Abstract
Hemorrhagic coagulopathy (without neurological injuries) constitutes 40% of injury-related death in civilian hospitals and on the battlefield, and the underlying contributing mechanisms remain unclear. The purpose of this study is to investigate the effects of fibrinogen availability on coagulation function after hemorrhage in pigs. Sixteen crossbred commercial Yorkshire swine were randomized into the control group (group C) (n = 8) and hemorrhage group (group H) (n = 8). Hemorrhage was induced in group H by bleeding 35% of the estimated total blood volume, followed by resuscitation with lactated Ringer solution at three times the bled volume. Pigs in group C were not hemorrhaged or resuscitated. Blood samples were withdrawn at baseline, 15 min, 3 h, 6 h, and 24 h after hemorrhage and lactated Ringer (LR) resuscitation (H–LR). Coagulation was assessed by using thrombelastography. All baseline measurements were similar between groups C and H. Hemorrhage caused a decrease in mean arterial pressure and an increase in heart rate in group H, but LR resuscitation corrected these changes within 1 h. Compared to baseline values, fibrinogen concentrations in group H decreased at 15 min, 3 h and 6 h after H–LR, but increased to double that of the baseline value at 24 h; platelet counts decreased throughout the study; clot strength was decreased at 15 min, 3 h and 6 h, but returned to baseline value at 24 h after H–LR. Hemorrhage caused decreases in fibrinogen and platelets, and compromised clot strength. The rebound of fibrinogen at 24 h restored clot strength despite platelet deficit. These data suggest the potential compensatory role of fibrinogen in restoring coagulation function in vivo after hemorrhagic shock.
INTRODUCTION
Normal hemostasis involves complex interactions of fibrinogen, platelets, coagulation factors and enzymes. The interactions include the initiation of thrombin generation by the activation of FVIIa/TF complex and FXa, the propagation of thrombin generation from the production of prothrombinase complex on the surface of activated platelets, fibrin formation and stabilization from fibrinogen by thrombin and FXIII, and fibrinolysis (1,2). Following trauma injury and blood loss, all components involved in the coagulation process are depleted and further diluted by resuscitation of crystalloid or colloid fluids. Consequently, hemostatic function is compromised and different approaches have been explored to restore coagulation function. In the United States, blood products, such as platelet concentrates, cryoprecipitate, or fresh frozen plasma have been used in patients with bleeding complications (3,4). To observe the effects of fibrinogen on survival, Stinger et al. performed a retrospective analysis in massive transfused trauma patients at a United States Army combat support hospital and reported that the amount of fibrinogen administered from transfused blood products correlates with survival (5). The mortality rates in patients receiving high amounts of fibrinogen (≥0.2 g from transfused blood products per unit red blood cells) and low amounts of fibrinogen (<0.2 g) were 24% and 52%, respectively (P < 0.001) (5). In central Europe, fibrinogen concentrates and prothrombin complex concentrate (PCC) have been used to treat acquired bleeding complications in surgical and trauma patients with success (6–10). Although the beneficial effects of fibrinogen on clotting function are indicated in recent literature, the role of fibrinogen on coagulation function in a trauma setting, such as hemorrhage and resuscitation, remains to be clarified.
Evaluation of hemostasis restoration requires a valid and comprehensive assessment of coagulation function. Normal coagulation assays, such as pro-thrombin time (PT) and activated partial thromboplastin time (aPTT), are performed in plasma, and, therefore, cannot reflect the interaction of platelet and fibrinogen. Activated clotting time (ACT) is performed in whole blood, however, it only detects the clotting times. Thromboelastography (TEG) (Hemoscope, Niles, IL, USA) and rotational thromboelastometry (ROTEM) (Pentapharm GmbH, Munich, Germany) have been recognized as global assessments of coagulation function owing to their being able to track clot initiation, clot growth, platelet activation and fibrinolysis in whole blood (11–13). The importance of thrombelastography measurements in treating trauma patients has been described by Plotkin et al. (14). In patients with penetration injuries, Plotkin et al. have shown that reduced clot formation rate and clot strength by ROTEM are indicative of transfusion requirements (14).
The purpose of this study was to investigate changes of endogenous fibrinogen availability in relation to changes of coagulation function after hemorrhagic shock and resuscitation in a swine model. Hemorrhagic shock was induced by bleeding 35% of total estimated blood volume, followed by lactated Ringer (LR) resuscitation fluids in pigs. Changes of endogenous fibrinogen availability were assessed at baseline, 15 minutes, 3 hours, 6 hours and 24 hours after hemorrhage and resuscitation. Corresponding changes in coagulation were evaluated by using PT, aPTT and TEG measurements.
MATERIALS AND METHODS
This study was approved by the Institutional Animal Care and Use Committee of the United States Army Institute of Surgical Research and has been conducted in compliance with the Animal Welfare Act, the implementing Animal Welfare Regulations and in accordance with the principles of the Guide for the Care and Use of Laboratory Animals.
Animal Groups and Surgical Procedures
A total of 16 crossbred commercial Yorkshire swine (39 ± 1 kg) were randomized into two groups: the sham control group (group C) (n = 8) and the hemorrhage and LR resuscitation (H–LR) (group H, n = 8). After an overnight fast, the animals were pre-anesthetized with glycopyrrolate (0.1 mg/kg) and Telazol (6mg/kg) and intubated by 1.0–1.5% isoflurane in 100% oxygen by mask for the surgical procedures. Polyvinyl chloride catheters were inserted into the thoracic aorta via the carotid artery to measure mean arterial pressures, heart rates and temperatures. The right femoral artery was cannulated for arterial blood sampling and the left femoral artery for induction of bleeding. The left femoral vein was cannulated for resuscitation of LR solution. The right femoral vein was cannulated for intravenous (i.v.) anesthesia of ketamine during the study.
Animal Experimental Protocol
Upon completion of catheter cannulation, anesthesia was switched to a combination of isoflurane (0.5 %) and continuous i.v. drip of ketamine (0.15 mL/kg/h of 100 mg/mL) in all pigs for the remainder of the study period. After a 10-min stabilization period, mean arterial pressure and heart rate were recorded and blood samples were taken for baseline measurements (baseline samples). Hemorrhagic shock was then induced in the hemorrhage group by bleeding approximately 35% of the estimated total blood volume (24.5 ± 0.1 mL/kg) over about a 30-min period from the left femoral artery to a preweighed canister on a balance. The rate of bleeding was controlled by adjusting the clamp on the left femoral artery catheter to maintain mean arterial pressure above 40 mmHg. Afterward, the pigs were resuscitated with LR solution at approximately three times the bled volume over approximately 30 min. Pigs in the control group were not bled or resuscitated. After 15-min stabilization after the resuscitation, mean arterial pressure and heart rate were recorded and blood samples were taken for coagulation measurements (15-min samples). The same measurements were made at 3 h and 6 h after hemorrhage and resuscitation (3-h and 6-h samples). Afterward, all catheters inserted during the surgery procedures were taped securely on the backs of the pigs. They were then allowed to awaken and were transferred to an environmentally controlled room. They stayed in appropriately sized runs or pens within the vivarium. During the night, the pigs were fed with laboratory-grade commercial swine feed by trained animal care staff. Water was provided ad libitum to all animals via an automated water delivery system.
On the next morning (24 h after hemorrhage and resuscitation), the pigs were tranquilized with diazepam (0.5 mg/kg intramuscular [IM]) before being transferred to the study room. All catheters were untied and connected to instruments or flushed for blood withdrawal. After 15-min stabilization, mean arterial pressure and heart rate were recorded, and blood samples were taken for coagulation measurements (24-h samples). Upon the completion of the study, the pigs were euthanized with sodium pentobarbital (FatalPlus, Fort Dodge, IA, USA) given intravenously by veterinary staff.
Analytical Methods
Platelet counts were measured from citrated blood by using an ABX Pentra 120 Hematology Analyzer (ABX Diagnostics Inc., Irvine, CA, USA). Blood gas measurements (lactate) were determined by the Omni-9 Blood Gas Analyzer (AVL, Montpellier, France). Blood chemistries (total protein and albumin) were measured by the Dimension Clinical Chemistry System (Dade Behring, Newark, DE, USA). Plasma fibrinogen concentration, PT, aPTT and coagulation factors were measured with the blood coagulation system (BCS) (Dade Behring, Deerfield, IL, USA). TEG (TEG 5000 Hemostasis Analyzer, Haemoscope Corp, Niles, IL, USA) was performed by using blood samples taken at baseline, 15 min, 3 h, 6 h and 24 h after hemorrhage and resuscitation.
Statistical Analysis
Data were expressed as mean ± SEM and analyzed by using SAS statistical software. In each group, comparisons were made in all measurements on a pre-or postbasis by using one-way analysis of variance (ANOVA). Between-group comparisons were made with appropriate adjustments for multiplicity by using Tukey adjustment. The statistically significant level was set at P < 0.05.
RESULTS
All animals from both groups survived to the end of the study (24 h after H–LR). Baseline measurements were similar between group C and group H. There were no significant changes in hemodynamics or coagulation function observed in group C during the study period.
Hemodynamic Measures
In group H, mean arterial pressure was decreased immediately by hemorrhage from the baseline value of 95 ± 4 mmHg to 53 ± 4 mmHg (P < 0.05), but returned to baseline values within 1 h following LR resuscitation. Cardiac output was decreased from baseline value of 3.8 ± 0.3 L/min to 2.9 ± 0.4 L/min (P < 0.05), but returned to baseline values after resuscitation. There were no significant changes in mean arterial pressure or cardiac output during the remainder of study.
Hematocrit (Hct) was decreased by H–LR from the baseline value of 28 ± 1% to 20 ± 1%, and remained at the decreased value during the remainder of the study. Lactate level was increased by hemorrhage from the baseline value of 1.8 ± 0.1 mmol/L to 2.4 ± 0.2 mmol/L (P < 0.05) and returned to 1.7 ± 0.2 mmol/L following LR resuscitation. There were no significant changes of lactate subsequently.
Fibrinogen Concentrations, Platelet Counts and Coagulation Factor Levels
Plasma fibrinogen concentration was decreased at 15 min, 3 h and 6 h after H–LR (Figure 1). However, at 24 h after H–LR, fibrinogen concentration increased to double that of the baseline values (see Figure 1). Platelet count was decreased at 15 min, 3 h, 6 h and 24 h after H–LR (see Figure 1). H–LR caused 30% to 65% decreases in coagulation factor II, V, VII, VIII, IX, X, XI, XII and XIII at 15 min after H–LR, and the decreases remained at 6 h and 24 h (Table 1).
Figure 1.
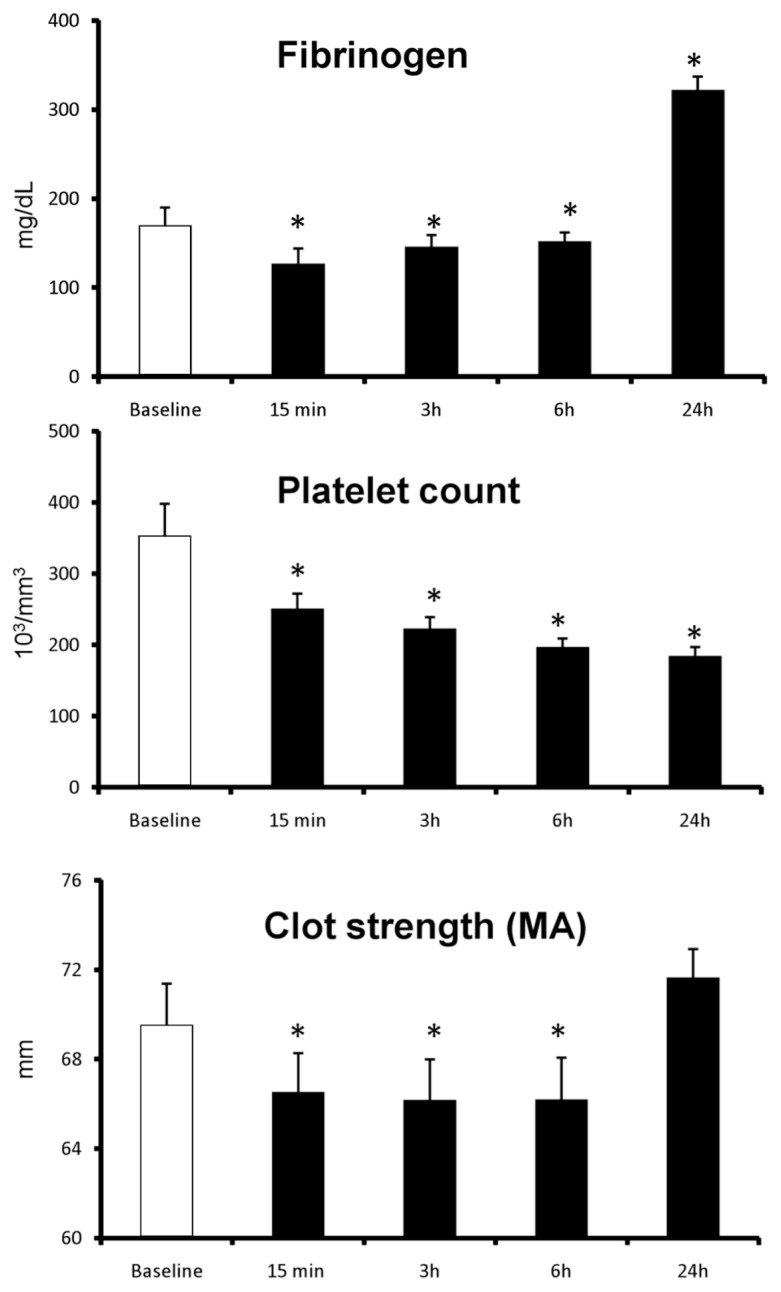
Changes in fibrinogen, platelet counts and clot strength following H–LR in pigs. Data represent mean ± SEM. * P < 0.05 compared with corresponding baseline values.
Table 1.
Changes of coagulation factors following H–LR.
| Coagulation factors | 15 min after H–LR, % | 6 h after H–LR,% | 24 h after H–LR, % |
|---|---|---|---|
| II | 58 ± 2 | 67 ± 3 | 70 ± 1 |
| V | 65 ± 11 | 69 ± 5 | 70 ± 5 |
| VII | 59 ± 3 | 57 ± 3 | 65 ± 5 |
| VIII | 51 ± 12 | 66 ± 11 | 75 ± 13 |
| IX | 41 ± 5 | 54 ± 6 | 62 ± 3 |
| X | 60 ± 7 | 62 ± 6 | 64 ± 7 |
| XI | 33 ± 6 | 37 ± 5 | 43 ± 7 |
| XII | 53 ± 9 | 64 ± 7 | 62 ± 8 |
| XIII | 65 ± 5 | 70 ± 4 | 68 ± 7 |
Data are expressed as percentages of the corresponding baseline values in the hemorrhage group. P < 0.05 compared with the corresponding baseline values.
Coagulation Functional Assessments
There were no significant changes observed in any coagulation measurement in group C during the study. In group H, clot strength (maximum amplitude) was decreased at 15 min, 3 h and 6 h after H–LR, but returned to baseline value at 24 h after H–LR (see Figure 1). Clot rapidity (angle, α) did not change from the baseline values (76 ± 1°) at 15 min, 3 h and 6 h after H–LR, but was decreased to 68 ± 1° at 24 h after H–LR (P < 0.05). Similarly, the initial clotting time (R time) did not change from the baseline values (3.1 ± 0.2 min) at 15 min, 3 h and 6 h after H–LR, but was prolonged to 4.6 ± 0.2 min at 24 h after H–LR (P < 0.05).
There were no significant changes in PT or aPTT observed in any group during the study.
DISCUSSION
Using a swine model, this study investigated changes of fibrinogen in relation to changes of coagulation function. A 35% blood loss and LR resuscitation decreased fibrinogen, platelet and coagulation factors. Consequently, hemostatic function was compromised. Twenty-four hours after H–LR, fibrinogen concentration was increased, but platelet counts and coagulation factors remained depleted. The increase of fibrinogen returned clot strength to its baseline value, despite the deficits of platelet counts and other coagulation factors. Thus, compromised clot strength by H–LR was corrected with the elevation of fibrinogen content. This finding suggests the potential role of fibrinogen to restore coagulation function under bleeding complications.
TEG has been used to assess and monitor coagulation status. In TEG or ROTEM measurements, clot strength (maximum amplitude in TEG, maximum clot firmness in ROTEM) has been demonstrated as an indicator of clotting status with sensitivity and specificity and as a predictor of blood loss and transfusion requirements (12,14). Clinical studies have shown that changes in clot strength can be used to guide blood product administration and to reduce the usage of blood products (15,16). In this study, we observed a parallel changing pattern between fibrinogen concentration and clot strength in this study. When fibrinogen concentration was depleted at 15 minutes, 3 hours and 6 hours after hemorrhage and resuscitation, clot strength was similarly compromised at these time points. When fibrinogen concentration was increased at 24 hours after H–LR, clot strength was recovered to its baseline values at the same time, even though platelet counts and other coagulation components were still at decreased levels. It is clear that the increase of fibrinogen levels can compensate for the deficit of platelet and restore coagulation function in vivo. It is worth mentioning that although fibrinogen concentration was increased to double its baseline value, clot strength returned only to its baseline value. The lack of increase of clot strength above baseline is likely due to the decreased levels of platelet counts and other coagulation factors. Nevertheless, the fact that high fibrinogen levels did not cause an overshot of clot strength reflects a possible safe mechanism to prevent hypercoagulation from high fibrinogen levels.
In addition to forming clots, fibrinogen plays an important role in platelet activation and aggregation by binding to the platelet glycoprotein receptor GPIIb/IIa. The compensatory role of fibrinogen under low platelet counts (thrombocytopenia) has been suggested by in vitro experimental data. Li et al. have shown that the effect of platelet-blocking substances can be antagonized by increasing fibrinogen concentrations (17). When fibrinogen was added to plasma samples with different platelet counts (10 × 103/mm3, 50 × 103/mm3 and 100 × 103/mm3), Lang et al. (18) reported that clot strength increased as fibrinogen levels increased at all three levels of platelet counts. In addition, in samples with the same platelet counts but different fibrinogen levels, the contribution of platelets to clot strength was increased in a fibrinogen-dependent pattern (18). These in vitro findings have been confirmed by findings from animal studies. In pigs with induced thrombocytopenia (platelet counts <30 × 103/mm3), Velik-Salchner et al. (19) reported that fibrinogen supplementation improved clot firmness by ROTEM and reduced blood loss after liver injury. Consistently, fibrinogen’s compensatory role following H–LR has been shown in the present pig study. Further, in addition to platelet counts, H–LR in this study caused decreases of coagulation factors. Platelet counts and coagulation factors (except fibrinogen) remained depleted at 24 hours after H–LR. However, the increase of fibrinogen alone at 24 hours restored clot strength to the baseline value. Thus, the compensatory role of fibrinogen is present not only in depleted platelet counts, but also in depleted coagulation factors and platelet counts.
The benefit of high fibrinogen levels has been shown in clinical observations. In obstetric patients, fibrinogen concentration usually increases by 50% to 250% (13). This elevation is believed to be beneficial in limiting blood loss during delivery, because failure to increase fibrinogen concentration is associated with severe bleeding (20). On the other hand, low fibrinogen level at admission was found to be the only marker associated with the occurrence of severe postpartum hemorrhage (20). A fibrinogen level of less than 200 mg/dL has a 100% positive predictive value of postpartum hemorrhage (20). Thus, the increase of fibrinogen availability in pregnant women appears to be essential to prevent bleeding complications during delivery.
The elevation of fibrinogen concentration in this study was possibly due to the acute phase response from H–LR. As an acute phase protein, fibrinogen is commonly observed to increase under stressed situations (21–23). Metabolic changes from trauma injury or stress are generalized by an increase in whole body protein turnover rate, with a net loss of host body protein (21–23). Specifically, there is an increase of amino acid release from the muscle and an increase of amino acid uptake in the splenic bed (22). This shift of amino acid sources from muscle to the liver is hypothesized to be beneficial because it facilitates the liver’s synthesis of proteins, which are critical for survival (24). From a coagulation perspective, the increase of fibrinogen concentration restored coagulation as demonstrated in this study, even when platelets and other components remained at the depleted levels. The specific mechanisms contributing to the elevation of fibrinogen concentrations remain to be discovered. Nevertheless, the quick increase of fibrinogen in the absence of recovery in platelet counts or other coagulation factors reveals the importance and priority of increasing fibrinogen after insults.
Although both platelets and fibrinogen are important to the coagulation process, the individual contribution of platelets and fibrinogen to coagulation appears to be different. When platelet function was blocked in blood samples taken from pregnant women, clot strength was found to increase linearly with the increase of fibrinogen (13). However, at certain levels of fibrinogen, no correlation was found between platelet counts and platelet contribution to clot strength (13). Thus, the compensatory role appears to be from fibrinogen to platelet, not from platelet to fibrinogen.
In this study, clot rapidity did not change at 15 minutes, 3 hours or 6 hours after H–LR, but was decreased at 24 hours after H–LR. Similarly, clotting initiation did not change at 15 minutes, 3 hours or 6 hours, but was prolonged at 24 hours after H–LR. The decrease of clot rapidity or increase of clotting initiation is not likely due to hemodilution, because Hct remained unchanged during 3 hours, 6 hours and 24 hours after H–LR. These changes may possibly result from an unbalanced dynamic process of clot formation and fibrinolysis. Further investigation will provide definitive answers.
CONCLUSION
To summarize, this study investigated the contribution of endogenous fibrinogen availability to coagulation function following H–LR in a swine model. H–LR caused depletions of fibrinogen, platelet counts and coagulation factors and compromised the coagulation process. The increase of fibrinogen at 24 hours after H–LR was associated with the restoration of clot strength, despite the deficit of platelets and other coagulation components. These findings support the notion of acutely administering fibrinogen to restore coagulation function in bleeding complications.
ACKNOWLEDGMENTS
The author appreciates the support received from the Veterinary Support Division and the Laboratory Support Division at the United States Army Institute of Surgical Research in animal studies and coagulation measurements. The author thanks Mrs. Shavaughn Colvin for her excellent technical assistance during the study.
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
This study was supported by the United States Army Medical Research and Materiel Command. The opinions or assertions contained herein are the private views of the author and are not to be constructed as official or as reflecting the views of the United States Department of the Army or the United States Department of Defense.
REFERENCES
- 1.Mann KG, Brummel K, Butenas S. What is all that thrombin for. J Thromb Haemost. 2003;1:1504–14. doi: 10.1046/j.1538-7836.2003.00298.x. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman M, Monroe DM., 3rd A cell-based model of hemostasis. Thromb Haemost. 2001;85:958–65. [PubMed] [Google Scholar]
- 3.Stahel PF, Moore EE, Schreier SL, Flierl MA, Kashuk JL. Transfusion strategies in postinjury coagulopathy. Curr Opin Anaesthesiol. 2009;22:289–98. doi: 10.1097/ACO.0b013e32832678ed. [DOI] [PubMed] [Google Scholar]
- 4.Cotton BA, et al. Damage control hematology: the impact of a trauma exsanguination protocol on survival and blood product utilization. J Trauma. 2008;64:1177–82. doi: 10.1097/TA.0b013e31816c5c80. discussion 1182–3. [DOI] [PubMed] [Google Scholar]
- 5.Stinger HK, et al. The ratio of fibrinogen to red cells transfused affects survival in casualties receiving massive transfusions at an army combat support hospital. J Trauma. 2008;64:S79–85. doi: 10.1097/TA.0b013e318160a57b. discussion S85. [DOI] [PubMed] [Google Scholar]
- 6.Schochl H, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit. Care. 2010;14:R55. doi: 10.1186/cc8948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danes AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in higH–risk severe bleeding. Vox Sang. 2008;94:221–6. doi: 10.1111/j.1423-0410.2007.01024.x. [DOI] [PubMed] [Google Scholar]
- 8.Fenger-Eriksen C, et al. Fibrinogen substitution improves whole blood clot firmness after dilution with hydroxyethyl starch in bleeding patients undergoing radical cystectomy: a randomized, placebo-controlled clinical trial. J Thromb Haemost. 2009;7:795–802. doi: 10.1111/j.1538-7836.2009.03331.x. [DOI] [PubMed] [Google Scholar]
- 9.Schick KS, Fertmann JM, Jauch KW, Hoffmann JN. Prothrombin complex concentrate in surgical patients: retrospective evaluation of vitamin K antagonist reversal and treatment of severe bleeding. Crit. Care. 2009;13:R191. doi: 10.1186/cc8186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bruce D, Nokes TJ. Prothrombin complex concentrate (Beriplex P/N) in severe bleeding: experience in a large tertiary hospital. Crit. Care. 2008;12:R105. doi: 10.1186/cc6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kettner SC, et al. Use of abciximabmodified thrombelastography in patients undergoing cardiac surgery. Anesth Analg. 1999;89:580–4. doi: 10.1097/00000539-199909000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Ereth MH, et al. Does the platelet-activated clotting test (HemoSTATUS) predict blood loss and platelet dysfunction associated with cardiopulmonary bypass. Anesth Anal. 1997;85:259–64. doi: 10.1097/00000539-199708000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Gottumukkala VN, Sharma SK, Philip J. Assessing platelet and fibrinogen contribution to clot strength using modified thromboelastography in pregnant women. Anesth Anal. 1999;89:1453–5. doi: 10.1097/00000539-199912000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Plotkin AJ, et al. A reduction in clot formation rate and strength assessed by thrombelastography is indicative of transfusion requirements in patients with penetrating injuries. J Trauma. 2008;64:S64–8. doi: 10.1097/TA.0b013e318160772d. [DOI] [PubMed] [Google Scholar]
- 15.Nuttall GA, et al. Efficacy of a simple intraoperative transfusion algorithm for nonerythrocyte component utilization after cardiopulmonary bypass. Anesthesiology. 2001;94:773–81. doi: 10.1097/00000542-200105000-00014. discussion 775A–776A. [DOI] [PubMed] [Google Scholar]
- 16.Shore-Lesserson L, et al. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Anal. 1999;88:312–9. doi: 10.1097/00000539-199902000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Li YF, Spencer FA, Becker RC. Comparative efficacy of fibrinogen and platelet supplementation on the in vitro reversibility of competitive glycoprotein IIb/IIIa (alphaIIb/beta3) receptor-directed platelet inhibition. Am Heart J. 2001;142:204–10. doi: 10.1067/mhj.2001.116962. [DOI] [PubMed] [Google Scholar]
- 18.Lang T, et al. The effects of fibrinogen levels on thromboelastometric variables in the presence of thrombocytopenia. Anesth Anal. 2009;108:751–8. doi: 10.1213/ane.0b013e3181966675. [DOI] [PubMed] [Google Scholar]
- 19.Velik-Salchner C, et al. The effect of fibrinogen concentrate on thrombocytopenia. J Thromb Haemost. 2007;5:1019–25. doi: 10.1111/j.1538-7836.2007.02481.x. [DOI] [PubMed] [Google Scholar]
- 20.Charbit B, et al. The decrease of fibrinogen is an early predictor of the severity of postpartum hemorrhage. J Thromb Haemost. 2007;5:266–73. doi: 10.1111/j.1538-7836.2007.02297.x. [DOI] [PubMed] [Google Scholar]
- 21.Cuthbertson DP, Tompsett SL. Note on the effect of injury on the level of the plasma proteins. Br J Exp Pathol. 1935;16:471–5. [Google Scholar]
- 22.Clowes GH, Jr, Randall HT, Cha CJ. Amino acid and energy metabolism in septic and traumatized patients. JPEN J Parenter Enteral Nutr. 1980;4:195–205. doi: 10.1177/014860718000400225. [DOI] [PubMed] [Google Scholar]
- 23.Jahoor F, Desai M, Herndon DN, Wolfe RR. Dynamics of the protein metabolic response to burn injury. Metabolism. 1988;37:330–7. doi: 10.1016/0026-0495(88)90132-1. [DOI] [PubMed] [Google Scholar]
- 24.Fleck A. Clinical and nutritional aspects of changes in acute-phase proteins during inflammation. Proc Nutr Soc. 1989;48:347–54. doi: 10.1079/pns19890050. [DOI] [PubMed] [Google Scholar]


