Abstract
Erythema multiforme (EM) is an interesting dermatologic disease which has oral manifestations. EM is clinically characterized by a “minor” form and a “major” form. It presents a diagnostic dilemma because the oral cavity has the ability to produce varied manifestations. Infections (particularly herpes simplex and mycoplasma pneumonia) and drugs seem to predispose toward the development of EM. The range of possible etiologies for oral disease is immense. Therefore, an otolaryngologist or a dentist while treating such patients should have a differential diagnosis for all oral lesions. We report a case of erythema multiforme in which alcohol (ethanol) seems to be the precipitating factor and have also reviewed the English literature in the present context.
Keywords: Erythema multiforme, Mucocutaneous disorders, Drug reactions, Ethanol
Introduction
Erythema multiforme (EM) is a rare acute mucocutaneous condition caused by a hypersensitivity reaction with the appearance of cytotoxic T lymphocytes in the epithelium that induce apoptosis in keratinocytes, which leads to satellite cell necrosis [1]. Despite being often caused by, or at least associated with, infection or drug therapy, the pathogenic mechanism of EM remains unclear, and as a consequence there are no evidence-based, reliably effective therapies. EM and related disorders comprise a group of mucocutaneous disorders characterized by variable degrees of mucosal and cutaneous blistering and ulceration that occasionally can give rise to systemic upset and possibly compromise life. Vesiculobullous diseases are frequently encountered by a practicing dermatologist. However, the oral cavity may be overlooked as a source of diagnostic information. Oral manifestations of vesiculobullous diseases may occur independently or precede cutaneous involvement. In such situations, a patient presenting with acute oral cavity mucosal ulceration and blistering condition either to an otolaryngologist or a dentist needs to be carefully managed.
The present article reviews aspects of EM as relevance to otolaryngology and dental practice and highlights the associated potential etiologic agents, pathogenic mechanisms and therapies.
Case Report
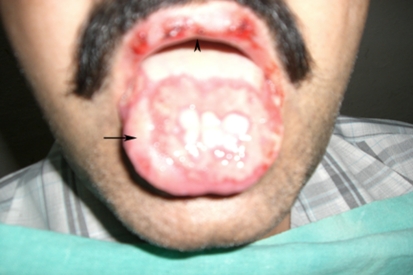
A 40-year old male patient presented to the out patient department of our institute with complaints of painful oral ulceration. History revealed that complaints started 3–4 days back. Initially to start with, there was redness in the oral cavity and over lips. Soon bleeding ulcers and bullae appeared at these sites. Bullae ruptured to form encrustations over lips. Odynophagia and dysarthria was present. No history of febrile episode was present. There was no history of drug intake before the onset of these lesions. No other mucosal surface involvement history was present. Only positive history was that patient was a chronic alcoholic and had drinking episode in which he had mixed different brands of alcohol, a day before start of his complaints. On clinical examination, dark brown encrustations were present on lips. Lips were edematous and erythema was present around encrustations. Bleeding ulcers were present on dorsum of tongue, hard palate, buccal mucosa, and gingivae. Few hyperemic papules and macules were also present (Fig. 1). Pharyngeal and laryngeal examination was normal. No neck nodes were palpable. A diagnostically significant finding was the presence of two target lesions on the palmar surface of left hand (Fig. 2). Other systemic examination was normal.
Fig. 1.
Photograph of the patient showing hemorrhagic bullae with ulcers over dorsum of tongue (arrow) and brown crusts over upper lip (arrowhead)
Fig. 2.
Photograph of the palmar surface of the left hand showing characteristic target lesions (arrows)
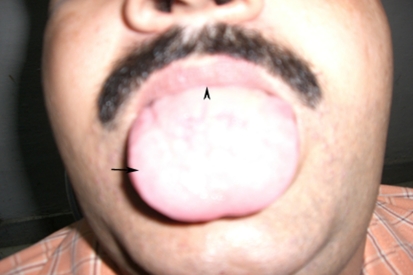
Routine hematological investigations were within normal range. Fasting ESR was 25 mm in first hour by Westergreen method. Liver function tests revealed slightly raised transaminases. Investigations for hepatitis B and C, and HIV were negative. Biopsy from the lesions on histopathological examination revealed intracellular edema, liquefaction degeneration in epithelial layer, dilatation of dermal capillaries and inflammatory cell infiltrate consisting predominantly of lymphocytes, neutrophils, and eosinophils. Clinically, diagnosis of erythema multiforme was made. Oral methylprednisolone at the dose of 32 mg/day was started. Within 5 days, all the mucosal lesions healed (Fig. 3) and methylprednisolone was stopped after tapering over next 7 days. Mouthwashes consisting of local anesthetics and antiseptics were added for symptomatic treatment. Patient was advised to abstain from alcohol during treatment period. After 10 days of treatment, while patient was still on oral steroids, patient again had a drinking episode in which he again mixed different brands. He had flare up of his oral cavity lesions. Oral steroids were increased in dosage and tapered once all lesions healed. Total alcohol abstinence was advised and 6 months into follow-up, there has been no recurrence.
Fig. 3.
Photograph of the patient 5 days post oral steroid treatment showing all lesions have healed
Discussion
EM has been classified into a number of variants, mainly minor and major forms. EM minor (EMm) is mainly a cutaneous disease. Typical and/or raised atypical target lesions are the hallmark. Disease involves less than 10% of the body surface area. Lesions are often symmetric in distribution, with a predilection for the extensor surfaces of the extremities. Mucosal involvement is uncommon, when present, only one site is affected, most commonly the mouth. EMm that only affects the oral mucosa may occasionally arise. EM major (EMM) typically involves two or more mucous membranes with more variable skin involvement. Symmetrically distributed typical cutaneous target lesions and/or atypical raised target lesions are the hallmark. Less than 10% of the body surface area but more severe than EMm is involved. Oral lesions are usually widespread and severe [2].
The oral manifestations of the spectrum of EM range from tender superficial erythematous and hyperkeratotic plaques to painful deep hemorrhagic bullae and erosions. The oral lesions initially manifest with edema, erythema, and erythematous macules of the lips and buccal mucosa, followed by the development of multiple vesicles and bullae that quickly rupture and result in pseudomembrane formation. The lips tend to become swollen and show diagnostically distinctive bloody excrustations (Fig. 1). Intact vesicles are rarely observed because they rapidly breakdown to form illdefined ulcers. In EMm, there is usually a mild extension of erythematous patches or superficial erosions of the oral mucosa and the lip. Target lesions may be seen on the lip but rarely on the intraoral mucosa [3]. The oral mucosa is the most commonly involved mucosal surface but any mucosal site can be affected in the course of EMM, including the epithelium of the trachea, bronchi or gastrointestinal tract [4, 5].
In EMM oral lesions are larger than that of EMm and in more than 50% of cases patients have ulceration of all mucosal surfaces. Multiple papules and vesicles are preceded by erythematous macules. The vesicles tend to rupture to leave multiple areas of superficial irregular erosions that are usually covered by a yellow fibrinous pseudomembrane [6]. Eventually, multiple, large, shallow, irregular, painful ulcers surrounded by an erythematous margin and covered by whitish plaques of desquamated epithelium occurs. They usually affect the lingual, buccal, and/or labial mucosa, and less frequently the floor of the mouth, palate and the gingivae. Affected patients may have trismus, dysphonia, dysarthria, and/or dysphagia. The oral lesions of EMM usually heal without scarring, and in some instances there can be hyperkeratotic plaques mixed with erythematous areas [3]. Other mucosal surfaces including ocular, nasal, pharyngeal, laryngeal, lower respiratory, and anogenital may be involved. Scarring sequelae from ocular and pharyngeal involvement cause morbidity. The oral EM variant is an under recognized form of EM. Most patients have chronic or recurrent oral lesions only, but one-third have oral and lip lesions and one quarter have oral, lip, and skin lesions. This variant is a reaction pattern similar to EMm and EMM [3].
Within the clinical spectrum of EM, two subgroups have been recently identified: recurrent EM and persistent EM [7]. In recurrent EM, multiple relapses occur every year. Mucosal involvement is present in only a minority of patients. Each attack lasts approximately 14 days, as in classic EM. Continuous or persistent EM is characterized by the uninterrupted occurrence of both typical and atypical target lesions. Lesions are often papulonecrotic or bullous and are widespread. These cases are exceedingly rare.
EM is a disorder that reacts primarily to antigens that are induced by exposure to microbes or drugs [3], and has been reported to be triggered by numerous microbial agents, particularly viruses, mainly herpes simplex virus (HSV), which is implicated in upto 70% of recurrent cases [8]. In one study, it has been reported that 71% of the EMm/EMM attacks were precipitated by a preceding HSV infection [9], particularly herpes labialis [10]. Typically, EMm/EMM lesions develop 10–14 days following clinical manifestations of HSV infection [11]. Aside from HSV infection, a wide range of other associations have been reported (Table 1; [12–14])
Table 1.
Most common associations with erythema multiforme
| 1. Micro-organisms |
| Viruses: herpes viruses (HSV, VZV, EBV), adenoviruses, enteroviruses, hepatitis viruses (A, B and C), HIV, influenza |
| Bacteria: Mycoplasma pneumoniae, Chlamydia, Corynebacterium diphtheria, Hemolytic streptococci, Legionellosis |
| Fungi and parasites: coccidioidomycosis, dermatophytes, histoplasmosis, sporotrichosis |
| 2. Drugs: allopurinol, barbiturates, cancer chemotherapeutic agents, carbamazepine, cephalosporins, non-steroidal anti-inflammatory drugs, penicillins, phenytoin, protease inhibitors |
| 3. Food additives or chemicals: benzoates, nitrobenzene, terpenes, ethanol |
| 4. Immune and other conditions: graft versus host disease, immunisation (BCG, hepatitis B), inflammatory bowel disease, pregnancy, sarcoidosis, systemic lupus erythematous |
The exact pathogenesis of EMm/EMM is unknown. It has been suggested that an immunologically mediated (i.e., lymphocytic) reaction to an infectious agent or drug leads to skin and mucosal lesions concentrated at the dermal-epithelial junction. In Herpes associated EM it is most likely that HSV-DNA fragments in the skin or mucosa precipitate the disease [15]. CD34+ cells transport fragments of HSV to the epithelium, and T cells accumulate in response to HSV antigens and damage cells. In contrast, drug-associated EM seems to involve CD8+ T-cell attack and expression of tumor necrosis factor alpha (TNFα) in lesional skin in the absence of HSV-DNA [16].
There is no reliable laboratory based mean of definitively diagnosing EM. The clinical appearance of diffuse and widespread oral ulceration can be difficult to differentiate from other vesiculobullous disorders such as pemphigus or pemphigoid. EM should also be differentiated from viral stomatitides and toxic epidermal necrolysis [3]. Diagnosis usually entails excluding other similar diseases by careful review of the clinical history and detailed clinical examination. Features more suggestive of EM are the acute onset (or recurrent nature), oral lesions typically located on the lip and anteriorly in the mouth, and pleomorphic skin lesions (typical and atypical target lesions).
The diagnosis is usually supported by peri-lesional tissue biopsy and exclusion of other causes. Histological examination and immunostaining often shows moderate to dense perivascular inflammatory infiltrate (CD4+ lymphocytes and histocytes) within the papillary dermis and along the dermoepidermal junction, dermal edema, intraepithelial/subepithelial vesicles and/or bullae, hydropic degeneration of basal keratinocytes and non-specific immune deposits of IgM, C3 and fibrin along basement membrane [3, 17]. Direct and indirect immunofluorescence is generally unhelpful other than to exclude other vesiculobullous disorders.
A full blood count is usually not helpful, although in severe EM, there is usually a rise in the erythrocyte sedimentation rate. The detection of intralesional HSV-DNA via polymerase chain reaction, as well as immunohistochemistry for IFN-γ and TNF-α, may be useful tests to differentiate herpes associated EM from drug-associated EM [18].
The management of EM can be difficult. There are no available systematic reviews, and randomized controlled trials are scarce. Any precipitants should be removed or treated. Casual drugs should be stopped and relevant infections treated. Antiviral agents may be indicated in herpes associated EM, and a 5 day course of acyclovir 200 mg five times daily at the first sign of lesions, or 400 mg four times daily for 6 months, or continuous treatment using valacyclovir, 500 mg twice a day, is useful for prophylaxis [1].
Tetracycline 250 mg four times a day for at least 1 week may be indicated in EM related to Mycoplasma pneumoniae. Mouthwashes containing local anesthetic and mild antiseptic compounds may help in relieving painful oral symptoms. Analgesics and a liquid diet may be necessary. In severe forms of EM, hospital and supportive care are often important.
Corticosteroids are the most commonly used drugs in the management of EM, despite the lack of evidence. EMm may respond to topical corticosteroids. Patients with EMM should be treated with systemic corticosteroids (prednisolone 0.5–1.0 mg/kg/day tapered over 7–10 days) or azathioprine, or both or other immunomodulatory drugs such as cyclophosphamide, dapsone, cyclosporine, levamisole, thalidomide or interferon-α [19]. Cyclosporine given intermittently may control recurrent EM [9].
Conclusions
EM should be kept in differential diagnosis while treating a patient of acute oro-mucosal ulceration. Histopathology aids in diagnosis by eliminating other differentials esp. pemphigus and pemphigoid. Systemic steroids are the mainstay of treatment. Supportive care, treatment of infections and withdrawal of precipitating agents are equally important. Recurrences, though rare, occur while patient is on steroids. Cyclosporine has a role in recurrent cases.
Glossary
- VZV
Varicella zoster virus
- EBV
Epstein bar virus
- HIV
Human immunodeficiency virus
- BCG
Bacillus calmette guerette
References
- 1.Siegel MA, Balciunas BA. Oral presentation and management of vesiculobullous disorders. Semin Dermatol. 1994;13:78–86. [PubMed] [Google Scholar]
- 2.Al-Johani K A, Fedele S, Porter SR. Erythema multiforme and related disorders. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:642–654. doi: 10.1016/j.tripleo.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Ayangco L, Rogers RS., 3rd Oral manifestations of erythema multiforme. Dermatol Clin. 2003;21:195–205. doi: 10.1016/S0733-8635(02)00062-1. [DOI] [PubMed] [Google Scholar]
- 4.Al-Ubaidy SS, Nally FF. Erythema multiforme: Review of twenty six cases. Oral Surg Oral Med Oral Pathol. 1976;41:601–606. doi: 10.1016/0030-4220(76)90312-1. [DOI] [PubMed] [Google Scholar]
- 5.Kenett S. Erythema multiforme affecting the oral cavity. Oral Surg Oral Med Oral Pathol. 1968;25:366–373. doi: 10.1016/0030-4220(68)90010-8. [DOI] [PubMed] [Google Scholar]
- 6.Gebel K, Hornestein OP. Drug induced oral erythema multiforme: Results of a long term retrospective study. Dermatologica. 1984;168:35–40. doi: 10.1159/000249663. [DOI] [PubMed] [Google Scholar]
- 7.Drago F, Parodi A, Rebora A, et al. Persistent erythema mutiforme: Report of two new cases and review of literature. J Am Acad Dermatol. 1995;33:366–369. doi: 10.1016/0190-9622(95)91435-8. [DOI] [PubMed] [Google Scholar]
- 8.Carrozzo M, Togliatto M, Gandolfo S. Eritema multiforme. Un fenotipo alterations in the HSV-specific T-cell response. Br J Dermatol. 1999;138:952–964. [Google Scholar]
- 9.Schofield JK, Tatnall FM, Leigh IM. Recurrent erythema multiforme: clinical features and treatment in a large series of patients. Br J Dermatol. 1993;128:542–545. doi: 10.1111/j.1365-2133.1993.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 10.Kokuba H, Imafuku S, Huang S, et al. Erythema multiforme lesions are associated with expression of a herpes simplex virus (HSV) gene and qualitative alterations in the HSV-specific T-cell response. Br J Dermatol. 1998;138:952–964. doi: 10.1046/j.1365-2133.1998.02260.x. [DOI] [PubMed] [Google Scholar]
- 11.Lemak MA, Duvic M, Bean SF. Oral acyclovir for the prevention of herpes associated erythema multiforme. J Am Acad Dermatol. 1986;15:50–54. doi: 10.1016/S0190-9622(86)70141-2. [DOI] [PubMed] [Google Scholar]
- 12.Farthing P, Bagan JV, Scully C. Mucosal disease series number IV. Erythema multiforme. Oral Dis. 2005;11:261–267. doi: 10.1111/j.1601-0825.2005.01141.x. [DOI] [PubMed] [Google Scholar]
- 13.Abdollahi M, Radfar M. A review of drug-induced oral reactions. J Contemp Dent Pract. 2003;3:10–31. [PubMed] [Google Scholar]
- 14.Scully C, Bagan JV. Adverse drug reactions in the orofacial region. Crit Rev Oral Biol Med. 2004;15:221–239. doi: 10.1177/154411130401500405. [DOI] [PubMed] [Google Scholar]
- 15.Imafuku S, Kokuba H, Aurelian L, et al. Expression of herpes simplex virus DNA fragments located in epidermal keratinocytes and germinative cells is associated with the development of erythema multiforme lesions. J Invest Dermatol. 1997;109:550–556. doi: 10.1111/1523-1747.ep12336800. [DOI] [PubMed] [Google Scholar]
- 16.Knowles SR, Uetrecht J, Sheas NH. Idiosyncratic drug reactions: the reactive metabolite syndromes. Lancet. 2000;356:1587–1591. doi: 10.1016/S0140-6736(00)03137-8. [DOI] [PubMed] [Google Scholar]
- 17.Howland WW, Golitz LE, Weston WL, et al. Erythema multiforme: clinical, histopathologic, and immunologic study. J Am Acad Dermatol. 1989;113:36–39. doi: 10.1016/s0190-9622(84)80090-0. [DOI] [PubMed] [Google Scholar]
- 18.Aurelian L, Ono F, Burnett J. Herpes simplex virus (HSV)-associated erythema multiforme (HAEM): a viral disease with an autoimmune component. Dermatol Online J. 2003;9:1. [PubMed] [Google Scholar]
- 19.Stewart MG, Duncan III NO, Franklin DJ, et al. Head and neck manifestations of erythema multiforme in children. Otolaryngol Head Neck Surg. 1994;111:236–242. doi: 10.1016/S0194-5998(94)70597-6. [DOI] [PubMed] [Google Scholar]