Abstract
Objective
To identify quantitative measurement variables that characterize mobility in older adults, meet reliability and validity criteria, distinguish fall-risk and predict future falls.
Design
Observational study with 1-year weekly falls follow-up
Setting
Mobility laboratory
Participants
Community-dwelling volunteers (n=74; 65–94 years old) categorized at entry as 27 ‘Non-fallers’ or 47 ‘Fallers’ by Medicare criteria (1 injury fall or >1 non-injury falls in the previous year).
Interventions
None
Outcome Measures
Test-retest and within-subject reliability, criterion and concurrent validity; predictive ability indicated by observed sensitivity and specificity to entry fall-risk group (Falls-status), Tinetti Performance Oriented Mobility Assessment (POMA), Computerized Dynamic Posturography Sensory Organization Test (SOT) and subsequent falls reported weekly.
Results
Measurement variables were selected that met reliability (ICC > 0.6) and/or discrimination (p<.01) criteria (Clinical variables- Turn- steps, time, Gait- velocity, Step-in-tub-time, and Downstairs- time; Force plate variables- Quiet standing Romberg ratio sway-area, Maximal lean- anterior-posterior excursion, Sit-to-stand medial-lateral excursion and sway-area). Sets were created (3 clinical, 2 force plate) utilizing combinations of variables appropriate for older adults with different functional activity levels and composite scores were calculated. Scores identified entry Falls-status and concurred with POMA and SOT. The Full clinical set (5 measurement variables) produced sensitivity/specificity (.80/.74) to Falls-status. Composite scores were sensitive and specific in predicting subsequent injury falls and multiple falls compared to Falls-status, POMA or SOT.
Conclusions
Sets of quantitative measurement variables obtained with this mobility battery provided sensitive prediction of future injury falls and screening for multiple subsequent falls using tasks that should be appropriate to diverse participants.
Keywords: Outcome measures, Elderly, Geriatric assessment, Mobility limitations
Mobility disorders compromise quality of life and limit an older person’s level of independence1. Maintenance of the center-of-gravity over the base-of-support2 or balance is required for safe functional activity. However, mobility function is complex, including such tasks as maintaining stance during limb movements, performing transfers and stepping up or down3.
Measurement of mobility is essential for developing and evaluating interventions to prevent chronic disability and acute morbidity. While existing screening tools are valuable to identify those not needing treatment, diagnostic and outcome measures are needed to assess efficacy. The most commonly used mobility assessments are self-reported capacity to climb stairs or walk one-half mile4 and rating scales such as the Tinetti Performance Oriented Mobility Assessment5 (POMA). Rating scales utilize subjective categorical determinations to create ordinal measurements, may be time-consuming, and are subject to inter-rater reliability concerns.
Objective performance indices (e.g. Short Physical Performance Battery6- ‘SPPB’, Timed-up-and-go- ‘TUG’7 or Sensory Organization Test-‘SOT’8) are also commonly used. These continuous measures offer interval or ratio measurements and therefore finer performance distinctions. However, the tasks included may offer limited challenge to high-functioning individuals or include elements that are too difficult for impaired older adults. To address these issues, we developed a mobility battery based on activities of daily living (ADLs) that includes tasks representing progressively more difficult mobility components (see Appendix for details). The tasks progress with increasing complexity9 and are designed to challenge performance abilities across the spectrum of older adults. Using progressively complex tasks including: standing balance, maximal leaning, reaching and pulling, sit-to-stand, gait, turns, stair descent and sideways step-in-tub offers the potential to avoid ceiling and floor effects10.
Since there is no accepted gold-standard for mobility measurement, we compared the proposed measures with several that have achieved broad use. A recent history of falls has been used as an indicator of functional decline11. Because falls may preceed or follow mobility changes12, we adopted the criteria of future injury or multiple falls as sentinel events for change in mobility status. Sensitivity rather than specificity was emphasized, as the focus was on recognizing individuals requiring intervention, rather than screening those who did not.
The purpose of this study was to identify quantitative measurement variables that characterize diverse mobility tasks in older adults, meet reliability and validity criteria comparing favorably with other approaches, distinguish entry fall-risk group (Falls-status) by Medicare criteria and offer sensitivity to changes in mobility status as evidenced by subsequent falls.
METHODS
Subjects and Procedure
This study was approved by the Institutional Review Board at the University of Connecticut Health Center (UCHC). Subjects were recruited by letter using a mailing list provided by the UCHC Center on Aging, and initially screened by telephone. On their first visit 74 community-dwelling elders (CDE) provided informed consent, mental status, medical and falls histories and underwent physical examination by a physician. Exclusion criteria were: cognitive impairment (Mini Mental Status Exam13, score <24), legally blind, obesity (BMI ≥ 30), and non-English speaking. To eliminate the influence of known pathology, volunteers with a diagnosis of neurological, orthopedic or visual disorders (e.g. Parkinson’s disease, knee replacement or macular degeneration) directly impairing mobility were excluded. Common orthopedic limitations (e.g. osteoarthritis, knee pain) were not considered exclusion criteria.
Utilizing Medicare14 fall-risk screening criteria, participants reporting ≥2 non-injury falls in the past year or ≥1 injury fall were categorized as ‘Fallers’; remaining subjects were considered ‘Non-Fallers’. Subjects also completed the Tinetti5 (POMA; Appendix) and the Sensory Organization Test8 (SOT; NeuroCom International, Appendix). The mobility battery was conducted on the subsequent visit, and Non-Fallers repeated the battery the same day to assess test-retest reliability. Test-retest data from Non-Fallers provided a preliminary reliability screen. Health changes and falls were reported weekly by postcard for up to a year or until ≥1 month of non-ambulatory status. Non-receipt of postcards, changes or falls triggered telephone inquiries. All follow-up participants were included in analyses of predictive validity.
Selection of Measurement Variables
We started with diverse measurement variables from the various tasks and sought to retain only those that were both reliable and repeatable. Measurement variables for each task derived from the biomechanics literature (Appendix) were assessed with semi-automated calculations using computer algorithms. Variables were evaluated for normality and normalized if necessary.
During mobility testing subjects practiced and rested as needed, then performed tasks at a self-selected pace 3 times (except where noted below) in their habitual manner. Tasks/variables with very low test-retest reliability (Non-Faller intraday Pearson r<0.3) were excluded from consideration. Within-subject reliability of variables in the first session for all subjects was evaluated by computing the intra-class correlation (ICC, herein defined as the ratio of across-subject to total variance) using a linear mixed model15 (LMM) with a random subject-specific intercept. Criterion-related validity was assessed with group means for each variable by ability to distinguish entry fall-risk group by Medicare criteria (Falls-status- Faller or Non-faller). Variables demonstrating moderate reliability (ICC>0.6)16, and/or those that clearly distinguish Falls-status (p<0.01) were selected for further examination and categorized as clinical or force plate measures.
Tasks are further described in the Appendix, those with variables meeting the aforementioned criteria (underlined) were assessed as follows:
Quiet standing
The clinical Romberg test17 of standing balance with eyes open and closed was conducted using a force plate (Advanced Mechanical Technology Inc). Data were collected for 1 minute and sway-area (enclosed center-of-pressure-COP path) was evaluated over the middle 30 seconds of 2 trials in each condition. The Romberg ratio compared eyes closed to open.
Maximal lean
Subjects leaned as far forward and backward as they could without bending their hips or knees or losing their balance. Anterior-posterior force plate COP excursion was calculated as the distance between the maximum forward and backward positions18.
Sit-to-stand
Sitting (seat 41.4cm height) with arms crossed below the sternum and feet on the force plate, subjects were asked to stand. Sit-to-stand time was measured from onset of anterior-posterior force until vertical force reached body-weight. Sway-area was calculated from this point until variance was <1SD for >5s. Medial-lateral and anterior-posterior excursion values were determined for anterior-posterior and vertical19 phases (Appendix).
Gait
Two self-paced out and back20 walks (8.1m) were performed, average velocity was calculated and velocity for the fastest performance was used.
Turn
Subjects started 2 strides (self-selected 1.8–2.8m) from a chair. Time from the first step until the subject began to sit, and number of steps to turn taken were assessed.
Step-in-tub
Subjects stepped sideways (hips perpendicular) into a simulated tub (33cm high), a vertical grab bar was used if needed. Time from initiation of weight-transfer until end of 1-legged stance was measured.
Downstairs
Subjects descended 3 steps (17.8cm) using the handrail, if desired. Time from initiation of descent to touchdown was measured.
Creating Composite Scores
To evaluate these variables as mobility criteria, we created composite scores by standardizing each measure and summing the Z scores of subsets of individual variables. Using 9 selected measurement variables (5 Clinical: Gait- velocity, Turn- steps, Turn- time, Downstairs- time, and Step-in-tub- time; and 4 Force plate: Quiet standing- Romberg ratio sway-area, Maximal lean-anterior- posterior excursion, Sit-to-stand- medial-lateral excursion, and Sit-to-stand- sway-area upon standing), 5 measurement ‘Sets’ were created. Three sets are appropriate for use in clinical settings, and 2 when a force plate is available. All 5 clinical measures comprised the ‘Full clinical set’. The ‘Intermediate clinical set’ excluded Step-in-tub- time and the ‘Brief clinical set’ further excluded Downstairs- time. There were 2 force plate sets; the ‘Intermediate force plate set’ included all 4 force plate measures, and the ‘Brief force plate set’ excluded Sit-to-stand-medial-lateral excursion and sway-area upon standing. By excluding more difficult tasks, ‘Brief’ sets may be appropriate for frail individuals; while elimination of tiring tasks for ‘Intermediate’ sets may enable assessment of those with marginal endurance.
Sensitivity and Specificity
Sensitivity and specificity were calculated by varying the composite score threshold and using Receiver Operator Characteristic (ROC) analysis to categorize individual performance. ROC analysis requires an established cut-off value or criterion21. Since no quantitative standards for mobility exist, we used published standards for POMA, SOT and entry Medicare14 fall-risk group (Falls-status) as criteria. POMA was considered normal if 1 item had a point deducted; and abnormal if 2 items had a point deducted, or one item had 2 points deducted22. SOT was considered normal23 if the participant scored above the 70–79 year old mean (>729), and abnormal if below (≤729). The ability of the composite scores to accurately determine Falls-status was compared to POMA and SOT using these values. Next, concurrence between composite scores and Falls-status, POMA and SOT criteria was evaluated. Finally, we examined the potential of the composite scores, POMA and SOT to predict sentinel events using 2 prospective criteria: an Injury fall or Multiple falls (≥ 2 non-injury falls OR ≥1 injury falls) Confidence intervals for observed prospective sensitivity and specificity were calculated on the logit scale.
RESULTS
Participants were separated into Falls-status entry groups: 27 ‘Non-fallers’ (age: range 65–87, 75.1±6.5 (mean±SD)) and 47 ‘Fallers’ (age: range 70–94, 80.1±6.2). Fallers were older than the Non-fallers (Mann-Whitney, p=.008). There was no difference between Falls-status groups by sex (Χ2=0.33, p=0.56). Four clinical and 2 force plate variables demonstrated both reliability (ICC>0.6) and fall-status discrimination (p<.01). Two force plate Sit-to-stand variables and the Number of Steps measure from the Turn task, which distinguished Falls-status (p<.01) were also included in further analyses. The distributional properties of Number of Steps (either 3 or 4) precluded calculation of reliability. Table 1 shows statistical profiles of these 9 variables. Prospective follow-up was completed by 62 participants, 12 declined weekly follow-up. Falls and medical changes were reported by postcard for up to 1 year or until subjects were non-ambulatory for a month. Five reached endpoint after sending postcards for 3–32 weeks due to stroke, serious illness or injury sequelae.
Table 1.
Characteristics of Clinical and Force Plate Measurement Variables
| Reliability | Mean (Standard Deviation) | p-value | ||
|---|---|---|---|---|
| Intra-Class Correlation |
Non-Fallers (n=27) |
Fallers (n=47) | Non-Fallers vs. Fallers* |
|
| Clinical Variables | ||||
| Gait- Velocity (meters/second) | 0.745 | 0.86 (0.13) | 0.64 (0.18) | <0.001 |
| Turn- Steps (number) | † | 3.04 (0.20) | 3.31(0.46) | <0.001‡ |
| Turn- Time (seconds) | 0.709 | 1.06 (0.32) | 1.48 (0.77) | 0.002 |
| Downstairs- Time (seconds) | 0.626 | 3.47 (0.69) | 4.45 (0.90) | <0.001 |
| Step-in-tub- Time (seconds) | 0.702 | 1.90 (0.52) | 2.93 (1.22) | <0.001 |
| Force Plate Variables§ | ||||
| Quiet Standing- Romberg ratio, sway-area (square centimeters) | 0.993 | 6.77 (1.35) | 11.55 (11.87) | 0.007 |
| Maximal Lean- Anterior-Posterior excursion (centimeters) | 0.754 | 16.28 (3.58) | 12.93 (3.48) | <0.001 |
| Sit-to-stand- sway-area (square centimeters) | 0.367 | 3.27 (6.58) | 7.47 (3.44) | 0.001 |
| Sit-to-stand- Medial-Lateral excursion (centimeters) | 0.562 | 2.54 (1.68) | 4.04 (3.66) | 0.006 |
Notes:
P values obtained with linear mixed models;
only two values observed, ICC cannot be calculated;
P values obtained using a logistic quasi-likelihood model.
There was no difference in Falls-status entry groups with 4/27(15%) Non-fallers and 8/47(17%) Fallers declining follow-up (p=0.80). During follow-up 3/23(13%) Non-fallers and 9/39(23%) Fallers sustained an Injury fall (p=0.51), while 17/23(74%) Non-fallers and 23/39(59%) Fallers were Multiple fallers (p=.24). Our community-dwelling volunteers were separated into 2 entry groups and statistical criteria were used to select measures that accurately identified their Falls-status. No treatment or intervention was undertaken; therefore in subsequent analyses of the proposed measures, no statistical inference was employed. We report the observed sensitivity/specificity of the measurement sets utilizing various criteria.
Entry Falls-status Criterion
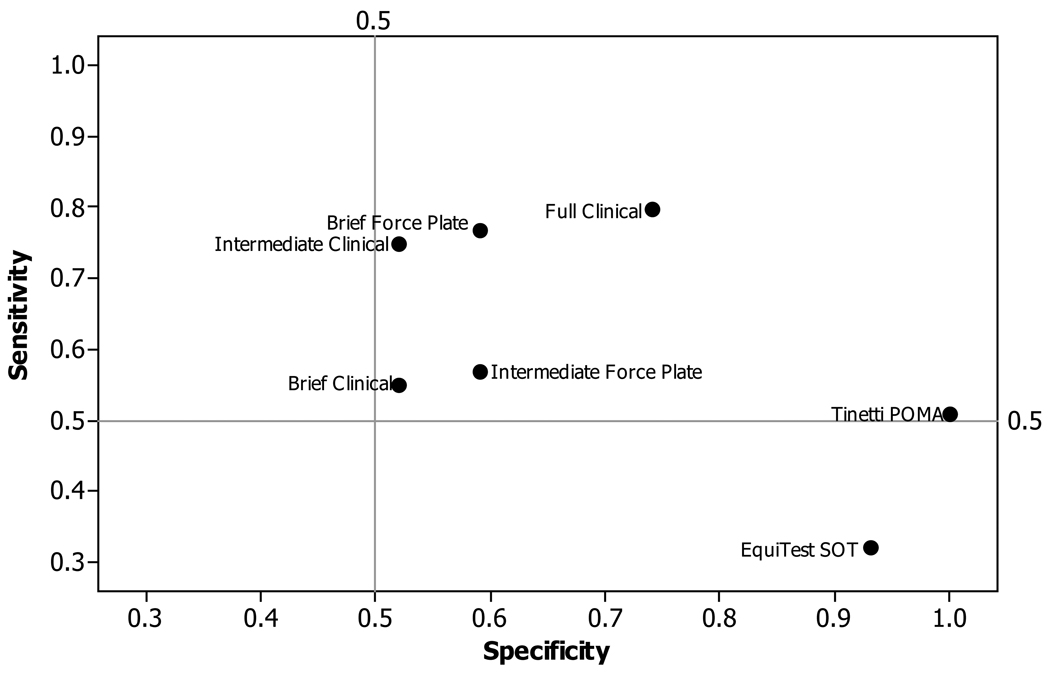
The sensitivity/specificity with which the 5 measurement sets identified entry Falls-status group was compared to POMA and SOT (Figure 1). The full clinical set produced both sensitivity and specificity (.80 and .74 respectively). Removing step-in-tub time (intermediate clinical set) markedly decreased specificity (from .74 to .52). When both downstairs and step-in-tub times were removed (brief clinical set), both sensitivity (from .80 to .57) and specificity (from .74 to .59) were decreased. The brief forceplate set provided higher sensitivity (.77) than both the intermediate (.75) and brief (.57) clinical sets. By comparison, both POMA and SOT scores showed lower sensitivity and high specificity (POMA, .51 and 1.00; and SOT, .32 and .93 respectively) to entry falls status.
Figure 1. Sensitivity and Specificity of Measurement Sets, POMA and SOT to Entry Fall-Risk Group.
Sensitivity and specificity values were calculated using entry Falls Status (Faller or Non-faller) as the criterion. Composite scores consisted of: Full Clinical set- Gait velocity, Turn steps, Turn time, Downstairs time, and Step-in-tub time; Intermediate Clinical set- Gait velocity, Turn steps, Turn time, Downstairs time; Brief Clinical set- Gait velocity, Turn steps, Turn time; Brief Force Plate set- Romberg ratio and Maximum lean; Tinetti POMA criterion- total of subscales where ≤26/28 was the threshold22; EquiTest SOT criterion- total score where ≤729 was the threshold23.
Concurrent Validity
Concurrence of the 5 measurement sets with Falls-status, POMA and SOT is shown in Table 2. Sets were sensitive to POMA, though less so to Falls-status and SOT. The Full clinical set was the most sensitive overall, identifying those who would fail the POMA criterion with 100% sensitivity, Falls-status 80% and SOT 79%. The Intermediate clinical set demonstrated 83–75% sensitivity to these criteria, while both Brief sets were sensitive to POMA (71%). Only the Full and Intermediate clinical sets offered concurrence with SOT.
Table 2.
Concurrent Validity of Quantitative Measurements with Existing Standards Sensitivity (lower limit of confidence interval, upper limit of confidence interval) Specificity (lower limit of confidence interval, upper limit of confidence interval)
| Entry Falls-status* |
POMA Score† | EquiTest SOT‡ | |
|---|---|---|---|
| Clinical Measurement Sets | |||
| Full Complement: Gait velocity, | |||
| Turn Time, Turn # of Steps, | A-0.8 (0.65, 0.9) | 1.0 | 0.79 (0.51, 0.93) |
| Down 3 Stairs, Step in Tub | B-0.74 (0.55, 0.87) | .57 (0.43, 0.7) | 0.66 (0.52, 0.77) |
| Intermediate: Gait velocity, | A-0.75 (0.59, 0.86) | 0.83 (0.59, 0.95) | 0.79 (0.51, 0.93) |
| Turn Time, Turn # of Steps, | B-0.52 (0.34, 0.7) | 0.71 (0.57, 0.82) | 0.74 (0.6, 0.84) |
| Down 3 Stairs | |||
| Brief: Gait velocity, Turn Time, | A-0.57 (0.43, 0.71) | 0.71 (0.5, 0.85) | 0.41 (0.21, 0.65) |
| Turn # of Steps | B-0.59 (0.4, 0.76) | 0.5 (0.36, 0.64) | 0.54 (0.41, 0.67) |
| Force Plate Measurement Sets | |||
| Intermediate: Quiet Standing, | A-0.55 (0.41, 0.69) | 0.63 (0.42, 0.79) | 0.53 (0.3, 0.74) |
| Maximal Leaning, Sway Area, | B-0.52 (0.34, 0.7) | 0.52 (0.38, 0.65) | 0.51 (0.38, 0.64) |
| Medial/Lateral Excursion | |||
| Brief: Quiet Standing, Maximal | A-0.77 (0.62, 0.87) | 0.75 (0.54, 0.88) | 0.47 (0.26, 0.7) |
| Leaning | B-0.59 (0.4, 0.76) | 0.5 (0.36, 0.64) | 0.58 (0.45, 0.7) |
NOTE: Values expressed as A-sensitivity (confidence interval) and B-specificity (confidence interval
Entry Falls-status - classification as Non-faller or Faller based on self-reported history of ≥1 injury fall or ≥2 falls14;
Tinetti POMA- total of Balance and Gait subscales, where ≤26/28 indicated problems22;
EquiTest SOT- total score for six conditions ≤729 (70–79 year old mean) was abnormal23.
Anterior-Posterior- A-P, Medial-Lateral- M-L.
Predictive Validity
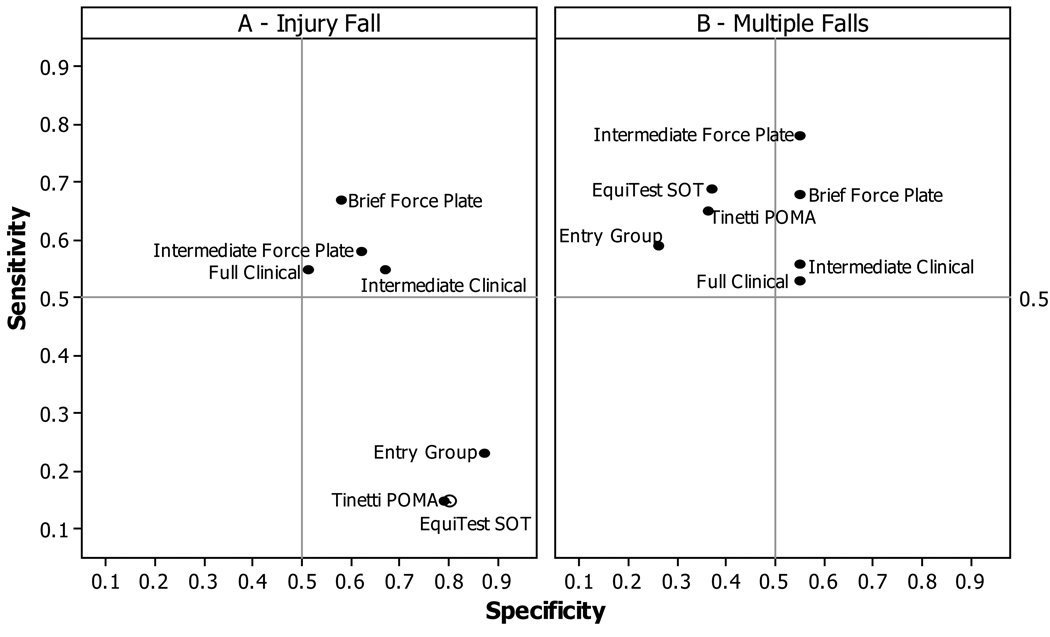
Predictive ability of measurement sets was evaluated by prospective criteria obtained during 1-year follow-up of 62 participants. The sensitivity/specificity with which each set predicted a participant’s subsequent Injury fall (12/62) or Multiple falls (40/62) during the follow-up period is compared to that of entry group Falls-status, POMA and SOT criteria in Figure 2. Entries in the upper right-hand quadrant demonstrate both sensitivity and specificity.
Figure 2. Predictive Value of Mobility Measurement Sets, Group, POMA and SOT.
Sensitivity and specificity values were calculated using two falls criteria (Injury fall or Multiple falls) from weekly reports during the 1-year follow-up period. Composite scores consisted of: Full Clinical set-Gait velocity, Turn steps, Turn time, Downstairs time, and Step-in-tub time; Intermediate Clinical set- Gait velocity, Turn steps, Turn time, Downstairs time; Brief Clinical set- Gait velocity, Turn steps, Turn time; Intermediate Force Plate set- Romberg ratio, Maximum lean, M-L excursion and Sit-to-stand sway-area; Brief Force Plate set- Romberg ratio and Maximum lean; Tinetti POMA criterion- total of subscales where ≤26/28 was the threshold22; EquiTest SOT criterion- total score where ≤729 was the threshold23.
Mobility measurement sets were more sensitive and less specific than Falls-status, POMA or SOT score when predicting an Injury fall (Figure 2a). The Brief force plate set yielded the highest sensitivity (.68) to Injury falls, and should allow testing of frail individuals. For Multiple falls (Figure 2b), SOT, POMA, and Falls-status showed sensitivity, but not specificity. Measurement sets offered both sensitivity and specificity, the Intermediate force plate set showing the highest sensitivity (.78).
DISCUSSION
A battery of common mobility activities was reduced to 9 physical performance measurement variables representing 7 tasks through evaluation of their reliability and ability to discriminate entry Falls-status. Measurement sets that would be appropriate to frail or easily-fatigued, as well as high-functioning individuals, were constructed with combinations of these 9 variables (5 clinical and 4 force plate). Composite scores created from the measurement sets identified Falls-status with superior sensitivity compared with POMA and SOT and concurred with these measures. Finally, these sets predicted individuals who would suffer an Injury fall and those who would not experience Multiple falls better than Falls-status, POMA or SOT criteria.
Maurer and Commenges24 emphasize the importance of sensitivity when measures are intended to assess changes and demonstrate its’ basis in validity and reliability. We deliberately set a moderate reliability16 standard because a measure lacking high reliability may discriminate if the differences between groups are sufficiently large, as was observed with Sit-to-stand variables. Whereas Full and Intermediate clinical sets had excellent concurrence with POMA and SOT, those excluding complex tasks (Brief clinical, Intermediate and Brief force plate) concur less well with POMA, and insufficiently with SOT. POMA and SOT demonstrated high specificity to Falls-status, supporting their value for screening those who may not require intervention. Both of these widely-used measures provide disappointing sensitivity to entry Falls-status and subsequent Injury falls. They were insufficient for situations requiring both sensitivity and specificity.
Raiche and colleagues25 found POMA to be sensitive (70/52 specificity) to 1 or more falls among 225 CDE. With a high cutoff score, 125 tested positive, but the sensitivity dropped rapidly with other cutoffs. They recommended including more challenging items or those addressing medical factors associated with falls. In a residential care facility26, POMA predicted those requiring further PT assessment (68/78) but not as well as simple gait velocity (80/89) in this population. We hoped to identify measures that could be used together to permit accurate evaluation in diverse situations. Quantitative measurement sets offer several advantages; with fewer components than POMA (3–5 compared to 16), clinical assessments take less time and qualitative judgment is eliminated. Turns, necessitating control of 3-dimensional movement, may provide an important addition to gait for frailer individuals. Inclusion of downstairs and step-in-tub tasks may eliminate the ceiling effect. In CDE, downstairs captured a wider spectrum of ADL limitations than climbing up27.
The SOT offers the potential to differentiate sensory deficits, but requires special equipment unavailable in many settings. DiFabio8 found that static posturography was more sensitive and equally specific when screening for vestibular deficits. We examined clinical and force plate variables separately and found that force plate measurements were especially good predictors. They provided tasks appropriate for older adults with existing impairments including standing balance, maximal leaning to stress the postural control system and sit-to-stand measures incorporating lower body strength.
Predictive ability is the hallmark of assessments that can identify individuals requiring intervention and sensitivity to change is critical to outcome evaluation. While appropriate for screening, Medicare Falls-status seems inadequate for these purposes, as very similar proportions of Fallers and Non-Fallers experienced an Injury fall or became Multiple fallers during follow-up. However, this may reflect self-selective enrollment by respondents who volunteered for mobility and falls studies because of underlying concerns.
Measurement sets offered superior sensitivity to participants who later sustained an Injury fall, as well as specificity to those who did not subsequently have Multiple falls. The personal cost of injury falls is significant, frequently resulting in ADLs assistance for longer than 6 months28. Whereas multiple falls increase fall-risk, one injury fall substantially increases risk14 and generates additional 1-year medical costs of $27,745–30,03829. Only quantitative measurement sets provided sensitivity to Injury falls. Sets requiring as few as 2 variables (when a force plate is available), may provide an opportunity to focus scarce resources by identifying and treating those at risk for injuries.
Each of the tasks selected using statistical criteria are individually important components of mobility. Gait has been called a “physical vital sign”30 and velocity may even be measured in the home-care setting31. A consensus report found preferred pace to predict adverse outcomes in community-dwellers32. Measurement of maximal lean33, sit-to-stand34 and stair descent35 offer opportunities to identify remediable deficits. Step-in-tub36, sit-to-stand37 or turning38 to sit tasks may highlight needed home safety modifications or unrealistic self-efficacy39. The Romberg ratio demonstrates visual and somatosensory contributions to quiet standing40.
Sets of quantitative measures are proposed to suit diverse older adults and avoid ceiling and floor effects that are commonly encountered. Stepping into a simulated tub presents the most complex task, included for high-functioning individuals. The time to complete this real-life task permits assessment of weight transfer and single-leg stance abilities without ceiling effects observed with 1-legged standing in SPPB6. Changes in SPPB cannot be detected clinically41, possibly because frail participants cannot complete repeated sit-to-stands, resulting in a floor effect. Brief sets omit this task and Intermediate sets, for the easily-fatigued, use 3 single performances with as-needed rest. Sit-to-stand, gait and turning are components of TUG7, which utilizes one combined score rather than single measurements. Our reliability and discrimination values were obtained for the individual measures and sets are composed of tasks considered appropriate for different settings and participant abilities.
Limitations
To establish the statistical underpinnings of the measurement variables we excluded frail older adults and those with existing disorders known to impair mobility. Our study was not intended to establish cut-off values for identification of mobility impairment and these healthy community-dwellers offer limited generalizability. Future studies must include a broader range of participants and patient cohorts.
Conclusions
The proposed battery offers diverse real-life mobility challenges that may accommodate different circumstances and varied levels of participant function. Mobility measurement variable sets distinguished Falls-status and concur with POMA and SOT. These quantitative measures offer superior sensitivity in predicting Injury falls and provide both sensitive and specific prediction of Multiple falls.
Supplementary Material
ACKNOWLEDGMENT
We would like to acknowledge the key contribution of Harrison Waite, Ph.D. to software technical development, without his important commitment, this work would not have been possible. We would also like to thank Jennifer Brindisi, M.A. who contributed significantly to testing, data analysis and recruitment. Finally we thank Veronica Smith, M.A., P.T. for her valuable suggestions to improve the manuscript.
FUNDING
This work was supported by the National Institute on Aging, National Institutes of Health (grant number AG022092 to LW and grant number AG02595 to VP); the New York Academy of Medicine, Mary & David Hoar Foundation (to VP); and the University of Connecticut Health Center, HCRAC (to VP).
Abbreviations
- ADLs
Activities of Daily Living
- BMI
Body Mass Index
- CDE
Community-Dwelling Elders
- ICC
Intra-class correlation
- LMM
Linear mixed model
- POMA
Performance Oriented Mobility Assessment
- ROC
Receiver operating characteristics
- SOT
Sensory Organization Test
- SPPB
Short Physical Performance Battery
- TUG
Timed Up and Go
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE
No party having a direct interest in the results of the research supporting this article has or will confer a benefit on me or on any organization with which I am associated AND, if applicable, I certify that all financial and material support for this research (eg, NIH or NHS grants) and work are clearly identified in the title page of the manuscript.
SUPPLIERS
NeuroCom International Inc., 9570 SE Lawnfield Road, Clackamas, OR 97015, (800) 767-6744
Advanced Mechanical Technology Inc (AMTI), 176 Waltham Street, Watertown, MA 02472, (617) 926-6700
Contributor Information
Victoria P. Panzer, Department of Neurology, University of Connecticut Health Center (UCHC), Farmington, CT.
Dorothy B. Wakefield, Department of Neurology, University of Connecticut Health Center (UCHC), Farmington, CT.
Charles B. Hall, Departments of Epidemiology & Population Health and Neurology, Albert Einstein College of Medicine of Yeshiva University, Bronx, NY.
Leslie I. Wolfson, Department of Neurology, University of Connecticut Health Center (UCHC), Farmington, CT.
REFERENCES
- 1.Wolfson L. Gait and balance dysfunction: A model of the interaction of age and disease. Neuroscientist. 2001;7:180–186. doi: 10.1177/107385840100700212. [DOI] [PubMed] [Google Scholar]
- 2.Panzer VP, Bandinelli S, Hallett M. Biomechanical assessment of quiet standing and changes associated with aging. Arch Phys Med Rehab. 1995;76:151–157. doi: 10.1016/s0003-9993(95)80024-7. [DOI] [PubMed] [Google Scholar]
- 3.Shumway-Cook A, Ciol MA, Yorkston KM, Hoffman JM, Chan L. Mobility limitations in the Medicare population: Prevalence and sociodemographic and clinical correlates. J Am Geriatr Soc. 2005;53:1217–1221. doi: 10.1111/j.1532-5415.2005.53372.x. [DOI] [PubMed] [Google Scholar]
- 4.Visser M, Simonsick EM, Colbert LH, Brach J, Rubin SM, Kritchevsky SB, Newman AB, Harris TB for the Health ABC Study. Type and intensity of activity and risk of mobility limitation. J Am Geriatr Soc. 2005;53:762–770. doi: 10.1111/j.1532-5415.2005.53257.x. [DOI] [PubMed] [Google Scholar]
- 5.Tinetti ME. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, Scherr PA, Wallace RB. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 7.Podsiadlo D, Richardson S. The timed "Up & Go": a test of basic functional for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 8.Di Fabio RP. Sensitivity and specificity of platform posturography for identifying patients with vestibular dysfunction. Phys Ther. 1995;75:290–305. doi: 10.1093/ptj/75.4.290. [DOI] [PubMed] [Google Scholar]
- 9.Lach HW, Reed AT, Arfken CL, Miller JP, Paige GD, Birge SJ, Peck WA. Falls in the elderly: Reliability of a classification system. J Am Geriatr Soc. 1991;3:197–202. doi: 10.1111/j.1532-5415.1991.tb01626.x. [DOI] [PubMed] [Google Scholar]
- 10.Hulley SB, Cummings SR. Planning the measurements: Precision and accuracy. In: Hulley SB, Cummings SR, editors. Designing Clinical Research: An Epidemiologic Approach. Baltimore, MD: Williams & Wilkins; 1988. pp. 31–40. [Google Scholar]
- 11.Ferrucci L, Guralnik JM, Studenski S, Fried LP, Cutler GB, Jr Interventions on Frailty Working Group. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons. J Am Geriatr Soc. 2004;52:625–634. doi: 10.1111/j.1532-5415.2004.52174.x. [DOI] [PubMed] [Google Scholar]
- 12.Alexander NB. [Accessed July 20, 2010];The Merck Manual Professional, Falls in the Elderly. [On-line] http://www.merck.com/mmpe/sec23/ch346/ch346a.html.
- 13.Folstein MF, Folstein SE, McHugh PR. "Mini-Mental State" A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services, U.S. Department of Health and Human Services. 2009 PQRI Measures List (measure #154) [Accessed April 27, 2010]; [On-line] http://www.cms.hhs.gov/PQRI/Downloads/2009_PQRI_MeasuresList_030409.pdf.
- 15.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 16.Zander RH. Mimimal values for reliability of bootstrapped and jackknife proportional decay index and Bayesian posterior probability. Phyloinformatics. 2004;2:1–13. [Google Scholar]
- 17.Newton R. Review of tests of standing balance abilities. Brain Inj. 1989;3:335–343. doi: 10.3109/02699058909004558. [DOI] [PubMed] [Google Scholar]
- 18.King MB, Judge JO, Wolfson L. Functional Base of Support decreases with age. J Gerontol. 1994;49:M258–M263. doi: 10.1093/geronj/49.6.m258. [DOI] [PubMed] [Google Scholar]
- 19.Schultz A, Alexander N, Ashton-Miller J. Biomechanical analysis of rising from a chair. J Biomech. 1992;25:1383–1391. doi: 10.1016/0021-9290(92)90052-3. [DOI] [PubMed] [Google Scholar]
- 20.Camicioli R, Panzer VP, Kaye J. Balance in the healthy elderly: posturography and clinical assessment. Arch Neurol. 1997;548:976–981. doi: 10.1001/archneur.1997.00550200040008. [DOI] [PubMed] [Google Scholar]
- 21.Fineberg HV. Evaluation of diagnostic tests and the role of diagnosis in therapeutic trials. In: Capildeo R, Orgogozo JM, editors. Methods in Clinical Trials in Neurology. London, UK: Macmillan Press; 1988. pp. 57–82. [Google Scholar]
- 22.Abrams WB, Beers MH, Berkow R, editors. Comprehensive Geriatric Assessment. The Merck Manual of Geriatrics. 2nd ed. Whitehouse Station, NJ: Merck Research Laboratories; 1995. pp. 231–234. [Google Scholar]
- 23.NeuroCom International, Inc. EquiTest User’s Manual. 1990. [Google Scholar]
- 24.Maurer W, Commenges D. Choice and analysis of judgement criteria. In: Capildeo R, Orgogozo JM, editors. Methods in Clinical Trials in Neurology. London, UK: Macmillan Press; 1988. pp. 29–55. [Google Scholar]
- 25.Raiche M, Hebert R, Prince F, Corriveau H. Screening older adults at-risk for falling with the Tinetti balance scale. Lancet. 2000;356:1000–1002. doi: 10.1016/S0140-6736(00)02695-7. [DOI] [PubMed] [Google Scholar]
- 26.Harada N, Chiu V, Damron-Rodriguez J, Fowler E, Siu A, Reuben DB. Screening for balance and mobility impairments in elderly individuals living in residential care facilities. Phys Ther. 1995;75:462–469. doi: 10.1093/ptj/75.6.462. [DOI] [PubMed] [Google Scholar]
- 27.Verghese J, Wang C, Xue X, Holtzer R. Self-reported difficulty in climbing up or down stairs in nondisabled elderly. Arch Phys Med Rehab. 2008;89:100–104. doi: 10.1016/j.apmr.2007.08.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Centers for Disease Control, Morbidity and Mortality Weekly. Self-Reported Falls and Fall-Related Injuries Among Persons Aged ≥65 Years –United States, 2006. [Accessed April 27, 2010]; [On-line] http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5709a1.htm.
- 29.Bohl AA, Fishman PA, Ciol MA, Williams B, LoGerfo J, Phelan EA. A longitudinal analysis of total 3-year health care costs for older adults who experience a fall requiring medical care. J Amer Geriatr Soc. 2010;58:853–860. doi: 10.1111/j.1532-5415.2010.02816.x. [DOI] [PubMed] [Google Scholar]
- 30.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, Fox M, Guralnik JM. Physical performance measures in the clinical setting. J Am Geriatr Soc. 2003;51:314–322. doi: 10.1046/j.1532-5415.2003.51104.x. [DOI] [PubMed] [Google Scholar]
- 31.Bohannon RW. Measurement of gait speed of older adults is feasible and informative in a home-care setting. J Ger Phys Ther. 2009;32:22–23. doi: 10.1519/00139143-200932010-00005. [DOI] [PubMed] [Google Scholar]
- 32.Abellan vanKan G, Rolland Y, Andrieu S, Bauer J, Beauchet O, Bonnefoy M, Cesari M, Donini LM, Gillette Guyonnet S, Inzitari M, Nourhashemi F, Onder G, Ritz P, Salva A, Visser M, Vellas B. Gait Speed at Usual Pace as a Predictor of Adverse Outcomes in Community-Dwelling Older People. J Nutr Health Aging. 2009;13:881–889. doi: 10.1007/s12603-009-0246-z. [DOI] [PubMed] [Google Scholar]
- 33.Faculjac PF, Panzer VP. Dynamic Limits of Stability in normal subjects and patients with brain injury. In: Woollacott M, Horack F, editors. Posture and Gait: Control Mechanisms. Eugene, OR: Univ Oregon Books; 1999. pp. 59–62. [Google Scholar]
- 34.Lord SR, March LM, Cameron ID, Cumming RG, Schwarz J, Zochling J, Chen JS, Makaroff J, Siton YY, Lau TC, Brnabic A, Sambrook PN. Differing risk factors for falls in nursing home and intermediate-care residents who can and cannot stand unaided. J Am Geriatr Soc. 2003;51:1645–1650. doi: 10.1046/j.1532-5415.2003.51518.x. [DOI] [PubMed] [Google Scholar]
- 35.Tiedemann AC, Sherrington C, Lord SR. Physical and psychological factors associated with stair negotiation in older people. J Gerontol A: Biol Sci. 2007;62:1259–1265. doi: 10.1093/gerona/62.11.1259. [DOI] [PubMed] [Google Scholar]
- 36.Mouchnino L, Aurenty R, Massion J, Pedotti A. Coordination between equilibrium and head-trunk orientation during leg movement. J Neurophysiol. 1992;67:1587–1598. doi: 10.1152/jn.1992.67.6.1587. [DOI] [PubMed] [Google Scholar]
- 37.Riley PO, Krebs DE, Popat RA. Biomechanical analysis of failed sit-to-stand. IEEE Trans Rehab Eng. 1997;5:353–359. doi: 10.1109/86.650289. [DOI] [PubMed] [Google Scholar]
- 38.Patla AE, Adkin A, Ballard T. Online steering: Coordination and control of body center of mass, head and body reorientation. and control of body center of mass, head and body reorientation. Exp Brain Res. 1999;129:629–634. doi: 10.1007/s002210050932. [DOI] [PubMed] [Google Scholar]
- 39.Fortinsky RH, Panzer V, Wakefield D, Into F. Fall Risk and Balance Confidence in Later Life: Has Over-confidence been Overlooked? Health Risk Soc. 2009;11:341–352. [Google Scholar]
- 40.Panzer VP, Zeffiro TA, Hallett M. Kinematics of Standing Posture Associated with Aging and Parkinson's Disease. In: Brandt T, Paulus W, Bles W, editors. Disorders of Posture and Gait. Stuggart, Germany: Thieme Verlag; 1990. pp. 390–393. [Google Scholar]
- 41.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.