Abstract
Aim
Previous studies suggest that circulating levels of interleukin-18 (IL-18) may be prospectively related to risk of coronary heart disease (CHD) in the general population. We report new data from the largest prospective study to date, which are combined with data from all published prospective studies in a meta-analysis.
Methods
We measured baseline IL-18 levels in stored serum samples of subjects from a case–control study nested within a prospective study of 5661 men aged 40–59 years recruited from general practices in 18 British towns in 1978–1980 and followed-up for up to 16 years (median time to event 8.4 years) for fatal CHD and non-fatal myocardial infarction (595 cases, 1238 controls).
Results
IL-18 concentrations were strongly related to cigarette smoking, triglyceride, HDL-cholesterol (inversely) and to circulating levels of several inflammatory and haemostatic markers. Men in the top third of baseline IL-18 levels had an age-adjusted odds ratio (OR) for CHD of 1.55 (95% CI 1.21, 1.98) compared with those in the lowest third; this was reduced to 1.30 (95% CI 0.99, 1.69) after additional adjustment for vascular risk factors and 1.12 (95% CI 0.84, 1.49) after further adjustment for CRP and IL-6. In meta-analyses of CVD, associations (or effect sizes) were consistent between studies; RRs were 1.63 (95% CI 1.46, 1.82) after age adjustment, 1.39 (95% CI 1.24, 1.55) after additional risk factor adjustment and 1.34 (95% CI 1.17, 1.54) after additional adjustment for inflammatory markers.
Conclusions
Circulating IL-18 is prospectively and independently associated with CVD risk.
Key words: Coronary heart disease, Epidemiology, Interleukin-18, Cohort, Meta-analysis
Atherosclerosis includes an important inflammatory component [1]; a substantial body of evidence demonstrates prospective associations between circulating acute-phase reactant proteins (particularly C-reactive protein [CRP] and fibrinogen) and coronary heart disease (CHD) risk [2,3]. More recently, growing evidence suggests that levels of circulating pro-inflammatory cytokines, particularly interleukin-6 (IL-6), are prospectively related to CHD risk [4]. However, evidence about the influence of circulating levels of other pro-inflammatory cytokines is more limited.
Interleukin-18 (IL-18) is a pleiotropic proinflammatory cytokine which plays an important role in the inflammatory cascade [5]. Some evidence suggests that its expression may be related to atherosclerotic plaque progression and vulnerability [6,7]. Circulating concentrations of IL-18 have been prospectively related to vascular events in patients with stable and unstable angina [8] or pre-existing CHD [9,10] and, more recently in nested case–control studies carried out within a population-based studies of healthy adults [11–14]. In order to clarify the strength and independence of this relationship further, we report here on the relationship between IL-18 and CHD risk in a large prospective study with considerably more events than in previous studies: a nested case–control study of middle-aged British men, nested within a large prospective study (the British Regional Heart Study), in which we have previously reported data on the relations of CRP and IL-6 to CHD [4,15]. To place our results in context, we have performed a systematic review and meta-analysis of all published prospective studies examining the relationship of IL-18 and CHD, also correcting for regression dilution bias.
1. Methods
1.1. British Regional Heart Study
In 1978–1980, 7735 men aged 40–59 years were randomly selected from general practice registers in each of 24 British towns and invited to take part in the British Regional Heart Study (response rate 78%). Nurses administered questionnaires, made physical measurements, recorded an electrocardiogram (ECG) and collected non-fasting venous blood samples; in 5661 men in 18 of the study towns, serum was stored at −20 °C for subsequent analysis [16]. Pre-existing CHD was defined on the basis of a history of a doctor diagnosis of CHD, a positive history of angina or possible MI on the Rose (WHO) chest pain questionnaire or an ECG consistent with definite or possible CHD. All men were monitored subsequently for all-cause mortality and for cardiovascular morbidity, with a follow up loss of <1% [17]. Fatal CHD cases were ascertained through National Health Service Central Registers on the basis of a death certificate with ICD-9 codes 410-414. The diagnosis of non-fatal myocardial infarction (MI) was based on reports from general practitioners, supplemented by regular reviews of general practice records, and diagnosed in accordance with World Health Organisation criteria [17]. A nested case–control study was established, based on all 643 CHD cases (279 CHD deaths and 364 cases of non-fatal MI) occurring between 1978 and 1995 and a total of 1278 controls (2 per case) “frequency matched” to cases on town of residence and age in 5-year bands was randomly selected from among men who had survived to the end of the study period (31/12/1995) free from incident CHD (the maximum follow-up time was 16 years and among the cases, the median time to event was 8.4 years). Serum samples were available for 595 of these cases and 1238 controls for IL-18 measurements to be carried out in 2004–2005. A sub-sample of 158 men had fasting serum samples drawn in both 1996 and 2000, and these were analysed for IL-18, in 2004–2005 in the same laboratory using the same methods [4], in order to correct associations for regression dilution bias.
1.2. Laboratory methods
Serum concentrations of IL-18 and IL-6 were assayed using enzyme immunoassays (R and D Systems, Oxford, UK) by laboratory personnel who were blind to the case–control status of samples. The intra- and inter-assay coefficients of variation for IL-18 were 5.6% and 10.4% respectively. CRP, serum amyloid A (SAA), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), E-selectin, P-selectin, von Willebrand factor (vWF), fibrin-D dimer and tissue plasminogen activator (t-PA) antigen were assayed as previously reported [15,18–21].
1.3. Statistical analysis
We pre-specified case–control analyses by thirds of IL-18 values in controls. Unmatched stratified logistic regression was fitted using unconditional maximum likelihood methods (SAS version 8.1). Models were fitted adjusted for (i) age (model 1), (ii) additionally for cigarette smoking (model 2), (iii) additionally for other established cardiovascular risk factors (blood pressure, total cholesterol, HDL cholesterol, body mass index [as continuous covariates], history of diabetes, history of CHD, social class and usual physical activity [as categorical covariates]) (model 3) and (iv) additionally for other inflammatory markers (IL-6 and C-reactive protein [continuous covariates, log transformed because of skewed distribution]) (model 4). Rosner's method for univariate models was used to calculate regression dilution ratios (RDRs) on a subset of men with repeated IL-18 measurements, using a linear regression model of the repeat measurement predicted by the first measurement [22].
1.4. Systematic review
A systematic review of all papers, letters, abstracts and review articles reporting on prospective associations between circulating interleukin-18 levels and CHD risk was conducted according to PRISMA guidelines. Relevant studies published between 1945 (Web of Science), 1970 (PubMed), 1974 (Embase) and October 2009 were identified from databases, by scanning reference lists and review articles and consulting experts in the field. Additionally, studies were sought using a combined text word and MESH/subject heading (for PubMed and Embase only) using the search strategy detailed in Web Table 1. The review was restricted to studies carried out on human subjects and written in the English language. Studies eligible for inclusion in the current review included prospective cohort studies or nested case–control studies based on approximately general populations (where participants were not selected on the basis of pre-existing disease) and prospective studies of patients with pre-existing coronary artery disease (CAD). Populations defined using other criteria (for example, pre-existing diabetes or metabolic syndrome) were excluded. Studies had to include at least one year of follow-up for CHD events following baseline IL-18 measurements. Relevant CHD events included fatal CHD, non-fatal CHD or all CHD (fatal and non-fatal). Studies including CHD and stroke outcome in a combined “cardiovascular disease” end point were included, although a sub-analysis was conducted to check for differences in the associations between IL-18 and CHD and CVD endpoints. Data were extracted by two researchers in accordance with a pre-specified fixed protocol; discrepancies were resolved by discussion. Odds ratios (ORs) or hazard ratios (HRs) providing estimates of relative risk (RR) for CHD for the highest third of the control IL-18 distribution compared with the lowest third were sought, in models adjusted for: (i) age (and gender where appropriate) (model 1), (ii) age and established cardiovascular risk factors (particularly blood lipids, cigarette smoking, blood pressure, diabetes) (model 2) and (iii) age, established cardiovascular risk factors and other inflammatory markers (IL-6 and C-reactive protein) (model 3). If estimates could not be obtained from published reports, they were sought directly from study authors, with supplementary descriptive data as required.
1.5. Statistical analysis
The meta-analysis was carried out using STATA 10. Estimates of log ORs or log HRs for different studies were combined using an inverse-variance weighted average in a fixed effects model: fixed effects were chosen over random effects because we did not have reason to expect different magnitude of results between studies. Between-study heterogeneity was assessed using the I2 statistic. Funnel plots were examined and tests described by Begg [23] and Egger [24] for small study bias were also performed. Pre-specified sensitivity analyses were undertaken using metaregression to investigate heterogeneity between studies with (i) CVD endpoints (combined CHD and other cardiovascular endpoints) compared to CHD and (ii) studies of approximately general populations compared with studies of patients with CAD.
2. Results
2.1. British Regional Heart Study
Established vascular risk factors showed highly significant differences between cases and controls in the expected directions (Table 1). Geometric mean IL-18 levels were higher among cases than controls (percentage difference 9.0%, 95% CI 4.0–14.0%, p <= 0.001). Baseline IL-18 levels in the control population were strongly related to cigarette smoking, triglyceride, HDL-cholesterol (inversely), acute phase proteins (CRP, SAA), IL-6, cellular adhesion molecules (ICAM-1, VCAM-1, E-selectin, P-selectin) and with haemostatic variables (vWF, fibrin D-dimer and t-PA). IL-18 levels were weakly related to diastolic BP, but were not appreciably related to age, BMI, total cholesterol, physical activity or to other established CHD risk factors (Table 2). In a comparison of men in the top third compared with those in the bottom third of baseline IL-18 levels, the age-adjusted OR for CHD was 1.55 (95% CI 1.21, 1.98) (Table 3). Adjustment for cigarette smoking slightly attenuated this OR, while additional adjustment for other vascular risk factors reduced the OR to 1.30 (95% CI 0.99, 1.69). Additional adjustment for CRP and IL-6 reduced the OR to 1.12 (95% CI 0.81, 1.55). In the last adjusted model, the OR for CVD for a doubling in CRP was 1.23 (95% CI 1.13, 1.33) and 1.15 (95% CI 1.00, 1.33) for a doubling in IL-6 (data not presented). Repeating the analysis excluding men with established CHD had little material impact on the results (Table 3). An RDR of 0.71 (95% CI 0.61, 0.81) was calculated for the 158 men with IL-18 levels measured 4 years apart [25]. The OR of CHD among men in the top third of IL-18 compared with the lowest third, adjusted for age and vascular risk factors, increased from 1.30 (95% CI 0.99, 1.69) to 1.45 (95% CI 0.99, 2.09) after correction for regression dilution.
Table 1.
Baseline characteristics of men with coronary heart disease and of age and town-matched controls. Mean (SD) or n (%).
| Cases | Controls | p (no difference) | |
|---|---|---|---|
| (n = 595) | (n = 1238) | ||
| Age (years) | 52.6 (5.2) | 52.5 (5.3) | Matched |
| Current smoker (n, %) | 304 (51.2) | 521 (42.2) | <0.001 |
| Evidence of coronary disease (n, %) | 247 (41.5) | 327 (26.4) | <0.001 |
| History of diabetes (n, %) | 16 (2.7) | 18 (1.5) | 0.091 |
| >2 drinks alcohol/day (n, %) | 120 (20.2) | 278 (22.5) | 0.262 |
| Non-manual occupation (n, %) | 184 (32.3) | 465 (38.7) | 0.009 |
| Physical activity (inactive/occasional) (n, %) | 275(46.7) | 493(40.4) | 0.011 |
| Physical measurements | |||
| Body mass index (kg/m2) | 25.9 (3.4) | 25.4 (3.3) | 0.001 |
| Systolic blood pressure (mm Hg) | 151.8 (21.8) | 146.7 (20.9) | <0.001 |
| Diastolic blood pressure (mm Hg) | 85.7 (13.8) | 82.8 (13.2) | <0.001 |
| FEV1 (L/min)a | 3.10 (0.65) | 3.21 (0.71) | 0.002 |
| Blood sample | |||
| Total cholesterol (mmol/L) | 6.64 (1.06) | 6.20 (0.99) | <0.001 |
| HDL cholesterol (mmol/L) | 1.09 (0.27) | 1.15 (0.27) | <0.001 |
| Triglyceride (mmol/L)b | 1.95 (0.69, 5.51) | 1.70 (0.57, 5.02) | <0.001 |
| IL-18 (pg/mL)b | 302.0 (117.1, 779.1) | 275.4 (106.8, 710.6) | 0.001 |
| C-reactive protein (mg/L)b | 2.51 (0.30, 20.95) | 1.45 (0.14, 15.11) | <0.001 |
| IL-6 (pg/mL)b | 2.75 (0.81, 9.32) | 2.29 (0.65, 8.11) | <0.001 |
FEV1 adjusted for [(cohort mean height)2/height2].
Geometric mean and 95% range.
Table 2.
Association between IL-18 (thirds) and cardiovascular risk factors in the coronary heart disease control sample (n = 1237).
| Thirds of IL-18 |
|||||||
|---|---|---|---|---|---|---|---|
| Low 29–233 pg/mL (n = 411) |
Middle 234–345 pg/mL (n = 413) |
High 346–2068 pg/mL (n = 414) |
p (trend), adjusted for age and town | ||||
| Mean or n | SE or % | Mean or n | SE or % | Mean or n | SE or % | ||
| Age | 52.5 | 0.3 | 52.1 | 0.3 | 52.8 | 0.3 | 0.609 |
| Current smoker (n, %) | 133 | 32.4 | 188 | 45.7 | 200 | 48.3 | <0.001 |
| >2 drinks alcohol/day (n, %) | 98 | 23.8 | 86 | 20.9 | 94 | 22.7 | 0.558 |
| Evidence of CHD at entry (n, %) | 106 | 25.8 | 113 | 27.4 | 108 | 26.1 | 0.773 |
| History of diabetes at baseline (n, %) | 7 | 1.7 | 6 | 1.5 | 6 | 1.5 | 0.770 |
| Non-manual occupations (n, %) | 181 | 45.1 | 145 | 36.4 | 139 | 34.3 | 0.016 |
| Physical Activity (inactive/occasional) (n, %) | 152 | 37.7 | 169 | 41.6 | 172 | 41.8 | 0.656 |
| Body mass index (kg/m2) | 25.1 | 0.2 | 25.5 | 0.2 | 25.6 | 0.2 | 0.052 |
| Systolic blood pressure (mm Hg) | 144.6 | 1.0 | 146.9 | 1.0 | 148.4 | 1.0 | 0.033 |
| Diastolic blood pressure (mm Hg) | 81.0 | 0.6 | 83.8 | 0.6 | 83.4 | 0.6 | 0.018 |
| FEV1 [adjusted] (L/min)a | 3.26 | 0.04 | 3.20 | 0.04 | 3.16 | 0.04 | 0.617 |
| Total cholesterol (mmol/L) | 6.14 | 0.05 | 6.23 | 0.05 | 6.21 | 0.05 | 0.394 |
| HDL cholesterol (mmol/L) | 1.20 | 0.01 | 1.15 | 0.01 | 1.11 | 0.01 | <0.001 |
| Triglycerides (mmol/L)b | 0.19 | 0.01 | 0.22 | 0.01 | 0.26 | 0.01 | <0.001 |
| Haematocrit (%) | 41.91 | 0.45 | 42.79 | 0.45 | 42.45 | 0.45 | 0.700 |
| Homocysteine (mmol/L)b | 1.10 | 0.01 | 1.12 | 0.01 | 0.16 | 0.01 | 0.011 |
| Insulin (mU/L)b | 1.07 | 0.02 | 1.05 | 0.02 | 1.13 | 0.02 | 0.032 |
| Glucose (mmol/L)b | 0.74 | 0.004 | 0.74 | 0.004 | 0.74 | 0.004 | 0.690 |
| C-reactive protein (mg/L)b | 0.05 | 0.03 | 0.15 | 0.03 | 0.30 | 0.03 | <0.001 |
| Serum amyloid A protein (mg/L)b | 0.81 | 0.02 | 0.83 | 0.02 | 0.88 | 0.02 | 0.003 |
| White cell count (109/L)b | 0.83 | 0.01 | 0.85 | 0.01 | 0.85 | 0.01 | 0.011 |
| Albumin (g/L) | 44.7 | 0.1 | 44.6 | 0.1 | 44.3 | 0.1 | 0.003 |
| Globulin (g/L) | 28.9 | 0.2 | 28.9 | 0.2 | 29.3 | 0.2 | 0.101 |
| E-selectin (ng/mL)b | 1.74 | 0.01 | 1.77 | 0.01 | 1.82 | 0.01 | <0.001 |
| P-selectin (ng/mL)b | 2.00 | 0.01 | 2.01 | 0.01 | 2.07 | 0.01 | 0.001 |
| ICAM 1 (ng/mL)b | 2.43 | 0.01 | 2.47 | 0.01 | 2.52 | 0.01 | <0.001 |
| VCAM 1 (ng/mL)b | 2.62 | 0.01 | 2.64 | 0.01 | 2.66 | 0.01 | 0.008 |
| von Willebrand factor (IU/dL)b | 2.00 | 0.01 | 2.01 | 0.01 | 2.05 | 0.01 | <0.001 |
| Fibrin D-dimer (ng/mL)b | 1.86 | 0.02 | 1.88 | 0.02 | 1.93 | 0.02 | 0.008 |
| t-PA (ng/mL)b | 0.97 | 0.01 | 1.01 | 0.01 | 1.06 | 0.01 | <0.001 |
| IL-6 (pg/mL)b | 0.31 | 0.01 | 0.36 | 0.01 | 0.41 | 0.01 | <0.001 |
FEV adjusted for [(cohort mean height)2/height2].
Values are transformed to log base 10.
Table 3.
Odds ratios for coronary heart disease in men by thirds of IL-18 and for continuous (doubling of) IL-18.
| IL-18 values | Cases | Controls | OR (95% CI) |
|||
|---|---|---|---|---|---|---|
| Thirds | N | N | Model 1a | Model 2b | Model 3c | Model 4d |
| All participants,n = 1833 (range pg/mL) | ||||||
| 346–2068 | 242 | 414 | 1.55 (1.21, 1.98) | 1.48(1.15, 1.90) | 1.30 (0.99, 1.69) | 1.12 (0.84, 1.49) |
| 234–345 | 193 | 413 | 1.24 (0.96, 1.59) | 1.19 (0.92, 1.54) | 1.06 (0.81, 1.39) | 1.04 (0.78, 1.38) |
| 29–233 | 160 | 411 | 1 | 1 | 1 | 1 |
| Total | 595 | 1238 | ||||
| Continuous doubling of IL-18e | 1.30(1.12,1.50) | 1.23(1.09, 1.47) | 1.21(1.03, 1.42) | 1.14(0.96, 1.34) | ||
| p trende | 0.003 | 0.008 | 0.032 | 0.191 | ||
| Participants without baseline CHD (BRHS definition),n = 1259 (range pg/mL) | ||||||
| 346–2068 | 150 | 306 | 1.63 (1.19 2.22) | 1.53 (1.12, 2.10) | 1.29 (0.92, 1.80) | 1.11 (0.77, 1.50) |
| 234–345 | 105 | 300 | 1.16 (0.84 1.60) | 1.09 (0.79, 1.52) | 1.03 (0.73, 1.46) | 1.05 (0.73, 1.51) |
| 47–233 | 93 | 305 | 1 | 1 | 1 | 1 |
| Total | 348 | 911 | ||||
| Continuous doubling of IL-18e | 1.31 (1.09, 1.57) | 1.27 (1.06, 1.53) | 1.16 (0.95, 1.41) | 1.09 (0.88, 1.35) | ||
| p trende | 0.030 | 0.056 | 0.343 | 0.758 | ||
Model 1 = age + town.
Model 2 = Model 1 + smoking.
Model 3 = Model 2 + blood pressure, total cholesterol, HDL, body mass index, history of diabetes, CHD, social class, physical activity.
Model 4 = Model 3 + log10 C-reactive protein (mg/L), log10 IL-6 (pg/mL).
OR of MI per 1 log2 increase in log2(IL-18), i.e. a doubling of IL-18, p value for trend using IL-18 as a continuous variable.
2.2. Meta-analysis
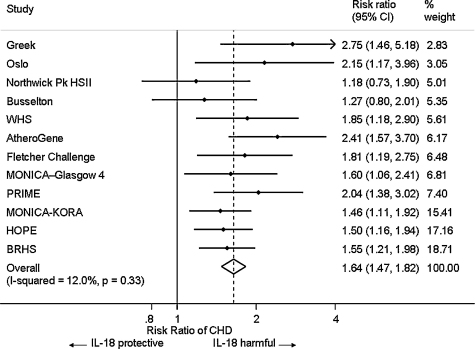
From 458 studies identified, eleven relevant prospective studies (flow diagram, Web Fig. 1), plus the BRHS were selected for the meta-analysis. Of the twelve included, eight were based on approximately general populations [11–14,26–28] and four on patients with CAD or at high risk of CHD [9,10,29,30]; in all, these studies included a total of 3047 CVD cases and 11,071 control individuals (Web Table 2). We were able to obtain relevant estimates for models 1 and 2 for all twelve studies and for model 3 for eleven studies (Web Table 3). In nine studies the outcome was CHD and in the other three CHD was combined with other cardiovascular disease (CVD) e.g. stroke, and reported here as CVD. All studies except one [26] used ELISAs produced by one of two manufacturers. Estimates of the age-adjusted ORs and HRs (Fig. 1) showed little evidence of heterogeneity between studies (I2, 12%, p = 0.327); there was no strong evidence of bias (examining the funnel plot for asymmetry and Egger test, p = 0.108; Begg test, p = 0.451, Web Fig. 2). The age and gender adjusted RR of CVD (for the highest third of IL-18 compared with the lowest third of IL-18) in a fixed-effect meta-analysis was 1.64 (95% CI 1.48, 1.83). Adjustment for established cardiovascular risk factors reduced the RR to 1.35 (95% CI 1.25, 1.51); additional adjustment for inflammatory markers (CRP and IL-6) somewhat reduced the RR to 1.34 (95% CI 1.17, 1.53), although the increase in RR remained statistically significant (Web Fig. 3). In age-adjusted analyses, RR estimates in approximately general populations (1.55 [95% CI 1.36, 1.77]) and patients with CAD (1.82 [95% CI 1.53, 2.18]) respectively were not appreciably different (F = 2.13, p = 0.176). Similarly, there was no evidence of a systematic difference in RR estimates between studies with the outcome of CHD (1.62 [95% CI 1.43, 1.81]) compared to CVD (RR 1.79 [95% CI 1.36, 2.34]) (F = 0.38, p = 0.552). Further meta-regressions did not find consistent evidence that RR estimated differed by gender, geographic location, sample storage temperature, study date or follow-up period (data not presented). The RR for CVD among participants in the top third of IL-18 compared with the lowest third and adjusted for age, gender, lipids, BMI and behavioural risk factors corrected using the RDR of 0.71 was 1.53 (95% CI 1.37, 1.79).
Fig. 1.
Associations of baseline IL-18 level and risk of onset of CHD in 12 studies, adjusted for age and gender. Models ordered by statistical weight.
3. Discussion
In this large population-based prospective study among mainly healthy middle-aged men, baseline circulating IL-18 concentrations were positively associated with CHD risk. Once conventional vascular risk factors were accounted for, the strength of the independent relationship was modest, with CHD risk in the highest third of the IL-18 distribution being approximately 30% higher than that in the lowest third. Positive associations between IL-18 and CHD are consistent with its postulated role in atherosclerotic plaque progression and rupture [6,7]. IL-18 is an important regulator of both innate and acquired immune responses [5]. It is present in human atherosclerotic lesions, and at higher concentrations in unstable plaques [6]. In animal models, IL-18 administration leads to increases in atherosclerotic lesion size and promotes increased numbers of T-lymphocytes in the lesion [31], suggesting that it may be of pathogenic importance in the tissue. We show that elevated IL-18 is associated with increased CVD risk, but whether this observation is indicative of IL-18 being causal in plaque rupture, or whether rupture prone plaques “leak” IL-18 into the circulation (reverse causality), or the association is coincidental, is currently unknown.
In addition to providing the largest prospective study of IL-18 and CHD risk reported to date (with almost twice as many CHD cases as the next largest study), the meta-analysis results, based on data from all published prospective studies, suggest that there is an independent association between IL-18 and CHD risk, independent of classical risk factors, with higher risk consistently observed in all studies. The effect is maintained even after adjustment for both established cardiovascular risk markers and for inflammatory markers. In the BRHS data the association of IL-18 with incident CHD was not significant after adjustment for CRP and IL-6, although CRP remained an independent predictor of CHD suggesting separate pathways from CRP and IL-18 to CHD risk. The RR adjusted for other inflammatory markers was of the order of 35% in the meta-analysis, so the strength of association with CHD is slightly smaller than that estimated for CRP in a recent meta-analysis, similar to that for erythrocyte sedimentation rate and stronger than that for von Willebrand factor [3]. However it is possible that intra-individual variability of IL-18 could lead to under-estimation of the strength of its association with CHD risk, as previously reported for IL-6 [4]. We report, for the first time, a serial study of IL-18 measurements over 4 years, and use this data to correct for regression dilution bias. The RDR-corrected RR adjusted for established risk factors from the meta-analysis strengthened somewhat from 1.35 to 1.53 (95% CI 1.37, 1.79); more similar to the magnitude of the uncorrected adjusted association of CRP with CHD [3].
However, in the present study population, after adjustment for established risk factors the strength of the association between IL-18 and CHD was weaker than both the associations with other established risk factors such as blood pressure and cholesterol [32], and the associations of other inflammatory markers [4,15]. These results therefore provide little support for the value of IL-18 as a predictor of risk in individual subjects. In the north Glasgow MONICA cohort study (included in the meta-analysis), IL-18 added less than 0.1% to the prediction of CVD using the ASSIGN risk score, once standard CVD risk factors and social class were accounted for. They also reported that CRP, a marker consistently associated with CVD events, did add to the prediction of CVD risk, although other studies have found moderate or little evidence for additional value of inflammatory markers as predictors of individual risk [27]. Nevertheless, IL-18 is still of potential interest as a causal factor in CHD, and potentially as a therapeutic target. In addition to testing of causality by Mendelian randomization studies [29], any trials of potential IL-18 antagonists would be of interest although we know of no such drugs currently in clinical trials.
Variation within the IL18 gene has been shown to influence circulating levels of IL-18 in both association studies [13,33,34], and in a genome wide association study [35]. These SNPs have been reported to be associated with clinical outcome in subjects with coronary artery disease [29,36,37] but this is not seen in all studies [38,39]. IL18 SNPs also are associated with differences in IL-18 production capability by monocytes [40–42]. While single SNPs in the gene are associated with modest effect on plasma levels, several groups have demonstrated that certain haplotypes defined by up to five SNPs in combination are strongly associated with lower levels of serum IL-18 in healthy individuals where for example subjects carrying two copies of the haplotype had IL-18 concentrations 15–20% lower than those carrying no copies [13,33], and with lower levels of serum IL-18 in individuals with cardiovascular disease, and with protection from future cardiovascular events [29]. No non-synonymous variation has been identified in IL18 despite exon sequencing in a number of individuals. It is therefore likely that the functional polymorphism(s) responsible for these observed associations cause differences in promoter activity, mRNA splicing or mRNA stability, but the precise mechanism is currently unknown.
It would therefore be possible to use IL18 genotypes to distinguish association and causation using a Mendelian randomization approach, as has previously been done for CRP [43] and fibrinogen [44], suggesting that these factors are not causally related to CHD. Future studies could also examine whether effect modifiers of any associations between IL-18 and CHD; previous studies have demonstrated interactions between IL-18 or IL-18 genotypes and factors related to CHD risk (e.g. metabolic syndrome [30], hypertension [45] or cigarette smoking [46]), any such interactions could change the predictive value of circulating IL-18 levels.
Compared with previous reports on IL-18, the present community-based study has considerably more CHD events and includes measurements of a wide range of potential confounding factors at study entry. For the first time, we report a meta-analysis of published studies and the potential impact of regression dilution bias. This study, like most others, is limited by having only a single measure of IL-18. However we estimated the intra-individual variability in serum IL-18 levels in a separate sub-study of men measured four years apart. Estimates suggested that the stability of serum IL-18 is similar to several established risk factors e.g. total cholesterol [25] and correction for within-person variability, somewhat strengthened the estimated associations with CHD or CVD. Despite prolonged sample storage before measurements (approximately 24 years at −20 °C, which was suboptimal for such a long time span), which might have resulted in underestimation of the association of IL-18 with CHD, the validity of our IL-18 levels is supported by their consistency with those in previously reported studies [11,12], by their associations with independently assessed inflammatory markers and by the consistency of the findings with those of the previously published prospective studies. The meta-analysis of prospective studies included data from all studies identified from three major databases and provided no strong evidence for small study bias. Additionally, sensitivity analyses excluding studies with CVD rather than CHD endpoints and based in high risk rather than general populations did not alter our conclusions. While the analysis of British Regional Heart Study data included only men, nine of the twelve studies in the meta-analysis included women, so our conclusions about the associations between IL-18 and CVD events are generalised to both men and women.
4. Conclusions
The results of the present study and the meta-analysis suggest that IL-18 is prospectively and independently associated with CHD risk, but the association is modest in strength.
Conflict of interest
The authors have no conflict of interest to declare.
Acknowledgments
We are very grateful to the following individuals for providing data in the format required for the meta-analysis for each study. Atherogene: S.Blankenberg, L.Tiret; PRIME: P. Ducimetière, S. Blankenberg, L. Tiret; HOPE: S. Blankenberg, L. Tiret; KORA/Augsberg: W. Koenig, B. Khuseyinova; NPHSII: S. Thompson, J. Cooper; Busselton: J. Hung, M. Knuiman; Greek case series: D. Tziakas, G. Chalikias, J.C. Kaski; 4th Glasgow MONICA: H. Tunstall Pedoe; Women's Health Study, B.M. Everett, P.M. Ridker; OSLO, M. Trøseid, I. Seljeflot and H. Arnesen.
This work was supported by the British Heart Foundation: Professor AG Shaper established the British Regional Heart Study, which is a British Heart Foundation Research Group. PHW, AR, GDOL and SEH are supported by programme grants from the British Heart Foundation [grants RG/04/003, RG/08/013/25942 and RG/08/008]. The views expressed in this paper are those of the authors and not necessarily those of the British Heart Foundation.
Footnotes
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.atherosclerosis.2011.03.015.
Contributor Information
Barbara J.M.H. Jefferis, Email: B.jefferis@ucl.ac.uk.
Olia Papacosta, Email: O.papacosta@ucl.ac.uk.
Christopher G. Owen, Email: c.owen@sgul.ac.u.
S. Goya Wannamethee, Email: G.wannamethee@ucl.ac.uk.
Steve E. Humphries, Email: s.humphries@ucl.ac.uk.
Mark Woodward, Email: markw@georgeinstitute.org.au.
Lucy T. Lennon, Email: l.lennon@ucl.ac.uk.
Andrew Thomson, Email: a.thomson@ucl.ac.uk.
Paul Welsh, Email: P.welsh@clinmed.gla.ac.uk.
Ann Rumley, Email: Ar4a@clinmed.gla.ac.uk.
Gordon D.O. Lowe, Email: Gdlowe@clinmed.gla.ac.uk.
Peter H. Whincup, Email: p.whincup@sgul.ac.uk.
Appendix A. Supplementary data
References
- 1.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 2.Danesh J., Lewington S., Thompson S.G. Plasma fibrinogen level and the risk of major cardiovascular diseases and nonvascular mortality: an individual participant meta-analysis. JAMA. 2005;294:1799–1809. doi: 10.1001/jama.294.14.1799. [DOI] [PubMed] [Google Scholar]
- 3.Danesh J., Wheeler J.G., Hirschfield G.M. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 4.Danesh J., Kaptoge S., Mann A.G. Long-term interleukin-6 levels and subsequent risk of coronary heart disease: two new prospective studies and a systematic review. PLoS Med. 2008;5:e78. doi: 10.1371/journal.pmed.0050078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gracie J.A., Robertson S.E., McInnes I.B. Interleukin-18. J Leukoc Biol. 2003;73:213–224. doi: 10.1189/jlb.0602313. [DOI] [PubMed] [Google Scholar]
- 6.Mallat Z., Corbaz A., Scoazec A. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–1603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 7.Gerdes N., Sukhova G.K., Libby P. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–257. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blankenberg S., Tiret L., Bickel C. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 9.Blankenberg S., McQueen M.J., Smieja M. Comparative impact of multiple biomarkers and N-terminal pro-brain natriuretic peptide in the context of conventional risk factors for the prediction of recurrent cardiovascular events in the Heart Outcomes Prevention Evaluation (HOPE) Study. Circulation. 2006;114:201–208. doi: 10.1161/CIRCULATIONAHA.105.590927. [DOI] [PubMed] [Google Scholar]
- 10.Tziakas D.N., Chalikias G.K., Kaski J.C. Inflammatory and anti-inflammatory variable clusters and risk prediction in acute coronary syndrome patients: a factor analysis approach. Atherosclerosis. 2007;193:196–203. doi: 10.1016/j.atherosclerosis.2006.06.016. [DOI] [PubMed] [Google Scholar]
- 11.Blankenberg S., Luc G., Ducimetiere P. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–2459. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 12.Hung J., Knuiman M.W., Divitini M.L. C-reactive protein and interleukin-18 levels in relation to coronary heart disease: prospective cohort study from Busselton Western Australia. Heart Lung Circ. 2008;17:90–95. doi: 10.1016/j.hlc.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Thompson S.R., Novick D., Stock C.J. Free interleukin (IL)-18 levels, and the impact of IL18 and IL18BP genetic variation, in CHD patients and healthy men. Arterioscler Thromb Vasc Biol. 2007;27:2743–2749. doi: 10.1161/ATVBAHA.107.149245. [DOI] [PubMed] [Google Scholar]
- 14.Everett B.M., Bansal S., Rifai N., Buring J.E., Ridker P.M. Interleukin-18 and the risk of future cardiovascular disease among initially healthy women. Atherosclerosis. 2009;202:282–288. doi: 10.1016/j.atherosclerosis.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danesh J., Whincup P., Walker M. Low grade inflammation and coronary heart disease: prospective study and updated meta-analyses. BMJ. 2000;321:199–204. doi: 10.1136/bmj.321.7255.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaper A.G., Pocock S.J., Walker M. British Regional Heart Study: cardiovascular risk factors in middle-aged men in 24 towns. Br Med J (Clin Res Ed) 1981;283:179–186. doi: 10.1136/bmj.283.6285.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker M., Whincup P.H., Shaper A.G. The British Regional Heart Study 1975–2004. Int J Epidemiol. 2004;33:1185–1192. doi: 10.1093/ije/dyh295. [DOI] [PubMed] [Google Scholar]
- 18.Malik I., Danesh J., Whincup P. Soluble adhesion molecules and prediction of coronary heart disease: a prospective study and meta-analysis. Lancet. 2001;358:971–976. doi: 10.1016/S0140-6736(01)06104-9. [DOI] [PubMed] [Google Scholar]
- 19.Danesh J., Whincup P., Walker M. Fibrin D-dimer and coronary heart disease: prospective study and meta-analysis. Circulation. 2001;103:2323–2327. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 20.Lowe G.D., Danesh J., Lewington S. Tissue plasminogen activator antigen and coronary heart disease. Prospective study and meta-analysis. Eur Heart J. 2004;25:252–259. doi: 10.1016/j.ehj.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 21.Whincup P.H., Danesh J., Walker M. von Willebrand factor and coronary heart disease: prospective study and meta-analysis. Eur Heart J. 2002;23:1764–1770. doi: 10.1053/euhj.2001.3237. [DOI] [PubMed] [Google Scholar]
- 22.Rosner B., Spiegelman D., Willett W.C. Correction of logistic regression relative risk estimates and confidence intervals for random within-person measurement error. Am J Epidemiol. 1992;136:1400–1413. doi: 10.1093/oxfordjournals.aje.a116453. [DOI] [PubMed] [Google Scholar]
- 23.Begg C.B., Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 24.Egger M., Davey S.G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emberson J.R., Whincup P.H., Morris R.W. Extent of regression dilution for established and novel coronary risk factors: results from the British Regional Heart Study. Eur J Cardiovasc Prev Rehabil. 2004;11:125–134. doi: 10.1097/01.hjr.0000114967.39211.e5. [DOI] [PubMed] [Google Scholar]
- 26.Koenig W., Khuseyinova N., Baumert J. Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol. 2006;26:2745–2751. doi: 10.1161/01.ATV.0000248096.62495.73. [DOI] [PubMed] [Google Scholar]
- 27.Woodward M., Welsh P., Rumley A., Tunstall-Pedoe H., Lowe G.D. Do inflammatory biomarkers add to the discrimination of cardiovascular disease after allowing for social deprivation? Results from a 10 year cohort study in Glasgow, Scotland. Eur Heart J. 2010;31:2669–2675. doi: 10.1093/eurheartj/ehp115. [DOI] [PubMed] [Google Scholar]
- 28.Welsh P., Woodward M., Rumley A., MacMahon S., Lowe G.D. Does interleukin-18 or tumour necrosis factor-a have an independent association with the risk of coronary heart disease? Results from a prospective study in New Zealand. Cytokine. 2010;50:94–98. doi: 10.1016/j.cyto.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 29.Tiret L., Godefroy T., Lubos E. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112:643–650. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- 30.Troseid M., Seljeflot I., Hjerkinn E.M., Arnesen H. Interleukin-18 is a strong predictor of cardiovascular events in elderly men with the metabolic syndrome: synergistic effect of inflammation and hyperglycemia. Diabetes Care. 2009;32:486–492. doi: 10.2337/dc08-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitman S.C., Ravisankar P., Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–E38. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 32.Emberson J.R., Whincup P.H., Morris R.W., Walker M. Re-assessing the contribution of serum total cholesterol, blood pressure and cigarette smoking to the aetiology of coronary heart disease: impact of regression dilution bias. Eur Heart J. 2003;24:1719–1726. doi: 10.1016/s0195-668x(03)00471-8. [DOI] [PubMed] [Google Scholar]
- 33.Thompson S.R., McCaskie P.A., Beilby J.P. IL18 haplotypes are associated with serum IL-18 concentrations in a population-based study and a cohort of individuals with premature coronary heart disease. Clin Chem. 2007;53:2078–2085. doi: 10.1373/clinchem.2007.092692. [DOI] [PubMed] [Google Scholar]
- 34.Presta I., Andreozzi F., Succurro E. IL-18 gene polymorphism and metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:e5–e6. doi: 10.1016/j.numecd.2008.10.002. [DOI] [PubMed] [Google Scholar]
- 35.Liu W., Tang Q., Jiang H. Promoter polymorphism of interleukin-18 in angiographically proven coronary artery disease. Angiology. 2009;60:180–185. doi: 10.1177/0003319708319939. [DOI] [PubMed] [Google Scholar]
- 36.He M., Cornelis M.C., Kraft P. Genome-wide association study identifies variants at the IL18-BCO2 locus associated with interleukin-18 levels. Arterioscler Thromb Vasc Biol. 2010;30:885–890. doi: 10.1161/ATVBAHA.109.199422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pei F., Han Y., Zhang X. Association of interleukin-18 gene promoter polymorphisms with risk of acute myocardial infarction in northern Chinese Han population. Clin Chem Lab Med. 2009;47:523–529. doi: 10.1515/CCLM.2009.130. [DOI] [PubMed] [Google Scholar]
- 38.Grisoni M.L., Proust C., Alanne M. Lack of association between polymorphisms of the IL18R1 and IL18RAP genes and cardiovascular risk: the MORGAM Project. BMC Med Genet. 2009;10:44. doi: 10.1186/1471-2350-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hernesniemi J.A., Anttila K., Nieminen T. IL-18 gene polymorphism, cardiovascular mortality and coronary artery disease. Eur J Clin Invest. 2010;40:994–1001. doi: 10.1111/j.1365-2362.2010.02356.x. [DOI] [PubMed] [Google Scholar]
- 40.Arimitsu J., Hirano T., Higa S. IL-18 gene polymorphisms affect IL-18 production capability by monocytes. Biochem Biophys Res Commun. 2006;342:1413–1416. doi: 10.1016/j.bbrc.2006.02.096. [DOI] [PubMed] [Google Scholar]
- 41.Barbaux S., Poirier O., Godefroy T. Differential haplotypic expression of the interleukin-18 gene. Eur J Hum Genet. 2007;15:856–863. doi: 10.1038/sj.ejhg.5201842. [DOI] [PubMed] [Google Scholar]
- 42.Khripko O.P., Sennikova N.S., Lopatnikova J.A. Association of single nucleotide polymorphisms in the IL-18 gene with production of IL-18 protein by mononuclear cells from healthy donors. Mediators Inflamm. 2008;2008:309721. doi: 10.1155/2008/309721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Casas J.P., Shah T., Cooper J. Insight into the nature of the CRP-coronary event association using Mendelian randomization. Int J Epidemiol. 2006;35:922–931. doi: 10.1093/ije/dyl041. [DOI] [PubMed] [Google Scholar]
- 44.Davey S.G., Harbord R., Ebrahim S. Fibrinogen C-reactive protein and coronary heart disease: does Mendelian randomization suggest the associations are non-causal? QJM. 2004;97:163–166. doi: 10.1093/qjmed/hch025. [DOI] [PubMed] [Google Scholar]
- 45.Grisoni M.L., Proust C., Alanne M. Haplotypic analysis of tag SNPs of the interleukin-18 gene in relation to cardiovascular disease events: the MORGAM Project. Eur J Hum Genet. 2008;16:1512–1520. doi: 10.1038/ejhg.2008.127. [DOI] [PubMed] [Google Scholar]
- 46.Hernesniemi J.A., Karhunen P.J., Oksala N. Interleukin 18 gene promoter polymorphism: a link between hypertension and pre-hospital sudden cardiac death: the Helsinki Sudden Death Study. Eur Heart J. 2009;30:2939–2946. doi: 10.1093/eurheartj/ehp316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.