Abstract
Despite improvements in prevention and management of colorectal cancer (CRC), uncontrolled tumor growth with metastatic spread to distant organs remains an important clinical concern. Genetic deletion of CD39, the dominant vascular and immune cell ectonucleotidase, has been shown to delay tumor growth and blunt angiogenesis in mouse models of melanoma, lung and colonic malignancy. Here, we tested the influence of CD39 on CRC tumor progression and metastasis by investigating orthotopic transplanted and metastatic cancer models in wild-type BALB/c, human CD39 transgenic and CD39 deficient mice. We also investigated CD39 and P2 receptor expression patterns in human CRC biopsies. Murine CD39 was expressed by endothelium, stromal and mononuclear cells infiltrating the experimental MC-26 tumors. In the primary CRC model, volumes of tumors in the subserosa of the colon and/or rectum did not differ amongst the treatment groups at day 10, albeit these tumors rarely metastasized to the liver. In the dissemination model, MC-26 cell line-derived hepatic metastases grew significantly faster in CD39 over-expressing transgenics, when compared to CD39 deficient mice. Murine P2Y2 was significantly elevated at both mRNA and protein levels, within the larger liver metastases obtained from CD39 transgenic mice where changes in P2X7 levels were also noted. In clinical samples, lower levels of CD39 mRNA in malignant CRC tissues appeared associated with longer duration of survival and could be linked to less invasive tumors. The modulatory effects of CD39 on tumor dissemination and differential levels of CD39, P2Y2 and P2X7 expression in tumors suggest involvement of purinergic signalling in these processes. Our studies also suggest potential roles for purinergic-based therapies in clinical CRC.
Keywords: CD39, NTPDase1, P2 receptors, Colorectal cancer, MC-26 cancer cell line
Introduction
Colorectal cancer (CRC) is an important malignant disease, being the second most common cause of cancer-related death in the USA [1]. Although significant improvements in care in patients with early disease have followed clinical advances with better screening, diagnosis and treatment modalities, pre-existing tumor growth and metastatic spread still limit long-term patient survival [1, 2]. Targeted surgical resection, in combination with cutting edge type interventions, e.g. anti-angiogenic modalities (e.g. bevacizumab (Avastin®)) have positive but limited impacts upon survival in patients with metastatic disease [3].
Suitable animal models are required to test further therapeutic interventions that might preclude spread or potentially treat established metastases. Limited numbers of studies describe feasible, practical and reliable experimental models that mimic both primary and metastatic colorectal cancer in the same animal [2, 4–7]. In the present studies, subserosal colorectal tumor models were developed in mice, in parallel with protocols aimed at provoking dissemination of cancer cells. This initially involved injecting MC-26 CRC cells [8] directly in the subserosal space of the colorectum, therefore allowing deposition of MC-26 CRC at a possible primary anatomical site. However, this model has been characterized by limited metastatic spread [4, 6]. Hence, to study the metastatic potential of MC-26 CRC cells, in parallel we injected these cells in the spleen, followed by splenectomy to ensure controlled and limited CRC dissemination into the portal system [7].
Tumor invasion and spread are thought to be influenced by genetic alterations in the tumor, angiogenesis, lymphatic dissemination, host matrix dysregulation and other factors [9, 10]. Extracellular nucleotides are hydrolyzed by ecto-enzymes such as nucleoside triphosphate diphosphohydrolase-1 (CD39/NTPDase-1). CD39 is the dominant vascular endothelial and immune ectonucleotidase and hydrolyzes both ATP and ADP in the plasma to AMP which is hydrolyzed to adenosine by CD73 [11]. Extracellular nucleotides regulate inflammation and immunity via scavenging of receptors for purinergic/pyrimidinergic P2 receptors (P2-R, P2X and P2Y) [12, 13]. NTPDases functionally interact with P2Y receptors [11]. For example, combinations of NTPDases have the capacity to terminate P2 receptor signalling, modulate receptor desensitization, alter specificities of the response or even generate signalling molecules (ADP) from precursors (ATP) [11]. Activation of P2 receptors appears to influence endothelial cell chemotactic and mitogenic responses in vitro [14]. Therefore, aberrant regulation of nucleotide P2 can influence angiogenesis in CD39-null mice [14] and therefore may play a significant role in the process of tumor progression and metastases formation. Recently, we reported the impact of CD39 and certain P2-R in human pancreatic cancer [15]. Other experimental studies have suggested putative involvement of CD39 and purinergic signalling in dissemination of melanoma and other cancer cell types in vivo [16, 17].
In this study, we explored the effects of human CD39 transgenic over-expression and gene deficiency on tumor progression in the above models of primary and metastatic CRC in BALB/c mice. Lastly, the distribution and location of CD39, P2X7 and P2Y2 in human colorectal cancer samples were investigated and expression levels were studied at different stages of cancer and disease. Our studies demonstrate clear connections between elements of purinergic signalling with tumor progression in mouse models and in human CRC.
Materials and methods
Materials and methods
Pathogen-free, wild-type transgenic and mice heterozygous for CD39 were studied on the BALB/c background. Homozygous CD39 gene deletion precludes homozygosity BALB/c mice as intense breeding efforts which involved crossing heterozygous CD39 deficient BALB/c mice culminated in only 37 homozygous CD39-null BALB/c mice out of 408 pups (9% vs. expected frequency of 25%). All homozygous CD39-null BALB/c mice were unfortunately runted and died at an early time (not shown). Hence, only heterozygous mice that are haploinsufficient were studied in this series of experiments in parallel to the over-expressing CD39 transgenics. Male mice, weighing 18–26 g at 8–10 weeks, were used in accordance with standard Institutional Animal Welfare guidelines, Beth Israel Deaconess Medical Center, Harvard University, Boston, USA and after IACUC approval of the relevant protocols.
CRC tumor models
A transplantable BALB/c mouse colon cancer cell line, classified as CRC MC-26, was cultured under standard cell culture conditions, as described [8]. The colon and rectum were accessed through a standard midline incision of 1.5 cm in the lower abdomen, then mobilized with wet cotton tips and exposed. To induce primary colorectal cancer, we injected (0.5 × 106) MC-26 cancer cells dissolved in 50 μl 1× PBS under microscopic guidance with an ultra fine insulin syringe (BD Ultra-FineTM II insulin syringe (31 gauge), Beckton Dickinson, Franklin Lakes, NJ, USA) into the subserosal layer of the lower colorectum. For the dissemination model to the liver, MC-26 cancer cells (1 × 106 in 50 μl RPMI1640 cell culture medium without FCS) were injected in the splenic parenchyma to allow hepatic spread. Five minutes after tumor injection, the spleen was removed and hemostasis secured by a single ligation of the splenic vessel axis.
Mice were sacrificed at day 10 after tumor injection (locally into colon or into spleen) by pentobarbital overdose. Venous blood was immediately collected after induction of anesthesia by direct intracardiac puncture. For more precision in comparing tumor loads in primary CRC colonic masses, we determined the three-dimensional tumor volume under microscopic measurement (2.5× magnification) and consecutive tumor volumes were calculated (mm3). To further determine the extent of potential liver metastases, whole liver weights were measured after exsanguination. Standard liver weights were assumed to be equal within the same batch of animals. We did not evaluate bowel weights of affected colon because the infiltration of the implanted tumors within the colon wall was heterogeneous.
In mice, colectomy (for primary CRC studies) or total hepatectomy (for liver metastasis studies) were performed, tissue biopsies measured and weighed, respectively, and embedded in TBS-Tissue freezing medium™ (American Mastertech, Lodi, CA, USA).
Human materials and clinical studies
All control (non-malignant) human colorectal samples (13 male and four female, median age 62 years; range, 41–81 years) were obtained from benign colorectal surgical specimen, e.g. after sigmoid resection for diverticulitis. Colorectal specimens with malignancy were obtained from a total of 63 patients (39 male and 24 female) with confirmed colorectal cancer were divided in seven subgroups for further analysis (Table 1). Samples were blinded to patient identities, and informed consent was obtained in all cases. The human studies were approved by the Human Subject Committee of the Technische Universität München (Munich, Germany).
Table 1.
Colorectal specimens with malignancy were obtained from a total of 63 patients (24 female (f) and 39 male (m)) with confirmed colorectal cancer (CRC) further divided in seven subgroups according to their tumor stage (TNM)
| Group | Number of patients [n] | Tumor stage [TNM] | Median age (range) [years] | Number of patients [f/m] |
|---|---|---|---|---|
| 1 | 7 | T3N + M0 (18–36 months) | 73 (66–87) | 2/5 |
| 2 | 6 | T3N + M0 (72–110 months) | 77 5(53–84) | 1/5 |
| 3 | 17 | T3N + M1 (synchronous) | 64 (26–86) | 8/9 |
| 4 | 17 | T3N + M1 (metachronous) | 72 (39–83) | 7/10 |
| 5 | 3 | TxN2M0 | 71 (56–86) | 3/0 |
| 6 | 4 | T2N0M1 | 63 (56–68 | 1/3 |
| 7 | 9 | T4N0M0 | 78 (51–91) | 2/7 |
Group 1 describes T3 CRC tumors with lymph node metastases (N+) but no distant metastases (M0) with a postoperative survival between 18 and 36 months. Group 2 consisted of patients with T3N + M0 CRC tumors with a postoperative survival between 72 and 110 months. Group 3 and 4 display patients with advanced CRC (T3N+) having distant metastases (M1) either of synchronous (group 3) or metachronous (group 4) type. Group 5 included CRC with extended lymph node metastases (N2), whereas group 6 included CRC tumors with early distant metastases (T2N0M1). Group 7 displays locally advanced CRC tumors (T4) with no lymph node or distant metastases (N0 and M0)
Human colorectal tumor samples and control samples were obtained immediately after surgical removal and equally processed to ensure uniformity of data. Tissues were immediately snap frozen in isopentane, transferred into liquid nitrogen and also processed for immunohistochemistry. Tissues for RNA extraction and protein analyses were also snap frozen in liquid nitrogen and/or maintained at −80°C until use.
Expression of CD39, P2X7 and P2Y2 purinergic receptors
RNA (RT-PCR) studies
The mRNA samples were extracted from mouse livers and colorectal specimen, as well as the human colorectal samples, and were DNAase-treated and reverse-transcribed using the SuperScript kit (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s recommendations using random hexamer primers.
Q-PCR for (mouse and human) CD39, P2X7 and P2Y2 (Applied Biosystems, Foster City, CA, USA) was performed using the 7700 Sequence Detector (Applied Biosystems) and TaqMan technology as previously described [15]. Reactions (of 25 μl) were set up using the 2× Universal PCR Master Mix (Applied Biosystems), template cDNA and adequate concentrations of primers and probes.
Data were analyzed using the relative standard curve method with serial dilutions of cDNA derived from human fetal brain RNA (Clontech, Mountain View, CA, USA), and expression levels were normalized to the expression of 18S ribosomal subunit as internal controls. To ensure the sensitivity and authenticity of the primers and probes, all PCR-derived products were subcloned into the TA-cloning Vector (Invitrogen) and authenticity confirmed by sequencing.
Western blots
Cell lysates were prepared as previously described [15, 18]. In brief, equal amounts of protein were employed with sodium dodecyl sulfate–polyacrylamide gel electrophoresis techniques to separate proteins (40 μg/lane) on 4% to 15% linear gradient gel, both under-reducing and non-reducing (only CD39) conditions. Proteins were then transferred to polyvinylidene difluoride membranes (Immobilon-P, Millipore, Bedford, MA, USA) by semi-dry electroblotting and probed with either CD39 rabbit polyclonal for mice (C9F) and the monoclonal anti-human CD39 (BU61, Ancell Corporation, Bayport, MN, USA) for human studies. P2X7 (APR-004) and P2Y2 (APR-010; P2X7, P2Y2; Alomone Labs Ltd., Jerusalem, Israel), or for control anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Amblion Inc., Woodward, TX, USA), primary antibodies were used, as previously described [15]. Bands were visualized using horseradish peroxidase-conjugated secondary antibodies donkey-anti-rabbit for (CD39, P2X7, P2Y2) and human-anti-mouse for GAPDH (1:85,000; Pierce, Rockford, IL, USA). The loading control for the human samples was performed with GAPDH (1:5,000; rabbit IgG, Santa Cruz, CA, USA.) Equal levels of gel loading were confirmed by stripping blots and then reprobing with GAPDH antibodies, respectively.
To validate P2 receptor protein expression (P2X7, P2Y2), primary antibodies against P2X7 and P2Y2 were blocked with the specific antigen, as provided by the manufacturer (Alomone labs). Internal controls show complete negative blots (not shown) indicating signals detected were specific.
Immunohistochemistry
Immunohistochemistry was performed as previously described [15, 18]. In brief, 5-μm serial cryostat sections from mouse and human samples were fixed in ice-cold acetone (Sigma). Mouse and human sections were then incubated overnight at 4°C with primary antibodies for CD39 (C9F) for mouse and CD39 (BU61, Ancell Corporation, Bayport, MN, USA) for human studies. After incubation with the appropriate biotinylated secondary antibody, staining was performed with the Vectastatin ABC elite kit (Vector Laboratories, Burlingame, CA, USA). Sections were counterstained with Mayer’s hematoxylin. Optimal antibody concentrations were determined by serial dilution in all cases.
Statistical analyses
Data in this study are presented as means ± standard deviation of values (obtained from at least four specimens per group and/or at least three independent experiments). All histology, Western blots and immunohistochemical images are representative of at least four specimens per group. Statistical analysis of data was performed using the GraphPad PRISM4 software (GraphPad Software Inc., San Diego, CA, USA). Mean values of the experimental groups were compared using nonparametric testing (Mann–Whitney U test) for pair wise comparison and one-way or two-way ANOVA analysis for multiple comparisons. Values of P < 0.05 are considered statistically significant.
Results
Morphology—primary tumor size and tumor loads in the liver
Ten days after the injection of MC-26 CRC cells either in the subserosal space (0.5 × 106 cells) of the colorectum (primary colorectal cancer model) or under the spleen capsule (1.0 × 106 cells; metastatic tumor model), tumor formation was quantified by direct measurements of the tumor volume (quantification of a three-dimensional tumor bulk [mm3] under microscope) and by liver weights. Data were compared between wild-type (wt) and mice heterozygous for mouse CD39 (hz) and mice transgenic for human CD39 (htCD39) mice (Fig. 1a, b).
Fig. 1.
Experimental assessments of tumor growth and dissemination. a Representative tumor growth of primary MC-26 orthotopic transplant cancer tumor formation in the colorectum. The dotted lines indicate the tumor border. b Liver metastases grew significantly larger in human CD39 transgenic mouse livers, compared to heterozygous animals after intrasplenic injections. c In the colorectal orthotopic model, no differences in the three-dimensional tumor bulk were calculated amongst the groups. d In the dissemination liver metastases model, significant differences in liver weights (due to metastatic growth) were observed, comparing livers from hz and htCD39 livers (P < 0.05). e In the orthotopic colorectal cancer model, macroscopic liver metastases could not be detected within the three groups. Microscopic metastases were excluded by histological assessment (not shown). f Expression patterns of CD39, CD39L1, P2X7 and P2Y2 mRNA were determined. MC-26 colorectal cancer cells do not express CD39
Primary colorectal cancer
The calculated tumor volumes, of those cancers within the colorectum, were not statistically different between wild-type, heterozygous and htCD39 mice (Fig. 1c). Tumors grew excentrically and did not obstruct the bowel lumen. The tumor growth was observed mainly extraluminally within the subserosal space. Nevertheless, tumor cells could be found in all subepithelial layers as well as circumferentially (Fig. 2a–c). Along the mesentery, there were infrequent single lymph node metastases (no differences were noted between the individual groups; not shown). No overt macroscopic metastases or peritoneal tumor seedings were detected. Stool passage did not appear impaired up to day 10 of experiments, and no tumor-related bowel obstruction was detected. No signs of gross bleeding were encountered. Furthermore, the body weights did not differ significantly amongst the treatment groups and decreases in body weight never exceeded 10% of the initial body weight.
Fig. 2.
Immunopathology of CD39 in CRC. a Immunohistochemical staining for mouse CD39 revealed expression in peritumoral stroma cells, endothelial and mononuclear cells but not in MC-26 tumor cells in orthotopic tumors. b The tumor border in orthotopic tumors is strongly CD39 positive, comprising mononuclear, stromal and endothelial cells. c Invading tumor clusters of MC-26 cells show strong staining for CD39 at the highly proliferative endothelial and stromal cells at the tumor border. d–f In liver metastases, specific staining for CD39 was displayed by hepatic fibroblasts, endothelial and mononuclear cells. The formation of spreading ductal cancer clusters (metastases) was only observed in d wild-type and e heterozygous livers. f Large solid CRC metastases that comprised the majority of the liver tissue sections were observed in htCD39 liver tissue slides
To examine for tumor dissemination to the liver, the livers were weighed and examined morphologically for tumor. No significant differences were found between the wt, hz and htCD39 liver weights (Fig. 1d) or with tumor spread (not shown).
Liver metastases
After injection of 0.5 mio MC-26 cells in the subserosal layer of the spleen, the spleen was removed after 5 min with full hemostasis then secured. Ten days after tumor cell injection, terminal hepatectomy of all mice was performed and livers weighed after exsanguination. Significant differences in liver weights were detected: tumor metastases had grown to significantly greater size in htCD39 livers. Livers of heterozygous and wild-type mice also displayed metastases in most of the liver lobes, but these appeared decreased in the CD39 deficient livers. In terms of tumor bulk, there were significant differences between deficient vs. over-expressing htCD39 liver weights (P < 0.05; Fig. 1d). Macroscopically, htCD39 livers were replaced largely by metastatic MC-26 CRC cells (Fig. 1b). The extent of cholestasis and other markers of liver injury (bilirubin, ALAT, alkaline phosphatase and other parameters), secondary to tumor-related biliary obstruction and cholestasis, was more pronounced in htCD39 mice when compared to CD39 deficient mice (P < 0.05).
In contrast to the primary tumor sites in the colon, distant metastases other than the liver occurred in the pancreas (two metastases) and the kidney (one subcapsular metastasis) in wt and htCD39 mice. No distant metastases were observed in CD39 heterozygous mice. Tumor cells did not express CD39 at any time but did have the following: CD39L1, P2X7 and P2Y2 (Fig. 1f).
Specific localization of CD39 in primary CRC and liver metastases
Orthotopic colorectal cancer model
CD39 was highly expressed in stromal cells and endothelial cells, but not in cancer cells (Fig. 2a–c). Invasive cancer (Fig. 2c) expressed high levels of CD39 in local environment and at tumor margins.
Liver metastases
In all liver tissue sections of hz, wt and htCD39 mice, CD39 was highly expressed in tumor infiltrates, in associated endothelial cells, immune cells (e.g. macrophages (Kupffer cells)) and in stromal cells (Fig. 2d–f). Interestingly, high CD39 expression was found generally at the tumor border, where cancerous cells were adjacent to normal liver parenchymal cells. Cancer cells did not express CD39 neither at mRNA nor at protein level (Figs. 1f and 2a–f). Around each and every metastasis, there was a CD39 positive rim of spindle-like cells, probably stromal cells (Fig. 2e, f).
Quantification of mRNA of CD39, P2X7 and P2Y2 transcripts
Mouse primary colorectal cancer and liver metastases
RT-PCR was performed from tumors arising from MC-26 cells implanted as primary mouse CRC samples to determine mRNA expression of CD39, P2X7 and P2Y2. In the primary CRC model, no significant differences in mRNA levels were observed between tumors implanted in wild-type, heterozygous and/or htCD39 mice (Fig. 3a). In liver metastases, the mouse CD39 mRNA levels were significantly higher in wild-type and htCD39 tissues, when compared to the heterozygous tissues (P ≤ 0.05). P2X7 was also expressed at higher levels in htCD39 implanted tumors (P ≤ 0.05), compared to those in heterozygous tissues. P2Y2 mRNA levels were also significantly higher in htCD39 compared to wild-type murine tumors (Fig. 3b).
Fig. 3.
CD39 and P2 receptors in CRC. a In orthotopic tumors, CD39, P2X7 and P2Y2 mRNA appeared at comparable levels to those seen in control livers. b As expected, mouse CD39 mRNA was significantly lower expressed in genetically deficient over wild-type and htCD39 tissues. P2X7 mRNA was significantly over-expressed in htCD39, compared to wt and deficient, heterozygous tissues. P2Y2 mRNA expression was significantly upregulated in the more substantive htCD39 liver metastases, whereas P2Y2 mRNA was expressed at low levels in wt and hz tissues. c Significant decreases in human CD39 mRNA expression occurred in T3N + M0 (18–36 months), T3N + M1 hep (synchron) and T4N0M0 tumors, when compared to control tissues. d Significantly less expression of P2X7 mRNA was observed in T3N + M0 (72–110 months) and T3N + M1 hep (metachron) tumors vs. normal colon. e P2Y2 mRNA expression was significantly lower in T3N + M1 hep (synchron) and T3N + M1 hep (metachron) colorectal cancer specimens, compared to normal colon
Human colorectal tissues
Human control (non-malignant) colon and colorectal cancer specimen mRNA expression profiles were determined while investigating seven different subgroups of human colorectal cancer (Table 1). In general, CRC was associated with lower levels of CD39 than control colonic and non-neoplastic samples. Specifically, T3N + M0 (18–36 months) colorectal cancer (P < 0.001), T3N + M1 (synchronous) colorectal cancer (P < 0.05) and T4N0M0 colorectal cancer samples (P < 0.05) had significantly lower levels of CD39 mRNA expression, whereas tumors from T3N ± M1 carcinomas expressed CD39 mRNA at comparable levels to normal colon (Fig. 3c).
Unlike the more aggressive mouse tumors, levels of P2Y2 mRNA were significantly less in synchronous and metachronous liver metastases of T3N + M1 colorectal cancer samples, when compared to normal colon (P < 0.05 and P < 0.01, respectively; Fig. 3e). P2X7 mRNA levels were noted to be at low levels in T3N + M0 (72–110 months) and metachronous metastatic colorectal (T3N + M1) cancer samples when compared to normal colonic biopsies (P < 0.05; Fig. 3d).
Western blot analyses
Orthotopic mouse tumors
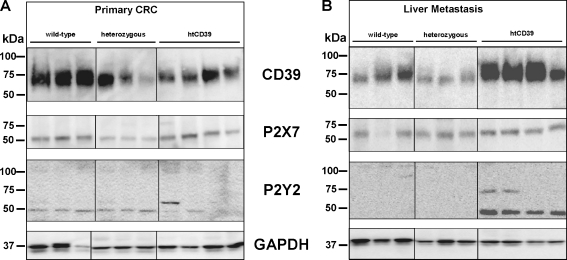
Mouse CD39 (78 kDa) was clearly expressed in primary CRC from wt mice bearing tumors. There were decreased signal intensities for mouse CD39 in both heterozygous CD39 and htCD39 CRC tissue lysates (Fig. 4a). P2X7 (60 kDa) was at low levels in lysates of tumors obtained from CD39 deficient mice. P2Y2 (47 and 105 kDa) was equivalently distributed at low levels in all CRC lysates (Fig. 4a).
Fig. 4.
Western blot analysis of CRC. a In primary colorectal cancer, mouse CD39 was lower in heterozygous (hz) and htCD39 CRC cells compared to wild-type (wt). P2X7 expression was decreased in CRC tumors in CD39 deficient mice. No changes in protein expression were noted for P2Y2. b Distinct and significant increases of mouse CD39 was observed in liver metastases of CRC in htCD39 mice. P2X7 was slightly over-expressed in htCD39 liver metastases only. P2Y2 mRNA was significantly over-expressed in htCD39 mice, compared to wt and heterozygous samples
Liver metastasis
In liver metastases, human CD39 antigen was over-expressed in htCD39 tissues (Fig. 4b), in accordance with noted differences at the mRNA level (Fig. 3b). P2Y2 proteins were highly expressed in htCD39 but were not detected in wild-type and CD39 deficient tumor tissues, in accordance with the P2Y2 mRNA levels. P2X7 was slightly increased in htCD39 liver metastases. GAPDH was used as internal control (Fig. 4a, b).
Protein expression and localization in human colorectal cancer
Significant changes in levels of CD39 and P2X7 protein levels relative to control samples were not noted in human CRC samples (Fig. 5a, b). P2Y2 protein expression levels were significantly decreased in all but T3N + M1hep (synchron) colorectal cancers. T3N + M0 (72–110 months; P < 0.01) and metachronous metastasis of T3N + M1 hep (metachron; P < 0.05) tumor samples displayed significantly low expression of P2Y2 proteins (Fig. 5c). Generally, there was a trend for lower levels of P2Y2 in all cancerous tissues, when compared to normal colonic control tissues. In tissues, P2Y2 was localized within mononuclear cells, peripheral nerves and in mesenchymal tissues such as fibroblasts (Fig. 5e). No P2Y2 expression was confirmed in normal or cancerous colonic epithelial cells. Stromal cells within the tumor stroma displayed less stromal P2Y2 staining than those in close vicinity to normal epithelial colonic epithelial cells. Fibroblasts around normal and cancerous colonic epithelial cells revealed prominent P2Y2 protein expression in tissues (Fig. 5e).
Fig. 5.
Purinergic studies in human CRC. Protein expression levels of CD39, P2X7 and P2Y2 (a–c) and localization of P2Y2 (d, e) was determined in human CRC. Changes in protein levels were observed for P2Y2 (c), which was significantly less in T3N + M0 (72–110 months; P < 0.01) and T3N + M1 hep (metachron; P < 0.05), respectively over controls. No such changes were noted for CD39 (a) and P2X7 (b). P2Y2 expression was confirmed in human normal tissue (d) and colorectal cancer (e) to be localized to mononuclear cells, stromal cells such as fibroblasts and on nerves (e) but was not noted on normal or cancerous epithelial cells
Discussion
Liver metastases secondary to primary colorectal cancer profoundly limit long-term survival. Our current study illustrates the involvement of purinergic signalling in regulating growth of colorectal metastases in the liver. The differential patterns of expression of CD39 and P2Y2 further suggest roles for purinergic signalling in tumor dissemination. We have previously shown that soluble ectonucleotidases are associated with promotion of primary tumor growth and that deletion of the dominant vascular ectonucleotidase CD39 results in abrogation of angiogenesis. This causes decreased growth of implanted tumors and inhibits development of pulmonary metastases after systemic injection of tumor cells [16].
In this present study, we have focused on the potential effects of purinergic signalling on tumor metastases of CRC to the liver and have also tested the hypothesis whether P2 receptors, as previously reported [19], and/or CD39 influence cancer progression [15, 16]. A major limitation in experimental studies has been a lack of CRC liver metastases formation in small animal models. In this study, primary tumor implantation in the colorectum did not result in dissemination of tumor. Crucially, no significant differences in tumor mass formation were noted in the wild-type, hemizygous or human transgenic (ht)CD39 mice. In contrast, when injected through the splenic system, MC-26 CRC cells formed significantly larger tumor masses in the livers of htCD39 mice, compared to mice heterozygous for CD39.
Our present findings also indicate effects of changes in CD39 and aberrant purinergic signalling on metastatic tumor progression in animal models that are seen in human CRC. In the small animal model, high levels of CD39 expression in htCD39 mice resulted in significant increases in metastatic CRC in the liver. Livers from htCD39 mice develop large tumor masses, whereas those from mice with heterozygous or wild-type CD39 background displayed much less liver metastases. In human CRC, low CD39 mRNA expression levels within the tumors appeared to correlate with less or delayed CRC spread and better long-term survival. A previous study [15] had suggested heightened levels of expression of CD39 in pancreatic cancer were linked to longer-term survival after surgical resection of primary tumors. These differing findings may arise from the distinct cancer phenotypes with different hematogenous metastatic potential and the local extent of desmoplastic reactions linked to CD39 in pancreatic cancer where the findings were somewhat unexpected [15].
Immunological phenotypes, such as the Th1 phenotype in CD39-null mice, are altered with genetic deletions and decreases in CD39 expression [20]. Lack of CD39 is associated with a predominant Th1 phenotype, with an increased effect of interferon-γ (IFN-γ). High IFN-γ serum concentrations clearly impact immunobiology and affect tumor growth. Further, pericellular adenosine the product of CD39 and CD73 interacts with T cell (A2A) adenosine receptors leading to T cell hyporesponsiveness, which is also noted in tumors [17, 21]. Inhibition of CD39 by ARL 67156 can, at least in part, overcome T cell hyporesponsiveness in a subset of patients with follicular lymphoma [21].
In our present study, it is plausible that high levels of mouse CD39 might provide a mechanism enabling the escape from immune surveillance by suppressing tumor cell lysis by cytotoxic T-lymphocytes or NK cells [17]. These factors could lead to the phenotype of human transgenic mice that show greater spread of malignancy within the liver.
Populations of CD39 expressing CD4+ and CD8+ T cells are overrepresented in follicular lymphoma as compared to normal reactive lymph nodes [21]. These published data from another group suggest that CD39 and the NTP-ectonucleotidase-adenosinergic system mediate T cell anergy in human tumors. A recent study in head and neck cancer also revealed that CD39 expressing regulatory T cell (Treg) frequency and activity are increased in cancer patients and as such may play a major role in tumor growth [22]. In short, tumor escape is favoured by the presence of Treg and heightened CD39, but mechanisms used to suppress anti-tumor immunity are mostly unknown.
We note that P2Y2 expression is lower in human CRC tissues when compared to normal colonic biopsies. Our own studies have shown that P2Y2 is localized to subepithelial cells, such as stromal and inflammatory cells. The loss of anatomical crypts might explain, at least in part, why P2Y2 expression is globally lower in primary CRC samples, when compared to normal colon. However, this does not explain the apparent differences in P2Y2 expression, particularly with respect to the more aggressive types of CRC liver metastases in hCD39 transgenic mice where the model does not fully mimic human disease.
As previously reported, extracellular nucleotides induce apoptosis and inhibit growth of cancer cells and P2Y2 signalling has been linked to antiproliferative and apoptosis-inducing effects in two human colorectal cancer cell lines [19]. Höpfner et al. have demonstrated in HT-29 cell lines that ATP and UTP elicit increases in intracellular Ca2+ concentrations [23] and impact tumor proliferation. Another study has shown upregulation of P2Y2 in both HT-29 and human colon cancer cells [24]. There remain controversies as to how P2Y2 might also positively influence tumor proliferation and bolster progression. The proliferative activity of ATP and UTP via P2Y2 receptors in A549 cell downstream of phospholipase C depends on Ca2+/calmodulin-dependent protein kinase II and nuclear factor-kappaB, but is independent of protein kinase C [25]. Schafer et al. [25] have postulated a novel pathway where extracellular nucleotides regulate proliferation. Interestingly, ATP antagonizes antiproliferative effects of the anticancer drugs paclitaxel and etoposide, but enhances the activity of cisplatin [25].
Our own data here suggest that CD39 and alterations in purinergic signalling have modulatory effects on CRC dissemination. Importantly, low levels of tumor-associated CD39 mRNA appear to correlate with increased survival and late or delayed formation of hepatic CRC metastases. Future investigations are needed to indicate possible roles for CD39 antagonistic therapies in CRC.
Acknowledgements
We would like to thank Dr. Kirk Ives and Dr. CM Townsend (UTMB, Galveston, USA) for their support in the present study and for providing our institution with the MC-26 mouse colorectal cancer cell line. Grants—this work was supported by the German Research Foundation Grants (DFG KU 1957/1-1 and DFG KU 1957/3-1 to B.M.K.) and the National Institute of Health (NIH HL63972 and HL076540 to S.C.R).
References
- 1.Brenner H, Stegmaier C, Ziegler H. Long-term survival of cancer patients in Germany achieved by the beginning of the third millenium. Ann Oncol. 2005;16(6):981–986. doi: 10.1093/annonc/mdi186. [DOI] [PubMed] [Google Scholar]
- 2.Takahashi T, Morotomi M, Nomoto K. A novel mouse model of rectal cancer established by orthotopic implantation of colon cancer cells. Cancer Sci. 2004;95(6):514–519. doi: 10.1111/j.1349-7006.2004.tb03242.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kramer I, Lipp HP. Bevacizumab, a humanized anti-angiogenic monoclonal antibody for the treatment of colorectal cancer. J Clin Pharm Ther. 2007;32(1):1–14. doi: 10.1111/j.1365-2710.2007.00800.x. [DOI] [PubMed] [Google Scholar]
- 4.Bresalier RS, Hujanen ES, Raper SE, Roll FJ, Itzkowitz SH, Martin GR, Kim YS. An animal model for colon cancer metastasis: establishment and characterization of murine cell lines with enhanced liver-metastasizing ability. Cancer Res. 1987;47(5):1398–1406. [PubMed] [Google Scholar]
- 5.Morikawa K, Walker SM, Nakajima M, Pathak S, Jessup JM, Fidler IJ. Influence of organ environment on the growth, selection, and metastasis of human colon carcinoma cells in nude mice. Cancer Res. 1988;48(23):6863–6871. [PubMed] [Google Scholar]
- 6.Tsutsumi S, Kuwano H, Morinaga N, Shimura T, Asao T. Animal model of para-aortic lymph node metastasis. Cancer Lett. 2001;169(1):77–85. doi: 10.1016/S0304-3835(00)00622-4. [DOI] [PubMed] [Google Scholar]
- 7.Daruwalla J, Christophi C. The effect of hyperbaric oxygen therapy on tumour growth in a mouse model of colorectal cancer liver metastases. Eur J Cancer. 2006;42(18):3304–3311. doi: 10.1016/j.ejca.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 8.Singh P, Walker JP, Townsend CM, Jr, Thompson JC. Role of gastrin and gastrin receptors on the growth of a transplantable mouse colon carcinoma (MC-26) in BALB/c mice. Cancer Res. 1986;46(4 Pt 1):1612–1616. [PubMed] [Google Scholar]
- 9.Achen MG, Stacker SA. Molecular control of lymphatic metastasis. Ann NY Acad Sci. 2008;1131:225–234. doi: 10.1196/annals.1413.020. [DOI] [PubMed] [Google Scholar]
- 10.Royston D, Jackson DG. Mechanisms of lymphatic metastasis in human colorectal adenocarcinoma. J Pathol. 2009;217(5):608–619. doi: 10.1002/path.2517. [DOI] [PubMed] [Google Scholar]
- 11.Enjyoji K, Sevigny J, Lin Y, Frenette PS, Christie PD, Esch JS, 2nd, Imai M, Edelberg JM, Rayburn H, Lech M, Beeler DL, Csizmadia E, Wagner DD, Robson SC, Rosenberg RD. Targeted disruption of cd39/ATP diphosphohydrolase results in disordered hemostasis and thromboregulation. Nat Med. 1999;5(9):1010–1017. doi: 10.1038/12447. [DOI] [PubMed] [Google Scholar]
- 12.Buell G, Lewis C, Collo G, North RA, Surprenant A. An antagonist-insensitive P2X receptor expressed in epithelia and brain. EMBO J. 1996;15(1):55–62. [PMC free article] [PubMed] [Google Scholar]
- 13.Burnstock G, Knight GE. Cellular distribution and functions of P2 receptor subtypes in different systems. Int Rev Cytol. 2004;240:31–304. doi: 10.1016/S0074-7696(04)40002-3. [DOI] [PubMed] [Google Scholar]
- 14.Goepfert C, Sundberg C, Sevigny J, Enjyoji K, Hoshi T, Csizmadia E, Robson S. Disordered cellular migration and angiogenesis in cd39-null mice. Circulation. 2001;104(25):3109–3115. doi: 10.1161/hc5001.100663. [DOI] [PubMed] [Google Scholar]
- 15.Kunzli BM, Berberat PO, Giese T, Csizmadia E, Kaczmarek E, Baker C, Halaceli I, Buchler MW, Friess H, Robson SC. Upregulation of CD39/NTPDases and P2 receptors in human pancreatic disease. Am J Physiol Gastrointest Liver Physiol. 2007;292(1):G223–230. doi: 10.1152/ajpgi.00259.2006. [DOI] [PubMed] [Google Scholar]
- 16.Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, Cszimadia E, Sundberg C, Robson SC. Disordered purinergic signalling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol. 2007;171(4):1395–1404. doi: 10.2353/ajpath.2007.070190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sun X, Wu Y, Gao W, Enjyoji K, Csizmadia E, Muller CE, Murakami T, Robson SC. CD39/ENTPD1 expression by CD4(+)Foxp3(+) regulatory T cells promotes hepatic metastatic tumor growth in mice. Gastroenterology. 2010;139:1030–1040. doi: 10.1053/j.gastro.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kunzli BM, Nuhn P, Enjyoji K, Banz Y, Smith RN, Csizmadia E, Schuppan D, Berberat PO, Friess H, Robson SC. Disordered pancreatic inflammatory responses and inhibition of fibrosis in CD39-null mice. Gastroenterology. 2008;134(1):292–305. doi: 10.1053/j.gastro.2007.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hopfner M, Maaser K, Barthel B, Lampe B, Hanski C, Riecken EO, Zeitz M, Scherubl H. Growth inhibition and apoptosis induced by P2Y2 receptors in human colorectal carcinoma cells: involvement of intracellular calcium and cyclic adenosine monophosphate. Int J Colorectal Dis. 2001;16(3):154–166. doi: 10.1007/s003840100302. [DOI] [PubMed] [Google Scholar]
- 20.Deaglio S, Dwyer KM, Gao W, Friedman D, Usheva A, Erat A, Chen JF, Enjyoji K, Linden J, Oukka M, Kuchroo VK, Strom TB, Robson SC. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204(6):1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hilchey SP, Kobie JJ, Cochran MR, Secor-Socha S, Wang JC, Hyrien O, Burack WR, Mosmann TR, Quataert SA, Bernstein SH. Human follicular lymphoma CD39+-infiltrating T cells contribute to adenosine-mediated T cell hyporesponsiveness. J Immunol. 2009;183:6157–6166. doi: 10.4049/jimmunol.0900475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandapathil M, Szczepanski MJ, Szajnik M, Ren J, Lenzner DE, Jackson EK, Gorelik E, Lang S, Johnson JT, Whiteside TL. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15(20):6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hopfner M, Lemmer K, Jansen A, Hanski C, Riecken EO, Gavish M, Mann B, Buhr H, Glassmeier G, Scherubl H. Expression of functional P2-purinergic receptors in primary cultures of human colorectal carcinoma cells. Biochem Biophys Res Commun. 1998;251(3):811–817. doi: 10.1006/bbrc.1998.9555. [DOI] [PubMed] [Google Scholar]
- 24.Nylund G, Hultman L, Nordgren S, Delbro DS. P2Y2- and P2Y4 purinergic receptors are over-expressed in human colon cancer. Auton Autacoid Pharmacol. 2007;27(2):79–84. doi: 10.1111/j.1474-8673.2007.00389.x. [DOI] [PubMed] [Google Scholar]
- 25.Schafer R, Sedehizade F, Welte T, Reiser G. ATP- and UTP-activated P2Y receptors differently regulate proliferation of human lung epithelial tumor cells. Am J Physiol Lung Cell Mol Physiol. 2003;285(2):L376–385. doi: 10.1152/ajplung.00447.2002. [DOI] [PubMed] [Google Scholar]