Abstract
In this study, the distribution patterns of P2Y1, P2Y2 P2Y4, P2Y6, P2Y12, and P2Y13 receptors in the anterior pituitary cells of rat were studied with double-labeling immunofluorescence and Western blot. The results showed that P2Y receptors were widely expressed in the anterior pituitary. P2Y1 and P2Y4 receptors were found to be expressed in the majority of gonadotrophs and thyrotrophs, P2Y2 receptors were expressed in a small subpopulation of lactotrophs and almost all the folliculo-stellate cells, that were also stained with S100 protein immunoreactivity. P2Y6 receptors were expressed in macrophages. P2Y13 receptors were expressed in a small subpopulation of cells in the rat anterior pituitary, the identity of which needs to be clarified. P2Y1 and P2Y4 receptors are co-expressed in some gonadotrophs and thyrotrophs. Corticotrophs and somatotrophs were found not to express P2Y receptors in this study. FSH and TSH were shown to coexist in the same endocrine cells in rat anterior pituitary. The present data suggests that purines and/or pyrimidines could be involved in regulating the functions of gonadotrophs and thyrotrophs via P2Y1 and P2Y4 receptors, some lactotrophs via P2Y2 receptors, and folliculo-stellate cells via P2Y2 receptors in the rat anterior pituitary.
Keywords: P2Y receptors, Pituitary, Immunohistochemistry, Rat
Introduction
Extracellular purines and pyrimidines act as messengers via purinergic receptors on the plasma membrane. There are two purinergic receptors: P1 receptors (adenosine receptor [AR]) activated by adenosine and P2 receptors activated by ATP-, ADP-, UTP- and/or UDP. There are four AR subtypes (A1, A2A, A2B and A3), which are all G protein-coupled receptors. There are two groups of P2 receptors: P2X and P2Y receptors, which are ligand-gated ion channels and G protein-coupled receptors, respectively. So far, seven P2Y receptors and seven P2X receptors have been cloned and are denoted P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, P2Y14, and P2X1 to P2X7 receptors [1].
There is increasing evidence to show that extracellular purines and pyrimidines, via P2 receptors, have important physiological functions in the regulation of anterior pituitary cell secretion. Using the technique of single-cell calcium measurement, the initial characterization of purinoceptors in anterior pituitary cells were identified [2–6]. These experiments revealed that functional P2X receptors are operative in all secretory cell types. With molecular biology techniques, P2X2, P2X3, P2X4, and P2X7 mRNA transcripts were detected in a mixed population of anterior pituitary cells. Lactotrophs and immortalized GH3 pituitary cells expressed transcripts for P2X3, P2X4, and P2X7 subunits, somatotrophs expressed P2X2 receptor, and thyrotrophs and corticotrophs also express P2X receptors [7] Single-cell patch-clamp analysis in gonadotrophs from embryonic, neonatal, and adult rats revealed that P2X2 receptors are operative and involved in release of luteinizing hormone (LH) and regulation of gonadotrophin-releasing hormone (GnRH) controlled electrical activity and secretion [8]. Lactotrophs express P2X4 receptors, which facilitate Ca2+ influx and hormone secretion [9].
Compared with data for P2X receptors on the anterior pituitary cells, there is less available data for P2Y receptors on these cells. Using calcium image analysis, sheep and rat anterior pituitary cells were found to express P2Y receptors [10–12]. Following RT-PCR analysis, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y12 receptor transcripts were detected in mixed anterior pituitary cells [13, 14]. The identified cells in the anterior pituitary with P2Y receptors were also found in rat pituitary folliculo-stellate cells in primary culture [15], gonadotrophs [16] and lactotrophs [17]. No detailed immunofluorescence, Western blot or in situ hybridization studies about P2Y receptor subtype expression on the individual cell types in the anterior pituitary are currently available.
In this study, we used single-labeling and double-labeling immunofluorescence, in situ hybridization and Western blot analyses to study the distribution patterns of P2Y1, P2Y2 P2Y4, P2Y6, P2Y12, and P2Y13 receptors in anterior pituitary cells of rat and found that gonadotrophs and thyrotrophs express P2Y1 and P2Y4 receptors, lactotrophs and folliculo-stellate cells express P2Y2, and macrophages express P2Y6.
Materials and methods
Animals and tissue preparation
All experimental procedures were approved by the Institutional Animal Care and Use Committee at Second Military Medical University. Five Sprague–Dawley rats were used. Animals were anesthetized by intraperitoneal injection with chloral hydrate and perfused through the aorta with 0.9% NaCl solution and 4% paraformaldehyde in 0.1 mol/l phosphate buffer, pH 7.4. The pituitaries were dissected out and refixed in 4% paraformaldehyde in 0.1 mol/l phosphate buffer pH 7.4 for 4–6 h, then transferred to 25% sucrose in PBS and kept in the solution until they sank to the bottom. Thereafter, the segment blocks were rapidly frozen and crosscut sections (10 μm in thickness) were cut with a Leica cryostat and thawed on slides covered with gelatin.
Immunohistochemistry
Table 1 shows the primary antibodies used in this study.
Table 1.
Primary antibodies used in this study
| Primary antibodies | Host | Manufacturer | Catalog no. | Dilution | |
|---|---|---|---|---|---|
| IHC | TSA | ||||
| P2Y1 | Rabbit | Alomone | APR-009 | 1:200 | 1:4,000 |
| P2Y2 | Rabbit | Alomone | APR-010 | 1:400 | 1:8,000 |
| P2Y4 | Rabbit | Alomone | APR-006 | 1:400 | 1:8,000 |
| P2Y6 | Goat | Santa Cruz | SC-15215 | 1:100 | |
| P2Y12 | Goat | Santa Cruz | SC-27152 | 1:50 | |
| P2Y13 | Goat | Santa Cruz | SC-69526 | 1:100 | |
| ACTH | Rabbit | Sigma | A1927 | 1:600 | 1:10,000 |
| FSH | Rabbit | Chemicon | AB928 | 1:600 | 1:10,000 |
| PRL | Rabbit | Chemicon | AB960 | 1:200 | 1:5,000 |
| TSH | Rabbit | Chemicon | AB976 | 1:400 | 1:10,000 |
| S-100 | Mouse | Abcam | ab4066 | 1:200 | |
| ED1 | Mouse | Abcam | ab31630 | 1:200 | |
| Digoxigenin | Mouse | Jackson | 200-002-156 | 1:200 | |
The following protocol is a double immunofluorescence technique for two primary antibodies from different hosts. The section slides were washed 3 × 5 min in PBS, and then preincubated in antiserum solution 1 (10% normal bovine serum, 0.2% Triton-X-100, 0.4% sodium azide in 0.01 mol/l PBS pH7.2) for 30 min, followed by incubation with two primary antibody dilutions, one a P2Y antibody (P2Y6, P2Y12, and P2Y13) and the second either a hormonal antibody adrenocorticotropin (ACTH), follicle-stimulating hormone (FSH), prolactin (PRL), and thyroid-stimulating hormone (TSH) or a cell type marker (ED1 [macrophage marker] and S-100 [folliculo-stellate cell marker]) at room temperature, overnight. Subsequently, the sections were incubated at room temperature for 2 h with a mixed secondary antibody solution of Cy3-conjugated donkey anti-rabbit IgG diluted 1:400 for P2Y antibodies and either FITC-conjugated donkey anti-goat IgG diluted 1:200 for hormonal antibodies or FITC-conjugated donkey anti-mouse IgG diluted 1:200 for cell type marker antibodies. All the incubations and reactions were separated by 3 × 10 min washes in PBS.
The following protocol is a double immunofluorescence technique for two primary antibodies from the same hosts. Simultaneous detection of two antigens by immunostaining usually requires primary antibodies from two different species. A novel double-labeling immunostaining method for immunodetection of two independent antigens has been described [18]. The principle of the method is that the first antigen is detected by the first primary antibody that is diluted so extensively that it cannot be detected with conventional methods; a highly sensitive tyramide signals amplification (TSA) system is used to identify this antibody; the second antigen is stained with the secondary primary antibody and detected by conventional immunostaining. We have used this double-labeling protocol of fluorescence immunohistochemistry successfully [19, 20]. The following protocol was modified from this protocol. Endogenous peroxidase was blocked by 1% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% normal horse serum (NHS), 0.2% Triton X-100 in PBS for 30 min, followed by incubation with P2Y antibodies diluted in antibody dilution solution (10% NHS, 0.2% Triton X-100 and 0.4% sodium azide in PBS) overnight at 4°C. Subsequently, the sections were incubated with biotinylated donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a dilution of 1:500 in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1,000 in PBS for 30 min at room temperature. P2Y immunoreactivity was visualized by the TSA Fluorescein system (NEL701, NEN, USA). After visualization the sections were incubated with the second primary antibody of either a hormone or a cell type marker, diluted in the antiserum dilution solution overnight at 4°C. Subsequently, the sections were incubated with Cy3 conjugated donkey–anti–rabbit or mouse (Jackson ImmunoResearch) diluted 1:400 in antiserum dilution solution for 1 h at room temperature. All the incubations and reactions were separated by 3 × 10 min washes in PBS. Some sections were counter-stained with 5 μg/ml Hoechst 33342.
Combined use of in situ hybridization and immunofluorescence
As the proper antibody for growth hormone could not be obtained for this study, sense and antisense digoxigenin-labeled oligonucleotide probes were synthesised (Sagon, Shanghai, China). The sense and antisense nucleotide sequences are AGGGCATCCAGGCTCTGATGCAGGAGCTGG, CCAGCTCCTGCATCAGAGC CTGGATGCCCT (GenBank Accession: U62779.1), respectively. In situ hybridization was carried out as following. The sections were heated in 0.01 mol/l citrate buffer pH6.8 in a 92°C water bath for 15 min and cool to room temperature in the same buffer. The sections were rinsed in PBS for 3 min for two times, then incubated in 0.25% acetic anhydride with 0.1 M triethanolamine (pH 8.0) for 10 min at room temperature, followed by washing in 2× saline sodium citrate (SSC) for 10 min. Digoxigenin-labeled oligonucleotides (0.5 μg/ml) of either antisense or sense probe was added to the hybridization solution containing 50% formamide, 10% dextrans sulphate, 0.3 M NaCl, 1× Denhardt’s solution, 0.05 M Tris–HCl (pH 8.0), 1 mM EDTA and 250 mg/ml Herring sperm DNA (Sigma). Hybridization was carried out for 12–16 h at 37°C in a hybridization oven. The sections were washed in 4× SSC for 20 min, in 2× SSC for 20 min, in 1× SSC for 20 min, in 0.5× SSC for 20 min at 37°C.
The following method was used to detect hybridization signals and P2Y receptors. Endogenous peroxidase was blocked by 3% H2O2 in PBS for 30 min. The sections were pre-incubated in 10% NHS, 0.2% Triton X-100 in PBS for 30 min, followed by incubation with two primary antibodies (mouse anti-digoxigenin IgG and one of the P2Y antibodies) diluted in antibody dilution solution overnight at 4°C. Subsequently, the sections were incubated with two secondary antibodies of biotinylated donkey anti-mouse IgG and FITC-conjugated donkey anti-rabbit IgG (Jackson ImmunoResearch) at a dilution of 1:500 and 1:200, respectively, in PBS containing 1% NHS for 1 h. The sections were then incubated in extravidin peroxidase (Sigma) diluted 1:1,000 in PBS for 30 min at room temperature. The digoxigenin immunoreactivity was visualized by the TSA Cy3 system (NEL704A, NEN life science, USA). All the incubations and reactions were separated by 3 × 10 min washes in PBS. Some sections were counter-stained with 5 μg/ml Hoechst 33342.
Western blot
Sprague–Dawley rats were deeply anesthetized by sodium pentobarbital (60 mg/kg) and killed by decapitation. The pituitaries were rapidly removed and lysed with 20 mM Tris–HCl buffer, pH 8.0, containing 1% NP-40, 150 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% mercaptoethanol, 0.5 mM dithiothreitol, and a mixture of proteinase and phosphatase inhibitors (Sigma). Protein concentration was determined by the BCA protein assay method using bovine serum albumin as standard (BCA protein assay kit from Beyotime). Protein samples (100 μg) were loaded in each lane, separated by SDS-PAGE (10% polyacrylamide gels) and then electrotransferred onto nitrocellulose membranes. The membranes were blocked with 10% non-fat dry milk in Tris-buffered saline for 1 h and incubated overnight at 4°C with the P2Y antibodies diluted 1:200–400 in PBS. The membranes were then incubated with alkaline phosphatase-conjugated goat anti-Goat or Rabbit IgG (Beyotime) diluted 1:1,000 in 2% BSA in PBS for 1 h at room temperature. The color development was performed with 400 μg/ml nitro-blue tetrazolium, 200 μg/ml 5-bromo-4-chloro-3-indolyl phosphate and 100 mg/ml levamisole in TSM2 (0.1 mol/l Tris–HCl2 buffer, pH 9.5, 0.1 mol/l NaCl and 0.05 mol/l MgCl2) in the dark. Bands were scanned using a densitometer (GS-700; Bio-Rad Laboratories).
Photomicroscopy
Images were taken with the Nikon digital camera DXM1200 (Nikon, Japan) attached to a Nikon Eclipse E600 microscope (Nikon). Images were imported into a graphics package (Adobe Photoshop). The two-channel readings for green and red fluorescence were merged by using Adobe Photoshop. The focal plane on the microscope was not adjusted whilst determining whether a particular cell co-localized both P2Y receptors and other markers. Only cells that demonstrated the same morphology, orientation and position when viewed under the two different filters (in the same focal plane) for the detection of Cy3 and FITC were deemed to co-localize both P2Y receptor and other markers.
Quantitative analysis
Quantitative analysis for the P2Y receptors immunostaining in the anterior pituitary was performed as follows: five random fields (each area was 0.36 mm2) for one section were chosen and the number of positive cells was counted and expressed as the positive cell numbers per square millimeter. Five fields for each of five sections from each of five rats were used. The mean number of positive cells/mm2 from each rat was calculated and data are expressed as the mean ± standard error of the mean (n = number of rat).
Results
With double-labeling immunofluorescence and combined in situ hybridization and immunofluorescence, P2Y1, P2Y2, P2Y4, P2Y6, and P2Y13 receptors were found to be expressed in the different cell types of the rat anterior pituitary. Control experiments and Western blot confirmed the specificity of P2Y1, P2Y2 P2Y4, P2Y6, P2Y12, P2Y13 receptor immunoreactivity in the anterior pituitary cells of rat.
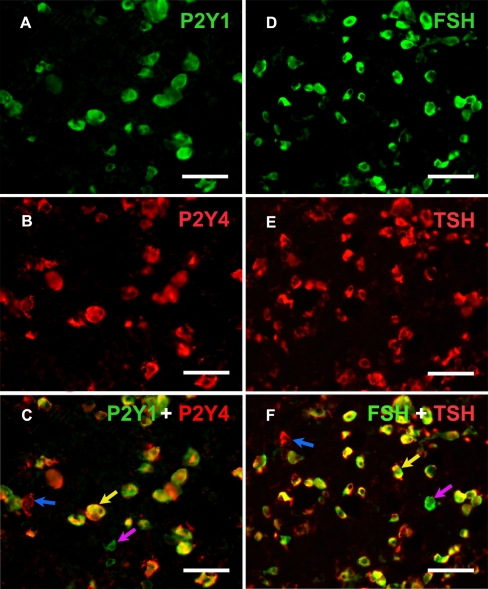
P2Y1 receptor-immunoreactive (−ir) cells were generally ovoid in shape with moderate to strong immunostaining, some of P2Y1 receptor-ir cells were angular in shape with processes (Fig. 1). Double-labeling immunofluorescence showed that 90% and 93% of P2Y1 receptor-ir cells were also immunoreactive for FSH and TSH, respectively, but none of them were immunoreactive for ACTH, PRL or S100 in the anterior pituitary of rat (Fig. 1). There were still about 32% of FSH-ir endocrine cells and 35% of TSH-ir endocrine cells, which were not found to express P2Y1 receptors. Growth hormone (GH) mRNA hybridization signals were also not detected in the P2Y1 receptor-ir cells in the anterior pituitary (Fig. 1c). P2Y2 receptor-ir cells were distributed widely in the rat anterior pituitary, and some of the positive cells with long processes were strongly immunostained and angular in shape (Fig. 2). Double-labeling immunofluorescence showed that about 38% of P2Y2 receptor-ir cells were also immunoreactive for PRL, but none of them were immunoreactive for ACTH, FSH, or TSH. GH mRNA hybridization signals were also not detected in the P2Y2 receptor-ir cells in the anterior pituitary (Fig. 2c). About 61% of P2Y2 receptor-ir cells with strong immuno-labeling were also labeled by the S100 antibody in the anterior pituitary. Almost all of the S100-ir cells were also labeled by the P2Y2 antibody (Fig. 2f). The distribution pattern of P2Y4 receptor-ir cells was very similar with that of the P2Y1 receptor-ir cells. P2Y4 receptor-ir cells were also generally ovoid in shape with strong immunostaining (Fig. 3). Double-labeling immunofluorescence showed that almost all of the P2Y4 receptor-ir cells were also labeled by FSH or TSH antibodies, but none of them were labeled by ACTH, GH, PRL or S100 antibodies (Fig. 3a, c, d, f). There were about 29% FSH-ir endocrine cells and 36% TSH-ir endocrine cells, which were found not to express P2Y4 receptors, respectively (Fig. 3b, e). GH mRNA hybridization signals were not detected in the P2Y4 receptor-ir cells in the anterior pituitary (Fig. 3c). P2Y6 receptor-ir cells were also found in the rat anterior pituitary, but they were very small in size and scattered in the anterior pituitary. These positive cells were not labeled by ACTH, FSH, PRL, TSH or S100 antibodies, or GH oligonucleotide probes, but were labeled by ED1 (a macrophage marker) (Fig. 4a, b, c). No P2Y12 receptor-ir cells were detected in the rat pituitary (data not showed). P2Y13 receptor-ir cells were detected in the anterior and intermediate lobes of the rat pituitary. In the anterior lobe, the number of P2Y13 receptor-ir cells was relatively low. They were found in clusters formed by a few positive cells. These P2Y13 receptor-ir cells were not labeled by ACTH, FSH, PRL, TSH, ED1 or S100 antibodies or GH oligonucleotide probes. P2Y13 receptor-ir cells were also found in the intermediate lobe of the rat pituitary and these P2Y13 receptor-ir cells were also labeled by the ACTH antibody (Fig. 4d, e, f). Of the P2Y receptor family members examined in this study, only the P2Y13 receptor was found to be expressed in the intermediate lobe of the rat pituitary. Table 2 summarizes the expression of P2Y1, P2Y2 P2Y4, P2Y6, and P2Y13 receptors in the different cell types of the rat anterior pituitary cells. Tables 3, 4 and 5 summarize the data of the quantitative analysis of expressions of P2Y1, P2Y2, and P2Y4 receptors in gonadotrophs, lactotrophs, thyrotrophs and folliculo-stellate cells in the anterior pituitary. As P2Y1 and P2Y4 receptors were found to coexist with FSH or TSH, it implies that P2Y1 and P2Y4 receptors, and FSH and TSH may coexist in the same endocrine cells. As such, double-labeling immunostaining experiments were carried out to clarify this issue. The results showed that almost all of the P2Y1 receptor-ir cells were also labeled by the P2Y4 receptor antibody and vice versa (Fig. 5a, b, c); in addition, almost all of the FSH-ir cells were also labeled by the TSH antibody, and vice versa (Fig. 5d, e, f). Tables 6 and 7 summarize the data of the coexistence of P2Y1 and P2Y4 receptors, and FSH and TSH in endocrine cells of the rat anterior pituitary, respectively.
Fig. 1.
Expression of P2Y1 receptors and co-localization with pituitary hormones in the anterior pituitary of the rat. a Merged image of P2Y1 receptor-immunoreactive (ir) (red) and ACTH-ir (green) cells. Note that there are no colocalisation (yellow) of P2Y1 receptor- and ACTH-ir cells. b Merged image of P2Y1 receptor-ir (red) and FSH-ir cells (green). Note that many P2Y1 receptor-ir cells are labeled with FSH immunoreactivity. Colocalised cells are yellow/orange in color, such as the one indicated by a yellow arrow. Single-labeled FSH-ir and P2Y1-ir cells are also observed. A pink arrow and a blue arrow each indicate a single labeled FSH-ir and P2Y1-ir cell, respectively. c Merged image of P2Y1 receptor-ir (green) and GH mRNA (red) cells. Note that there is no colocalisation of P2Y1 receptor-ir and GH mRNA stained cells. d Merged image of P2Y1 receptor-ir (red) and PRL-ir cells (green). Note that there is no colocalisation of P2Y1 receptor- and PRL-immunoreactive cells. e Merge image of P2Y1 receptor-ir (red) and TSH-ir (green) cells. Note that many P2Y1 receptor-ir cells are also labeled with TSH-immunoreactivity. Double-labeled cells are yellow in color, such as the one indicated by a yellow arrow. Single labeled FSH-ir and P2Y1-ir cells are also observed. A pink arrow and a blue arrow each indicate a single labeled FSH-ir and P2Y1-ir cell, respectively. f Merged image of P2Y1 receptor-ir (red) and S100-ir (green) cells. Note that there were no cells doubled labeled (yellow) with P2Y1 receptor- and S100- immunoreactivity. All scale bars = 60 μm
Fig. 2.
Expression of P2Y2 receptors and co-localization with pituitary hormones in the anterior pituitary of the rat. a Merged image of P2Y2 receptor-immunoreactive (ir) (red) and ACTH-ir (green) cells. Note that there was no colocalisation between P2Y2 receptor- and ACTH-ir cells. b Merged image of P2Y2 receptor-ir (red) and FSH-ir cells (green). Note that there was no colocalisation between P2Y2 receptor- and FSH-ir cells. c Merged image of P2Y2 receptor-ir (green) and GH mRNA stained (red) cells. Note that no colocalised cells with P2Y2 receptors and GH mRNA signals are observed. d Merged image of P2Y2 receptor-ir (red) and PRL-ir cells (green). Note that some P2Y2 receptor-ir cells are also immunoreactive for PRL. One of the double-labeled cells (yellow in color) is indicated by a yellow arrow. Single-labeled FSH-ir and P2Y2-ir cells are also observed. A pink arrow and a blue arrow indicate a single labeled FSH-ir and P2Y2-ir cell, respectively. e Merged image of P2Y2-ir and TSH-ir cells. Note that there is no colocalisation. f a merged image of P2Y2 receptor-ir (red) and S100-ir (green) cells. Note that almost all of the S100-ir cells are also labeled with P2Y2 immunoreactivity, but a proportion of the P2Y2-ir cells are not labeled by S100 antibody, such as the one indicated by a red arrow. A yellow arrow indicates a double-labeled cell. All scale bars = 60 μm
Fig. 3.
Expression of P2Y4 receptors and co-localization with pituitary hormones in the anterior pituitary of the rat. a Merged image of P2Y4 receptor-ir (red) and ACTH-ir (green) cells. Note that no cells double-labeled (yellow) with P2Y4 receptor- and ACTH-immunoreactivity are observed. b Merged image of P2Y4 receptor-ir (red) and FSH-ir cells (green). Note that almost all P2Y4 receptor-cells are labeled with FSH immunoreactivity. Double-labeled cells are yellow in color, such as the one indicated by a yellow arrow. Single-labeled FSH-ir cells are also observed, such as the one indicated by a pink arrow. c Merged image of P2Y4 receptor-ir (green) and GH mRNA stained (red) cells. Note that there are no cells double labeled for P2Y4 receptors and GH mRNA signals. d Merged image of P2Y4 receptor-ir (red) and PRL-ir cells (green). Note that there are no cells double labeled with P2Y4 receptor- and PRL-immunoreactivity. e Merge image of P2Y4 receptor-ir (red) and TSH-ir (green) cells. Note that all P2Y4 receptor-ir cells are also labeled with TSH immunoreactivity. Double-labeled cells are yellow in color, such as the one indicated by a yellow arrow. Single-labeled FSH-ir cells are also observed, such as the one indicated by a pink arrow. f Merged image of P2Y4 receptor-ir (red) and S100-ir (green) cells. Note that there are no cells double labeled (yellow) with P2Y4 receptor- and S100 immunoreactivity. All scale bars = 60 μm
Fig. 4.
Expression of P2Y6 and P2Y13 receptors in the rat pituitary. a Expression of P2Y6 receptors in the anterior pituitary. Note that the positive cells are small in size. b ED1 expression in the same area of a. c Merged image of a and b. Note that all the P2Y6 receptor-ir cells are also labeled with ED1 immunoreactivity, such as the one indicated by a yellow arrow. d Expression of P2Y13 receptors in the intermediate lobe of the rat pituitary. Note that numerous positive cells are detected in the intermediate lobe, while there are only scattered positive cells in the anterior lobe. e ACTH expression in the same area of d. f Merged image of d and e. Note that almost all of the P2Y13 receptor-ir cells in the intermediate lobe are also labeled with ACTH immunoreactivity, such as the one indicated by a yellow arrow, but not labeled with ACTH immunoreactivity in the anterior lobe, such as the one indicated by a pink arrow. All scale bars = 60 μm
Table 2.
Coexistence between P2Y receptors and pituitary hormones or S100
| P2Y | ACTH | FSH | GH | PRL | TSH | S100 |
|---|---|---|---|---|---|---|
| P2Y1 | − | + | − | − | + | − |
| P2Y2 | − | − | − | + | − | + |
| P2Y4 | − | + | − | − | + | − |
| P2Y6 | − | − | − | − | − | − |
| P2Y12 | − | − | − | − | − | − |
| P2Y13 | − | − | − | − | − | − |
+ coexistence, − no coexistence
Table 3.
Quantitative analysis of the coexistence between P2Y1 receptor-ir cells and FSH, PRL, TSH and S100 (the positive cell number/0.36 mm2)
| P2Y+1 | P2Y1− | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| FSH+ | 46 ± 15 | 90 (p1) | 22 ± 8 | 32 (p9) |
| FSH− | 5 ± 3 | 10 (P2) | NA | NA |
| PRL+ | 0 | 0 (p3) | 52 ± 15 | 100 (p10) |
| PRL− | 43 ± 10 | 100 (p4) | NA | NA |
| TSH+ | 52 ± 16 | 93 (p5) | 28 ± 10 | 35 (p11) |
| TSH− | 4 ± 2 | 7 (p6) | NA | NA |
| S100+ | 0 | 0 (p7) | 36 ± 9 | 100 (p12) |
| S100− | 42 ± 12 | 100 (p8) | NA | NA |
P2Y+1FSH+, P2Y1 receptor-ir cells also expressing FSH; P2Y1+FSH−, P2Y1 receptor-ir cells not expressing FSH; P2Y1−FSH+, FSH-ir cells not expressing P2Y1; P2Y1−FSH−, cells expressing neither P2Y1 receptors nor FSH; NA, not assayed; p1 = number of P2Y+1FSH+/total number of P2Y+1 cells × 100; p2 = the number of P2Y+1FSH−/total number of P2Y+1 cells × 100; p3 = the number of P2Y+1PRL+/total number of P2Y+1 cells × 100; p4 = number of P2Y+1PRL−/total number of P2Y+1 cells × 100; p5 = number of P2Y+1TSH+/total number of P2Y+1 cells × 100; p6 = number of P2Y+T1TSH−/total number of P2Y+1 cells × 100; p7 = number of P2Y+1S100+/total number of P2Y+1 cells × 100; p8 = the number of P2Y+1S100–/total number of P2Y+1 cells × 100; p9 = number of FSH+ P2Y−1/total number of TSH+ cells × 100; p10 = number of PRL+P2Y1−/total number of PRL+ cells × 100; p11 = number of P2Y1−TSH+ cells/total number of TSH+ cells × 100; p12 = number of P2Y1−S100+ cells/total number of S100+ cells × 100
Table 4.
Quantitative analysis of the coexistence of P2Y2 receptor-ir cells and FSH, PRL, TSH and S100 (positive cell number/0.36 mm2)
| P2Y+2 | P2Y2− | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| FSH+ | 0 | 0 (p1) | 73 ± 25 | 100 (p9) |
| FSH− | 63 ± 21 | 100 (p2) | NA | NA |
| PRL+ | 21 ± 8 | 38 (p3) | 37 ± 11 | 64 (p10) |
| PRL− | 34 ± 10 | 62 (p4) | NA | NA |
| TSH+ | 0 | 0 (p5) | 75 ± 26 | 100 (p11) |
| TSH− | 59 ± 19 | 100 (p6) | NA | NA |
| S100+ | 38 ± 15 | 61 (p7) | 0 | 0 (p12) |
| S100− | 24 ± 7 | 39 (p8) | NA | NA |
P2Y+2FSH+, P2Y2 receptor-ir cells also expressing FSH; P2Y2+FSH−, P2Y2 receptor-ir cells not expressing FSH; P2Y2−FSH+, FSH-ir cells not expressing P2Y2; P2Y2−FSH−, cells expressing neither P2Y2 receptor nor FSH; NA, not assayed; p1 = number of P2Y+2FSH+/total number of P2Y+2 cells × 100; p2 = number of P2Y+2FSH−/total number of P2Y+2 cells × 100; p3 = number of P2Y+2PRL+/total number of P2Y+2 cells × 100; p4 = the number of P2Y+2PRL−/total number of P2Y+2 cells × 100; p5 = number of P2Y+2TSH+/total number of P2Y+2 cells × 100; p6 = number of P2Y+2TSH−/total number of P2Y+2 cells × 100; p7 = number of P2Y+2S100+/total number of P2Y+2 cells × 100; p8 = number of P2Y+2S100−/total number of P2Y+2 cells × 100; p9 = number of FSH+ P2Y2−/total number of TSH+ cells × 100; p10 = number of PRL+P2Y2−/total number of PRL+ cells × 100; p11 = number of P2Y2−TSH+ cells/total number of TSH+ cells × 100; p12 = number of P2Y2−S100+/total number of S100+ cells × 100
Table 5.
Quantitative analysis of the coexistence between P2Y4 receptor-ir cells and FSH, PRL, TSH and S100 (positive cell number/0.36 mm2)
| P2Y+4 | P2Y4− | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| FSH+ | 52 ± 17 | 95 (p1) | 21 ± 7 | 29 (p9) |
| FSH− | 3 ± 2 | 5 (p2) | NA | NA |
| PRL+ | 0 | 0 (p3) | 47 ± 15 | 100 (p10) |
| PRL− | 62 ± 18 | 100 (p4) | NA | NA |
| TSH+ | 56 ± 21 | 97 (p5) | 31 ± 14 | 36 (p11) |
| TSH− | 2 ± 1 | 3 (p6) | NA | NA |
| S100+ | 0 | 0 (p7) | 42 ± 12 | 100 (p12) |
| S100− | 49 ± 22 | 100 (p8) | NA | NA |
P2Y+4FSH+, P2Y4 receptor-ir cells also expressing FSH; P2Y+4FSH−, P2Y4 receptor-ir cells not expressing FSH; P2Y4−FSH+, FSH-ir cells not expressing P2Y4; P2Y4−FSH−, expressing neither P2Y4 receptor-ir nor FSH-ir cells; NA, not assayed; p1 = number of P2Y+4FSH+/total number of P2Y+4 cells × 100; p2 = number of P2Y+4FSH−/total number of P2Y+4 cells × 100; p3 = number of P2Y+4PRL+/total number of P2Y+4 cells × 100; p4 = number of P2Y+4PRL−/total number of P2Y+4 cells × 100; p5 = number of P2Y+4TSH+/total number of P2Y+4 cells × 100; p6 = number of P2Y+4TSH−/total number of P2Y+4 cells × 100; p7 = number of P2Y+4S100+/total number of P2Y+4 cells × 100; p8 = number of P2Y+4S100−/total number of P2Y+4 cells × 100; p9 = number of FSH+ P2Y4−/total number of TSH+ cells × 100; p10 = number of PRL+P2Y4−/total number of PRL+ cells × 100; p11 = number of P2Y4−TSH+ cells/total number of TSH+ cells × 100; p12 = number of P2Y4−S100+ cells/total number of S100+ cells × 100
Fig. 5.
Colocalization of P2Y11 and P2Y4 receptors, and FSH and TSH in the rat anterior pituitary. a Expression of P2Y1 receptors in the anterior pituitary. b P2Y4 expression in the same area of a. c Merged image of a and b. Note that most of the P2Y1 receptor-ir cells are also labeled with P2Y4 immunoreactivity, such as the one indicated by a yellow arrow, but single P2Y1 and P2Y4 receptor-ir cells are also observed, such as the ones indicated by a pink arrow and a blue arrow, respectively. d Expression of FSH in the anterior pituitary. e TSH expression in the same area of d. f Merged image of d and e. Note that most of the FSH-ir cells are also labeled with TSH immunoreactivity, such as the one indicated by a yellow arrow, but single FSH and TSH-ir cells are also observed, such as the ones indicated by a pink arrow and a blue arrow, respectively. All scale bars = 60 μm
Table 6.
Quantitative analysis of the coexistence between P2Y1 and P2Y4 receptors in the rat anterior pituitary (positive cell number/0.36 mm2)
| P2Y+1 | P2Y1− | |||
|---|---|---|---|---|
| Number | % | Number | % | |
| P2Y+4 | 42 ± 12 | 93 (p1) | 4 ± 2 | 91 (p3) |
| P2Y4− | 3 ± 1 | 7 (p2) | NA | NA |
P2Y+1P2Y+4, P2Y1 receptor-ir cells also expressing P2Y4 receptors; P2Y1+P2Y4−, P2Y1 receptor-ir cells not expressing P2Y4−; P2Y1−P2Y4+, P2Y4-ir cells not expressing P2Y1 receptors; P2Y1− P2Y4−, cells expressing neither P2Y1 nor P2Y4 receptors; NA, not assayed; p1 = number of P2Y+1P2Y+4/total number of P2Y+1 cells × 100; p2 = number of P2Y+1P2Y4−/total number of P2Y+1 cells × 100; p3 = number of P2Y1−P2Y+4/total number of P2Y+4 cells × 100
Table 7.
Quantitative analysis of the coexistence between FSH and TSH in the rat anterior pituitary (positive cell number/0.36 mm2)
| FSH+ | FSH− | |||
|---|---|---|---|---|
| Number | % | Number | %# | |
| TSH+ | 59 ± 17 | 94 (p1) | 5 ± 3 | 92 (p3) |
| TSH− | 4 ± 2 | 6 (p2) | NA | NA |
FSH+TSH+, FSH-ir cells also expressing TSH; FSH+TSH−, FSH-ir cells not expressing TSH; FSH−TSH+, TSH-ir cells not expressing FSH; FSH−TSH−, cells expressing neither FSH nor TSH; NA, not assayed; p1 = number of FSH+TSH+ cells/total number of FSH+ cells × 100; p2 = the number of FSH+TSH− cells/total number of FSH+ cells × 100; p3 = number of FSH−TSH+ cells/total number of TSH+ cells × 100
Western blotting, performed on tissue extracts derived from the rat pituitary (Fig. 6a), assessed the specificity of the polyclonal P2Y receptor antibodies. The immunoreactive bands were detected at different molecular weight levels: one band for the P2Y1 receptor at about 48 kDa (Fig. 6a, lane 1), two bands for the P2Y2 receptor at about 36, 90 kDa (Fig. 6a, lane 3), one band for the P2Y4 at about 49 kDa (Fig. 6a, lane 5), one band for the P2Y13 receptor at about 37 kDa (Fig. 6a, lane 9), but no clear bands for P2Y6 and P2Y12 receptors were found (Fig. 6a, lanes 7 and 8). Preadsorption of the antiserums with the peptide antigens resulted in the absence of the bands (Fig. 6a, lanes 2, 4, 6 and 10), indicating that the antibodies detected the appropriate antigen sequences.
Fig. 6.
Results of Western blotting (WB) and control experiments. a WB results. The color ladder is the molecular marker of protein; lanes 1, 3, 5, 7, 8, 9 are the results of P2Y1, P2Y2, P2Y4, P2Y6, P2Y12, and P2Y13 WB, respectively. Note that immunoreactive bands were detected at different molecular weight levels: one band for P2Y1 receptors at about 48 kDa (lane 1), two bands for P2Y2 receptors at about 36, 90 kDa (lane 3), one band for P2Y4 at about 49 kDa (lane 5), no clear band for P2Y6 or P2Y12 receptors (lane 7, lane 8), one band for P2Y13 at about 37 kDa (lane 9); lanes 2, 4, 6 and 10 are the results of P2Y1, P2Y2, P2Y4, and P2Y13 peptide pre-absorption experiments. Note that pre-adsorption of the antiserums with the peptide antigens resulted in the absence of bands (lanes 2, 4, 6, 10). Control experiments were carried out with the P2Y antibodies pre-absorbed with their respective peptides. No staining was observed in those preparations incubated with antisera solutions pre-absorbed with their respective peptides, as shown for P2Y2 and P2Y4 receptor antisera (b, c). Scale bars = 60 μm
Control experiments were carried out with the P2Y antibodies preabsorbed with their own peptides. No staining was observed in those preparations incubated with antisera solutions preabsorbed with their own peptides, as shown for the P2Y1 and P2Y2 receptors (Fig. 6b, c). A further negative control, of omitting the primary antibodies, was also carried out. No staining was also observed in those preparations.
Discussion
The present study showed that corticotroph, gonadotroph, somatotroph, lactotroph, thyrotroph and follico-stellate cells in the anterior pituitary express at least one of the P2Y receptor family: the corticotroph, somatotroph, lactotroph and follico-stellate cells express P2Y2 receptors; gonadotroph and thyrotroph express P2Y1, P2Y2 and P2Y4 receptors. The identity of a small subpopulation of cells with P2Y13 receptors in the rat anterior pituitary is confirmed in this study. The results presented in this study are credible as strict control experiments were carried out. Firstly, no immunostaining in the pituitary sections was obtained in the control experiments where the P2Y antibodies were preabsorbed with their corresponding peptides or by omitting the P2Y antibodies. Secondly, preadsorption of the P2Y receptor antiserums with their corresponding peptide antigens resulted in the absence of bands, indicating that the antibody detects the appropriate antigen sequence, further confirming the specificity of these P2Y receptors.
There are eight receptors of the P2Y family that have been cloned, namely P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14, all of which are G protein-coupled receptors. There are two subgroups of G proteins which P2Y receptors are coupled with, namely Gq and Gi. P2Y1, P2Y2, P2Y4 and P2Y6 receptors are linked to activation of phospholipase C (PLC), inositol lipid signaling and the mobilization of intracellular Ca2+, P2Y12, P2Y13 and P2Y14 receptors are coupled with Gi protein resulting in inhibition of cAMP formation and P2Y11 (only in human) are coupled with Gs and Gq, resulting in increased cAMP [21]. Within the family of P2Y receptors, P2Y1, P2Y12 and P2Y13 receptors respond to ADP. P2Y2 and P2Y4 receptors respond to both ATP and UTP, and P2Y6 receptors to UDP [1]. One question must be asked, where are the endogenous purines (ATP and ADP) and pyrimidines (UTP and UDP) released from in the anterior pituitary? Currently, the physiological sources of extracellular purines and pyrimidines required for activation of purinergic receptors in pituitary cells remains unknown.
In general, neurons, neuroendocrine cells and platelets release ATP by Ca2+-controlled exocytosis of nucleotides stored within synaptic vesicles or dense core granules. The magnocellular neuroendocrine cells in the hypothalamus, that control release of vasopressin and oxytocin, also contain secretory ATP and could control the release of ATP [22]. Recently, the data was presented showing that ATP is also released by normal and immortalized anterior pituitary cells at resting conditions. Such basal ATP release was enhanced in cells simulated by ARL67156, an inhibitor of ectonucleotidases [13]. GnRH-induced stimulation of gonadotropin release was accompanied by elevation in basal ATP release, raising the possibility that ATP is stored in the secretory vesicles of these cells [2]. This is consistent with an earlier study showing calcium dependence of ATP release [23]. The roles of ATP as a neurotransmitter or co-transmitter are well established in the peripheral and central nervous systems [24]. Several reports have also suggested that ATP is secreted by skeletal muscle, adrenal chromaffin cells, mast cells, blood cells, fibroblasts and endothelial cells, pyrimidines are secreted by endothelial, epithelial and astrocytoma cells [24, 25]. The duration and distance of ATP actions are limited by several ecto-ATPases, which ensure that circulating levels of ATP are below that required for the global activation of purninceptors [7]. These previous data suggested that purines and pyrimidines could be released by the anterior pituitary cells and act as an autocrine/paracrine factor to be involved in the regulation of physiological functions of anterior pituitary endocrine cells.
Rat gonadotrophs show a potency order of ATP = ADP = UTP, suggesting the presence of P2Y1, P2Y12, P2Y13 (ADP) and P2Y2 and P2Y4 (ATP = UTP) receptors [26]. UTP-sensitive receptors have been suggested to be present in gonadotrophs [16, 26] and lactotrophs [17], but these results were not confirmed by other reports [2, 14]. The present results showed that gonadotrophs and lactotrophs express P2Y4 and P2Y2 receptors, respectively, the ligand for which is UTP, confirming that gonadotrophs and lactotrophs express UTP-sensitive receptors.
In this study, 91% and 95% of P2Y1 receptor-ir cells in the rat anterior pituitary were labeled with FSH and TSH immunoreactivity, respectively. Almost all the P2Y4 receptor-ir cells were labeled with FSH and TSH immunoreactivity. This result implies that at least a subpopulation of gonadotrophs and thyrotrophs co-express FSH and TSH in the rat anterior pituitary. In order to confirm this, we studied the coexistence of FSH and TSH in the anterior pituitary and found that almost all the gonadotrophs labeled by the FSH antibody were also labeled by the TSH antibody, which means that FSH and TSH coexist in the same endocrine cells in the anterior pituitary. A report showing that FSH and TSH coexist in the same endocrine cells of the musk shrew anterior pituitary has been published [27]. To our knowledge, this study is the first report that FSH and TSH coexist in the same endocrine cells of the rat pituitary. As the majority of gonadotrophs or thyrotrophs were found to express P2Y1 or P2Y4 receptors, this implies that P2Y1 and P2Y4 receptors may be co-expressed in a subpopulation of gonadotrophs and thyrotrophs. Further, double-labeling immunostaining confirmed that P2Y1 and P2Y4 receptors actually coexpress in the same endocrine cells in the anterior pituitary of the rat. As the endogenous ligands for P2Y1 and P2Y4 receptors are ADP and UTP, purines and pyrimidines may act on gonadotrophs and thyrotrophs at the same time if P2Y1 and P2Y4 receptors were functionally expressed in these cells. This suggestion needs to be confirmed.
The folliculo-stellate cells are glia-like cells in the anterior pituitary, which express nervous tissue-specific S100 protein [28]. The physiological functions of the folliculo-stellate cells are heterogeneous. They are involved in regulating the activity of the pituitary endocrine cells, act as immune cells such as macrophages and dendritic cells, and represent an adult stem cell population of the pituitary [29]. Previous data showed that both ATP and UTP increased the intracellular Ca2+ concentration of primary cultured folliculo-stellate cells of the pituitary in a concentration-dependent manner in a range between 0.1 and 10 μM. The response was completely suppressed by thapsigargin, an inhibitor of endoplasmic reticulum Ca2+-ATPase, and was significantly suppressed by U-73122, an inhibitor of PLC. These results indicate that ATP increases the intracellular Ca2+ concentration of folliculo-stellate cells by activating PLC via P2Y2 receptors [15]. In this study, strong immunostaining signals for P2Y2 receptors were detected in the folliculo-stellate cells that were also stained with S100 in sections of the rat anterior pituitary. This result further confirmed the previous report that ATP/UTP, via P2Y2 receptors released by autocrine or paracrine mechanisms, is involved in modulating the activity of folliculo-stellate cells.
In conclusion, the present study has shown that P2Y receptors are widely expressed in the anterior pituitary. P2Y1 and P2Y4 receptors are found to be expressed in the majority of gonadotrophs and thyrotrophs, P2Y2 receptors are expressed in a small subpopulation of lactotrophs and almost all of the folliculo-stellate cells that are stained with S100 protein. The identity of a small subpopulation of P2Y13 receptor-ir cells in the rat anterior pituitary still needs to be confirmed. The present results show that P2Y1 and P2Y4 receptors are co-expressed in a subpopulation of gonadotrophs and thyrotrophs. Purines and pyrimidines may act on gonadotrophs and thyrotrophs at the same time via P2Y1 and P2Y4 receptors. This result further confirmed the previous report that ATP/UTP, via P2Y2 receptors released by an autocrine or paracrine mechanism, is involved in modulating the activity of folliculo-stellate cells.
Footnotes
Q. Yu and W. Guo contributed equally to this work.
References
- 1.Burnstock G. Purine and pyrimidine receptors. Cell Mol Life Sci. 2007;64:1471–1483. doi: 10.1007/s00018-007-6497-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tomic M, Jobin RM, Vergara LA, Stojilkovic SS. Expression of purinergic receptor channels and their role in calcium signaling and hormone release in pituitary gonadotrophs. Integration of P2 channels in plasma membrane- and endoplasmic reticulum-derived calcium oscillations. J Biol Chem. 1996;271:21200–21208. doi: 10.1074/jbc.271.35.21200. [DOI] [PubMed] [Google Scholar]
- 3.Nunez L, Villalobos C, Frawley LS. Extracellular ATP as an autocrine/paracrine regulator of prolactin release. Am J Physiol. 1997;272:E1117–E1123. doi: 10.1152/ajpendo.1997.272.6.E1117. [DOI] [PubMed] [Google Scholar]
- 4.Koshimizu TA, Tomic M, Wong AO, Zivadinovic D, Stojilkovic SS. Characterization of purinergic receptors and receptor-channels expressed in anterior pituitary cells. Endocrinology. 2000;141:4091–4099. doi: 10.1210/en.141.11.4091. [DOI] [PubMed] [Google Scholar]
- 5.Villalobos C, Alonso-Torre SR, Nunez L, Garcia-Sancho J. Functional ATP receptors in rat anterior pituitary cells. Am J Physiol. 1997;273:C1963–C1971. doi: 10.1152/ajpcell.1997.273.6.C1963. [DOI] [PubMed] [Google Scholar]
- 6.Chung HS, Park KS, Cha SK, Kong ID, Lee JW. ATP-induced [Ca(2+)](i) changes and depolarization in GH3 cells. Br J Pharmacol. 2000;130:1843–1852. doi: 10.1038/sj.bjp.0703253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stojilkovic SS, Koshimizu T. Signaling by extracellular nucleotides in anterior pituitary cells. Trends Endocrinol Metab. 2001;12:218–225. doi: 10.1016/S1043-2760(01)00387-3. [DOI] [PubMed] [Google Scholar]
- 8.Zemkova H, Balik A, Jiang Y, Kretschmannova K, Stojilkovic SS. Roles of purinergic P2X receptors as pacemaking channels and modulators of calcium-mobilizing pathway in pituitary gonadotrophs. Mol Endocrinol. 2006;20:1423–1436. doi: 10.1210/me.2005-0508. [DOI] [PubMed] [Google Scholar]
- 9.Zemkova H, Kucka M, Li S, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Characterization of purinergic P2X4 receptor channels expressed in anterior pituitary cells. Am J Physiol Endocrinol Metab. 2010;298:E644–E651. doi: 10.1152/ajpendo.00558.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Merwe PA, Wakefield IK, Fine J, Millar RP, Davidson JS. Extracellular adenosine triphosphate activates phospholipase C and mobilizes intracellular calcium in primary cultures of sheep anterior pituitary cells. FEBS Lett. 1989;243:333–336. doi: 10.1016/0014-5793(89)80156-5. [DOI] [PubMed] [Google Scholar]
- 11.Davidson JS, Wakefield IK, Sohnius U, Merwe PA, Millar RP. A novel extracellular nucleotide receptor coupled to phosphoinositidase-C in pituitary cells. Endocrinology. 1990;126:80–87. doi: 10.1210/endo-126-1-80. [DOI] [PubMed] [Google Scholar]
- 12.Chen ZP, Kratzmeier M, Poch A, Xu S, McArdle CA, Levy A, Mukhopadhyay AK, Lightman SL. Effects of extracellular nucleotides in the pituitary: adenosine triphosphate receptor-mediated intracellular responses in gonadotrope-derived alpha T3-1 cells. Endocrinology. 1996;137:248–256. doi: 10.1210/en.137.1.248. [DOI] [PubMed] [Google Scholar]
- 13.He ML, Gonzalez-Iglesias AE, Tomic M, Stojilkovic SS. Release and extracellular metabolism of ATP by ecto-nucleotidase eNTPDase 1–3 in hypothalamic and pituitary cells. Purinergic Signal. 2005;1:135–144. doi: 10.1007/s11302-005-6208-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.He ML, Gonzalez-Iglesias AE, Stojilkovic SS. Role of nucleotide P2 receptors in calcium signaling and prolactin release in pituitary lactotrophs. J Biol Chem. 2003;278:46270–46277. doi: 10.1074/jbc.M309005200. [DOI] [PubMed] [Google Scholar]
- 15.Uchiyama M, Nakajima Y, Sakuma Y, Kato M. Purinergic regulation of intracellular Ca2+ concentration of rat pituitary folliculo-stellate cells in primary culture. J Neuroendocrinol. 2001;13:378–385. doi: 10.1046/j.1365-2826.2001.00639.x. [DOI] [PubMed] [Google Scholar]
- 16.Chen ZP, Krull N, Xu S, Levy A, Lightman SL. Molecular cloning and functional characterization of a rat pituitary G protein-coupled adenosine triphosphate (ATP) receptor. Endocrinology. 1996;137:1833–1840. doi: 10.1210/en.137.5.1833. [DOI] [PubMed] [Google Scholar]
- 17.Carew MA, Wu ML, Law GJ, Tseng YZ, Mason WT. Extracellular ATP activates calcium entry and mobilization via P2U-purinoceptors in rat lactotrophs. Cell Calcium. 1994;16:227–235. doi: 10.1016/0143-4160(94)90025-6. [DOI] [PubMed] [Google Scholar]
- 18.Teramoto N, Szekely L, Pokrovskaja K, Hu LF, Yoshino T, Akagi T, Klein G. Simultaneous detection of two independent antigens by double staining with two mouse monoclonal antibodies. J Virol Meth. 1998;73:89–97. doi: 10.1016/S0166-0934(98)00048-2. [DOI] [PubMed] [Google Scholar]
- 19.Xiang Z, Burnstock G. Distribution of P2Y2 receptors in the guinea pig enteric nervous system and its coexistence with P2X2 and P2X3 receptors, neuropeptide Y, nitric oxide synthase and calretinin. Histochem Cell Biol. 2005;124:379–390. doi: 10.1007/s00418-005-0043-7. [DOI] [PubMed] [Google Scholar]
- 20.Xiang Z, He C, Burnstock G. P2X5 receptors are expressed on neurons containing arginine vasopressin and nitric oxide synthase in the rat hypothalamus. Brain Res. 2006;1099:56–63. doi: 10.1016/j.brainres.2006.04.126. [DOI] [PubMed] [Google Scholar]
- 21.Abbracchio MP, Burnstock G, Boeynaems J-M, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology. Update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Troadec JD, Thirion S. Multifaceted purinergic regulation of stimulus-secretion coupling in the neurohypophysis. Neuroendocrinol Lett. 2002;23:273–280. [PubMed] [Google Scholar]
- 23.Chen ZP, Kratzmeier M, Levy A, McArdle CA, Poch A, Day A, Mukhopadhyay AK, Lightman SL. Evidence for a role of pituitary ATP receptors in the regulation of pituitary function. Proc Natl Acad Sci USA. 1995;92:5219–5223. doi: 10.1073/pnas.92.11.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnstock G. Cotransmission. Curr Opin Pharmacol. 2004;4:47–52. doi: 10.1016/j.coph.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 25.Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- 26.Chen ZP, Levy A, McArdle CA, Lightman SL. Pituitary ATP receptors: characterization and functional localization to gonadotropes. Endocrinology. 1994;135:1280–1283. doi: 10.1210/en.135.3.1280. [DOI] [PubMed] [Google Scholar]
- 27.Hirano N, Shiino M. Co-existence of gonadotrophins (FSH, LH) and thyrotrophin (TSH) in single anterior pituitary cells of the musk shrew, Suncus murinus. Cell Tissue Res. 1993;272:315–320. doi: 10.1007/BF00302736. [DOI] [PubMed] [Google Scholar]
- 28.Nakajima T, Yamaguchi H, Takahashi K. S100 protein in folliculostellate cells of the rat pituitary anterior lobe. Brain Res. 1980;191:523–531. doi: 10.1016/0006-8993(80)91300-1. [DOI] [PubMed] [Google Scholar]
- 29.Allaerts W, Vankelecom H. History and perspectives of pituitary folliculo-stellate cell research. Eur J Endocrinol. 2005;153(1):1–12. doi: 10.1530/eje.1.01949. [DOI] [PubMed] [Google Scholar]