Abstract
Preclinical studies have shown that diets supplemented with or deficient in n-3 polyunsaturated fatty acids (PUFAs) could influence serotonergic neurotransmission but information about their effects on the serotonergic function of humans is scant. Therefore simultaneous assessments of n-3 PUFAs and of the ACTH and cortisol responses to challenges with the serotonin (5-HT) probe, d,l-fenfluramine (FEN) were performed in 25 cocaine abusing men and 12 control subjects. Cocaine abusers were tested 18 days after their admission to a closed ward. ACTH and cortisol were measured in plasma samples collected on 2 testing days separated by 48 hours following the random administration of 60 mg of FEN or placebo. Fatty acids were measured in the first test day samples. Patients’ FEN-induced ACTH rises were significantly and positively correlated with docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA). Patients’ cortisol rises were positively and significantly correlated with EPA but not with DHA. There were no significant correlations between hormonal responses and pre-hospitalization cocaine use parameters. Control subjects’ responses to FEN were not correlated with any PUFA. In conclusion, higher EPA and DHA levels were associated with a more intense FEN-induced ACTH response and higher EPA levels with a more intense cortisol response in cocaine abusing men withdrawn from cocaine but not in control subjects. These findings support and expand existing evidence that EPA and DHA could influence 5-HT function in some human subgroups.
Keywords: serotonin probe, hormonal responses, EPA, DHA
1. INTRODUCTION
A body of evidence indicates that serotonergic neurotransmission could be influenced by n-3 polyunsaturated fatty acids (PUFAs). Our current knowledge of the relationship between n-3 PUFAs and serotonin (5-HT) is largely based on studies of their manipulations in animal models. In these models, diets deficient in or supplemented with n-3 PUFAs were shown to modify the lipid composition and the neurochemical function of various brain areas. Dietary deficiencies of n-3 PUFAs led to decreases in the concentrations of 5-HT and its metabolite 5-Hydroxyindole Acetic Acid (5-HIAA) in several brain regions (Olson et al., 1998), to decreases in the 5-HT content and turnover in the frontal cortex and to increases in 5-HT receptor density in the frontal cortex with no changes in binding activity (Delion et al., 1996). On the other hand, the supplementation of arachidonic acid (AA, 20:4n-6) and docosahexaenoic acid (DHA, 22:6n-3) in the diets of piglets increased the frontal cortex concentrations of 5-HT and dopamine (de la Pressa Owens and Innis, 2000). Supplements of n-3 PUFAs were also found to reverse a stress induced reduction in 5-HT in mice brain (Van Cassel et al., 2008).
In humans, the precise mechanisms linking the supply of n-3 PUFAs and 5-HT function have not yet been totally elucidated and are probably complex. There is however a growing literature showing that some n-3 PUFAs, whether obtained from the diet or from supplementation, prevent or improve disorders where 5-HT has been implicated, namely depression, suicide, impulsivity and aggressive disorders. This literature has been extensively reviewed (Alessandri et al., 2004; Peet, 2005; Young, and Conquer, 2005; Berger et al., 2006; Freeman et al., 2006; Parker et al., 2006; Riediger et al., 2009). A more direct evidence linking the n-3 PUFA, DHA to serotonergic neurotransmission has been provided by Hibbeln et al. (1998; 2004) who observed that higher cerebrospinal (CSF) concentrations of 5-HIAA were associated with higher concentrations of plasma DHA in healthy subjects and late- onset alcoholics as well as in violent individuals. These studies did not, however, assess the functional responsivity of the 5-HT system in the subjects studied. In contrast to basal measurements, pharmacological challenges measure dynamic changes in neurotransmitter systems and can uncover and amplify underlying alterations. This technique was used in one preclinical study aimed at assessing the effect of one n-3 PUFA on the 5-HT function. In this study, rats were fed either a balanced diet or were made chronically deficient in the n-3 PUFA precursor, alpha linolenic acid (ALA, 18:3n-3). When challenged with the 5-HT releaser/reuptake inhibitor fenfluramine (FEN), a lower 5-HT stimulated release was found in the hippocampus of the deficient rats by comparison with rats fed a balanced diet (Kodas et al., 2004).
In order to further explore in humans the functional link previously suggested between PUFA status and serotonergic processes, the study described below was performed in healthy control subjects and in cocaine abusing men. These patients frequently present problems in mood, impulse and aggression control believed to be linked to a dysregulated 5-HT function. Moreover some evidence suggests a role for the 5-HT system in cocaine addiction. Craving for cocaine was observed to decrease after the administration of a single dose of 60 mg of FEN (Buydens-Branchey et al., 1998) and a study of the serotonergic responsiveness to FEN of cocaine users suggested the existence of changes in 5-HT transmission during early cocaine abstinence (Ghitza et al., 2007).
Patients’ and healthy controls’ responsivity to the challenging agent d,l-FEN was explored as a function of their plasma PUFA levels. When administered acutely, d,l-FEN induces the release of 5-HT from the hypothalamic 5-HT neurons nerve terminals. This provokes the secretion of adrenocorticotropic hormone (ACTH) from the anterior pituitary and the secretion of cortisol from the adrenals. The PUFAs of interest were two n-3 PUFAs, eicosapentaenoic acid (EPA, 20:5n-3) and DHA as well as one n-6 PUFA, AA. DHA and EPA, found to have psychotropic effects, have different mechanisms of action (Sinclair et al., 2007). DHA is concentrated in synaptic neuronal membranes, can alter the fluidity of these membranes and can influence the conformation of embedded proteins (Salem et al., 2001). These proteins can act as receptors or transporters and can alter the passage of ions. DHA can thus affect the function of neurotransmitter systems. EPA is not concentrated in neuronal membranes but may affect neuronal function via neuroimmunological and vascular effects (Sinclair et al., 2007). It competes with AA for conversion to potent eicosanoids (Simopoulos, 2002). It competitively attenuates the formation of n-6 eicosanoids that mediate immune inflammatory responses. It can also, as well as DHA, inhibit the production of proinflammatory cytokines (Song et al., 1998; Salem et al., 2001). In addition, EPA could counteract the vasoconstrictive effects of AA, thereby increasing blood flow in the brain.
It was hypothesized that the intensity of the patients’ ACTH and cortisol responses to the acute administration of challenging doses of 60 mg of d,l-FEN would be influenced by circulating levels of n-3 PUFAs and/or by the n-6/n-3 PUFA ratio. It was also hypothesized that this would not be the case for the healthy controls’ responses. It should be pointed out that the amounts of ACTH and cortisol measured following the pharmacological stimulation with FEN reflect the responsivity of the serotonergic system and are different from amounts resulting from the spontaneous activity of the HPA axis.
2. METHODS
2.1. Subjects
2.1.1 Patients
Patients selected for participation in this study were 25 cocaine abusing men, aged 39.0±6.1years (Mean±SD). They were studied while hospitalized on an inpatient drug rehabilitation unit. To ensure that patients admitted to this unit were kept in a drug free environment, they underwent a thorough search upon admission, were prevented from receiving visitors and were not allowed to leave the premises unless accompanied by a staff member. The unit was kept locked at all times. Urines were collected upon admission and in a random fashion throughout patients’ hospitalization. They were tested for the presence of substances of abuse.
Participating patients were physically healthy. The absence of medical problems was confirmed by physical examination and clinical chemistry laboratory tests. Patients did not receive any medication during their stay in the hospital. They were screened with the Structured Clinical Interview for DSM IV or SCID (First et al., 1997). Additional information was obtained about number of years of regular use of substances and amounts of substances consumed during the month preceding admission. Patients were enrolled in the study if they met DSM IV criteria for cocaine dependence but did not meet criteria for any other Axis I disorder (including dependence on any substance besides cocaine) as determined by the SCID. Patients with a history of intravenous use of any substance, patients who had used opiates in any form during the year preceding their admission and patients who had used on a daily basis more than 0.8 g/kg BW of pure ethanol during the year preceding their admission were excluded from the study. The presence of Axis II disorders was not an exclusionary criterion. All patients participating in the study had signed an informed consent.
Patients were administered the Buss-Durkee Hostility Inventory (Buss and Durkee, 1957), the Baratt Impulsiveness Scale, version 10 (Barratt, 1985) and the Eysenck IVE questionnaire (Eysenck and Eysenck, 1978). The Buss-Durkee score was arrived at by adding the scores on seven hostility and one suspicion subscales, the Barratt score by adding the scores on motor impulsiveness, cognitive impulsiveness and nonplanning impulsiveness subscales, and the Eysenck score by adding the scores on an impulsiveness, a venturesomeness and an empathy subscales. A life-long history of violent and criminal behavior was also obtained.
2.1.2 Control subjects
Twelve healthy male subjects, aged 33.9±7.3 years (Mean±SD) were selected following a psychiatric interview and the administration of the SCID to ensure that they did not have a history of abuse of alcohol or other substances and did not present any Axis I or Axis II diagnosis. After having been selected and having signed an informed consent, controls were administered the same psychological test battery as the patients.
2.2. Challenge procedure and blood collection
The study was conducted prior to 1997, when FEN was still an FDA-approved medication. Patients’ data presented below were obtained 18.44±1.33 days after they had been admitted to the hospital and had been kept in a drug free environment. Testing was delayed for 18 days because cortisol responses that have been shown to be altered during early cocaine abstinence normalize within 2 weeks (Contoreggi et al., 2003).
After having signed informed consent, patients and controls participated in two test sessions separated by 48 hours during which either 60 mg of FEN in its d,l-form or placebo were given orally as identically looking capsules. The order of FEN and placebo administration was random and capsules given according to a double blind design. Subjects were instructed to fast after midnight on test days and continued to fast until the end of the test sessions. During these sessions, they stayed awake and abstained from smoking. Blood was drawn from an antecubital vein through an intravenous catheter kept patent by a slow infusion of normal saline solution. The catheter was inserted at 8:00 am and blood samples drawn for ACTH and cortisol determinations, 30 and 15 minutes before and 30, 60, 90, 120, 180, 210, 240 and 270 minutes after the administration of FEN or placebo that took place at 9:00 am. A blood aliquot collected 15 minutes before the administration of the FEN or placebo capsules on the first study day was used for fatty acids (FAs) and basal ACTH and cortisol measurements. Blood was centrifuged and biochemical measurements done in plasma samples kept frozen at −80° C until assayed.
2.3. Follow-up assessments
Following discharge, patients’ new hospitalizations for cocaine-related problems were monitored for a period of 6 months.
2.4. Biochemical determinations
Plasma ACTH levels were measured with radioimmunoassay kits from “Nichols, San Juan Capistrano, CA ”. Intra-assay and inter-assay coefficients of variance were 5.5 and 8.3%. Plasma cortisol levels were measured with radioimmunoassay kits from “ICM Pharmaceuticals, Costa Mesa, CA”. Intra-assay and inter-assay coefficients of variance were 6.7% and 8.9%.
Plasma FEN levels were quantified by gas chromatography using a nitrogen selective detector according to the method of Krebs et al. (1984) with minor modifications. Intra-assay and inter-assay coefficients of variance were 2.7% and 3.7% at 15 ng/ml.
FAs were measured at the NIAAA Laboratory of Membrane Biophysics and Biochemistry. The method used involved liquid transmethylation followed by gas chromatographic determination (Masood et al., 2005). Measurements included total FAs; total saturated fatty acids (SFAs), comprising 14:0, 16:0, 17:0, 18:0, 20:0, 22:0 and 24:0 FAs; total monounsaturated fatty acids (MUFAs), comprising 14:1n-5, 16:1n-7, 18:1n-7, 18:1n-9, 20:1n-9, 22:1n-9 and 24:1n-9 FAs; total PUFAs, comprising all individual PUFAs along the n-3 and n-6 PUFAs elongation and desaturation pathways. Alpha-linolenic acid (ALA, 18:3n-3), EPA and DHA as well as linoleic acid (LA, 18:2n-6), AA and docosapentaenoic acid (DPA, 22:5n-6) were among these measurements.
2.5. Data analysis
Unless specified otherwise, values expressed as means are given with SDs.
In order to assess the intensity of the ACTH and cortisol responses to FEN, double Deltas (ΔΔs) were calculated for each subject. This measure is defined as the difference between the maximum change from baseline following FEN and the change from baseline at the corresponding time following placebo.
2.5.1. Patients
The level of statistical association between patients’ ΔΔs and individual patients’ age, weight, peak fenfluramine plasma levels and age at onset of cocaine use as well as number of days of use and total amount of cocaine used during the month preceding admission was assessed with Pearson correlation coefficients. Only age was found to be significantly associated with the ACTH and cortisol ΔΔs. The level of statistical association between PUFA variables and the ACTH and cortisol ΔΔs was then assessed with partial correlation coefficients, with age as a control variable. These partial correlation coefficients were obtained using the “Regression” program of the SPSS Statistics software (version 17.0). This program provided “partial regression plots”, namely scatterplots of the residuals of the dependent variable (in the present study, the ACTH ΔΔ or the cortisol ΔΔ) and one independent variable (in the present study, one of the PUFA levels) when both of these variables are regressed on the rest of the independent variables (in the present study, age).
2.5.2. Control subjects
The level of statistical association between subjects’ΔΔs and individual subjects’ age, weight and peak FEN plasma levels was assessed with Pearson correlation coefficients. Only age was found to be significantly associated with the ACTH and cortisol ΔΔs. The level of statistical association between PUFA variables and the ACTH and cortisol ΔΔs was assessed with partial correlation coefficients, with age as a control variable.
2.5.3. Group comparisons
Student’s t-tests were used to compare the two groups’ psychological tests scores and their plasma FAs levels, after having tested the equality of variances with the Levene test. Comparisons between the groups’ hormonal responses to FEN were done with univariate analyses of covariance, using age as a covariate.
2.5.4. Relapse
Student’s t-tests were used to compare the FAs measured on the first study day (prior to the administration of FEN or placebo) of the patients who were rehospitalized for cocaine-related problems to those of patients who were not rehospitalized.
3. RESULTS
3.1. Prechallenge assessments
3.1.1 Intake of substances
The 25 patients, who were 39.0±6.1 years of age when tested, had been 28.0±6.3 years old when they started using cocaine on a regular basis. They had used cocaine 21.1±9.3 days during the month preceding their admission and had used 32.6±25.4 grams of cocaine during that month. Admission urine toxicology screens were positive for cocaine. Additional screens performed while patients were hospitalized were all negative.
Control subjects had no history of use of substances other than alcohol. Their total consumption of ethanol during the month preceding the study was equivalent to 4.04±6.63 oz. of pure ethanol or an average daily consumption of 0.044±0.073 g of ethanol/kg of body weight.
3.1.2. Psychological scales / Violent and criminal behavior
Scores on the Buss-Durkee Hostility Inventory, the Baratt Impulsiveness Scale and the Eysenck IVE questionnaire are shown on table 1. Patients’ scores on the three scales were significantly higher than the scores of the control subjects (p<0.001 for the three scales).
Table 1.
Comparisons of patients’ and healthy subjects’ psychological scales scores
| Patients N=25 |
Healthy subjects N=12 |
P value | |
|---|---|---|---|
| Buss-Durkee | 29.9±9.8 | 18.7±9.2 | <0.001 |
| Baratt 10 | 56.8±15.5 | 37.4±9.9 | <0.001 |
| Eysenck IVE | 30.3±6.0 | 20.9±6.7 | <0.001 |
Values are Means±SD. Patients’ and healthy subjects’ values were compared with Student’s t-tests.
Ten patients (40%) had displayed assaultive behaviors. These behaviors included hitting and kicking others, assaulting others with guns, knives and pipes, raping and beating girlfriends, and armed robberies. Other violent behaviors involved the breaking up of car windows, of radios, of TV sets and of furniture. Six patient s (24%) had served jail terms. Eleven patients (44%) reported crimes that did not involve violence such as thefts without confrontation of the victims, forgeries and receiving stolen property. Controls did not report displaying these behaviors.
3.2. ACTH and cortisol responses
The administration of 60 mg of d.l-FEN induced rises in ACTH and cortisol. The ACTH ΔΔ value was lower in patients than in control subjects (22.6±18.8 vs. 24.6±14.5 pg/ml) but not significantly so. The cortisol ΔΔ value was also lower in patients than in control subjects (6.9±5.5 vs. 11.0±3.6 μg/dl) but the difference did not reach a conventional level of significance when age was taken into account (F1,34=2.9, p=0.098).
3.3. Cocaine effects
Eighteen days after admission, baseline ACTH and cortisol as well as these hormones response to FEN were not significantly associated with any of the cocaine use parameters.
3.4. Fatty acids plasma levels
Table 2 shows the mean FA values of patients and control subjects, expressed as percent of total FAs. Comparisons between the two groups revealed that patients had significantly higher plasma levels of DPA n-6 and significantly lower levels of total n-3 PUFAs, ALA and DHA. The n-6/n-3 PUFA ratio was significantly higher in the patients.
Table 2.
Comparisons of plasma fatty acid levels of patients and control subjects
| Fatty acids | Patients | Control subjects |
P value |
|---|---|---|---|
| Total FAs | 2346.78±516.90 | 2290.82±673.34 | N.S. |
| Total SFAs | 31.92±1.92 | 31.33±1.94 | N.S. |
| Total MUFAs | 23.09±2.75 | 23.98±2.85 | N.S. |
| Total n-6 PUFAs | 42.10±3.87 | 41.30±4.00 | N.S. |
| LA | 30.19±3.39 | 30.76±3.19 | N.S. |
| AA | 8.02±1.29 | 7.66±1.08 | N.S. |
| DPA | 0.30±0.07 | 0.20±0.04 | 0.001 |
| Total n-3 PUFAs | 2.89±0.62 | 3.39±0.85 | 0.049 |
| ALA | 0.55±0.20 | 0.70±0.20 | 0.039 |
| EPA | 0.56±0.17 | 0.60±0.29 | N.S. |
| DHA | 1.21±0.29 | 1.51±0.52 | 0.036 |
| n-6/n-3 PUFA ratio | 15.12±3.19 | 12.77±2.92 | 0.038 |
All values are Means±SD. Total FAs are expressed in μg/ml. All other values, except for the ratio, are expressed as % of total FAs. Comparisons between patients and control subjects were made with Student’s t-tests.
3.5. Correlational analyses
None of the correlations between basal ACTH and cortisol concentrations and FAs or FA ratios were significant.
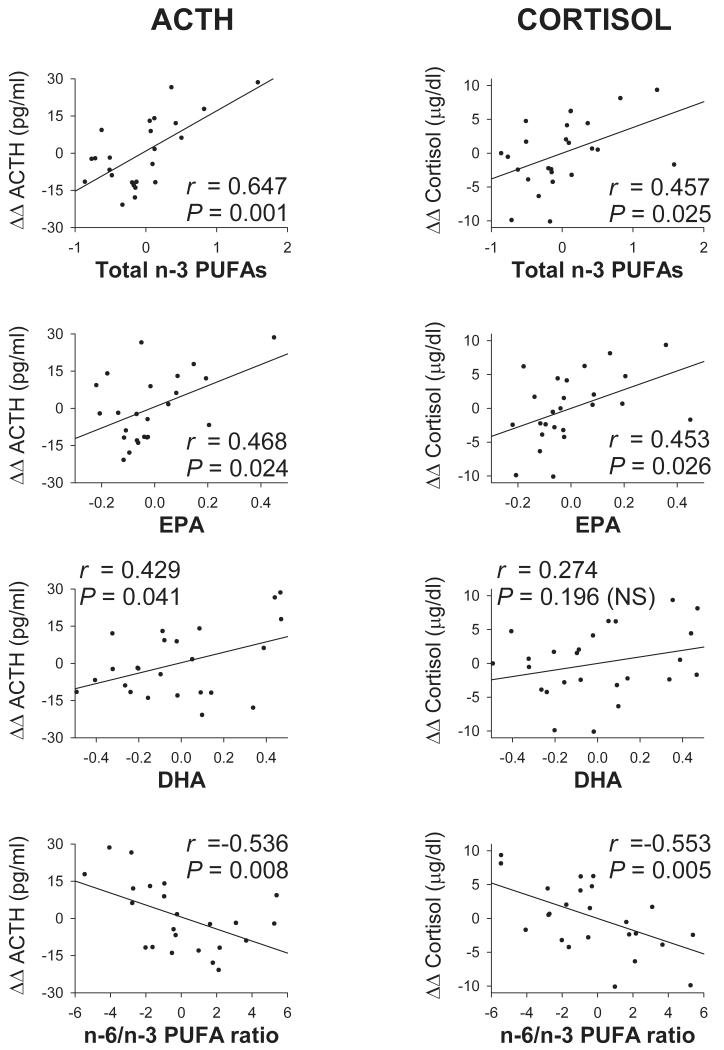
Table 3 shows the patients’ partial correlation coefficients (with age as control variable) between FA values, expressed as percent of total FAs and the ACTH and cortisol rises that followed the administration of FEN. The table also shows the levels of significance of the correlations. In the patient group, the ACTH ΔΔ was significantly and positively correlated with total n-3 PUFAs, ALA, EPA and DHA and significantly but negatively correlation with the n-6/n-3 PUFA ratio. The cortisol ΔΔ was significantly and positively correlated with total n-3 PUFAs and EPA and negatively correlated with the n-6/n-3 PUFA ratio. The ACTH and cortisol responses were not correlated with total SFA, total MUFA, total PUFAs, total n-6 PUFAs or individual n-6 PUFAs.
Table 3.
Partial correlation coefficients between patients’ fatty acids and their responses to FEN
| ΔΔ ACTH | ΔΔ Cortisol | |||
|---|---|---|---|---|
| Fatty acids. | Partial CC. | P value | Partial CC. | P value |
| Total SFAs | 0.058 | N.S. | 0.145 | N.S. |
| Total MUFAs | −0.118 | N.S. | 0.282 | N.S. |
| Total n-6 PUFAs | −0.046 | N.S. | −0.351 | N.S. |
| LA | −0.019 | N.S. | −0.319 | N.S. |
| AA | −0.035 | N.S. | −0.149 | N.S. |
| DPA | −0.120 | N.S. | −0.364 | N.S. |
| Total n-3 PUFAs | 0.647 | 0.001 | 0.457 | 0.025 |
| ALA | 0.408 | 0.048 | 0.384 | N.S. |
| EPA | 0.468 | 0.024 | 0.453 | 0.026 |
| DHA | 0.429 | 0.041 | 0.274 | N.S. |
| n-6/n-3 PUFA ratio | −0.536 | 0.008 | −0.553 | 0.005 |
The partial correlation coefficients between fatty acid levels (in % of total FAs) and hormonal responses to FEN were calculated with age as a control variable.
Figure 1 displays the scattergrams and regression lines for patients’ ACTH and cortisol responses to FEN as a function of total n-3 PUFAs, EPA, DHA and the n-6/n-3 PUFA ratio.
Figure 1.
Patients’ partial regression plots of ACTH and cortisol responses (ΔΔs) to fenfluramine as a function of some PUFA values, using age as a control variable. PUFA values are expressed as % of total FAs. Regression lines, partial correlation coefficients (r) and their levels of significance are shown in each plot. Correlations are positive for total n-3 PUFAs, EPA and DHA. They are negative for the n-6/n-3 PUFA ratio.
None of the control subjects’ partial correlation coefficients (with age as control variable) between FA values and responses to FEN reached a conventional level of significance.
3.6. Relapse
Only DHA measured prior to the administration of FEN or placebo was found to be significantly lower in the patients who needed to be rehospitalized when compared to those who did not. Differences between relapsers and non relapsers were significant 3 months after discharge (22.0±0.6 vs. 29.4±7.7 μg/ml, p=0.004) and 6 months after discharge (22.9±2.1 vs. 29.2±8.3 μg/ml, p=0.029).
4. DISCUSSION
Several mechanisms have been proposed to explain the impact of n-3 PUFAs in psychiatric disorders. According to one theory, an increase in DHA that is selectively concentrated in synaptic neuronal membranes would facilitate serotonergic neurotransmission but evidence linking DHA and 5-HT in humans is still scant. In the present study of cocaine abusers and controls, a challenge technique was used that allowed the assessment of the functional responsiveness of hypothalamic 5-HT neurons nerve terminals to a serotonergic probe as a function of PUFA levels. In order to allow normalization of cortisol responses, known to be altered during early abstinence and to normalize within 2 weeks (Contoreggi et al., 2003), testing started 18 days after patients were admitted to the Medical Center. None of the cocaine use parameters were found to be significantly associated with baseline cortisol or cortisol responses at that time.
In the patients, a significant positive correlation was found between DHA levels and the ACTH response to the probe. These data give support in some humans to the observation made by Kodas et al. (2004) of a decrease in FEN-stimulated 5-HT release in the hippocampus of rats subjected to a chronic n-3 PUFAs dietary deficiency. Explanations for the observations made in the present study of an association between higher DHA levels and an increased ACTH response to FEN can be derived from preclinical studies that have shown that DHA is readily incorporated into neuronal membranes phospholipids, increases these membranes fluidity and affects the function of receptors, transporters and ion channels as well as enzyme activity, signal transmission and the regulation of neurotransmitters including 5-HT (Tassoni et al., 2008).
A more robust ACTH response to the probe was also found to be significantly associated with EPA levels. EPA is not concentrated in neuronal membranes but cell cultures and animal studies have demonstrated that it can, as well as DHA, inhibit the production of several proinflammatory cytokines, such as interleukins 1-beta and 6, and tumor necrosis factor alpha (Song et al., 1998; Salem et al., 2001), that are potent inducers of the enzyme indole-amine 2,3-dioxygenase (IDO) (Weiss et al., 1999). An increase in IDO leads to a decrease in tryptophan, the 5-HT precursor that crosses the blood-brain barrier. Tryptophan hydroxylase, the rate-limiting enzyme along the 5-HT pathway is unsaturated and a decrease in tryptophan leads to a decrease in brain 5-HT (Grohmann et al., 2003; Babcock and Carlin, 2000). Through their inhibition of cytokines production, EPA and DHA can thus influence brain 5-HT levels. The increased ACTH response of individuals with higher EPA levels could also be due to an increase in the levels of neuronal synaptic membranes that were found by Cansev and Wurtman (2007) to be enhanced following the administration of both EPA and DHA. It has also been suggested that EPA could play a role in brain function by counteracting the AA-mediated signaling. EPA could, for example, attenuate the formation of n-6 derived eicosanoids that mediate immune-inflammatory responses or could counteract the vasoconstrictive effects of AA, thereby increasing blood flow in the brain. Meta-analyses and several clinical trials have reported an antidepressant effect of n-3 PUFAs and although the relative roles of DHA and EPA are still unknown, it has been argued that EPA might be more effective as an antidepressant than DHA (Lin and Su, 2007) but this conclusion needs to be corroborated by additional studies.
The cortisol response to an increase in the serotonergic tone was significantly and positively correlated with EPA levels. The correlation between the cortisol response and DHA was positive but did not reach a conventional level of significance. The suppressive action of DHA on the adrenocortical function, demonstrated in animal studies, could have played a role in this observation. Carsia et al. (2008) studied the corticosterone responses to ACTH of the adrenocortical cells of rats fed diets that contained 10% of different FAs as the predominant lipids. Some of the diets contained butter fat (high saturated fat), EPA or DHA. Compared to the butter fat, both n-3 PUFAs dampened the corticosterone responses. The suppression efficacies of EPA and DHA depended on the duration of administration of the diets, with DHA being more effective than EPA after 6 months. It should be kept in mind that in the Carsia et al. study, equal proportions of the two PUFAs had been administered whereas, in the present study, participants’ EPA levels were smaller than their levels of DHA and might have been less effective in dampening the cortisol response.
Unlike patients, control subjects did not show significant correlations between PUFAs and responses to the probe. This could be attributed to the small number of subjects and thus to a lack of power of the statistical analysis. It could also be hypothesized that controls did not present 5-HT deficits that could have been influenced by n-3 PUFAs. Past history and psychological scales indicated that their levels of hostility and impulsiveness, known to be associated with deficits in serotonergic neurotransmission, were much lower than those of patients. Their n-6/n-3 PUFA ratio was lower than that of patients. Whether this could have contributed to the present finding cannot be determined because the ideal ratio has not yet been established.
In a study of perpetrators of domestic violence, Hibbeln et al. (2004) observed that low basal levels of DHA were correlated to higher levels of corticotrophin releasing hormone (CRH). In this study, ACTH and cortisol were not measured. These data could appear to be in contradiction with the present observation of a more robust ACTH response in subjects with higher DHA levels. It should be kept in mind that Hibbeln et al. assessed basal levels of CRH whereas in the present study, ACTH and cortisol were measured following a pharmacological challenge aimed at inducing a transient increase in the serotonergic tone. Moreover, the activity of the HPA axis is modulated by a number of feed-back mechanisms, one of which could involve an increase in neuroactive steroids. An association was recently observed between lower plasma n-3 PUFAs and increases in neuroactive steroid concentrations, which could indicate an increased feedback of the HPA axis (Nieminen et al., 2006).
A low DHA was significantly associated with patients’ rate of rehospitalization for cocaine-related problems up to 6 months following their discharge but resumption of cocaine use that did not lead to rehospitalization was not monitored. This can be considered as being one of the limitations of the present study. Nevertheless, in another study of substance abusers treated as outpatients, baseline FAs were measured when patients were enrolled and every 3 months for one year thereafter. Relapsers were also found to have significantly lower DHA levels at the start of the study when compared to non-relapsers (Buydens-Branchey et al., 2009).
In the present study, FAs were measured at one point in time and one could wonder whether these measurements are representative of FA levels over longer periods. An animal study of depletion and repletion of DHA showed that the brain required 12 weeks for its DHA deficiency to be restored (Moriguchi et al., 2001). One could thus speculate that the central nervous system (CNS) PUFA levels of this study participants were determined in part by preadmission dietary intakes. In another study of a comparable group of substance abusers who were followed for one year, plasma n-3 and n-6 levels were assessed at baseline and every 3 months thereafter. These levels remained remarkably stable throughout the follow-up period, indicating that patients’ diets changed little over time (Buydens-Branchey et al., 2009). In addition, dietary intake is probably not the only contributing factor to plasma and CNS PUFA levels. As yet unidentified metabolic variations could play a role as well (Freeman et al., 2006).
In conclusion, an acute and transient increase in the serotonergic tone following the administration of FEN provoked a rise in ACTH that, in cocaine abusers, was significantly correlated with EPA and DHA levels. It also resulted in a rise in cortisol that was significantly correlated with EPA levels. These findings support and expand existing evidence indicating that EPA and DHA could possibly influence serotonergic neurotransmission in humans presenting impairments in the serotonergic function, as might have been the case for the cocaine abusing individuals who were participants in the present study. No correlations were found in a group of control subjects. This could reflect a lack of alterations in their 5-HT pathways but this hypothesis is speculative and in need of corroboration.
ACKNOWLEDGMENTS
The work was supported by grant R01-DA15630 from the National Institute on Drug Abuse and by the National Institute on Alcohol Abuse and Alcoholism.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alessandri JM, Guesnet P, Vancassel S, Astorg P, Denis I, Langelier B, Aïd S, Poumès-Ballihaut C, Champeil-Potokar G, Lavialle M. Polyunsaturated fatty acids in the central nervous system: evolution of concepts and nutritional implications throughout life. Reproduction, Nutrition, Development. 2004;44:509–538. doi: 10.1051/rnd:2004063. [DOI] [PubMed] [Google Scholar]
- Babcock TA, Carlin JM. Transcriptional activation of indoleamine dioxygenase by interleukin 1 and tumor necrosis factor alpha in interferon-treated epithelial cells. Cytokine. 2000;12:588–594. doi: 10.1006/cyto.1999.0661. [DOI] [PubMed] [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Itard CE, editors. Motivation, emotion and personality. Elsevier; Amsterdam: 1985. pp. 137–146. [Google Scholar]
- Berger GE, Smesny S, Amminger GP. Bioactive lipids in schizophrenia. International Review of Psychiatry. 2006;18:85–98. doi: 10.1080/09540260600583072. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Effect of fenfluramine challenge on cocaine craving in addicted male users. American Journal on Addictions. 1998;7:142–155. [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hibbeln JR. Low plasma levels of docosahexaenoic acid are associated with an increased relapse vulnerability in substance abusers. American Journal on Addictions. 2009;18:73–80. doi: 10.1080/10550490802544003. [DOI] [PubMed] [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of consulting psychology. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Cansev M, Wurtman RJ. Chronic administration of docosahexaenoic acid or eicosapentaenoic acid, alone or in combination with uridine, increases brain phosphatiede and synaptic protein levels in gerbils. Neuroscience. 2007;148:421–431. doi: 10.1016/j.neuroscience.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carsia RV, Weber H, McIlroy PJ, Hock CE. Long-term dietary lipid regimen alters adrenocortical function at the cellular level. Hormone and Metabolic Research. 2008;40:848–853. doi: 10.1055/s-0028-1086025. [DOI] [PubMed] [Google Scholar]
- Contoreggi C, Herning RI, Koeppl B, Simpson PM, Negro PJ, Jr., Fortner-Burton C, Hess J. Treatment-seeking inpatient cocaine abusers show hypothalamic dysregulation of both basal prolactin and cortisol secretion. Neuroendocrinology. 2003;78:154–162. doi: 10.1159/000072797. [DOI] [PubMed] [Google Scholar]
- de la Pressa Owens S, Innis SM. Diverse, region-specific effects of addition of arachidonic and docosahexaenoic acids to formula with low or adequate linoleic and α-linolenic acids on piglet brain monoaminergic neurotransmitters. Pediatric Research. 2000;48:125–130. doi: 10.1203/00006450-200007000-00022. [DOI] [PubMed] [Google Scholar]
- Delion S, Chalon S, Guilloteau D, Besnard JC, Durand G. α-Linolenic acid dietary deficiency alters age-related changes of dopaminergic and serotoninergic neurotransmission in the rat frontal cortex. Journal of Neurochemistry. 1996;66:1582–1591. doi: 10.1046/j.1471-4159.1996.66041582.x. [DOI] [PubMed] [Google Scholar]
- Eysenck SBG, Eysenck HJ. Impulsiveness and Venturesomeness: Their position in a dimensional system of personality description. Psychological Reports. 1978;43:1247–1255. doi: 10.2466/pr0.1978.43.3f.1247. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM IV Axis I Disorders (SCID-I) American Psychiatric Press; Washington, DC: 1997. [Google Scholar]
- Freeman MP, Hibbeln JR, Wisner KL, Davis JM, Mischoulon D, Peet M, Keck PE, Jr, Marangell L,B, Richardson A,J, Lake J, Stoll AL. Omega-3 fatty acids: evidence basis for treatment and future research in psychiatry. Journal of Clinical Psychiatry. 2006;67:1954–1967. doi: 10.4088/jcp.v67n1217. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Rothman RB, Gorelick DA, Henningfield JE, Baumann MH. Serotonergic responsiveness in human cocaine users. Drug and Alcohol Dependence. 2007;86:207–213. doi: 10.1016/j.drugalcdep.2006.06.007. [DOI] [PubMed] [Google Scholar]
- Grohmann U, Fallarino F, Puccetti P. Tolerance, DCs and tryptophan: much ado about IDO. Trends in Immunology. 2003;24:242–248. doi: 10.1016/s1471-4906(03)00072-3. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Bissette G, Umhau JC, George DT. Omega-3 status and cerebrospinal fluid corticotrophin releasing hormone in perpetrators of domestic violence. Biological Psychiatry. 2004;56:895–897. doi: 10.1016/j.biopsych.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Hibbeln JR, Linnoila M, Umhau C, Rawlings R, George DT, Salem N., Jr. Essential fatty acids predict metabolites of serotonin and dopamine in cerebrospinal fluid among healthy control subjects, and early- and late-onset alcoholics. Biological Psychiatry. 1998;44:235–242. doi: 10.1016/s0006-3223(98)00141-3. [DOI] [PubMed] [Google Scholar]
- Kodas E, Galineau L, Bodard S, Vancassel L, Guilloteau D, Besnard JC, Chalon S. Serotonergic transmission is affected by n-3 polyunsaturated fatty acids in the rat. Journal of Neurochemistry. 2004;89:695–702. doi: 10.1111/j.1471-4159.2004.02401.x. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Cheng LK, Wright GH. Determination of fenfluramine and norfenfluramine in plasma using a nitrogen sensitive detector. Journal of Chromatography B: Biomedical Sciences and Applications. 1984;310:412–417. doi: 10.1016/0378-4347(84)80109-7. [DOI] [PubMed] [Google Scholar]
- Lin PY, Su KP. A meta-analytic review of double-blind, placebo-controlled trials of antidepressant efficacy of omega-3 fatty acids. Journal of Clinical Psychiatry. 2007;68:1056–1061. doi: 10.4088/jcp.v68n0712. [DOI] [PubMed] [Google Scholar]
- Masood A, Stark KD, Salem N., Jr. A simplified and efficient method for the analysis of fatty acid methyl esters suitable for large clinical studies. Journal of Lipid Research. 2005;46:2299–2305. doi: 10.1194/jlr.D500022-JLR200. [DOI] [PubMed] [Google Scholar]
- Moriguchi T, Loewke J, Garrison M, Catalan JN, Salem N., Jr. Reversal of docosahexaenoic acid deficiency in the rat brain, retina, liver, and serum. Journal of Lipid Research. 2001;42:419–427. [PubMed] [Google Scholar]
- Nieminen LRG, Makino KK, Mehta N, Virkkunen M, Kim HY, Hibbeln JR. Relationship between omega-3 fatty acids and plasma neuroactive steroids in alcoholism, depression and controls. Prostaglandins, Leukotrienes and Essential Fatty Acids. 2006;75:309–314. doi: 10.1016/j.plefa.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Olsson NU, Shoaf SE, Salem N., Jr. The effect of dietary polyunsaturated fatty acids and alcohol on neurotransmitters levels in rat brain. Nutritional Neuroscience. 1998;1:133–140. doi: 10.1080/1028415X.1998.11747222. [DOI] [PubMed] [Google Scholar]
- Parker G, Gibson NA, Brotchie H, Heruc G, Rees AM, Hadzi-Pavlovic D. Omega-3 fatty acids and mood disorders. American Journal of Psychiatry. 2006;163:969–978. doi: 10.1176/ajp.2006.163.6.969. [DOI] [PubMed] [Google Scholar]
- Peet M. Nutrition and schizophrenia. World Review of Nutrition and Dietetics. 2005;95:17–28. doi: 10.1159/000088267. [DOI] [PubMed] [Google Scholar]
- Riediger ND, Othman RA, Suh M, Moghadasian MH. A systemic review of the roles of n-3 fatty acids in health and disease. Journal of the American Dietetic Association. 2009;109:668–679. doi: 10.1016/j.jada.2008.12.022. [DOI] [PubMed] [Google Scholar]
- Salem N, Jr., Litman B, Kim HY, Gawrisch K. Mechanisms of action of docosahexaenoic acid in the nervous system. Lipids. 2001;36:945–959. doi: 10.1007/s11745-001-0805-6. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. Omega-3 fatty acids in inflammation and autoimmune diseases. Journal of the American College of Nutrition. 2002;21:495–505. doi: 10.1080/07315724.2002.10719248. [DOI] [PubMed] [Google Scholar]
- Sinclair AJ, Begg D, Mathai M, Weisinger RS. Omega 3 fatty acids and the brain: review of studies in depression. Asia Pacific Journal of Clinical Nutrition. 2007;16(Suppl 1):391–397. [PubMed] [Google Scholar]
- Song C, Lin A, Bonaccorso S, Heide C, Verkerk R, Kenis G, Bosmans E, Scharpe S, Whelan A, Cosyns P, de Jongh R, Maes M. The inflammatory response system and the availability of plasma tryptophan in patients with primary sleep disorders and major depression. Journal of Affective Disorders. 1998;49:211–219. doi: 10.1016/s0165-0327(98)00025-1. [DOI] [PubMed] [Google Scholar]
- Tassoni D, Kaur G, Weisinger RS, Sinclair AJ. The role of eicosanoids in the brain. Asia Pacific Journal of Clinical Nutrition. 2008;17(Suppl 1):220–228. [PubMed] [Google Scholar]
- Vancassel S, Leman S, Hanonick L, Denis S, Roger J, Nollet M, Bodard S, Kousignian I, Belzung C, Chalon S. n-3 Polyunsaturated fatty acid supplementation reverses stress-induced modifications on brain monoamine levels in mice. Journal of Lipid Research. 2008;49:340–348. doi: 10.1194/jlr.M700328-JLR200. [DOI] [PubMed] [Google Scholar]
- Young GS, Conquer J. Omega-3 fatty acids and neuropsychiatric disorders. Reproduction, Nutrition, Development. 2005;45:1–28. doi: 10.1051/rnd:2005001. [DOI] [PubMed] [Google Scholar]
- Weiss G, Murr C, Zoller H, Haun M, Widner B, Ludescher C, Fuchs D. Modulation of neopterin formation and tryptophan degradation by Th1- and Th2-derived cytokines in human monocytic cells. Clinical and Experimental Immunology. 1999;116:435–440. doi: 10.1046/j.1365-2249.1999.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]