Abstract
Fatty acid synthase (FASN), the enzyme responsible for de novo synthesis of fatty acids, has been shown to be deregulated in several cancers, including epithelial ovarian carcinoma (EOC). In this study, we investigated the function of the FASN signaling pathway in a large series of Middle Eastern EOC patient samples, a panel of cell lines and nude mouse model. Using immunohistochemistry, we detected overexpression of FASN in 75.5% (114/151) of the tumor samples. Overexpression of FASN was associated significantly with tumor proliferative marker Ki-67 (P = 0.0009), activated AKT (P = 0.0117) and XIAP (P = 0.0046). Treatment of EOC cell lines with C-75, a selective inhibitor of FASN, caused inhibition of EOC cell viability via induction of apoptosis. Inhibition of FASN by C-75 led apoptosis via the mitochondrial pathway. FASN inhibition caused downregulation of activated AKT and its downstream targets. In addition, inhibition by FASN siRNA caused downregulation of FASN and activation of caspases, suggesting the role of FASN in C-75 mediated apoptosis. Furthermore, treatment of EOC cells with subtoxic doses of C-75 augmented the effect of cisplatin-mediated induction of apoptosis. Finally, treatment of EOC cell line xenografts with a combination of C-75 and cisplatin resulted in growth inhibition of tumors in nude mice through downregulation of FASN and activation of caspases. Altogether, our results show overexpression of FASN in Middle Eastern EOC, suggesting that FASN may be a potential therapeutic target in a subset of EOC, alone or in combination with other conventional chemotherapeutic agents.
INTRODUCTION
Ovarian cancer is one of the lethal gynecologic malignancies and this is due in large part to the resistance of recurrent ovarian cancer cells to standard chemotherapeutic strategies (1). Resistance to apoptotic cell death is a fundamental characteristic of cancer cells, and a primary cause of treatment failure. Of the different chemotherapeutic agents in use for treating cancer, platinum-based chemotherapy is often used to treat recurrent ovarian cancers, but many of the ovarian cancer cells are resistant to the platinum-based agents (2). This resistance to chemotherapy results in recurrence and ultimately the loss of life. Therefore, there is an urgent need to improve the effectiveness of platinum-based chemotherapy.
Fatty acid synthase (FASN) is a multi-functional enzyme that catalyzes the terminal steps in the synthesis of the long-chain saturated fatty acid palmitate in normal cells (3). In normal cells, FASN expression levels are relatively low, since fatty acid is generally supplied by dietary fatty acid. In contrast, FASN is expressed at significantly higher levels in a variety of human epithelial cancers including breast, thyroid, colon, ovary, lung and prostate (4–9). Moreover, several reports have shown that FASN expression levels correlate with tumor progression, aggressiveness and metastasis (10–11). FASN appears to provide a selective proliferative advantage since its overexpression is shown to correlate with poor prognosis in breast and prostate cancers and is found to be elevated in the blood of cancer patients (9,12–13). Furthermore, inhibition of FASN activity is selectively cytotoxic to cancer cells in vitro and in vivo (14–16). Upregulation of FASN expression in cancer cells has been linked to phosphatidylinositol-3-kinase (PI3K)/ AKT signaling pathway (17–19). Activation of PI3-kinase pathway recruits a number of signaling proteins including AKT. During recruitment, AKT becomes phosphorylated/activated and exerts its antiapoptotic activity through phosphorylation of downstream targets such as Bad, FOXO and GSK3 (20–23). In addition, PI3K pathway has been shown to be capable of negatively regulating FASN-induced cell death (19).
In the current study, we investigated the expression of FASN and its correlation to other clinicopathological parameters in a large cohort of Saudi EOC using tissue microarray (TMA) technology. We next examined the effect of C-75, a synthetic slow-binding inhibitor of FASN activity on a panel of EOC cell lines. In addition, we investigated whether subtoxic doses of C-75 can potentiate the anti-cancer effects of cisplatin in vitro and in vivo. Our findings strongly suggest that a tight functional association between FASN and AKT is taking place in a subset of EOC, and that FASN expression can be a useful biomarker. Furthermore, inhibition of FASN activity by C-75 induces apoptosis in EOC cells and combination treatment with C-75 and cisplatin augmented the apoptotic effects in vitro and in vivo, implicating therapeutic usefulness of FASN targeting in EOC.
MATERIALS AND METHODS
Patient Selection and Tissue Microarray Construction
One hundred fifty-six patients with ovarian carcinoma diagnosed between 1991–2007 were selected from the files of the King Faisal Specialist Hospital and Research Centre. All samples were analyzed in a tissue microarray (TMA) format. TMA construction was performed as described earlier (24). Two cores of ovarian carcinoma were arrayed from each case. The Institutional Review Board of the King Faisal Specialist Hospital and Research Centre approved the study. The patients were diagnosed histologically and received follow-up care in the Departments of Obstetrics and Gynecology and Oncology at King Faisal Specialist Hospital and Research Centre. The histological subtype of each ovarian tumor sample was determined by pathologist (PB) according to accepted criteria (25). Department of Obstetrics and Gynecology, King Faisal Specialist Hospital and Research Center provided long-term follow-up data for these patients. The primary pathological diagnosis was: serous in 125 patients (80.1%), endometriod in 22 (14.1%), clear cell in 4 (2.6%) and undifferentiated/mixed epithelial in 5 (3.2%). The ages of the patients ranged from 19 to 86 years, with a median age of 56 years. The majority of patients underwent primary surgical staging or cytoreduction. In some patients who were not fit for primary surgery, primary neoadjuvant chemotherapy was followed by interval debulking surgery. The distribution by FIGO stage at diagnosis was: stage I–II in 8 patients (5.1%), stage III–IV in 137 (87.8%), and unknown in 11 (6.1%). The median follow-up time was 14.9 months (range, 2 to 130 months). Progression-free survival was computed from date of surgery for patients who underwent primary cytoreduction and from date of diagnosis by biopsy or cytology in those who underwent primary neoadjuvant chemotherapy. Since the majority of patients are lost to follow-up as their disease reaches its terminal stages, it was impossible to determine overall survival in this specific patient population.
Immunohistochemistry (IHC)
TMA slides were processed and stained manually. The streptavidin-biotin peroxidase technique with diaminobenzidine as chromogen was applied. For antigen retrieval, Dako Target Retrieval Solution pH 9.0 (Number S2368) was used, and the slides were boiled in a pressure cooker (Pascal Pressure Cooker, model: S2800, Dako Cytomation, Carpinteria, CA, USA). Primary antibodies used, their dilutions and antigen retrieval are listed in Supplementary Table 1. Endogenous peroxidase activity was quenched using 3% hydrogen peroxidase. Endogenous biotin was blocked and all slides were counterstained with hematoxylin, dehydrated, cleared, and cover slipped with premount. Only fresh cut slides were stained simultaneously to minimize the influence of slide aging and maximize repeatability and reproducibility of the experiment. p-AKT scoring was done as described earlier (26). For purposes of statistical analysis, all cases staining at Level 0 or 1 were grouped as p-AKT negative and all cases staining at Level 2 and Level 3 were grouped as p-AKT positive. Two types of negative controls were used for p-AKT, one was the negative control in the kit in which the primary antibody was omitted and a preabsorption experiment using p-AKT Ser 473 blocking peptide (Cell Signaling Technology, Beverly, MA, USA, No. 1140) was used as the second negative control. Each TMA spot was assigned an intensity score from 0–3 (I0, I1–3) and proportions of the tumor staining for that intensity were recorded as 5% increments from a range of 0–100 (P0, P1–3). A final H score (range 0–300) was obtained by adding the sum of scores obtained for each intensity and proportion of area stained (H score = P1 + I2XP2 + I3XP3). Ovarian tumors were grouped into two groups using X-tile bioinformatics software: low FAS-N expression (H score δ 110) and the other group showed high FAS-N expression (H score >110) (27).
Statistics
All statistical analysis were performed using the Statview JMP software (version 7.0). The Fisher exact chi-square [χ2] test was used to assess associations between categorical variables. Kaplan-Meier survival analyses were carried out for progression free survival, using the log-rank test for differences between groups. Results were considered statistically significant when P from a two-tailed test was < 0.05.
Cell Culture
EOC cell lines MDAH2774 and SKOV3, OVCAR3 cells (purchased from American Type Culture Collection [ATCC, Manassas, VA, USA]), OVTOKO and OVISE (from Japanese Collection of Research Bioresources [Osaka, Japan]) were cultured in RPMI 1640 supplemented with 10% (v/v) fetal bovine serum (ATCC), 100 units/mL penicillin, and 100 units/mL streptomycin (Sigma, St. Louis, MO, USA) at 37°C in humidified atmosphere containing 5% CO2. All experiments were performed in RPMI 1640(ATCC) containing 5% serum.
Reagents and Antibodies
C-75 was purchased from Calbiochem (San Diego, CA, USA) and cisplatin was purchased from Sigma. Antibodies against caspase 3, cleaved caspase 3, AKT, pAKT, FOXO1 and GSK3 antibodies were purchased from Cell Signaling Technologies (Beverly, MA, USA). FASN, cytochrome c, β-actin, and poly (ADP-ribose) polymerase (PARP) antibodies were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). XIAP, cIAP1, and caspase 8 antibodies were purchased from R&D (Minneapolis MN, USA). Annexin V was purchased from Molecular Probes (Eugene, OR, USA). Apoptotic DNA-ladder kit was obtained from Roche (Penzberg, Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assays
One hundred four cells were incubated in triplicate in a 96-well plate in the presence or absence of indicated test doses of C-75, cerulenin and C-75 in combination with cisplatin, a final volume of 0.20 mL for 48 h. The ability of C-75, cerulenin and C-75 in combination with cisplatin to suppress cell growth was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assays, as described previously (28). Replicates of three wells for each dosage, including vehicle control, were analyzed for each experiment.
Annexin V/Propidium Iodide Dual Staining-Apoptotic Assay
EOC cell lines were treated with the indicated concentrations of C-75, cerulenin and C-75 in combination with cisplatin. The cells were harvested and the percentage of cells undergoing apoptosis was measured by flow cytometry after staining with fluorescein-conjugated annexin V and propidium iodide (Molecular Probes) as described previously (28).
DNA Laddering
A DNA laddering assay was performed as described earlier (28). Briefly, cells (2 × 106) were treated with cisplatin alone, C-75 alone and C-75 in combination with cisplatin for 48 h. The cells were then harvested and resuspended in 200 mL 1 × PBS. Then, 200 mL lysis buffer containing 6 mol/L guanidine HCl, 10 mmol/L urea, 10 mmol/L Tris-HCl, and 20% Triton × (v/v; pH 4.4), were added to the cells and incubated for 10 min at room temperature. Isopropanol (100 mL) was added and shaken for 30 s on a vortex. Then, samples were passed through a filter and spun at 4,500g for 1 min, and the supernatant was discarded. The pellets were washed 3x with wash buffer containing 20 mmol/L NaCl, 2 mmol/L Tris-HCl, and 80% ethanol. The pellets then were transferred into a new 1.5-mL tube and eluted with 200 mL prewarmed elution buffer. After measuring the DNA, 2 mg DNA were electrophoresed on a 1.5% agarose gel containing ethidium bromide at 75V for 2 h and visualized using an ultraviolet light source.
Gene Silencing Using siRNA
FASN siRNA and scrambled control siRNA were purchased from Qiagen (Valencia, CA, USA). Cells were transfected by Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) for 6 h after which the lipid and siRNA complex were removed and fresh medium was added. Cells were lysed 48 h after transfection and specific protein levels were determined by Western blot analysis with specific antibodies.
Cell Lysis and Immunoblotting
Cells were treated with FASN inhibitor C-75 as described in the legends and lysed as described previously (4). Proteins (15–20 mg) were separated by SDS-PAGE and transferred to polyvinylidene difluoride (PVDF) membrane (Immobilon, Millipore, Billerica, MA, USA). Immunoblotting was done with different antibodies and visualized by the enhanced chemiluminescence (Amersham) method.
Measurement of Mitochondrial Membrane Potential
Cells were treated with C-75 for 48 h, washed twice with PBS, and suspended in mitochondrial incubation buffer. JC1 staining and flow cytometry was done as described previously (26).
Assays for Cytochrome c Release
EOC cells were treated with C-75 as described in figure legends and assayed for cytochrome c as described previously (26).
In Vivo Tumor Xenograft Studies
Six-week-old nude mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and maintained in a pathogen-free animal facility at least 1 wk before use. All animal studies were done in accordance with institutional guidelines. For xenograft study, mice were inoculated subcutaneously into the right abdominal quadrant with 5 million MDAH2774 cells in 200 mL PBS. After 1 wk, mice were assigned randomly into four groups receiving 3 mg/kg cisplatin, 10 mg/kg C-75, combination of cisplatin and C-75 or only 0.9% saline. The body weight and tumor volume of each mouse were monitored weekly. The tumor volume was measured as described previously (29). After 5 wks of treatment, mice were euthanized by cervical dislocation according to Animal Care and Use Committee (ACUC) guidelines (NIH Policy Manual: 3040-2 Animal Care and Use in the Intramural Program; http://oacu.od.nih.gov/ARAC/index.htm). Individual tumors were weighed, then snap frozen in liquid nitrogen for storage.
All supplementary materials are available online at www.molmed.org.
RESULTS
FASN Expression and its Correlation with Clinicopathological Parameters and p-AKT
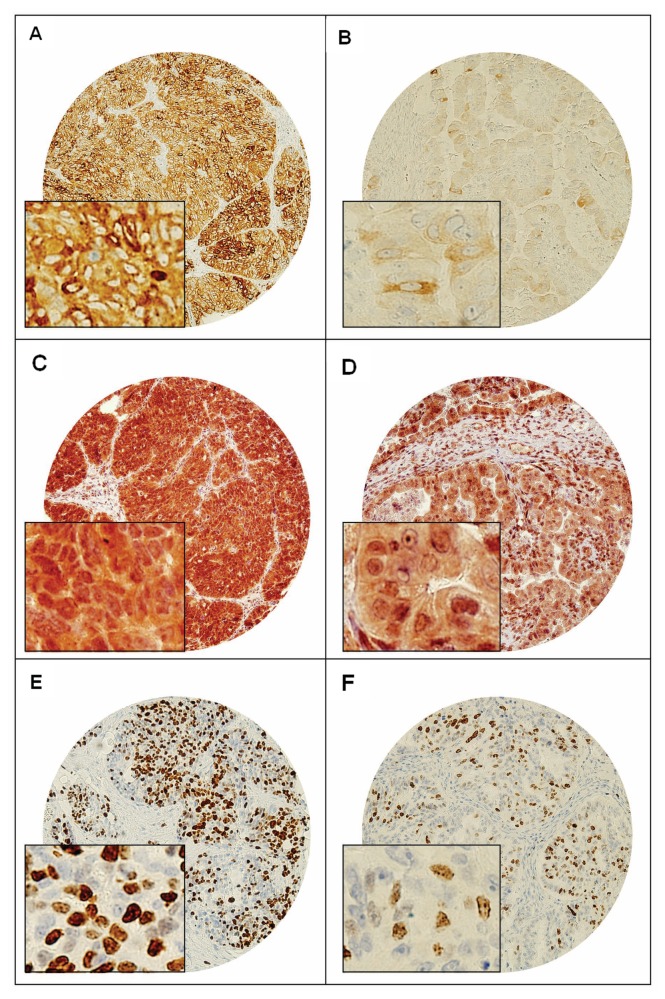
Expression levels of FASN were examined by immunohistochemistry in a large cohort of 156 EOC patient samples. High levels of FASN expression were seen in 75.5% (114/151) of the epithelial ovarian carcinomas (Figure 1). Representative information for FASN expression were observed in 151 spots, and immunohistochemical analysis failure of the remaining five cases was due to missing spots or fixation artifacts. FASN overexpression was associated significantly with overexpression of p-AKT (P = 0.0117), XIAP (P = 0.0046) and proliferative marker Ki-67 (P = 0.0009) (Table 1). However, FASN overexpression was not associated with age, American Joint Cancer Committee (AJCC) stage, FIGO grade and histopathological subtype. FASN expression was also not associated with outcome (P = 0.1784). Ovarian cancer with high FASN had an overall survival of 17.7 months as compared with 12.3 months for EOC with low FASN.
Figure 1.
Tissue microarray based immunohistochemical analysis of FASN, p-AKT and Ki-67 in EOC patients. An EOC tissue microarray spot showing over expression of (A) FASN-N, (C) p-AKT, (E) and Ki-67. In contrast, another EOC tissue microarray spot showing low expression of (B) FASN-N, (D) p-AKT and (F) Ki-67. 20 X/0.70 objectives on an Olympus BX 51 microscope (Olympus America Inc., Center Valley, PA, USA) with the inset showing a 40X/ 0.85 aperture magnified view of the same.
Table 1.
Clinico-pathological correlation of immunohistochemical expression of FASN in epithelial ovarian carcinomas.
| Total
|
High FAS-N
|
Low FAS-N
|
|||||
|---|---|---|---|---|---|---|---|
| Epithelial group | N | % | N | % | N | % | P value |
| Total number of cases | 151 | 114 | 75.5 | 37 | 24.5 | ||
| Age | |||||||
| ≤50 years | 59 | 39.1 | 46 | 78.0 | 13 | 22.0 | 0.5703 |
| >50 years | 92 | 60.9 | 68 | 73.9 | 24 | 26.1 | |
| Tumor Stage | |||||||
| Stage I-II | 8 | 5.7 | 6 | 75.0 | 2 | 25.0 | 0.9521 |
| Stage III-IV | 133 | 94.3 | 101 | 75.9 | 32 | 24.1 | |
| Histopathology | |||||||
| Clear cell | 4 | 2.6 | 3 | 75.0 | 1 | 25.0 | 0.4235 |
| Endometriod | 21 | 13.9 | 17 | 81.0 | 4 | 19.0 | |
| Serous | 122 | 80.8 | 90 | 73.8 | 32 | 26.2 | |
| Undifferentiated | 4 | 2.6 | 4 | 100.0 | 0 | 0.0 | |
| FIGO Grade | |||||||
| Well differentiated | 26 | 17.2 | 18 | 69.2 | 8 | 30.8 | 0.6596 |
| Moderately differentiated | 82 | 54.3 | 62 | 75.6 | 20 | 24.4 | |
| Poorly differentiated | 43 | 28.5 | 34 | 79.1 | 9 | 20.9 | |
| Ki-67 | |||||||
| Above 50 | 54 | 36.7 | 49 | 90.7 | 5 | 9.3 | 0.0009 |
| Below = 50 | 93 | 63.3 | 63 | 67.7 | 30 | 32.3 | |
| PAKT (Ser473) | |||||||
| High (2–3) | 75 | 52.4 | 64 | 85.3 | 11 | 14.7 | 0.0117 |
| Low (0–1) | 68 | 47.6 | 46 | 67.7 | 22 | 32.3 | |
| PFS-Median (months) | 17.7 | 12.3 | 0.1784 | ||||
Inhibition of FASN-Induced Loss of Cell Viability and Loss of Cell Proliferation and Apoptosis in EOC Cell Lines
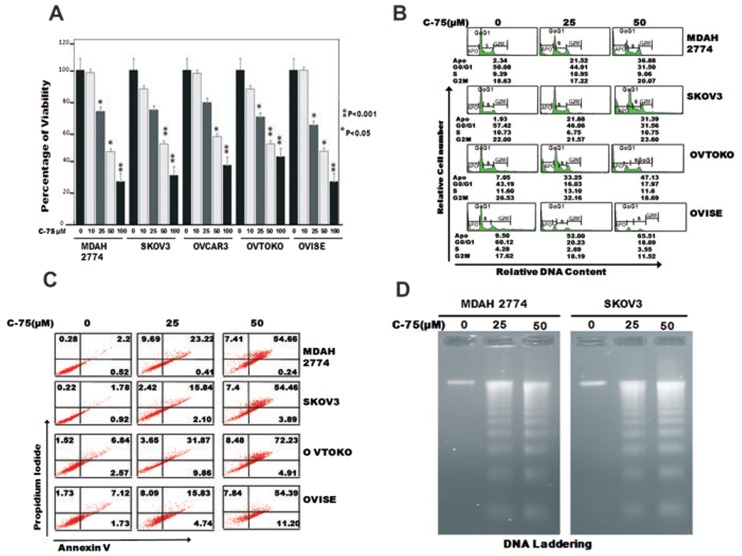
We first sought to determine whether C-75, a selective FASN inhibitor, caused dose dependent inhibition of viability in EOC cells using MTT assay. Treatment of C-75 at increasing doses from 10 to 100 mmol/L caused significant loss of viability and was found to be statistically significant at most of the doses tested (P < 0.05, Student t test) (Figure 2A). We further sought to determine whether the observed effect on C-75 growth inhibition was due to induction of cell cycle arrest or apoptosis. We, therefore, treated EOC cell lines with different doses of C-75 and determined cell cycle fractions by flow cytometry. As shown in (Figure 2B) the sub-G1 population of cells increased from 2.34% in the control to 54.66% at 50 mmol/L dosage in MDAH2774 cells. This increase in sub-G1 population was accompanied by loss of cells in G0/G1, S and G2/M phases, suggesting that the treated EOC cells were dying of apoptosis. Similar observation was made in like SKOV3, OVTAKO and OVISE cell lines.
Figure 2.
C-75 inhibits cell growth, induces apoptosis and causes G2-M arrest in EOC cell lines (A). MDAH2774, SKOV3, OVCAR3, OVTAKO and OVISE cells were incubated with 10–100 mmol/L of C-75 for 48 h. Cell proliferation assays were performed using MTT as described in Materials and Methods. The graph displays the mean ± standard deviation (SD) of three independent experiments for all the doses and vehicle control for each experiment. *P ≤ 0.05; **P ≤ 0.001, statistically significant (Student t test). (B) C-75 causes G2-M arrest after 48-h treatment leading to Apo fraction of cell cycle in EOC cells after 48 h. MDAH2774 and SKOV3 cells were incubated with 25 and 50 mmol/L of C-75 for 48 h. Thereafter the cells were washed, fixed and stained with propidium iodide, and analyzed by flow cytometry for DNA content as described in Materials and Methods. (C) MDAH2774, SKOV3, OVTOKO and OVISE cells were incubated with 25 and 50 mmol/L of C-75 and apoptosis detected by annexin V/propidium iodide dual staining. (D) MDAH2774, and SKOV3 were incubated with C-75 as indicated for 48 h, and DNA extracted and separated by electrophoresis on 1.5% agarose gel.
C-75 treatment-induced apoptosis in EOC cells was confirmed further by annexin/PI dual staining assay (Figure 2C) suggesting that suppression of growth by C-75 in EOC cells was through apoptosis. To further confirm whether the cells were dying of apoptosis, DNA fragmentation status was confirmed by the DNA laddering assay (Figure 2D). Cerulenin, another FASN inhibitor, caused inhibition of proliferation via induction of apoptosis (Supplementary Figure 1).
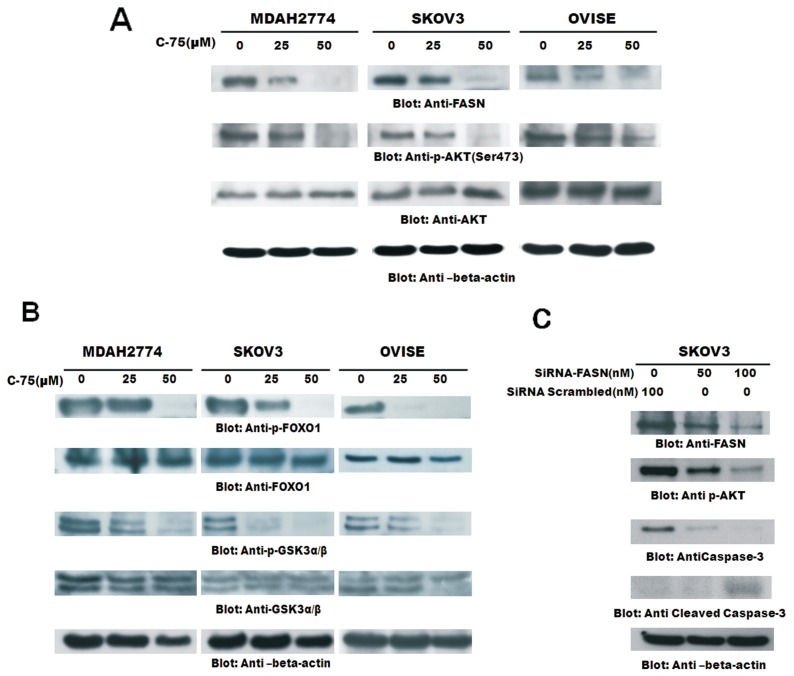
Constitutive Expression of FASN Associated with AKT Signaling Pathways in EOC Cell Lines
Activation of AKT and overexpression of fatty acid synthase (FASN) frequently are observed in human ovarian cancer (19). To explore a possible connection between AKT and FASN, we have investigated the inhibition of FASN activity by C-75 on MDAH2774, SKOV3 and OVISE cells treated with 50 mmol/L of C-75 whose proteins were analyzed for Western blotting. All the cell lines expressed constitutive FASN expression and activated AKT, and C-75 treatment suppressed FASN and inactivated AKT in a dose-dependent manner (Figure 3A). Since FOXO1 transcription factors have been reported to be a downstream target of AKT and are known to promote transcription of genes involved in cell cycle arrest and apoptosis (30), we investigated the level of FOXO1 phosphorylation in C-75 treated and untreated EOC cells by Western blotting. Constitutive phosphorylation of FOXO1 was observed in all EOC cell lines and C-75 dephosphorylated in a dose-dependent manner (Figure 3B). We next determined the activation of GSK3 in these cells, as it has been reported to be a downstream target of AKT involved in promoting cell survival (31). GSK3 dephosphorylation was seen more in EOC cells treated with C-75 (see Figure 3B). These observations suggest that FASN inhibition suppresses AKT activation and its downstream targets activated FOXO1 and GSK3 thereby inducing apoptosis. We sought to determine, by FASN-specific siRNA-targeting FASN, inhibited expression of FASN, dephosphorylation of AKT and induced apoptosis by cleavage of caspase 3 (Figure 3C). We next sought to determine whether FASN downregulation by FASN-specific siRNA caused inactivation of AKT. Inhibition of FASN by siRNA transfection downregulated FASN and dephosphorylated AKT and induced apoptosis by cleavage of caspase 3.
Figure 3.
(A) Downregulation of FASN by C-75 inhibition causes downregulation of the activated form of p-AKT, p- FOXO1 and p-GSK3. (A) MDAH2774, SKOV3 and OVISE cells were incubated with 25 and 50 mmol/L of C-75 for 48 h. Cells were lysed with equal amount of proteins, separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with antibodies against FASN, p-AKT (ser 473) and AKT. (B) MDAH2774, SKOV3 and OVISE cells incubated with 25 and 50 mmol/L of C-75 for 48 h. Cells were lysed and equal amount of proteins were separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with antibodies against p-FOXO1, FOXO1, p-GSK3 and GSK3. The blot was stripped and reprobed with an antibody against β-actin for equal loading. (C) SKOV3 cells were transfected with scrambled siRNA and FASN siRNA (50 and 100 nmol/L) in triplicates. After 48 h, cells were lysed and proteins were immunoblotted with antibodies against FASN, p-AKt-Ser473, caspase 3, cleaved caspase 3 and β-actin; 1 of the 3 experiments is depicted in this figure.
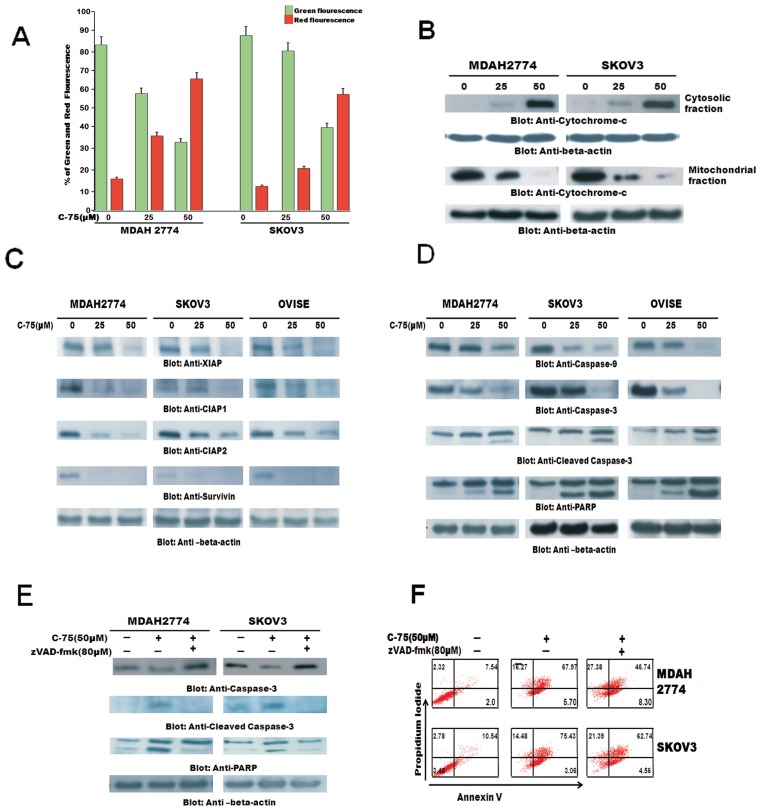
C-75 Induced Apoptosis Through AKT Signaling via the Mitochondrial Pathway and Activation of Caspases
Inactivation of AKT has been shown to induce apoptosis via the mitochondrial apoptotic pathway. We therefore sought to determine whether the observed apoptotic effect on EOC cells of C-75 involved the mitochondrial pathway. First, we tested the effect of C-75 therapy on the mitochondrial membrane potential in EOC cells. We treated EOC cells with 50 mmol/L of C-75 for 48 h, labeled with JC-1 dye and measured mitochondrial membrane potential by flow cytometry. Inhibition of FASN resulted in loss of mitochondrial membrane potential as measured by JC-1 red fluorescence depicting apoptotic cells (Figure 4A). The number of apoptotic cells increased in a dose dependent manner in cells undergoing C-75 treatment. Next, we examined release of cytochrome c from the mitochondria. For this, mitochondria-free cytosolic lysates and mitochondrial extracts were prepared as described in Materials and Methods. Cytochrome c was released to the cytosol in treated cells, conferred by the increase in intensity of bands in the cytosolic fractions after C-75 treatment and concurrently, there was a decrease in mitochondrial fraction after C-75 treatment (Figure 4B).
Figure 4.
C-75-induced mitochondrial apoptotic pathway in EOC cells. (A) Loss of mitochondrial membrane potential by C-75 treatment in MDAH2774 and SKOV3 cells incubated with 25 and 50 mmol/L of C-75 for 48 h. Live cells with intact mitochondrial membrane potential (green bar) and dead cells with lost mitochondrial potential (red bar) were measured by JCI staining and analyzed by flow cytometry as described in Materials and Methods. (B) C-75-induced release of cytochrome c. MDAH2774 and SKOV3 cells incubated with 25 and 50 mmol/L of C-75 for 48 h. Mitochondrial and cytoplasmic fractions were isolated and cell extracts were immunoblotted with antibodies against cytochrome c and β-actin. (C) Downregulation by FASN by C-75 inhibition causes downregulation of IAP1s, in MDAH2774, SKOV3and OVISE cells incubated with 25 and 50 mmol/L of C-75 for 48 h. Cells were lysed and immunoblotted with antibodies against cIAP1, cIAP2, XIAP and survivin as described in Materials and Methods. (D) Activation of caspases induced by C-75 treatment in EOC cells. Cells incubated with 25 and 50 mmol/L of C-75 for 48 h. Cells were lysed, and immunoblotted with antibodies against caspase 9, caspase 3 and cleaved caspase 3 and PARP as described in Materials and Methods. (E) MDAH2774 and SKOV3 cells were pretreated with 80 mmol/L of z-VAD-fmk and without for 2 h and subsequently with 25 and 50 mmol/L C-75 for 48 h. Cells were lysed and immunoblotted with antibodies against caspase 3, cleaved caspase 3 and PARP as described in Materials and Methods. (F) Effect of z-VAD/fmk on C-75-induced apoptosis in EOC cells. MDAH2774 and SKOV3 cells were pretreated with 80 mmol/L of z-VAD/fmk and without for 2 h and subsequently with 25 and 50 mmol/L C-75 for 48 h. Apoptosis was measured by annexin V/PI staining. z-VAD-fmk abrogates C-75-induced activation of caspase 3 in EOC cells.
Additionally, in our clinical samples, XIAP expression was found to be associated with FASN expression (P = 0.0046) (Table 1). We therefore examined whether C-75 induced cell death by modulating the expression of IAP family members that ultimately determine the cell response to apoptotic stimuli. EOC cells were treated with C7-5 for 48 h and the expression of XIAP, CIAP1 and CIAP2 and survivin was determined using Western blotting. C-75 caused downregulation of XIAP, CIAP1, CIAP2 in a dose- dependent manner (Figure 4C). Inhibitors of the apoptotic proteins have been shown to affect the caspases directly (32). Cytochrome c release has been shown to cause activation of caspases and cleavage of PARP. C-75 treatment resulted in activation of caspase 9, caspase 3 and cleavage of caspase 3 and PARP in MDAH2774, SKOV3 and OVISE cells (Figure 4D). These results are consistent with the data on cytochrome c release, and indicate that the activation of effector caspases are involved in C-75- induced apoptosis in EOC cells. In addition, pretreatment of MDAH2774 and SKOV3 cells with 80 mmol/L of z-VAD-fmk, a universal inhibitor of caspases, abrogated apoptosis and prevented apoptosis by caspase 3 and PARP activation induced by C-75-induced apoptosis in EOC cells (Figure 4E). This was further confirmed by annexin/PI staining (Figure 4F).
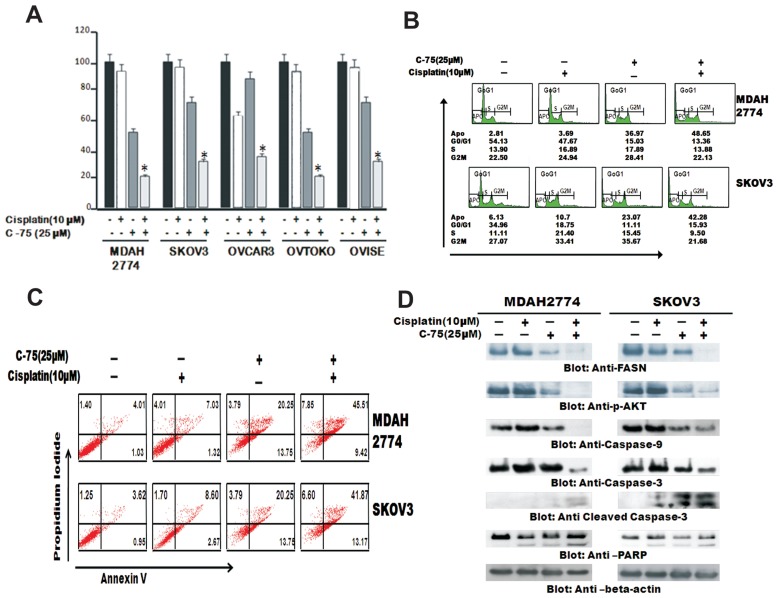
Inhibition of FASN Augments Antiproliferative Effects of Cisplatin in EOC Cells
The standard chemotherapy for EOC patients currently is a combination of taxane and platinum cisplatin (CDDP). CDDP is a well-known anticancer agent that also is active against many types of cancer (2). However cisplatin toxicity is a major concern in treatment of EOC. Therefore, we sought to determine whether C-75 augmented the antiproliferative effect of cisplatin and the induction of apoptosis in EOC cells. A panel of EOC cells, MDAH2774 and SKOV3, were treated with subtoxic doses of C-75 in combination with subtoxic doses of cisplatin for 48 h, and cell viability was assayed using MTT assay (Figure 5A). Combination treatment of 25 mmol/L C-75 and 10 mmol/L cisplatin induced growth inhibition, which was found to be statistically significant (P < 0.05) (Student t test) in all cell lines. We further sought to determine if the observed growth inhibition by MTT assay was due to induction of cell cycle arrest and apoptosis. We treated EOC cells with 25 mmol/L C-75 and 10 mmol/L cisplatin for 48 h, and cell cycle fractions were determined by flow cytometry. The sub-G1 population of cells increased from 2.81% in the control to 3.69% with cisplatin alone and 36.97% with C-75 alone, however, combination treatment increased it to 48.65% in MDAH2774 cells (Figure 5B.) This increase in sub-G1 population was accompanied by a loss of cells in G0/G1, S and G2/M phases, suggesting that the treated EOC cells were dying of apoptosis. Similar observation was also made in SKOV3 cells.
Figure 5.
C-75 enhances the cisplatin-mediated antitumor effects in EOC cells. (A) MDAH2774 and SKOV3, OVCAR3, OVTAKO and OVISE cells were incubated with 25 mmol/L of C-75 and 10 mmol/L cisplatin alone and in combination and were incubated for 48 h. Cell viability assays were performed using MTT as described in Materials and Methods. The graph displays the mean ± SD of three independent experiments for all the doses and vehicle control for each experiment. *P ≤ 0.05; **P ≤ 0.001, statistically significant (Student t test). (B) C-75 causes G2-M arrest after 48 h treatment leading to Apo fraction of cell cycle in EOC cells after 48 h. MDAH2774 and SKOV3 cells were incubated with 25 mmol/L of C-75 and 10 mmol/L cisplatin for 48 h. Thereafter the cells were washed, fixed and stained with propidium iodide and analyzed by flow cytometry for DNA content as described in Materials and Methods. (C) MDAH2774 and SKOV3 cells were incubated with 25 mmol/L of C-75 and 10 mmol/L cisplatin and apoptosis was detected by annexinV/propidium iodide dual staining. (D) Downregulation of FASN by C-75 inhibition alone and in combination with cisplatin causes downregulation of activated AKT, FOXO1 and GSK3. MDAH2774 and SKOV3 cells were incubated with 25 mmol/L of C-75 and 10 mmol/L cisplatin alone, and in combination for 48 h. Cells were lysed and equal amount of proteins were separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with antibodies against FASN, p-AKT, p- FOXO1 and p-GSK3. The blot was stripped and reprobed with an antibody against β-actin for equal loading.
Combination treatment–induced apoptosis in EOC cells was further confirmed by annexin/PI dual staining assay (Figure 5C), suggesting that suppression of growth by combination treatment in EOC cells is through apoptosis. To investigate whether inhibition of FASN activity by the subtoxic doses of C-75 in combination with cisplatin could be via inactivation of AKT pathway, MDAH2774, SKOV3 and OVISE cells were incubated with subtoxic doses of C-75 in combination with cisplatin for 48 h, cells were lysed and proteins were analyzed for Western blotting. As shown in Figure 5D, suppression of FASN expression and dephosphorylation of AKT was more effective when EOC cells were treated with a combination of subtoxic doses of C-75 and cisplatin rather than when treated alone, thereby potentiating the effect of C-75.
We next investigated the activation of caspases in cells treated with subtoxic doses of 25 mmol/L C-75 in combination with 10 mmol/L cisplatin in EOC cells by Western blotting. Combination treatment resulted in activation of caspase 9, caspase 3 and subsequent cleavage of caspase 3 and PARP in MDAH2774 and SKOV3 cells (see Figure 5D).
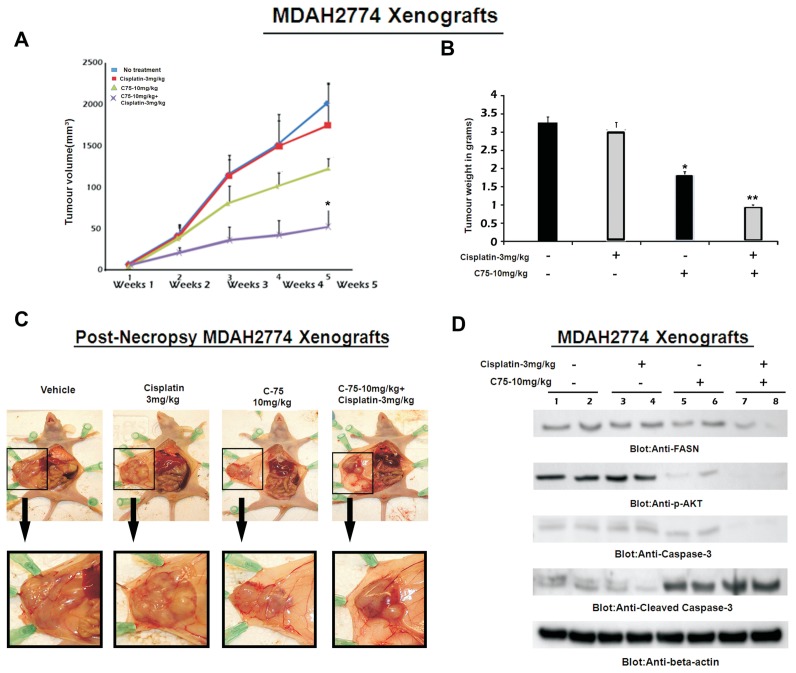
FASN Inhibition Enhanced Cisplatin-Mediated Antitumor Effects in Mice Xenografts
To confirm whether C-75 in combination with cisplatin can inactivate AKT and its downstream targets, inducing efficient apoptosis, we sought to determine whether combination of C-75 with cisplatin potentiates the inhibition of EOC xenograft tumor in nude mice as described in Materials and Methods. After 5 wks of treatment, mice were euthanized and the tumors were collected. Combination treatment caused significant regression of tumor volume at the end of the fifth week (P < 0.05) (Figure 6A). A significant reduction in the tumor weight (Figure 6B) was observed in the combination-treated mice when compared with mice treated with C-75 or with cisplatin alone. Furthermore, images of the tumor after necropsy showed more shrinkage of tumor size in combination-treated tumors than in those tumors treated alone (Figure 6C). Our data from in vitro experiments showed FASN inhibition following combination treatment with FASN inhibitor C-75 and cisplatin and subsequent dephosphorylation of AKT of activated AKT. We therefore examined whether FASN inhibition in combination with cisplatin altered the expression of these genes in vivo. Western blot analysis was carried out to analyze FASN, activated AKT and the caspase 3 levels in the primary tumors derived from vehicle-treated mice and in mice treated with C-75 alone or with cisplatin alone or the two in combination. FASN and its downstream target activated AKT and the apoptotic marker caspase 3 was downregulated substantially in the combination-treated xenograft tumors (Figure 6D).
Figure 6.
FASN inhibition enhances cisplatin-mediated antitumor effects in mouse xenografts. Nude mice at 6 wks of age were injected i.p. with 5 × 106 MDAH2774 cells. After 1 wk, mice were treated with 3 mg/kg per dose of cisplatin and 10 mg/kg per dose of C-75 alone and in combination or 5% DMSO in PBS as a vehicle control. (A) Inhibition of MDAH2774 tumor growth with C-75. The volume of each tumor was measured every week after the start of treatment. (B) The average (n = 5) tumor volume in vehicle-treated control mice and treated with 3 mg/kg per dose of cisplatin and 10 mg/kg per dose of C-75 alone and in combination was plotted. *P < 0.05. After 4 wks of treatment, mice were euthanized and tumor weights were measured. *P < 0.05 compared with vehicle-treated mice by Student t test. (C) Representative tumor images after necropsy of mice treated with vehicle, cisplatin alone, C-75 alone and the two in combination. The lower panel shows images with 10× magnification. (D) Whole-cell homogenates of individual tumor were separated on SDS-PAGE, transferred to PVDF membrane and immunoblotted with antibodies against FASN, p-AKT, caspase 3, cleaved caspase 3 and β-actin.
DISCUSSION
In light of recent evidence that links FASN activity and AKT activation for the promotion of tumorigenesis in various tumors (33–34), we sought to explore the relationship between FASN and AKT and its associated pathways in a cohort of Saudi EOC samples in a TMA format. Immunohistochemistry analysis of a large cohort of EOC samples showed an overexpression of FASN (75.5%) and its significant association with activated AKT and XIAP, linking its pathogenic role in tumorigenesis of Middle Eastern EOC. Sehdev et al. also have shown a higher incidence of FASN expression in ovarian carcinoma (7). Recently, it has been shown that AKT modulates the expression of FASN in a positive feedback manner in ovarian cancer cells (19).
In this study, we have aimed to clarify this issue by investigating the effect of FASN inhibition on cell growth, proliferation and FASN/PI3K/AKT signal transduction in a panel of EOC cell lines. We demonstrated that inhibition of FASN activity by C-75, a selective inhibitor, resulted in downregulation of FASN, inactivation (dephosphorylation) of AKT, as well as downregulation of its downstream target, GSK3 and FOXO1, leading to induction of apoptosis. Our pharmacological inhibition and gene silencing studies suggest that inhibition of AKT does not affect the expression of FASN. On the other hand, C-75 treatment of EOC cell lines, as well as gene silencing of FASN, inactivated AKT activity. These findings suggest that FASN is an upstream effector of AKT and its downregulation induces cell death via modulation of AKT-mediated antiapoptotic genes such as XIAP, CIAPs and survivin in ovarian cancer cell lines. Apoptosis is a multistep process, and an increasing number of genes have been identified that are involved in the control or execution of apoptosis (33). Our study shows that FASN inhibition by C-75 in EOC cells caused apoptosis via disruption of the mitochondrial membrane, allowing activation of proapoptotic proteins and the release of cytochrome c into cytosol. Released cytochrome c results in the formation of apoptosome by interaction with APAF1 and caspase 9, leading to the activation of caspase 3, eventually resulting in cleavage of PARP in apoptotic cells, a hallmark of apoptosis by various antitumor agents (34). Furthermore, pretreatment of EOC cells with a broad-spectrum caspase inhibitor abrogated the C-75-induced apoptosis. These data suggest that inhibition of the FASN/AKT pathway in EOC-induced apoptosis via caspase cascade activation.
Cisplatin has been used to treat a variety of malignancies, however, acquired resistance prevents use of this chemotherapeutic agent owing to the escalation of doses to overcome cellular resistance (35). The increased doses of cisplatin can cause severe cytotoxicity to normal tissues, thereby posing major clinical challenges. The use of subtoxic doses of dual agents that target distinct molecules can be a useful alternative strategy of effective treatment with less toxicity. Our data showed that subtoxic doses of C-75 augmented the apoptotic response of cisplatin in EOC cells. Furthermore, combination treatment of C-75 and cisplatin significantly regressed the Xenograft tumor in the Nude mouse model, suggesting that FASN and cisplatin combination treatment has more effective anti-tumor effects in vivo.
Altogether, these findings show that the FASN/AKT signaling pathway plays a key role in the pathogenesis of Middle Eastern EOC. Inhibition of FASN/AKT sensitizes EOC cells to cisplatin-induced cell death via apoptosis in vitro and in vivo. Thus, effectiveness of the combination of inhibitors of FASN with conventional chemotherapeutic agents such as cisplatin offers a promising targeted therapy for the treatment of EOC.
Supplemental Data
ACKNOWLEDGMENTS
We thank Saeeda O Ahmed, Sarita Prabhakran, Valorie Balde, Thangavel Saravanan and Hassan Al-Dossari for technical assistance and Thara George and Zeeshan Qadri for data analysis. The work was supported by King Abdulaziz City for Science and Technology (KACST) and the National Comprehensive Plan for Science and Technology (NCPST) resulting from KACST Project # 08-MED478–20.
Footnotes
Online address: http://www.molmed.org
DISCLOSURES
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Cannistra SA. Cancer of the ovary. N Eng J Med. 2004;351:2519–29. doi: 10.1056/NEJMra041842. [DOI] [PubMed] [Google Scholar]
- 2.Peluso JJ, Liu X, Saunders MM, Claffey KP, Phoenix K. Regulation of ovarian cancer cell viability and sensitivity to cisplatin by progesterone receptor membrane component-1. J Clin Endocrinol Metab. 2008;93:1592–9. doi: 10.1210/jc.2007-2771. [DOI] [PubMed] [Google Scholar]
- 3.Asturias FJ, et al. Structure and molecular organization of mammalian fatty acid synthase. Nat Struct Mol Biol. 2005;12:225–32. doi: 10.1038/nsmb899. [DOI] [PubMed] [Google Scholar]
- 4.Milgraum LZ, Witters LA, Pasternack GR, Kuhajda FP. Enzymes of the fatty acid synthesis pathway are highly expressed in in-situ breast carcinoma. Clin Cancer Res. 1997;3:2115–20. [PubMed] [Google Scholar]
- 5.Uddin S, et al. Fatty acid synthase and AKT pathway signaling in a subset of papillary thyroid cancers. J Clin Endocrinol Metab. 2008;93:4088–97. doi: 10.1210/jc.2008-0503. [DOI] [PubMed] [Google Scholar]
- 6.Rashid A, et al. Elevated expression of fatty acid synthase and fatty acid synthetic activity in colorectal neoplasia. Am J Pathol. 1997;150:201–8. [PMC free article] [PubMed] [Google Scholar]
- 7.Sehdev AS, Kurman RJ, Kuhn E, Shih IeM. Serous tubal intraepithelial carcinoma upregulates markers associated with high-grade serous carcinomas including Rsf-1 (HBXAP), cyclin E and fatty acid synthase. Mod Pathol. 2010;23:844–55. doi: 10.1038/modpathol.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cerne D, Zitnik IP, Sok M. Increased fatty acid synthase activity in non-small cell lung cancer tissue is a weaker predictor of shorter patient survival than increased lipoprotein lipase activity. Arch Med Res. 2010;41:405–9. doi: 10.1016/j.arcmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Pizer ES, et al. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–10. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 10.Kuhajda FP. Fatty-acid synthase and human cancer: new perspectives on its role in tumor biology. Nutrition. 2000;16:202–8. doi: 10.1016/s0899-9007(99)00266-x. [DOI] [PubMed] [Google Scholar]
- 11.Kuhajda FP. Fatty acid synthase and cancer: new application of an old pathway. Cancer Res. 2006;66:5977–80. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 12.Ueda SM, et al. Expression of fatty acid synthase depends on NAC1 and is associated with recurrent ovarian serous carcinomas. J. Oncol. 2010;28:5191. doi: 10.1155/2010/285191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pizer ES, et al. Increased fatty acid synthase as a therapeutic target in androgen-independent prostate cancer progression. Prostate. 2001;47:102–10. doi: 10.1002/pros.1052. [DOI] [PubMed] [Google Scholar]
- 14.Pizer ES, et al. Malonyl-coenzyme-A is a potential mediator of cytotoxicity induced by fatty-acid synthase inhibition in human breast cancer cells and xenografts. Cancer Res. 2000;60:213–8. [PubMed] [Google Scholar]
- 15.De Schrijver E, Brusselmans K, Heyns W, Verhoeven G, Swinnen JV. RNA interference-mediated silencing of the fatty acid synthase gene attenuates growth and induces morphological changes and apoptosis of LNCaP prostate cancer cells. Cancer Res. 2003;63:3799–804. [PubMed] [Google Scholar]
- 16.Pizer ES, et al. Inhibition of fatty acid synthesis delays disease progression in a xenograft model of ovarian cancer. Cancer Res. 1996;56:1189–93. [PubMed] [Google Scholar]
- 17.Bandyopadhyay S, et al. FASN expression inversely correlates with PTEN level in prostate cancer and a PI 3-kinase inhibitor synergizes with FASN siRNA to induce apoptosis. Oncogene. 2005;24:5389–95. doi: 10.1038/sj.onc.1208555. [DOI] [PubMed] [Google Scholar]
- 18.Porstmann T, et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene. 2005;24:6465–81. doi: 10.1038/sj.onc.1208802. [DOI] [PubMed] [Google Scholar]
- 19.Wang HQ, et al. Positive feedback regulation between AKT activation and fatty acid synthase expression in ovarian carcinoma cells. Oncogene. 2005;24:3574–3582. doi: 10.1038/sj.onc.1208463. [DOI] [PubMed] [Google Scholar]
- 20.Franke TF, Hornik CP, Segev L, Shostak GA, Sugimoto C. PI3K/Akt and apoptosis: size matters. Oncogene. 2003;22:8983–98. doi: 10.1038/sj.onc.1207115. [DOI] [PubMed] [Google Scholar]
- 21.Nicholson KM, Anderson NG. The protein kinase B/Akt signalling pathway in human malignancy. Cell Signal. 2002;14:381–95. doi: 10.1016/s0898-6568(01)00271-6. [DOI] [PubMed] [Google Scholar]
- 22.Häusler P, et al. Protection of CD95-mediated apoptosis by activation of phosphatidylinositide 3-kinase and protein kinase B. Eur J Immunol. 1998;1:57–69. doi: 10.1002/(SICI)1521-4141(199801)28:01<57::AID-IMMU57>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 23.Di Cristofano A, et al. Impaired FASN response and autoimmunity in Pten+/− mice. Science. 1999;285:21. doi: 10.1126/science.285.5436.2122. [DOI] [PubMed] [Google Scholar]
- 24.Bavi P, et al. Prognostic significance of TRAIL death receptors in Middle Eastern colorectal carcinomas and their correlation to oncogenic K-RAs alterations. Mol. Cancer. 2010;9:203. doi: 10.1186/1476-4598-9-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russel P. Surface epithelial-stromal tumors of the ovary. In: Kurman RJ, editor. Blaustein’s Pathology of the Female Genital Tract. 4th edition. Springer-Verlag; New York: 1994. pp. 705–82. [Google Scholar]
- 26.Uddin S, et al. Role of phosphatidylinositol 3′-kinase/AKT pathway in diffuse large B-cell lymphoma survival. Blood. 2006;108:4178–86. doi: 10.1182/blood-2006-04-016907. [DOI] [PubMed] [Google Scholar]
- 27.Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio-informatics tool for biomarker assessment and outcome based cut-point optimization. Clin Cancer Res. 2004;10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713. [DOI] [PubMed] [Google Scholar]
- 28.Hussain AR, et al. Sanguinarine-dependent induction of apoptosis in primary effusion lymphoma cells. Cancer Res. 2007;67:3888–97. doi: 10.1158/0008-5472.CAN-06-3764. [DOI] [PubMed] [Google Scholar]
- 29.Uddin S, et al. High prevalence of fatty acid synthase expression in colorectal cancers in Middle Eastern patients and its potential role as a therapeutic target. Am J Gastroenterol. 2009;104:1790–801. doi: 10.1038/ajg.2009.230. [DOI] [PubMed] [Google Scholar]
- 30.Ciechomska I, Pyrzynska B, Kazmierczak P, Kaminska B. Inhibition of Akt kinase signalling and activation of Forkhead are indispensable for upregulation of FasL expression in apoptosis of glioma cells. Oncogene. 2003;22:7617–27. doi: 10.1038/sj.onc.1207137. [DOI] [PubMed] [Google Scholar]
- 31.Cohen P, Alessi DR, Cross DA. PDK1, one of the missing links in insulin signal transduction. FEBS Lett. 1997;410:3–10. doi: 10.1016/s0014-5793(97)00490-0. [DOI] [PubMed] [Google Scholar]
- 32.Dan HC, et al. Akt phosphorylation and stabilization of X-linked inhibitor of apoptosis protein (XIAP) J Biol Chem. 2004;279:5405–12. doi: 10.1074/jbc.M312044200. [DOI] [PubMed] [Google Scholar]
- 33.Gastman BR. Apoptosis and its clinical impact. Head Neck. 2001;23:409–25. doi: 10.1002/hed.1052. [DOI] [PubMed] [Google Scholar]
- 34.Duriez PJ, et al. Characterization of anti-peptide antibodies directed towards the auto-modification domain and apoptotic fragment of poly (ADP-ribose) polymerase. Biochim Biophys Acta. 1997;1334:65–72. doi: 10.1016/s0304-4165(96)00077-3. [DOI] [PubMed] [Google Scholar]
- 35.Piccart MJ, Lamb H, Vermorken JB. Current and future potential roles of the platinum drugs in the treatment of ovarian cancer. Ann Oncol. 2001;12:1195–203. doi: 10.1023/a:1012259625746. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.