Abstract
Hepatitis C virus (HCV) infection is a major cause of chronic liver disease, cirrhosis, and death; it is estimated that 180 million persons are infected with HCV worldwide. The consequences of HCV are worse in those who are coinfected with human immunodeficiency virus 1 (HIV-1), which is unfortunately a common scenario because of shared risk factors of the viruses. More studies into effects of HCV/HIV-1 coinfection are needed, but efforts have been hampered by limitations in our understanding of the combined pathogenesis of the 2 viruses. Gaining insight into the mechanisms that underlie the immunopathogenesis of these persistent viral infections could lead to new therapeutic strategies for patients with HCV/HIV-1 coinfection.
The abilities of human immunodeficiency virus 1 (HIV-1) and HCV to establish persistent infections present unique challenges to the immune system. A shared characteristic of these infections is that they result in high-grade chronic viremia within the host that, unless treated, lasts indefinitely. The disease course is initially asymptomatic but then, over years to decades, results in destruction of the immune system (in the case of HIV-1 infection) or advancing liver fibrosis (in the case of HCV infection). Each virus causes considerable global health problems, and the two often meet in the same host because of shared risk factors for infection.
The effects of HIV-1 on the pathogenesis of HCV infection are deleterious and include a higher rate of viral persistence, increased viral loads, a faster rate of fibrosis progression, and higher rates of hepatic decompensation.1 HIV-1/HCV–coinfected individuals have worse treatment outcomes following interferon (IFN)-based therapies compared with their HCV–mono-infected counterparts; moreover, liver transplantation is complicated for this population.2 These clinical observations are accompanied by a suboptimal understanding of the pathogenesis of coinfection.
We review what is known about how these viruses interact with their respective host immune responses in coinfected individuals, with a dual focus on pathways to viral persistence and mechanisms that might underlie accelerated liver disease. In particular, we examine the potential role of the order of infection. Although the lack of a suitable animal model of coinfection has hindered studies of the mechanisms that underlie the interactions between the viruses and their immune responses, data from human and simian studies have yielded a variety of recent insights that could improve our understanding of the pathogenesis of persistent viral infections and the links between infection, inflammation, and fibrosis.
Pathways to Viral Persistence
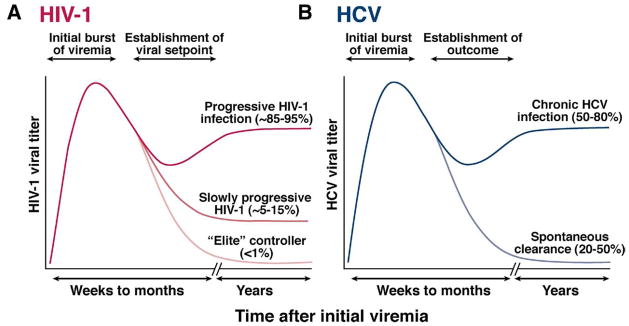
After host entry, certain viruses have found ways to persist indefinitely. Some persist in a latent phase, with periods of reactivation and viremia (eg, herpes viruses). By contrast, HIV-1 and HCV have each evolved the ability to achieve an immensely high rate of replication (~109 particles/day for HIV-1 and ~1012 for HCV) that generally continues for years. The HIV-1 life cycle is fundamentally different from that of HCV; HIV-1 is a retrovirus that integrates into host DNA via the viral reverse transcriptase enzyme and the integrase complex, whereas HCV replicates via an RNA-dependent RNA polymerase and does not have a DNA intermediate. HIV-1 replication can be spontaneously controlled in a minority of individuals after infection, resulting in low or even undetectable plasma levels of virus and delayed progression to acquired immunodeficiency syndrome (AIDS) (Figure 1A). Understanding the mechanisms of spontaneous control and these so-called “elite controllers” of HIV-1 could contribute to the development of a prophylactic or therapeutic vaccine.3
Figure 1.
Schematic representation of the outcomes of HIV-1 and HCV infection. Persistent viruses such as HIV-1 and HCV achieve high levels of chronic viral replication. The ultimate outcomes of HIV-1 and HCV infection depend on host-viral interactions. (A) After initial HIV-1 infection, the viral set point can vary considerably and is related to the ultimate speed of progression to AIDS. About 5%–15% of individuals experience slow progression, while a very small subset of individuals are termed “elite controllers” of HIV-1. (B) After HCV infection, a subset is able to control the virus over a sustained period, termed “spontaneous clearance” or “spontaneous control,” but the majority (~50%– 80%) progress to chronic viremia.
After HCV infection, viremia occurs within days after infection with large inoculums of virus (eg, via a blood transfusion) or 6 – 8 weeks after the more common exposure to a smaller volume of virus (eg, that contained within a contaminated needle).4 Ultimately, approximately 50%– 85% of infected individuals develop long-term persistent infections, characterized by a viral “set point” that generally does not vary by more than 10-fold (Figure 1B). A subset of hosts is able to clear the virus spontaneously. Viral clearance versus persistence is almost always determined during the first several months following infection, although cases of late spontaneous clearance have been documented.5–7 Factors associated with clearance of HCV include female gender, younger age at acquisition, jaundice during acute disease, and nonblack race.4
The host immune system plays a role in the control of these 2 pathogens, evidenced by the mechanisms that each virus has evolved to establish persistent infection. A partial list of mechanisms for persistence of HIV-1 and simian immunodeficiency virus (SIV) is summarized in Table 1. While a detailed consideration of the immunopathogenesis of HIV-1 is beyond the present scope of this discussion, we refer the interested reader to several recent reviews.3,8,9 As we turn to a more detailed discussion emphasizing the immunopathogenesis of HCV, we will highlight recent insights from studies on HIV-1 or SIV that are relevant.
Table 1.
Immune Responses and Postulated Viral Evasion Mechanisms or Interactions
| Host immune response | HCV | HIV-1 |
|---|---|---|
| Innate immunity | ||
| Activation of pathogen-associated molecular pattern via TLRs | Direct stimulation by single-stranded RNA elements17 | |
| Interferon signaling pathways | Core-mediated induction of SOCS3 NS3/4-mediated cleavage of CARDIF and TRIF NS5- or E2-mediated inhibition of PKR activity Reviewed in Chung et al11 |
Tat-mediated interference with PKR activity |
| NK cells | HCV E2 protein interference with NK receptors21 Aberrant cross talk with dendritic cells Reviewed in Golden-Mason and Rosen242 |
Altered expression of NK receptors Aberrant or decreased cytokine secretion Aberrant cross talk with dendritic cells Enhanced apoptosis Reviewed in Ianello et al20,243 |
| Regulators of adaptive immunity | ||
| Dendritic cells | Direct infection and altered function244 | Direct infection Decline in number and function Reviewed in Noursadeghi et al245 |
| Regulatory T cells | Suppression of antiviral responses Reviewed in Billerbeck and Thimme80 |
Suppression of antiviral responses Reviewed in de St Groth and Landay90 |
| Adaptive immunity | ||
| Neutralizing antibody responses | Mutational escape Reviewed in Stamataki et al246 |
Mutational escape Glycan shielding Altered complement Loss of potency with CD4 decline Suppressed class switching Reviewed in Moir and Fauci247 |
| Cell-mediated responses | ||
| T-helper cells (CD4) | Decreased function Exhaustion Apoptosis |
Direct killing Exhaustion Immune activation (producing more targets) Interference with MHC class II processing |
| Cytotoxic T cells (CD8) | Mutational escape Exhaustion Core-mediated down-regulation140 Reviewed in Bowen and Walker248 |
Mutational escape Exhaustion Nef-mediated endocytosis of MHC class I249 Vpu-mediated destabilization of MHC class I250 Reviewed in Goulder and Watkins251 |
NOTE. This table shows various examples of viral evasion mechanisms and does not detail every possible relevant finding or cite every relevant publication; we have included reviews for the reader when appropriate for further reading.
NS, nonstructural protein; MHC, major histocompatibility complex.
Role of the Innate Immune System in Containing Viruses
Viral RNA is recognized by the innate immune system, initially via Toll-like receptors (TLRs) or the retinoic acid inducible gene I helicase. This initial interaction activates downstream signaling pathways that up-regulate the transcription factors IFN regulatory factor 3 and nuclear factor κB, leading to production of type I IFNs and induction of other antiviral effects that form the first line of defense against intracellular pathogens (reviewed in Gale and Foy10 and Chung et al11). TLR7 agonism in particular has been pursued as a potential therapeutic intervention against HCV infection.12 Interestingly, HCV proteins have been shown to interfere with these pathways, suggesting direct evasion mechanisms to circumvent the innate immune system.13 Most intriguing is the observation that the HCV NS3/4 protease cleaves proteins that are required for innate immune signaling; this evasion strategy may be reversed by selective inhibitors of the HCV protease, such as telaprevir and boceprevir.14 –16 HIV-1 similarly engages these pathways, because it contains motifs within its nucleotide sequence that are recognized by TLR7 and TLR8.17
Innate effectors such as natural killer (NK) cells and natural killer T (NKT) cells also mediate antiviral defenses. For HCV, a genetic study showed that specific NK inhibitory receptor polymorphisms were associated with spontaneous clearance, but only in individuals with low initial inoculums (eg, injection drug users).18 This study suggested that large inoculums, such as that received from a blood transfusion, can overwhelm these defenses. Furthermore, disruption of NK cell function could promote chronicity by alterations induced by chronic HIV-1 infection (such as increased activation and/or decreased cytokine secretion of NK cells induced by HIV-1)19,20 or via direct interference by the HCV E2 protein,21 although this latter result has not been verified in all in vitro systems.22 NK and NKT cells constitute a significant proportion of liver-infiltrating lymphocytes during HCV infection,23 promote virus-specific adaptive responses,24 and in the case of NK cells can develop memory-like features.25 A recent murine study in a liver fibrosis model demonstrated a role of NK cells in clearing senescent hepatic stellate cells,26 which are activated by transforming growth factor (TGF)-β and are the primary profibrotic cell type within the liver.27 More studies of NK and NKT cells and their relationship to dendritic cell function, the clearance of hepatic stellate cells, and the development and maintenance of T-cell function in the setting of HIV-1 are needed.
Role of the Adaptive Immune System in Containing Viruses
The next line of host defense is the adaptive immune system, which often controls or eradicates viruses but, in most cases, ultimately fails to contain HCV. With regard to humoral responses, antibodies capable of neutralizing HCV in vitro have been recognized28; however, their initial production in vivo is delayed compared with responses against other viruses. By the time successful neutralizing antibodies are produced, it is likely that the virus has mutated beyond recognition by this arm of the immune system.29,30 Thus, HCV-infected individuals harbor antibodies that do not automatically provide immunity, as would be the case for antibody responses to other viruses, such as measles virus and varicella zoster virus. HCV reinfection of chimpanzees and humans is well documented, suggesting sterilizing immunity is rarely naturally acquired.31,32 Nonetheless, as part of a preventive vaccine, induction of strong neutralizing antibodies that target conserved and immunogenic epitopes within the HCV envelope protein might achieve sterilizing immunity (abrogation of infection after exposure) if their presence is established before infection.33
There is growing consensus on the importance of T-cell responses in determining HCV clearance. First, genetic studies provide evidence for the importance of cell-mediated responses; polymorphisms in the HLA class I and class II molecules on chromosome 6, which determine the specificities of the CD8 and CD4 responses, respectively, are associated with spontaneous clearance.34 –37 Second, high-level T-cell responses (both virus-specific CD4+ and CD8+ cells in particular) have been detected during acute disease compared with chronic disease. These cells are responsible for identification and clearance of virus, either by cytolysis of virus-infected cells or noncytolytic clearance via cytokine or chemokine-mediated effects. Their appearance in the peripheral blood and at the site of infection is temporally associated with control of viremia; their disappearance is correlated with loss of control.38–40 Third, longitudinal studies suggest the development of protective immunity, because those with evidence of spontaneous viral clearance are more likely to clear the virus upon reexposure41,42; depletion experiments in the chimpanzee model showed prolonged viremia if CD4+ or CD8+ T-cell memory responses were removed.43,44 These types of immune responses could represent a prime goal for prophylactic vaccines that elicit protective immunity45 and therapeutic vaccines (or immunotherapies) that reverse immune defects and boost immune function in infected individuals.46 A parallel body of literature supports the role of T cells in control of HIV-1 or SIV infection, including genetic studies associating certain HLA alleles with spontaneous control,3 studies of acutely infected individuals,47 and depletion experiments in simian models.48 Ultimately, T-cell responses fail to control replication of either virus in the majority of cases.
These 2 viruses mutate to escape the adaptive immune response via their high replicative capacities and error-prone polymerases. Mutational adaptation to the T-cell response has been shown longitudinally within an individual in humans and in simian models of HCV and HIV-1 infection.49 –54 Changes in or around recognized viral epitopes lead to lack of intracellular processing, loss of peptide binding to the HLA, and/or reduction of binding affinity of the HLA-peptide complex to the T-cell receptor.55 This phenomenon may be one of the major driving forces underlying the evolution of both HIV-1 and HCV (especially outside of the envelope proteins), because population studies have shown that key escape mutations are enriched in individuals with a corresponding class I HLA allele.56 –58 Given the significant differences between HCV genotypes at the amino acid level (cited as up to 30%), cross-genotype immunity may be difficult to achieve.32,59 – 61
T-cell exhaustion occurs as effector T cells become progressively less functional over time in the face of continued antigenic load, despite preservation of the cognate antigen. Cross-sectional studies have shown decreases in total numbers of T cells and functionality (largely their capacities to proliferate and secrete cytokines) in cases of persistent HCV infection.62– 64 Several T-cell markers have been identified in the mouse model of chronic lymphochoriomeningitis virus infection, including high levels of programmed death 1 (PD-1).65 Recent studies have extended these findings into human viral infections, including HIV-166 – 68 and HCV.69 –73 Because PD-1 is characteristically up-regulated on virus-specific CD8+ T cells in patients with chronic viremia for both HIV and HCV, it is hypothesized that modulation of PD-1 or its ligands increases T-cell function and perhaps helps control viral load in chronic disease.74,75 Whether these observations from in vitro or ex vivo experiments can be exploited in vivo without inducing autoimmunity in humans remains to be determined. Moreover, PD-1 is not the only marker that signals dysfunction in virus-specific T cells (other examples may be CTLA-4 and TIM-3),76,77 and modulation of a single pathway may not be enough to reverse T-cell dysfunction74 and consequently lower in vivo viral loads after established chronic infection. Despite these caveats, reversing counterregulatory pathways induced during chronic infections remains a promising avenue for immunotherapeutic strategies, as evidenced by a recent proof-of-principle study in the SIV model.78
In addition to the internal pathways within virus-specific T cells described previously, a variety of other cell subsets are responsible for their regulation, including dendritic cells responsible for their generation79 and T regulatory cells that counteract inflammatory responses.80 The function of plasmacytoid dendritic cells that secrete IFN-β in chronic infection may be impaired, although data on dendritic cell impairment in HCV infection are conflicting81– 84 and more studies of dendritic cell function during acute infection and in the setting of coinfection are needed. Regulatory T cells are induced by each of these chronic viral infections and produce inhibitory cytokines such as interleukin (IL)-10 85 that reduce proinflammatory cytokine secretion. Regulatory CD4 T-cell subsets that express the transcription factor forkhead box P3 (FOXP3) play key roles in both autoimmunity and viral infection, including that caused by HCV.86 – 88 FOXP3+ regulatory T-cell subsets are altered in HIV-1 infection, but the directionality of the effect is controversial89,90; further investigations are needed to understand how they may influence the pathogenesis of HCV, especially within the liver. Furthermore, CD8 T cells that secrete IL-10 and TGF-β in an antigen-specific manner have been detected.91,92 While the ability of these cells to dampen the immune system is beneficial in the short term to avoid overzealous cellular destruction, they may also play a role in the establishment of persistence and fibrosis within the liver, especially because TGF-β serves as an activator of hepatic stellate cells, the principal source of fibrinogen within the liver.
Ultimately, generation of an adaptive immune response against HCV is relatively delayed compared with that of other acute infections, possibly because of the tolerizing effect of adaptive responses primed in the liver.93 This delay in adaptive immunity has been linked to outcome in the chimpanzee model94 and may be linked to events of the innate immune response. Pathways linking innate and adaptive immunity are incompletely understood in chronic viral infections but will likely yield insights into the constitution of effective immune responses.
Parallels between the specific immune response to either HCV or HIV-1 abound, but several differences should be noted. First, specific T-cell responses are found at much higher frequencies against HIV-1 compared with HCV, especially when compared within the same individuals.95 A surprisingly high frequency of activated HIV-1–specific cells can be found in the setting of high HIV-1 viral loads,96,97 whereas HCV-specific cells are relatively infrequent in those with chronic HCV. Second, the tropism of the viruses as well as the sites of infection likely influence the respective T-cell responses, because HCV does not primarily infect CD4 T cells and HIV-1 has not been conclusively shown to replicate within the liver. Studies have suggested that HIV-1– and HCV-specific CD8 T cells have distinct phenotypes (with HCV-specific cells skewed toward a “central memory” phenotype).98 Further understanding of these differences may be highly relevant to what makes up an effective immune response.
HCV/HIV-1 Coinfection: The Sequence of Events
After reviewing how persistent RNA viruses such as HCV and HIV-1 evade the immune system, we next discuss why HIV-1 coinfection is associated with a higher likelihood of persistent outcome. The immune response in the context of coinfection with HIV and HCV is complex and differs based on the order of infection.
HCV Followed by HIV-1: Injection Drug Users and Blood-borne Infection
Epidemiologic studies have shown that HCV has a greater likelihood of transmission via a blood-borne route (such as by exposure to blood products or injection drug use) than HIV-1. There is an approximately 10-fold greater risk of HCV transmission after needlestick exposure compared with HIV-199 and a much higher prevalence of HCV than HIV-1 among injection drug users, possibly related to the greater concentrations of HCV than HIV-1 in a given volume of blood. Therefore, while cotransmission of both viruses can occur, coinfected individuals with blood-borne exposures were usually infected first with HCV, often years before HIV-1 infection.
Early cross-sectional studies that included mostly injection drug users showed that there is a higher rate of chronic HCV viremia among individuals who are coinfected with HIV-1 compared with those infected with only HCV. Higher rates of HCV persistence were inversely correlated with CD4+ T-cell counts.100 However, if the injection drug users were infected with HCV years before HIV-1, how could spontaneous clearance versus chronicity of HCV be affected by HIV-1, a much later event?
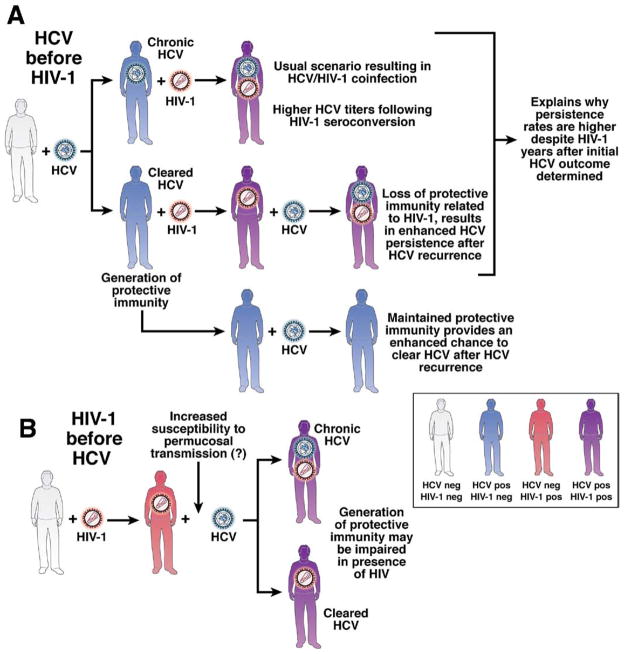
This question was addressed by a cross-sectional study that included 47 individuals who had spontaneously cleared HCV in the absence of anti-HCV therapy, 30 of whom were coinfected with HIV, and analyzed HCV-specific CD4+ T-cell proliferative responses,101 a hallmark of spontaneous clearance62 that is absent in HIV-1/HCV–coinfected persons (particularly in those who have had very low nadir CD4+ T-cell counts). When 6 of the coinfected individuals faced recurrent HCV infections, their eventual outcome was chronicity. Virus-specific responses declined when measured longitudinally and the degree of immunosuppression at the time of recurrent HCV was related to the eventual viral load.101 It was concluded that the secondary (or protective) immunity observed in other longitudinal cohorts41,42 was missing from those with HIV-1 infection because of significant immunosuppression (determined by CD4+ cell depletion). Although the mechanisms of CD4+ cell depletion were not perfectly analogous between studies, these findings were consistent with those from a HCV rechallenge study in chimpanzees; depletion of all CD4+ cells (and thus CD4+ cell-mediated responses) using monoclonal antibodies resulted in prolonged hepatitis C viremia and viral escape from CD8+ cell responses upon rechallenge.43 Thus, loss of secondary immunity against HCV provides a possible explanation for how coinfection with HIV-1 results in a higher persistence rate of HCV, even in patients who were not infected with HIV-1 when they were first infected with HCV (Figure 2A). Therefore, coinfected patients who are negative for HCV RNA and have been given antiretroviral therapy at an earlier stage in their HIV disease should be able to preserve HCV-specific immune responses and be partially protected against reinfection.
Figure 2.
Influence of the order of infection promoting the persistence of HCV in the setting of HIV-1 coinfection. Uninfected individuals are represented in gray, HCV antibody–positive individuals in blue, HIV-1 antibody–positive individuals in red, and dually HCV/ HIV-1 antibody–positive individuals in purple. (A) Most blood-borne exposures result in HCV infection well before HIV-1. The usual outcome is chronicity, represented in the first line. HCV titers become progressively higher after HIV-1 seroconversion. The second line represents the situation where protective immunity is generated. Progressive HIV-1 infection, signified by progressive CD4 T-cell dysfunction and depletion, may abrogate this protective immunity. When rechallenged with HCV, the likely outcome is chronicity. The third line represents a situation where HCV is cleared and HIV-1 is never contracted, and thus protective immunity is preserved. (B) When HIV-1 precedes HCV infection, the generation of protective immunity may be primarily impaired, thus resulting in a greater likelihood of persistent outcome. Together, this schematic may help to explain why there are higher persistence rates in coinfected individuals compared with mono-infected individuals.
Coinfected patients with chronic HCV infections have higher HCV titers when compared with individuals infected with only HCV. A study of sera collected from hemophiliac patients before and after HIV-1 seroconversion showed that viral loads increased by a significant amount following HIV-1 infection and more rapidly than in those who remained HIV-1 negative.102 This increase occurred before CD4+ T-cell numbers began to decline significantly, so other early effects on the immune system mediated by HIV-1 infection might be responsible for higher hepatitis C viremia in coinfected individuals. Later, as peripheral CD4+ cells counts decline, there could be a loss of HCV-specific antibody responses103,104 as well as functional HCV-specific CD8+ T-cell responses.105,106 However, HCV-specific CD4+ and CD8+ T cells, as currently measured, might not be linked to the viral set point. This is because HCV-specific T-cell responses are already significantly reduced in patients with chronic HCV and the magnitude of the specific immune responses against HCV in patients in the chronic phase does not correlate with viral load. The lack of correlation may relate to difficulties in measuring immune function at the site of infection (the hepatocyte), because most studies have relied on measurements either on intrahepatic lymphocytes that were expanded in vitro or on peripheral blood mononuclear cells. Ultimately, the basis for the magnitude of the HCV titer set point in each patient with a chronic infection is unknown107; it is likely to be related to contributions from the innate and adaptive immune systems, which in the case of coinfection are then modulated by many factors, including both HIV-1 and aging.108,109 Further investigations understanding the perturbations induced by HIV-1 infection will improve our understanding of the mechanisms that lead to the high viral titers in coinfected patients, an issue of prime importance because these affect responsiveness to IFN-based treatments.2
HIV-1 Then HCV: HCV Transmission in HIV-1–Infected Men Who Have Sex With Men
Transmission of HCV is far less efficient via sexual exposure than via blood-borne exposure, whereas HIV-1 is adapted to cross the mucosa found at genital and rectal surfaces. Thus, the prevalence of HCV is far lower among those who become infected with HIV-1 by sexual risk behaviors than blood-borne exposure.110 Numerous out- breaks of HCV infection have been reported throughout Europe and the United States among HIV-1–infected men who have sex with men (MSM).111–117 Studies of these outbreaks have provided insights into the natural history of HCV in the context of preexisting HIV-1 infection, which may be relevant to the immunopathogenesis of coinfection.
These outbreaks were initially reported from sexually transmitted disease clinics, based on results of liver function testing. Epidemiologic studies have shown that HCV infection is associated with sexual risk-taking behaviors that include a high number of partners, a greater likelihood for engaging in group sex, sexual acts that involve trauma, and higher rates of past sexually transmitted infections.116 One study reported that only 17% of HIV-1–infected MSM in London who were infected with HCV reported injection drug use, suggesting that percutaneous transmission is not primarily responsible as a route of transmission.116 HCV acquisition is also associated with higher rates of noninjection drug use (ie, 3,4-methylenedioxymethamphetamine [Ecstasy], crystal methamphetamine); it is not clear whether use of “party” drugs increases viral transmission independently of sexual risk behaviors.
The epidemic of HCV infection in MSM has occurred among HIV-1–infected individuals rather than HIV-1–negative MSM. Although HIV-1–infected MSM might engage in higher-risk practices, there are additional biological explanations for the increased HCV infection rate in this population. Source-related issues might increase transmission, because HIV-1/HCV– coinfected individuals have higher HCV viral loads than those with HCV alone. Recipient-related issues such as practices that cause trauma to mucosal surfaces and changes in local mucosal immunity that are present during early stages of HIV-1 infection might also facilitate HCV transmission. Furthermore, HIV-1 and SIV deplete CD4+ T cells of the gastrointestinal tract before those of the peripheral blood.118,119 This lack of mucosal defense could promote HCV transmission, even when those exposed are receiving antiretroviral therapy, which only partially reverses this defect.120 Depletion of CD4+ T cells from the mucosa could account for the increase in HCV infection among HIV-1–positive MSM, as compared with lower rates of infection in those who are HIV-1 negative but engage in similar practices.
Although HIV-1 infection seems to promote transmission of HCV, data on spontaneous clearance rates in HIV-1–positive MSM vary, ranging from 5% to 25%.121,122 Higher CD4+ T-cell counts were associated with spontaneous clearance of HCV121 and HCV-specific T-cell responses were reported in 4 of 4 individuals tested,123 suggesting no major defects in the ability of HIV-1–infected individuals to generate immunity against HCV. Although coinfected individuals usually have persistent HCV infections, spontaneous HCV clearance has been observed in these patients, indicating that HIV-1 infection alone does not preclude vaccine-induced HCV-specific immunity. However, one study showed that reduced CD4+ T-cell responses against HCV in patients with HIV-1 were associated with a high rate of HCV persistence (Figure 2B).122 In a small study, biopsy analyses revealed a high prevalence and degree of liver fibrosis among HIV-1–positive individuals who were recently infected with HCV.124 Studies of larger cohorts are needed to define the progression and pathogenesis of HCV-related fibrosis in HIV-1–infected MSM, especially due to potential confounding factors such as exposure to concomitant illicit drugs, alcohol, and antiretroviral medications.
HIV-1–negative4,125 and HIV-1–positive patients with acute HCV infections have high rates of response to IFN-based therapies (59%–90%).114,115,121,126,127 Among coinfected individuals, this differs from the treatment response rates of those with chronic-phase HCV infections, especially for genotypes 1 or 4 (sustained virological response rates to the combination of pegylated interferon and ribavirin range from 14% to 44%), despite the more intensive and prolonged regimens used to treat patients with chronic compared with acute disease.2 The mechanisms that underlie the increased treatment response among patients in the acute phase remain unclear but could be related to certain unique characteristics of acute infection, such as the presence of more vigorous immune responses, lower HCV viral set points, and lower diversity and complexity of viral quasispecies.4,128
Secondary Immunity or Therapeutic Vaccines for HCV/HIV-1–Coinfected Individuals
Preventive and therapeutic vaccines could be developed for coinfected individuals (whether first infected with HIV-1 or HCV). Therapeutic vaccines, which are designed to increase immunity against a virus, would activate CD4+ helper T-cell responses; CD8+ T cells are also likely to be necessary. For a therapeutic vaccine to be effective, it would need to overcome the high HCV titers observed in coinfected patients and be potent enough to avoid immediate viral escape via mutations. For preventive vaccines, the best strategy might be to increase anti-HCV immunity in patients who have cleared the virus or to induce protective immunity against HCV in those who are HIV-1 positive and at risk for acquiring HCV.
The recent failure of an adenovirus-based vaccine strategy designed to elicit CD8+ T-cell immunity against HIV-1 infection could dampen enthusiasm for similar approaches to prevent HCV infection. Despite demonstrated immunogenicity, a higher number of HIV-1 infections were observed in the vaccinated group compared with the placebo group.129 A recently developed adenoviral vector– based prophylactic vaccine against HCV could be more effective than the HIV-1 vaccine,45 because spontaneous HCV clearance is achieved in a greater proportion of infected individuals (20%–50%); in contrast, the “elite controllers” represent only ~0.3%– 0.5% of HIV-1–infected individuals.3 Moreover, the levels of cell-mediated immunity by IFN-γ ELISpot between individuals who have cleared HCV infection and those with chronic infection are clearly different in magnitude from each other, whereas between patients with HIV-1 that progress to AIDS and those that control the infection levels of cell-mediated immunity are often similar.63,130
The level of cell-mediated immunity might not be the only factor that determines HCV vaccine efficacy; CD4+ and CD8+ T-cell responses are required, but viruses can still escape immune detection via mutation. Due to the immense diversity of HCV (even greater than that of HIV-1), it is likely that prophylactic or therapeutic vaccines based on T cells will be genotype specific. In addition to quantity of T-cell responses, their quality, timing, and location are each of likely importance.94,131–133 An ideal T cell may need to produce multiple antiviral cytokines, have ample proliferative capacity, and be at the “right time” and “right place” to be effective. In regard to the timing and location of T-cell responses, improving our understanding of the mechanisms that underlie the delay in the onset of the acquired immune response against HCV (the link between innate immunity and acquired immunity) and the mechanisms that govern the homing of vaccine-induced T cells (including route of administration) after viral challenge are also relevant to effective vaccines. Ultimately, the ideal HCV vaccine for HIV-1–infected individuals would probably need to be administered to patients on highly active antiretroviral therapy, so that immunity may be generated without direct interference from HIV-1 infection and in the presence of ample CD4 T-cell help.
Pathogenesis of Accelerated Liver Disease in Patients With HIV-1/HCV Coinfection
Many studies have shown that fibrosis progresses more rapidly in coinfected than mono-infected patients, leading to increased rates of cirrhosis and complications.134,135 A recent longitudinal study supported these findings, although the phenomenon of accelerated fibrosis is not universal, because progression of fibrosis by serial biopsies is highly variable.136 Although the immune system is likely to be involved in the fibrosis related to HCV, immunosuppression does not ameliorate fibrosis but accelerates it. Thus, HIV-1–induced immunosuppression parallels the accelerated fibrosis observed in patients with HCV following liver transplantation, who are also immunosuppressed.
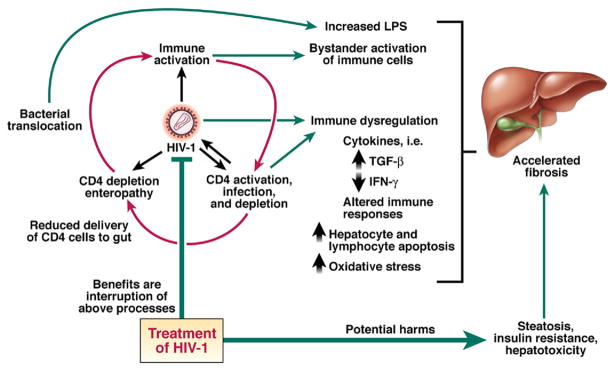
The acceleration of liver disease in coinfected patients is a challenge to study because of the lack of a suitable animal model for HCV-related fibrosis; only some HCV-infected chimpanzees develop fibrosis over an extremely long period.137 Human liver studies have been hampered by the limited frequency of sample collection and small amounts of liver tissue available, except in postmortem or explant situations. Experiments measure only a limited set of parameters, although there are many interrelated factors that potentially contribute to pathogenesis. Moreover, a biopsy represents a single snapshot of the liver; the events occurring at the time the sample was collected do not represent the whole course of a disease, which takes years to decades to develop. A parameter measured at one point in time, in a cross-sectional fashion, may not be expected to statistically correlate with an outcome that takes years to decades to develop. With these experimental and conceptual limitations in mind, Figure 3 shows pathways that have been proposed to accelerate fibrosis in coinfected patients. These include direct viral effects, dysregulation of the immune system toward a profibrotic state, and other metabolic pathways that lead to liver toxicity and processes such as steatosis and insulin resistance.
Figure 3.
Influence of HIV-1 replication and its treatment on the liver in HCV coinfection. The red arrows represent the “vicious cycle” of immune activation and CD4 activation, infection, and depletion, as well as effects on the gut mucosa promoting microbial translocation that are proposed to be central to the immunopathogenesis on the liver. These may affect liver fibrosis by a variety of mechanisms. Treatment of HIV-1 by antiretroviral therapy may interrupt the above processes but may introduce additional issues that are on balance negative to liver disease.
Direct Viral Effects
Although HCV viral titers are particularly high in the presence of HIV-1, a direct correlation of fibrosis with viral titers has not been confirmed.138 Histologic variants in liver biopsy specimens of coinfected individuals have been observed that indicate direct cytopathic activity of HCV in patients with HIV-1, although this histology is unusual.139 Some studies indicate that HCV can interact directly with the immune system; the HCV core, NS3, and NS5 proteins exert a variety of effects, in vitro, on the innate or adaptive immune system.14,16,17,140 –142 It is not clear if these interactions occur in vivo or how they would be altered in coinfected patients. Hepatocytes have not been found to be coinfected by the 2 viruses, so a “unified theory” that would explain the pathogenesis of coinfection via intracellular interactions between HIV-1 and HCV proteins is not likely.
It has been proposed that HIV-1 is present in the liver and can promote fibrosis,143,144 although it is more likely to act through indirect effects on hepatocytes. CCR5 and CXCR4 are coreceptors for HIV-1 that are expressed on hepatocytes and other liver resident cells. Polymorphisms in these receptors may affect the pathogenesis of HCV,145,146 but the HIV-1 receptor CD4 is not highly expressed on hepatocytes. Experiments using in vitro models have suggested that the HIV-1 viral envelope glycoprotein gp120 can directly promote hepatocyte apoptosis147 and result in higher viral loads by induction of TGF-β, a cytokine that dampens the immune response and promotes fibrosis and transformation toward hepatocellular carcinoma.148,149
Dysregulation of the Immune System
Shifts in the balance between T-helper (Th) 1 and Th2 cell activities have been postulated to be relevant to the pathogenesis of HCV. In one model, the relationship between the immune response and fibrosis involves induction of a Th2 cell response.150 This response is mediated by cytokines such as IL-4, IL-5, and IL-13, which together with TGF-β promote collagen deposition by fibro-blasts. To counteract this process, IFN-γ, despite being proinflammatory, is antiviral and antifibrotic, whereas IL-10 is anti-inflammatory and antifibrotic. However, the perturbations of the immune system induced by HIV-1 are myriad and cannot be defined as predominant Th1 or Th2 responses.151 We will review several aspects of the immune system that are dysregulated during HIV-1 infection and that could be relevant to accelerated liver disease (see Figure 3).
The hallmark of HIV-1 disease progression is CD4+ T-cell depletion, which correlates with the risk for opportunistic infections and with worsened liver disease. Accelerated fibrosis is most pronounced when peripheral blood CD4+ T-cell counts are decreased.152–156 Because CD4+ T cells have critical roles in the support of both humoral104 and the cytotoxic T-cell responses, their loss signals dysregulation of these arms of the immune system. Chronic antigen stimulation leads to T-cell exhaustion and direct killing of CD4+ T cells (especially those with an activated phenotype, which would be expected at the site of infection and are preferentially infected by HIV-1) results in a “double hit” to antigen-specific CD4+ T cells. The end result is extremely low or absent ex vivo HCV-specific CD4+ T-cell responses in coinfected individuals with chronic HCV outcomes.95,101,157–159 Canchis et al observed profound periportal loss of CD4+ T cells in liver resident lymphocytes when examining the histology in coinfected individuals,160 and other studies have suggested that HCV is associated with a greater degree of lymphocyte apoptosis in coinfected patients compared with those with HIV-1 alone.161,162 Despite the hypothesized higher susceptibility to apoptosis of CD4 cells when resident in the liver, these cells can be recovered from liver-infiltrating lymphocyte specimens after antigen stimulation and culture for weeks in vitro, indicating that they are not necessarily completely deleted.163,164
Measurement of IFN-γ secretion of peripheral blood mononuclear cells in coinfected patients, via an ELISpot assay with peptides from an HCV genotype 1a strain, revealed a strong positive correlation between numbers of peripheral CD4+ T cells and the total number of functional CD8+ T cells specific for HCV (but not HIV-1 or Epstein–Barr virus).105 Similarly, another study that utilized an analogous assay found reductions in IFN-γ secretion by CD8+ T cells as CD4+ T counts declined.106 Together, these studies suggest that HCV-specific CD8+ T cells are more sensitive to the helper cell environment than those specific to other viruses, and the relative lack of production of virus-specific IFN-γ (an antifibrotic cytokine) could be related to disease pathogenesis.165,166 Other defects in CD8+ T-cell function may also be present in coinfected individuals.133,167
In addition to the hypothesis that HIV-1 could have a direct role in promoting fibrosis in the liver, it is likely that immune activation by chronic HIV-1 infection could adversely affect the liver. HIV-1 activates the immune system by several mechanisms, such as direct activation by single-stranded HIV RNA of TLR, which then activate lymphocyte or dendritic cell subsets.17,168,169 The results include polyclonal B-cell activation, increased T-cell turnover, and increased expression of various activation markers on CD4+ and CD8+ T cells.170 This immune activation can predict CD4+ T-cell depletion independently of viral load and is therefore believed to be crucial for HIV pathogenesis and disease progression.171–173 In models of SIV infection, immune activation occurs in infected macaques but not in sooty mangabeys, which usually do not experience progressive immune destruction.174 Changes in the cytokine milieu induced by immune activation could have relevance to inflammation and fibrosis in the liver. One cytokine that may be important is IL-7. IL-7 is required for maintenance of T-cell memory in the mouse model of lymphochoriomeningitis virus infections, where it sustains continued T-cell proliferation in the absence of antigen (termed homeostatic proliferation). In mouse hepatocytes, IL-7 has been recently described to be differentially regulated, challenging the previous notion that IL-7 was constitutively expressed in tissues.175 Intrahepatic variation in IL-7 secretion and/or disruption of this IL-7 signal could therefore be linked to T-cell dysfunction.71,176 –180
Some researchers propose that bystander activation of T cells that are not specific for HCV could be relevant to coinfection. Coinfected individuals have a greater proportion of their peripheral blood CD8+ T cells specific to HIV-1 than to HCV95,105; these cells are present at relatively high numbers in coinfected patients, even when CD4+ T cells are depleted during progressive HIV-1 infection.97 HIV-1–specific T cells may be particularly susceptible to CD95/Fas-mediated cell death.181 Activated HIV-1–specific CD8+ T cells have been linked to the distortion of lymph node architecture and fibrosis observed in patients with progressive HIV-1 infection182; one recent study reported finding these cells in the livers of coinfected individuals.183 Therefore, activated cells that are not specific to HCV might be attracted to the liver via a bystander effect of chronic inflammation and contribute to fibrosis after apoptosis (see the following text) and release of profibrotic cytokines.184
A Punch to the Gut by HIV-1
As mentioned, recent models of HIV-1 immunopathogenesis have focused on the massive depletion of lymphoid tissue associated with the gastrointestinal tract, the largest lymphoid organ, leading to disrupted epithelium and increased microbial translocation.9 The recent “rediscovery” of this enteropathy has led to further seminal insights into the pathogenesis of HIV-1. This effect is detected early in disease118,185 and results in loss of local CD4+ T cells.186 Furthermore, in SIV models, there is selective loss of CD4+ cells that secrete IL-17 (termed Th17 cells), a proinflammatory cytokine that has an important role in host defenses against intestinal bacteria187,188 and possibly viruses such as HCV.189
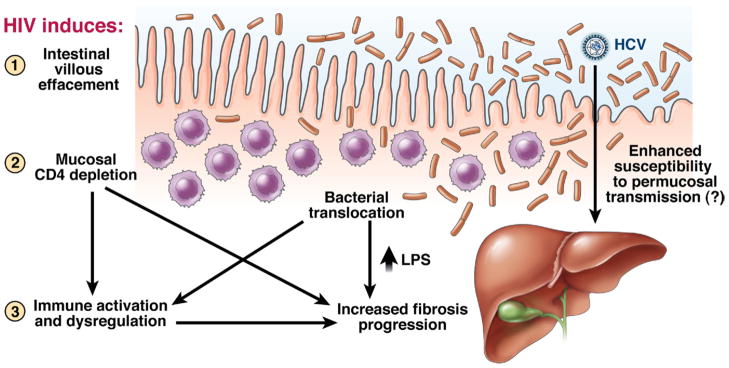
In the gastrointestinal tract, this CD4+ cell depletion enteropathy leads to direct immune activation as microbial products, particularly lipopolysaccharide (LPS), enter the bloodstream and correlates with eventual disease progression.190 LPS can increase hepatic fibrosis in animal models and has effects on Kupffer cells within the liver that would promote fibrosis.191 A recent study extrapolated these findings to HIV-1/HCV–coinfected individuals with paired biopsies, finding a correlation between increased levels of LPS and other associated markers with liver disease progression (see Figure 4).156 If increased LPS levels are related to the cause rather than the effect of advancing fibrosis, these findings could support novel approaches to treating liver disease in coinfected individuals, such as by eliminating harmful bacteria (such as with probiotics) and targeting LPS or its downstream effects.
Figure 4.
Loss of mucosal lymphoid tissue related to HIV-1 infection could promote HCV-related disease. A recent study in GASTROENTEROLOGY highlights the potential role of bacterial translocation in promoting liver disease, perhaps by direct effects of LPS or indirectly by enhancing immune activation.156 In addition, the loss of mucosal integrity can be hypothesized to enhance permucosal transmission of HCV in cases of sexual transmission, as has been detected among sexual networks in Europe.117 Adapted with permission from Balagopal et al.156
Apoptosis and Liver Fibrosis
Inflammation within the liver can increase the susceptibility of intrahepatic lymphocytes, hepatocytes, and/or hepatic stellate cells to apoptosis. While induction of apoptosis of activated hepatic stellate cells, the predominant fibrogenic cell in the liver, may be beneficial,26 overall the continuous cycle of cell death and regeneration among lymphocytes and hepatocytes is likely to promote fibrogenesis. This process involves tumor necrosis factor α activation of the Apo-1/Fas pathway, which likely contributes to HIV-1 and HCV pathogenesis.192,193
Another pathway that could mediate the negative effects of HIV-1/HCV coinfection on liver fibrosis is the tumor necrosis factor–related apoptosis-inducing ligand (TRAIL), also called Apo-2L, which induces apoptosis in transformed cells. TRAIL, depending on its subtype, induces apoptosis in normal human hepatocytes,194 but its effects are more pronounced in inflammatory conditions, such as during HCV infection195; TRAIL is up-regulated by TGF-β.196 CD4+ T cells are more susceptible to TRAIL-induced cell death, possibly induced by IFN-β in HIV-1–infected individuals,197 which is in turn associated with increased levels of circulating TRAIL.198 The combination of chronic HIV-1 infection and HCV-mediated inflammation could produce an environment of increased levels of TRAIL and susceptibility of cells (stellate cells, lymphocytes, and hepatocytes) to TRAIL-mediated apoptosis within the liver.199 –201 Human hepatoma cell lines that sustain HCV subgenomic replication and express the E2 protein are protected from TRAIL-mediated apoptosis, suggesting a viral mechanism to spare HCV-infected cells.202
Studies in mice that are depleted of CD4+ cells and infected with lymphochoriomeningitis virus have shown that a subset of CD8+ T cells secrete soluble TRAIL and are of a “helpless” phenotype, displaying reduced proliferative capacity when they encounter secondary antigen.203 If rendered TRAIL deficient, CD8+ T cells no longer exhibit this phenotype, suggesting that blockade of TRAIL or its downstream pathways could be beneficial to the function of these helpless CD8+ cells.204 It will be interesting to determine whether CD8+ T cells primarily express TRAIL within the intracellular inflammatory milieu of an HCV-infected liver and whether TRAIL blockade can restore T-cell function and reverse hepatotoxicity.205
Other Factors That Promote Fibrosis: Steatosis, Apoptosis, and Oxidative Stress
Metabolic factors have been proposed to have important roles in the pathogenesis of HCV-mediated liver disease, particularly cases of insulin resistance and nonalcoholic liver steatosis (Figure 3).206 Direct viral effects and chronic inflammation could contribute to the development of these metabolic syndromes,207 although the exact mechanisms have not been elucidated. HCV infection has an independent association with the development of insulin resistance and type 2 diabetes.208 –210 Factors that lead to steatosis in the absence of alcohol could include the direct interference of HCV with lipid metabolism211–213 and the increased sensitivity of intrahepatic lymphocytes and/or hepatocytes to apoptosis.214 –218
To complicate the picture, chronic HIV-1 infection itself induces a host of metabolic abnormalities, including glutathione deficiency, which could predispose T cells to apoptosis via enhanced susceptibility to oxidative stress219 and could have relevance during chronic HCV infection of the liver.220 Furthermore, steatosis and diabetes are each potential side effects of HIV-1 therapy.221 In coinfected patients, risk factors for hepatic steatosis include use of certain nucleoside analogues (particularly stavudine), HCV genotype 3 infection, high body mass index, and the degree of inflammation score.222–224 Because steatosis and insulin resistance are each associated with disease progression and poor treatment outcomes, future studies will focus on further understanding of mechanisms that promote and interventions that address these cofactors.225
Addressing Coinfection by Treating HIV-1
Regardless of the mechanisms that contribute to the accelerated fibrosis observed in patients with HIV-1/ HCV coinfection, one available therapeutic approach is to reverse the CD4+ cell depletion, apoptosis, and immune dysregulation that accompany chronic HIV-1 infection. HIV-1 can be suppressed via antiretroviral drugs; these therapeutics do not usually reduce HCV viremia,226 which is not surprising because most immunocompetent individuals cannot control HCV. Nevertheless, antiretroviral combinations that suppress replication of HIV-1 restore CD4+ T cells (and possibly HCV-specific T-cell responses), reduce immune activation, preserve Th17 responses,227 and reduce HIV-1–specific CD8+ T-cell responses.97 Lower plasma levels of HIV-1 have been associated with reduced hepatocyte proliferation in histologic samples160 and lower soluble levels of TRAIL.198 Although more studies are needed, HCV/HIV-1– coinfected individuals who are long-term nonprogressors (have not experienced a progressive decline in the CD4+ cell count) or have factors associated with this phenotype (such as concurrent infection with another flavivirus, GBV-C) might have more vigorous immune responses228 or improved outcomes.229 Interrupting the processes that mediate HIV-1 progression might be beneficial for coinfected patients (Table 2).
Table 2.
Relationship of Clinical Observations With Possible Mechanisms and Modulation by Antiretroviral Therapy
| Clinical observation | Possible mechanism | Influence of HIV-1 | Influence of antiretroviral therapy |
|---|---|---|---|
| Increased susceptibility to HCV infection | Donor-related | ||
| Higher viral loads | Increased viral load if donor is HIV-1/HCV coinfected | Antiretroviral therapy does not decrease HCV viral titers on average | |
| Recipient-related | |||
| Loss of mucosal integrity and perimucosal CD4+CCR5+ T cells | Massive gut CD4 depletion early in HIV infection | Gut-related CD4 depletion only partially restored | |
| Increased rate of persistence | HCV before HIV-1 | ||
| CD4+ T-cell depletion, loss of secondary (protective) immunity | Direct killing by HIV | Reversal of CD4 depletion, preservation of secondary immunity | |
| HIV-1 before HCV | |||
| CD4+ T-cell dysfunction | Direct killing by HIV | Reversal of CD4 depletion | |
| CD8+ T-cell dysfunction | Related to CD4 T-cell depletion | Reversal of CD4 depletion | |
| Increased viral loads | Loss of innate or adaptive immunity | Unclear mechanism | Antiretroviral therapy does not decrease HCV viral titers on average |
| Accelerated liver disease | Direct HIV-1 protein interactions | gp120 interactions | Removal of circulating HIV-1 protein |
| Altered cytokine profile | Related to progressive CD4 deficiency | Reversal of CD4 depletion | |
| Loss/dysfunction of IFN-γ secreting HCV-specific T cells | |||
| Enhanced TGF-β | |||
| Loss of Th17 function | |||
| Compartmentalization of HCV- specific cells to the liver | Unknown effect | Unknown effect | |
| Immune activation; LPS secretion | Microbial translocation from gut | Partially restored gut mucosa | |
| HIV-specific T cells | Increased levels in presence of viral replication | Decreased levels of HIV-1–specific T cells | |
| Enhanced hepatocyte apoptosis | Up-regulation of secreted soluble proapoptotic molecules | Decreased proapoptotic molecules, | |
| Glutathione deficiency | Oxidative stress induced by antiretrovirals | ||
| Hepatotoxicity | Worsened particularly by nevirapine | ||
| Steatosis and metabolic abnormalities | Possible relationship to certain antiretrovirals (ie, stavudine) | ||
By a combination of mechanisms, suppression of HIV-1 via antiretroviral therapy could slow fibrosis progression rates230 and prevent adverse liver-related outcomes.231 Recent HIV-1 treatment guidelines suggest that HCV coinfection might present a situation under which antiretroviral therapy should be considered, independent of CD4+ cell criteria, which are otherwise used to determine initiation of treatment.232 By contrast, it should be noted that antiretroviral therapies do not lower HCV titers,226 can induce steatosis, and are often hepatotoxic, particularly in coinfected patients.233,234 Postulated mechanisms of hepatotoxicity include increased hepatocyte sensitivity to direct drug effects and/or inflammatory mediators related to immune reconstitution against HCV.235
Is It a Two-Way Street—Possible Effects of HCV on HIV-1 Disease Progression?
Although there are many effects of HIV-1 on HCV disease progression, what are the reciprocal effects of HCV on HIV-1 disease progression? HCV coinfection has been reported to have a modest negative effect on the magnitude of CD4+ T-cell reconstitution following antiretroviral therapy in some cohorts236; this might result from a direct effect of HCV on CD4+ cell apoptosis,161 cirrhosis-induced effects on CD4+ T cells that are independent of HCV,237 or increased immune activation.170 Ultimately, the degree of this effect might not be of substantial clinical significance; studies have shown mixed results regarding the overall effect of HCV on HIV-1 disease progression.238–240 At present, the overall literature suggests that the major contribution of HCV to mortality of coinfected individuals is attributable to accelerated liver disease and not increased AIDS-related complications.
Future Directions
Better tools and conceptual advances are needed to determine the interactions between HIV-1 and HCV and the immune responses to each pathogen. Although studies in chimpanzees have yielded many insights into protective immunity, the slow development of fibrosis in these animals137 reduces their relevance to human infection and HIV infection of chimpanzees does not result in progressive disease. Primate models that are susceptible to infection with SIV are not susceptible to HCV. Until a robust small animal model of coinfection is developed, only human studies can be performed.
In the short term, research can prioritize the effects of HIV-1 on the liver, especially on the various populations of liver-resident cells described in this review. Broader studies regarding the genetic determinants of HCV and HIV-1 clearance could identify additional mechanisms of control of persistent viruses. Longer-term, larger clinical studies are needed to follow up findings from pilot studies and should include longitudinal sampling, innovative in vitro models simulating coinfection, and improved techniques. These techniques should include noninvasive markers of fibrosis, microarray analyses of gene expression patterns in liver, and improved imaging techniques (ie, confocal microscopy) to examine directly the interaction between immune cells, hepatocytes, and stellate cells.
Yearly, the HIV/AIDS vaccine budget at the National Institutes of Health exceeds $1 billion.241 These immense resources are devoted to improving our understanding of HIV-1 disease, and many of the findings from these studies apply to HIV-1/HCV coinfection. In the field of HCV research, important topics for study include gaining better insight into the early events of HCV infection (good cell culture models are now available to answer these questions), activation of the innate immune system, the relationship between innate and adaptive immunity, the counterregulatory mechanisms that viruses use to reduce immune responses, and age-related changes in the immune system (given the effect of age on fibrosis). The development of coculture systems that model the effects of combined HCV and HIV infection of hepatocytes will advance our understanding of the pathogenesis of coinfection. Many important questions also remain about the effects of antiretrovirals, such as whether highly active antiretroviral therapy and CD4+ cell repletion restore HCV-specific immune responses and reverse profibrotic processes, in addition to mechanisms of antiretroviral hepatotoxicity and associated steatosis.
Because of the expense and difficulties of current therapies directed against HCV in coinfected patients, it would be beneficial to develop interventions that can induce the immune system to lower HCV viral titers before treatment with IFN-based treatments (thereby increasing the likelihood of sustained virological response) and/or retard the progression of fibrosis. Such interventions may first be tested in cancer immunotherapy trials or against HIV-1, and thus investments in these realms may benefit our ability to harness the immune system against HCV. In developed nations, coinfected patients have received the benefits of improved antiretroviral therapies and fewer have progressive HIV-1; the next frontiers include the challenges of accelerated fibrosis, poor response to IFN-based therapies, and system-related barriers that occur in HCV/HIV-1 coinfection.
Conclusions
Insights from clinical studies of HIV-1/HCV coinfection have revealed complex interactions that parallel challenges faced by health care providers. Coinfected patients experience accelerated fibrosis; expanding knowledge of the perturbations of host immunity induced by HIV-1 infection and the deleterious effects of the virus itself have relevance to the pathogenesis of HCV-related liver disease. These 2 viruses present immense challenges to the host immune response, yet some patients have been found to control both viruses. Gaining a greater understanding of the mechanisms of viral control and persistence could lead to therapeutic approaches to control these viruses in coinfected patients.
Acknowledgments
The authors thank Daniel Kaufmann and Joerg Timm for careful review of the manuscript.
Funding
Supported by National Institutes of Health grants U19 AI066345 and K23 AI054379 (to A.Y.K.) and National Institutes of Health grants R01 AI069939 and K24 DK078772-02 (to R.T.C.).
Abbreviations used in this paper
- AIDS
acquired immune deficiency syndrome
- FOXP3
forkhead box P3
- HIV-1
human immunodeficiency virus 1
- IFN
interferon
- IL
interleukin
- LPS
lipopolysaccharide
- MSM
men who have sex with men
- NK
natural killer
- NKT cell
natural killer T cell
- PD-1
programmed death 1
- SIV
simian immunodeficiency virus
- TGF
transforming growth factor
- Th
T helper
- TLR
Toll-like receptor (TLR)
- TRAIL
tumor necrosis factor–related apoptosis-inducing ligand
Footnotes
Conflicts of interest
The authors disclose the following: R.T.C. receives research grant support from Roche Labs and Schering-Plough. A.Y.K. discloses no conflicts.
References
- 1.Sulkowski MS, Thomas DL. Hepatitis C in the HIV-infected person. Ann Intern Med. 2003;138:197–207. doi: 10.7326/0003-4819-138-3-200302040-00012. [DOI] [PubMed] [Google Scholar]
- 2.Koziel MJ, Peters MG. Viral hepatitis in HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity. 2007;27:406–416. doi: 10.1016/j.immuni.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Maheshwari A, Ray S, Thuluvath PJ. Acute hepatitis C. Lancet. 2008;372:321–332. doi: 10.1016/S0140-6736(08)61116-2. [DOI] [PubMed] [Google Scholar]
- 5.Jauncey M, Micallef JM, Gilmour S, et al. Clearance of hepatitis C virus after newly acquired infection in injection drug users. J Infect Dis. 2004;190:1270–1274. doi: 10.1086/423943. [DOI] [PubMed] [Google Scholar]
- 6.Cox AL, Netski DM, Mosbruger T, et al. Prospective evaluation of community-acquired acute-phase hepatitis C virus infection. Clin Infect Dis. 2005;40:951–958. doi: 10.1086/428578. [DOI] [PubMed] [Google Scholar]
- 7.Scott JD, McMahon BJ, Bruden D, et al. High rate of spontaneous negativity for hepatitis C virus RNA after establishment of chronic infection in Alaska Natives. Clin Infect Dis. 2006;42:945–952. doi: 10.1086/500938. [DOI] [PubMed] [Google Scholar]
- 8.Walker BD, Burton DR. Toward an AIDS vaccine. Science. 2008;320:760–764. doi: 10.1126/science.1152622. [DOI] [PubMed] [Google Scholar]
- 9.Douek DC, Roederer M, Koup RA. Emerging concepts in the immunopathogenesis of AIDS. Annu Rev Med. 2009;60:471–484. doi: 10.1146/annurev.med.60.041807.123549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gale M, Jr, Foy EM. Evasion of intracellular host defence by hepatitis C virus. Nature. 2005;436:939–945. doi: 10.1038/nature04078. [DOI] [PubMed] [Google Scholar]
- 11.Chung RT, Gale M, Jr, Polyak SJ, et al. Mechanisms of action of interferon and ribavirin in chronic hepatitis C: summary of a workshop. Hepatology. 2008;47:306–320. doi: 10.1002/hep.22070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee J, Wu CC, Lee KJ, et al. Activation of anti-hepatitis C virus responses via Toll-like receptor 7. Proc Natl Acad Sci U S A. 2006;103:1828–1833. doi: 10.1073/pnas.0510801103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foy E, Li K, Wang C, et al. Regulation of interferon regulatory factor-3 by the hepatitis C virus serine protease. Science. 2003;300:1145–1148. doi: 10.1126/science.1082604. [DOI] [PubMed] [Google Scholar]
- 14.Foy E, Li K, Sumpter R, Jr, et al. Control of antiviral defenses through hepatitis C virus disruption of retinoic acid-inducible gene-I signaling. Proc Natl Acad Sci U S A. 2005;102:2986–2991. doi: 10.1073/pnas.0408707102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li K, Foy E, Ferreon JC, et al. Immune evasion by hepatitis C virus NS3/4A protease-mediated cleavage of the Toll-like receptor 3 adaptor protein TRIF. Proc Natl Acad Sci U S A. 2005;102:2992–2997. doi: 10.1073/pnas.0408824102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meylan E, Curran J, Hofmann K, et al. Cardif is an adaptor protein in the RIG-I antiviral pathway and is targeted by hepatitis C virus. Nature. 2005;437:1167–1172. doi: 10.1038/nature04193. [DOI] [PubMed] [Google Scholar]
- 17.Heil F, Hemmi H, Hochrein H, et al. Species-specific recognition of single-stranded RNA via toll-like receptor 7 and 8. Science. 2004;303:1526–1529. doi: 10.1126/science.1093620. [DOI] [PubMed] [Google Scholar]
- 18.Khakoo SI, Thio CL, Martin MP, et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science. 2004;305:872–874. doi: 10.1126/science.1097670. [DOI] [PubMed] [Google Scholar]
- 19.Alter G, Teigen N, Davis BT, et al. Sequential deregulation of NK cell subset distribution and function starting in acute HIV-1 infection. Blood. 2005;106:3366–3369. doi: 10.1182/blood-2005-03-1100. [DOI] [PubMed] [Google Scholar]
- 20.Iannello A, Debbeche O, Samarani S, et al. Antiviral NK cell responses in HIV infection: II. viral strategies for evasion and lessons for immunotherapy and vaccination. J Leukoc Biol. 2008;84:27–49. doi: 10.1189/jlb.0907649. [DOI] [PubMed] [Google Scholar]
- 21.Tseng CT, Klimpel GR. Binding of the hepatitis C virus envelope protein E2 to CD81 inhibits natural killer cell functions. J Exp Med. 2002;195:43–49. doi: 10.1084/jem.20011145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yoon JC, Shiina M, Ahlenstiel G, et al. Natural killer cell function is intact after direct exposure to infectious hepatitis C virions. Hepatology. 2009;49:12–21. doi: 10.1002/hep.22624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Durante-Mangoni E, Wang R, Shaulov A, et al. Hepatic CD1d expression in hepatitis C virus infection and recognition by resident proinflammatory CD1d-reactive T cells. J Immunol. 2004;173:2159–2166. doi: 10.4049/jimmunol.173.3.2159. [DOI] [PubMed] [Google Scholar]
- 24.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 25.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature. 2009;457:557–561. doi: 10.1038/nature07665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krizhanovsky V, Yon M, Dickins RA, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134:657–667. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bataller R, Paik YH, Lindquist JN, et al. Hepatitis C virus core and nonstructural proteins induce fibrogenic effects in hepatic stellate cells. Gastroenterology. 2004;126:529–540. doi: 10.1053/j.gastro.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 28.Farci P, Shimoda A, Wong D, et al. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc Natl Acad Sci U S A. 1996;93:15394–15399. doi: 10.1073/pnas.93.26.15394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Hahn T, Yoon JC, Alter H, et al. Hepatitis C virus continuously escapes from neutralizing antibody and T-cell responses during chronic infection in vivo. Gastroenterology. 2007;132:667–678. doi: 10.1053/j.gastro.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan DE, Sugimoto K, Newton K, et al. Discordant role of CD4 T-cell response relative to neutralizing antibody and CD8 T-cell responses in acute hepatitis C. Gastroenterology. 2007;132:654–666. doi: 10.1053/j.gastro.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 31.Farci P, Alter HJ, Govindarajan S, et al. Lack of protective immunity against reinfection with hepatitis C virus. Science. 1992;258:135–140. doi: 10.1126/science.1279801. [DOI] [PubMed] [Google Scholar]
- 32.Micallef JM, Macdonald V, Jauncey M, et al. High incidence of hepatitis C virus reinfection within a cohort of injecting drug users. J Viral Hepat. 2007;14:413–418. doi: 10.1111/j.1365-2893.2006.00812.x. [DOI] [PubMed] [Google Scholar]
- 33.Law M, Maruyama T, Lewis J, et al. Broadly neutralizing antibodies protect against hepatitis C virus quasispecies challenge. Nat Med. 2008;14:25–27. doi: 10.1038/nm1698. [DOI] [PubMed] [Google Scholar]
- 34.Thio CL, Gao X, Goedert JJ, et al. HLA-Cw*04 and hepatitis C virus persistence. J Virol. 2002;76:4792–4797. doi: 10.1128/JVI.76.10.4792-4797.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKiernan SM, Hagan R, Curry M, et al. Distinct MHC class I and II alleles are associated with hepatitis C viral clearance, originating from a single source. Hepatology. 2004;40:108–114. doi: 10.1002/hep.20261. [DOI] [PubMed] [Google Scholar]
- 36.Thursz M, Yallop R, Goldin R, et al. Influence of MHC class II genotype on outcome of infection with hepatitis C virus. The HENCORE group. Hepatitis C European Network for Cooperative Research. Lancet. 1999;354:2119–2124. doi: 10.1016/s0140-6736(99)91443-5. [DOI] [PubMed] [Google Scholar]
- 37.Harris RA, Sugimoto K, Kaplan DE, et al. Human leukocyte antigen class II associations with hepatitis C virus clearance and virus-specific CD4 T cell response among Caucasians and African Americans. Hepatology. 2008;48:70–79. doi: 10.1002/hep.22287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerlach JT, Diepolder HM, Jung MC, et al. Recurrence of hepatitis C virus after loss of virus-specific CD4(+) T-cell response in acute hepatitis C [see comments] Gastroenterology. 1999;117:933–941. doi: 10.1016/s0016-5085(99)70353-7. [DOI] [PubMed] [Google Scholar]
- 39.Lechner F, Wong DK, Dunbar PR, et al. Analysis of successful immune responses in persons infected with hepatitis C virus. J Exp Med. 2000;191:1499–1512. doi: 10.1084/jem.191.9.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Thimme R, Oldach D, Chang KM, et al. Determinants of viral clearance and persistence during acute hepatitis C virus infection. J Exp Med. 2001;194:1395–1406. doi: 10.1084/jem.194.10.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grebely J, Conway B, Raffa JD, et al. Hepatitis C virus reinfection in injection drug users. Hepatology. 2006;44:1139–1145. doi: 10.1002/hep.21376. [DOI] [PubMed] [Google Scholar]
- 42.Mehta SH, Cox A, Hoover DR, et al. Protection against persistence of hepatitis C. Lancet. 2002;359:1478–1483. doi: 10.1016/S0140-6736(02)08435-0. [DOI] [PubMed] [Google Scholar]
- 43.Grakoui A, Shoukry NH, Woollard DJ, et al. HCV persistence and immune evasion in the absence of memory T cell help. Science. 2003;302:659–662. doi: 10.1126/science.1088774. [DOI] [PubMed] [Google Scholar]
- 44.Shoukry NH, Grakoui A, Houghton M, et al. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J Exp Med. 2003;197:1645–1655. doi: 10.1084/jem.20030239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Folgori A, Capone S, Ruggeri L, et al. A T-cell HCV vaccine eliciting effective immunity against heterologous virus challenge in chimpanzees. Nat Med. 2006;12:190–197. doi: 10.1038/nm1353. [DOI] [PubMed] [Google Scholar]
- 46.Strickland GT, El-Kamary SS, Klenerman P, et al. Hepatitis C vaccine: supply and demand. Lancet Infect Dis. 2008;8:379–386. doi: 10.1016/S1473-3099(08)70126-9. [DOI] [PubMed] [Google Scholar]
- 47.Altfeld M, Rosenberg ES, Shankarappa R, et al. Cellular immune responses and viral diversity in individuals treated during acute and early HIV-1 infection. J Exp Med. 2001;193:169 –180. doi: 10.1084/jem.193.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schmitz JE, Kuroda MJ, Santra S, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 49.Weiner A, Erickson AL, Kansopon J, et al. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc Natl Acad Sci U S A. 1995;92:2755–2759. doi: 10.1073/pnas.92.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cox AL, Mosbruger T, Mao Q, et al. Cellular immune selection with hepatitis C virus persistence in humans. J Exp Med. 2005;201:1741–1752. doi: 10.1084/jem.20050121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tester I, Smyk-Pearson S, Wang P, et al. Immune evasion versus recovery after acute hepatitis C virus infection from a shared source. J Exp Med. 2005;201:1725–1731. doi: 10.1084/jem.20042284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Timm J, Lauer GM, Kavanagh DG, et al. CD8 epitope escape and reversion in acute HCV infection. J Exp Med. 2004;200:1593–1604. doi: 10.1084/jem.20041006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Allen TM, O’Connor DH, Jing P, et al. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature. 2000;407:386–390. doi: 10.1038/35030124. [DOI] [PubMed] [Google Scholar]
- 54.McMichael AJ, Rowland-Jones SL. Cellular immune responses to HIV. Nature. 2001;410:980–987. doi: 10.1038/35073658. [DOI] [PubMed] [Google Scholar]
- 55.Bowen DG, Walker CM. Mutational escape from CD8+ T cell immunity: HCV evolution, from chimpanzees to man. J Exp Med. 2005;201:1709–1714. doi: 10.1084/jem.20050808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gaudieri S, Rauch A, Park LP, et al. Evidence of viral adaptation to HLA class I-restricted immune pressure in chronic hepatitis C virus infection. J Virol. 2006;80:11094–11104. doi: 10.1128/JVI.00912-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Timm J, Li B, Daniels MG, et al. Human leukocyte antigen-associated sequence polymorphisms in hepatitis C virus reveal reproducible immune responses and constraints on viral evolution. Hepatology. 2007;46:339–349. doi: 10.1002/hep.21702. [DOI] [PubMed] [Google Scholar]
- 58.Kawashima Y, Pfafferott K, Frater J. Adaptation of HIV-1 to human leukocyte antigen class I. Nature. 2009;458:641–645. doi: 10.1038/nature07746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lanford RE, Guerra B, Chavez D, et al. Cross-genotype immunity to hepatitis C virus. J Virol. 2004;78:1575–1581. doi: 10.1128/JVI.78.3.1575-1581.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sugimoto K, Kaplan DE, Ikeda F, et al. Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection. J Virol. 2005;79:6976–6983. doi: 10.1128/JVI.79.11.6976-6983.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schulze Zur Wiesch J, Lauer GM, Timm J, et al. Immunologic evidence for lack of heterologous protection following resolution of HCV in subjects with non-genotype 1 infection. Blood. 2007;110:1559–1569. doi: 10.1182/blood-2007-01-069583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Takaki A, Wiese M, Maertens G, et al. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat Med. 2000;6:578–582. doi: 10.1038/75063. [DOI] [PubMed] [Google Scholar]
- 63.Lauer GM, Barnes E, Lucas M, et al. High resolution analysis of cellular immune responses in resolved and persistent hepatitis C virus infection. Gastroenterology. 2004;127:924–936. doi: 10.1053/j.gastro.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 64.Wedemeyer H, He XS, Nascimbeni M, et al. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J Immunol. 2002;169:3447–3458. doi: 10.4049/jimmunol.169.6.3447. [DOI] [PubMed] [Google Scholar]
- 65.Sharpe AH, Wherry EJ, Ahmed R, et al. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat Immunol. 2007;8:239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 66.Day CL, Kaufmann DE, Kiepiela P, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 67.Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 69.Urbani S, Amadei B, Tola D, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Golden-Mason L, Palmer B, Klarquist J, et al. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Radziewicz H, Ibegbu CC, Fernandez ML, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kasprowicz V, Schulze Zur Wiesch J, Kuntzen T, et al. High level of PD-1 expression on hepatitis C virus (HCV)-specific CD8+ and CD4+ T cells during acute HCV infection, irrespective of clinical outcome. J Virol. 2008;82:3154–3160. doi: 10.1128/JVI.02474-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rutebemberwa A, Ray SC, Astemborski J, et al. High-programmed death-1 levels on hepatitis C virus-specific T cells during acute infection are associated with viral persistence and require preservation of cognate antigen during chronic infection. J Immunol. 2008;181:8215–8225. doi: 10.4049/jimmunol.181.12.8215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nakamoto N, Kaplan DE, Coleclough J, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. e1–2. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Urbani S, Amadei B, Tola D, et al. Restoration of HCV-specific T cell functions by PD-1/PD-L1 blockade in HCV infection: effect of viremia levels and antiviral treatment. J Hepatol. 2008;48:548 –558. doi: 10.1016/j.jhep.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 76.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 77.Jones RB, Ndhlovu LC, Barbour JD, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Velu V, Titanji K, Zhu B, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nattermann J, Zimmermann H, Iwan A, et al. Hepatitis C virus E2 and CD81 interaction may be associated with altered trafficking of dendritic cells in chronic hepatitis C. Hepatology. 2006;44:945–954. doi: 10.1002/hep.21350. [DOI] [PubMed] [Google Scholar]
- 80.Billerbeck E, Thimme R. CD8+ regulatory T cells in persistent human viral infections. Hum Immunol. 2008;69:771–775. doi: 10.1016/j.humimm.2008.07.016. [DOI] [PubMed] [Google Scholar]
- 81.Dolganiuc A, Chang S, Kodys K, et al. Hepatitis C virus (HCV) core protein-induced, monocyte-mediated mechanisms of reduced IFN-alpha and plasmacytoid dendritic cell loss in chronic HCV infection. J Immunol. 2006;177:6758–6768. doi: 10.4049/jimmunol.177.10.6758. [DOI] [PubMed] [Google Scholar]
- 82.Decalf J, Fernandes S, Longman R, et al. Plasmacytoid dendritic cells initiate a complex chemokine and cytokine network and are a viable drug target in chronic HCV patients. J Exp Med. 2007;204:2423–2437. doi: 10.1084/jem.20070814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yonkers NL, Rodriguez B, Milkovich KA, et al. TLR ligand-dependent activation of naive CD4 T cells by plasmacytoid dendritic cells is impaired in hepatitis C virus infection. J Immunol. 2007;178:4436–4444. doi: 10.4049/jimmunol.178.7.4436. [DOI] [PubMed] [Google Scholar]
- 84.Shiina M, Rehermann B. Cell culture-produced hepatitis C virus impairs plasmacytoid dendritic cell function. Hepatology. 2008;47:385–395. doi: 10.1002/hep.21996. [DOI] [PubMed] [Google Scholar]
- 85.Kaplan DE, Ikeda F, Li Y, et al. Peripheral virus-specific T-cell interleukin-10 responses develop early in acute hepatitis C infection and become dominant in chronic hepatitis. J Hepatol. 2008;48:903–913. doi: 10.1016/j.jhep.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Manigold T, Shin EC, Mizukoshi E, et al. Foxp3+ CD4+ CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood. 2006;107:4424–4432. doi: 10.1182/blood-2005-09-3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ward SM, Fox BC, Brown PJ, et al. Quantification and localisation of FOXP3+ T lymphocytes and relation to hepatic inflammation during chronic HCV infection. J Hepatol. 2007;47:316–324. doi: 10.1016/j.jhep.2007.03.023. [DOI] [PubMed] [Google Scholar]
- 88.Smyk-Pearson S, Golden-Mason L, Klarquist J, et al. Functional suppression by FoxP3+ CD4+ CD25(high) regulatory T cells during acute hepatitis C virus infection. J Infect Dis. 2008;197:46 –57. doi: 10.1086/523651. [DOI] [PubMed] [Google Scholar]
- 89.Belkaid Y. Regulatory T cells and infection: a dangerous necessity. Nat Rev Immunol. 2007;7:875–888. doi: 10.1038/nri2189. [DOI] [PubMed] [Google Scholar]
- 90.de St Groth BF, Landay AL. Regulatory T cells in HIV infection: pathogenic or protective participants in the immune response? AIDS. 2008;22:671–683. doi: 10.1097/QAD.0b013e3282f466da. [DOI] [PubMed] [Google Scholar]
- 91.Accapezzato D, Francavilla V, Paroli M, et al. Hepatic expansion of a virus-specific regulatory CD8+ T cell population in chronic hepatitis C virus infection. J Clin Invest. 2004;113:963–972. doi: 10.1172/JCI20515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alatrakchi N, Graham CS, van der Vliet HJ, et al. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor beta that can suppress HCV-specific T-cell responses. J Virol. 2007;81:5882–5892. doi: 10.1128/JVI.02202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crispe IN. Hepatic T cells and liver tolerance. Nat Rev Immunol. 2003;3:51–62. doi: 10.1038/nri981. [DOI] [PubMed] [Google Scholar]
- 94.Major ME, Dahari H, Mihalik K, et al. Hepatitis C virus kinetics and host responses associated with disease and outcome of infection in chimpanzees. Hepatology. 2004;39:1709 –1720. doi: 10.1002/hep.20239. [DOI] [PubMed] [Google Scholar]
- 95.Lauer GM, Nguyen TN, Day CL, et al. Human immunodeficiency virus type 1-hepatitis C virus coinfection: intraindividual comparison of cellular immune responses against two persistent viruses. J Virol. 2002;76:2817–2826. doi: 10.1128/JVI.76.6.2817-2826.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gea-Banacloche JC, Migueles SA, Martino L, et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol. 2000;165:1082–1092. doi: 10.4049/jimmunol.165.2.1082. [DOI] [PubMed] [Google Scholar]
- 97.Draenert R, Verrill CL, Tang Y, et al. Persistent recognition of autologous virus by high-avidity CD8 T cells in chronic, progressive human immunodeficiency virus type 1 infection. J Virol. 2004;78:630–641. doi: 10.1128/JVI.78.2.630-641.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Appay V, Dunbar PR, Callan M, et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 99.Gerberding JL. Incidence and prevalence of human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and cytomegalovirus among health care personnel at risk for blood exposure: final report from a longitudinal study. J Infect Dis. 1994;170:1410–1417. doi: 10.1093/infdis/170.6.1410. [DOI] [PubMed] [Google Scholar]
- 100.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284:450–456. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 101.Kim AY, Schulze zur Wiesch J, Kuntzen T, et al. Impaired hepatitis C virus-specific T cell responses and recurrent hepatitis C virus in HIV coinfection. PLoS Med. 2006;3:e492. doi: 10.1371/journal.pmed.0030492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Eyster ME, Fried MW, Di Bisceglie AM, et al. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84:1020–1023. [PubMed] [Google Scholar]
- 103.Cribier B, Rey D, Schmitt C, et al. High hepatitis C viraemia and impaired antibody response in patients coinfected with HIV [see comments] AIDS. 1995;9:1131–1136. doi: 10.1097/00002030-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 104.Netski DM, Mosbruger T, Astemborski J, et al. CD4+ T cell-dependent reduction in hepatitis C virus-specific humoral immune responses after HIV infection. J Infect Dis. 2007;195:857–863. doi: 10.1086/511826. [DOI] [PubMed] [Google Scholar]
- 105.Kim AY, Lauer GM, Ouchi K, et al. The magnitude and breadth of hepatitis C virus-specific CD8+ T cells depend on absolute CD4+ T-cell count in individuals coinfected with HIV-1. Blood. 2005;105:1170–1178. doi: 10.1182/blood-2004-06-2336. [DOI] [PubMed] [Google Scholar]
- 106.Dutoit V, Ciuffreda D, Comte D, et al. Differences in HCV-specific T cell responses between chronic HCV infection and HIV/HCV co-infection. Eur J Immunol. 2005;35:3493–3504. doi: 10.1002/eji.200535035. [DOI] [PubMed] [Google Scholar]
- 107.Thomas DL, Astemborski J, Vlahov D, et al. Determinants of the quantity of hepatitis C virus RNA. J Infect Dis. 2000;181:844–851. doi: 10.1086/315314. [DOI] [PubMed] [Google Scholar]
- 108.Effros RB, Fletcher CV, Gebo K, et al. Aging and infectious diseases: workshop on HIV infection and aging: what is known and future research directions. Clin Infect Dis. 2008;47:542–553. doi: 10.1086/590150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lichterfeld M, Mou D, Cung TD, et al. Telomerase activity of HIV-1-specific CD8+ T cells: constitutive up-regulation in controllers and selective increase by blockade of PD ligand 1 in progressors. Blood. 2008;112:3679–3687. doi: 10.1182/blood-2008-01-135442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sulkowski MS. Is there a point at which you would recommend discontinuing treatment due to liver toxicity, short of liver failure? What, for instance, would be the numbers to look for with liver enzyme tests? How high is too high? My doctor has said they are seeing more people in their practice die from liver disorders than from the virus itself nowadays. This has me very concerned since I am co-infected with HCV The Hopkins HIV Report: a Bimonthly Newsletter for. Healthcare Providers. 2001;13:11. [PubMed] [Google Scholar]
- 111.Ghosn J, Pierre-Francois S, Thibault V, et al. Acute hepatitis C in HIV-infected men who have sex with men. HIV Med. 2004;5:303–306. doi: 10.1111/j.1468-1293.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 112.Gambotti L, Batisse D, Colin-de-Verdiere N, et al. Acute hepatitis C infection in HIV positive men who have sex with men in Paris, France, 2001–2004. Euro Surveill. 2005;10:115–117. [PubMed] [Google Scholar]
- 113.Gotz HM, van Doornum G, Niesters HG, et al. A cluster of acute hepatitis C virus infection among men who have sex with men—results from contact tracing and public health implications. AIDS. 2005;19:969–974. doi: 10.1097/01.aids.0000171412.61360.f8. [DOI] [PubMed] [Google Scholar]
- 114.Vogel M, Bieniek B, Jessen H, et al. Treatment of acute hepatitis C infection in HIV-infected patients: a retrospective analysis of eleven cases. J Viral Hepat. 2005;12:207–211. doi: 10.1111/j.1365-2893.2005.00580.x. [DOI] [PubMed] [Google Scholar]
- 115.Luetkemeyer A, Hare CB, Stansell J, et al. Clinical presentation and course of acute hepatitis C infection in HIV-infected patients. J Acquir Immune Defic Syndr. 2006;41:31–36. doi: 10.1097/01.qai.0000191281.77954.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Danta M, Brown D, Bhagani S, et al. Recent epidemic of acute hepatitis C virus in HIV-positive men who have sex with men linked to high-risk sexual behaviours. AIDS. 2007;21:983–991. doi: 10.1097/QAD.0b013e3281053a0c. [DOI] [PubMed] [Google Scholar]
- 117.van de Laar T, Pybus O, Bruisten S, et al. Evidence of a large, international network of HCV transmission in HIV-positive men who have sex with men. Gastroenterology. 2009;136:1609–1617. doi: 10.1053/j.gastro.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mattapallil JJ, Douek DC, Hill B, et al. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature. 2005;434:1093–1097. doi: 10.1038/nature03501. [DOI] [PubMed] [Google Scholar]
- 119.Mehandru S, Poles MA, Tenner-Racz K, et al. Mechanisms of gastrointestinal CD4+ T-cell depletion during acute and early human immunodeficiency virus type 1 infection. J Virol. 2007;81:599–612. doi: 10.1128/JVI.01739-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Guadalupe M, Sankaran S, George MD, et al. Viral suppression and immune restoration in the gastrointestinal mucosa of human immunodeficiency virus type 1-infected patients initiating therapy during primary or chronic infection. J Virol. 2006;80:8236–8247. doi: 10.1128/JVI.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Gilleece YC, Browne RE, Asboe D, et al. Transmission of hepatitis C virus among HIV-positive homosexual men and response to a 24-week course of pegylated interferon and ribavirin. J Acquir Immune Defic Syndr. 2005;40:41–46. doi: 10.1097/01.qai.0000174930.64145.a9. [DOI] [PubMed] [Google Scholar]
- 122.Danta M, Semmo N, Fabris P, et al. Impact of HIV on host-virus interactions during early hepatitis C virus infection. J Infect Dis. 2008;197:1558–1566. doi: 10.1086/587843. [DOI] [PubMed] [Google Scholar]
- 123.van den Berg CH, Ruys TA, Nanlohy NM, et al. Comprehensive longitudinal analysis of hepatitis C virus (HCV)-specific T cell responses during acute HCV infection in the presence of existing HIV-1 infection. J Viral Hepat. 2009;16:239–248. doi: 10.1111/j.1365-2893.2009.01076.x. [DOI] [PubMed] [Google Scholar]
- 124.Fierer DS, Uriel AJ, Carriero DC, et al. Liver fibrosis during an outbreak of acute hepatitis C virus infection in HIV-infected men: a prospective cohort study. J Infect Dis. 2008;198:683–686. doi: 10.1086/590430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jaeckel E, Cornberg M, Wedemeyer H, et al. Treatment of acute hepatitis C with interferon alfa-2b. N Engl J Med. 2001;345:1452–1457. doi: 10.1056/NEJMoa011232. [DOI] [PubMed] [Google Scholar]
- 126.Dominguez S, Ghosn J, Valantin MA, et al. Efficacy of early treatment of acute hepatitis C infection with pegylated interferon and ribavirin in HIV-infected patients. AIDS. 2006;20:1157–1161. doi: 10.1097/01.aids.0000226956.02719.fd. [DOI] [PubMed] [Google Scholar]
- 127.Matthews GV, Hellard M, Haber P, et al. Characteristics and treatment outcomes among HIV-infected individuals in the Australian Trial in Acute Hepatitis C. Clin Infect Dis. 2009;48:650–658. doi: 10.1086/596770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Morishima C, Polyak SJ, Ray R, et al. Hepatitis C virus-specific immune responses and quasi-species variability at baseline are associated with nonresponse to antiviral therapy during advanced hepatitis C. J Infect Dis. 2006;193:931–940. doi: 10.1086/500952. [DOI] [PubMed] [Google Scholar]
- 129.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–1893. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Addo MM, Yu XG, Rathod A, et al. Comprehensive epitope analysis of human immunodeficiency virus type 1 (HIV-1)-specific T-cell responses directed against the entire expressed HIV-1 genome demonstrate broadly directed responses, but no correlation to viral load. J Virol. 2003;77:2081–2092. doi: 10.1128/JVI.77.3.2081-2092.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Seder RA, Darrah PA, Roederer M. T-cell quality in memory and protection: implications for vaccine design. Nat Rev Immunol. 2008;8:247–258. doi: 10.1038/nri2274. [DOI] [PubMed] [Google Scholar]
- 132.Badr G, Bedard N, Abdel-Hakeem MS, et al. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J Virol. 2008;82:10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ciuffreda D, Comte D, Cavassini M, et al. Polyfunctional HCV-specific T-cell responses are associated with effective control of HCV replication. Eur J Immunol. 2008;38:2665–2677. doi: 10.1002/eji.200838336. [DOI] [PubMed] [Google Scholar]
- 134.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30:1054–1058. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 135.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33:562–569. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 136.Sulkowski MS, Mehta SH, Torbenson MS, et al. Rapid fibrosis progression among HIV/hepatitis C virus-co-infected adults. AIDS. 2007;21:2209–2216. doi: 10.1097/QAD.0b013e3282f10de9. [DOI] [PubMed] [Google Scholar]
- 137.Lanford RE, Chavez D, Guerra B, et al. Hepatocellular carcinoma as a long term outcome of HCV infection in chimpanzees. Presented at: 15th International Symposium on Hepatitis C Virus & Related Viruses; October 5–9, 2008; San Antonio, TX.. [Google Scholar]
- 138.Heller T, Seeff LB. Viral load as a predictor of progression of chronic hepatitis C? Hepatology. 2005;42:1261–1263. doi: 10.1002/hep.20982. [DOI] [PubMed] [Google Scholar]
- 139.Rosenberg PM, Farrell JJ, Abraczinskas DR, et al. Rapidly progressive fibrosing cholestatic hepatitis—hepatitis C virus in HIV coinfection. Am J Gastroenterol. 2002;97:478–483. doi: 10.1111/j.1572-0241.2002.05459.x. [DOI] [PubMed] [Google Scholar]
- 140.Kittlesen DJ, Chianese-Bullock KA, Yao ZQ, et al. Interaction between complement receptor gC1qR and hepatitis C virus core protein inhibits T-lymphocyte proliferation. J Clin Invest. 2000;106:1239–1249. doi: 10.1172/JCI10323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chou AH, Tsai HF, Wu YY, et al. Hepatitis C virus core protein modulates TRAIL-mediated apoptosis by enhancing Bid cleavage and activation of mitochondria apoptosis signaling pathway. J Immunol. 2005;174:2160–2166. doi: 10.4049/jimmunol.174.4.2160. [DOI] [PubMed] [Google Scholar]
- 142.Lin W, Kim SS, Yeung E, et al. Hepatitis C virus core protein blocks interferon signaling by interaction with the STAT1 SH2 domain. J Virol. 2006;80:9226–9235. doi: 10.1128/JVI.00459-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Blackard JT, Hiasa Y, Smeaton L, et al. Compartmentalization of hepatitis C virus (HCV) during HCV/HIV coinfection. J Infect Dis. 2007;195:1765–1773. doi: 10.1086/518251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Blackard JT, Sherman KE. HCV/ HIV co-infection: time to reevaluate the role of HIV in the liver? J Viral Hepat. 2008;15:323–330. doi: 10.1111/j.1365-2893.2008.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Mangia A, Santoro R, D’Agruma L, et al. HCV chronic infection and CCR5-delta32/delta32. Gastroenterology. 2003;124:868–869. doi: 10.1053/gast.2003.50134. author reply 869–870. [DOI] [PubMed] [Google Scholar]
- 146.Hellier S, Frodsham AJ, Hennig BJ, et al. Association of genetic variants of the chemokine receptor CCR5 and its ligands, RANTES and MCP-2, with outcome of HCV infection. Hepatology. 2003;38:1468–1476. doi: 10.1016/j.hep.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 147.Munshi N, Balasubramanian A, Koziel M, et al. Hepatitis C and human immunodeficiency virus envelope proteins cooperatively induce hepatocytic apoptosis via an innocent bystander mechanism. J Infect Dis. 2003;188:1192–1204. doi: 10.1086/378643. [DOI] [PubMed] [Google Scholar]
- 148.Matsuzaki K, Murata M, Yoshida K, et al. Chronic inflammation associated with hepatitis C virus infection perturbs hepatic transforming growth factor beta signaling, promoting cirrhosis and hepatocellular carcinoma. Hepatology. 2007;46:48 –57. doi: 10.1002/hep.21672. [DOI] [PubMed] [Google Scholar]
- 149.Lin W, Weinberg EM, Tai AW, et al. HIV increases HCV replication in a TGF-beta1-dependent manner. Gastroenterology. 2008;134:803–811. doi: 10.1053/j.gastro.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 150.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Graziosi C, Pantaleo G, Gantt KR, et al. Lack of evidence for the dichotomy of TH1 and TH2 predominance in HIV-infected individuals. Science. 1994;265:248–252. doi: 10.1126/science.8023143. [DOI] [PubMed] [Google Scholar]
- 152.Rockstroh JK, Spengler U, Sudhop T, et al. Immunosuppression may lead to progression of hepatitis C virus-associated liver disease in hemophiliacs coinfected with HIV. Am J Gastroenterol. 1996;91:2563–2568. [PubMed] [Google Scholar]
- 153.Di Martino V, Rufat P, Boyer N, et al. The influence of human immunodeficiency virus coinfection on chronic hepatitis C in injection drug users: a long-term retrospective cohort study. Hepatology. 2001;34:1193–1199. doi: 10.1053/jhep.2001.29201. [DOI] [PubMed] [Google Scholar]
- 154.Goedert JJ, Eyster ME, Lederman MM, et al. End-stage liver disease in persons with hemophilia and transfusion-associated infections. Blood. 2002;100:1584–1589. [PubMed] [Google Scholar]
- 155.Monto A, Kakar S, Dove LM, et al. Contributions to hepatic fibrosis in HIV-HCV coinfected and HCV monoinfected patients. Am J Gastroenterol. 2006;101:1509–1515. doi: 10.1111/j.1572-0241.2006.00613.x. [DOI] [PubMed] [Google Scholar]
- 156.Balagopal A, Philp FH, Astemborski J, et al. Human immunodeficiency virus-related microbial translocation and progression of hepatitis C. Gastroenterology. 2008;135:226–233. doi: 10.1053/j.gastro.2008.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Valdez H, Anthony D, Farukhi F, et al. Immune responses to hepatitis C and non-hepatitis C antigens in hepatitis C virus infected and HIV-1 coinfected patients. AIDS. 2000;14:2239–2246. doi: 10.1097/00002030-200010200-00004. [DOI] [PubMed] [Google Scholar]
- 158.Graham CS, Wells A, Liu T, et al. Antigen-specific immune responses and liver histology in HIV and hepatitis C coinfection. AIDS. 2005;19:767–773. doi: 10.1097/01.aids.0000168970.80551.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Harcourt G, Gomperts E, Donfield S, et al. Profound loss of HCV-specific interferon-gamma secreting CD4+ T cells in HIV/ HCV co-infected patients. Gut. 2006;55:1484–1487. doi: 10.1136/gut.2005.083758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Canchis PW, Yee HT, Fiel MI, et al. Intrahepatic CD4+ cell depletion in hepatitis C virus/HIV-coinfected patients. J Acquir Immune Defic Syndr. 2004;37:1125–1131. doi: 10.1097/01.qai.0000131937.52106.92. [DOI] [PubMed] [Google Scholar]
- 161.Nunez M, Soriano V, Lopez M, et al. Coinfection with hepatitis C virus increases lymphocyte apoptosis in HIV-infected patients. Clin Infect Dis. 2006;43:1209–1212. doi: 10.1086/508355. [DOI] [PubMed] [Google Scholar]
- 162.Korner C, Kramer B, Schulte D, et al. Effects of HCV co-infection on apoptosis of CD4+ T cells in HIV-positive patients. Clin Sci (Lond) 2009;116:861–870. doi: 10.1042/CS20080532. [DOI] [PubMed] [Google Scholar]
- 163.Graham CS, Curry M, He Q, et al. Comparison of HCV-specific intrahepatic CD4+ T cells in HIV/HCV versus HCV. Hepatology. 2004;40:125–132. doi: 10.1002/hep.20258. [DOI] [PubMed] [Google Scholar]
- 164.Alatrakchi N, Graham CS, He Q, et al. CD8+ cell responses to hepatitis C virus (HCV) in the liver of persons with HCV-HIV coinfection versus HCV monoinfection. J Infect Dis. 2005;191:702–709. doi: 10.1086/427778. [DOI] [PubMed] [Google Scholar]
- 165.Smith MW, Walters KA, Korth MJ, et al. Gene expression patterns that correlate with hepatitis C and early progression to fibrosis in liver transplant recipients. Gastroenterology. 2006;130:179–187. doi: 10.1053/j.gastro.2005.08.015. [DOI] [PubMed] [Google Scholar]
- 166.Walters KA, Smith MW, Pal S, et al. Identification of a specific gene expression pattern associated with HCV-induced pathogenesis in HCV- and HCV/HIV-infected individuals. Virology. 2006;350:453–464. doi: 10.1016/j.virol.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 167.Yonkers NL, Rodriguez B, Post AB, et al. HIV coinfection impairs CD28-mediated costimulation of hepatitis C virus-specific CD8 cells. J Infect Dis. 2006;194:391–400. doi: 10.1086/505582. [DOI] [PubMed] [Google Scholar]
- 168.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Alter G, Suscovich TJ, Teigen N, et al. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- 170.Kovacs A, Al-Harthi L, Christensen S, et al. CD8(+) T cell activation in women coinfected with human immunodeficiency virus type 1 and hepatitis C virus. J Infect Dis. 2008;197:1402–1407. doi: 10.1086/587696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Giorgi JV, Ho HN, Hirji K, et al. CD8+ lymphocyte activation at human immunodeficiency virus type 1 seroconversion: development of HLA-DR+ CD38+ CD8+ cells is associated with subsequent stable CD4+ cell levels. The Multicenter AIDS Cohort Study. Group J Infect Dis. 1994;170:775–781. doi: 10.1093/infdis/170.4.775. [DOI] [PubMed] [Google Scholar]
- 172.Liu Z, Cumberland WG, Hultin LE, et al. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 173.Deeks SG, Kitchen CM, Liu L, et al. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood. 2004;104:942–947. doi: 10.1182/blood-2003-09-3333. [DOI] [PubMed] [Google Scholar]
- 174.Silvestri G, Feinberg MB. Turnover of lymphocytes and conceptual paradigms in HIV infection. J Clin Invest. 2003;112:821–824. doi: 10.1172/JCI19799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Sawa Y, Arima Y, Ogura H, et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity. 2009;30:447–457. doi: 10.1016/j.immuni.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 176.Wherry EJ, Day CL, Draenert R, et al. HIV-specific CD8 T cells express low levels of IL-7Ralpha: implications for HIV-specific T cell memory. Virology. 2006;353:366–373. doi: 10.1016/j.virol.2006.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Golden-Mason L, Burton JR, Jr, Castelblanco N, et al. Loss of IL-7 receptor alpha-chain (CD127) expression in acute HCV infection associated with viral persistence. Hepatology. 2006;44:1098–1109. doi: 10.1002/hep.21365. [DOI] [PubMed] [Google Scholar]
- 178.Penna A, Pilli M, Zerbini A, et al. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology. 2007;45:588–601. doi: 10.1002/hep.21541. [DOI] [PubMed] [Google Scholar]
- 179.Soriano-Sarabia N, Vallejo A, Molina-Pinelo S, et al. HIV-hepatitis C virus co-infection is associated with decreased plasmatic IL-7 levels. AIDS. 2007;21:253–255. doi: 10.1097/QAD.0b013e328011ec76. [DOI] [PubMed] [Google Scholar]
- 180.Bengsch B, Spangenberg HC, Kersting N, et al. Analysis of CD127 and KLRG1 expression on hepatitis C virus-specific CD8+ T cells reveals the existence of different memory T-cell subsets in the peripheral blood and liver. J Virol. 2007;81:945–953. doi: 10.1128/JVI.01354-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Mueller YM, De Rosa SC, Hutton JA, et al. Increased CD95/ Fas-induced apoptosis of HIV-specific CD8(+) T cells. Immunity. 2001;15:871–882. doi: 10.1016/s1074-7613(01)00246-1. [DOI] [PubMed] [Google Scholar]
- 182.Schacker TW, Nguyen PL, Beilman GJ, et al. Collagen deposition in HIV-1 infected lymphatic tissues and T cell homeostasis. J Clin Invest. 2002;110:1133–1139. doi: 10.1172/JCI16413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vali B, Yue FY, Jones RB, et al. HIV-specific T-cells accumulate in the liver in HCV/HIV co-infection. PLoS ONE. 2008;3:e3454. doi: 10.1371/journal.pone.0003454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Crispe IN, Dao T, Klugewitz K, et al. The liver as a site of T-cell apoptosis: graveyard, or killing field? Immunol Rev. 2000;174:47–62. doi: 10.1034/j.1600-0528.2002.017412.x. [DOI] [PubMed] [Google Scholar]
- 185.Mehandru S, Poles MA, Tenner-Racz K, et al. Primary HIV-1 infection is associated with preferential depletion of CD4+ T lymphocytes from effector sites in the gastrointestinal tract. J Exp Med. 2004;200:761–770. doi: 10.1084/jem.20041196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Li Q, Duan L, Estes JD, et al. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 187.Raffatellu M, Santos RL, Verhoeven DE, et al. Simian immuno-deficiency virus-induced mucosal interleukin-17 deficiency promotes Salmonella dissemination from the gut. Nat Med. 2008;14:421–428. doi: 10.1038/nm1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Rowan AG, Fletcher JM, Ryan EJ, et al. Hepatitis C virus-specific Th17 cells are suppressed by virus-induced TGF-beta. J Immunol. 2008;181:4485–4494. doi: 10.4049/jimmunol.181.7.4485. [DOI] [PubMed] [Google Scholar]
- 190.Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 191.Seki E, De Minicis S, Osterreicher CH, et al. TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat Med. 2007;13:1324–1332. doi: 10.1038/nm1663. [DOI] [PubMed] [Google Scholar]
- 192.Westendorp MO, Frank R, Ochsenbauer C, et al. Sensitization of T cells to CD95-mediated apoptosis by HIV-1 Tat and gp120. Nature. 1995;375:497–500. doi: 10.1038/375497a0. [DOI] [PubMed] [Google Scholar]
- 193.Macias J, Japon MA, Saez C, et al. Increased hepatocyte fas expression and apoptosis in HIV and hepatitis C virus coinfection. J Infect Dis. 2005;192:1566–1576. doi: 10.1086/491736. [DOI] [PubMed] [Google Scholar]
- 194.Jo M, Kim TH, Seol DW, et al. Apoptosis induced in normal human hepatocytes by tumor necrosis factor-related apoptosis-inducing ligand. Nat Med. 2000;6:564–567. doi: 10.1038/75045. [DOI] [PubMed] [Google Scholar]
- 195.Volkmann X, Fischer U, Bahr MJ, et al. Increased hepatotoxicity of tumor necrosis factor-related apoptosis-inducing ligand in diseased human liver. Hepatology. 2007;46:1498 –1508. doi: 10.1002/hep.21846. [DOI] [PubMed] [Google Scholar]
- 196.Herzer K, Ganten TM, Schulze-Bergkamen H, et al. Transforming growth factor beta can mediate apoptosis via the expression of TRAIL in human hepatoma cells. Hepatology. 2005;42:183–192. doi: 10.1002/hep.20757. [DOI] [PubMed] [Google Scholar]
- 197.Herbeuval JP, Grivel JC, Boasso A, et al. CD4+ T-cell death induced by infectious and noninfectious HIV-1: role of type 1 interferon-dependent, TRAIL/DR5-mediated apoptosis. Blood. 2005;106:3524–3531. doi: 10.1182/blood-2005-03-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Herbeuval JP, Boasso A, Grivel JC, et al. TNF-related apoptosis-inducing ligand (TRAIL) in HIV-1-infected patients and its in vitro production by antigen-presenting cells. Blood. 2005;105:2458–2464. doi: 10.1182/blood-2004-08-3058. [DOI] [PubMed] [Google Scholar]
- 199.Zhu H, Dong H, Eksioglu E, et al. Hepatitis C virus triggers apoptosis of a newly developed hepatoma cell line through antiviral defense system. Gastroenterology. 2007;133:1649–1659. doi: 10.1053/j.gastro.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 200.Lan L, Gorke S, Rau SJ, et al. Hepatitis C virus infection sensitizes human hepatocytes to TRAIL-induced apoptosis in a caspase 9-dependent manner. J Immunol. 2008;181:4926–4935. doi: 10.4049/jimmunol.181.7.4926. [DOI] [PubMed] [Google Scholar]
- 201.Babu CK, Suwansrinon K, Bren GD, et al. HIV induces TRAIL sensitivity in hepatocytes. PLoS ONE. 2009;4:e4623. doi: 10.1371/journal.pone.0004623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee SH, Kim YK, Kim CS, et al. E2 of hepatitis C virus inhibits apoptosis. J Immunol. 2005;175:8226–8235. doi: 10.4049/jimmunol.175.12.8226. [DOI] [PubMed] [Google Scholar]
- 203.Janssen EM, Droin NM, Lemmens EE, et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature. 2005;434:88–93. doi: 10.1038/nature03337. [DOI] [PubMed] [Google Scholar]
- 204.Hamilton SE, Wolkers MC, Schoenberger SP, et al. The generation of protective memory-like CD8+ T cells during homeo-static proliferation requires CD4+ T cells. Nat Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 205.Kuerten S, Asaad RJ, Schoenberger SP, et al. The TRAIL of helpless CD8+ T cells in HIV infection. AIDS Res Hum Retroviruses. 2008;24:1175–1183. doi: 10.1089/aid.2008.0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Semple RK, Sleigh A, Murgatroyd PR, et al. Postreceptor insulin resistance contributes to human dyslipidemia and hepatic steatosis. J Clin Invest. 2009;119:315–322. doi: 10.1172/JCI37432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Petta S, Camma C, Di Marco V, et al. Insulin resistance and diabetes increase fibrosis in the liver of patients with genotype 1 HCV infection. Am J Gastroenterol. 2008;103:1136 –1144. doi: 10.1111/j.1572-0241.2008.01813.x. [DOI] [PubMed] [Google Scholar]
- 208.Caronia S, Taylor K, Pagliaro L, et al. Further evidence for an association between non-insulin-dependent diabetes mellitus and chronic hepatitis C virus infection. Hepatology. 1999;30:1059–1063. doi: 10.1002/hep.510300416. [DOI] [PubMed] [Google Scholar]
- 209.Mehta SH, Brancati FL, Sulkowski MS, et al. Prevalence of type 2 diabetes mellitus among persons with hepatitis C virus infection in the United States. Ann Intern Med. 2000;133:592–599. doi: 10.7326/0003-4819-133-8-200010170-00009. [DOI] [PubMed] [Google Scholar]
- 210.Mehta SH, Brancati FL, Strathdee SA, et al. Hepatitis C virus infection and incident type 2 diabetes. Hepatology. 2003;38:50–56. doi: 10.1053/jhep.2003.50291. [DOI] [PubMed] [Google Scholar]
- 211.Shintani Y, Fujie H, Miyoshi H, et al. Hepatitis C virus infection and diabetes: direct involvement of the virus in the development of insulin resistance. Gastroenterology. 2004;126:840 – 848. doi: 10.1053/j.gastro.2003.11.056. [DOI] [PubMed] [Google Scholar]
- 212.Mirandola S, Realdon S, Iqbal J, et al. Liver microsomal triglyceride transfer protein is involved in hepatitis C liver steatosis. Gastroenterology. 2006;130:1661–1669. doi: 10.1053/j.gastro.2006.02.035. [DOI] [PubMed] [Google Scholar]
- 213.Piodi A, Chouteau P, Lerat H, et al. Morphological changes in intracellular lipid droplets induced by different hepatitis C virus genotype core sequences and relationship with steatosis. Hepatology. 2008;48:16–27. doi: 10.1002/hep.22288. [DOI] [PubMed] [Google Scholar]
- 214.Feldstein AE, Canbay A, Angulo P, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–443. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 215.Seidel N, Volkmann X, Langer F, et al. The extent of liver steatosis in chronic hepatitis C virus infection is mirrored by caspase activity in serum. Hepatology. 2005;42:113–120. doi: 10.1002/hep.20747. [DOI] [PubMed] [Google Scholar]
- 216.Mundt B, Wirth T, Zender L, et al. Tumour necrosis factor related apoptosis inducing ligand (TRAIL) induces hepatic steatosis in viral hepatitis and after alcohol intake. Gut. 2005;54:1590–1596. doi: 10.1136/gut.2004.056929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Malhi H, Barreyro FJ, Isomoto H, et al. Free fatty acids sensitise hepatocytes to TRAIL mediated cytotoxicity. Gut. 2007;56:1124–1131. doi: 10.1136/gut.2006.118059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wedemeyer I, Bechmann LP, Odenthal M, et al. Adiponectin inhibits steatotic CD95/Fas up-regulation by hepatocytes: therapeutic implications for hepatitis C. J Hepatol. 2009;50:140–149. doi: 10.1016/j.jhep.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 219.Herzenberg LA, De Rosa SC, Dubs JG, et al. Glutathione deficiency is associated with impaired survival in HIV disease. Proc Natl Acad Sci U S A. 1997;94:1967–1972. doi: 10.1073/pnas.94.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Barbaro G, Di Lorenzo G, Soldini M, et al. Hepatic glutathione deficiency in chronic hepatitis C: quantitative evaluation in patients who are HIV positive and HIV negative and correlations with plasmatic and lymphocytic concentrations and with the activity of the liver disease. Am J Gastroenterol. 1996;91:2569–2573. [PubMed] [Google Scholar]
- 221.Howard AA, Klein RS, Schoenbaum EE. Association of hepatitis C infection and antiretroviral use with diabetes mellitus in drug users. Clin Infect Dis. 2003;36:1318–1323. doi: 10.1086/374838. [DOI] [PubMed] [Google Scholar]
- 222.Bani-Sadr F, Carrat F, Bedossa P, et al. Hepatic steatosis in HIV-HCV coinfected patients: analysis of risk factors. AIDS. 2006;20:525–531. doi: 10.1097/01.aids.0000210606.63138.f5. [DOI] [PubMed] [Google Scholar]
- 223.Sulkowski MS, Mehta SH, Torbenson M, et al. Hepatic steato-sis and antiretroviral drug use among adults coinfected with HIV and hepatitis C virus. AIDS. 2005;19:585–592. doi: 10.1097/01.aids.0000163935.99401.25. [DOI] [PubMed] [Google Scholar]
- 224.McGovern BH, Ditelberg JS, Taylor LE, et al. Hepatic steatosis is associated with fibrosis, nucleoside analogue use, and hepatitis C virus genotype 3 infection in HIV-seropositive patients. Clin Infect Dis. 2006;43:365–372. doi: 10.1086/505495. [DOI] [PubMed] [Google Scholar]
- 225.Lonardo A, Adinolfi LE, Loria P, et al. Steatosis and hepatitis C virus: mechanisms and significance for hepatic and extrahepatic disease. Gastroenterology. 2004;126:586–597. doi: 10.1053/j.gastro.2003.11.020. [DOI] [PubMed] [Google Scholar]
- 226.Cooper CL, Cameron DW. Review of the effect of highly active antiretroviral therapy on hepatitis C virus (HCV) RNA levels in human immunodeficiency virus and HCV coinfection. Clin Infect Dis. 2002;35:873–879. doi: 10.1086/342388. [DOI] [PubMed] [Google Scholar]
- 227.Ndhlovu LC, Chapman JM, Jha AR, et al. Suppression of HIV-1 plasma viral load below detection preserves IL-17 producing T cells in HIV-1 infection. AIDS. 2008;22:990–992. doi: 10.1097/QAD.0b013e3282ff884e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Alatrakchi N, Martino VD, Thibault V, et al. Strong CD4 Th1 responses to HIV and hepatitis C virus in HIV-infected long-term non-progressors co-infected with hepatitis C virus. AIDS. 2002;16:713–717. doi: 10.1097/00002030-200203290-00006. [DOI] [PubMed] [Google Scholar]
- 229.Berzsenyi MD, Bowden DS, Kelly HA, et al. Reduction in hepatitis C-related liver disease associated with GB virus C in human immunodeficiency virus coinfection. Gastroenterology. 2007;133:1821–1830. doi: 10.1053/j.gastro.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 230.Brau N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44:47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 231.Qurishi N, Kreuzberg C, Luchters G, et al. Effect of antiretroviral therapy on liver-related mortality in patients with HIV and hepatitis C virus coinfection. Lancet. 2003;362:1708–1713. doi: 10.1016/S0140-6736(03)14844-1. [DOI] [PubMed] [Google Scholar]
- 232.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2008 recommendations of the International AIDS Society-USA panel. JAMA. 2008;300:555–570. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 233.McGovern BH, Birch C, Zaman MT, et al. Managing symptomatic drug-induced liver injury in HIV-hepatitis C virus-coinfected patients: a role for interferon. Clin Infect Dis. 2007;45:1386–1392. doi: 10.1086/522174. [DOI] [PubMed] [Google Scholar]
- 234.Moodie EE, Pant Pai N, Klein MB. Is antiretroviral therapy causing long-term liver damage? A comparative analysis of HIV-mono-infected and HIV/hepatitis C co-infected cohorts. PLoS ONE. 2009;4:e4517. doi: 10.1371/journal.pone.0004517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Stone SF, Lee S, Keane NM, et al. Association of increased hepatitis C virus (HCV)-specific IgG and soluble CD26 dipeptidyl peptidase IV enzyme activity with hepatotoxicity after highly active antiretroviral therapy in human immunodeficiency virus-HCV-coinfected patients. J Infect Dis. 2002;186:1498 –1502. doi: 10.1086/344892. [DOI] [PubMed] [Google Scholar]
- 236.Miller MF, Haley C, Koziel MJ, et al. Impact of hepatitis C virus on immune restoration in HIV-infected patients who start highly active antiretroviral therapy: a meta-analysis. Clin Infect Dis. 2005;41:713–720. doi: 10.1086/432618. [DOI] [PubMed] [Google Scholar]
- 237.McGovern BH, Golan Y, Lopez M, et al. The impact of cirrhosis on CD4+ T cell counts in HIV-seronegative patients. Clin Infect Dis. 2007;44:431–437. doi: 10.1086/509580. [DOI] [PubMed] [Google Scholar]
- 238.Sulkowski MS, Moore RD, Mehta SH, et al. Hepatitis C and progression of HIV disease. JAMA. 2002;288:199–206. doi: 10.1001/jama.288.2.199. [DOI] [PubMed] [Google Scholar]
- 239.Dorrucci M, Valdarchi C, Suligoi B, et al. The effect of hepatitis C on progression to AIDS before and after highly active antiretroviral therapy. AIDS. 2004;18:2313–2318. doi: 10.1097/00002030-200411190-00012. [DOI] [PubMed] [Google Scholar]
- 240.El-Serag HB, Giordano TP, Kramer J, et al. Survival in hepatitis C and HIV co-infection: a cohort study of hospitalized veterans. Clin Gastroenterol Hepatol. 2005;3:175–183. doi: 10.1016/s1542-3565(04)00620-2. [DOI] [PubMed] [Google Scholar]
- 241.Fauci AS. HIV vaccines: science and policy, keynote lecture at the AIDS vaccine meeting; Cape Town, South Africa. October 2008. [Google Scholar]
- 242.Golden-Mason L, Rosen HR. Natural killer cells: primary target for hepatitis C virus immune evasion strategies? Liver Transpl. 2006;12:363–372. doi: 10.1002/lt.20708. [DOI] [PubMed] [Google Scholar]
- 243.Iannello A, Debbeche O, Samarani S, et al. Antiviral NK cell responses in HIV infection: I. NK cell receptor genes as determinants of HIV resistance and progression to AIDS. J Leukoc Biol. 2008;84:1–26. doi: 10.1189/jlb.0907650. [DOI] [PubMed] [Google Scholar]
- 244.Bain C, Fatmi A, Zoulim F, et al. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 245.Noursadeghi M, Katz DR, Miller RF. HIV-1 infection of mononuclear phagocytic cells: the case for bacterial innate immune deficiency in AIDS. Lancet Infect Dis. 2006;6:794–804. doi: 10.1016/S1473-3099(06)70656-9. [DOI] [PubMed] [Google Scholar]
- 246.Stamataki Z, Grove J, Balfe P, et al. Hepatitis C virus entry and neutralization. Clin Liver Dis. 2008;12:693–712. x. doi: 10.1016/j.cld.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 247.Moir S, Fauci AS. B cells in HIV infection and disease. Nat Rev Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Bowen DG, Walker CM. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature. 2005;436:946–952. doi: 10.1038/nature04079. [DOI] [PubMed] [Google Scholar]
- 249.Schwartz O, Marechal V, Le Gall S, et al. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat Med. 1996;2:338–342. doi: 10.1038/nm0396-338. [DOI] [PubMed] [Google Scholar]
- 250.Kerkau T, Bacik I, Bennink JR, et al. The human immunodeficiency virus type 1 (HIV-1) Vpu protein interferes with an early step in the biosynthesis of major histocompatibility complex (MHC) class I molecules. J Exp Med. 1997;185:1295–1305. doi: 10.1084/jem.185.7.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Goulder PJ, Watkins DI. Impact of MHC class I diversity on immune control of immunodeficiency virus replication. Nat Rev Immunol. 2008;8:619–630. doi: 10.1038/nri2357. [DOI] [PMC free article] [PubMed] [Google Scholar]