Abstract
Research into the topic of the role of cholesterol and prostate disease has been ongoing for many years, however our mechanistic and translational understanding is still poor. Recent evidence indicates that cholesterol lowering drugs reduce the risk of aggressive prostate cancer, however the studies in this area, performed over many years, reflect much controversy and uncertainty. Here we explore the entire literature on the relationship between circulating cholesterol and prostate cancer, with consideration and criticism of the older as well as the newer studies. We consider why low cholesterol is associated with both increased and decreased risk of advanced prostate cancer, and explain why both observations are probably correct. We discuss the conflicting results of randomized placebo-controlled trials of statin drugs vs. observational studies and demonstrate that a predominance of pravastatin in the randomized trials paints a distorted view of statin effects. Lastly, we discuss new data suggesting that a critical aspect of the role of cholesterol in prostate cancer progression is through its role in intratumoral steroidogenesis. With these points addressed, the data strongly point to hypercholesterolemia as a risk factor for prostate cancer progression and suggest clinical opportunities for the use of cholesterol lowering therapies to alter disease course.
Keywords: prostate cancer, cholesterol, statins, steroidogenesis, castration resistance
“…it seems that the balance of evidence is…in favor of cholesterol’s playing at least some part in the growth of malignant tumors, and… in benign enlargement of the prostate.”.
—G. I. M. Swyer 1942
Cholesterol and prostate cancer: deconstructing a complex relationship
After over a hundred years of research into the topic of cholesterol and abnormal cell growth(1), much debate and substantial doubt remain concerning the effect of hypercholesterolemia on prostate disorders. These unresolved issues include whether drugs used to treat hypercholesterolemia alter the risk of prostate cancer. Much of the controversy stems from limitations in the existing literature and interpretations thereof that go beyond the data. In this review, we will highlight some of these points of debate and draw contrasts between established findings, inconsistencies between randomized trials and observational studies, and what we believe to be misinterpretations of published data sets. What emerges from a careful analysis of a large body of literature, going back many decades, is an integration of older findings and data in newer reports that support a conclusion that hypercholesterolemia is a risk factor for aggressive prostate cancer.
The prostate synthesizes cholesterol at rates equivalent to the liver and an age-dependent shift in cholesterol homeostasis allows cholesterol to accumulate in the prostate at high levels in older individuals(2). Cholesterol makes up about 30% of the lipid content of plasma membranes and is an absolute requirement for new membrane synthesis. This neutral lipid also contributes to the physiological properties of cell membranes by regulating membrane fluidity, promoting negative membrane curvature, and by interdigitating with acyl chains of phospholipids to create “liquid ordered” membrane microdomains. Cholesterol-rich microdomains are believed to play an important role in signal transduction and in other physiologic mechanisms such as solute transport. Because of the multiple roles of cholesterol in the cell, perturbations in cholesterol metabolism might conceivably alter epithelial, stromal and inflammatory cell infiltrates in the prostate. In a scenario involving rapid cell proliferation, the requirement for cholesterol assembly into new membranes may be a rate-limiting step in the process of tissue growth. Tumor cell proliferation, in turn, may affect circulating cholesterol levels. Here we review various aspects of this complex relationship.
Cancer and its effects on circulating cholesterol: the U-shaped curve
The concept of the “U shaped curve” refers to the higher levels of mortality found on either end of the cholesterol level spectrum that many investigators discovered when studies were performed to assess the potential relationship between circulating cholesterol and mortality. Although it is well established that the increase in mortality at the high end of the cholesterol spectrum is due to cardiovascular disease, the higher level of mortality at the low end of the spectrum is somewhat more mysterious and appears to emanate from a variety of sources. These include an unexplained higher frequency of accidental deaths in individuals with low cholesterol and an association between endemic hepatitis and low cholesterol. However, another important reason for higher mortality at the low end of the U arises from the cholesterol-lowering effect of cancer itself; therefore, the low end of the curve likely includes an excess of subjects with pre-existing cancer.
Although earlier papers speculated on this point, evidence implicating a direct effect of cholesterol lowering on increased cancer risk was first reported in 1971 by Pearce & Dayton(3). These investigators examined cancer incidence and cancer-specific deaths in two patient cohorts, one that received a control diet and the other an experimental cholesterol-lowering diet. The experimental diet was essentially the same as control, but with less cholesterol and with additional polyunsaturated fats. These authors found that within 10 years of the study’s initiation there was more total cancer (81 vs. 66), more cancer deaths (31 vs. 17) and, for our purposes, more prostate cancers (12 vs. 10) in the experimental diet cohort, although the prostate cancer differences are small. A review of 5 diet trials published in 1971(4) did not support a cholesterol-lowering diet and cancer association (OR for cancer incidence was 1.15 95% CI (0.81–1.63); for cancer death it was 1.08 95% CI (0.71–1.69)) and other, subsequent, cholesterol-lowering diet trials did not confirm the observations of Pearce and Dayton(5). A review of pre-statin cholesterol lowering trials in 1988(6), in which 22 randomized trials including ≈40,000 individuals were discussed (only 2 of which are cited), found no evidence suggesting an association between low cholesterol and cancer risk. This contrasts with a 1990 meta-analysis(7) of 6 cholesterol lowering trials, which used stringent criteria for selection of included studies. This review demonstrated a significant increase in overall cancer mortality (OR 1.43 95% CI (1.08–1.90)) that remained even when studies not including drugs (2 studies) were used in the analysis (OR 1.62 95% (CI 1.03–2.57)).
Randomized, placebo- or diet-controlled cholesterol lowering trials were largely equivocal in their conclusions concerning a cholesterol-cancer association, and population studies were equally ambiguous. Rose et al.(8) (1974) pooled data from 6 prospective population studies (more on these studies below) to demonstrate a low cholesterol-colon cancer link, with colon cancer patients having on average a 10 mg/dL reduction in total cholesterol vs. the population mean. The conclusions of this early study were buttressed by a number of additional population studies that showed excess cancers in the cohorts with the lowest cholesterol.
We have reviewed 52(9–60) population studies that reported on cholesterol and total cancer incidence and/or mortality that were published from 1972–2009. In total, these reports cover 79.5 million men and women ages 15–99 (average 38–63) from Finland, Yugoslavia, USA, New Zealand, Italy, France, Japan, China, Scotland, England, Norway, Israel, Australia and Sweden. 32 studies report an inverse association between cancer risk and cholesterol level, 16 show no association, 2 provided no statistics, 1 was a follow-up report, and 1 study was largely equivocal. Studies that demonstrate excess risk usually find this association more prominently in men and, most frequently, the associated cancers are those of the liver, colon, and lung (probably due to the high rates of mortality seen with these cancers). Many of these studies were long in duration (as long as 40 years); consequently, a number of the authors transformed the data by removing cancer cases that appeared in the studies’ early years (defined by authors as those occurring anywhere from 2–20 years after study initiation). In 12 of a total of 30 of these reports, removal of cancers that appeared early in the study either diminished or eliminated the significance of the low cholesterol-increased cancer risk association, suggesting that lower cholesterol was not the cause but the result of cancer.
One intriguing aspect of the above population studies that seems to strongly support the hypothesis that the inverse correlation between cancer and cholesterol level is due to an effect of cancer on cholesterol level is that there is no absolute ‘low level’ of cholesterol associated with cancer. For example, an association is found when the low cholesterol level for a population is ≤230(26) or ≤134(25), i.e., the 20% of any cohort with the lowest cholesterol in any population seems to have a greater prevalence of cancer than groups within that population with higher cholesterol. Logically, one would predict that if low cholesterol triggered cancer it would do so at a relatively uniform cholesterol level, and that if such an association existed, populations with low cholesterol would show an excess of certain cancers, an outcome that population studies do not support. Instead, if cancer reduces cholesterol level we would expect the 20% of the population with the lowest cholesterol to have excess cancer regardless of the endemic cholesterol level of the population, a prediction that is supported by the population studies when considered in aggregate.
A second interesting point that appears to demonstrate that low cholesterol is the result of the effect of cancer on the host, and not the cause of cancer, arises from studies that measured cholesterol proximal to time of death. Keys et al. show that men who died of cancer within 2 years of study onset had cholesterol values 9.48% lower than the average of all men at entry(25). Sherwin et al. demonstrated that men who developed cancer exhibited a 22.7 mg/dL drop in cholesterol level vs. matched survivors(44). The International Collaborative Group showed that individuals dying from cancer 1 year after cholesterol measurement had cholesterol levels that were 24–35 mg/dL lower than controls (i.e. those not dying); those dying 2–5 years after cholesterol measure demonstrated values that were 4–5 mg/dL lower than controls; and those dying of cancer 6–10 years after cholesterol measure had cholesterol values 2 mg/dL lower than controls(10). These findings likely reflect the tendency of cancer to depress circulating cholesterol levels.
Particularly revealing are studies of cholesterol level variation over time in relation to cancer incidence or death. There are only a few of these reports because they require multiple cholesterol measures over time and few studies were designed to capture these data. The first such report we analyzed is by Sorlei and Feinleib (1982)(46), which used Framingham Heart Study data and showed that in some individuals who develop cancer, cholesterol levels are lower than the mean value up to 18 years prior to cancer diagnosis, while in other individuals cholesterol levels decline near the time of diagnosis. The robustness of the analysis is hampered by the fact that the authors split the group by decades of age and sex, thus making each cohort quite small. The authors write “Although the Framingham data are not conclusive, they do suggest that in some cancer cases where the serum cholesterol level was lower than that expected at as much as 16--18 years before cancer diagnosis, the depressed level was likely to be a precursor to the tumor growth. However, consistent with the metabolic consequences of tumor growth, the data show that in some cancer cases, serum cholesterol had decreased at measurements made close to the time of cancer diagnosis.” Pekkanen et al. 1992(33), studying Finnish men (aged 55–74), analyzed the change in cholesterol levels from 1959–1974 (every 5 years) in individuals that did not have cardiovascular disease in 1974. The authors found that older men (aged 65–74 in 1974) with the steepest decline in cholesterol had a higher risk of dying from cancer. In 1992 Pocock and Seed(37) reported cholesterol time trend data from British men and women with hypertension (aged 65–74) demonstrating that cholesterol levels fell an average of 11.2 mg/dL in men within a year prior to a cancer death. Sharp and Pocock (1997)(42), again using Framingham Heart Study data, demonstrate a number of highly relevant findings: 1) 61% of individuals demonstrate a 12.6 [95% CI (8.46–16.70)] mg/dL decline in cholesterol within 2 years prior to a cancer death; 2) The mean level of cholesterol 2–4 years prior to death from cancer is 10 mg/dL lower than the population norm; 3) The odds of dying of cancer within 4 years were increased (2.11 95% CI (1.41.–3.14)) in individuals whose decline in cholesterol was greater than 38 mg/dL.
Whether low cholesterol causes cancer or low cholesterol is the result of cancer has important implications for public health. Not surprisingly, the National Institutes of Health was concerned, especially in light of the large body of evidence that high cholesterol is associated with death from cardiovascular disease (nearly all the population studies of cholesterol and mortality verified this), and the international reaction to reduce cholesterol levels broadly in the human population. At least 3 NIH conferences(61,62) were held to explore the relationship between cholesterol and mortality, and cancer mortality, specifically: the first was held in February of 1980, and the second soon after in May of the same year(61); a third was held in October of 1990(62). Since the report of Jacobs(62) is the most thorough, with its inclusion of 19 population studies in its analysis, we will briefly explore this report with regards to cancer. After elimination of cancer deaths occurring within 5 years of the onset of each individual study, the cancer rate ratio for men with cholesterol <160 mg/dL was between 1.18 (p<0.05) and 1.23 (p<0.001), depending on how the studies were analyzed. For women the cancer rate ratio was 1.05 (not significant). Significant associations between low cholesterol and cancer were found for lung cancer in both men and women, and other cancers (not specified) in women. No association was found between low cholesterol and colon cancer. The summarized conclusion from the 3 NIH sponsored conferences was that there was insufficient data regarding the association between cholesterol and cancer. They cite multiple concerns, including: 1) the modest increase in cancer among the low cholesterol population, 2) the lack of a clear association in women, 3) the presence of the cancer-cholesterol association in some populations but not others, 4) the presence of a presumptive effect in some but not all studies, and 5) the absence of a plausible biological mechanism that would explain why “lower,” but still physiological levels of cholesterol might trigger cancer. These considerations, as well as an absence of information about the effect of disease on cholesterol levels over time, prevented the conferees at any of the 3 meetings mentioned above from adopting specific recommendations on cholesterol reduction therapy. The recommendation of all 3 NIH conferences on the subject of low cholesterol and mortality was not to alter the prescription of lowering cholesterol levels to improve public health, but to continue to study the issue.
The concern that low cholesterol might increase cancer risk persisted until the early 1990’s, however it has almost entirely disappeared in the post-statin era. Multiple meta-analyses demonstrate that statins, which potently reduce cholesterol levels, do not cause an increase in cancer(63–65). This leaves the conclusion that cancer itself reduces circulating cholesterol as the only plausible explanation for the presumptive associations reported in the population studies described above.
What do the large population studies of overall disease incidence, mortality and cholesterol level, where prostate cancer was not an exclusive focus, indicate about prostate cancer, specifically? A small minority of the studies (10 out of 52 publications) we examined for this review include prostate cancer in the analysis, and the total number of prostate cancer cases combined in the studies is only 1,652. The following summarizes all of the major population studies that include prostate cancer as an endpoint. Knect et al.(26) reported a positive association between low cholesterol and increased prostate cancer risk (n=45), but did not follow their cohort for greater than 5 years. Kark et al. (24) found that individuals with prostate cancer (n=12) had significantly reduced cholesterol levels. Hiatt et al.(19) reported on 601 prostate cancers and found that removing cases occurring within the first two years after study inception eliminated the positive correlation between low cholesterol and cancer incidence. Williams et al.(53) reported on 44 prostate cancer cases, but do not comment specifically on a cholesterol-prostate cancer association. Wingard et al.(54) reported on 49 prostate cancer cases, but found no cholesterol-cancer association. Morris et al.(31) found a significant low cholesterol-cancer association (n=26). These investigators show that the 5-year prostate cancer incidence rates decreased step-wise with lower to higher cholesterol levels (6.9 deaths/1000 cases when cholesterol was ≤204 mg/dL; 6.5/1000 in the 205–230 mg/dL quartile; 2.2/1000 in the 231–260 mg/dL quartile and 1.7 deaths/1000 cases in the ≥261 mg/dL quartile). Schatzkin et al.(40) reported on 95 prostate cancer deaths and found no cholesterol-cancer association. Tulinius et al.(49) describe no statistically significant association between cholesterol level and 524 incident prostate cancer cases. Davey-Smith et al.(45) reported on 92 prostate cancer deaths and in quintile analysis demonstrate a non-significant trend, with lower levels of cholesterol associated with reduced cancer risk; the HR corresponding to a 1 SD decline in cholesterol was 0.85 95% CI (0.69–1.04). Iso et al. found no low cholesterol-prostate cancer association in their 164 cases; instead they report a significant trend of higher levels of cholesterol associated with increased cancer risk (p for trend 0.0023), an association that disappeared when advanced cancers were removed from the analysis (p for trend 0.12). Collectively, these studies point to a modest association between low cholesterol and increased prostate cancer risk.
In contrast to the above reports that include limited numbers of prostate cancer cases, for the most part were of relatively short duration, and almost never report or comment on late stage disease (except as it pertains to death), studies that specifically address the potential association between serum cholesterol level and prostate cancer have large enough cohort sizes to allow a more thorough analysis. Thompson et al. 1989(66) found no cholesterol-prostate cancer association (n=100); the Asia Pacific Cohort Studies Collaboration 2007(67) (Huxley R.) reported on 308 prostate cancer deaths and found a greater number of deaths in the population with the highest cholesterol based on tertile analysis, but the difference was not significant (possibly due to small numbers). Platz et al. 2008(68) in a case-control analysis of men in the Health Professionals Follow-up Study demonstrated that men (n=698) with low cholesterol (Q1 in quartile analysis; actual cholesterol level is undefined because of assay complications) had a lower risk of high-grade prostate cancer (OR, 0.61 95% CI (0.39–0.98)). Platz et al. 2009(69) examined 1,251 incident prostate cancers and found that men with low cholesterol (<200 mg/dL) had a lower risk of high-grade disease (Gleason 8–10; OR, 0.41 95% CI (0.22–0.77)). Mondul et al. 2010(70) examined 438 incident prostate cancers and determined that men with cholesterol <240 mg/dL were less likely to develop high grade prostate cancer then men with cholesterol >240 mg/dL, results that were unchanged after eliminating cholesterol-lowering drug users. Batty et al. 2011(71), in a study that included 578 prostate cancer deaths, reported a greater number of cancer-specific deaths in the highest cholesterol tertile (<175 mg/dL HR 1.0 (reference population); 175–214 mg/dL HR 1.07 95% CI (0.87–1.32); >214 mg/dL HR 1.35 95% CI (1.11–1.65) p-value for trend = 0.003). Clearly, these reports do not support a low cholesterol-increased prostate cancer risk association, but instead suggest that men with high cholesterol are either at increased risk for prostate cancer or castrate-resistant disease.
A question of PSA
The answer to the question of why some studies support an association between low cholesterol and prostate cancer, while others support an association with high cholesterol, most likely reflects when the study was conducted. The FDA approved serum prostate specific antigen (PSA) as a prostate cancer biomarker in 1994, forever changing the diagnostic landscape in the field. With PSA testing, men now generally present clinically with early stage disease, years before any clinical symptoms would otherwise appear. Thus, cancer populations considered in studies published prior to 1994 include many more advanced cancers than studies published in the last 10 years. This important milestone also suggests that cholesterol readings in the pre-PSA era have a greater chance of being a product of tumor metabolism, leading to a low cholesterol-cancer association, whereas cholesterol measures in post-PSA studies are more likely to reflect the cholesterol environment prior to the development of cancer. This would lead to a positive correlation between high cholesterol and prostate cancer risk.
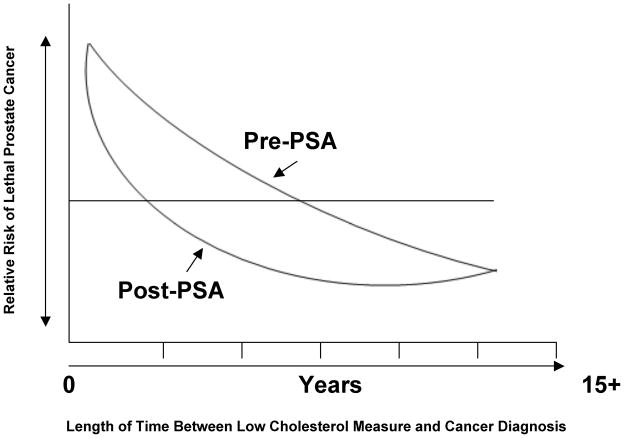
We propose a unifying model that reconciles the data from the pre- and post-PSA studies (Fig. 1). The most recent evidence indicates that high circulating cholesterol is a risk factor for prostate cancer(72). In the pre-PSA era a low cholesterol reading is more likely to be associated with a higher risk of a prostate cancer death; in the post-PSA era this association is reversed. This contradiction can be explained by the vastly different patient cohorts (with regard to extent of disease progression) analyzed in the older vs. the new studies, by preclinical findings that high circulating cholesterol promotes prostate cancer growth(73,74) and, by epidemiological data showing higher cholesterol levels increase prostate cancer risk. Additional evidence includes the apparent protective effects of long-term statin drug therapy on prostate cancer risk (considered below).
Figure 1.
Theoretical representation of the relationship between low cholesterol and the risk of prostate cancer death in the pre- and post-PSA eras.
However, there are important caveats that alter this simple equation, and these are best understood by considering the period of time between a “low” cholesterol measure and a prostate cancer diagnosis vs. the relative risk of prostate cancer death (Fig. 1). In both the pre- and post PSA eras a low cholesterol measure within 1 year of a prostate cancer diagnosis raises the risk of a prostate cancer death, while in both the pre- and post-PSA eras a low cholesterol measure >6 years prior to a prostate cancer diagnosis reduces the risk of a prostate cancer death (Fig. 1, left end of the curves vs. the right end of the curves). Between 1 and approximately 6 years prior to a prostate cancer diagnosis the tendency for low cholesterol to correlate with increased risk of prostate cancer death is different in the pre- and post-PSA eras. In the pre-PSA era a low cholesterol measure about 1–6 years prior to prostate cancer diagnosis raises the risk of a prostate cancer death, while in the post-PSA era a low cholesterol measure ~1–6 years prior to a prostate cancer diagnosis decreases the risk of prostate cancer death.
The statin controversy
Recent epidemiologic studies from a number of groups have shown that cholesterol-lowering drugs (primarily 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, known as “statins”) may lower prostate cancer risk, and in particular, the risk of advanced disease(75–87). Platz et al.(84) assessed potential statin drug effects specifically on prostate cancer in an analysis powered to measure differences in cancer incidence and progression in a total of 2,579 cancer cases, with 316 cases of advanced disease. The adjusted relative risk of castration resistant cancer among statin users in this study was 0.51 95% CI (0.30–0.86)) and of metastatic or fatal disease was 0.39 95% CI (0.19–0.77)) for statin users vs. nonusers. These authors also demonstrated that risk of advanced disease was lower with longer statin use (the relative risk for statin users of <5 years of use was 0.60 95% CI (0.35 –1.03)) and for ≥5 years of use was 0.26 95% CI (0.08–0.83)). In contrast to advanced disease, this study found no association between statin use and prostate cancer risk, suggesting that incidence alone is inadequate for evaluating potential chemopreventives in this disease.
Several more recent prospective studies from independent groups have largely confirmed the conclusions of Platz et al.(79,82–84) that statins reduce the risk of aggressive prostate cancer. Flick et al. performed a case-control analysis using a population of 69,047 participants in the California Men’s Health Study that included 888 total cases of prostate cancer, with 131 advanced cases. They concluded that use of statins for ≥5 yrs was associated with a 28% lower disease risk (adjusted rate ratio of 0.72 95% CI (0.53–0.99)). Jacobs et al. reported on a case-control study using 55,454 men from the Cancer Prevention Study II Nutrition Cohort, which included 3,413 cases of prostate cancer, with 317 cases of advanced disease. This group did not show a change in overall prostate cancer risk in the statin group; however, they found a marginally significant effect on advanced disease among the statin users (adjusted rate ratio of 0.60 95% CI (0.36–1.00)). Murtola et al. presented a case-control study of a large study population using data from the Finnish Cancer Registry, the Population Register Center, and the Social Insurance Institution of Finland (24,723 cases with an equal number of matched controls). This group found a significant reduction of risk of advanced prostate cancer in users of atorvastatin, lovastatin, and simvastatin (approximately 77% of the statin drug usage in the cohort), with an adjusted odds ratio of 0.61 95% CI (0.37–0.98); 0.61 95% CI (0.43–0.85); and 0.78 95% CI (0.61–1.01), respectively (the overall odds ratio for all statins was 0.75 95% CI (0.62–0.91)). Murtola et al. also noted that non-statin cholesterol-reducing drugs (fibrates specifically) modestly reduced the risk of advanced prostate cancer, but these data did not reach significance, possibly a result of a small sample size. This latter point is interesting. We have proposed that the chemopreventive effects of statin drugs arise predominantly or exclusively from cholesterol lowering, not from other statin effects, such as inhibition of isoprenoid synthesis(72); consequently, if this hypothesis is true, we would expect that other methods of cholesterol reduction would also modify risk. In summary, recent observational studies of statin effects on prostate cancer risk, which contain large numbers of subjects, are largely supportive of the hypothesis that statins reduce the risk of advanced prostate cancer.
Although the recent literature indicates that long-term statin therapy is chemopreventive against aggressive prostate cancer, large randomized trials of statin drugs that report on cancer (including prostate cancer) do not support this claim(63–65). There are a number of reasons that these two types of studies tend to present very different pictures regarding prostate cancer risk and statin use.
Large randomized placebo controlled trials of statins include small numbers of prostate cancers. We reviewed 49(88–136) trial reports that included 134,516 individuals and identified only 5 prostate cancer deaths and 1,142 incident prostate cancer cases, and these trials were of relatively short duration (4.2 years on average). Observational studies of prostate cancer risk and statin use include many more prostate cancer cases (77,325 in a total study population of 4,168,049), and they include follow-up to 14 years. This explains some of the discrepancy between randomized, placebo-controlled studies and observational studies, but there are some additional important differences that deserve attention.
Too much pravastatin?
Of the 34 separate randomized placebo-controlled trials that we analyzed that reported on the LDL reduction, including all the major statin trials (some of the 49 reports we evaluated were various analyses of the same initial trial, and others did not report on LDL reduction), 14 were trials of pravastatin(90–93,101,102,105,110–112,114,116,119,121–123,127,128,135,136), 6 were simvastatin trials(88,89,94,96,98,100,118,129,130,133,134), 6 were atorvastatin trials(99,113,115,117,120,126,131), 4 were trials of fluvastatin(107–109,124,125), 3 were lovastatin trials(97,103,106,132) and 1 was a rosuvaststin trial(104) (Table 1). Of the 56,095 individuals randomized to any statin, 46.5% were randomized to pravastatin. This contrasts greatly with statins used as reported in the observational studies, in which only 1.6% of the patients took pravastatin. This lower percentage of prevastatin is more similar to overall statin sales in which pravastatin only represents about 6% of the total(72). It is important to note this large difference in the use of a specific statin between these two types of reports. Statins are usually considered as an undifferentiated group in population studies, as if one statin drug was chemically and physiologically equivalent to another. However, statins are chemically diverse and have significantly different potencies with respect to cholesterol lowering. This large difference in pravastatin use in the various studies may have important consequences with regards to outcomes.
Table 1. Number of randomized, placebo-controlled statin trials that recorded LDL reduction, and percentage of LDL reduction achieved.
LDL reduction was calculated by subtracting the levels post-treatment with pre-treatment values in the statin cohort(88–93,95–97,99–102,104,106,107,109,110,113–117,119–122,124–128,130–132,135,136). In some studies, the placebo group also demonstrated LDL reduction. In these cases this value was subtracted from the reduction achieved in the treatment group.
| Statin Trials | Number of studies | LDL reduction (%) |
|---|---|---|
|
| ||
| Pravastatin | 14 | 24.3 ± 6.5 |
| Simvastatin | 6 | 32.6 ± 8.8 |
| Atorvastatin | 6 | 33.4 ± 8.9 |
| Fluvastatin | 4 | 25.5 ± 9.3 |
| Lovastatin | 3 | 30.6 ± 8.6 |
| Rosuvastatin | 1 | 41 |
Pravastatin is the weakest of the statin drugs in terms of its ability to inhibit HMG-CoA reductase (IC50 6.93 nM; Ki 23 × 10−10)(72) and consequently does not reduce total or LDL cholesterol to the same extent as other statins. This difference in potency seems to be reflected in the results of randomized studies of statin effects on cardiovascular disease. Although all statins seem to be similarly effective in reducing risk of cardiovascular disease, pravastatin was less effective than any other statin used in reducing LDL cholesterol (Table 1). In our analysis of these published data, pravastatin reduced LDL by an average of 24.3%. In contrast, simvastatin reduced LDL cholesterol by 32.5%, a difference that is statistically significant (p=0.01). Moreover, we calculated that the total percentage decline in LDL from all the randomized studies was 28.5%. However, if the patients randomized to take a statin were given statins in the percentage used in observational studies, we estimate that the decrease in LDL cholesterol would be about 32%, a potential difference of 3.5 mg/dL in LDL levels. Because so many patients were randomized to pravastatin, pharmacological cholesterol lowering in the study population is not as great as would be expected if the patients were taking statins outside of the study setting (i.e., in the proportions used by the general public). The consequences of this skewed mix of statins for detecting a statin effect on cancer incidence or progression is unknown. However, coupled with the short duration of most randomized studies, patient crossover from placebo to statin (as required by usual care), and patient selection bias (as we discussed in prior works)(72,137), this could entirely explain the differences in statin association with prostate cancer risk in randomized, placebo-controlled studies and observational studies.
How does cholesterol increase the risk of lethal prostate cancer?
As mentioned above, cholesterol plays a vital role in establishing the properties of functional animal cell membranes. It also affects various signaling pathways and proteins, either by direct conjugation to proteins, i.e. sonic hedgehog, or by modifying the activities of membrane proximal signaling pathways and proteins such as the cell survival kinase, AKT(138,139). Certain signal transduction pathways appear to be highly sensitive to manipulations in circulating cholesterol levels(73,74). In vivo, hypercholesterolemia causes increased tumor angiogenesis, reduces tumor apoptosis and increases tumor cell proliferation(73,74). The relative contribution(s) of these mechanisms to prostate cancer progression is unknown. We have extensively reviewed the above mechanisms in previous commentaries(72,140–143).
Recent studies have provided another mechanism for tumor-promoting effects of cholesterol that is highly relevant to prostate cancer. Prostate tumors respond to circulating androgen through the action of the androgen receptor (AR), a nuclear receptor that drives prostate cancer cell proliferation and survival even under conditions of hormone suppression during late-stage disease(144–146). Androgen Deprivation Therapy (ADT) remains the primary treatment strategy for advanced prostate cancer(144,147). Despite widespread early responses to therapy, prostate cancer almost invariably becomes castration resistant and the tumor cells continue to grow despite circulating androgen at castrate levels(144,145). In this phase of disease (termed CRPC), tumors become more aggressive. There are several theories about how this castration-resistant phenotype comes about: 1) gene amplification and/or mutation of the AR, allowing the receptor to be sensitive to low levels of androgen(148–154); 2) residual androgen production from the adrenal glands(155); and 3) promiscuous receptor-ligand interactions(150,156).
Although it was 30 years ago that Geller et al.(157) first reported that sufficient androgen to drive the AR remained in the prostate after ADT(157,158), the significance of this observation was partially obscured because other work appeared to demonstrate that co-administration of the anti-androgen flutamide, along with castration, totally ablated DHT activity, suggesting that any residual disease is not driven by androgen, per se. Notably, this latter finding was based on an n=4 and employed an assay that did not detect DHT levels <300pg/g tissue(159). The combination of castration with an anti-androgen is commonly used to treat advanced prostate cancer. Indeed, results of a phase III trial using the CYP17 inhibitor, abiraterone, in men with CRPC showing a significant overall survival benefit, strongly suggesting that for many men, CRPC is still driven, at least in part, by androgen(160–167). Several groups have presented data showing that prostate tumors in men receiving ADT contain androgen levels high enough to activate the AR(157,159–166,168–175).
Cholesterol and androgen synthesis
One major biological role of cholesterol is as the precursor for the synthesis of steroid hormones, including androgens. Enzymes and other proteins are essential in converting cholesterol into testosterone and dihydrotestosterone including STAR, obligatory for cholesterol transport into the mitochondria, CYP11A1 to convert cholesterol to pregnenolone, CYP17A & HSD3B to form androstenedione, and a 17β-hydroxysteroid dehydrogenase (e.g. AKR1C3 or HSD17B3) to convert androstenedione to testosterone.
Several lines of evidence indicate that prostate cancer cells in vivo synthesize their own androgens (i.e. de novo steroidogenesis), including in the castrate environment(173,176,177), in sufficient quantities to be biologically active. Locke et al.(176) demonstrated that all of the enzymes necessary for de novo androgen synthesis are expressed in LNCaP tumor xenografts, and that androgen-starved prostate cancer cells are capable of synthesizing DHT from acetic acid, suggesting that the entire pathway from acetate→cholesterol→DHT is intact in this model system(176). Montgomery et al. demonstrated that the full complement of enzymes comprising the steroidogenic pathways is present in the majority of human primary and metastatic prostate cancers examined(173), implying that de novo androgen synthesis is not merely an experimental phenomenon, but rather a potential underlying cause of disease progression in the CRPC phase of the disease.
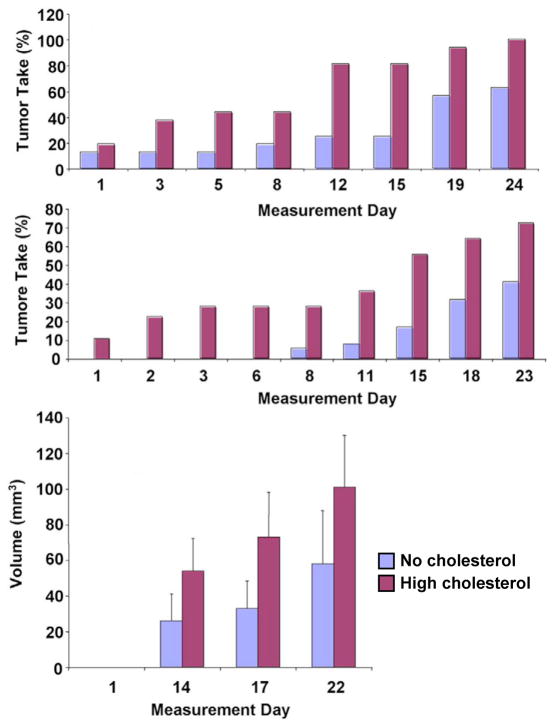
The ability of prostate cancer cells to synthesize androgen from cholesterol may actually increase following hormone suppression because castration raises serum cholesterol levels(172,178–181), and because cholesterol synthesis increases in experimental prostatic tumors in the castrate condition(182). We have noted this increase in serum cholesterol in castrated mice. We find that hypercholesterolemia accelerates the growth of human prostate cancer xenografts following castration (Fig. 2). New data from our group (Mostaghel et al manuscript in preparation) suggest that hypercholesterolemia contributes to androgen synthesis in prostate tumors, even in the castrate environment. Thus, the combination of castration and a rise in circulating cholesterol may actually increase the risk of CRPC progression.
Figure 2. Tumor growth in castrated mice.
Castrated mice were fed isocaloric no cholesterol or high cholesterol diets for 80 days. LNCaP cells were subsequently injected subcutaneously and the animals continued on their respective diets for 22 days. (A) Tumor take. The numbers of tumor in each diet group were counted and the data are plotted as tumor take (% of all implantation sites) vs. Time (days). Significance was determined by logistic regression analysis. At all time points where tumors were present, the 2 cohorts differed significantly. P<0.05. n=40/group. (B) Longitudinal volume measurements. Tumors were measured at various time points by calipers starting at first appearance (day 1) and continued until tumor burden required the animals to be sacrificed (day 22). Data are plotted as tumor volume (mm3) per site vs. Time (days) ± SE. n=40/group.
In humans, there are two potential sources of intratumoral androgens in the castrate environment; they are either products of the adrenal glands (potentially as both precursors and as actual androgens), or they are created de novo through intratumoral steroidogenesis. After day 14.5 of embryogenesis(183), adrenal glands in the mouse begin to lose expression of 17 α-hydroxylase (CYP17) and express little to none of the enzyme into adulthood. CYP17 is an essential enzyme required to convert pregnenolone to androstenedione, and consequently the murine adrenals produce little to no androgen or androgen precursors (e.g. androstenediol)(176,184). The lack of CYP17 in the adrenals of mice also explains why the major glucocorticoid present in the mouse is corticosterone instead of cortisol(185). In total, these data suggest, provocatively, that in the murine castrate environment prostatic tumors acquire the ability to synthesize T and DHT from intratumoral cholesterol, and do not require adrenal contributions. Whether this is also true in human patients remains to be established.
Cholesterol plays an essential role in steroidogenesis through its place as the precursor for all steroid hormones. However, we propose that cholesterol also plays an additional role as a steroidogenesis pathway agonist. Free cholesterol is cytotoxic and cells have multiple methods of reducing excess free cholesterol including: 1) upregulating the cholesterol efflux transporters, ABCA1, ABCG1, and ABCA7, 2) upregulating enzymes catalyzing the non-hepatic ‘acid’ pathway of bile acid synthesis, CYP27A1 and CYP7B1(186), 3) reducing expression of cholesterol receptors LDLR & SR-B1, 4) increasing cholesterol esterification through acyl-CoA cholesterol acyl transferase (ACAT), and 5) reducing cholesterol synthesis through regulation of HMG-CoA reductase expression. In addition, we propose that an additional mechanism for reducing the pool of free cholesterol is through the upregulation of the proteins/enzymes responsible for synthesizing androgens from cholesterol, i.e., de novo steroidogenesis within the tumor microenvironment. The relevance of these diverse mechanisms to prostate cancer remains to be tested in experimental models and in humans.
In summary, circulating cholesterol is a likely risk factor for aggressive prostate cancer. The multifaceted nature of the complex metabolic pathways in which cholesterol participates allows this lipid to play multiple roles in cancer progression. Further studies on the detailed mechanisms of cholesterol effects on prostate and other cancers are warranted, and could lead to new avenues for therapeutic intervention, particularly in controlling progression to late-stage disease.
Acknowledgments
This study was supported by grants from the NIH (R01CA101046 to K.R.S.; R01CA43777 to M.R.F.) and the US Department of Defense (W81XWH-08-1-0150 to M.R.F.).
Footnotes
Conflict of interest: The authors have nothing to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.White RM. On the occurance of crystals in tumours. J Pathol Bacteriol. 1909;13:3–10. [Google Scholar]
- 2.Schaffner CP. Prostatic cholesterol metabolism: regulation and alteration. Prog Clin Biol Res. 1981;75A:279–324. [PubMed] [Google Scholar]
- 3.Pearce ML, Dayton S. Incidence of cancer in men on a diet high in polyunsaturated fat. Lancet. 1971;1(7697):464–7. doi: 10.1016/s0140-6736(71)91086-5. [DOI] [PubMed] [Google Scholar]
- 4.Ederer F, Leren P, Turpeinen O, Frantz ID., Jr Cancer among men on cholesterol-lowering diets: Experience from five clinical trials. Lancet. 1971;2(7717):203–6. doi: 10.1016/s0140-6736(71)90911-1. [DOI] [PubMed] [Google Scholar]
- 5.Miettinen M, Turpeinen O, Karvonen MJ, Elosuo R, Paavilainen E. Effect of cholesterol-lowering diet on mortality from coronary heart-disease and other causes. A twelve-year clinical trial in men and women. Lancet. 1972;2(7782):835–8. doi: 10.1016/s0140-6736(72)92208-8. [DOI] [PubMed] [Google Scholar]
- 6.Yusuf S, Wittes J, Friedman L. Overview of results of randomized clinical trials in heart disease. II. Unstable angina, heart failure, primary prevention with aspirin, and risk factor modification. Jama. 1988;260(15):2259–63. [PubMed] [Google Scholar]
- 7.Muldoon MF, Manuck SB, Matthews KA. Lowering cholesterol concentrations and mortality: a quantitative review of primary prevention trials. Bmj. 1990;301(6747):309–14. doi: 10.1136/bmj.301.6747.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rose G, Blackburn H, Keys A, Taylor HL, Kannel WB, Paul O, Reid DD, Stamler J. Colon cancer and blood-cholesterol. Lancet. 1974;1(7850):181–3. doi: 10.1016/s0140-6736(74)92492-1. [DOI] [PubMed] [Google Scholar]
- 9.A co-operative trial in the primary prevention of ischaemic heart disease using clofibrate. Report from the Committee of Principal Investigators. Br Heart J. 1978;40(10):1069–118. doi: 10.1136/hrt.40.10.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Circulating cholesterol level and risk of death from cancer in men aged 40 to 69 years. Experience of an international collaborative group. Jama. 1982;248(21):2853–9. doi: 10.1001/jama.1982.03330210035031. [DOI] [PubMed] [Google Scholar]
- 11.Anderson KM, Castelli WP, Levy D. Cholesterol and mortality. 30 years of follow-up from the Framingham study. Jama. 1987;257(16):2176–80. doi: 10.1001/jama.257.16.2176. [DOI] [PubMed] [Google Scholar]
- 12.Beaglehole R, Foulkes MA, Prior IA, Eyles EF. Cholesterol and mortality in New Zealand Maoris. Br Med J. 1980;280(6210):285–7. doi: 10.1136/bmj.280.6210.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cambien F, Ducimetiere P, Richard J. Total serum cholesterol and cancer mortality in a middle-aged male population. Am J Epidemiol. 1980;112(3):388–94. doi: 10.1093/oxfordjournals.aje.a113004. [DOI] [PubMed] [Google Scholar]
- 14.Cowan LD, O’Connell DL, Criqui MH, Barrett-Connor E, Bush TL, Wallace RB. Cancer mortality and lipid and lipoprotein levels. Lipid Research Clinics Program Mortality Follow-up Study. Am J Epidemiol. 1990;131(3):468–82. doi: 10.1093/oxfordjournals.aje.a115521. [DOI] [PubMed] [Google Scholar]
- 15.Dyer AR, Stamler J, Paul O, Shekelle RB, Schoenberger JA, Berkson DM, Lepper M, Collette P, Shekelle S, Lindberg HA. Serum cholesterol and risk of death from cancer and other causes in three Chicago epidemiological studies. J Chronic Dis. 1981;34(6):249–60. doi: 10.1016/0021-9681(81)90030-8. [DOI] [PubMed] [Google Scholar]
- 16.Farchi G, Menotti A, Conti S. Coronary risk factors and survival probability from coronary and other causes of death. Am J Epidemiol. 1987;126(3):400–8. doi: 10.1093/oxfordjournals.aje.a114671. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Palmieri MR, Sorlie PD, Costas R, Jr, Havlik RJ. An apparent inverse relationship between serum cholesterol and cancer mortality in Puerto Rico. Am J Epidemiol. 1981;114(1):29–40. doi: 10.1093/oxfordjournals.aje.a113171. [DOI] [PubMed] [Google Scholar]
- 18.Gerhardsson M, Rosenqvist U, Ahlbom A, Carlson LA. Serum cholesterol and cancer--a retrospective case-control study. Int J Epidemiol. 1986;15(2):155–9. doi: 10.1093/ije/15.2.155. [DOI] [PubMed] [Google Scholar]
- 19.Hiatt RA, Fireman BH. Serum cholesterol and the incidence of cancer in a large cohort. J Chronic Dis. 1986;39(11):861–70. doi: 10.1016/0021-9681(86)90034-2. [DOI] [PubMed] [Google Scholar]
- 20.Isles CG, Hole DJ, Gillis CR, Hawthorne VM, Lever AF. Plasma cholesterol, coronary heart disease, and cancer in the Renfrew and Paisley survey. Bmj. 1989;298(6678):920–4. doi: 10.1136/bmj.298.6678.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iso H, Ikeda A, Inoue M, Sato S, Tsugane S. Serum cholesterol levels in relation to the incidence of cancer: the JPHC study cohorts. Int J Cancer. 2009;125(11):2679–86. doi: 10.1002/ijc.24668. [DOI] [PubMed] [Google Scholar]
- 22.Jancar J, Eastham RD, Carter G. Hypocholesterolaemia in cancer and other causes of death in the mentally handicapped. Br J Psychiatry. 1984;145:59–61. doi: 10.1192/bjp.145.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Kagan A, McGee DL, Yano K, Rhoads GG, Nomura A. Serum cholesterol and mortality in a Japanese-American population: the Honolulu Heart program. Am J Epidemiol. 1981;114(1):11–20. doi: 10.1093/oxfordjournals.aje.a113157. [DOI] [PubMed] [Google Scholar]
- 24.Kark JD, Smith AH, Hames CG. The relationship of serum cholesterol to the incidence of cancer in Evans County, Georgia. J Chronic Dis. 1980;33(5):311–32. doi: 10.1016/0021-9681(80)90026-0. [DOI] [PubMed] [Google Scholar]
- 25.Keys A, Aravanis C, Blackburn H, Buzina R, Dontas AS, Fidanza F, Karvonen MJ, Menotti A, Nedeljkovic S, Punsar S, et al. Serum cholesterol and cancer mortality in the Seven Countries Study. Am J Epidemiol. 1985;121(6):870–83. doi: 10.1093/oxfordjournals.aje.a114057. [DOI] [PubMed] [Google Scholar]
- 26.Knekt P, Reunanen A, Aromaa A, Heliovaara M, Hakulinen T, Hakama M. Serum cholesterol and risk of cancer in a cohort of 39,000 men and women. J Clin Epidemiol. 1988;41(6):519–30. doi: 10.1016/0895-4356(88)90056-x. [DOI] [PubMed] [Google Scholar]
- 27.Kozarevic D, McGee D, Vojvodic N, Gordon T, Racic Z, Zukel W, Dawber T. Serum cholesterol and mortality: the Yugoslavia Cardiovascular Disease Study. Am J Epidemiol. 1981;114(1):21–8. doi: 10.1093/oxfordjournals.aje.a113170. [DOI] [PubMed] [Google Scholar]
- 28.Kromhout D, Bosschieter EB, Drijver M, de Lezenne Coulander C. Serum cholesterol and 25-year incidence of and mortality from myocardial infarction and cancer. The Zutphen Study. Arch Intern Med. 1988;148(5):1051–5. [PubMed] [Google Scholar]
- 29.Menotti A, Keys A, Kromhout D, Nissinen A, Blackburn H, Fidanza F, Giampaoli S, Karvonen M, Pekkanen J, Punsar S, et al. All cause mortality and its determinants in middle aged men in Finland, The Netherlands, and Italy in a 25 year follow up. J Epidemiol Community Health. 1991;45(2):125–30. doi: 10.1136/jech.45.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Menotti A, Kromhout D, Blackburn H, Jacobs D, Lanti M. Forty-year mortality from cardiovascular diseases and all causes of death in the US Railroad cohort of the Seven Countries Study. Eur J Epidemiol. 2004;19(5):417–24. doi: 10.1023/b:ejep.0000027354.00742.c1. [DOI] [PubMed] [Google Scholar]
- 31.Morris DL, Borhani NO, Fitzsimons E, Hardy RJ, Hawkins CM, Kraus JF, Labarthe DR, Mastbaum L, Payne GH. Serum cholesterol and cancer in the Hypertension Detection and Follow-up Program. Cancer. 1983;52(9):1754–9. doi: 10.1002/1097-0142(19831101)52:9<1754::aid-cncr2820520933>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 32.Panagiotakos DB, Pitsavos C, Polychronopoulos E, Chrysohoou C, Menotti A, Dontas A, Stefanadis C. Total cholesterol and body mass index in relation to 40-year cancer mortality (the Corfu cohort of the seven countries study) Cancer Epidemiol Biomarkers Prev. 2005;14(7):1797–801. doi: 10.1158/1055-9965.EPI-04-0907. [DOI] [PubMed] [Google Scholar]
- 33.Pekkanen J, Nissinen A, Punsar S, Karvonen MJ. Short- and long-term association of serum cholesterol with mortality. The 25-year follow-up of the Finnish cohorts of the seven countries study. Am J Epidemiol. 1992;135(11):1251–8. doi: 10.1093/oxfordjournals.aje.a116231. [DOI] [PubMed] [Google Scholar]
- 34.Pekkanen J, Nissinen A, Vartiainen E, Salonen JT, Punsar S, Karvonen MJ. Changes in serum cholesterol level and mortality: a 30-year follow-up. The Finnish cohorts of the seven countries study. Am J Epidemiol. 1994;139(2):155–65. doi: 10.1093/oxfordjournals.aje.a116977. [DOI] [PubMed] [Google Scholar]
- 35.Peterson B, Trell E, Sternby NH. Low cholesterol level as risk factor for noncoronary death in middle-aged men. Jama. 1981;245(20):2056–7. [PubMed] [Google Scholar]
- 36.Peto R, Boreham J, Chen J, Li J, Campbell TC, Brun T. Plasma cholesterol, coronary heart disease, and cancer. Bmj. 1989;298(6682):1249. doi: 10.1136/bmj.298.6682.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pocock SJ, Seed PT. Cholesterol in elderly women. Lancet. 1992;339(8806):1426. doi: 10.1016/0140-6736(92)91254-6. [DOI] [PubMed] [Google Scholar]
- 38.Salmond CE, Beaglehole R, Prior IA. Are low cholesterol values associated with excess mortality? Br Med J (Clin Res Ed) 1985;290(6466):422–4. doi: 10.1136/bmj.290.6466.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salonen JT. Risk of cancer and death in relation to serum cholesterol. A longitudinal study in an eastern Finnish population with high overall cholesterol level. Am J Epidemiol. 1982;116(4):622–30. doi: 10.1093/oxfordjournals.aje.a113445. [DOI] [PubMed] [Google Scholar]
- 40.Schatzkin A, Hoover RN, Taylor PR, Ziegler RG, Carter CL, Albanes D, Larson DB, Licitra LM. Site-specific analysis of total serum cholesterol and incident cancer in the National Health and Nutrition Examination Survey I Epidemiologic Follow-up Study. Cancer Res. 1988;48 (2):452–8. [PubMed] [Google Scholar]
- 41.Schatzkin A, Hoover RN, Taylor PR, Ziegler RG, Carter CL, Larson DB, Licitra LM. Serum cholesterol and cancer in the NHANES I epidemiologic followup study. National Health and Nutrition Examination Survey. Lancet. 1987;2(8554):298–301. doi: 10.1016/s0140-6736(87)90890-7. [DOI] [PubMed] [Google Scholar]
- 42.Sharp SJ, Pocock SJ. Time trends in serum cholesterol before cancer death. Epidemiology. 1997;8(2):132–6. doi: 10.1097/00001648-199703000-00002. [DOI] [PubMed] [Google Scholar]
- 43.Sharper AG, Phillips AN, Pocock SJ. Plasma cholesterol, coronary heart disease, and cancer. BMJ. 1989;298:1381. [Google Scholar]
- 44.Sherwin RW, Wentworth DN, Cutler JA, Hulley SB, Kuller LH, Stamler J. Serum cholesterol levels and cancer mortality in 361,662 men screened for the Multiple Risk Factor Intervention Trial. Jama. 1987;257(7):943–8. [PubMed] [Google Scholar]
- 45.Smith GD, Shipley MJ, Marmot MG, Rose G. Plasma cholesterol concentration and mortality. The Whitehall Study. Jama. 1992;267(1):70–6. [PubMed] [Google Scholar]
- 46.Sorlie PD, Feinleib M. The serum cholesterol-cancer relationship: an analysis of time trends in the Framingham Study. J Natl Cancer Inst. 1982;69(5):989–96. [PubMed] [Google Scholar]
- 47.Stemmermann GN, Chyou PH, Kagan A, Nomura AM, Yano K. Serum cholesterol and mortality among Japanese-American men. The Honolulu (Hawaii) Heart Program. Arch Intern Med. 1991;151(5):969–72. [PubMed] [Google Scholar]
- 48.Toshima H, Koga Y, Menotti A, Keys A, Blackburn H, Jacobs DR, Seccareccia F. The seven countries study in Japan. Twenty-five-year experience in cardiovascular and all-causes deaths. Jpn Heart J. 1995;36(2):179–89. doi: 10.1536/ihj.36.179. [DOI] [PubMed] [Google Scholar]
- 49.Tulinius H, Sigfusson N, Sigvaldason H, Bjarnadottir K, Tryggvadottir L. Risk factors for malignant diseases: a cohort study on a population of 22,946 Icelanders. Cancer Epidemiol Biomarkers Prev. 1997;6(11):863–73. [PubMed] [Google Scholar]
- 50.Vartiainen E, Du DJ, Marks JS, Korhonen H, Geng GY, Guo ZY, Koplan JP, Pietinen P, We GL, Williamson D, et al. Mortality, cardiovascular risk factors, and diet in China, Finland, and the United States. Public Health Rep. 1991;106(1):41–6. [PMC free article] [PubMed] [Google Scholar]
- 51.Wald NJ, Thompson SG, Law MR, Densem JW, Bailey A. Serum cholesterol and subsequent risk of cancer: results from the BUPA study. Br J Cancer. 1989;59(6):936–8. doi: 10.1038/bjc.1989.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westlund K, Nicolaysen R. Ten-year mortality and morbidity related to serum cholesterol. A follow-up of 3.751 men aged 40–49. Scand J Clin Lab Invest Suppl. 1972;127:1–24. [PubMed] [Google Scholar]
- 53.Williams RR, Sorlie PD, Feinleib M, McNamara PM, Kannel WB, Dawber TR. Cancer incidence by levels of cholesterol. Jama. 1981;245(3):247–52. [PubMed] [Google Scholar]
- 54.Wingard DL, Criqui MH, Holdbook MJ, Barrett-Connor E. Plasma cholesterol and cancer morbidity and mortality in an adult community. J Chronic Dis. 1984;37(5):401–6. doi: 10.1016/0021-9681(84)90107-3. [DOI] [PubMed] [Google Scholar]
- 55.Yaari S, Goldbourt, Even-Zohar S, Neufeld HN. Associations of serum high density lipoprotein and total cholesterol with total, cardiovascular, and cancer mortality in a 7-year prospective study of 10 000 men. Lancet. 1981;1(8228):1011–5. doi: 10.1016/s0140-6736(81)92184-x. [DOI] [PubMed] [Google Scholar]
- 56.Chen Z, Keech A, Collins R, Slavin B, Chen J, Campbell TC, Peto R. Prolonged infection with hepatitis B virus and association between low blood cholesterol concentration and liver cancer. Bmj. 1993;306(6882):890–4. doi: 10.1136/bmj.306.6882.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen Z, Peto R, Collins R, MacMahon S, Lu J, Li W. Serum cholesterol concentration and coronary heart disease in population with low cholesterol concentrations. Bmj. 1991;303(6797):276–82. doi: 10.1136/bmj.303.6797.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schuit AJ, Van Dijk CE, Dekker JM, Schouten EG, Kok FJ. Inverse association between serum total cholesterol and cancer mortality in Dutch civil servants. Am J Epidemiol. 1993;137(9):966–76. doi: 10.1093/oxfordjournals.aje.a116769. [DOI] [PubMed] [Google Scholar]
- 59.Tornberg SA, Holm LE, Carstensen JM, Eklund GA. Cancer incidence and cancer mortality in relation to serum cholesterol. J Natl Cancer Inst. 1989;81(24):1917–21. doi: 10.1093/jnci/81.24.1917. [DOI] [PubMed] [Google Scholar]
- 60.Petersson B, Trell E, Henningsen NC, Hood B. Risk factors for premature death in middle aged men. Br Med J (Clin Res Ed) 1984;288(6426):1264–8. doi: 10.1136/bmj.288.6426.1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feinleib M. Summary of a workshop on cholesterol and noncardiovascular disease mortality. Prev Med. 1982;11(3):360–7. doi: 10.1016/0091-7435(82)90059-7. [DOI] [PubMed] [Google Scholar]
- 62.Jacobs D, Blackburn H, Higgins M, Reed D, Iso H, McMillan G, Neaton J, Nelson J, Potter J, Rifkind B, et al. Report of the Conference on Low Blood Cholesterol: Mortality Associations. Circulation. 1992;86(3):1046–60. doi: 10.1161/01.cir.86.3.1046. [DOI] [PubMed] [Google Scholar]
- 63.Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, Kirby A, Sourjina T, Peto R, Collins R, Simes R. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet. 2005;366(9493):1267–78. doi: 10.1016/S0140-6736(05)67394-1. [DOI] [PubMed] [Google Scholar]
- 64.Browning DR, Martin RM. Statins and risk of cancer: a systematic review and metaanalysis. Int J Cancer. 2007;120(4):833–43. doi: 10.1002/ijc.22366. [DOI] [PubMed] [Google Scholar]
- 65.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. Jama. 2006;295(1):74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 66.Thompson MM, Garland C, Barrett-Connor E, Khaw KT, Friedlander NJ, Wingard DL. Heart disease risk factors, diabetes, and prostatic cancer in an adult community. Am J Epidemiol. 1989;129(3):511–7. doi: 10.1093/oxfordjournals.aje.a115162. [DOI] [PubMed] [Google Scholar]
- 67.Huxley R. The impact of modifiable risk factors on mortality from prostate cancer in populations of the Asia-Pacific region. Asian Pac J Cancer Prev. 2007;8(2):199–205. [PubMed] [Google Scholar]
- 68.Platz EA, Clinton SK, Giovannucci E. Association between plasma cholesterol and prostate cancer in the PSA era. Int J Cancer. 2008;123(7):1693–8. doi: 10.1002/ijc.23715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Platz EA, Till C, Goodman PJ, Parnes HL, Figg WD, Albanes D, Neuhouser ML, Klein EA, Thompson IM, Jr, Kristal AR. Men with low serum cholesterol have a lower risk of high-grade prostate cancer in the placebo arm of the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2009;18(11):2807–13. doi: 10.1158/1055-9965.EPI-09-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mondul AM, Clipp SL, Helzlsouer KJ, Platz EA. Association between plasma total cholesterol concentration and incident prostate cancer in the CLUE II cohort. Cancer Causes Control. 2010;21(1):61–8. doi: 10.1007/s10552-009-9434-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Batty GD, Kivimaki M, Clarke R, Davey Smith G, Shipley MJ. Modifiable risk factors for prostate cancer mortality in London: forty years of follow-up in the Whitehall study. Cancer Causes Control. 2011;22(2):311–8. doi: 10.1007/s10552-010-9691-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Solomon KR, Freeman MR. Do the cholesterol-lowering properties of statins affect cancer risk? Trends Endocrinol Metab. 2008;19(4):113–21. doi: 10.1016/j.tem.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 73.Solomon KR, Pelton K, Boucher K, Joo J, Tully C, Zurakowski D, Schaffner CP, Kim J, Freeman MR. Ezetimibe is an inhibitor of tumor angiogenesis. Am J Pathol. 2009;174(3):1017–26. doi: 10.2353/ajpath.2009.080551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhuang L, Kim J, Adam RM, Solomon KR, Freeman MR. Cholesterol targeting alters lipid raft composition and cell survival in prostate cancer cells and xenografts. J Clin Invest. 2005;115(4):959–68. doi: 10.1172/JCI200519935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Moyad MA. Heart healthy equals prostate healthy equals statins: the next cancer chemoprevention trial. Part I. Curr Opin Urol. 2005;15(1):1–6. doi: 10.1097/00042307-200501000-00002. [DOI] [PubMed] [Google Scholar]
- 76.Moyad MA, Merrick GS. Statins and cholesterol lowering after a cancer diagnosis: why not? Urol Oncol. 2005;23(1):49–55. doi: 10.1016/j.urolonc.2005.03.017. [DOI] [PubMed] [Google Scholar]
- 77.Moyad MA, Merrick GS. Cholesterol, cholesterol lowering agents/statins, and urologic disease: part I - knowing your numbers. Urol Nurs. 2006;26(2):156–9. [PubMed] [Google Scholar]
- 78.Moyad MA, Merrick GS, Butler WM, Wallner KE, Galbreath RW, Kurko B, Adamovich E. Statins, especially atorvastatin, may favorably influence clinical presentation and biochemical progression-free survival after brachytherapy for clinically localized prostate cancer. Urology. 2005;66(6):1150–4. doi: 10.1016/j.urology.2005.08.053. [DOI] [PubMed] [Google Scholar]
- 79.Flick ED, Habel LA, Chan KA, Van Den Eeden SK, Quinn VP, Haque R, Orav EJ, Seeger JD, Sadler MC, Quesenberry CP, Jr, Sternfeld B, Jacobsen SJ, Whitmer RA, Caan BJ. Statin Use and Risk of Prostate Cancer in the California Men’s Health Study Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2218–25. doi: 10.1158/1055-9965.EPI-07-0197. [DOI] [PubMed] [Google Scholar]
- 80.Friis S, Poulsen AH, Johnsen SP, McLaughlin JK, Fryzek JP, Dalton SO, Sorensen HT, Olsen JH. Cancer risk among statin users: a population-based cohort study. Int J Cancer. 2005;114(4):643–7. doi: 10.1002/ijc.20758. [DOI] [PubMed] [Google Scholar]
- 81.Graaf MR, Beiderbeck AB, Egberts AC, Richel DJ, Guchelaar HJ. The risk of cancer in users of statins. J Clin Oncol. 2004;22(12):2388–94. doi: 10.1200/JCO.2004.02.027. [DOI] [PubMed] [Google Scholar]
- 82.Jacobs EJ, Rodriguez C, Bain EB, Wang Y, Thun MJ, Calle EE. Cholesterol-lowering drugs and advanced prostate cancer incidence in a large u.s. Cohort. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2213–7. doi: 10.1158/1055-9965.EPI-07-0448. [DOI] [PubMed] [Google Scholar]
- 83.Murtola TJ, Tammela TL, Lahtela J, Auvinen A. Cholesterol-Lowering Drugs and Prostate Cancer Risk: A Population-based Case-Control Study. Cancer Epidemiol Biomarkers Prev. 2007;16(11):2226–32. doi: 10.1158/1055-9965.EPI-07-0599. [DOI] [PubMed] [Google Scholar]
- 84.Platz EA, Leitzmann MF, Visvanathan K, Rimm EB, Stampfer MJ, Willett WC, Giovannucci E. Statin drugs and risk of advanced prostate cancer. J Natl Cancer Inst. 2006;98(24):1819–25. doi: 10.1093/jnci/djj499. [DOI] [PubMed] [Google Scholar]
- 85.Breau RH, Karnes RJ, Jacobson DJ, McGree ME, Jacobsen SJ, Nehra A, Lieber MM, St Sauver JL. The association between statin use and the diagnosis of prostate cancer in a population based cohort. J Urol. 184(2):494–9. doi: 10.1016/j.juro.2010.03.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gutt R, Tonlaar N, Kunnavakkam R, Karrison T, Weichselbaum RR, Liauw SL. Statin use and risk of prostate cancer recurrence in men treated with radiation therapy. J Clin Oncol. 28(16):2653–9. doi: 10.1200/JCO.2009.27.3003. [DOI] [PubMed] [Google Scholar]
- 87.Murtola TJ. Statin use is associated with improved prostate cancer survival: is it time for a clinical trial? Expert Rev Anticancer Ther. 10(10):1563–7. doi: 10.1586/era.10.137. [DOI] [PubMed] [Google Scholar]
- 88.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–9. [PubMed] [Google Scholar]
- 89.Effect of simvastatin on coronary atheroma: the Multicentre Anti-Atheroma Study (MAAS) Lancet. 1994;344(8923):633–8. [PubMed] [Google Scholar]
- 90.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339(19):1349–57. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 91.Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell’Infarto Miocardico) Ital Heart J. 2000;1(12):810–20. [PubMed] [Google Scholar]
- 92.Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT) Jama. 2002;288(23):2998–3007. doi: 10.1001/jama.288.23.2998. [DOI] [PubMed] [Google Scholar]
- 93.Long-term effectiveness and safety of pravastatin in 9014 patients with coronary heart disease and average cholesterol concentrations: the LIPID trial follow-up. Lancet. 2002;359(9315):1379–87. doi: 10.1016/S0140-6736(02)08351-4. [DOI] [PubMed] [Google Scholar]
- 94.The effects of cholesterol lowering with simvastatin on cause-specific mortality and on cancer incidence in 20,536 high-risk people: a randomised placebo-controlled trial [ISRCTN48489393] BMC Med. 2005;3:6. doi: 10.1186/1741-7015-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Beishuizen ED, van de Ree MA, Jukema JW, Tamsma JT, van der Vijver JC, Meinders AE, Putter H, Huisman MV. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2004;27(12):2887–92. doi: 10.2337/diacare.27.12.2887. [DOI] [PubMed] [Google Scholar]
- 96.Bestehorn HP, Rensing UF, Roskamm H, Betz P, Benesch L, Schemeitat K, Blumchen G, Claus J, Mathes P, Kappenberger L, Wieland H, Neiss A. The effect of simvastatin on progression of coronary artery disease. The Multicenter coronary Intervention Study (CIS) Eur Heart J. 1997;18(2):226–34. doi: 10.1093/oxfordjournals.eurheartj.a015224. [DOI] [PubMed] [Google Scholar]
- 97.Blankenhorn DH, Azen SP, Kramsch DM, Mack WJ, Cashin-Hemphill L, Hodis HN, DeBoer LW, Mahrer PR, Masteller MJ, Vailas LI, Alaupovic P, Hirsch LJ. Coronary angiographic changes with lovastatin therapy. The Monitored Atherosclerosis Regression Study (MARS) Ann Intern Med. 1993;119(10):969–76. doi: 10.7326/0003-4819-119-10-199311150-00002. [DOI] [PubMed] [Google Scholar]
- 98.Chonchol M, Cook T, Kjekshus J, Pedersen TR, Lindenfeld J. Simvastatin for secondary prevention of all-cause mortality and major coronary events in patients with mild chronic renal insufficiency. Am J Kidney Dis. 2007;49(3):373–82. doi: 10.1053/j.ajkd.2006.11.043. [DOI] [PubMed] [Google Scholar]
- 99.Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, Thomason MJ, Mackness MI, Charlton-Menys V, Fuller JH. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364(9435):685–96. doi: 10.1016/S0140-6736(04)16895-5. [DOI] [PubMed] [Google Scholar]
- 100.Collins R, Peto R, Armitage J. The MRC/BHF Heart Protection Study: preliminary results. Int J Clin Pract. 2002;56(1):53–6. [PubMed] [Google Scholar]
- 101.Crouse JR, 3rd, Byington RP, Bond MG, Espeland MA, Craven TE, Sprinkle JW, McGovern ME, Furberg CD. Pravastatin, Lipids, and Atherosclerosis in the Carotid Arteries (PLAC-II) Am J Cardiol. 1995;75(7):455–9. doi: 10.1016/s0002-9149(99)80580-3. [DOI] [PubMed] [Google Scholar]
- 102.Crouse JR, Byington RP, Bond MG, Espeland MA, Sprinkle JW, McGovern M, Furberg CD. Pravastatin, lipids, and atherosclerosis in the carotid arteries: design features of a clinical trial with carotid atherosclerosis outcome. Control Clin Trials. 1992;13(6):495–506. doi: 10.1016/0197-2456(92)90206-f. [DOI] [PubMed] [Google Scholar]
- 103.Downs JR, Clearfield M, Tyroler HA, Whitney EJ, Kruyer W, Langendorfer A, Zagrebelsky V, Weis S, Shapiro DR, Beere PA, Gotto AM. Air Force/Texas Coronary Atherosclerosis Prevention Study (AFCAPS/TEXCAPS): additional perspectives on tolerability of long-term treatment with lovastatin. Am J Cardiol. 2001;87(9):1074–9. doi: 10.1016/s0002-9149(01)01464-3. [DOI] [PubMed] [Google Scholar]
- 104.Fellstrom BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Gronhagen-Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Suleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wuthrich RP, Gottlow M, Johnsson E, Zannad F. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360(14):1395–407. doi: 10.1056/NEJMoa0810177. [DOI] [PubMed] [Google Scholar]
- 105.Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM. Long-term follow-up of the West of Scotland Coronary Prevention Study. N Engl J Med. 2007;357(15):1477–86. doi: 10.1056/NEJMoa065994. [DOI] [PubMed] [Google Scholar]
- 106.Furberg CD, Adams HP, Jr, Applegate WB, Byington RP, Espeland MA, Hartwell T, Hunninghake DB, Lefkowitz DS, Probstfield J, Riley WA, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation. 1994;90(4):1679–87. doi: 10.1161/01.cir.90.4.1679. [DOI] [PubMed] [Google Scholar]
- 107.Herd JA, Ballantyne CM, Farmer JA, Ferguson JJ, 3rd, Jones PH, West MS, Gould KL, Gotto AM., Jr Effects of fluvastatin on coronary atherosclerosis in patients with mild to moderate cholesterol elevations (Lipoprotein and Coronary Atherosclerosis Study [LCAS]) Am J Cardiol. 1997;80(3):278–86. doi: 10.1016/s0002-9149(97)00346-9. [DOI] [PubMed] [Google Scholar]
- 108.Holdaas H, Fellstrom B, Cole E, Nyberg G, Olsson AG, Pedersen TR, Madsen S, Gronhagen-Riska C, Neumayer HH, Maes B, Ambuhl P, Hartmann A, Staffler B, Jardine AG. Long-term cardiac outcomes in renal transplant recipients receiving fluvastatin: the ALERT extension study. Am J Transplant. 2005;5(12):2929–36. doi: 10.1111/j.1600-6143.2005.01105.x. [DOI] [PubMed] [Google Scholar]
- 109.Holdaas H, Fellstrom B, Jardine AG, Holme I, Nyberg G, Fauchald P, Gronhagen-Riska C, Madsen S, Neumayer HH, Cole E, Maes B, Ambuhl P, Olsson AG, Hartmann A, Solbu DO, Pedersen TR. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361(9374):2024–31. doi: 10.1016/S0140-6736(03)13638-0. [DOI] [PubMed] [Google Scholar]
- 110.Jukema JW, Bruschke AV, van Boven AJ, Reiber JH, Bal ET, Zwinderman AH, Jansen H, Boerma GJ, van Rappard FM, Lie KI, et al. Effects of lipid lowering by pravastatin on progression and regression of coronary artery disease in symptomatic men with normal to moderately elevated serum cholesterol levels. The Regression Growth Evaluation Statin Study (REGRESS) Circulation. 1995;91(10):2528–40. doi: 10.1161/01.cir.91.10.2528. [DOI] [PubMed] [Google Scholar]
- 111.Kobashigawa JA, Katznelson S, Laks H, Johnson JA, Yeatman L, Wang XM, Chia D, Terasaki PI, Sabad A, Cogert GA, et al. Effect of pravastatin on outcomes after cardiac transplantation. N Engl J Med. 1995;333(10):621–7. doi: 10.1056/NEJM199509073331003. [DOI] [PubMed] [Google Scholar]
- 112.Kobashigawa JA, Moriguchi JD, Laks H, Wener L, Hage A, Hamilton MA, Cogert G, Marquez A, Vassilakis ME, Patel J, Yeatman L. Ten-year follow-up of a randomized trial of pravastatin in heart transplant patients. J Heart Lung Transplant. 2005;24(11):1736–40. doi: 10.1016/j.healun.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 113.Koren MJ, Hunninghake DB. Clinical outcomes in managed-care patients with coronary heart disease treated aggressively in lipid-lowering disease management clinics: the alliance study. J Am Coll Cardiol. 2004;44(9):1772–9. doi: 10.1016/j.jacc.2004.07.053. [DOI] [PubMed] [Google Scholar]
- 114.Mercuri M, Bond MG, Sirtori CR, Veglia F, Crepaldi G, Feruglio FS, Descovich G, Ricci G, Rubba P, Mancini M, Gallus G, Bianchi G, D’Alo G, Ventura A. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. Am J Med. 1996;101(6):627–34. doi: 10.1016/s0002-9343(96)00333-6. [DOI] [PubMed] [Google Scholar]
- 115.Mohler ER, 3rd, Hiatt WR, Creager MA. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation. 2003;108(12):1481–6. doi: 10.1161/01.CIR.0000090686.57897.F5. [DOI] [PubMed] [Google Scholar]
- 116.Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, Nakaya N, Nishimoto S, Muranaka M, Yamamoto A, Mizuno K, Ohashi Y. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368(9542):1155–63. doi: 10.1016/S0140-6736(06)69472-5. [DOI] [PubMed] [Google Scholar]
- 117.Newman CB, Szarek M, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HA, Demicco DA, Auster S, Fuller JH. The safety and tolerability of atorvastatin 10 mg in the Collaborative Atorvastatin Diabetes Study (CARDS) Diab Vasc Dis Res. 2008;5(3):177–83. doi: 10.3132/dvdr.2008.029. [DOI] [PubMed] [Google Scholar]
- 118.Pedersen TR, Wilhelmsen L, Faergeman O, Strandberg TE, Thorgeirsson G, Troedsson L, Kristianson J, Berg K, Cook TJ, Haghfelt T, Kjekshus J, Miettinen T, Olsson AG, Pyorala K, Wedel H. Follow-up study of patients randomized in the Scandinavian simvastatin survival study (4S) of cholesterol lowering. Am J Cardiol. 2000;86(3):257–62. doi: 10.1016/s0002-9149(00)00910-3. [DOI] [PubMed] [Google Scholar]
- 119.Pitt B, Mancini GB, Ellis SG, Rosman HS, Park JS, McGovern ME. Pravastatin limitation of atherosclerosis in the coronary arteries (PLAC I): reduction in atherosclerosis progression and clinical events. PLAC I investigation. J Am Coll Cardiol. 1995;26(5):1133–9. doi: 10.1016/0735-1097(95)00301-0. [DOI] [PubMed] [Google Scholar]
- 120.Pitt B, Waters D, Brown WV, van Boven AJ, Schwartz L, Title LM, Eisenberg D, Shurzinske L, McCormick LS. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. Atorvastatin versus Revascularization Treatment Investigators. N Engl J Med. 1999;341(2):70–6. doi: 10.1056/NEJM199907083410202. [DOI] [PubMed] [Google Scholar]
- 121.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. Cholesterol and Recurrent Events Trial investigators. N Engl J Med. 1996;335(14):1001–9. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 122.Salonen R, Nyssonen K, Porkkala-Sarataho E, Salonen JT. The Kuopio Atherosclerosis Prevention Study (KAPS): effect of pravastatin treatment on lipids, oxidation resistance of lipoproteins, and atherosclerotic progression. Am J Cardiol. 1995;76(9):34C–39C. doi: 10.1016/s0002-9149(99)80468-8. [DOI] [PubMed] [Google Scholar]
- 123.Sato S, Ajiki W, Kobayashi T, Awata N. Pravastatin use and the five-year incidence of cancer in coronary heart disease patients: from the prevention of coronary sclerosis study. J Epidemiol. 2006;16(5):201–6. doi: 10.2188/jea.16.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Serruys PW, de Feyter P, Macaya C, Kokott N, Puel J, Vrolix M, Branzi A, Bertolami MC, Jackson G, Strauss B, Meier B. Fluvastatin for prevention of cardiac events following successful first percutaneous coronary intervention: a randomized controlled trial. Jama. 2002;287(24):3215–22. doi: 10.1001/jama.287.24.3215. [DOI] [PubMed] [Google Scholar]
- 125.Serruys PW, Foley DP, Jackson G, Bonnier H, Macaya C, Vrolix M, Branzi A, Shepherd J, Suryapranata H, de Feyter PJ, Melkert R, van Es GA, Pfister PJ. A randomized placebo-controlled trial of fluvastatin for prevention of restenosis after successful coronary balloon angioplasty; final results of the fluvastatin angiographic restenosis (FLARE) trial. Eur Heart J. 1999;20(1):58–69. doi: 10.1053/euhj.1998.1150. [DOI] [PubMed] [Google Scholar]
- 126.Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, Collins R, Kjeldsen SE, Kristinsson A, McInnes GT, Mehlsen J, Nieminen M, O’Brien E, Ostergren J. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361(9364):1149–58. doi: 10.1016/S0140-6736(03)12948-0. [DOI] [PubMed] [Google Scholar]
- 127.Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, Ford I, Gaw A, Hyland M, Jukema JW, Kamper AM, Macfarlane PW, Meinders AE, Norrie J, Packard CJ, Perry IJ, Stott DJ, Sweeney BJ, Twomey C, Westendorp RG. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623–30. doi: 10.1016/s0140-6736(02)11600-x. [DOI] [PubMed] [Google Scholar]
- 128.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333(20):1301–7. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 129.Strandberg TE, Pyorala K, Cook TJ, Wilhelmsen L, Faergeman O, Thorgeirsson G, Pedersen TR, Kjekshus J. Mortality and incidence of cancer during 10-year follow-up of the Scandinavian Simvastatin Survival Study (4S) Lancet. 2004;364(9436):771–7. doi: 10.1016/S0140-6736(04)16936-5. [DOI] [PubMed] [Google Scholar]
- 130.Teo KK, Burton JR, Buller CE, Plante S, Catellier D, Tymchak W, Dzavik V, Taylor D, Yokoyama S, Montague TJ. Long-term effects of cholesterol lowering and angiotensin-converting enzyme inhibition on coronary atherosclerosis: The Simvastatin/Enalapril Coronary Atherosclerosis Trial (SCAT) Circulation. 2000;102(15):1748–54. doi: 10.1161/01.cir.102.15.1748. [DOI] [PubMed] [Google Scholar]
- 131.Wanner C, Krane V, Marz W, Olschewski M, Mann JF, Ruf G, Ritz E. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353(3):238–48. doi: 10.1056/NEJMoa043545. [DOI] [PubMed] [Google Scholar]
- 132.Waters D, Higginson L, Gladstone P, Kimball B, Le May M, Boccuzzi SJ, Lesperance J. Effects of monotherapy with an HMG-CoA reductase inhibitor on the progression of coronary atherosclerosis as assessed by serial quantitative arteriography. The Canadian Coronary Atherosclerosis Intervention Trial. Circulation. 1994;89(3):959–68. doi: 10.1161/01.cir.89.3.959. [DOI] [PubMed] [Google Scholar]
- 133.Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Krobot K, Steinbeck G, Seidel D, Reichart B. Simvastatin initiated early after heart transplantation: 8-year prospective experience. Circulation. 2003;107(1):93–7. doi: 10.1161/01.cir.0000043241.32523.ee. [DOI] [PubMed] [Google Scholar]
- 134.Wenke K, Meiser B, Thiery J, Nagel D, von Scheidt W, Steinbeck G, Seidel D, Reichart B. Simvastatin reduces graft vessel disease and mortality after heart transplantation: a four-year randomized trial. Circulation. 1997;96(5):1398–402. doi: 10.1161/01.cir.96.5.1398. [DOI] [PubMed] [Google Scholar]
- 135.Zanchetti A, Crepaldi G, Bond MG, Gallus G, Veglia F, Mancia G, Ventura A, Baggio G, Sampieri L, Rubba P, Sperti G, Magni A. Different effects of antihypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering by pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS--a randomized double-blind trial. Stroke. 2004;35(12):2807–12. doi: 10.1161/01.STR.0000147041.00840.59. [DOI] [PubMed] [Google Scholar]
- 136.Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. The Pravastatin Multinational Study Group for Cardiac Risk Patients. Am J Cardiol. 1993;72(14):1031–7. doi: 10.1016/0002-9149(93)90858-a. [DOI] [PubMed] [Google Scholar]
- 137.Freeman MR, Solomon KR, Moyad M. Statins and the risk of cancer. Jama. 2006;295(23):2720–1. doi: 10.1001/jama.295.23.2720-b. author reply 2721–2. [DOI] [PubMed] [Google Scholar]
- 138.Adam RM, Mukhopadhyay NK, Kim J, Di Vizio D, Cinar B, Boucher K, Solomon KR, Freeman MR. Cholesterol sensitivity of endogenous and myristoylated Akt. Cancer Res. 2007;67(13):6238–46. doi: 10.1158/0008-5472.CAN-07-0288. [DOI] [PubMed] [Google Scholar]
- 139.Zhuang L, Lin J, Lu ML, Solomon KR, Freeman MR. Cholesterol-rich lipid rafts mediate akt-regulated survival in prostate cancer cells. Cancer Res. 2002;62(8):2227–31. [PubMed] [Google Scholar]
- 140.Di Vizio D, Solomon KR, Freeman MR. Cholesterol and cholesterol-rich membranes in prostate cancer: an update. Tumori. 2008;94(5):633–9. doi: 10.1177/030089160809400501. [DOI] [PubMed] [Google Scholar]
- 141.Freeman MR, Cinar B, Kim J, Mukhopadhyay NK, Di Vizio D, Adam RM, Solomon KR. Transit of hormonal and EGF receptor-dependent signals through cholesterol-rich membranes. Steroids. 2007;72(2):210–7. doi: 10.1016/j.steroids.2006.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Freeman MR, Solomon KR. Cholesterol and prostate cancer. J Cell Biochem. 2004;91(1):54–69. doi: 10.1002/jcb.10724. [DOI] [PubMed] [Google Scholar]
- 143.Hager MH, Solomon KR, Freeman MR. The role of cholesterol in prostate cancer. Curr Opin Clin Nutr Metab Care. 2006;9(4):379–85. doi: 10.1097/01.mco.0000232896.66791.62. [DOI] [PubMed] [Google Scholar]
- 144.Harris WP, Mostaghel EA, Nelson PS, Montgomery B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat Clin Pract Urol. 2009;6(2):76–85. doi: 10.1038/ncpuro1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Singer EA, Golijanin DJ, Messing EM. Androgen deprivation therapy for advanced prostate cancer: why does it fail and can its effects be prolonged? Can J Urol. 2008;15(6):4381–7. [PubMed] [Google Scholar]
- 146.Singer EA, Golijanin DJ, Miyamoto H, Messing EM. Androgen deprivation therapy for prostate cancer. Expert Opin Pharmacother. 2008;9(2):211–28. doi: 10.1517/14656566.9.2.211. [DOI] [PubMed] [Google Scholar]
- 147.Huggins C, Hodges C. Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Research. 1941;1:293–97. doi: 10.3322/canjclin.22.4.232. [DOI] [PubMed] [Google Scholar]
- 148.Fenton MA, Shuster TD, Fertig AM, Taplin ME, Kolvenbag G, Bubley GJ, Balk SP. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin Cancer Res. 1997;3(8):1383–8. [PubMed] [Google Scholar]
- 149.Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, Sartor O, Taplin ME, Kantoff PW, Oh WK. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112(6):1247–53. doi: 10.1002/cncr.23304. [DOI] [PubMed] [Google Scholar]
- 150.Taplin ME. Drug insight: role of the androgen receptor in the development and progression of prostate cancer. Nat Clin Pract Oncol. 2007;4(4):236–44. doi: 10.1038/ncponc0765. [DOI] [PubMed] [Google Scholar]
- 151.Taplin ME, Balk SP. Androgen receptor: a key molecule in the progression of prostate cancer to hormone independence. J Cell Biochem. 2004;91(3):483–90. doi: 10.1002/jcb.10653. [DOI] [PubMed] [Google Scholar]
- 152.Taplin ME, Bubley GJ, Ko YJ, Small EJ, Upton M, Rajeshkumar B, Balk SP. Selection for androgen receptor mutations in prostate cancers treated with androgen antagonist. Cancer Res. 1999;59(11):2511–5. [PubMed] [Google Scholar]
- 153.Taplin ME, Bubley GJ, Shuster TD, Frantz ME, Spooner AE, Ogata GK, Keer HN, Balk SP. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N Engl J Med. 1995;332(21):1393–8. doi: 10.1056/NEJM199505253322101. [DOI] [PubMed] [Google Scholar]
- 154.Taplin ME, Rajeshkumar B, Halabi S, Werner CP, Woda BA, Picus J, Stadler W, Hayes DF, Kantoff PW, Vogelzang NJ, Small EJ. Androgen receptor mutations in androgen-independent prostate cancer: Cancer and Leukemia Group B Study 9663. J Clin Oncol. 2003;21(14):2673–8. doi: 10.1200/JCO.2003.11.102. [DOI] [PubMed] [Google Scholar]
- 155.Labrie F. Adrenal androgens and intracrinology. Semin Reprod Med. 2004;22(4):299–309. doi: 10.1055/s-2004-861547. [DOI] [PubMed] [Google Scholar]
- 156.Veldscholte J, Ris-Stalpers C, Kuiper GG, Jenster G, Berrevoets C, Claassen E, van Rooij HC, Trapman J, Brinkmann AO, Mulder E. A mutation in the ligand binding domain of the androgen receptor of human LNCaP cells affects steroid binding characteristics and response to anti-androgens. Biochem Biophys Res Commun. 1990;173(2):534–40. doi: 10.1016/s0006-291x(05)80067-1. [DOI] [PubMed] [Google Scholar]
- 157.Geller J, Albert J, Loza D. Steroid levels in cancer of the prostate--markers of tumour differentiation and adequacy of anti-androgen therapy. J Steroid Biochem. 1979;11(1B):631–6. doi: 10.1016/0022-4731(79)90092-x. [DOI] [PubMed] [Google Scholar]
- 158.Geller J, Albert J, Nachtsheim D, Loza D, Lippman S. Steroid levels in cancer of the prostate--markers of tumor differentiation and adequacy of anti-androgen therapy. Prog Clin Biol Res. 1979;33:103–11. [PubMed] [Google Scholar]
- 159.Belanger B, Belanger A, Labrie F, Dupont A, Cusan L, Monfette G. Comparison of residual C-19 steroids in plasma and prostatic tissue of human, rat and guinea pig after castration: unique importance of extratesticular androgens in men. J Steroid Biochem. 1989;32(5):695–8. doi: 10.1016/0022-4731(89)90514-1. [DOI] [PubMed] [Google Scholar]
- 160.Shah S, Ryan C. Abiraterone acetate for castration resistant prostate cancer. Expert Opin Investig Drugs. doi: 10.1517/13543781003639427. [DOI] [PubMed] [Google Scholar]
- 161.Ang JE, Olmos D, de Bono JS. CYP17 blockade by abiraterone: further evidence for frequent continued hormone-dependence in castration-resistant prostate cancer. Br J Cancer. 2009;100(5):671–5. doi: 10.1038/sj.bjc.6604904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Scher HI, Logothetis C, Molina A, Goodman OB, Sternberg CN, Chi KN, Kheoh TS, Haqq CM, Fizazi K, De Bono JS. Improved survival outcomes in clinically relevant patient subgroups from COU-AA-301, a phase III study of abiraterone acetate (AA) plus prednisone (P) in patients with metastatic castration-resistant prostate cancer (mCRPC) progressing after docetaxel-based chemotherapy. J Clin Oncol; 2011 Genitourinary Cancers Symposium; Orlando, FL: 2011. [Google Scholar]
- 163.Antonarakis ES, Eisenberger MA. Is abiraterone acetate well tolerated and effective in the treatment of castration-resistant prostate cancer? Nat Clin Pract Oncol. 2009;6(1):12–3. doi: 10.1038/ncponc1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Attard G, Reid AH, Yap TA, Raynaud F, Dowsett M, Settatree S, Barrett M, Parker C, Martins V, Folkerd E, Clark J, Cooper CS, Kaye SB, Dearnaley D, Lee G, de Bono JS. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J Clin Oncol. 2008;26(28):4563–71. doi: 10.1200/JCO.2007.15.9749. [DOI] [PubMed] [Google Scholar]
- 165.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, Martins V, Lee G, Kheoh T, Kim J, Molina A, Small EJ. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 28(9):1481–8. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed]
- 166.Danila DC, Morris MJ, de Bono JS, Ryan CJ, Denmeade SR, Smith MR, Taplin ME, Bubley GJ, Kheoh T, Haqq C, Molina A, Anand A, Koscuiszka M, Larson SM, Schwartz LH, Fleisher M, Scher HI. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J Clin Oncol. 28(9):1496–501. doi: 10.1200/JCO.2009.25.9259. [DOI] [PMC free article] [PubMed]
- 167.Attard G, Reid AH, A’Hern R, Parker C, Oommen NB, Folkerd E, Messiou C, Molife LR, Maier G, Thompson E, Olmos D, Sinha R, Lee G, Dowsett M, Kaye SB, Dearnaley D, Kheoh T, Molina A, de Bono JS. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J Clin Oncol. 2009;27(23):3742–8. doi: 10.1200/JCO.2008.20.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Madan RA, Arlen PM. Abiraterone. Cougar Biotechnology. IDrugs. 2006;9(1):49–55. [PubMed] [Google Scholar]
- 169.Reid AH, Attard G, Danila DC, Oommen NB, Olmos D, Fong PC, Molife LR, Hunt J, Messiou C, Parker C, Dearnaley D, Swennenhuis JF, Terstappen LW, Lee G, Kheoh T, Molina A, Ryan CJ, Small E, Scher HI, de Bono JS. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J Clin Oncol. 28(9):1489–95. doi: 10.1200/JCO.2009.24.6819. [DOI] [PMC free article] [PubMed]
- 170.Forti G, Salerno R, Moneti G, Zoppi S, Fiorelli G, Marinoni T, Natali A, Costantini A, Serio M, Martini L, et al. Three-month treatment with a long-acting gonadotropin-releasing hormone agonist of patients with benign prostatic hyperplasia: effects on tissue androgen concentration, 5 alpha-reductase activity and androgen receptor content. J Clin Endocrinol Metab. 1989;68(2):461–8. doi: 10.1210/jcem-68-2-461. [DOI] [PubMed] [Google Scholar]
- 171.Mohler JL, Gregory CW, Ford OH, 3rd, Kim D, Weaver CM, Petrusz P, Wilson EM, French FS. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10(2):440–8. doi: 10.1158/1078-0432.ccr-1146-03. [DOI] [PubMed] [Google Scholar]
- 172.Nishiyama T, Hashimoto Y, Takahashi K. The influence of androgen deprivation therapy on dihydrotestosterone levels in the prostatic tissue of patients with prostate cancer. Clin Cancer Res. 2004;10(21):7121–6. doi: 10.1158/1078-0432.CCR-04-0913. [DOI] [PubMed] [Google Scholar]
- 173.Montgomery RB, Mostaghel EA, Vessella R, Hess DL, Kalhorn TF, Higano CS, True LD, Nelson PS. Maintenance of intratumoral androgens in metastatic prostate cancer: a mechanism for castration-resistant tumor growth. Cancer Res. 2008;68(11):4447–54. doi: 10.1158/0008-5472.CAN-08-0249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mostaghel EA, Nelson PS. Intracrine androgen metabolism in prostate cancer progression: mechanisms of castration resistance and therapeutic implications. Best Pract Res Clin Endocrinol Metab. 2008;22(2):243–58. doi: 10.1016/j.beem.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Titus MA, Schell MJ, Lih FB, Tomer KB, Mohler JL. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin Cancer Res. 2005;11(13):4653–7. doi: 10.1158/1078-0432.CCR-05-0525. [DOI] [PubMed] [Google Scholar]
- 176.Locke JA, Guns ES, Lubik AA, Adomat HH, Hendy SC, Wood CA, Ettinger SL, Gleave ME, Nelson CC. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 2008;68(15):6407–15. doi: 10.1158/0008-5472.CAN-07-5997. [DOI] [PubMed] [Google Scholar]
- 177.Dillard PR, Lin MF, Khan SA. Androgen-independent prostate cancer cells acquire the complete steroidogenic potential of synthesizing testosterone from cholesterol. Mol Cell Endocrinol. 2008;295(1–2):115–20. doi: 10.1016/j.mce.2008.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Fillios LC, Kaplan R, Martin RS, Stare FJ. Some aspects of the gonadal regulation of cholesterol metabolism. Am J Physiol. 1958;193(1):47–51. doi: 10.1152/ajplegacy.1958.193.1.47. [DOI] [PubMed] [Google Scholar]
- 179.Haug A, Hostmark AT, Spydevold O, Eilertsen E. Hypercholesterolaemia, hypotriacylglycerolaemia and increased lipoprotein lipase activity following orchidectomy in rats. Acta Endocrinol (Copenh) 1986;113(1):133–9. doi: 10.1530/acta.0.1130133. [DOI] [PubMed] [Google Scholar]
- 180.Leblanc M, Belanger MC, Julien P, Tchernof A, Labrie C, Belanger A, Labrie F. Plasma lipoprotein profile in the male cynomolgus monkey under normal, hypogonadal, and combined androgen blockade conditions. J Clin Endocrinol Metab. 2004;89(4):1849–57. doi: 10.1210/jc.2003-031233. [DOI] [PubMed] [Google Scholar]
- 181.Pick R, Stamler J, Rodbard S, Katz LN. Effects of testosterone and castration on cholesteremia and atherogenesis in chicks on high fat, high cholesterol diets. Circ Res. 1959;7(2):202–4. doi: 10.1161/01.res.7.2.202. [DOI] [PubMed] [Google Scholar]
- 182.Leon CG, Locke JA, Adomat HH, Etinger SL, Twiddy AL, Neumann RD, Nelson CC, Guns ES, Wasan KM. Alterations in cholesterol regulation contribute to the production of intratumoral androgens during progression to castration-resistant prostate cancer in a mouse xenograft model. Prostate. 70(4):390–400. doi: 10.1002/pros.21072. [DOI] [PubMed] [Google Scholar]
- 183.Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143(11):4358–65. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- 184.van Weerden WM, Bierings HG, van Steenbrugge GJ, de Jong FH, Schroder FH. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992;50(12):857–61. doi: 10.1016/0024-3205(92)90204-3. [DOI] [PubMed] [Google Scholar]
- 185.Spackman DH, Riley V. Corticosterone concentrations in the mouse. Science. 1978;200(4337):87. doi: 10.1126/science.635580. [DOI] [PubMed] [Google Scholar]
- 186.An S, Jang YS, Park JS, Kwon BM, Paik YK, Jeong TS. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp Mol Med. 2008;40(4):407–17. doi: 10.3858/emm.2008.40.4.407. [DOI] [PMC free article] [PubMed] [Google Scholar]