Abstract
Nucleotides released upon brain injury signal to astrocytes and microglia playing an important role in astrogliosis, but the participation of microglia in the purinergic modulation of astrogliosis is still unclear. Highly enriched astroglial cultures and co-cultures of astrocytes and microglia were used to investigate the influence of microglia in the modulation of astroglial proliferation mediated by nucleotides. In highly enriched astroglial cultures, adenosine-5’-triphosphate (ATP), adenosine 5’-O-(3-thio)-triphosphate (ATPγS), adenosine 5’-O-(3-thio)-diphosphate (ADPβS; 0.01–1 mM), and adenosine-5’-diphosphate (ADP; 0.1–1 mM) increased proliferation up to 382%, an effect abolished in co-cultures containing 8% of microglia. The loss of ATP proliferative effect in co-cultures is supported by its fast metabolism and reduced ADP accumulation, an agonist of P2Y1,12 receptors that mediate astroglial proliferation. No differences in ADPβS and ATPγS metabolism or P2Y1,12 receptors expression were found in co-cultures that could explain the loss of their proliferative effect. However, conditioned medium from microglia cultures or co-cultures treated with ADPβS, when tested in highly enriched astroglial cultures, also prevented ADPβS proliferative effect. None of the uracil nucleotides tested had any effect in proliferation of highly enriched astroglial cultures, but uridine-5′-triphosphate (UTP; 0.1–1 mM) inhibited proliferation up to 66% in co-cultures, an effect that was dependent on uridine-5’-diphosphate (UDP) accumulation, coincident with a co-localization of P2Y6 receptors in microglia and due to cell apoptosis. The results indicate that microglia control astroglial proliferation by preventing the proliferative response to adenine nucleotides and favouring an inhibitory effect of UTP/UDP. Several microglial P2Y receptors may be involved by inducing the release of messengers that restrain astrogliosis, a beneficial effect for neuronal repair mechanisms following brain injury.
Keywords: Astroglial proliferation; P2Y receptors; Nucleotide metabolism; P2Y1,6,12 expression; P2Y1,6,12 cell-type localization; Astrocyte–microglia communication
Introduction
Astrogliosis and microglia activation are common features of neurodegenerative diseases and acute pathological episodes of trauma, stroke, seizure or infection [1, 2]. ATP and other nucleotides are massively released under these conditions and by activation of P2Y receptors initiate astrogliosis, a response that is characterised by an increase in glial fibrillary acidic protein (GFAP) expression, cell stellation and astroglial proliferation [3, 4]. P2Y receptors also mediate astrocyte migration [5] and modulate the release of cytokines [6, 7] and prostaglandins [8], supporting the astroglial reactive phenotype observed during astrogliosis. In injury models, astroglial proliferation was found to be mediated by P2Y1 receptors [9, 10], and recently, we have shown that P2Y12 receptors may also be involved in this response [11]. Additionally, nucleotides activate microglia P2 receptors which induce chemotaxis [12, 13], phagocytosis [14], the release of trophic factors [15] and cytokines [16] that may have protective effects in cerebral injury [17, 18]. Several cytokines and growth factors released by microglia, such as interleukin-1β, interleukin-6, interferon-γ, tumour necrosis factor-α and fibroblast growth factor-2 stimulate astrogliosis [19–21] whereas others, such as interleukin-10, attenuate astroglial reactivity through a decrease in microglia activation [22, 23]. Furthermore, the in vitro and in vivo demonstration that microglia activation precedes astrogliosis lead to the proposal that these cells are of major relevance in the modulation of this response [24].
Most of the known effects of nucleotides in astrogliosis are based on results obtained from studies in astroglial cultures which, regardless of the protocols used, contained microglia in different proportions, but the influence of microglia in the purinergic trophic effects was rarely addressed [25]. Microglia, even when present in small amounts, may regulate astroglial responses and may be responsible for some of the effects attributed to astrocytes [26].
In this study, we investigated the influence of microglia in the modulation of astroglial proliferation mediated by nucleotides using two types of primary astroglial cultures: highly enriched astroglial cultures and co-cultures of astrocytes and microglia. In a first approach to understand the differences observed in the effects of nucleotides in both types of cultures, several factors that could offer an immediate explanation were investigated: (1) the metabolism of nucleotides, (2) the expression and cellular localization of the P2Y receptors potentially involved in the modulation of astroglial proliferation and (3) the release of soluble messengers by microglia that could have influenced astroglial proliferation. With this experimental approach, we aimed to start disclosing the purinergic mechanisms that influence the astrocyte–microglia communication during astrogliosis, a hallmark of brain injury.
Materials and methods
Drugs and antibodies
The following antibodies and drugs were used: goat anti-mouse IgG conjugated to Alexa Fluor 488 from Invitrogen (Barcelona, Spain); rabbit polyclonal anti-P2Y1 and anti-P2Y6 from Alomone Laboratories (Jerusalem, Israel); mouse monoclonal anti-CD11b, rabbit polyclonal anti-actin and goat anti-rabbit IgG conjugated to horseradish peroxidase from Santa Cruz Biotechnology (Santa Cruz, CA, USA); rabbit polyclonal anti-P2Y12, rabbit and mouse anti-glial fibrillary acidic protein (anti-GFAP), goat anti-rabbit IgG conjugated to crystalline tetramethylrodamine isothiocyanate (TRITC), adenosine, adenosine-5’-monophosphate (AMP), adenosine-5’-diphosphate tetrasodium (ADP), adenosine 5’-O-(3-thio)-diphosphate tetralithium (ADPβS), adenosine-5’-triphosphate disodium (ATP), adenosine 5’-O-(3-thio)-triphosphate tetralithium (ATPγS), cytosine β-d-arabinofuranoside (Ara-C), 2′-(4-hydroxyphenyl)-5-(4-methyl-1-piperazinyl)-2,5′-bi-1 H-benzimidazole trihydrochloride hydrate (Hoechst 33258), hypoxanthine, inosine, l-leucine methyl ester hydrochloride (LME), penicillin, streptomycin, uracil, uridine, uridine-5’-monophosphate disodium (UMP), uridine-5’-diphosphate sodium (UDP), uridine-5’-triphosphate trisodium (UTP) and uridine 5′-diphosphoglucose disodium (UDP-glucose) and methyl green from Sigma-Aldrich (Sintra, Portugal); methyl-[3H]-thymidine (specific activity 80–86 Ci.mmol-1) and enhanced chemiluminescence Western blotting system from Amersham Biosciences (Lisbon, Portugal); Sulfo-NHS-SS-Biotin and Ultralink Immobilized Neutravidin from Pierce (Rockford, IL, USA). Stock solutions of drugs were prepared with dimethylsulphoxide or distilled water and kept at −20°C. Solutions of drugs were prepared from stock solutions diluted in culture medium immediately before use.
Cell cultures
Animal handling and experiments were conducted according to the guidelines of the Directive 2010/63/EU of the European Parliament and the Council of the European Union. Primary cortical astroglial cultures were prepared from offspring of Wistar rats (Charles River, Barcelona, Spain) as previously described [27], with minor modifications. Briefly, the brains were placed in ice-cold Dulbecco’s phosphate buffered calcium-free saline solution (DPBS) containing 0.2% glucose. The hemispheres were free of meninges and blood vessels, and after washing twice with ice-cold DPBS, they were cut into small pieces in culture medium, i.e., Dulbecco’s modified Eagle medium containing 3.7 g/L NaHCO3, 1.0 g/L d-glucose and stable glutamine, supplemented with 50 U/ml penicillin and 50 μg/ml streptomycin. Tissue from two hemispheres was dissociated by triturating in 10 ml culture medium. The cell suspension obtained was passed through a 40-μm pore nylon mesh and then centrifuged at 200×g for 5 min and the supernatant discharged. Centrifugation followed by cell suspension was repeated twice, and the pellet obtained was suspended in culture medium supplemented with 10% foetal bovine serum (FBS) and seeded at a density of 2 × 105cells/ml. Cultures were incubated at 37°C in a humidified atmosphere of 95% air, 5% CO2 and the medium was replaced 1 day after preparation and subsequently, twice a week.
Highly enriched astroglial cultures were obtained by treating confluent cultures, after 20 days in vitro (DIV), with 8 μM Ara-C for 4 days followed by treatment with 50 mM l-LME for 90 min [28]. At DIV28, two types of cultures were obtained: co-cultures of astrocytes and microglia, when no treatment was applied and highly enriched astroglial cultures, when cultures were treated with Ara-C plus LME. In both types of cultures, astrocytes were the main cell type, but the number of microglia present differed between the two types of cultures (see below). Cultures were synchronised to a quiescent phase of the cell cycle, by shifting serum concentration to 0.1% FBS for 48 h, being used in experiments at DIV30.
Cultures of microglia were obtained from confluent co-cultures that were shaken overnight at 200 rpm. Supernatants containing detached cells were centrifuged at 290×g for 10 min. The pellet obtained was suspended in culture medium containing 10% FBS at a density of 3 × 104 cells/ml. Cells were seeded in 24-well plates, and the medium was changed 1 h later, allowing a selective attachment of microglia [29]. After cell synchronisation for 48 h, microglia cultures and co-cultures were treated with solvent or ADPβS (0.1 mM) for 8 h. After this period of incubation, the medium was discarded and replaced by fresh medium, which was collected 24 h later to be tested in highly enriched astroglial cultures. This medium was named microglia conditioned medium (MCM) or co-cultures conditioned medium (CCCM). Conditioned medium obtained from cells treated with solvent (MCM-S or CCCM-S) or with ADPβS (MCM-ADPβS or CCCM-ADPβS) was tested in proliferation assays of highly enriched astroglial cultures.
Immunocytochemistry
Cell cultures were fixed with a solution containing 4% formaldehyde and 4% sucrose in phosphate buffered saline (PBS; 100 mM NaH2PO4, 50 mM NaCl, pH adjusted to 7.3) and then treated with PBS containing 0.3% Triton X-100. For double-labelling astrocytes and microglia, cultures were incubated with the primary antibodies rabbit anti-GFAP (1:600) and mouse anti-CD11b (1:50), overnight at 4°C. For P2Y receptors localization, cultures were incubated with the primary antibodies mouse anti-GFAP (1:300) or mouse anti-CD11b (1:50) and rabbit anti-P2Y1 (1:400), anti-P2Y6 (1:200) or anti-P2Y12 (1:400), overnight at 4°C. Visualisation of GFAP, CD11b and P2Y receptors positive cells was accomplished upon 1 h incubation, at room temperature, with the secondary antibodies anti-rabbit IgG conjugated to crystalline TRITC (1:100 and 1:400 for GFAP and P2Y receptors detection, respectively) and anti-mouse IgG conjugated to Alexa Fluor 488 (1:400). In negative controls, the primary antibody was omitted. Cell nuclei were labelled with Hoechst 33258 (5 μg/ml) for 30 min at room temperature. To evaluate the percentage of microglia, the two types of cultures were processed in parallel, and about 200 cells were counted in each culture. The number of CD11b-positive cells was expressed as percentage of the total number of cells counted. Images were captured with a Digital Sight DS-5Mc camera (Nikon, Japan) coupled to an Eclipse E400 fluorescence microscope (Nikon, Japan).
DNA synthesis
At DIV30, the cultures grown in 24-well plates were incubated with nucleotides or solvent for 48 h (tested in duplicate in each plate), and methyl-[3H]-thymidine was added in the last 24 h, at a concentration of 1 μCi/ml. When MCMs and CCCMs were tested in highly enriched astroglial cultures, they were added simultaneously with the nucleotides. Cells were then rinsed with PBS, fixed with 10% of trichloroacetic acid (TCA) for 30 min at 4°C, washed with ice-cold 5% TCA and rinsed again with PBS. Protein content and methyl-[3H]-thymidine incorporation were evaluated after cell lysis with 0.2 M NaOH. The effect of drugs in cell proliferation was determined by methyl-[3H]-thymidine incorporation which was quantified by liquid scintillation spectrometry (Beckman LS 6500, Beckman Instruments, Fullerton, USA) and normalised by the protein content determined by the Bradford method.
Metabolism of nucleotides
Cultures were rinsed three times with buffer at 37°C with the following composition (mM): 135 NaCl, 5 KCl, 0.8 MgSO4, 1.8 CaCl2, 10 HEPES, 10 glucose and 1 sodium pyruvate, with pH adjusted to 7.4 with NaOH (1 M). Nucleotides were added at zero time at concentration of 0.1 mM, and samples collected at 0, 1, 3, 8, 24 and 48 h were immediately stored at −20°C. Nucleotides and their metabolites were separated by ion-pair-reverse-phase high performance liquid chromatography with UV detection (HPLC-UV), as previously described [30]. Standards were analysed in the same conditions, and the retention time identified was (minutes): uracil (0.95), hypoxanthine (1.19), uridine (1.32), inosine (1.86), UMP (2.15), adenosine (3.93), UDP (4.40), AMP (4.76), UTP (6.40), ADP (6.63), ADPβS (7.70), ATP (7.87) and ATPγS (8.10). The concentration of nucleotides and metabolites was calculated by peak area integration followed by interpolation in calibration curves obtained with standards.
Western blot analysis
Cells were rinsed with ice-cold PBS and total cell protein extracted in lysis buffer with protease inhibitors (1 mM Na3VO4, 1 mM NaF, 1 mM PMSF, 2 μg/ml aprotinin and 2 μg/ml leupeptin). After a brief sonication (10 s), the lysate was incubated on ice for 1 h and then centrifuged at 20,000×g for 45 min at 4°C. The protein concentration was determined in the supernatant, and equal amounts of protein (50 μg) were boiled at 95°C for 5 min in 6× sample buffer (0.35 M Tris–HCl at pH 6.8, 10% sodium dodecyl sulfate (SDS), 30% glycerol, 9.3% dithiothreitol and 0.01% bromphenol blue) and subjected to 12% SDS-PAGE (SDS-polyacrylamide gel electrophoresis). Proteins were electrotransferred onto nitrocellulose membranes for 2 h at 40 V in a transfer buffer. Membranes were blocked overnight at 4°C with 5% of non-fat dry milk in PBS and then probed for 2 h at room temperature with appropriately diluted primary polyclonal antibodies: rabbit anti-P2Y1, rabbit anti-P2Y6 (both at 1:300) and rabbit anti-P2Y12 (1:400) followed by secondary antibody goat anti-rabbit IgG conjugated to horseradish peroxidase (1:10,000). Immunoblots were then stripped by incubation in stripping buffer (62.5 mM Tris–HCl, 100 mM 2-mercaptoethanol and 2% SDS, pH adjusted to 6.8) for 15 min at 50°C and blocked overnight with 5% of non-fat dry milk in PBS. Subsequently, membranes were re-probed with the primary polyclonal antibody rabbit anti-actin (1:200) for 2 h at room temperature, followed by the secondary antibody. Immunocomplexes were detected by enhanced chemiluminescence system. Quantification of P2Y protein levels, obtained in arbitrary density units, was performed by densitometric analysis using Bio-Rad’s Quantity One software (Basic version 4.6.5), and total P2Y receptors expression was normalised to actin.
Cell surface biotinylation
Cell surface protein biotinylation was performed to determine membrane expression of P2Y1 and P2Y6 receptors. Briefly, cultures were rinsed twice with ice-cold PBS with 0.1 mM CaCl2 and 1.0 mM MgCl2 (PBS-Ca-Mg). The apical surface was then exposed to 1 mg/ml of Sulfo-NHS-SS-biotin in biotinylation buffer (10 mM triethanolamine, 2 mM CaCl2, 150 mM NaCl at pH 8.0) for 50 min with horizontal motion at 4°C. After labelling, the cells were rinsed with quenching solution (PBS-Ca-Mg with 100 mM glycine) and then extracted in lysis buffer with protease inhibitors. Precipitation of biotinylated proteins was attained by adding Neutravidin-agarose beads to approximately 850 μg of total cell protein, with end-over-end rotation overnight at 4°C. Then, beads were centrifuged three times at 6,000×g for 2 min at 4°C, washed with PBS and bound proteins solubilised with SDS sample buffer (0.0625 M Tris–HCl at pH 6.8, 2% SDS, 10% glycerol, 2.5% 2-mercaptoethanol and 0.01% bromphenol blue). Samples were subjected to SDS-PAGE and blotting as described in the previous section (see Western blot analysis).
Lactate dehydrogenase assays
Necrotic cell death was assessed by measuring the lactate dehydrogenase (LDH) release with an enzymatic assay according to the manufacturer’s instructions (Sigma Aldrich). The assay was based in the oxidation of lactate to pyruvate by LDH with the formation of NADH, which reduces tetrazolium to coloured formazan that was measured at a wavelength of 490 nm. Following incubation with nucleotides or solvent for 48 h, culture supernatants were collected, and the respective extracts were obtained upon incubation of astrocytes with a lysis solution for 45 min at 37°C. Samples were then centrifuged at 250×g for 4 min, and LDH activity was determined in the collected supernatants and respective extracts. The LDH released into the culture medium was expressed as percentage of total LDH.
Terminal transferase-mediated dUTP nick end-labelling assays
Apoptotic cell death was evaluated using the indirect terminal transferase-mediated dUTP-digoxigenin nick end-labelling (TUNEL) to detect DNA fragmentation using an ApopTag peroxidase detection kit (Millipore, Madrid, Spain). Cultures treated with nucleotides or solvent for 48 h were fixed in 4% paraformaldehyde in PBS pH 7.4, for 10 min at room temperature and subsequently post-fixed in pre-cooled ethanol/acetic acid (2:1, v/v) for 15 min at −20°C. The endogenous peroxidase activity was quenched with 3% hydrogen peroxide. Cultures were incubated with equilibration buffer and treated with terminal deoxynucleotidyltransferase plus digoxigenin-dNTPs for 1 h at 37°C. The anti-digoxigenin antibody conjugated to peroxidase was added for 30 min at room temperature, and colour was developed with 3,3′-diaminobenzidine substrate. Counterstaining of the nuclei was accomplished with 0.5% methyl green, and cells were visualised by bright field microscopy. The cell bodies were also labelled with Hoechst 33258 (5 μg/ml) to confirm the results obtained with the TUNEL assay. The number of TUNEL-positive cells was evaluated by analysing eight high-power fields (×400) in each culture and expressed as percentage of total cell number counted.
Statistical analysis
Data are expressed as means ± standard errors of the mean (SEM) from n number of experiments, unless otherwise stated. Statistical analysis was carried out using the unpaired Student’s t test or one-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. The Western blot data analysis was performed by ANOVA repeated measures followed by Bonferroni’s multiple comparison test. P values lower than 0.05 were considered to indicate significant differences.
Results
Characterization of cell cultures
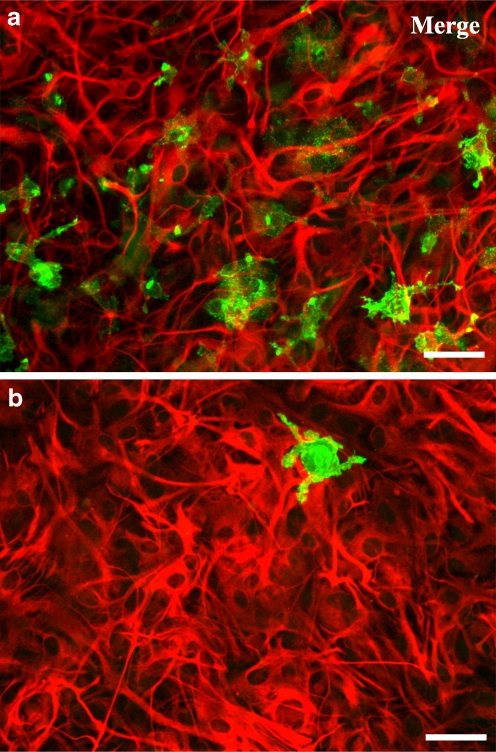
Cultures that grown without any treatment were named co-cultures and could be described as monolayers of astrocytes exhibiting a flattened, polygonal morphology, containing 8.0 ± 0.8% (n = 5) of microglia (Fig. 1a). Highly enriched astroglial cultures were obtained by applying a very effective treatment to eliminate microglia from astroglial cultures [28], which resulted in highly enriched astrocytes cultures containing only 1.0 ± 0.3% (n = 5) of microglia, which was considered negligible (Fig. 1b). The two types of primary cultures, highly enriched astroglial cultures and co-cultures were used in the experiments.
Fig. 1.
Characterization of primary astroglial cultures containing different percentage of microglia. Astrocytes were labelled with anti-GFAP (TRITC, red) and microglia with anti-CD11b (Alexa Fluor 488, green). Representative immunofluorescent micrographs of the two types of cultures: a co-cultures and b highly enriched astroglial cultures, double-labelled for GFAP and CD11b. In co-cultures, the number of microglia present was 8.0 ± 0.8% (n = 5) and in highly enriched astroglial cultures was 1.0 ± 0.3% (n = 5). Scale bar: 50 μm
Effect of nucleotides in cell proliferation
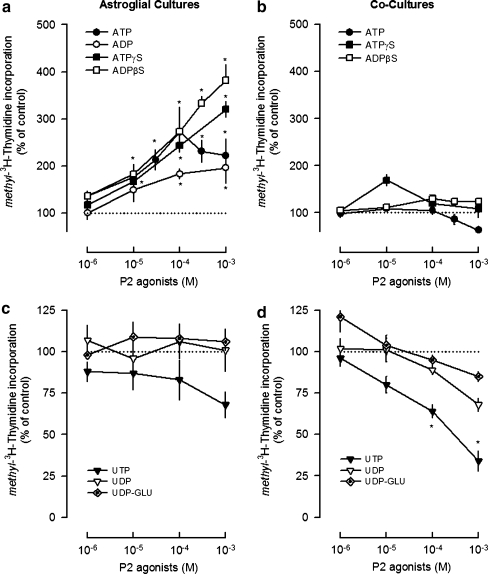
Nucleotides that activate different P2Y receptor subtypes were tested in the cultures previously described: ATP and ATPγS which are non-selective P2 agonists, ADPβS and ADP that have higher affinity for the P2Y1,12,13 subtypes, UTP that activates the P2Y2,4 subtypes, UDP that is selective for the P2Y6 receptors and UDP-glucose, the selective agonist of the P2Y14 receptors [31]. The nucleotides tested mediated opposite effects in cell proliferation, and their effects were influenced by the presence of microglia (Fig. 2).
Fig. 2.
Modulation of astroglial proliferation by nucleotides in highly enriched astroglial cultures (a, c) and co-cultures (b, d). Cultures were incubated with nucleotides or solvent for 48 h and methyl-[3H]-thymidine (1 μCi/ml) was added in the last 24 h. Cell proliferation was estimated by methyl-[3H]-thymidine incorporation and expressed in percentage of control. Values are means ± SEM from five to seven experiments. *P < 0.05, significant differences from control (solvent)
In highly enriched astroglial cultures, the adenine nucleotides ADP (0.1–1 mM), ATPγS and ADPβS (0.01–1 mM) increased cell proliferation in a concentration-dependent manner up to 382 ± 33% (Fig. 2a). ATP increased cell proliferation up to a concentration of 0.1 mM and then the effect declined (Fig. 2a). In co-cultures, neither ATP nor ATPγS or ADPβS had any effect in cell proliferation (Fig. 2b). ADP was not tested in co-cultures, since ADPβS, which is a more stable analogue [32], caused no effect. The absence of proliferative effects of adenine nucleotides in co-cultures was not due to the inability of cells to proliferate, since 10% FBS increased cell proliferation by 398 ± 54% (n = 5, P < 0.05). The uracil nucleotides UDP and UDP-glucose (0.001–1 mM) had no effect in both types of cultures (Fig. 2c, d), but UTP (0.1–1 mM) inhibited cell proliferation up to 66 ± 6% (n = 4, P < 0.05) in co-cultures (Fig. 2d). The results indicate that the presence of microglia in the cultures prevents the astroglial proliferative response to the endogenous nucleotide ATP and to the stable adenine nucleotide analogues ATPγS and ADPβS [32] and favours the inhibition of proliferation caused by UTP.
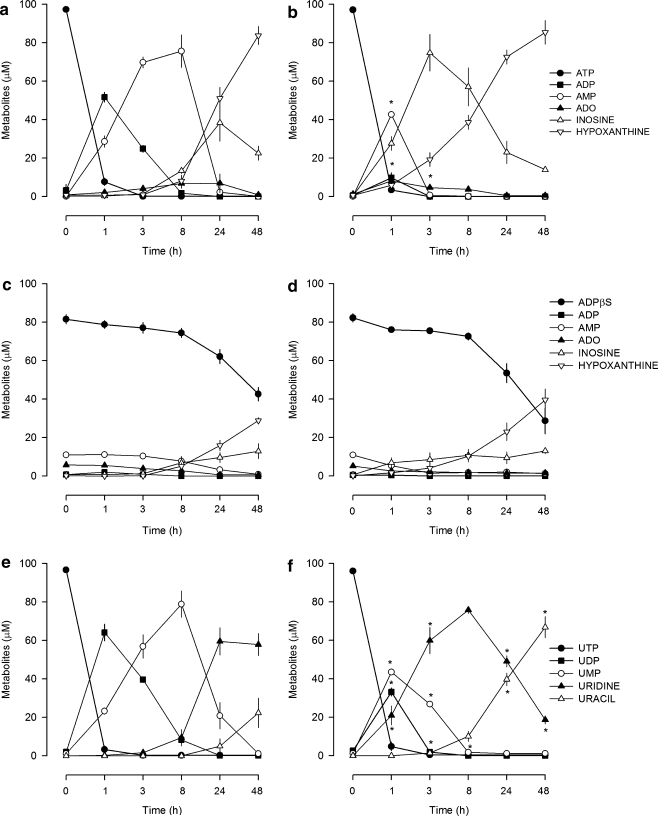
Extracellular metabolism of nucleotides
In highly enriched astroglial cultures, ATP was rapidly metabolised into ADP, which was the main metabolite formed in the first hour of incubation and remained detectable in the following 7 h (Fig. 3a), whereas in co-cultures ATP was directly converted into AMP in the first hour, and ADP was hardly detected (Fig. 3b). ATPγS was more stable than ATP, being slowly metabolised in both types of cultures. The half-life for ATPγS was of 8.5 ± 0.3 h (n = 4) in highly enriched astroglial cultures and 7.9 ± 0.4 h (n = 4) in co-cultures. ADPβS was also metabolically stable in the first 8 h; then its concentration slowly declined without being completely metabolised after 48 h of incubation (Fig. 3c, d). Results indicate that in highly enriched astroglial cultures the effect of ATP was predominantly mediated by ADP, whereas, in co-cultures, its effect in cell proliferation was lost (Fig. 2b) because the metabolism of ADP was faster. ADPβS and ATPγS metabolism was much slower and similar in both types of cultures, indicating that other mechanisms need to be addressed to explain their loss of proliferative effect in co-cultures (Fig. 2b). In both types of cultures, UTP was rapidly metabolised into UDP, which attained higher concentrations in highly enriched astroglial cultures (Fig. 3e) than in co-cultures (Fig. 3f). Even though an inhibitory effect mediated by UTP was only observed in co-cultures (Fig. 2d). Therefore, besides the differences found in the metabolism, other mechanisms come into play to explain the inhibitory effect of UTP in co-cultures.
Fig. 3.
Metabolism of nucleotides in highly enriched astroglial cultures (a, c, e) and co-cultures (b, d, f). Cultures were incubated with 0.1 mM of ATP, ADPβS or UTP, and samples were collected at 0, 1, 3, 8, 24 and 48 h. Nucleotides and their metabolites were quantified by HPLC-UV as described in Materials and methods. Values are means ± SEM from four experiments. *P < 0.05, significant differences from highly enriched astroglial cultures
Expression and cellular localization of P2Y1, P2Y12 and P2Y6 receptors
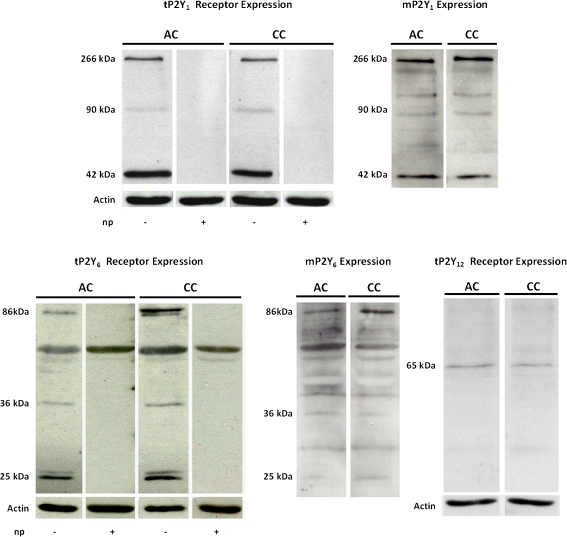
The loss of ADPβS proliferative effect in co-cultures could not be explained by differences in its metabolic profile. Therefore, based on previous studies indicating that ADPβS mediates astroglial proliferation by activation of P2Y1 and P2Y12 receptors [11], the possibility that differences in the expression of these P2Y receptor subtypes could explain the attenuation of ADPβS proliferative effect observed in co-cultures was investigated. Additionally, the rapid conversion of UTP into UDP, which is selective for P2Y6 receptors [33], also raised the question of whether P2Y6 receptor expression could be increased in cultures with microglia, thus favouring the inhibitory effect mediated by UTP/UDP in co-cultures.
These two hypotheses were tested by evaluating the relative expression of P2Y1, P2Y12 and P2Y6 receptor subtypes in both types of cultures. The P2Y1 receptor expression was represented by three immunoreactive bands of 42, 90 and 266 kDa that reacted with the anti-P2Y1 antibody. These bands were absent in the presence of the P2Y1 neutralising peptide indicating the P2Y1 receptor specificity (Fig. 4). Total P2Y1 receptor expression (tP2Y1) and the membrane fraction (mP2Y1) were similar in the two types of cultures (Table 1). Total P2Y12 receptor (tP2Y12) expression was represented by a single band of 65 kDa (Fig. 4), with a similar expression in both types of cultures (Table 1), but, because the expression was low, the respective membrane fraction was not evaluated.
Fig. 4.
Representative Western blots showing P2Y1, P2Y6 and P2Y12 receptors expression in highly enriched astroglial cultures (AC) and co-cultures (CC). Total P2Y1 (tP2Y1), P2Y6 (tP2Y6) and P2Y12 (tP2Y12) receptors and actin (43 kDa) expression were obtained from whole cell lysates. Expression of membrane P2Y1 (mP2Y1) and P2Y6 (mP2Y6) receptors was obtained from surface biotinylated and immunoprecipitated proteins. Three immunoreactive bands of 42, 90 and 266 kDa specifically reacted with rabbit anti-P2Y1 antibody, and those of 25, 36 and 86 kDa specifically reacted with rabbit anti-P2Y6 antibody. These bands were absent in the presence of the respective neutralising peptides. A single band of ~65 KDa was detected with the rabbit anti-P2Y12 antibody
Table 1.
Relative expression of P2Y1, P2Y6 and P2Y12 receptors in astroglial cultures
| % Highly enriched astroglial cultures | |||||||
|---|---|---|---|---|---|---|---|
| tP2Y1 | mP2Y1 | tP2Y6 | tP2Y6 86 kDa | mP2Y6 | mP2Y6 86 kDa | tP2Y12 | |
| Co-cultures | 93 ± 14 (5) | 121 ± 22 (3) | 129 ± 19 (4) | 245 ± 20* (4) | 176 ± 12* (3) | 200 ± 57* (3) | 93 ± 11 (3) |
Quantification by densitometry of P2Y1, P2Y6 and P2Y12 receptors total expression (tP2Y1, tP2Y6, tP2Y12) and the membrane fractions of P2Y1 and P2Y6 receptors (mP2Y1, mP2Y6). The expression of the 86 kDa band of P2Y6 receptors was also compared between cultures. Values are means ± SEM from (n) cultures represented in percentage of the expression observed in highly enriched astroglial cultures. Statistical analysis was performed using one-way repeated measures analysis of variance followed by Bonferroni multiple comparison test
*P < 0.05, significant differences from highly enriched astroglial cultures
The P2Y6 receptor comprised four bands, two of approximately 25, one of 36 and other of 86 kDa, which were all absent in the presence of the P2Y6 neutralising peptide (Fig. 4), indicating they all represent the P2Y6 receptor. When the four protein bands were quantified, total P2Y6 receptor expression (tP2Y6) was similar in both types of cultures (Table 1). However, the analysis of individual bands revealed that expression of the 86 kDa band was higher in co-cultures than in highly enriched astroglial cultures (Table 1). Moreover, when the membrane fraction (mP2Y6) was quantified, a higher expression was detected in co-cultures, considering either the four bands or only that of 86 kDa (Table 1).
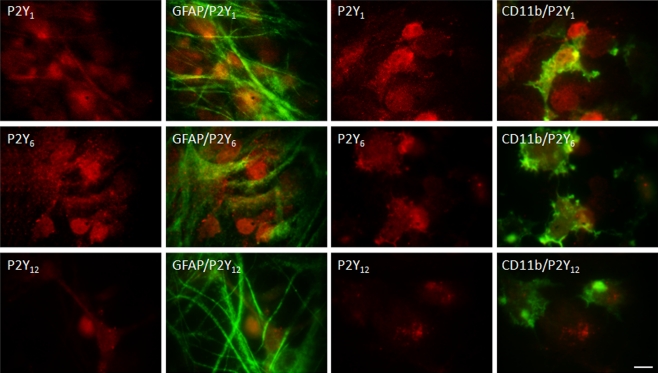
The immunocytochemical studies designed to identify a preferential cellular localization of these receptors in co-cultures revealed that P2Y1 and P2Y12 receptors were localised in both astrocytes and microglia, without any predominant cell-type localization (Fig. 5). However, the pattern of P2Y6 receptors distribution was different, revealing a higher co-localization with microglia (Fig. 5), which is in agreement with the higher mP2Y6 receptor expression observed in co-cultures.
Fig. 5.
Representative immunofluorescent micrographs of co-cultures double-labelled for P2Y receptors and GFAP or CD11b. P2Y receptors were labelled with anti-P2Y1, anti-P2Y6 and anti-P2Y12 (TRITC, red), astrocytes and microglia with anti-GFAP and with anti-CD11b (Alexa Fluor 488, green), respectively. P2Y1, P2Y6 and P2Y12 receptors are expressed both by astrocytes (first and second panels) and microglia (third and fourth panels). A stronger immunoreactivity for P2Y6 receptors co-localised with microglia. Scale bar = 10 μm
Microglia soluble factors and cell death
Attenuation of the proliferative response to adenine nucleotides in co-cultures could not be explained by differences in the P2Y1 and P2Y12 receptors expression or by a preferential cellular localization of these receptors in astrocytes or microglia. However, P2Y receptors localised in microglia may still play a role in these effects. Since ADPβS activates P2Y1,12,13 receptors subtypes, which are expressed by microglia [34, 35], it was investigated if ADPβS mediates the release of messengers from microglia that might interfere with its proliferative effect. This hypothesis was tested by comparing the effect of ADPβS (0.1 mM) in highly enriched astroglial cultures, in the absence and in the presence of conditioned medium obtained from microglia cultures or co-cultures that were previously treated with solvent (MCM-S or CCCM-S, respectively) or with ADPβS (MCM-ADPβS or CCCM-ADPβS, respectively).
The proliferative effect of ADPβS (0.1 mM) was not modified by MCM-S but was abolished in the presence of MCM-ADPβS (Table 2). Furthermore, in the presence of CCCM-S the proliferative effect of ADPβS (0.1 mM) was attenuated, an effect even more evident when the cultures were treated with CCCM-ADPβS (Table 2). In none of the conditions tested was astroglial proliferation near its maximum, as previously demonstrated the proliferative effect mediated by ADPβS (0.3–1 mM) in highly enriched astroglial cultures (see Fig. 2a). These results suggest that ADPβS activates microglia P2Y1,12 and/or P2Y13 receptors, which induce the release of diffusible messengers that attenuate its proliferative effect in astrocytes. They further suggest that the co-presence of astrocytes and microglia, and eventually their physical contact, facilitates the release of these inhibitory messengers since CCCM-S, but not MCM-S, attenuated ADPβS proliferative effect (Table 2). The same experimental approach was adopted for UTP, but no effects could be detected (not shown), possibly because of its higher metabolic instability, but, since UTP caused an inhibition of proliferation in co-cultures, its contribution to cell death was investigated. UTP (1 mM) caused no change in LDH release (not shown), but it increased the number of cells presenting DNA fragmentation, detected by the TUNEL assay, from 1.25 ± 0.13% (n = 3) in solvent treated cultures to 6.21 ± 0.72% (n = 3, P < 0.05). Results suggest that, in co-cultures, the presence of microglia may favour UTP/UDP-induced cell apoptosis, contributing to the inhibition of cell proliferation.
Table 2.
Influence of conditioned medium from microglia cultures or co-cultures in the ADPβS-induced astroglial proliferation in highly enriched astroglial cultures
| Methyl-[3H]-thymidine incorporation (% of control) | |||||
|---|---|---|---|---|---|
| Medium/drugs | No treatment | MCM-S | MCM-ADPβS | CCCM-S | CCCM-ADPβS |
| ADPβS 0.1 mM | 222 ± 10* (16) | 193 ± 17* (6) | 114 ± 18**, *** (6) | 157 ± 7*, *** (8) | 128 ± 5*, **, *** (8) |
Conditioned medium from microglia cultures or co-cultures treated with solvent (MCM-S and CCCM-S, respectively) or with 0.1 mM ADPβS (MCM-ADPβS and CCCM-ADPβS, respectively) was tested in highly enriched astroglial cultures in combination with 0.1 mM ADPβS for 48 h and methyl-[3H]-thymidine (1 μCi/ml) was added in the last 24 h. The effects in cell proliferation were estimated by methyl-[3H]-thymidine incorporation and expressed in percentage of respective control (solvent, MCM-S or CCCM-S). Values are means ± SEM from (n) experiments
*P < 0.05, significant differences from respective control
**P<0.05, from the effect of MCM-S or CCCM-S
***P < 0.05, from the effect of ADPβS alone
Discussion
Astrogliosis and microglia activation are two closely related responses involved in the brain repairing mechanisms to injury, and research in the field has been focusing the attention on how astrocytes and microglia influence each other activity during this process [24]. Astroglial proliferation induced by nucleotides was previously demonstrated both in vitro [3, 4] and in vivo [9]. However, whereas the in vivo studies did not discriminate the influence of microglia, the in vitro studies were performed in astroglial cultures, with the special concern of working without microglia interference. When the influence of microglia was to be explored, the mostly used strategy consisted in the evaluation of microglia conditioned medium influence in astroglial cultures [36, 37]. Although being an interesting and valid approach, it only evaluates astroglial response to soluble factors released by microglia, considering a unidirectional communication between both types of cells, which excludes the possibility that astrocytes may also release soluble factors that regulate and/or influence microglia responses [38, 39]. In the present study, the influence of microglia in the astroglial proliferative response to nucleotides was investigated by comparing their effects in two types of cultures: highly enriched astroglial cultures with a negligible presence of microglia and co-cultures of astrocytes containing 8% of microglia. This approach has the advantage of preserving the physical contact between astrocytes and microglia, facilitating the action of soluble messengers and a bidirectional communication between both cell types.
As expected, in highly enriched astroglial cultures, the adenine nucleotides increased astroglial proliferation in a concentration-dependent manner [3, 4]. The concentration–response curve to ATP was biphasic; it caused an increase in astroglial proliferation, mainly by activation of P2Y1, A2A and A2B receptors [11], but at the highest concentrations tested (0.3–1 mM), the proliferative response declined, which may be explained by activation of inhibitory adenosine A1 receptors [3] or by activation of P2X7 receptors that have opposite effects to those of P2Y receptors in cell proliferation [40]. Several uracil nucleotides were also tested but did not change astroglial proliferation. Even the P2Y14 receptors that are highly expressed in glial cells [41] were not involved in the modulation of astroglial proliferation since the selective agonist at these receptors, UDP-glucose, had no effect. Previous studies have shown that UTP may contribute to astroglial proliferation but only in synergism with the fibroblast growth factor-2 [40].
In co-cultures of astrocytes and microglia, the proliferative effect of adenine nucleotides was abolished, whereas UTP or its metabolite UDP inhibited astroglial proliferation.
This study demonstrates that microglia modify the astroglial response to nucleotides. In order to explain the differences observed in the proliferative responses to nucleotides in the two types of cultures, several hypotheses were considered: (1) the nucleotides metabolic profile was different in the presence of microglia, (2) the P2Y receptors expression could be different in the presence of microglia, (3) P2Y receptors could have a preferential cellular localization or (4) microglia P2Y receptors induced the release of messengers that modified the astroglial response to nucleotides and/or reduced cell viability.
The time course of ATP and UTP metabolism was similar in both types of cultures, but the accumulation of ADP and UDP differed. The presence of microglia in cultures accelerated the metabolism of the intermediates, having a higher influence on ADP than on UDP metabolism. Therefore, in co-cultures, ADP was hardly detected, which can explain the loss of ATP proliferative effect, whereas in highly enriched astroglial cultures the accumulation of ADP favoured the activation of P2Y1 receptors, which mediate astroglial proliferation [11]. However, the differences observed in the metabolism of UTP are insufficient to explain why it only caused inhibition of proliferation in co-cultures. Additionally, the metabolic profile of the stable nucleotides ADPβS and ATPγS failed to explain the loss of their proliferative effects in co-cultures.
The nucleotide ADPβS induced astroglial proliferation through the activation of P2Y1 and P2Y12 receptors, and this effect was lost in co-cultures, therefore the relative expression of these receptors in both types of cultures and their cell-type localization were evaluated. P2Y1 receptor expression presented a multiple band pattern: a band of 42 kDa that corresponds to the P2Y1 receptor monomer [42] and additional bands with molecular weights of 90 and 266 kDa. Similar bands were previously described and may represent homomeric forms of the P2Y1 receptor, or heteromers with A1 receptors [43–45]. P2Y1 and P2Y12 receptors expression was similar in both types of cultures (Table 1), as well as the pattern of distribution in astrocytes (not shown), arguing against these two factors as relevant contributors to the loss of proliferative effects mediated by the stable nucleotides in co-cultures. The most feasible explanation for the differences in proliferation found in both types of cultures resides in the influence mediated by the microglia P2Y receptors. Our findings show that microglia exert an inhibitory influence in the P2Y receptor-mediated proliferative effects in astrocytes. Furthermore, this inhibitory influence of microglia is more pronounced when in contact with astrocytes, since CCCM-S, but not MCM-S, attenuated the proliferative effect of ADPβS in highly enriched astroglial cultures. In agreement with this observation, previous studies have demonstrated that in co-cultures the spontaneous release of ATP by astrocytes may enhance the release of messengers by microglia, which may be involved in the regulation/control of astroglial proliferation [38]. The CCCM-ADPβS and MCM-ADPβS prevented ADPβS-induced astroglial proliferation in highly enriched astroglial cultures. Therefore, besides the inhibitory influence provided by the presence of microglia, activation of microglia P2Y receptors sensitive to ADPβS seem to contribute to the release of messengers that interfere with the proliferative effects mediated by astroglial P2Y receptors. Microglia express P2Y1, P2Y12 and P2Y13 receptors [34, 35], which are activated by ADPβS and may regulate the release of messengers, such as interleukin-10 [46]. These messengers may regulate astroglial proliferation mediated by P2Y receptors through a functional interaction occurring at the receptor level and/or at the intracellular signal transduction pathways. For example, interleukin-1β was shown to decrease the activity of P2Y1 receptors by a mechanism that involves an interaction with connexin 43 [47, 48], but interleukin-1β and tumour necrosis factor-α may also activate intracellular signalling pathways shared by P2Y receptors [49] and consequently regulate astroglial response. Other messengers such as transforming growth factor-β or interleukin-4 mediate opposite effects to adenine nucleotides [50, 51] and may prevent astroglial proliferation. Therefore, several candidates exist to mediate this interaction between astrocytes and microglia in the modulation of astroglial proliferation and it is likely that several mediators participate in this process.
A comparative study of the P2Y6 receptors expression and their cell-type localization helped to have clearer picture of the differences found in the UTP effect between the two types of cultures. P2Y6 receptor expression also revealed a multiple band pattern; besides the band of 36 kDa predicted for this receptor [52], additional bands of lower molecular weight were also detected, which may correspond to degradation products, and a band of 86 kDa that was previously reported to represent a homomeric association of P2Y6 receptors or a heterodimeric association between P2Y6 and P2Y4 receptors [53]. The tP2Y6 expression was similar in both types of cultures, but the 86 kDa band and the mP2Y6 had a higher expression in co-cultures. Additionally, P2Y6 receptors were highly localised in microglia with a more diffuse and less intense distribution in the astrocyte net. These results indicate that UTP metabolism with UDP formation and a higher expression of P2Y6 receptors by microglia may have favoured activation of microglia P2Y6 receptors, mediating UTP/UDP-induced apoptosis through the release of inhibitory messengers. These messengers may include several cytokines described to inhibit proliferation (see above); a short-lived messenger such as nitric oxide [54] or other messengers not yet identified that may be released by microglia P2Y6 receptor-stimulation.
Our results indicate that microglia present in these co-cultures are sufficient to influence the effects of modulators of astroglial proliferation and underline the importance of studying the contribution of microglia P2Y receptors to the modulation of astroglial proliferation induced by nucleotides. This modulation is mediated through the release of messengers, not yet identified, but whose identity is currently under investigation. Activation of P2Y1, P2Y12 and/or P2Y13 receptors attenuates the proliferative effect of adenine nucleotides, and activation of P2Y6 receptors mediates cell apoptosis triggered by uracil nucleotides. In addition to the roles previously described for P2Y6 receptors, i.e. secretion of cytokines and phagocytosis [55], these receptors also modulate astroglial proliferation, a mechanism that is important to prevent excessive astrogliosis that may compromise neuronal repair.
Acknowledgements
This study is supported by Fundação para a Ciência e a Tecnologia Projects (PTDC/SAU-TOX/115597/2009 and REQUIMTE/CEQUP) and Grant SFRH/BD/23907/2005.
References
- 1.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lucin KM, Wyss-Coray T. Immune activation in brain aging and neurodegeneration: too much or too little? Neuron. 2009;64:110–122. doi: 10.1016/j.neuron.2009.08.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccarelli R, Iorio P, Ballerini P, Ambrosini G, Giuliani P, Tiboni GM, Caciagli F. Effects of exogenous ATP and related analogues on the proliferation rate of dissociated primary cultures of rat astrocytes. J Neurosci Res. 1994;39:556–566. doi: 10.1002/jnr.490390507. [DOI] [PubMed] [Google Scholar]
- 4.Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Kong Q, Gonzalez FA, Sun G, Erb L, Seye C, Weisman GA. P2Y2 nucleotide receptor interaction with αv integrin mediates astrocyte migration. J Neurochem. 2005;95:630–640. doi: 10.1111/j.1471-4159.2005.03408.x. [DOI] [PubMed] [Google Scholar]
- 6.Kucher BM, Neary JT. Bi-functional effects of ATP/P2 receptor activation on tumor necrosis factor-alpha release in lipopolysaccharide-stimulated astrocytes. J Neurochem. 2005;92:525–535. doi: 10.1111/j.1471-4159.2004.02885.x. [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Tozaki-Saitoh H, Inoue K. P2Y1 receptor signaling enhances neuroprotection by astrocytes against oxidative stress via IL-6 release in hippocampal cultures. Glia. 2009;57:244–257. doi: 10.1002/glia.20749. [DOI] [PubMed] [Google Scholar]
- 8.Xu J, Chalimoniuk M, Shu Y, Simonyi A, Sun AY, Gonzalez FA, Weisman GA, Wood WG, Sun GY. Prostaglandin E2 production in astrocytes: regulation by cytokines, extracellular ATP, and oxidative agents. Prostaglandins Leukot Essent Fatty Acids. 2003;69:437–448. doi: 10.1016/j.plefa.2003.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Franke H, Krügel U, Grosche J, Heine C, Härtig W, Allgaier C, Illes P. P2Y receptor expression on astrocytes in the nucleus accumbens of rats. Neuroscience. 2004;127:431–441. doi: 10.1016/j.neuroscience.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 10.Neary JT, Kang Y, Willoughby KA, Ellis EF. Activation of extracellular signal-regulated kinase by stretch-induced injury in astrocytes involves extracellular ATP and P2 purinergic receptors. J Neurosci. 2003;23:2348–2356. doi: 10.1523/JNEUROSCI.23-06-02348.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quintas C, Fraga S, Goncalves J, Queiroz G. Opposite modulation of astroglial proliferation by adenosine 5'-O-(2-thio)-diphosphate tetralithium and 2-methylthioadenosine-5'-diphosphate trisodium: mechanisms involved. Neuroscience. 2011 doi: 10.1016/j.neuroscience.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Honda S, Sasaki Y, Ohsawa K, Imai Y, Nakamura Y, Inoue K, Kohsaka S. Extracellular ATP or ADP induce chemotaxis of cultured microglia through Gi/o-coupled P2Y receptors. J Neurosci. 2001;21:1975–1982. doi: 10.1523/JNEUROSCI.21-06-01975.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Simone R, Niturad CE, Nuccio C, Ajmone-Cat MA, Visentin S, Minghetti L. TGF-β and LPS modulate ADP-induced migration of microglial cells through P2Y1 and P2Y12 receptor expression. J Neurochem. 2010;115:450–459. doi: 10.1111/j.1471-4159.2010.06937.x. [DOI] [PubMed] [Google Scholar]
- 14.Koizumi S, Shigemoto-Mogami Y, Nasu-Tada K, Shinozaki Y, Ohsawa K, Tsuda M, Joshi BV, Jacobson KA, Kohsaka S, Inoue K. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature. 2007;446:1091–1095. doi: 10.1038/nature05704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ulmann L, Hatcher JP, Hughes JP, Chaumont S, Green PJ, Conquet F, Buell GN, Reeve AJ, Chessell IP, Rassendren F. Up-regulation of P2X4 receptors in spinal microglia after peripheral nerve injury mediates BDNF release and neuropathic pain. J Neurosci. 2008;28:11263–11268. doi: 10.1523/JNEUROSCI.2308-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Färber K, Kettenmann H. Purinergic signaling and microglia. Pflugers Arch. 2006;452:615–621. doi: 10.1007/s00424-006-0064-7. [DOI] [PubMed] [Google Scholar]
- 17.Choi HB, Ryu JK, Kim SU, McLarnon JG. Modulation of the purinergic P2X7 receptor attenuates lipopolysaccharide-mediated microglial activation and neuronal damage in inflamed brain. J Neurosci. 2007;27:4957–4968. doi: 10.1523/JNEUROSCI.5417-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanagisawa D, Kitamura Y, Takata K, Hide I, Nakata Y, Taniguchi T. Possible involvement of P2X7 receptor activation in microglial neuroprotection against focal cerebral ischemia in rats. Biol Pharm Bull. 2008;31:1121–1130. doi: 10.1248/bpb.31.1121. [DOI] [PubMed] [Google Scholar]
- 19.Eclancher F, Kehrli P, Labourdette G, Sensenbrenner M. Basic fibroblast growth factor (bFGF) injection activates the glial reaction in the injured adult rat brain. Brain Res. 1996;737:201–214. doi: 10.1016/0006-8993(96)00732-9. [DOI] [PubMed] [Google Scholar]
- 20.Balasingam V, Tejada-Berges T, Wright E, Bouckova R, Yong VW. Reactive astrogliosis in the neonatal mouse brain and its modulation by cytokines. J Neurosci. 1994;14:846–856. doi: 10.1523/JNEUROSCI.14-02-00846.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein MA, Moller JC, Jones LL, Bluethmann H, Kreutzberg GW, Raivich G. Impaired neuroglial activation in interleukin-6 deficient mice. Glia. 1997;19:227–233. doi: 10.1002/(SICI)1098-1136(199703)19:3<227::AID-GLIA5>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 22.Balasingam V, Yong VW. Attenuation of astroglial reactivity by interleukin-10. J Neurosci. 1996;16:2945–2955. doi: 10.1523/JNEUROSCI.16-09-02945.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woiciechowsky C, Schöning B, Stoltenburg-Didinger G, Stockhammer F, Volk HD. Brain-IL-1β triggers astrogliosis through induction of IL-6: inhibition by propranolol and IL-10. Med Sci Monit. 2004;10:BR325–BR330. [PubMed] [Google Scholar]
- 24.Zhang D, Hu X, Qian L, O'Callaghan JP, Hong JS. Astrogliosis in CNS pathologies: is there a role for microglia? Mol Neurobiol. 2010;41:232–241. doi: 10.1007/s12035-010-8098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ciccarelli R, Iorio P, D'Alimonte I, Giuliani P, Florio T, Caciagli F, Middlemiss PJ, Rathbone MP. Cultured astrocyte proliferation induced by extracellular guanosine involves endogenous adenosine and is raised by the co-presence of microglia. Glia. 2000;29:202–211. doi: 10.1002/(SICI)1098-1136(20000201)29:3<202::AID-GLIA2>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 26.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Queiroz G, Gebicke-Haerter PJ, Schobert A, Starke K, Kugelgen I. Release of ATP from cultured rat astrocytes elicited by glutamate receptor activation. Neuroscience. 1997;78:1203–1208. doi: 10.1016/S0306-4522(96)00637-9. [DOI] [PubMed] [Google Scholar]
- 28.Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 29.Giulian D, Baker TJ. Characterization of ameboid microglia isolated from developing mammalian brain. J Neurosci. 1986;6:2163–2178. doi: 10.1523/JNEUROSCI.06-08-02163.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunha RA, Sebastiao AM, Ribeiro JA. Inhibition by ATP of hippocampal synaptic transmission requires localized extracellular catabolism by ecto-nucleotidases into adenosine and channeling to adenosine A1 receptors. J Neurosci. 1998;18:1987–1995. doi: 10.1523/JNEUROSCI.18-06-01987.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gendaszewska-Darmach E, Maszewska M, Zaklos M, Koziolkiewicz M. Degradation of extracellular nucleotides and their analogs in HeLa and HUVEC cell cultures. Acta Biochim Pol. 2003;50:973–984. [PubMed] [Google Scholar]
- 33.Communi D, Parmentier M, Boeynaems JM. Cloning, functional expression and tissue distribution of the human P2Y6 receptor. Biochem Biophys Res Commun. 1996;222:303–308. doi: 10.1006/bbrc.1996.0739. [DOI] [PubMed] [Google Scholar]
- 34.Fumagalli M, Brambilla R, D'Ambrosi N, Volonte C, Matteoli M, Verderio C, Abbracchio MP. Nucleotide-mediated calcium signaling in rat cortical astrocytes: role of P2X and P2Y receptors. Glia. 2003;43:218–230. doi: 10.1002/glia.10248. [DOI] [PubMed] [Google Scholar]
- 35.Fumagalli M, Trincavelli L, Lecca D, Martini C, Ciana P, Abbracchio MP. Cloning, pharmacological characterisation and distribution of the rat G-protein-coupled P2Y13 receptor. Biochem Pharmacol. 2004;68:113–124. doi: 10.1016/j.bcp.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 36.Röhl C, Lucius R, Sievers J. The effect of activated microglia on astrogliosis parameters in astrocyte cultures. Brain Res. 2007;1129:43–52. doi: 10.1016/j.brainres.2006.10.057. [DOI] [PubMed] [Google Scholar]
- 37.Tilleux S, Berger J, Hermans E. Induction of astrogliosis by activated microglia is associated with a down-regulation of metabotropic glutamate receptor 5. J Neuroimmunol. 2007;189:23–30. doi: 10.1016/j.jneuroim.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Bianco F, Pravettoni E, Colombo A, Schenk U, Moller T, Matteoli M, Verderio C. Astrocyte-derived ATP induces vesicle shedding and IL-1β release from microglia. J Immunol. 2005;174:7268–7277. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 39.Saura J, Angulo E, Ejarque A, Casadó V, Tusell JM, Moratalla R, Chen JF, Schwarzschild MA, Lluis C, Franco R, Serratosa J. Adenosine A2A receptor stimulation potentiates nitric oxide release by activated microglia. J Neurochem. 2005;95:919–929. doi: 10.1111/j.1471-4159.2005.03395.x. [DOI] [PubMed] [Google Scholar]
- 40.Neary JT, Shi YF, Kang Y, Tran MD. Opposing effects of P2X7 and P2Y purine/pyrimidine-preferring receptors on proliferation of astrocytes induced by fibroblast growth factor-2: implications for CNS development, injury, and repair. J Neurosci Res. 2008;86:3096–3105. doi: 10.1002/jnr.21765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abbracchio MP, Verderio C. Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 2006;276:91–103. doi: 10.1002/9780470032244.ch8. [DOI] [PubMed] [Google Scholar]
- 42.Ayyanathan K, Webbs TE, Sandhu AK, Athwal RS, Barnard EA, Kunapuli SP. Cloning and chromosomal localization of the human P2Y1 purinoceptor. Biochem Biophys Res Commun. 1996;218:783–788. doi: 10.1006/bbrc.1996.0139. [DOI] [PubMed] [Google Scholar]
- 43.Wang L, Karlsson L, Moses S, Hultgardh-Nilsson A, Andersson M, Borna C, Gudbjartsson T, Jern S, Erlinge D. P2 receptor expression profiles in human vascular smooth muscle and endothelial cells. J Cardiovasc Pharmacol. 2002;40:841–853. doi: 10.1097/00005344-200212000-00005. [DOI] [PubMed] [Google Scholar]
- 44.Yoshioka K, Saitoh O, Nakata H. Agonist-promoted heteromeric oligomerization between adenosine A1 and P2Y1 receptors in living cells. FEBS Lett. 2002;523:147–151. doi: 10.1016/S0014-5793(02)02965-4. [DOI] [PubMed] [Google Scholar]
- 45.Tonazzini I, Trincavelli ML, Montali M, Martini C. Regulation of A1 adenosine receptor functioning induced by P2Y1 purinergic receptor activation in human astroglial cells. J Neurosci Res. 2008;86:2857–2866. doi: 10.1002/jnr.21727. [DOI] [PubMed] [Google Scholar]
- 46.Seo DR, Kim SY, Kim KY, Lee HG, Moon JH, Lee JS, Lee SH, Kim SU, Lee YB. Cross talk between P2 purinergic receptors modulates extracellular ATP-mediated interleukin-10 production in rat microglial cells. Exp Mol Med. 2008;40:19–26. doi: 10.3858/emm.2008.40.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Striedinger K, Scemes E. Interleukin-1β affects calcium signaling and in vitro cell migration of astrocyte progenitors. J Neuroimmunol. 2008;196:116–123. doi: 10.1016/j.jneuroim.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Scemes E. Modulation of astrocyte P2Y1 receptors by the carboxyl terminal domain of the gap junction protein Cx43. Glia. 2008;56:145–153. doi: 10.1002/glia.20598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu JS, John GR, Sikora A, Lee SC, Brosnan CF. Modulation of interleukin-1β and tumor necrosis factor α signaling by P2 purinergic receptors in human fetal astrocytes. J Neurosci. 2000;20:5292–5299. doi: 10.1523/JNEUROSCI.20-14-05292.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Estes ML, Iwasaki K, Jacobs BS, Barna BP. Interleukin-4 down-regulates adult human astrocyte DNA synthesis and proliferation. Am J Pathol. 1993;143:337–341. [PMC free article] [PubMed] [Google Scholar]
- 51.Lindholm D, Castrén E, Kiefer R, Zafra F, Thoenen H. Transforming growth factor-β1 in the rat brain: increase after injury and inhibition of astrocyte proliferation. J Cell Biol. 1992;117:395–400. doi: 10.1083/jcb.117.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang K, Hanaoka K, Kumada M, Takuwa Y. Molecular cloning and functional analysis of a novel P2 nucleotide receptor. J Biol Chem. 1995;270:26152–26158. doi: 10.1074/jbc.270.44.26152. [DOI] [PubMed] [Google Scholar]
- 53.D'Ambrosi N, Iafrate M, Saba E, Rosa P, Volonte C. Comparative analysis of P2Y4 and P2Y6 receptor architecture in native and transfected neuronal systems. Biochim Biophys Acta. 2007;1768:1592–1599. doi: 10.1016/j.bbamem.2007.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Ohtani Y, Minami M, Satoh M. Expression of inducible nitric oxide synthase mRNA and production of nitric oxide are induced by adenosine triphosphate in cultured rat microglia. Neurosci Lett. 2000;293:72–74. doi: 10.1016/S0304-3940(00)01478-6. [DOI] [PubMed] [Google Scholar]
- 55.Virgilio F, Ceruti S, Bramanti P, Abbracchio MP. Purinergic signalling in inflammation of the central nervous system. Trends Neurosci. 2009;32:79–87. doi: 10.1016/j.tins.2008.11.003. [DOI] [PubMed] [Google Scholar]