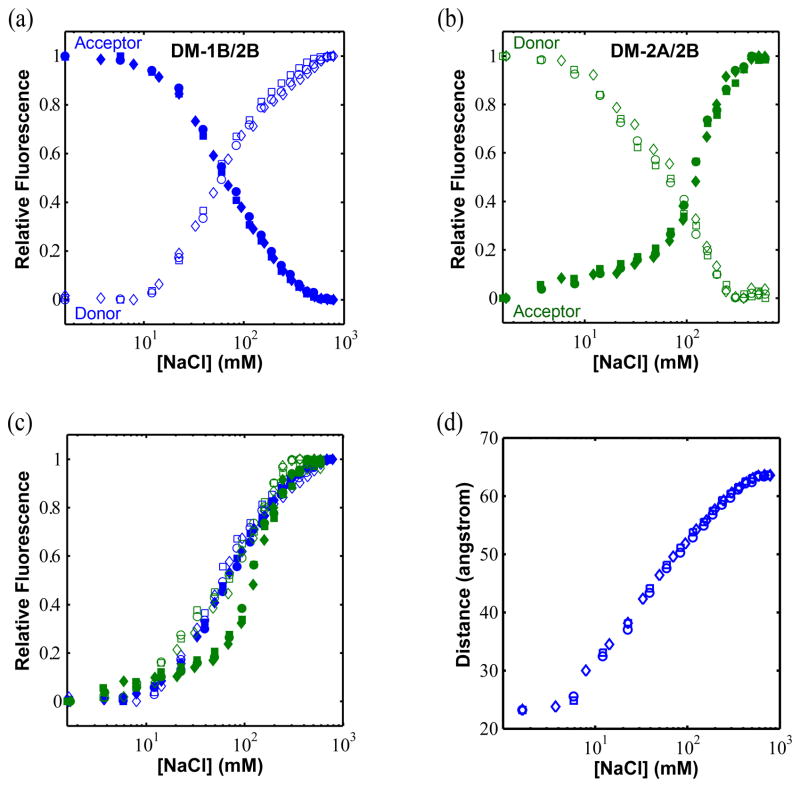
Superposition of the apo UvrD crystal structure reported here and the UvrD-DNA crystal structures34 (Figure 1c) indicates that the 2B sub-domain can undergo a ~160° rotation about a hinge region connected to the 2A sub-domain. To determine whether the 2B sub-domain orientation could be influenced by solution conditions we first examined the relative fluorescence of Cy3 donor and Cy5 acceptor in ensemble FRET studies of Cy3/Cy5 labeled UvrD mutants DM-1B/2B and DM-2A/2B as a function of [NaCl] in the absence of DNA. These experiments were carried out in 10 mM Tris-HCl (pH 8.3 at 25 °C), 20%(v/v) glycerol (100 μg/ml BSA was included to reduce protein sticking to the cuvette). Figure 4 shows fluorescence emission spectra for the Cy3/Cy5 labeled UvrD DM-1B/2B mutant at different NaCl concentrations from 20 mM to 600 mM. In these experiments, the Cy3 donor fluorescence was excited at 515 nm and both donor (Cy3) and acceptor (Cy5) fluorescence emission were monitored. For the DM-1B/2B mutant, the donor fluorescence emission intensity increases while the acceptor fluorescence intensity decreases concomitantly as the [NaCl] is increased as shown in Figure 5a. Since we show below that these fluorescence changes are due only to FRET, the decrease in FRET observed for the DM-1B/2B mutant indicates that the 2B sub-domain moves from a relatively closed orientation to a more open conformation as the [NaCl] increases. Similar experiments performed with the Cy3/Cy5 labeled DM-2A/2B mutant (Figure 5b) shows the opposite effect, namely a decrease in donor fluorescence intensity and an increase in acceptor fluorescence intensity as the [NaCl] is increased which is expected for this mutant if the 2B sub-domain swivels open as the [NaCl] increases. Thus, the two UvrD mutants, DM-1B/2B and DM-2A/2B give qualitatively consistent results indicating that the 2B sub-domain of a UvrD monomer is able to freely rotate in solution and that it moves from a closed to a more open conformation as the [NaCl] increases.
Figure 4. Cy3/Cy5 FRET changes accompany rotation of the 2B sub-domain of UvrD(DM-1B/2B).
Cy3 (donor) and Cy5 (acceptor) labeled UvrD(DM-1B/2B) (20 nM) in 10 mM Tris-HCl (pH 8.3 at 25 °C) and 20% (v/v) glycerol was titrated with concentrated NaCl in the same buffer. The fluorescence emission spectra of labeled UvrD(DM-1B/2B) (excitation at 515 nm) are plotted at several [NaCl]. The arrows indicate the increase in fluorescence intensity of the Cy3 (donor) and the concomitant decrease in fluorescence of the Cy5 (acceptor) with increasing [NaCl], demonstrating the transition from a closed 2B sub-domain conformation to an open conformation. The cartoons showing the relative orientation of the 4 sub-domains are colored yellow for sub-domains 1A, 1B, and 2A and orange for sub-domain 2B.
Figure 5. NaCl concentration influences the 2B sub-domain rotational conformation.
Normalized fluorescence changes of donor (open symbols, excitation at 515 nm/emission at 566 nm) and acceptor (filled symbols, excitation at 515 nm/emission at 670 nm) for (a) UvrD(DM-1B/2B) labeled with Cy3/Cy5 and (b) UvrD(DM-2A/2B) labeled with Cy3/Cy5. For each, three experimental data sets are shown (squares, circles, and diamonds). (c) Replot of the donor and acceptor signals for DM-1B/2B and DM-2A/2B so that all normalized donor and acceptor changes are shown as increasing from zero to one. (d) The FRET changes were used to calculate distances between the FRET pairs for UvrD(DM-1B/2B) as a function of [NaCl]. The distances between the two alanines (A100 and A473) in the crystal structures of the open (apo) and closed34 conformations shown in Figure 1 were used to calibrate the end points of the titrations.
To determine whether the fluorescence changes plotted in Figure 5a and 5b are due solely to FRET changes, we compared all four transitions (acceptor and donor for both DM-1B/2B and DM-2A/2B) directly in Figure 5c by replotting them so that all normalized donor and acceptor changes are shown as increasing from zero to one. As is clear in Figure 5c, three of the transitions (donor and acceptor for UvrD(DM-1B/2B) and donor for UvrD(DM-2A/2B)) are nearly identical with a midpoint of ~65 mM NaCl, whereas the acceptor transition for UvrD(DM-2A/2B) is offset to higher [NaCl] (midpoint of ~120 mM NaCl). Since the donor and acceptor fluorescence changes observed for UvrD(DM-1B/2B) mutant are quantitatively anti-correlated, these changes appear to be due solely to FRET effects. However, although the donor and acceptor fluorescence changes for the UvrD(DM-2A/2B) mutant show qualitative anti-correlation, they differ quantitatively suggesting that some fluorescence changes other than FRET occur for the Cy5 acceptor fluorescence in UvrD(DM-2A/2B).
We infer that this non FRET effect is due to DM-2A/2B molecules that are labeled with the Cy5 acceptor at position 370 within the 2A sub-domain for two reasons. First, it is not likely due to molecules labeled with Cy5 in the 2B sub-domain, since the same labeling position was used for the DM-1B/2B protein for which the results suggest all fluorescence changes are due to FRET. Second, since the Cy3 donor fluorescence transition of DM-2A/2B overlays quantitatively with both the donor and acceptor transitions observed with DM-1B/2B, the changes in donor (Cy3) fluorescence would appear to be due only to FRET changes. Hence, we surmise that the Cy5 fluorescence of DM-2A/2B molecules labeled with Cy5 at the Cys370 position within the 2A sub-domain is either quenched or enhanced additionally when the 2B sub-domain is in either the closed or open conformation. Based on the position of amino acid 370, it seems most likely that the problem occurs when the 2B sub-domain is in the open conformation since the 2B sub-domain comes in close proximity to A370 (see Figure 2a). In any event, because of this, all remaining experiments reported here were performed with the DM-1B/2B mutant since the fluorescence changes that occur with both donor and acceptor fluorophores appear to be due solely to FRET changes and thus result from true distance changes, at least for the monomeric apo UvrD. However, our experiments with both mutants support the qualitative conclusion that the 2B domain swivels to a more open conformation at high salt and that the open and closed forms are in equilibrium in solution. In the absence of single molecule studies, we cannot conclude whether the salt induced transition represents gradual changes in the rotational conformation of the 2B sub-domain or changes in the relative population of two or a few conformational states.
We have further examined the origins of the effect of salt on the 2B sub-domain orientation by comparing the effects a series of other salts (KCl, NaBr, NaCH3CO2 and MgCl2) on the closed to open transition. We find that all salts induce the transition; however, the midpoints of the transitions are sensitive to both the type of cation and anion (Supplementary Figure S1). These results indicate that the salt induced transition to the open form observed here is accompanied by the direct binding of both cations and anions and is not due to a simple screening effect of ionic strength. Examination of the UvrD-DNA crystal structure in the closed form shows a number of potentially important electrostatic interactions between charged groups of sub-domains 1B and 2B that could explain these salt effects. A pair of salt bridges (D115–K389 and D118–R396) appear able to form between 1B and 2B. There are also several positively charged residues in sub-domain 1B (R121, K124, R125, K128, and R183) and negatively charged residues in sub-domain 2B (D404, E408, D420, D424, and D432). Since these residues are far apart in the open state, it makes sense that high salt would favor the open conformation.
In the UvrD crystal structures, the α-carbons of the two cysteines at positions 473 (2B) and 100 (1B) in UvrD(DM-1B/2B) are separated by 64 Å for the apo UvrD in the open conformation and 23 Å when UvrD is in the closed conformation in the UvrD-DNA structure (see Figure 2b). Examination of the UvrD crystal structures indicates that the open form in the UvrD apo structure and the closed form in the UvrD-DNA structure34 represent the extremes of the possible rotational states that the 2B sub-domain can occupy, hence further rotations are not possible. Thus it is not possible that the low salt plateau FRET value corresponds to a 2B rotational state in which Cys473 and Cy100 are closer together than in the UvrD-DNA crystal structure. Thus, we assume that the orientation of the 2B sub-domain in the high NaCl concentration plateau region corresponds to the open form of the apo crystal structure (Figure 1a) and that the low NaCl concentration plateau region corresponds to the closed form of the UvrD-DNA crystal structure (Figures 1b). We can then use the Förster equation assuming R0 = 54Å for the Cy3/Cy5 pair35 (see Materials and Methods) to infer distance changes from the FRET changes that we observe in the DNA and nucleotide binding experiments described below. Alternatively we could assume that the high and low salt plateaus correspond to the apo form of UvrD in solution and the UvrD-DNA structure in solution, respectively. However, this assignment seems less reasonable since as we show below, the FRET value of the UvrD-DNA complex is near the midpoint of the high and low salt transition.
UvrD binding to ssDNA induces opening of the 2B sub-domain
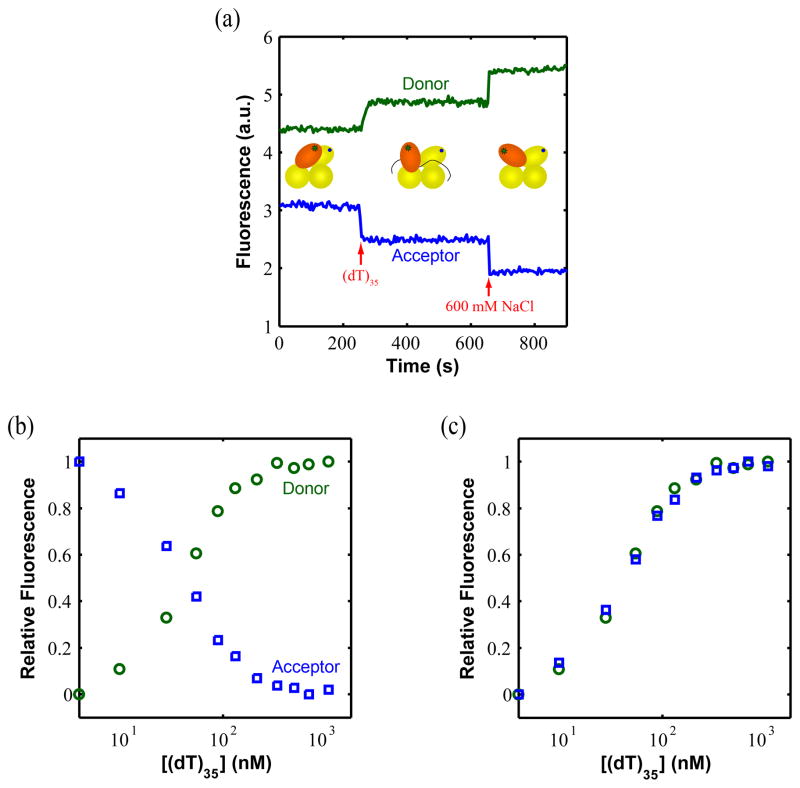
We next used UvrD(DM-1B/2B) to examine whether DNA binding affects the rotational orientation of its 2B sub-domain. These experiments were performed in Buffer T20 including BSA (100 μg/ml) and thus before the addition of DNA, the 2B sub-domain of UvrD is near its fully closed low [NaCl] conformation, although not completely closed (~35Å vs ~23Å in the fully closed conformation). Figure 6a shows that upon addition of saturating concentrations of (dT)35 (500 nM as shown in Figure 6b) to DM-1B/2B (20 nM), the Cy3 (donor) fluorescence increases while the Cy5 (acceptor) fluorescence decreases, indicating that binding of (dT)35, induces an opening of the 2B sub-domain of UvrD (to ~50 Å), although not as fully open as at high [NaCl] (~64 Å). Figure 6b shows the relative donor and acceptor fluorescence changes accompanying a full titration of DM-1B/2B with (dT)35. The normalized forms of these two transition curves (Figure 6c) are identical suggesting that the FRET changes result only from distance changes. The titration curve with (dT)70 is similar (data not shown).
Figure 6. Binding of UvrD to ssDNA induces opening of the 2B sub-domain.
(a) Changes in the donor and acceptor signals when 500 nM dT35 and 600 mM NaCl are added to 20 nM DM-1B/2B. The final signal at 600 mM NaCl is used as a control since at this condition 2B sub-domain is fully open. (b) The relative Cy3 (donor) and Cy5 (acceptor) fluorescence intensities of DM-1B/2B (20 nM) upon titration with dT35 in buffer T20. The fluorescence signal increases for the donor and decreases for the acceptor until saturation is reached indicating a relative opening of the 2B sub-domain upon binding dT35. (c) Replot to normalize the donor and acceptor changes as increasing from zero to one indicating that all fluorescence signals changes upon binding dT35 result from FRET changes due to distance changes.
Binding of UvrD to duplex DNA with a 3′ ss-DNA tail induces 2B sub-domain opening
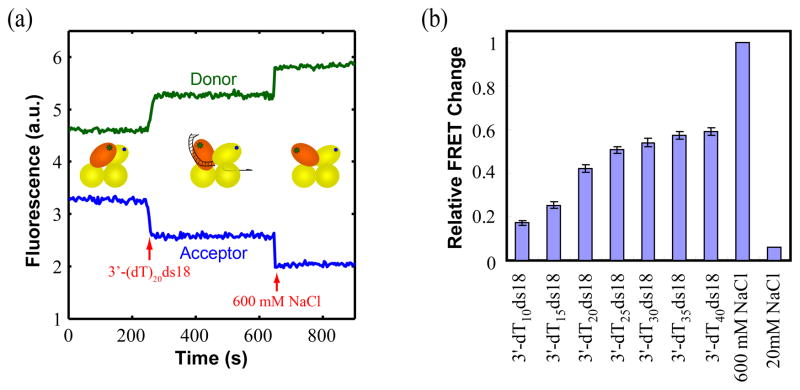
When saturating amounts (50 nM) (Supplementary Figure S2) of a ss-duplex DNA junction (18 bp duplex) with a 20 nucleotide 3′ tail are added to UvrD(DM-1B/2B) in buffer T20, the donor signal increases and the acceptor signal decreases, indicating that UvrD binding to this DNA also causes the 2B sub-domain to become more open (Figure 7a). When saturating amounts of a partial duplex with a short 3′ ssDNA tail is added, UvrD(DM-1B/2B) shows relatively little FRET change, indicating that the 2B sub-domain remains mostly in the closed form. It therefore appears that the 2B sub-domain adopts a partially open conformation when bound to a ss-duplex DNA junction in solution, not fully closed as is observed in the UvrD-DNA junction crystal structures (see Figure 1b).30 As DNA with longer ssDNA tails are examined (at saturating concentrations), the degree of 2B sub-domain opening increases with the largest change observed for ss-DNA lengths larger than 25 (Figure 7b).
Figure 7. Binding of a partial DNA duplex with a 3′-ssDNA tail promotes 2B sub-domain opening.
(a) An increase in Cy3 (donor) fluorescence and decrease in Cy5 (acceptor) fluorescence occur upon binding a 3′-ssDNA duplex junction (50nM) and addition of 600 mM NaCl to DM-1B/2B (20 nM). (b) Relative FRET changes indicate an opening of the 2B sub-domain as the length of the 3′ ssDNA tail of the partial duplex increases from 10 to 40 nucleotides. The FRET signals at low [NaCl] (20mM) and high [NaCl] (600mM) are used as references, respectively.
ATP binding and hydrolysis are coupled to swiveling of the 2B sub-domain of UvrD
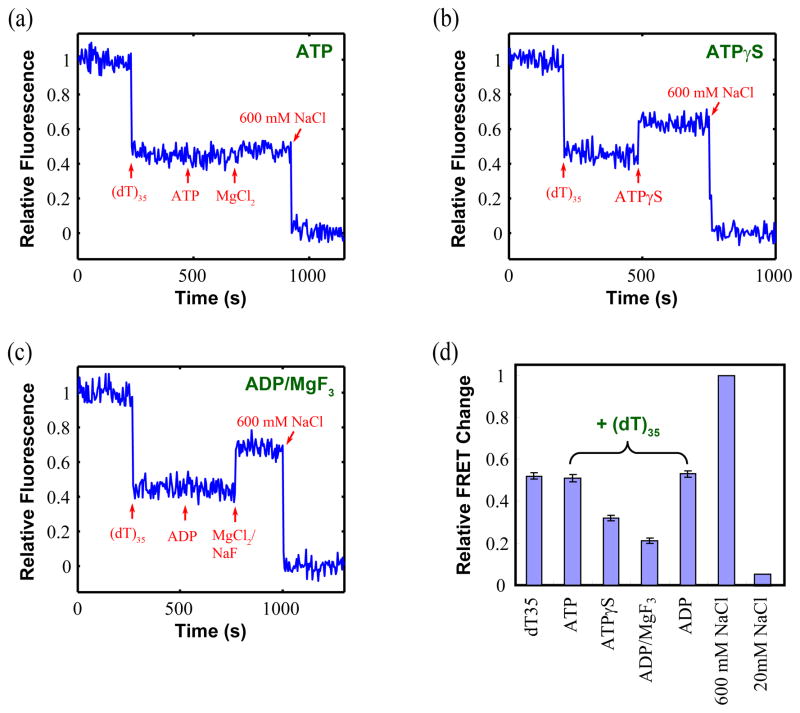
To further investigate the functional significance of the 2B sub-domain movement, we measured FRET changes within UvrD(DM-1B/2B) upon binding ATP and nucleotide analogues that mimic some of the reaction intermediates during the ATP hydrolysis cycle. Figure 8 shows the results of ensemble FRET experiments performed with DM-1B/2B in the presence of saturating concentrations of (dT)35 (500 nM) and several different nucleotides in buffer T20. The relative FRET change, and by inference the 2B sub-domain conformation, is at an intermediate position between fully open (600 mM NaCl) and closed (20 mM NaCl) when bound to ssDNA alone and remains nearly the same upon further binding of ATP or ADP (Figure 8a, c and d). However the FRET signal change is considerably reduced when ATPγS (a slowly hydrolyzable ATP analog) or ADP-MgF3 (which is believed to mimic an ADP-Pi intermediate)36 is added with the ssDNA (Figure 8b, c and d). Therefore, the nucleotide ligation state has a clear effect on the average rotational conformational state of the UvrD 2B sub-domain. These results suggest that the 2B sub-domain of a UvrD monomer exists predominantly in several partially open conformations in all of these ligation states when bound to ssDNA. Thus, the 2B sub-domain of a UvrD monomer likely moves among a series of partially open states during ATP-driven ssDNA translocation. Movement of the 2B sub-domain is clearly modulated by interactions with DNA, as well as ATP binding and hydrolysis.
Figure 8. ATP analogues affect the rotational conformation of the 2B sub-domain differently in the presence of ssDNA.
The relative acceptor fluorescence of Cy3/Cy5 labeled UvrD(DM-1B/2B) (20nM) are shown when interacting with (a)- ATP, (b)- ATPγS, and (c)-ADP·MgF3 in the presence of saturating concentration of (dT)35. The arrows indicate the addition of 500 nM (dT)35, 0.2 mM ATP, 0.5 mM MgCl2, 600 mM NaCl, 0.2 mM ATPγS, 0.2 mM ADP, and 0.5 mM MgCl2/1.5 mM NaF, respectively. (d) The relative FRET signals reflecting different degrees of opening of the 2B sub-domain are compared in the presence of saturating concentration of (dT)35 only, (dT)35 + ATP, (dT)35 +ATPγS, (dT)35 + ADP·MgF3, (dT)35 + ADP, and high/low salt (as references).
Discussion
We report a crystal structure of a monomeric apo form of E. coli UvrD with its 2B sub-domain in an open conformation. Previous crystal structures of a UvrD monomer bound to a series of 3′-ssDNA-duplex junctions show the 2B sub-domain in a quite different closed conformation.30 Thus, the 2B sub-domain of a UvrD monomer can exist in both an open and closed form (differing by a rotation of ~160 degrees), similar to what has been observed for the structurally similar E. coli Rep29 and B. stearothermophilus PcrA SF1 monomers.27; 28 We also show, using ensemble FRET studies, that the open and closed forms can interconvert in solution and that the rotational conformational state of the 2B sub-domain is sensitive to the salt concentration as well as anion and cation type, with low salt concentration favoring the closed conformation and high salt concentration favoring the open conformation in the absence of DNA. With increasing [NaCl], the 2B sub-domain rotates from a closed orientation to an open conformation with its maximum opening occurring near 600 mM NaCl, with a transition midpoint near 65 mM NaCl. The rotational conformational state of the 2B sub-domain is also affected by DNA binding as well as ATP binding and hydrolysis. In the previous structural studies of UvrD34 and PcrA28, only the closed conformations were considered as the active forms of the enzyme. Within this closed form, small conformational changes corresponding to the opening and closing of the cleft between the 1A and 2A sub-domains upon ATP binding and hydrolysis were proposed to be coupled to translocation. The FRET experiments reported here suggest that binding of UvrD to ssDNA or partial duplex DNA induces swiveling of the 2B sub-domain, which adopts an intermediate state that is only partially open. Thus, the 2B sub-domain can populate intermediate rotational conformational states between the most open and most closed states and DNA and nucleotide binding affect the relative populations of these states.
The need for rotation of the 2B sub-domain can be understood, at least partially, by examining its conformations in the extreme closed and open states represented by the crystal structures of the UvrD-DNA complex and the apo UvrD, which either block the ssDNA binding site (closed form) or partially block the ss- and ds-DNA binding sites (open form). Hence, movement of the 2B sub-domain away from these extreme conformations is needed to allow DNA binding. The relative orientations of the 2B sub-domains within the apo and DNA-bound forms of UvrD and PcrA as well as the two conformations of Rep when bound to ssDNA are illustrated in Figure 9. The 2B sub-domain in the open structure of Rep is intermediate between the two extreme states, exemplified by the fully closed and open states observed for PcrA and UvrD. This intermediate state (Rep open state) is the only conformation among all of the UvrD, PcrA and Rep crystal structures in which both DNA binding sites (within the 1A and 2A sub-domains) appear to be fully accessible to ssDNA binding. In contrast, in the fully open forms of PcrA and UvrD, the ssDNA binding site of the 2A sub-domain appears partially blocked by the 2B sub-domain, and the dsDNA binding sites within the 2B and 1B sub-domains are involved in a 1B/2B interaction. In all of the closed forms, the ssDNA bound to the 1A sub-domain is completely occluded by the 1A, 1B and 2B sub-domains, suggesting that the initial binding of the 3′ ssDNA tail to the 1A sub-domain is likely to precede any closing of the 2B sub-domain. These results suggest that the conformation of the 2B sub-domain of UvrD may predominantly exist in a partially open form for most of its ATP cycle during ssDNA translocation. Only the ATP bound (ATPγS or ADP-MgF3) and fully unligated UvrD states exist in a more closed conformation in solution. This suggests that ADP or Pi release should induce a reopening of the 2B sub-domain, possibly allowing UvrD to translocate along ssDNA.
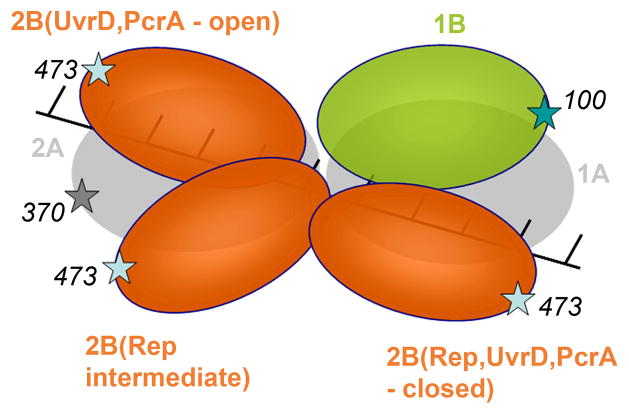
Figure 9. Relative orientations of the 2B sub-domain in the crystal structures of the open and closed forms of UvrD, PcrA, and Rep.
The relative positions of the 2B sub-domains in the apo and DNA-bound forms of UvrD and PcrA compared with the positions of the 2B sub-domain in the open and closed conformations of Rep in complex with ssDNA. The six structures were compared by superimposing sub-domains 1A and 2A (shown in grey) and 1B (green) for all structures. The 2B sub-domains are in red. The positions of the fluorophores are also indicated. The closed conformations of UvrD, PcrA, and Rep are similar, while the open conformation of Rep shows the 2B sub-domain in an intermediate position relative to the fully open conformations of UvrD and PcrA.
The FRET methods described here have previously been used to monitor rotational conformational changes of the 2B sub-domains within Rep monomers37 and PcrA monomers32 during translocation along ssDNA. Single molecule FRET studies of Rep37 doubly labeled with Cy3/Cy5 showed that the 2B sub-domain of a Rep monomer closes gradually as the enzyme reaches a blockade that it cannot bypass (e.g., a duplex DNA region that the monomer cannot unwind). More recent single molecule FRET studies of PcrA doubly labeled with Cy3/Cy5 showed that when PcrA initially binds the junction of 5′-ss-ds DNA, its 2B sub-domain adopts a closed conformation, but then moves to a more open conformation and maintains the open conformation during ssDNA translocation that is coupled with repetitive ssDNA looping.32 Those studies as well as the results reported here suggest that Rep, PcrA and UvrD monomers adopt a more open 2B sub-domain conformation during ss-DNA translocation.
The FRET studies reported here suggest that in solution the fully closed conformation of UvrD is not highly populated when UvrD is bound to a 3′-ss-ds DNA junction. In all UvrD-DNA crystal structures, the 2B sub-domain adopts a closed conformation, similar to the closed structure observed in the PcrA-DNA complexes.28 However, our FRET results suggest that the 2B sub-domain adopts a partially open conformation when it interacts with the 3′-ss-ds DNA junction in Buffer T20. The addition of DNA to UvrD(DM-1B/2B) induces an opening of the 2B sub-domain (Figure 7a). However, when UvrD(DM-1B/2B) interacts with both the 3′-ss-ds DNA and ATPγS (Mg2+), the 2B sub-domain moves to a more closed conformation. In the ternary complex of UvrD-DNA-ADP·MgF3, the 2B sub-domain moves to an even yet more closed conformation (data not shown), which is more consistent with the crystal structures of UvrD-DNA-AMPPNP and UvrD-DNA-ADP·MgF3 ternary complexes.34 Interestingly, while the 2B sub-domain also adopts a partially open conformation upon binding a 5′-ss-ds DNA junction, it returns to a more closed conformation when ATP is added (data not shown). Further titration with Mg2+ causes a reopening of the 2B sub-domain (data not shown) as expected since under these conditions UvrD can initiate translocation along ss-DNA from the junction in the 3′ to 5′ direction along the ss-DNA tail.38 When UvrD is bound to dsDNA alone, the 2B sub-domain closes even further relative to its position at 20 mM NaCl (data not shown), indicating that the ssDNA tails induce an opening of the 2B sub-domain.
It is not clear whether rotation of the 2B sub-domain is important for translocation or DNA unwinding is not known. It was shown previously that the 2B sub-domain of E. coli Rep inhibits the helicase activity of the Rep monomer in vitro and that removal of the 2B sub-domain to form RepΔ2B activates monomer helicase activity.19; 39 This suggests that the 2B sub-domain of Rep may play a role in regulating its activities.19 In this regard it is interesting that although RepΔ2B and wtRep monomers can both translocate rapidly along ssDNA with 3′ to 5′ directionality, removal of the 2B sub-domain increases the rate of ssDNA translocation of the RepΔ2B monomer by roughly two-fold.19 Hence, the 2B sub-domain of Rep modulates its rate of ssDNA translocation in vitro. As mentioned above, the open form of the 2B sub-domain of Rep observed in crystal structures is not in a fully open position compared to all of other UvrD and PcrA open structures. Furthermore, in this open Rep state, both DNA binding sites (1A and 2A sites) appear to be fully accessible to ssDNA binding. Since in the fully open and closed conformations of UvrD the 2B sub-domain partly blocks both ssDNA binding sites, our results suggest that during ssDNA translocation the 2B sub-domain of UvrD would be maintained in a partially open state, as has been observed for PcrA translocation.32 The fact that the translocation rate for wtRep is slightly faster (about 50%) than for wtUvrD,19; 20 suggests the possibility that the fully open position of the 2B sub-domain in UvrD may inhibit translocation.
The focus of this study has been on the conformational changes that occur due to rotation of the 2B sub-domain of UvrD, an SF1 helicase/translocase. However, crystallographic studies of PcrA28 and UvrD34 suggest that ATP binding causes the cleft between the two RecA-like sub-domains 1A and 2A to close around the nucleotide and that the cleft reopens following ATP hydrolysis and ADP releasing, and that this may drive ssDNA translocation.2; 40 Single-molecule FRET experiments also show that the interdomain cleft of the Bacillus subtilis DEAD box helicase YxiN closes upon binding both RNA and ATP, whereas it opens in the ADP bound state.41; 42 Those movements are relatively small and by themselves would not cause the large FRET changes due to 2B sub-domain rotation that we report here. The experiments reported here and previous studies32; 37 suggest that rotational movement of the 2B sub-domain also occurs during ATP-driven ssDNA translocation.
Materials and Methods
Buffers and Reagents
Buffers were prepared with reagent grade chemicals using distilled water that was also deionized using a Milli-Q water purification system (Millipore Corp., Bedford, MA). Spectrophotometric-grade glycerol (99.5% purity) was from Aldrich (Milwaukee, WI). Buffer T20 is 10 mM Tris-HCl (pH 8.3 at 25 °C), 20 mM NaCl, and 20% (v/v) glycerol. Storage Buffer is 20 mM Tris-HCl (pH 8.3 at 25 °C), 200 mM NaCl, 50% (v/v) glycerol, 1 mM EDTA, 0.5 mM EGTA, 25 mM 2-mercaptoethanol. Storage minimal buffer is 20 mM Tris-HCl (pH 8.3 at 25 °C), 200 mM NaCl, 50 % (v/v) glycerol. Buffer A is 20 mM Tris-HCl (pH 7.5 at 25 °C), 500 mM NaCl, 20% (v/v) glycerol, 5 mM 2-mercaptoethanol. Buffer B is 20 mM Tris-HCl (pH 7.5 at 25 °C), 500 mM NaCl, 20% (v/v) glycerol. Buffer C is 20 mM Tris-HCl (pH 8.3 at 25 °C), 20% (v/v) glycerol, 2 mM EDTA. ATP (Sigma-Aldrich) stock solutions were prepared in 50 mM NaOH (pH 7.5), and 500 μl aliquots were stored at −20°C. ATP concentrations were determined spectrophotometrically using an extinction coefficient (259 nm) of 15.4 × 103 M−1 cm−1.
Double-cysteine UvrD mutant plasmids
Site-directed mutagenesis was performed with the QuickChange kit (Stratagene, Cedar Creek, TX). Plasmids expressing all UvrD mutants were constructed by starting with plasmid pGG209 which contains the wtUvrD coding sequence cloned into plasmid pET-9d (kanamycin resistance and under control of the T7 promoter, Novagen, Madison, WI). Mutations were first made to the DNA sequences encoding all six native Cysteine residues in the UvrD gene (C52, C181, C322, C350, C441, and C640) to replace them with Ser in order to create a plasmid encoding a Cys-less UvrD plasmid (pGG209ΔCys). We did not anticipate this to be a problem for enzyme activity since none of the naturally occurring Cys residues are conserved among UvrD, Rep, and PcrA. The plasmid pGG209ΔCys was digested with restriction enzymes (Nco I and BstX I), and inserted with the PCR fragment amplified using primers AN55 and AN56 to generate an expression vector (pA10) to introduce a hexa-histidine (6xHis) tag and thrombin cleavage site (MGSSHHHHHHSSGLVPRGSH, 20 aa) at the N-terminal end of the Cys-less UvrD mutant to aid in purification and labeling. Two different UvrD mutants, each containing two Cys residues, were then constructed, 6xHis-UvrDΔCys[A100C, A473C] (called DM-1B/2B) and 6xHis-UvrDΔCys[A370C, A473C] (called DM-2A/2B), by substituting Cys for Ala at the indicated positions within UvrDΔCys. Site-directed mutagenesis was carried out using plasmid pA10 and primers (AN70 and AN71 for A100C, AN72 and AN73 for A370C, AN74 and AN75 for A473C) to generate the plasmids expressing these double-cysteine mutants (named pA20 and pA21, respectively). All mutations were confirmed by DNA sequencing.
UvrDΔ73 protein expression and purification
A plasmid, pGG221 overexpressing UvrDΔ73 in which the last 73 C-terminal amino acids have been removed from UvrD was constructed as follows. The Xba I restriction fragment of pGG209 containing the wtUvrD coding region was ligated into the Xba I site of pET28a to generate plasmid pGG219. To remove the DNA encoding the last 73 C-terminal amino acids from Ala648 on, primers were designed to replace the Ala648 codon with a stop codon (TAA) and the coding sequence after this position was deleted. Plasmid pGG219 was digested with Xho I and BsrG I to generate subclone 1. The C-terminal portion of pGG209 was amplified using primers RSG-P4 and GHG72, digested with Xho I and BsrG I, ligated into subclone 1 to generate the UvrDΔ73 overexpression plasmid (called pGG221). All ORFs were confirmed by DNA sequencing.
UvrDΔ73 protein under control of T7 promoter was overexpressed in strain E. coli BL21(DE3) ΔUvrD (with a deletion of wtUvrD gene, tetracycline resistant) and purified as described for wild type UvrD43 with the following modifications. First, the single stranded DNA cellulose column was loaded in buffer containing a lower [NaCl] (100 mM). Second, due to the C-terminal deletion, UvrDΔ73 did not show the usual extent of nuclease contamination as we find with wild type UvrD, hence the double stranded DNA cellulose column was not needed to remove the nuclease contamination. This suggests that the nuclease may interact with the unstructured C-terminus of UvrD.
DNA
The oligodeoxynucleotides used in this study were synthesized using an ABI model 391 (Applied Biosystems, Foster City, CA) and purified by electroelution of the DNA from denaturing polyacrylamide gels as described.44 The concentrations of the DNA strands were determined by spectrophotometric analysis of the mixture of mononucleotides after digestion of the DNA with snake venom phosphodiesterase I in 100 mM Tris-HCl (pH 9.2 at 25 °C), 3 mM MgCl2.45 The oligodeoxynucleotides were dialyzed against 10 mM Tris-HCl (pH 7.5 at 25 °C) and stored at −20 °C. Duplex DNA was formed by annealing the top strand with an equal molar of complementary bottom strand in 10 mM Tris-HCl (pH 7.5 at 25 °C) plus 50 mM NaCl by heating to 95°C for 5 min and then slowly cooling to room temperature over a period of 2 hours. Duplex DNA formation was confirmed by native PAGE on 10% acrylamide gel in 1× TBE buffer.
UvrDΔ73 crystallization, data collection and refinement
Crystals of the UvrD C-terminal truncation, UvrDΔ73, were obtained by the hanging drop method. 1 μl of protein solution, containing 30 mg/ml UvrDΔ73 in 20 mM Tris-HCl (pH 8.0), 0.1 M NaCl, 2 μM βME, was mixed with 1 μl of reservoir buffer, containing 0.1 M BICINE 9.1, 1.0 M (NH4)2SO4 and 2% PEG 400. Crystals of ~ 0.5 × 0.3 × 0.3 mm size were grown at room temperature in 1 week. They were gradually transferred into 3.0 M (NH4)2SO4, 0.1 M BICINE 9.1, 2% PEG 400, 5% glycerol and flash frozen at 100° K in a stream of nitrogen vapor.
Data were collected at beam line 19ID, SBC at Argonne National Laboratory, and processed by HKL200046 program and truncated by CCP4 programs.47 Reflections from three thin resolution layers corresponding to weak ice rings were excluded from the data set. An initial solution was found by the molecular replacement (MR) method, using the AMoRe program48 and a poly-alanine structure of PcrA helicase (PDB entry code 1PJR) as a search model. The MR solution was subjected to rigid body refinement using program CNS0.549 for the whole molecule and subsequently for each domain separately. The side chains and the loop regions were built in a few rounds in program O50 using electron density maps calculated with 2Fo-Fc coefficient as well as simulated annealing omit maps. The structure was refined by a simulated annealing protocol with a maximum likelihood target. Final refinement was performed with the program REFMAC51 and the parameters of refinement are shown in Table 1. The residues 1-2, 160-163, 521-525, 542 were omitted due to lack of density and residues 3, 157-168, 520, 526, 543, 545, 547 were build as alanines because of poor density. 92.7% of residues are in the core of the Ramachandran plot, 7.3 % in allowed regions. The coordinates were deposited into the PDB database (PDB ID 3LFU).
Table 1.
Refinement statistics.
| Resolution | 20-1.8 Å |
| Non-H protein atoms | 5059 |
| Sulfates | 4 |
| Water | 721 |
| Reflection (compl.(%))a | 67970 (91/99) |
| R factor (%)b | 19.9 (27.0) |
| R-free (%)b,c | 24.8 (31.1) |
| Average B (Å2) | |
| Prot. | 25.8 |
| Sulf. | 30.5 |
| Water | 36.0 |
| R.m.s. deviations | |
| Bond lengths (Å) | 0.011 |
| Bond angles (°) | 1.7 |
Numbers for reflections with F/σ(F) > 0.0; value for completeness for overall/high (1.87-1.80 Å) resolution shell in parentheses. Overall completeness is low due to removal of ice rings shells.
Numbers for R factor and R-free in highest resolution shell are shown in parentheses.
R-free was calculated on the bases of 5% of the observed reflections that were randomly omitted from the refinement.
Fluorescence measurements
Fluorescence measurements, except for the [NaCl] titrations, were generally carried out in Buffer T20 with BSA (otherwise indicated) at 25 °C using a PTI QM-4 fluorometer (Photon Technology International, Lawrenceville, NJ) equipped with a 75 W Xenon lamp. The slit widths were set at 0.5 mm for excitation and 1 mm for emission, respectively. The sample temperature in the cuvette was controlled using a Neslab RTE-111 recirculation water-bath (Neslab, Newington, NH). The [NaCl] titrations were started in Buffer T20 with no added NaCl (including BSA). The addition of BSA was necessary to prevent UvrD protein from sticking to the cuvette walls. Non-specific sticking of BSA to the cuvette walls was monitored by the decrease of BSA Trp fluorescence with excitation at 295 nm and emission at 336 nm. The cuvette was placed in a thermostatted sample holder and equilibrated by constant stirring with a magnetic stirrer for at least 1 h. When the Trp fluorescence of BSA reached a constant value, the double labeled UvrD mutant was added to a final concentration of 20 nM and the fluorescence signal was measured after an additional 30 min equilibration. Fluorescence emission measurements using excitation/emission wavelengths of 515 nm/566 nm, 515 nm/670 nm and 620 nm/670 nm were made to obtain the donor fluorescence, the acceptor fluorescence, and the acceptor fluorescence without energy transfer as an internal control, respectively. For titration experiments, the solution was allowed to equilibrate with stirring for 10 min after each addition of titrant. For each fluorescence measurement, the sample was excited for 15 s with integration time of 2 s so that eight data points were taken, after which the shutter was closed. Fluorescence measurements were repeated every two to three minutes until the signal was constant. Data points from three measurements were averaged to obtain the final fluorescence. Stirring was maintained at a constant speed throughout each experiment. FRET occurs between two dyes when the emission spectrum of an excited donor fluorophore overlaps the absorption spectrum of a nearby acceptor fluorophore. FRET efficiency, E, depends on the inverse sixth power of the intermolecular separation, E = R06/(R6 + R06), where R0 is the Förster radius (defined as the distance between donor and acceptor when E = 50%). The donor-acceptor pair used here (Cy3/Cy5) has a Förster radius R0 of 54 Å.35
Supplementary Material
Acknowledgments
This work was supported in part by NIH (GM045948 to T.M.L.); (GM065367 to T.H.); (GM073837 to S.K.). We thank T. Ho for synthesis and purification of the DNA, and R. Galletto and E. Antony for discussions and comments on the manuscript. TML, T. Ha, SM, and HJ designed the experiments. SM, AN-M and HJ designed and made the labeled UvrD mutants. NM and GG made the UvrDΔ73 used for the crystallography. SK and GW solved the UvrD73 crystal structure. HJ, SK, SM, TH and TML wrote the paper.
Footnotes
Accession Number
Coordinates and structure factors of apo UvrD have been deposited in the Protein Data Bank with accession number ID: 3LFU.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lohman TM, Bjornson KP. Mechanisms of helicase-catalyzed DNA unwinding. Annu Rev Biochem. 1996;65:169–214. doi: 10.1146/annurev.bi.65.070196.001125. [DOI] [PubMed] [Google Scholar]
- 2.Singleton MR, Dillingham MS, Wigley DB. Structure and mechanism of helicases and nucleic acid translocases. Annu Rev Biochem. 2007;76:23–50. doi: 10.1146/annurev.biochem.76.052305.115300. [DOI] [PubMed] [Google Scholar]
- 3.Patel SS, Picha KM. Structure and function of hexameric helicases. Annu Rev Biochem. 2000;69:651–97. doi: 10.1146/annurev.biochem.69.1.651. [DOI] [PubMed] [Google Scholar]
- 4.Lohman TM, Tomko EJ, Wu CG. Non-hexameric DNA helicases and translocases: mechanisms and regulation. Nat Rev Mol Cell Biol. 2008;9:391–401. doi: 10.1038/nrm2394. [DOI] [PubMed] [Google Scholar]
- 5.Berger JM. SnapShot: nucleic acid helicases and translocases. Cell. 2008;134:888–888. e1. doi: 10.1016/j.cell.2008.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorbalenya AE, Koonin EV. Helicases: amino acid sequence comparisons and structure-function relationships. Current Opinion in Structural Biology. 1993;3:419–429. [Google Scholar]
- 7.Yamaguchi M, Dao V, Modrich P. MutS and MutL Activate DNA Helicase II in a Mismatch-dependent Manner. J Biol Chem. 1998;273:9197–9201. doi: 10.1074/jbc.273.15.9197. [DOI] [PubMed] [Google Scholar]
- 8.Husain I, van Houten B, Thomas DC, Abdel-Monem M, Sancar A. Effect of DNA polymerase I and DNA helicase II on the turnover rate of UvrABC excision nuclease. Proc Natl Acad Sci, USA. 1985;82:6774–6778. doi: 10.1073/pnas.82.20.6774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flores MJ, Bidnenko V, Michel B. The DNA repair helicase UvrD is essential for replication fork reversal in replication mutants. EMBO Rep. 2004;5:983–8. doi: 10.1038/sj.embor.7400262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Payne BT, van Knippenberg IC, Bell H, Filipe SR, Sherratt DJ, McGlynn P. Replication fork blockage by transcription factor-DNA complexes in Escherichia coli. Nucleic Acids Res. 2006 doi: 10.1093/nar/gkl682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruand C, Ehrlich SD. UvrD-dependent replication of rolling-circle plasmids in Escherichia coli. Mol Microbiol. 2000;35:204–10. doi: 10.1046/j.1365-2958.2000.01700.x. [DOI] [PubMed] [Google Scholar]
- 12.Flores MJ, Sanchez N, Michel B. A fork-clearing role for UvrD. Mol Microbiol. 2005;57:1664–75. doi: 10.1111/j.1365-2958.2005.04753.x. [DOI] [PubMed] [Google Scholar]
- 13.Bidnenko V, Lestini R, Michel B. The Escherichia coli UvrD helicase is essential for Tus removal during recombination-dependent replication restart from Ter sites. Mol Microbiol. 2006;62:382–96. doi: 10.1111/j.1365-2958.2006.05382.x. [DOI] [PubMed] [Google Scholar]
- 14.Veaute X, Delmas S, Selva M, Jeusset J, Le Cam E, Matic I, Fabre F, Petit MA. UvrD helicase, unlike Rep helicase, dismantles RecA nucleoprotein filaments in Escherichia coli. Embo J. 2005;24:180–9. doi: 10.1038/sj.emboj.7600485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lestini R, Michel B. UvrD controls the access of recombination proteins to blocked replication forks. Embo J. 2007;26:3804–14. doi: 10.1038/sj.emboj.7601804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delagoutte E, von Hippel PH. Helicase mechanisms and the coupling of helicases within macromolecular machines. Part I: Structures and properties of isolated helicases. Q Rev Biophys. 2002;35:431–78. doi: 10.1017/s0033583502003852. [DOI] [PubMed] [Google Scholar]
- 17.Byrd AK, Raney KD. Displacement of a DNA binding protein by Dda helicase. Nucl Acids Res. 2006;34:3020–9. doi: 10.1093/nar/gkl369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dillingham MS, Wigley DB, Webb MR. Demonstration of unidirectional single-stranded DNA translocation by PcrA helicase: measurement of step size and translocation speed. Biochemistry. 2000;39:205–12. doi: 10.1021/bi992105o. [DOI] [PubMed] [Google Scholar]
- 19.Brendza KM, Cheng W, Fischer CJ, Chesnik MA, Niedziela-Majka A, Lohman TM. Autoinhibition of Escherichia coli Rep monomer helicase activity by its 2B subdomain. Proc Natl Acad Sci U S A. 2005;102:10076–81. doi: 10.1073/pnas.0502886102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fischer CJ, Maluf NK, Lohman TM. Mechanism of ATP-dependent translocation of E.coli UvrD monomers along single-stranded DNA. J Mol Biol. 2004;344:1287–309. doi: 10.1016/j.jmb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Cheng W, Hsieh J, Brendza KM, Lohman TM. E. coli Rep oligomers are required to initiate DNA unwinding in vitro. J Mol Biol. 2001;310:327–50. doi: 10.1006/jmbi.2001.4758. [DOI] [PubMed] [Google Scholar]
- 22.Maluf NK, Fischer CJ, Lohman TM. A Dimer of Escherichia coli UvrD is the active form of the helicase in vitro. J Mol Biol. 2003;325:913–35. doi: 10.1016/s0022-2836(02)01277-9. [DOI] [PubMed] [Google Scholar]
- 23.Niedziela-Majka A, Chesnik MA, Tomko EJ, Lohman TM. Bacillus stearothermophilus PcrA Monomer Is a Single-stranded DNA Translocase but Not a Processive Helicase in Vitro. J Biol Chem. 2007;282:27076–27085. doi: 10.1074/jbc.M704399200. [DOI] [PubMed] [Google Scholar]
- 24.Yang Y, Dou SX, Ren H, Wang PY, Zhang XD, Qian M, Pan BY, Xi XG. Evidence for a functional dimeric form of the PcrA helicase in DNA unwinding. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkm1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun B, Wei KJ, Zhang B, Zhang XH, Dou SX, Li M, Xi XG. Impediment of E. coli UvrD by DNA-destabilizing force reveals a strained-inchworm mechanism of DNA unwinding. EMBO J. 2008;27:3279–87. doi: 10.1038/emboj.2008.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Slatter AF, Thomas CD, Webb MR. PcrA helicase tightly couples ATP hydrolysis to unwinding double-stranded DNA, modulated by the initiator protein for plasmid replication, RepD. Biochemistry. 2009;48:6326–34. doi: 10.1021/bi900101h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Subramanya HS, Bird LE, Brannigan JA, Wigley DB. Crystal Structure of a DExx box DNA helicase. Nature. 1996;384:379–383. doi: 10.1038/384379a0. [DOI] [PubMed] [Google Scholar]
- 28.Velankar SS, Soultanas P, Dillingham MS, Subramanya HS, Wigley DB. Crystal Structures of Complexes of PcrA DNA Helicase with a DNA Substrate Indicate an Inchworm Mechanism. Cell. 1999;97:75–84. doi: 10.1016/s0092-8674(00)80716-3. [DOI] [PubMed] [Google Scholar]
- 29.Korolev S, Hsieh J, Gauss GH, Lohman TM, Waksman G. Major domain swiveling revealed by the crystal structures of complexes of E. coli Rep helicase bound to single-stranded DNA and ADP. Cell. 1997;90:635–47. doi: 10.1016/s0092-8674(00)80525-5. [DOI] [PubMed] [Google Scholar]
- 30.Lee JY, Yang W. UvrD Helicase Unwinds DNA One Base Pair at a Time by a Two-Part Power Stroke. Cell. 2006;127:1349–1360. doi: 10.1016/j.cell.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rasnik I, Myong S, Cheng W, Lohman TM, Ha T. DNA-binding orientation and domain conformation of the E. coli rep helicase monomer bound to a partial duplex junction: single-molecule studies of fluorescently labeled enzymes. J Mol Biol. 2004;336:395–408. doi: 10.1016/j.jmb.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 32.Park J, Myong S, Niedziela-Majka A, Lee KS, Yu J, Lohman TM, Ha T. PcrA helicase dismantles RecA filaments by reeling in DNA in uniform steps. Cell. 2010;142:544–55. doi: 10.1016/j.cell.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fischer CJ, Lohman TM. ATP-dependent translocation of proteins along single-stranded DNA: models and methods of analysis of pre-steady state kinetics. J Mol Biol. 2004;344:1265–86. doi: 10.1016/j.jmb.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Lee J, Yang W. UvrD Helicase Unwinds DNA One Base Pair at a Time by a Two-Part Power Stroke. Cell. 2006;127:1349–1360. doi: 10.1016/j.cell.2006.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ha T, Rasnik I, Cheng W, Babcock HP, Gauss GH, Lohman TM, Chu S. Initiation and re-initiation of DNA unwinding by the Escherichia coli Rep helicase. Nature. 2002;419:638–41. doi: 10.1038/nature01083. [DOI] [PubMed] [Google Scholar]
- 36.Graham DL, Lowe PN, Grime GW, Marsh M, Rittinger K, Smerdon SJ, Gamblin SJ, Eccleston JF. MgF(3)(-) as a transition state analog of phosphoryl transfer. Chem Biol. 2002;9:375–81. doi: 10.1016/s1074-5521(02)00112-6. [DOI] [PubMed] [Google Scholar]
- 37.Myong S, Rasnik I, Joo C, Lohman TM, Ha T. Repetitive shuttling of a motor protein on DNA. Nature. 2005;437:1321–5. doi: 10.1038/nature04049. [DOI] [PubMed] [Google Scholar]
- 38.Tomko EJ, Jia H, Park J, Maluf NK, Ha T, Lohman TM. 5′-Single-stranded/duplex DNA junctions are loading sites for E. coli UvrD translocase. Embo J. 2010;29:3826–39. doi: 10.1038/emboj.2010.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng W, Brendza KM, Gauss GH, Korolev S, Waksman G, Lohman TM. The 2B domain of the Escherichia coli Rep protein is not required for DNA helicase activity. Proc Natl Acad Sci USA. 2002;99:16006–11. doi: 10.1073/pnas.242479399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soultanas P, Wigley DB. DNA helicases: ‘inching forward’. Curr Opin Struct Biol. 2000;10:124–8. doi: 10.1016/s0959-440x(99)00059-7. [DOI] [PubMed] [Google Scholar]
- 41.Aregger R, Klostermeier D. The DEAD box helicase YxiN maintains a closed conformation during ATP hydrolysis. Biochemistry. 2009;48:10679–81. doi: 10.1021/bi901278p. [DOI] [PubMed] [Google Scholar]
- 42.Theissen B, Karow AR, Kohler J, Gubaev A, Klostermeier D. Cooperative binding of ATP and RNA induces a closed conformation in a DEAD box RNA helicase. Proc Natl Acad Sci U S A. 2008;105:548–53. doi: 10.1073/pnas.0705488105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Runyon GT, Wong I, Lohman TM. Overexpression, purification, DNA binding, and dimerization of the Escherichia coli uvrD gene product (helicase II) Biochemistry. 1993;32:602–12. doi: 10.1021/bi00053a028. [DOI] [PubMed] [Google Scholar]
- 44.Wong I, Chao KL, Bujalowski W, Lohman TM. DNA-induced dimerization of the Escherichia coli rep helicase. Allosteric effects of single-stranded and duplex DNA. J Biol Chem. 1992;267:7596–610. [PubMed] [Google Scholar]
- 45.Holbrook JA, Capp MW, Saecker RM, Record MT., Jr Enthalpy and heat capacity changes for formation of an oligomeric DNA duplex: interpretation in terms of coupled processes of formation and association of single-stranded helices. Biochemistry. 1999;38:8409–22. doi: 10.1021/bi990043w. [DOI] [PubMed] [Google Scholar]
- 46.Otwinowski Z, Minor W. Processing of X-ray diffraction data collected in oscillation mode. Macromolecular Crystallography, Pt A. 1997;276:307–326. doi: 10.1016/S0076-6879(97)76066-X. [DOI] [PubMed] [Google Scholar]
- 47.Collaborative Computational Project, N. The CCP4 Suite: Programs for protein crystallography. Acta Crystallographica Section D Biological Crystallography. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- 48.Navaza J. AMoRe: an automated package for molecular replacement. Acta Crystallographica Section A. 1994;50:157–163. [Google Scholar]
- 49.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, Jiang JS, Kuszewski J, Nilges M, Pannu NS, Read RJ, Rice LM, Simonson T, Warren GL. Crystallography & NMR system: A new software suite for macromolecular structure determination. Acta Crystallogr D Biol Crystallogr. 1998;54:905–21. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 50.Jones TA, Zou JY, Cowan SW, Kjeldgaard M. Improved methods for building protein models in electron density maps and the location of errors in these models. Acta Crystallogr A. 1991;47 (Pt 2):110–9. doi: 10.1107/s0108767390010224. [DOI] [PubMed] [Google Scholar]
- 51.Murshudov GN, Vagin AA, Dodson EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallographica Section D-Biological Crystallography. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.