Abstract
The case of a patient with visceral leishmaniasis showing inadequate response to amphotericin B from a region where leishmaniasis is not endemic is reported, with the Leishmania donovani isolate showing increased tolerance to amphotericin B in vivo. Four single nucleotide polymorphisms (SNPs) detected in the cysteine proteinase B gene resulted in changes to the deduced amino acid sequence: valine→alanine and arginine→leucine. Overexpression and underexpression of proteins were observed in the 65- to 80-kDa range and at 20 kDa, respectively.
CASE REPORT
A 69-year-old male from Lucknow City, Uttar Pradesh, India, presented to his local tertiary hospital with a history of fever with rigor and chills and weight loss. He had no significant past medical history and had never traveled to any regions where visceral leishmaniasis (VL) is endemic. On clinical examination, he was found to have splenomegaly, and hematological investigations revealed pancytopenia. Diagnosis of visceral leishmaniasis was confirmed by splenic smear, and the patient was treated with a total dose of 15 mg/kg amphotericin B (AB) on alternate days for 30 days. No test of cure was performed, but the patient improved symptomatically during the course of treatment, with resolution of fever, regression of spleen, and return of hematological parameters toward normal.
Three months later following discharge, however, there was relapse of his signs and symptoms, and he reported again to the tertiary hospital in Lucknow with high-grade fever, chills, and splenomegaly. He had not traveled outside Lucknow during this time. At this point, he was referred to our institution, Sir Sunderlal Hospital, Banaras Hindu University, Varanasi, India, for specialist management. On clinical examination, he had pallor and splenomegaly of 6 cm below the costal margin. Hematologic examination revealed a hemoglobin level of 8.8 g/dl and a total leukocyte count of 2,400/mm3. His hepatic enzymes were within normal limits, and his total protein was 9 g/dl, with albumin at 2.8 g/dl. Splenic biopsy was repeated, confirming the presence of Leishmania parasites both as observed on splenic smear and grown in culture (strain BHU-402). The patient was subsequently treated with liposomal amphotericin B (Ambisome; Gilead Sciences) in a dose of 3 mg/kg of body weight for 7 days and made a full recovery.
The timing of this patient's return of symptoms and the absence of a travel history made the possibility of reinfection unlikely and raised the question of increased parasite tolerance to AB. Therefore, it was decided that this possibly resistant parasite strain, BHU-402, should be further analyzed in comparison to a representative sensitive clinical parasite isolate, BHU-313, taken from a group of 60 sensitive isolates from patients cured with AB at our field site at the Kala-azar Medical Research Center, Muzaffarpur, Bihar, India. An in vivo sensitivity assay, PCR sequencing of the intragenic cysteine proteinase B (cpb) gene, and SDS-PAGE of crude soluble antigen (CSA) to assess protein production were performed. The data presented below were analyzed by analysis of variance (ANOVA) and Bonferroni's post hoc test, as appropriate.
Ethical clearance to conduct the project was obtained from the Ethical Committee of the Institute of Medical Sciences, Banaras Hindu University.
For in vivo assessment of sensitivity, 44 BALB/c mice (35 to 40 g) were infected by intracardiac injection with 1 × 108 stationary-stage promastigotes: 32 mice with the BHU-402 isolate and 12 mice with the BHU-313 isolate. These promastigotes had been grown in vitro in culture for a maximum of three passages before injection and other downstream analysis. Patency of infection was checked on day 30 postinoculation (p.i.) by sacrificing four randomly selected mice from each group for splenic smears, which were prepared and stained with Giemsa stain for analysis and confirmation of infection.
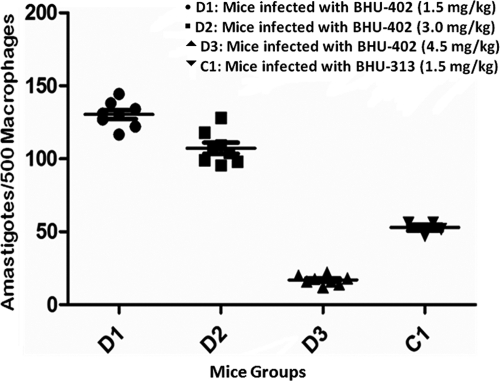
The infected animals from the BHU-402-infected group were randomly divided into four subgroups of 8 animals (three drug groups and one control group) and were either administered with a 5-day course of intraperitoneal injections of AB in doses of 1.5 mg/kg body weight (group D1), 3.0 mg/kg body weight (group D2), 4.5 mg/kg body weight (group D3), or phosphate-buffered saline (PBS) (11). The mice infected with BHU-313 clinical isolates were divided into two groups of four, and one group was treated with a 5-day course of intraperitoneal injections of AB at 1.5 mg/kg body weight (C1) and the control received PBS. Autopsies were conducted on day 7 posttreatment (p.t.). Dabbed imprints of the spleen were taken immediately after autopsy on glass slides. Visceral infection of amastigote parasites was analyzed microscopically by using Giemsa-stained imprints, in which parasite burdens were measured by counting the number of amastigotes per 500 nuclei of macrophage cells. The percentages of inhibition and suppression of replication of parasites were calculated according to the numbers of amastigotes and macrophages by the formulae (5, 6) PI = [(PP − PT)/PP] × 100 and PS = [(PC − PT)/PC] × 100, where PI is the percentage of inhibition, PP is the number of amastigotes per 500 nuclei in spleen before treatment, PT is the number of amastigotes per 500 nuclei after treatment, PS is the percentage of suppression of parasite replication, and PC is the number of amastigotes per 500 nuclei in spleen tissue after treatment in the control group.
There was more than three times greater inhibition of AB-sensitive (BHU-313) parasites compared to parasites with AB relapse (BHU-402) (P ≥ 0.0001) at a normal AB dose in mice of 1.5 mg/kg body weight. Evaluation of the in vivo sensitivity of the assay with different groups is shown in Table 1 and Fig. 1.
Table 1.
Results from in vivo sensitivity assays of BALB/c micea
| Parameter | Result for group |
|||||
|---|---|---|---|---|---|---|
| Untreated |
Infected with: |
|||||
| Resistant strain BHU-402 |
Sensitive strain BHU-313 (C1; 1.5 mg/kg [n = 4]) | |||||
| Resistant (n = 4) | Sensitive (n = 4) | D1 (1.5 mg/kg [n = 8]) | D2 (3.0 mg/kg [n = 8]) | D3 (4.5 mg/kg [n = 8]) | ||
| No. of amastigotes/500 nuclei | 164.91 ± 5.02 | 164.64 ± 6.4 | 130.37 ± 8.78 | 107.25 ± 11.03 | 16.94 ± 3.19 | 52.84 ± 4.26 |
| % inhibition in spleen | 20.94 ± 5.33 | 34.96 ± 6.68 | 89.73 ± 1.94 | 67.91 ± 2.58 | ||
| % suppression of parasite replication | 37.82 ± 4.19 | 48.85 ± 5.26 | 91.92 ± 1.52 | 75.65 ± 1.96 | ||
BALB/c mice infected with BHU-402 parasites were treated with different doses of amphotericin B. The killing of amastigotes was defined in terms of the percentages of inhibition and suppression of parasite replication in the spleen tissue of mice. For all comparisons between the group C1 and D1 drug doses, P < 0.0001.
Fig. 1.
Responses to amphotericin B of the relapse and sensitive strains in different mouse groups. The splenic parasite burden is shown. The BALB/c mice were divided into groups receiving different amphotericin B drug doses. The number of amastigotes per 500 macrophages represents the spleen tissue parasitic burden after treatment. D1, D2, and D3 represent mice infected with BHU-402 and treated with 1.5, 3.0, and 4.5 mg/kg body weight amphotericin B, respectively. C1 represents mice infected with BHU-313 and treated with 1.5 mg/kg body weight amphotericin B.
As part of previous work in our laboratory, we have been exploring genetic heterogeneity in L. donovani clinical isolates, looking at polymorphisms in three genes: Hsp70, ITS, and cpb (unpublished data). In this unique case, we screened BHU-402 for polymorphisms in these genes and found SNPs presented here within the cpb gene only. Total DNA was extracted from 1 × 107 stationary-stage cultured promastigotes, which were subjected to a maximum of three passages, by using the QIAamp DNA minikit (Qiagen, Germany). The cultured strains were subjected to PCR-direct sequencing assays that target the gene locus of intragenic region of cysteine proteinase B (cpb) gene (7). A single band of 1,080 bp was observed on agarose gel electrophoresis.
Sequencing of PCR products was conducted using the ABI BigDye terminator 3.1 cycle sequencing kit (Applied Biosystems, Inc., Foster City, CA) according to the manufacturer's instructions. Sequencing of isolate BHU-402 was done with both forward and reverse primers, and the experiment was repeated to ensure results could be replicated. On comparison of the sequencing data from the cpb gene in isolate BHU-313 with those from the cpb gene in BHU-402, 99% identity between both strains was observed, but mutations revealed four base substitutions affecting alignments in deduced amino acids. No sequence variation in the cpb gene had been previously observed among 60 different sensitive lines (our unpublished data). The positions of substitution single nucleotide polymorphisms (SNPs) were +497T/C, +795C/T, +976A/C, and +977G/T. Of these, two were nonsynonymous SNPs—+497T/C and +977G/T—which resulted in the amino acid substitutions valine→alanine and arginine→leucine.
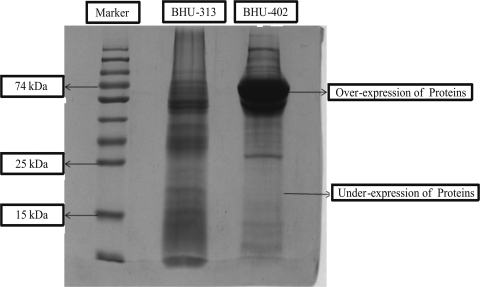
For preparation of CSA, L. donovani parasites grown in Complete RPMI (cRPMI) 1640 medium were centrifuged and washed with PBS two or three times. An approximately equal volume (0.7 ml) of the lysis buffer (pH 7.4, containing 20 mM Tris HCl, 40 mM NaCl, 10 mM EDTA, 2mM phenylmethylsulfonyl fluoride [PMSF] prepared in methanol, 10 μg/ml leupeptin, 2% Nonidet-40, and 10% SDS at 0.4% maintained in triple-distilled water [TDW]) was added to the pelllet and mixed thoroughly. The solution was then sonicated (Soniprep 150) at an amplitude of 12 for 10 cycles of 30 s each in a cold chain. Furthermore, the suspension was centrifuged at 3,000 rpm and 4°C for 20 min. The CSA supernatant was collected and stored at −80°C until use. Forty-five micrograms of CSA from the relapse and sensitive strains was loaded in each well to perform SDS-PAGE. The SDS-PAGE profile of CSA isolated from relapsed strain is markedly different from that of AB-sensitive strains, showing over- and underexpression of proteins in bands in the range of 65 to 80 kDa and 20 kDa, respectively, in relapse strain BHU-402 (Fig. 2).
Fig. 2.
SDS-PAGE profile representing the overexpression and underexpression of proteins in isolate BHU-402 compared with expression of crude soluble protein from isolate BHU-313.
Visceral leishmaniasis (VL), caused by Leishmania donovani, is known to be endemic in the state of Bihar, and in this region less than 40% of patients respond to pentavalent antimonials (10). Amphotericin B (AB) deoxycholate is highly effective in antimony-resistant cases for the treatment of VL and is now used widely in Bihar, with good results (4). In this case report, a parasite strain (BHU-402) with poor responsiveness to AB, isolated from a patient with VL from a region in Uttar Pradesh in which VL is not endemic, is reported. The strain was characterized by in vivo sensitivity assays, PCR sequencing of intragenic cysteine proteinase B (cpb) genes, and SDS-PAGE of CSA to assess protein production.
Resistance to AB is unknown even after nearly 2 decades of its use in India and elsewhere and even after the same strains have been repeatedly exposed to AB (4). Despite the widespread use of AB against fungal infections and leishmaniasis, resistance to this polyene antifungal agent tends to be species dependent and emerges uncommonly and slowly in isolates from patients treated with AB (3). Nonresponsiveness to AB in the context of visceral leishmaniasis has not previously been reported in patients with intact immune systems, and in coinfected HIV patients with multiple relapses treated with AB, no resistance to AB was seen in clinical isolates analyzed in vitro (4). Our patient is unique: coming from a region where VL is not endemic, he had a parasite isolate showing 3 times decreased responsiveness to AB in in vivo compared to the responsiveness of susceptible parasites.
Although, sporadic cases of VL have been reported from the Indian states of Himachal Pradesh and Madhya Pradesh, and a recent outbreak was reported in eastern Uttar Pradesh, there are no reports from central Uttar Pradesh, particularly from an urban area, i.e., the city of Lucknow (1, 2, 8). Due to the rarity of AB resistance, little is known about potential mechanisms, especially in clinical isolates. In a previous report on laboratory-induced AB resistance in Leishmania tarentolae, two independent gene amplification events were observed, and the amplicons consisted of extrachromosomal circles (9). Four novel SNPs were encountered only in our AB relapse isolate, which were not present in 60 other isolates from AB-responsive patients. Moreover, characterization of CSA by SDS-PAGE revealed an interesting overexpression of proteins in the range of a 65- to 80-kDa band and underexpression of 20-kDa proteins that were also not present in 60 responsive isolates.
This first report of VL from central Uttar Pradesh combined with a poor response to AB, confirmed by increased drug tolerance in vivo, is unique. Since there is no mechanistic linkage known between cpb and AB resistance, we speculate that this polymorphism might be a result of normal geographical variation. The protein analysis performed, however, may provide more information as to potential resistance mechanisms in L. donovani, and this warrants further investigation and characterization.
Nucleotide sequence accession numbers.
The sequences obtained from the cpb genes from sensitive strain BHU-313 and relapse strain BHU-402 have been submitted to GenBank under accession no. GU143558 and HQ159842, respectively.
Acknowledgments
This work was supported by NIAID-NIH grant no. 1P50AI074321-01. V.K.P. is thankful to the Indian Council of Medical Research, New Delhi, for financial support.
Footnotes
Published ahead of print on 25 May 2011.
REFERENCES
- 1. Barnett P. G., Singh S. P., Bern C., Hightower A. W., Sundar S. 2005. Virgin soil: the spread of visceral leishmaniasis into Uttar Pradesh, India. Am. J. Trop. Med. Hyg. 73: 720–725 [PubMed] [Google Scholar]
- 2. Dey A., Sharma U., Singh S. 2007. First case of indigenous visceral leishmaniasis from central India. Am. J. Trop. Med. Hyg. 77: 95–98 [PubMed] [Google Scholar]
- 3. Ellis D. 2002. Amphotericin B: spectrum and resistance. J. Antimicrob. Chemother. 49(Suppl. 1):7–10 [DOI] [PubMed] [Google Scholar]
- 4. Lachaud L., et al. 2009. Parasite susceptibility to amphotericin B in failures of treatment for visceral leishmaniasis in patients coinfected with HIV type 1 and Leishmania infantum. Clin. Infect. Dis. 48: 16–22 [DOI] [PubMed] [Google Scholar]
- 5. Manandhar K. D., et al. 2008. Antileishmanial activity of nano-amphotericin B deoxycholate. J. Antimicrob. Chemother. 62: 376–380 [DOI] [PubMed] [Google Scholar]
- 6. Prajapati V. K., et al. 2011. Targeted killing of Leishmania donovani in vivo and in vitro with amphotericin B attached to functionalized carbon nanotubes. J. Antimicrob. Chemother. 66: 874–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Quispe Tintaya K. W., et al. 2004. Antigen genes for molecular epidemiology of leishmaniasis: polymorphism of cysteine proteinase B and surface metalloprotease glycoprotein 63 in the Leishmania donovani complex. J. Infect. Dis. 189: 1035–1043 [DOI] [PubMed] [Google Scholar]
- 8. Sharma N. L., et al. 2009. Characteristics of Leishmania spp. isolated from a mixed focus of cutaneous and visceral leishmaniasis in Himachal Pradesh (India). Internet J. Third World Med. 7:8 [Google Scholar]
- 9. Singh A. K., Ouellette P. M. 2001. Gene amplification in amphotericin B resisitance Leishmania tarentolae. Exp. Parasitol. 99: 141–147 [DOI] [PubMed] [Google Scholar]
- 10. Sundar S., et al. 2000. Failure of pentavalent antimony in visceral leishmaniasis in India: report from the center of the Indian epidemic. Clin. Infect. Dis. 31: 1104–1107 [DOI] [PubMed] [Google Scholar]
- 11. Yardley V., Croft S. L. 2000. A comparison of the activities of three amphotericin B lipid formulations against experimental visceral and cutaneous leishmaniasis. Int. J. Antimicrob. Agents 13: 243–248 [DOI] [PubMed] [Google Scholar]