Abstract
Pneumonia is the leading cause of mortality in children in developing countries and is also the leading infectious cause of death in adults. The most important cause of pneumonia is the Gram-positive bacterial pathogen, Streptococcus pneumoniae, also known as the pneumococcus. It has thus become the leading vaccine-preventable cause of death and is a successful and diverse human pathogen. The development of conjugate pneumococcal vaccines has made possible the prevention of pneumococcal disease in infants, but has also elucidated aspects of pneumococcal biology in a number of ways. Use of the vaccine as a probe has increased our understanding of the burden of pneumococcal disease in children globally. Vaccination has also elucidated the clinical spectrum of vaccine-preventable pneumococcal infections; the identification of a biological niche for multiple pneumococcal serotypes in carriage and the differential invasiveness of pneumococcal serotypes; the impact of pneumococcal transmission among children on disease burden in adults; the role of carriage as a precursor to pneumonia; the plasticity of a naturally transformable pathogen to respond to selective pressure through capsular switching and the accumulation of antibiotic-resistance determinants; and the role of pneumococcal infections in hospitalization and mortality associated with respiratory viral infections, including both seasonal and pandemic influenza. Finally, there has been a recent demonstration that pneumococcal pneumonia in children may be an important cause of hospitalization for those with underlying tuberculosis.
Keywords: vaccine, pneumonia, pneumococcus
1. Introduction
Pneumonia is the leading cause of mortality in children [1]. As the bacterial pathogen Streptococcus pneumoniae has been known for over a century to be a major cause of that mortality, the development of a new vaccine to combat the disease has allowed new insight into the biology of this important human pathogen. The pneumococcus, which has at least 93 different polysaccharide capsules, is a leading bacterial cause of pneumonia, meningitis, otitis media and sinusitis. The usefulness of capsules to vaccinate humans against these pneumococcal diseases has been known for over 50 years [3]. It was an observation by Oswald Avery in 1931 that lead the way, however, to a new generation of pneumococcal conjugate vaccines (PCVs) that were introduced for immunization of infants in the year 2000. He showed that the type III pneumococcal capsule retained its specific immunogenicity when conjugated to a foreign protein [4]. Polysaccharide antigens are poorly immunogenic in children less than 2 years of age, but conjugation of the polysaccharide to a protein overcomes this problem and makes the combined polysaccharide–protein complex immunogenic in young children less than 2 years of age, The development and introduction of PCVs have led researchers to a number of recent observations that have enhanced our understanding of pneumococcal disease. The first licensed vaccine comprised seven capsular types of the pneumococcus (serotypes) each conjugated to a mutant diphtheria toxoid called CRM197. This vaccine is known as PCV7 and has now been replaced by higher valency vaccines containing 10 or 13 serotypes conjugated to a variety of proteins (PCV10 and PCV13, respectively).
2. The clinical spectrum and burden of pneumococcal disease
Attempts to elucidate the global burden of pneumococcal disease have been frustrated by a lack of sensitivity of current diagnostic methods. As pneumococcal pneumonia is the main contributor to the global burden of pneumococcal disease mortality, its diagnosis is key to measure that burden. Diagnosis may be made in adults by microscopy or culture of good-quality sputum specimens, but children with pneumonia rarely produce purulent sputum. Blood culture is a highly specific diagnostic method but is positive only in a small fraction of presumed pneumococcal pneumonia cases in children. Attempts to culture the lungs themselves are invasive and rarely performed. There is also a paucity of diagnostic laboratories in developing countries and a lack of utilization of the meagre resources available in those countries [5,6]. The millennium development goal 4, which aims to reduce the infant mortality rate (IMR) by two-thirds to under 40 deaths per thousand live births globally by 2015, is dependent on interventions to reduce pneumonia deaths, as the fraction of pneumonia deaths increases with increasing IMR [1,7], but the contribution of the pneumococcus to that burden cannot be determined because of the insensitivity of these current diagnostic tests.
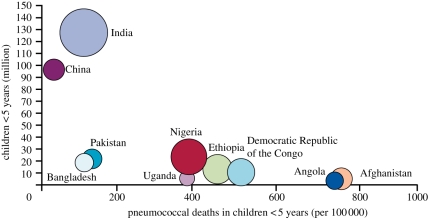
The contribution of the pneumococcus to the pneumonia fraction of the IMR has recently been elucidated by observations from three pivotal clinical trials in Africa and Asia, which showed that PCV can reduce chest radiograph-confirmed pneumonia [8–10]. Based on these clinical trials and the distribution of pneumococcal serotypes globally, it has been possible to better define the global burden of pneumococcal mortality. This study emphasizes the burden of disease in Africa, but also the significant number of pneumococcal deaths in highly populated countries such as India and China [2] (figure 1).
Figure 1.
Pneumococcal deaths in children less than 5 years of age [2].
These trials also demonstrated that the burden of hospitalized lower respiratory tract infection extends beyond alveolar consolidation on X-ray [11] to include clinical pneumonias that do not have wheezing, but have a raised C-reactive protein [12]. Extension of these data from efficacy trials to the demonstration of PCV effectiveness in the prevention of pneumonia in countries where vaccination has been part of routine childhood immunization has been a challenge. Although pneumonia is considered to be the leading cause of childhood deaths globally [1], there are few, if any, systematic population-based pneumonia surveillance programmes even in developed countries, let alone the poorest developing countries in which most of the pneumonia deaths occur. In the USA, surveillance based on rates of hospitalizations, which are coded in the International Statistical Classification of Diseases and Related Health Problems, v. 9 (ICD9), has been used to show a significant reduction in ICD9 code 481, which includes pneumococcal pneumonia and lobar pneumonia in children less than 2 years of age, since the introduction of PCV7 [13]. The direct effect on pneumonia has been confirmed by review of records in children less than 1 year of age in one group health setting in Washington State [14]. Long-term trends from Quebec have also confirmed a significant reduction in lobar pneumonia in infants following the introduction of PCV7 in that Canadian province [15]. A similar impact has been demonstrated in Australia in children [16]. A recent long-term analysis of more than a million hospitalizations in 24 per cent of the US population has demonstrated a 47 per cent reduction (95% CI 38–54%) by 2006 in the rate of pneumococcal or lobar pneumonia coded as ICD9 481, among children less than 2 years of age, compared with a pre-PCV7 baseline of 1997–1999 [17].
Other insights from the conjugate pneumococcal vaccine trials include the significant contribution of pneumococcal disease to the hospitalization of HIV-infected children and the potential of vaccination to reduce that burden [9]. Over 90 per cent of the pneumococcal disease burden of pneumonia and meningitis associated with HIV is in Africa [2], which makes the case for the introduction of vaccination into this at risk group particularly compelling. There are also data from a recent randomized trial in adults showing that conjugate vaccination may be protective against vaccine-type invasive pneumococcal disease (IPD) among HIV-infected adults in Malawi [18].
A further insight from vaccination into the clinical spectrum of pneumococcal disease is the identification of the role played by the pneumococcus in the aetiology of culture-negative empyema in the antibiotic era. It has been observed that the increase in invasive disease owing to pneumococcal serotypes not included in PCV7 has been temporally associated with an increase in culture-negative empyema [19]. Where there has been extensive use of the polymerase chain reaction to detect pneumococcal DNA in empyema specimens, the role of these non-vaccine types as the leading cause of culture-negative empyema has been clearly established [20,21].
The randomized trial of a 9-valent PCV in The Gambia [8] showed that rural children with less access to care have a greater burden of pneumococcal-attributed pneumonia than children from urban [9] or peri-urban settings [10]. The Gambian study also demonstrated a 16 per cent reduction in all-cause mortality among recipients of the conjugate vaccine, which reduced IPD by 45 per cent [8], suggesting that the true contribution of the pneumococcus to mortality may be in excess of 30 per cent in that community.
3. Children play a significant role in pneumococcal disease transmission to susceptible adults
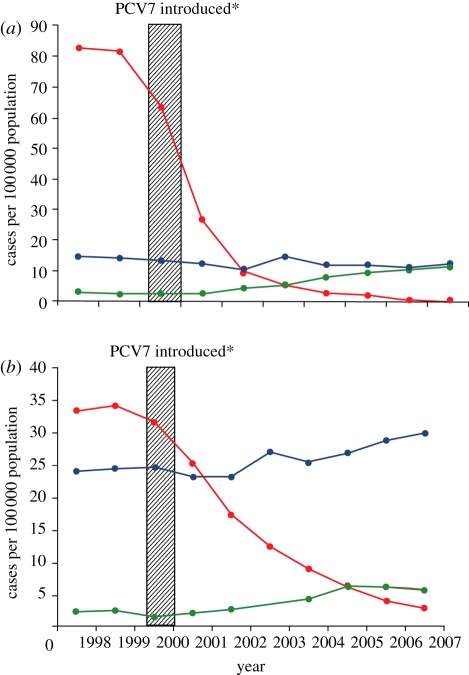
The presence of a toddler in day care is recognized as a risk for IPD in household adults [22]. Similarly, contact with a child has been recognized as a risk for IPD in HIV-infected adults [23]. The fraction of IPD in adults owing to serotypes recognized as ‘paediatric’ types because their natural habitat is the nasopharynx of healthy children, such as those belonging to serogroups 6, 9, 19 and 23, as well as serotype 14, increases as a cause of IPD with increasing age such that these strains caused more than 50 per cent of IPD in over 85 year olds in the USA prior to introduction of PCV7 [24]. There was also a spike in IPD among elderly persons in the first week of the year following the holiday season and this spike has been eliminated by the introduction of PCV7 in the USA, suggesting that it was transmission of these types from young children to their grandparents that led to the winter spike in adult disease [25]. Up to 2007, there had been a 92 per cent reduction in IPD among adults over 65 years of age owing to the seven serotypes in PCV given to infants [26] (figure 2). This phenomenon, attributed to herd protection, has also translated into reductions in the incidence of pneumococcal meningitis across all age groups [27].
Figure 2.
Changes in invasive pneumococcal disease (IPD) incidence by serotype group among (a) children aged less than 5 years and (b) adults aged more than 65 years, 1998–2007. Asterisk denotes 7-valent pneumococcal conjugate vaccine (PCV7) which was introduced in the USA for routine use among young children and infants in the second half of 2000 [26]. Red circles, PCV7 type; violet circles, non-PCV7 type; green circles, 19A.
Although it is biologically logical to assume that herd protection afforded to adults by immunization of infants will extend to protection from pneumonia, the confirmation that this is so has taken more time. The original study of vaccine effectiveness in reducing pneumonia hospitalization in children in the USA [13] also found a significant reduction in pneumococcal pneumonia ICD9 coding admissions among young adults of child-bearing age, suggesting protection through herd effects in those expected to spend most time with young children. Although the trends were towards reductions in lobar pneumonia in other age groups, these reductions were not statistically significant. The study which demonstrated the effectiveness of PCV7 in young children in Washington State found no significant reductions in hospital admissions with pneumonia in other age groups [14], demonstrating perhaps the lack of sensitivity of broad-based hospitalization data over a short time period, during which fluctuations in respiratory admissions for other reasons (for example, a severe influenza season) may make these non-specific longitudinal data difficult to interpret. Two more recent studies, however, based on large-scale documentation of hospitalization data in Australia [16] and the USA [17], have confirmed that the reductions in IPD seen in adults after infant immunization have extended to reductions in adult pneumococcal pneumonia. Indeed, in the USA over 90 per cent of the reduction in hospitalizations attributable to pneumococcal conjugate vaccination of infants, occurs through prevention of pneumonia in adults [17]. Conjugate vaccines are currently in clinical trial in adults—their effectiveness is of great interest but may be limited in countries where the same vaccines are given to children [28].
In developing countries, there are studies underway to establish the extent of herd protection resulting from the immunization of infants—it is likely that the patterns of pneumococcal transmission differ between countries and also likely that a greater fraction of the pneumonia prevented will be in children compared with the US data. The basis for this herd protection is the impact of PCV on carriage, and the vaccine impact on carriage has provided a number of further insights into pneumococcal biology.
4. The occurrence of a biological niche in the human nasopharynx for carriage of multiple pneumococcal serotypes
The impact of an early 5-valent PCV on the dynamics of nasopharyngeal carriage of pneumococci not included in the vaccine was first documented in The Gambia with the observation of a replacement phenomenon [29], later confirmed with the 9-valent PCV in Soweto, South Africa [30]. These studies showed little impact of the vaccine on the overall nasopharyngeal carriage, but a reduction in the carriage of vaccine types which was met with almost complete replacement of those vaccine types by non-vaccine types. The timing of the effect (no impact on carriage a month after immunization), but a 50 per cent reduction in the carriage of vaccine types and increase in non-vaccine types six months later [30], suggests that PCV-induced antibody does not eradicate the existing carriage but that these antibodies are able to reduce the acquisition of new carriage because of vaccine types. The drop in acquisition of vaccine types seems to create a biological niche allowing the unmasking of subdominant non-vaccine types not previously detected by nasopharyngeal culture, or an increased acquisition of non-vaccine types owing to lack of opposition from vaccine types. The replacement phenomenon observed in carriage post-PCV suggests that there are major differences in the invasiveness of pneumococcal types, as the complete replacement of vaccine types with non-vaccine types in carriage has not, in most instances, resulted in a return to pre-PCV levels of IPD (replacement disease), as would be expected if non-vaccine types were able to cause disease as effectively as vaccine types. In general, the replacing non-vaccine types appear to be less able to cause IPD than the vaccine types. Indeed, the extent of replacement disease has revealed further insights into the susceptibility of populations to pneumococcal serotypes. Replacement disease has been marked among HIV-infected adults in the USA exposed to types not included in the 7-valent PCV [31], and among indigenous communities in Alaska, where IPD has actually increased among adults post-PCV introduction [32]. The impact of serotype replacement may thus lead to an overall beneficial impact in both children and adults, but may negate these effects in some populations or even lead to deleterious effects [33]. The population at risk for non-vaccine type disease is different from the population of healthy children previously at risk of IPD, so that there may be particular risks for disease among the increasing numbers of adults and children living with chronic underlying diseases.
5. Nasopharyngeal carriage as a precursor to infection
In a longitudinal study of pneumococcal acquisition from birth, Gray et al. [34] showed that the risk of pneumococcal disease was greatest within a month of the acquisition of carriage of a new serotype. There is thus a link between acquisition of carriage and potential for disease. The 23-valent pneumococcal polysaccharide vaccine which has been given to adults for the past 30 years (it is not given to children less than 2 years of age as it is poorly immunogenic in that age group) does not appear to prevent carriage in most populations and also does not seem to prevent non-bacteraemic pneumonia in those same populations. In the two populations of young soldiers and gold miners in whom this vaccine protected against pneumonia [3,35], the vaccine also impacted on nasopharyngeal carriage ([3] and H. J. Koornhof 2011, personal communication). Conjugate pneumococcal vaccines reduce both carriage and pneumonia. It is therefore possible that the impact of conjugate vaccines on pneumonia is at least in part a function of their ability to prevent acquisition of carriage and/or to prevent the increase in density of carriage that may lead to micro-aspiration and pneumonia.
There are semi-quantitative data on the density of carriage that suggest a reduction in the density of carriage post-vaccination with PCV [36], but more studies using molecular methods are in progress. The usefulness of measurement of the density of pneumococcal carriage in the nasopharynx as a diagnostic tool for pneumonia is also of current interest. Bacterial densities are increased among pneumonic adults [37] and children [38] when compared with controls, but the serotype-specific densities remain unclear and the diagnostic usefulness in an individual patient remains to be clearly established. The relationship between density of pneumococcal colonization and microaspiration leading to pneumonia is a fruitful area for further clinical investigation.
6. Role of antimicrobial resistance in the persistence and serotype distribution of pneumococci
There has been a change in the serotype distribution of pneumococci causing invasive disease in adults over the past century, particularly in developed countries, with a gradual increase in the proportion of invasive disease strains among adults that have serotypes that are carried by children [39]. The reason for this change is not certain but it first became obvious after the introduction of antibiotics in the 1940s, suggesting that the selective advantage of antimicrobial resistance may be playing a role. Another factor may be the increasing age of the at-risk population in developed countries, with elderly patients at particular risk for invasive disease owing to ‘paediatric’ pneumococcal serotypes [24].
As in the pre-PCV era, antimicrobial resistance was mainly seen among PCV serotypes, the introduction of PCV was thought to be a potential tool to decrease pneumococcal resistance [40], and initial results during a clinical trial [9], and following PCV introduction, suggested that indeed there had been both an absolute [41,42] and relative [43] reduction in disease caused by resistant strains compared with susceptible strains of pneumococci. These reductions have been undermined by the rapid evolution of resistance in non-vaccine strains causing invasive disease such that the proportion of such strains resistant to antibiotics has now reached pre-vaccine levels [44,45], even if the absolute number of cases of disease has decreased. In particular, there has been a rapid expansion of antimicrobial-resistant serotype 19A, leading to the speculation that the resistance alone may be sufficient to explain the ascent in the prevalence of this serotype. In the context of a randomized clinical trial [46] of PCV, it has recently been shown that vaccinees had an excess of carriage of serotype 19A regardless of their drug susceptibility. It is therefore likely that both antimicrobial resistance and vaccine pressure have led to the selection of resistance in non-vaccine types.
7. Role of the pneumococcus in influenza-associated pneumonia hospitalization and mortality
It has been recognized for many years that viral infections, such as the common ‘cold’ can progress to severe bacterial pneumonia. The insensitivity of diagnostic methods to detect bacterial (including pneumococcal) pneumonia has meant that the extent of this interaction and the specific pathogens involved could not be accurately measured. The 9-valent PCV was shown to reduce not only X-ray confirmed pneumonia hospitalization in Soweto, South Africa, but also hospitalization associated with a number of respiratory viruses including RSV, influenza, para-influenza [47] and human metapneumovirus [48] (table 1). The impact of a 45 per cent reduction in influenza-associated pneumonia by a vaccine that protects against only around 50 per cent of the serotypes associated with IPD in that community, suggests that more than half of the morbidity owing to influenza in children in that community may be preventable by PCV. These observations have led not only to a renewal in interest both in the biology of the interaction between the pneumococcus and respiratory viruses, but also to a re-examination of the role of the pneumococcus in mortality during the 1918 influenza pandemic.
Table 1.
Reduction in hospitalization for pneumonia associated with co-infections among children immunized with three doses of 9-valent conjugate pneumococcal vaccine in infancy.
| associated infection | no. of vaccinees (n = 18 245) | no. of controls (n = 18 268) | vaccine efficacy (95% CI) | p | reference |
|---|---|---|---|---|---|
| influenza A | 31 | 56 | 45 (14,64) | 0.01 | [47] |
| parainfluenza types 1–3 | 24 | 43 | 44 (8,66) | 0.02 | [47] |
| RSV | 90 | 115 | 22 (−3,41) | 0.08 | [47] |
| human metapneumovirus | 26 | 62 | 58 (34,73) | 0.0001 | [48] |
| pulmonary tuberculosis | 30 | 53 | 43 (12,64) | 0.01 | [49] |
In an elegant experiment performed in monkeys 35 years ago, it was clearly shown that pre-exposure of monkeys to influenza virus increases the load of pneumococcal exposure in the blood from 55 to 30 000 colonies ml−1 [50]. Similarly, the pneumococcal load in the lungs of influenza pre-exposed monkeys was more than 100 000 colonies ml−1 compared with 90 colonies ml−1 in monkeys exposed to the pneumococcus alone [50]. The order of exposure to both the pathogens has been shown to be important in mouse studies in which influenza virus following pneumococcal exposure does not lead to the deleterious effects seen when the pneumococcus follows influenza virus [51]. It is thus the response to influenza virus (or other respiratory viruses) that down-regulates the innate immune response to the pneumococcus. There are a number of hypotheses for the mechanism of downregulation, including induction of gamma-interferon leading to downregulation of the MARCO receptor in alveolar macrophages [52], and type 1 interferon-mediated reduction in the production of chemo-attractants for neutrophils [53]. Influenza infection also increases susceptibility to pneumococcal acquisition in ferrets [54] and the role of respiratory viruses may be critical to the transmission of the pneumococcus in humans, with observations over the past 50 years suggesting enhanced isolation of pneumococci from individuals with symptomatic upper respiratory tract infections [55–57].
A re-analysis of the lung autopsy specimens from 58 patients who succumbed to pneumonia during the 1918 influenza pandemic revealed the evidence of bacterial infection in all the specimens [58], and likewise, an analysis of the blood, lung and pleural fluid cultures obtained during life from influenza patients who developed pneumonia, reveals a predominance of bacterial infections, particularly by pneumococci, followed by haemolytic streptococci [59]. Finally, although the effectiveness of whole cell bacterial vaccines directed against the pneumococcus has not been established, there is evidence from the 1918 pandemic that these types of vaccines may have contributed to a reduction in influenza-associated pneumonia and mortality [60].
PCV has thus made a major contribution to our understanding of the interaction of the pneumococcus with viral infections, and the role of the pneumococcus in viral-associated severe pneumonia.
8. Role of the pneumococcus in the hospitalization of children with pulmonary tuberculosis
It is well-recognized that African children who die of tuberculosis (TB) may have concomitant bacterial pneumonia [61]. TB may predispose to the development of another bacterial infection, or the pneumococcal infection may reactivate latent pulmonary TB. In a series of 138 children with culture-proven pulmonary TB, nearly half of them had less than 10 days duration of symptoms [62]. We therefore made use of the long-term follow-up of children enrolled in the South African 9-valent PCV trial to investigate the potential role of the vaccine in the prevention of pneumonia among children with culture-confirmed TB. Children from the trial were identified during 5.3 years of follow-up if they were hospitalized with lower respiratory tract infection; among 2439 hospitalizations there were 90 episodes of pulmonary TB identified in 83 children [49]. Among these 83 first episodes, 55 were in HIV-infected children and 28 in HIV-uninfected children. Fewer children in each group had received PCV9 than placebo—19 versus 36 in HIV-infected children (vaccine efficacy 47%, p = 0.02) and 11 versus 17 in HIV-uninfected children (vaccine efficacy 37%, p = 0.26). Overall, there was a reduction of 43% (p = 0.01) [49] (table 1). These data suggest that acute pneumococcal pneumonia may be a major reason for young children exposed to TB to present to hospital with acute pneumonia. The molecular basis of this interaction is unknown but it is possible that the proposed mechanism of susceptibility following respiratory viral infection, associated with interferon gamma response and its downregulation of alveolar macrophage activity, may also play a role in pneumococcal susceptibility following a new infection with TB.
9. Unresolved issues
A number of unresolved issues are amenable to investigation by analysis of the roll-out in developing countries of new PCVs—PCV10 and 13—which include three and six additional serotypes to PCV7, respectively (both have additional serotypes 1, 5 and 7F, with PCV13 also including serotypes 3, 6A and 19A). These will include the extent to which these vaccines can induce herd protection, and not lead to erosion of protection owing to non-vaccine serotypes. In developing countries much pneumococcal disease is seen in patients with underlying nutritional deficiency or immunological disease such as HIV which may make them susceptible to disease owing to less-invasive non-vaccine serotypes. The patterns of transmission of the pneumococcus in developing countries are less well understood than in the industrialized world so that vaccination of a limited cohort of children may be less able to induce herd immunity. It will be of particular interest to evaluate the impact of PCV10 and 13 in infants on the epidemiology of the type 1 pneumococcus, which is the predominant pneumococcal type in developing countries [63], but it appears to be rarely carried by children. Furthermore, immunity to this type could not be demonstrated in the trials of the 9-valent conjugate vaccines in Africa.
The biological insights that have been gained from conjugate vaccine trials raise further questions about future vaccine-use strategies. Surveillance of non-vaccine serotypes that increase after vaccination needs to be in place in developing countries to accurately measure the impact of vaccine and this may include the need for more sophisticated surveillance, such as identification of empyema. Surveillance of carriage is an essential tool in predicting the success or failure of vaccination strategies globally. The role of the pneumococcus in co-infections with respiratory viruses and TB suggests specific groups of patients who may be targeted for vaccination. It may be that the current strategy may succeed in the long term in that it allows unchanged pneumococcal carriage, but relies on lower virulence of the remaining colonizing strains. The issue of a broad protein-based approach to pneumococcal vaccination is, however, of interest. There are no data to date to suggest that protein-based vaccines may induce the same levels of protection and herd immunity seen with conjugate vaccines, so they are likely to be developed as adjuncts to the current conjugate strategy. This will make the vaccines even more complex and expensive. The addition of proteins also increases the possibility that eradication of all pneumococcal carriage may expose a niche for enhanced colonization and disease caused by other pathogens such as staphylococci or Gram-negative bacteria—these concerns remain theoretical at this time. There is renewed interest in whether simple interventions such as whole cell-killed pneumococcal vaccines may play a role in the induction of pneumococcal immunity [64,65]. A re-analysis of the attempts using killed bacterial vaccines to prevent death and pneumonia following influenza during the 1918 pandemic supports the idea that these vaccines may have had some efficacy [60].
The introduction of conjugate pneumococcal vaccines effective in the prevention of pneumococcal pneumonia has elucidated many aspects of pneumococcal biology and holds the key to reduction of this major vaccine-preventable cause of death in all age groups.
Acknowledgements
I wish to acknowledge the wonderful collaborators with whom I have worked and who have contributed in a large part to many of the studies described in this paper. I wish to acknowledge in particular, Hendrik Koornhof for his foresight in setting up pneumococcal surveillance in South Africa, Shabir Madhi for his insights into bacterial viral co-infections, Robin Huebner and Nontombi Mbelle for their work on the conjugate vaccine trial, Anne von Gottberg for her energetic expansion of respiratory pathogen surveillance and risk factor analysis, Yu Wen Chien for her careful and insightful assistance in the 1918 influenza studies.
References
- 1.Rudan I., et al. 2010. Causes of deaths in children younger than 5 years in China in 2008. Lancet 375, 1083–1089 10.1016/S0140-6736(10)60060-8 (doi:10.1016/S0140-6736(10)60060-8) [DOI] [PubMed] [Google Scholar]
- 2.O'Brien K. L., et al. 2009. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet 374, 893–902 10.1016/S0140-6736(09)61204-6 (doi:10.1016/S0140-6736(09)61204-6) [DOI] [PubMed] [Google Scholar]
- 3.Macleod C. M., Hodges R. G., Heidelberger M., Bernhard W. G. 1945. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J. Exp. Med. 82, 445–465 10.1084/jem.82.6.445 (doi:10.1084/jem.82.6.445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery O. T., Goebel W. F. 1931. Chemo-immunological studies on carbohydrate-proteins. V. The immunological specificity of an antigen prepared by combining the capsular polysaccharide of type III pneumococcus with foreign protein. J. Exp. Med. 54, 419–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berkelman R., Cassell G., Specter S., Hamburg M., Klugman K. 2006. The ‘Achilles heel’ of global efforts to combat infectious diseases. Clin. Infect. Dis. 42, 1503–1504 10.1086/504494 (doi:10.1086/504494) [DOI] [PubMed] [Google Scholar]
- 6.Klugman K. P., Madhi S. A., Albrich W. C. 2008. Novel approaches to the identification of Streptococcus pneumoniae as the cause of community-acquired pneumonia. Clin. Infect. Dis. 47(Suppl. 3), S202–S206 10.1086/591405 (doi:10.1086/591405) [DOI] [PubMed] [Google Scholar]
- 7.Williams B. G., Gouws E., Boschi-Pinto C., Bryce J., Dye C. 2002. Estimates of world-wide distribution of child deaths from acute respiratory infections. Lancet Infect. Dis. 2, 25–32 10.1016/S1473-3099(01)00170-0 (doi:10.1016/S1473-3099(01)00170-0) [DOI] [PubMed] [Google Scholar]
- 8.Cutts F. T., et al. 2005. Efficacy of nine-valent pneumococcal conjugate vaccine against pneumonia and invasive pneumococcal disease in The Gambia: randomised, double-blind, placebo-controlled trial. Lancet 365, 1139–1146 10.1016/S0140-6736(05)71876-6 (doi:10.1016/S0140-6736(05)71876-6) [DOI] [PubMed] [Google Scholar]
- 9.Klugman K. P., Madhi S. A., Huebner R. E., Kohberger R., Mbelle N., Pierce N. 2003. A trial of a 9-valent pneumococcal conjugate vaccine in children with and those without HIV infection. N. Engl. J. Med. 349, 1341–1348 10.1056/NEJMoa035060 (doi:10.1056/NEJMoa035060) [DOI] [PubMed] [Google Scholar]
- 10.Lucero M. G., et al. 2009. Efficacy of an 11-valent pneumococcal conjugate vaccine against radiologically confirmed pneumonia among children less than 2 years of age in the Philippines: a randomized, double-blind, placebo-controlled trial. Pediatr. Infect. Dis. J. 28, 455–462 10.1097/INF.0b013e31819637af (doi:10.1097/INF.0b013e31819637af) [DOI] [PubMed] [Google Scholar]
- 11.Madhi S. A., Kuwanda L., Cutland C., Klugman K. P. 2005. The impact of a 9-valent pneumococcal conjugate vaccine on the public health burden of pneumonia in HIV-infected and -uninfected children. Clin. Infect. Dis. 40, 1511–1518 10.1086/429828 (doi:10.1086/429828) [DOI] [PubMed] [Google Scholar]
- 12.Madhi S. A., Klugman K. P. 2007. World Health Organisation definition of ‘radiologically-confirmed pneumonia’ may under-estimate the true public health value of conjugate pneumococcal vaccines. Vaccine 25, 2413–2419 10.1016/j.vaccine.2006.09.010 (doi:10.1016/j.vaccine.2006.09.010) [DOI] [PubMed] [Google Scholar]
- 13.Grijalva C. G., Nuorti J. P., Arbogast P. G., Martin S. W., Edwards K. M., Griffin M. R. 2007. Decline in pneumonia admissions after routine childhood immunisation with pneumococcal conjugate vaccine in the USA: a time-series analysis. Lancet 369, 1179–1186 10.1016/S0140-6736(07)60564-9 (doi:10.1016/S0140-6736(07)60564-9) [DOI] [PubMed] [Google Scholar]
- 14.Nelson J. C., Jackson M., Yu O., Whitney C. G., Bounds L., Bittner R., Zavitkovsky A., Jackson L. A. 2008. Impact of the introduction of pneumococcal conjugate vaccine on rates of community acquired pneumonia in children and adults. Vaccine 26, 4947–4954 10.1016/j.vaccine.2008.07.016 (doi:10.1016/j.vaccine.2008.07.016) [DOI] [PubMed] [Google Scholar]
- 15.De Wals P., Robin E., Fortin E., Thibeault R., Ouakki M., Douville-Fradet M. 2008. Pneumonia after implementation of the pneumococcal conjugate vaccine program in the province of Quebec, Canada. Pediatr. Infect. Dis. J. 27, 963–968 10.1097/INF.0b013e31817cf76f (doi:10.1097/INF.0b013e31817cf76f) [DOI] [PubMed] [Google Scholar]
- 16.Jardine A., Menzies R. I., McIntyre P. B. 2010. Reduction in hospitalizations for pneumonia associated with the introduction of a pneumococcal conjugate vaccination schedule without a booster dose in Australia. Pediatr. Infect. Dis. J. 29, 607–612 10.1097/INF.0b013e3181d7d09c (doi:10.1097/INF.0b013e3181d7d09c) [DOI] [PubMed] [Google Scholar]
- 17.Simonsen L., Taylor R. J., Young-Xu Y., Haber M., May L., Klugman K. P. 2011. Impact of pneumococcal conjugate vaccination of infants on pneumonia and influenza hospitalization and mortality in all age groups in the United States. mBio 2, e00309–e00310 10.1128/mBio.00309-10 (doi:10.1128/mBio.00309-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.French N., et al. 2010. A trial of a 7-valent pneumococcal conjugate vaccine in HIV-infected adults. N. Engl. J. Med. 362, 812–822 10.1056/NEJMoa0903029 (doi:10.1056/NEJMoa0903029) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grijalva C. G., Nuorti J. P., Zhu Y., Griffin M. R. 2010. Increasing incidence of empyema complicating childhood community-acquired pneumonia in the United States. Clin. Infect. Dis. 50, 805–813 10.1086/650573 (doi:10.1086/650573) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blaschke A. J., et al. 2011. Molecular analysis improves pathogen identification and epidemiologic study of pediatric parapneumonic empyema. Pediatr. Infect. Dis. J. 30, 289–294 10.1097/INF.0b013e3182002d14 (doi:10.1097/INF.0b013e3182002d14) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Resti M., et al. 2010. Community-acquired bacteremic pneumococcal pneumonia in children: diagnosis and serotyping by real-time polymerase chain reaction using blood samples. Clin. Infect. Dis. 51, 1042–1049 10.1086/656579 (doi:10.1086/656579) [DOI] [PubMed] [Google Scholar]
- 22.Nuorti J. P., Butler J. C., Farley M. M., Harrison L. H., McGeer A., Kolczak M. S., Breiman R. F. 2000. Cigarette smoking and invasive pneumococcal disease. Active Bacterial Core Surveillance Team. N. Engl. J. Med. 342, 681–689 10.1056/NEJM200003093421002 (doi:10.1056/NEJM200003093421002) [DOI] [PubMed] [Google Scholar]
- 23.Breiman R. F., Keller D. W., Phelan M. A., Sniadack D. H., Stephens D. S., Rimland D., Farley M. M., Schuchat A., Reingold A. L. 2000. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch. Intern. Med. 160, 2633–2638 10.1001/archinte.160.17.2633 (doi:10.1001/archinte.160.17.2633) [DOI] [PubMed] [Google Scholar]
- 24.Feikin D. R., Klugman K. P., Facklam R. R., Zell E. R., Schuchat A., Whitney C. G. 2005. Increased prevalence of pediatric pneumococcal serotypes in elderly adults. Clin. Infect. Dis. 41, 481–487 10.1086/432015 (doi:10.1086/432015) [DOI] [PubMed] [Google Scholar]
- 25.Walter N. D., Taylor T. H., Jr, Dowell S. F., Mathis S., Moore M. R. 2009. Holiday spikes in pneumococcal disease among older adults. N. Engl. J. Med. 361, 2584–2585 10.1056/NEJMc0904844 (doi:10.1056/NEJMc0904844) [DOI] [PubMed] [Google Scholar]
- 26.Pilishvili T., et al. 2010. Sustained reductions in invasive pneumococcal disease in the era of conjugate vaccine. J. Infect. Dis. 201, 32–41 10.1086/648593 (doi:10.1086/648593) [DOI] [PubMed] [Google Scholar]
- 27.Hsu H. E., et al. 2009. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N. Engl. J. Med. 360, 244–256 10.1056/NEJMoa0800836 (doi:10.1056/NEJMoa0800836) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metersky M. L., Dransfield M. T., Jackson L. A. 2010. Determining the optimal pneumococcal vaccination strategy for adults: is there a role for the pneumococcal conjugate vaccine? Chest 138, 486–490 10.1378/chest.10-0738 (doi:10.1378/chest.10-0738) [DOI] [PubMed] [Google Scholar]
- 29.Obaro S. K., Adegbola R. A., Banya W. A., Greenwood B. M. 1996. Carriage of pneumococci after pneumococcal vaccination. Lancet 348, 271–272 10.1016/S0140-6736(05)65585-7 (doi:10.1016/S0140-6736(05)65585-7) [DOI] [PubMed] [Google Scholar]
- 30.Mbelle N., Huebner R. E., Wasas A. D., Kimura A., Chang I., Klugman K. P. 1999. Immunogenicity and impact on nasopharyngeal carriage of a nonavalent pneumococcal conjugate vaccine. J. Infect. Dis. 180, 1171–1176 10.1086/315009 (doi:10.1086/315009) [DOI] [PubMed] [Google Scholar]
- 31.Cohen A. L., et al. 2010. Prevention of invasive pneumococcal disease among HIV-infected adults in the era of childhood pneumococcal immunization. AIDS 24, 2253–2262 10.1097/QAD.0b013e32833d46fd (doi:10.1097/QAD.0b013e32833d46fd) [DOI] [PubMed] [Google Scholar]
- 32.Singleton R. J., Hennessy T. W., Bulkow L. R., Hammitt L. L., Zulz T., Hurlburt D. A., Butler J. C., Rudolph K., Parkinson A. 2007. Invasive pneumococcal disease caused by nonvaccine serotypes among Alaska native children with high levels of 7-valent pneumococcal conjugate vaccine coverage. J. Am. Med. Assoc. 297, 1784–1792 10.1001/jama.297.16.1784 (doi:10.1001/jama.297.16.1784) [DOI] [PubMed] [Google Scholar]
- 33.Melegaro A., Choi Y. H., George R., Edmunds W. J., Miller E., Gay N. J. 2010. Dynamic models of pneumococcal carriage and the impact of the heptavalent pneumococcal conjugate vaccine on invasive pneumococcal disease. BMC Infect. Dis. 10, 90. 10.1186/1471-2334-10-90 (doi:10.1186/1471-2334-10-90) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gray B. M., Converse G. M., III, Dillon H. C., Jr 1980. Epidemiologic studies of Streptococcus pneumoniae in infants: acquisition, carriage, and infection during the first 24 months of life. J. Infect. Dis. 142, 923–933 10.1093/infdis/142.6.923 (doi:10.1093/infdis/142.6.923) [DOI] [PubMed] [Google Scholar]
- 35.Austrian R., Douglas R. M., Schiffman G., Coetzee A. M., Koornhof H. J., Hayden-Smith S., Reid R. D. 1976. Prevention of pneumococcal pneumonia by vaccination. Trans. Assoc. Am. Phys. 89, 184–194 [PubMed] [Google Scholar]
- 36.O'Brien K. L., et al. 2007. Effect of pneumococcal conjugate vaccine on nasopharyngeal colonization among immunized and unimmunized children in a community-randomized trial. J. Infect. Dis. 196, 1211–1220 10.1086/521833 (doi:10.1086/521833) [DOI] [PubMed] [Google Scholar]
- 37.Yang S., et al. 2005. Quantitative PCR assay using sputum samples for rapid diagnosis of pneumococcal pneumonia in adult emergency department patients. J. Clin. Microbiol. 43, 3221–3226 10.1128/JCM.43.7.3221-3226.2005 (doi:10.1128/JCM.43.7.3221-3226.2005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vu H. T. T., et al. 2011. Association between nasopharyngeal load of Streptococcus pneumoniae, viral infection, and radiologically confirmed pneumonia in Vietnamese children. Pediatr. Infect. Dis. J. 30, 11–18 10.1097/INF.0b013e3181f111a2 (doi:10.1097/INF.0b013e3181f111a2) [DOI] [PubMed] [Google Scholar]
- 39.Feikin D. R., Klugman K. P. 2002. Historical changes in pneumococcal serogroup distribution: implications for the era of pneumococcal conjugate vaccines. Clin. Infect. Dis. 35, 547–555 10.1086/341896 (doi:10.1086/341896) [DOI] [PubMed] [Google Scholar]
- 40.Klugman K. P., Friedland I. R. 1995. Antibiotic-resistant pneumococci in pediatric disease. Microb. Drug Resist. 1, 5–8 10.1089/mdr.1995.1.5 (doi:10.1089/mdr.1995.1.5) [DOI] [PubMed] [Google Scholar]
- 41.Kyaw M. H., et al. 2006. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N. Engl. J. Med. 354, 1455–1463 10.1056/NEJMoa051642 (doi:10.1056/NEJMoa051642) [DOI] [PubMed] [Google Scholar]
- 42.Stephens D. S., et al. 2005. Incidence of macrolide resistance in Streptococcus pneumoniae after introduction of the pneumococcal conjugate vaccine: population-based assessment. Lancet 365, 855–863 10.1016/S0140-6736(05)71043-6 (doi:10.1016/S0140-6736(05)71043-6) [DOI] [PubMed] [Google Scholar]
- 43.Black S., Shinefield H., Baxter R., Austrian R., Elvin L., Hansen J., Lewis E., Fireman B. 2006. Impact of the use of heptavalent pneumococcal conjugate vaccine on disease epidemiology in children and adults. Vaccine 24(Suppl. 2), S279–S280 [DOI] [PubMed] [Google Scholar]
- 44.Moore M. R., et al. 2008. Population snapshot of emergent Streptococcus pneumoniae serotype 19A in the United States, 2005. J. Infect. Dis. 197, 1016–1027 10.1086/528996 (doi:10.1086/528996) [DOI] [PubMed] [Google Scholar]
- 45.Gertz R. E., Jr, Li Z., Pimenta F. C., Jackson D., Juni B. A., Lynfield R., Jorgensen J. H., Carvalho Mda G., Beall B. W. 2010. Increased penicillin nonsusceptibility of nonvaccine-serotype invasive pneumococci other than serotypes 19A and 6A in post-7-valent conjugate vaccine era. J. Infect. Dis. 201, 770–775 [DOI] [PubMed] [Google Scholar]
- 46.van Gils E. J., et al. 2010. Pneumococcal conjugate vaccination and nasopharyngeal acquisition of pneumococcal serotype 19A strains. J. Am. Med. Assoc. 304, 1099–1106 10.1001/jama.2010.1290 (doi:10.1001/jama.2010.1290) [DOI] [PubMed] [Google Scholar]
- 47.Madhi S. A., Klugman K. P. 2004. A role for Streptococcus pneumoniae in virus-associated pneumonia. Nat. Med. 10, 811–813 10.1038/nm1077 (doi:10.1038/nm1077) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Madhi S. A., Ludewick H., Kuwanda L., Niekerk N., Cutland C., Little T., Klugman K. P. 2006. Pneumococcal coinfection with human metapneumovirus. J. Infect. Dis. 193, 1236–1243 10.1086/503053 (doi:10.1086/503053) [DOI] [PubMed] [Google Scholar]
- 49.Moore D. P., Klugman K. P., Madhi S. A. 2010. Role of Streptococcus pneumoniae in hospitalisation for acute community-acquired pneumonia associated with culture-confirmed Mycobacterium tuberculosis in children: a pneumococcal conjugate vaccine probe study. Pediatr. Infect. Dis. J. 29, 1099–1104 10.1097/INF.0b013e3181eaefff (doi:10.1097/INF.0b013e3181eaefff) [DOI] [PubMed] [Google Scholar]
- 50.Berendt R. F., Long G. G., Walker J. S. 1975. Influenza alone and in sequence with pneumonia due to Streptococcus pneumoniae in the squirrel monkey. J. Infect. Dis. 132, 689–693 10.1093/infdis/132.6.689 (doi:10.1093/infdis/132.6.689) [DOI] [PubMed] [Google Scholar]
- 51.McCullers J. A., Rehg J. E. 2002. Lethal synergism between influenza virus and Streptococcus pneumoniae: characterization of a mouse model and the role of platelet-activating factor receptor. J. Infect. Dis. 186, 341–350 10.1086/341462 (doi:10.1086/341462) [DOI] [PubMed] [Google Scholar]
- 52.Sun K., Metzger D. W. 2008. Inhibition of pulmonary antibacterial defense by interferon-gamma during recovery from influenza infection. Nat. Med. 14, 558–564 10.1038/nm1765 (doi:10.1038/nm1765) [DOI] [PubMed] [Google Scholar]
- 53.Shahangian A., Chow E. K., Tian X., Kang J. R., Ghaffari A., Liu S. Y., Belperio J. A., Cheng G., Deng J. C. 2009. Type I IFNs mediate development of postinfluenza bacterial pneumonia in mice. J. Clin. Invest. 119, 1910–1920 10.1172/JCI35412 (doi:10.1172/JCI35412) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McCullers J. A., McAuley J. L., Browall S., Iverson A. R., Boyd K. L., Henriques Normark B. 2010. Influenza enhances susceptibility to natural acquisition of and disease due to Streptococcus pneumoniae in ferrets. J. Infect. Dis. 202, 1287–1295 10.1086/656333 (doi:10.1086/656333) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdullahi O., Nyiro J., Lewa P., Slack M., Scott J. A. 2008. The descriptive epidemiology of Streptococcus pneumoniae and Haemophilus influenzae nasopharyngeal carriage in children and adults in Kilifi district, Kenya. Pediatr. Infect. Dis. J. 27, 59–64 10.1097/INF.0b013e31814da70c (doi:10.1097/INF.0b013e31814da70c) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brimblecombe F. S., Cruickshank R., Masters P. L., Reid D. D., Stewart G. T. 1958. Family studies of respiratory infections. Br. Med. J. 1, 119–128 10.1136/bmj.1.5063.119 (doi:10.1136/bmj.1.5063.119) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gwaltney J. M., Jr, Sande M. A., Austrian R., Hendley J. O. 1975. Spread of Streptococcus pneumoniae in families. II. Relation of transfer of S. pneumoniae to incidence of colds and serum antibody. J. Infect. Dis. 132, 62–68 10.1093/infdis/132.1.62 (doi:10.1093/infdis/132.1.62) [DOI] [PubMed] [Google Scholar]
- 58.Morens D. M., Taubenberger J. K., Fauci A. S. 2008. Predominant role of bacterial pneumonia as a cause of death in pandemic influenza: implications for pandemic influenza preparedness. J. Infect. Dis. 198, 962–970 10.1086/591708 (doi:10.1086/591708) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chien Y. W., Klugman K. P., Morens D. M. 2009. Bacterial pathogens and death during the 1918 influenza pandemic. N. Engl. J. Med. 361, 2582–2583 10.1056/NEJMc0908216 (doi:10.1056/NEJMc0908216) [DOI] [PubMed] [Google Scholar]
- 60.Chien Y. W., Klugman K. P., Morens D. M. 2010. Efficacy of whole-cell killed bacterial vaccines in preventing pneumonia and death during the 1918 influenza pandemic. J. Infect. Dis. 202, 1639–1648 10.1086/657144 (doi:10.1086/657144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chintu C., et al. 2002. Lung diseases at necropsy in African children dying from respiratory illnesses: a descriptive necropsy study. Lancet 360, 985–990 10.1016/S0140-6736(02)11082-8 (doi:10.1016/S0140-6736(02)11082-8) [DOI] [PubMed] [Google Scholar]
- 62.Jeena P. M., Pillay P., Pillay T., Coovadia H. M. 2002. Impact of HIV-1 co-infection on presentation and hospital-related mortality in children with culture proven pulmonary tuberculosis in Durban, South Africa. Int. J. Tuberc. Lung Dis. 6, 672–678 [PubMed] [Google Scholar]
- 63.Johnson H. L., Deloria-Knoll M., Levine O. S., Stoszek S. K., Freimanis Hance L., Reithinger R., Muenz L. R., O'Brien K. L. 2010. Systematic evaluation of serotypes causing invasive pneumococcal disease among children under five: the pneumococcal global serotype project. PLoS Med. 7, e1000348. 10.1371/journal.pmed.1000348 (doi:10.1371/journal.pmed.1000348) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lu Y. J., et al. 2010. Options for inactivation, adjuvant, and route of topical administration of a killed, unencapsulated pneumococcal whole-cell vaccine. Clin. Vaccine Immunol. 17, 1005–1012 10.1128/CVI.00036-10 (doi:10.1128/CVI.00036-10) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lu Y. J., et al. 2010. GMP-grade pneumococcal whole-cell vaccine injected subcutaneously protects mice from nasopharyngeal colonization and fatal aspiration-sepsis. Vaccine 28, 7468–7475 10.1016/j.vaccine.2010.09.031 (doi:10.1016/j.vaccine.2010.09.031) [DOI] [PMC free article] [PubMed] [Google Scholar]