Abstract
Fibroblast growth factor-21 (FGF21) is a pleiotropic protein involved in glucose, lipid metabolism and energy homeostasis, with main tissues of expression being the liver and adipose tissue. Brown adipose tissue (BAT) is responsible for cold-induced thermogenesis in rodents. The role of FGF21 in BAT biology has not been investigated. In the present study, wild-type C57BL/6J mice as well as a brown adipocyte cell line were used to explore the potential role of cold exposure and β3-adrenergic stimulation in the expression of FGF21 in BAT. Our results demonstrate that short-term exposure to cold, as well as β3-adrenergic stimulation, causes a significant induction of FGF21 mRNA levels in BAT, without a concomitant increase in FGF21 plasma levels. This finding opens new routes for the potential use of pharmaceuticals that could induce FGF21 and, hence, activate BAT thermogenesis.
INTRODUCTION
Fibroblast growth factor (FGF)-21 belongs to the family of atypical FGFs that lack the conventional heparin-binding domain (1,2) and can thus diffuse away from their tissues of origin to function as hormones. FGF21 is abundantly expressed in liver, pancreas and white adipose tissue (WAT) (3,4). It signals through cell-surface complexes of FGF receptors with the transmembrane protein β-Klotho (5–8). The limited β-Klotho expression in metabolically competent liver, pancreas and adipose tissue permits FGF21 to selectively target these tissues, thus allowing this factor to influence glucose, lipid and body weight homeostasis (8,9).
FGF21 is involved in the adaptation of the body to starvation and acts to regulate fatty acid oxidation and ketone formation. The transcription factor peroxisome proliferator–activated receptor (PPAR)α is a critical regulator of FGF21 (10).
FGF21-infused mice have higher energy expenditure and elevated core body temperature, suggesting that FGF21 has catabolic functions (11,12). Centrally administered FGF21 induces energy expenditure and insulin sensitivity (13). Systemically administered FGF21 triggers a sustained lowering of blood glucose and triglycerides, improved insulin sensitivity, enrichment in brown adipocytes (14), preservation of β-cell function and mass (15), amelioration of obesity and hepatosteatosis (10,12).
In adipose tissue, FGF21 simultaneously induces lipid accumulation, uncoupling, biogenesis and inhibition of lipolysis, indicative of a state of futile cycling (11,16).
Brown adipose tissue (BAT) burns fatty acids for heat production to defend the body against cold and has recently been shown to be present in humans (17). Upon cold-triggered activation, BAT increases its energy demand and burns carbohydrates and lipids to produce heat using uncoupling protein-1 (UCP1) (18). β3-adrenergic receptors are expressed abundantly and predominantly in brown adipocytes and selective agonists of this receptor have been synthesized. Treatment of mice with such agonists doubles oxygen consumption, demonstrating the remarkable capacity of this thermogenic mechanism (19). PPARγ coactivator-1α (PGC-1α) is highly expressed in brown but not white fat, with marked and rapid induction in brown fat and skeletal muscle upon exposure of mice to cold. This cold induction of PGC-1α is largely due to sympathetic nervous system input through β-adrenergic receptors and cAMP action (20,21).
There are reports that FGF21 is expressed in BAT, but its role in metabolism has not been investigated (11,14,22). Instead, FGF21 produced in the liver promotes thermogenic activation of brown fat and is dispensable during starvation-induced torpor (23,24).
Here we demonstrate that BAT of mice not only responds to FGF21 produced in the liver, but also overexpresses FGF21 after cold exposure or selective β3-adrenergic stimulation. This FGF21 might act as an autocrine factor.
MATERIALS AND METHODS
Mice
Mice were bred and housed in the animal facility of the University of Patras Medical School at 22°C with ad libitum access to standard laboratory chow diet. We used male age-matched (24 weeks) C57BL/6J wild-type mice (The Jackson Laboratory, Bar Harbor, ME, USA). For the cold experiments, mice were individually housed and fasted for 12 h, and during the last 4 h of fasting, they were exposed to either control (22°C) or low temperature (4°C). At the end of the cold exposure, blood was collected and interscapular BAT, epididymal WAT and liver were harvested in RNA later solution. Similarly, the selective β3-adrenergic receptor agonist CL316243 (Sigma, Germany; 2 mg/kg body weight) was given by intraperitoneal injection 4 h before the end of the experiment. All animal procedures were approved by the institutional review board of the University of Patras Medical School and were in accordance with EC (European Commission) Directive 86/609/EEC.
Measurements of Hormones and Metabolites
Plasma was collected by using heparin as an anticoagulant and was centrifuged at 2,000g for 20 min at 4°C. Plasma measurements were conducted following the manufacturer’s instruction for each kit. Enzyme-linked immunosorbent assay (ELISA) kits were used for plasma leptin (ALPCO, Salem, NH, USA) and FGF21 (R&D, Minneapolis, MN, USA). Cholesterol and triglycerides were measured by using an Olympus AU640 analyzer (Hamburg, Germany).
Quantification of Gene Expression Levels
Liver, BAT and WAT were submerged immediately after collection in RNA later solution (Ambion, Foster City, CA, USA). Total RNA was isolated by using Trizol reagent (Invitrogen) and further purified by using the RNeasy mini kit (Qiagen, Hilden, Germany). A DNAse (Turbo -DNAse; Ambion) digestion step was included to prevent genomic DNA contamination. cDNA was synthesized by using the Superscript first-strand synthesis system (Invitrogen) and real-time (RT)- polymerase chain reactions (PCRs) were performed in triplicate on a Step One Plus instrument (Applied Biosystems, Foster City, CA) using Taqman Gene Expression assays on demand (Applied Biosystems): FGF21, Mm00840165_g1; PGC-1α, Mm00447183_m1; PPARα, Mm00440939_m1; GAPDH (glyceraldehyde-3-phosphate dehydrogenase), 4352339E. Relative mRNA levels were calculated by the comparative threshold cycle method using GAPDH as the housekeeping gene.
Cell Culture and Treatments
SV40T-immortalized brown adipocytes from the C57BL/6J strain of mice were provided by Prof. Johannes Klein (Lübeck, Germany) (25). Preadipocytes were grown to confluence in Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Paisley, Strathclyde, UK) supplemented with 20% fetal bovine serum, 4.5 g/L glucose, 20 nmol/L insulin, 1 nmol/L triiodothyronine (“differentiation medium”) and penicillin/streptomycin. Adipocyte differentiation was induced by complementing the medium further with 250 μmol/L indomethacin, 500 μmol/L isobutylmethylxanthine and 2 μg/mL dex-amethasone for 24 h when confluence was reached. After this induction period, cells were changed back to differentiation medium. Cell culture was continued for 5 more days before cells were starved for 24 h with serum-free medium prior to carrying out the experiments. Maximally differentiated cells were treated, when indicated, with 50 μmol/L of the β3-adrenergic receptor agonist CL316243 for 6 h or with 10 μmol/L of the PPARα antagonist GW6471 (Sigma, Germany) for 16 h before harvesting the cells.
Statistical Analyses
Experiments were performed three times by using at least triplicate samples per group. Data were expressed as the mean ± SEM. Student t test or one-way analysis of variance (ANOVA) followed by Tukey test was performed by using GraphPad Prism 5 (GraphPad Software, La Jolla, CA, USA). P < 0.05 was considered significant.
RESULTS
Cold Exposure Induces FGF21 Gene Expression in BAT
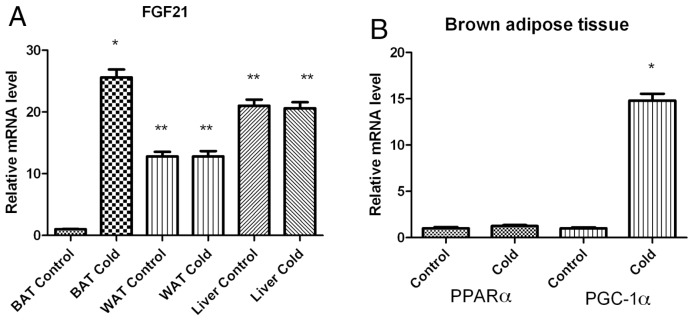
Exposure of wild-type C57BL/6J mice to 4°C for 4 h caused a significant fold increase in FGF21 mRNA levels in BAT over the baseline (25.6 ± 1.3, P < 0.01, Figure 1A). It is known that after cold exposure, PGC-1α mRNA levels of BAT are induced as part of the cold-induced thermogenesis program. In the same experiment, PGC-1α mRNA levels indeed increased by 15-fold, as expected (20). In contrast, cold did not change FGF21 mRNA levels in the liver or the WAT tissues, where FGF21 is known to be mainly expressed (see Figure 1A).
Figure 1.
FGF21 mRNA levels are induced in BAT of wild-type C57BL/6J mice after exposure to cold for 4 h. (A) FGF21 mRNA levels were measured by quantitative RT-PCR in BAT, WAT and liver obtained from wild-type C57BL/6J mice. Bars show means ± SEM of eight mice per group. Data were analyzed by one-way ANOVA. Statistically significant differences between control mice and mice exposed to 4°C for the same tissue are shown (*P < 0.001). **Statistically significant differences between control tissues. (B) PPARα and PGC-1α mRNA levels were measured by quantitative RT-PCR in BAT of mice. Data were analyzed by a t test for each gene separately. *Statistically significant differences between control mice and mice exposed to 4°C.
Bearing in mind that the induction of FGF21 with fasting is PPARα dependent (10), PPARα mRNA levels were tested in BAT. No difference however was found between control and cold-exposed animals (Figure 1B).
Plasma Chemistries after Exposure to Cold
Taking into account that FGF21 might be secreted and act as a hormone, plasma FGF21 levels were measured after exposure of mice to cold for 4 h. No differences were found between the two states. By contrast, plasma leptin and triglyceride levels dropped in accordance with other studies (Table 1) (26,27).
Table 1.
Plasma metabolic parameters of C57BL/6J mice maintained at 22°C or after exposure to 4°C for 4 h.
| 22°C | 4°C | |
|---|---|---|
| Triglycerides (mg/dL) | 92 ± 8 | 74 ± 6a |
| Cholesterol (mg/dL) | 94 ± 6 | 96 ± 6 |
| Leptin (pg/mL) | 5200 ± 78 | 3296 ± 70b |
| FGF21 (pg/mL) | 3620 ± 451.1 | 2755 ± 173.4 |
Data are means ± SEM; n = 10.
P < 0.05.
P < 0.001.
β3-Adrenergic Receptor Stimulation Induces FGF21 Gene Expression in BAT But Not in the Liver
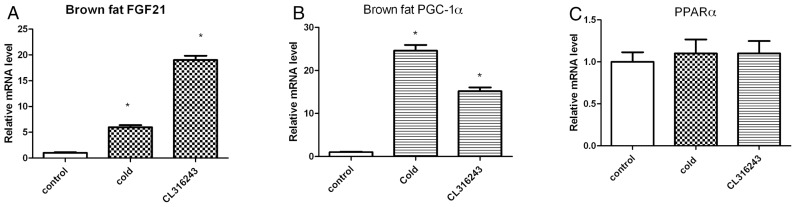
Because it is known that cold activates BAT via the sympathetic nervous system, a selective β3-agonist (CL316243) was tested for its ability to reproduce the above findings (28). The intraperitoneal administration of CL316243 led to a significant fold increase in FGF21 mRNA levels in BAT over the baseline (19 ± 0.8, P < 0.01, Figure 2A). As a control, PGC-1α mRNA levels were measured and found to be induced by both cold and CL316243, as expected (Figure 2B). No differences in the expression of PPARα were found (Figure 2C).
Figure 2.
FGF21 mRNA levels are induced in BAT of wild-type C57BL/6J mice after stimulation with the β3-adrenergic agonist CL316243. Mice were either exposed to 4°C for 4 h or injected intraperitoneally (2 mg/kg body weight) with CL316243, 4 h before harvesting BAT. mRNA levels for FGF21 (A), PGC-1α (B) and PPARα (C) were measured by quantitative RT-PCR. Bars show means ± SEM of eight mice per group. Data were analyzed by one-way ANOVA. Statistically significant differences between control mice and mice exposed to 4°C or CL316243 are shown (*P < 0.001).
Treatment of Differentiated Mouse Brown Adipocytes with the β3-Agonist Increases FGF21 mRNA Levels
To discriminate between cell autonomous or nonautonomous effect of β3-adrenergic stimulation on FGF21 expression, a brown fat cell line able to differentiate into mature brown adipocytes and respond to the β3-agonist was used (25). Differentiated brown adipocytes were treated with CL316243 (50 μmol/L) for 6 h. The levels of FGF21 mRNA were increased more than five-fold (5.1 ± 0.8, P < 0.001, Figure 3). No change in FGF21 mRNA was observed in undifferentiated brown preadipocytes (data not shown), since they do not express β3-adrenergic receptors (18). Treatment of the adipocytes with the PPARα antagonist GW6471 did not affect the response of these cells to the β3 agonist.
Figure 3.
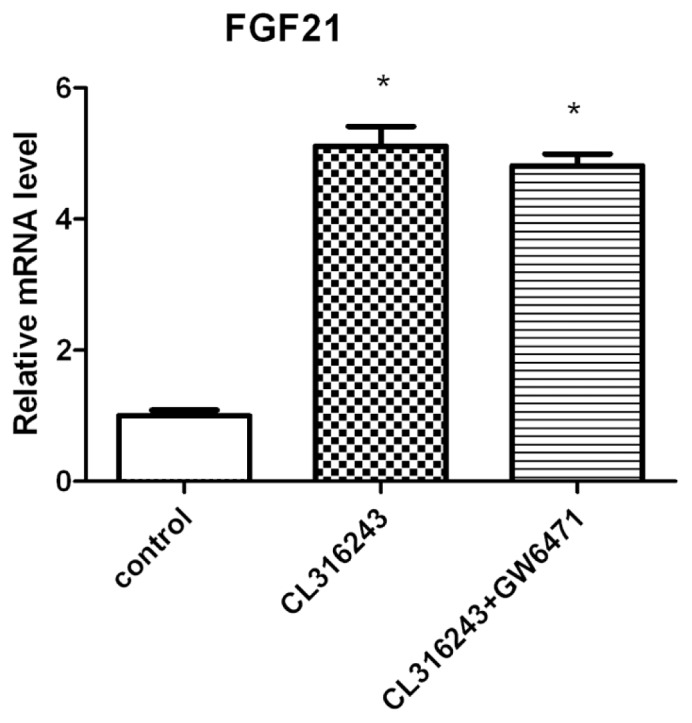
Induction of FGF21 mRNA levels with the β3-adrenergic agonist CL316243 is not hampered by coadministration of a PPARα antagonist. Differentiated brown adipocyte cells were treated with CL316243 for 6 h in the presence or absence of GW6471, and FGF21 mRNA levels were measured by quantitative RT-PCR. Data were analyzed by one-way ANOVA. Bars show means ± SEM of three independent experiments. Statistically significant differences between control and treated cells are denoted (*P < 0.01).
DISCUSSION
In the present study, we have shown that short-term exposure of mice to 4°C induced FGF21 mRNA levels in brown fat. This induction is restricted to BAT, whereas no change is observed in liver and WAT. The baseline expression level of FGF21 in BAT is lower than that in WAT, but after cold exposure, it is induced well above that in the latter. At the same time, plasma FGF21 was not found to increase accordingly by this short-term exposure to cold.
BAT is heavily innervated by sympathetic nerves and is responsible for thermogenesis during cold exposure. β3-adrenergic receptor agonists cause an increase in energy expenditure that is comparable to that induced by cold in both rodents and humans (19). In our experiments, stimulation of either mice or a brown adipocyte cell line with the β3 agonist CL316243 had the same affect on FGF21 expression as exposure to cold. This points to a novel mechanism of action of β3 agonists in brown fat through induction of FGF21 and suggests that some of the favorable effects of β3 agonists might be mediated via induction of FGF21 (11–13,19). PGC-1α, a known target of β3-adrenergic receptor stimulation in brown fat (19), was also induced in our experimental system. It is noteworthy that FGF21 induces PGC-1α and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response in liver (29). It remains to be seen if this applies to BAT as well.
Leptin levels fall after cold exposure, and this might be a signal for initiating a broad program of adaptation to starvation (30). FGF21 is considered a starvation factor as well (31). In this vein, the reduction of leptin levels observed after exposure to cold could be a permissive factor for the observed induction of FGF21 in BAT, although this does not explain the selectivity of the induction only in BAT.
In liver, FGF21 was shown to be induced after fasting in a PPARα-dependent manner. In our in vivo or in vitro experiments, no differences were found in the expression of PPARα that could account for the aforementioned increase of FGF21. Nevertheless, activation of PPARα without any change in its expression levels could lead to elevation of FGF21. This result is not supported by our findings, since the PPARα antagonist GW6471 did not change the response of brown adipocytes to β3 agonist regarding FGF21 induction.
The fact that FGF21 plasma levels did not change could be attributed to the small contribution of brown fat to the overall production of FGF21. In this case, it is conceivable that FGF21 could be induced in BAT after cold exposure and act in an autocrine fashion. A general model for this kind of action of FGF21 is favored by a recent review (31). Alternatively, the duration of cold exposure might have not been sufficient enough to reveal any differences. Direct action of the sympathetic nervous system on BAT and induction of FGF21 remains as the most plausible explanation. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat (23). It can be envisioned that medications that augment the expression of FGF21 in brown fat could activate BAT thermogenesis and be used in the treatment of obesity. Experiments are under way in our laboratory to delineate the mechanisms of FGF21 induction by cold/β3-adrenergic stimulation, as well as the significance of this induction in the thermogenic program of the brown fat adipocyte. We note that during finalizing this report, a paper addressing the same issue was published online, corroborating our main finding of FGF21 induction by cold in BAT (32).
Footnotes
Online address: http://www.molmed.org
DISCLOSURE
The authors declare that they have no competing interests as defined by Molecular Medicine, or other interests that might be perceived to influence the results and discussion reported in this paper.
REFERENCES
- 1.Itoh N, Ornitz DM. Functional evolutionary history of the mouse Fgf gene family. Dev Dyn. 2008;237:18–27. doi: 10.1002/dvdy.21388. [DOI] [PubMed] [Google Scholar]
- 2.Goetz R, et al. Molecular insights into the Klotho-dependent, endocrine mode of action of fibroblast growth factor 19 subfamily members. Mol Cell Biol. 2007;27:3417–28. doi: 10.1128/MCB.02249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nishimura T, Nakatake Y, Konishi M, Itoh N. Identification of a novel FGF, FGF-21, preferentially expressed in the liver. Biochim Biophys Acta. 2000;1492:203–6. doi: 10.1016/s0167-4781(00)00067-1. [DOI] [PubMed] [Google Scholar]
- 4.Muise ES, et al. Adipose fibroblast growth factor 21 is up-regulated by peroxisome proliferator-activated receptor gamma and altered metabolic states. Mol Pharmacol. 2008;74:403–12. doi: 10.1124/mol.108.044826. [DOI] [PubMed] [Google Scholar]
- 5.Kharitonenkov A, et al. FGF-21/FGF-21 receptor interaction and activation is determined by betaKlotho. J Cell Physiol. 2008;215:1–7. doi: 10.1002/jcp.21357. [DOI] [PubMed] [Google Scholar]
- 6.Kurosu H, et al. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem. 2007;282:26687–95. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suzuki M, et al. BetaKlotho is required for fibroblast growth factor (FGF) 21 signaling through FGF receptor (FGFR) 1c and FGFR3c. Mol Endocrinol. 2008;22:1006–14. doi: 10.1210/me.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa Y, et al. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Sci U S A. 2007;104:7432–7. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito S, et al. Molecular cloning and expression analyses of mouse betaklotho, which encodes a novel Klotho family protein. Mech Dev. 2000;98:115–9. doi: 10.1016/s0925-4773(00)00439-1. [DOI] [PubMed] [Google Scholar]
- 10.Badman MK, et al. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab. 2007;5:426–37. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Coskun T, et al. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology. 2008;149:6018–27. doi: 10.1210/en.2008-0816. [DOI] [PubMed] [Google Scholar]
- 12.Xu J, et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–9. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sarruf DA, et al. Fibroblast growth factor 21 action in the brain increases energy expenditure and insulin sensitivity in obese rats. Diabetes. 2010;59:1817–24. doi: 10.2337/db09-1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kharitonenkov A, et al. FGF-21 as a novel metabolic regulator. J Clin Invest. 2005;115:1627–35. doi: 10.1172/JCI23606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wente W, et al. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;55:2470–8. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- 16.Xu J, et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin resistant mouse models: association with liver and adipose tissue effects. Am J Physiol Endocrinol Metab. 2009 2009 Aug 25; doi: 10.1152/ajpendo.00348.2009. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–8. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 18.Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 19.Lowell BB, Spiegelman BM. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–60. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 20.Puigserver P, et al. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92:829–39. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 21.Boss O, et al. Role of the beta(3)-adrenergic receptor and/or a putative beta(4)-adrenergic receptor on the expression of uncoupling proteins and peroxisome proliferator-activated receptor-gamma coactivator-1. Biochem Biophys Res Commun. 1999;261:870–6. doi: 10.1006/bbrc.1999.1145. [DOI] [PubMed] [Google Scholar]
- 22.Fon Tacer K, et al. Research resource: comprehensive expression atlas of the fibroblast growth factor system in adult mouse. Mol Endocrinol. 2010;24:2050–64. doi: 10.1210/me.2010-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hondares E, et al. Hepatic FGF21 expression is induced at birth via PPARalpha in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 2010;11:206–12. doi: 10.1016/j.cmet.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oishi K, et al. FGF21 is dispensable for hypothermia induced by fasting in mice. Neuro Endocrinol Lett. 2010;31:198–202. [PubMed] [Google Scholar]
- 25.Klein J, et al. Beta(3)-adrenergic stimulation differentially inhibits insulin signaling and decreases insulin-induced glucose uptake in brown adipocytes. J Biol Chem. 1999;274:34795–802. doi: 10.1074/jbc.274.49.34795. [DOI] [PubMed] [Google Scholar]
- 26.Bartelt A, et al. Brown adipose tissue activity controls triglyceride clearance. Nat Med. 2011;17:200–5. doi: 10.1038/nm.2297. [DOI] [PubMed] [Google Scholar]
- 27.Trayhurn P, Duncan JS, Rayner DV. Acute cold-induced suppression of ob (obese) gene expression in white adipose tissue of mice: mediation by the sympathetic system. Biochem J. 1995;311:729–33. doi: 10.1042/bj3110729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomez-Ambrosi J, Fruhbeck G, Martinez JA. Rapid in vivo PGC-1 mRNA upregulation in brown adipose tissue of Wistar rats by a beta(3)-adrenergic agonist and lack of effect of leptin. Mol Cell Endocrinol. 2001;176:85–90. doi: 10.1016/s0303-7207(01)00451-8. [DOI] [PubMed] [Google Scholar]
- 29.Potthoff MJ, et al. FGF21 induces PGC-1alpha and regulates carbohydrate and fatty acid metabolism during the adaptive starvation response. Proc Natl Acad Sci U S A. 2009;106:10853–8. doi: 10.1073/pnas.0904187106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahima RS, et al. Role of leptin in the neu-roendocrine response to fasting. Nature. 1996;382:250–2. doi: 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- 31.Kharitonenkov A, Larsen P. FGF21 reloaded: challenges of a rapidly growing field. Trends Endocrinol Metab. 2011;22:81–6. doi: 10.1016/j.tem.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Hondares E, et al. Thermogenic activation induces FGF21 expression and release in brown adipose tissue. J Biol Chem. 2011;286:12983–90. doi: 10.1074/jbc.M110.215889. [DOI] [PMC free article] [PubMed] [Google Scholar]