Abstract
Adoption of new vaccines in developing countries is critical to reducing child mortality and meeting Millennium Development Goal 4. However, such introduction has historically suffered from significant delays that can be attributed to various factors including (i) lack of recognition of the value of a vaccine, (ii) factors related to weak health systems, and (iii) policy considerations. Recently, the Global Alliance for Vaccines and Immunization (GAVI) supported efforts to accelerate the introduction of Haemophilus influenzae type b (Hib) vaccines in developing countries, which resulted in a significant surge in vaccine adoption by these countries. The experience with Hib vaccines, as well as similar efforts by GAVI to support the introduction of new pneumococcal and rotavirus vaccines, provides a strategy for new vaccine adoption that is reviewed in this paper, providing a useful model to help accelerate the uptake of other life-saving vaccines. This strategy addresses barriers for vaccine adoption by focusing on three major areas: (i) communications to increase awareness about the various factors needed for evidence-based decisions that meet a country's health goals; (ii) research activities to answer key questions that support vaccine introduction and long-term programme sustainability; and (iii) coordination with the various stakeholders at global, regional and country levels to ensure successful programme implementation.
Keywords: vaccination, Haemophilus influenza type b, Hib initiative, Global Alliance for Vaccines and Immunization
1. Introduction
Despite the recent decline in child mortality worldwide, over 8 million children continue to die annually [1]. The majority of these deaths occur in developing countries, with a large proportion caused by vaccine-preventable diseases. Pneumonia is the leading cause of child mortality globally, causing approximately 18 per cent of deaths in children less than 5 years of age [1]. Haemophilus influenzae type b (Hib) is a common cause of bacterial meningitis and pneumonia in children under 5 years of age in developing countries. Hib disease is estimated to account for 5 per cent of clinical pneumonia cases and 21 per cent of radiologically defined pneumonia cases [2]. Globally, an estimated 8 million cases of pneumonia and meningitis and 371 000 deaths per year are attributed to Hib [2]. Safe and effective Hib conjugate vaccines have been available since the late 1980s, and where introduced, routine use of these vaccines has led to the virtual elimination of Hib disease. These vaccines have the potential to reduce overall childhood deaths by 4 per cent, and their incorporation into countries' routine immunization schedules has been recognized as an important indicator of progress towards the United Nations Millennium Development Goal 4 (MDG4), which aims to reduce childhood mortality by two-thirds between 1990 and 2015. However, the introduction of Hib vaccines, like most new vaccines, has been characterized by rapid uptake in developed countries, where the disease burden is least, and delayed uptake in developing countries where the disease burden is greatest [3]. Similar to many other effective interventions to reduce childhood mortality, global efforts to accelerate introduction of these vaccines have not been undertaken until recently.
The introduction of new vaccines is one of the most effective ways to improve health and wealth and help developing countries reach the MDGs [4]. The GAVI Alliance (formerly known as the Global Alliance for Vaccines and Immunization) is a public–private partnership formed in 1999 with the goal of ending inequity of access to vaccines, and accelerating the uptake and use of new and under utilized vaccines in the poorest countries of the world (http://www.gavialliance.org). GAVI partners include international organizations such as the World Health Organization (WHO), United Nations International Children's Emergency Fund (UNICEF), the World Bank, the Bill and Melinda Gates Foundation, donor governments and vaccine manufacturers in both developed and developing countries, civil society organizations, technical health institutions as well as independent individuals.
In 2005, 15 years after its introduction in many developed countries, Hib vaccine was still absent from the national immunizations programmes of most developing countries. Financial factors alone could not explain this delay in vaccine introduction, especially since, in 2000, Hib vaccine was offered by GAVI free of cost to eligible countries [5]. Nevertheless, in 2004, only 13 out of 75 (17%) GAVI-eligible countries had introduced the vaccine. In 2005, GAVI established the Hib Initiative (http://www.HibAction.org), a consortium that consisted of four academic and public health organizations: Johns Hopkins Bloomberg School of Public Health, the London School of Hygiene and Tropical Medicine, the WHO and the US Centers for Disease Control and Prevention. The mission of the Hib Initiative was to accelerate evidence-based decisions to introduce Hib vaccines, which led to a significant surge in Hib vaccine adoption [5] by 2010, when the number of GAVI-eligible countries that had introduced Hib vaccine increased to 66 (92%) out of 72 countries. This paper discusses the obstacles to vaccine introduction, and draws from the experience of the Hib Initiative to propose strategies that will help to accelerate the introduction of new vaccines in the future.
2. Barriers to new vaccine introduction
The delay in the introduction of new vaccines has been attributed to multiple factors. A recent study [6] provides a concise review of various papers, published over the past decade, that have tried to identify these barriers, mainly reviewing experiences of hepatitis B and Hib vaccines. These factors can be summarized under three major categories—understanding the value of vaccines, health system characteristics, and policy issues [7]. With regards to the value of a vaccine, multiple studies have highlighted the importance of the availability of local data on disease burden and cost-effectiveness [8,9]. Furthermore, these papers highlighted the importance of documenting the efficacy of the vaccine in various parts of the world, and not just in developed countries. Factors related to inadequate health systems were very important: many countries did not have a systematic decision-making process, such as a functional national immunizations advisory committee, and as such suffered from poor planning and lack of financial support. A recent analysis, conducted by WHO and UNICEF [10] between 2000 and 2006, reported a positive relationship between having a budget line for vaccination and increased expenditure on routine vaccines and overall immunization. In addition, DTP3 vaccine coverage, which is used as an indicator of the strength of national immunization programmes, was found to be an important factor for new vaccine introduction in various studies [6,11]. Policy considerations play an important role in supporting vaccine decisions as well, in particular, the absence of clear global recommendations is a strong negative factor. For example, in the case of Hib vaccine, the initial WHO position paper [12] did not provide a strong, supportive recommendation for vaccine use but was rather weak and permissive, and as a result, did not generate significant demand for the vaccine. The revision of this position paper in 2006 [13] into a firm recommendation calling for universal vaccine introduction in all countries sent a strong and clear message to countries about the value of Hib vaccines. Similarly, the absence of clear financing policies and commitments from donors can negatively impact vaccine decisions, as countries become reluctant to commit their limited resources. In addition to global factors, regional factors can play an important role; the study by Shearer et al. [6] found that the decision of neighbouring countries to introduce a vaccine was independently associated with accelerated decision-making. The Pan American Health Organization experience is an excellent example of the importance of strong regional leadership in generating political will and facilitating vaccine financing and procurement [14]. At the country level, the presence of a strong political will, as well as support for a vaccine by local paediatric and other medical associations, was observed to have an important influence on decision-making [8]. The Hib Initiative experience [15] confirmed that the previously recognized factors are important obstacles to new vaccine introduction. However, it also highlighted the important role of communications and advocacy in accelerating decision-making. At the country level, and especially among decision-makers, there was often a lack of awareness about the value of vaccines, their efficacy and safety, potential contribution to national health priorities and mortality reduction, and a poor understanding of local disease burden issues. Similarly, there was a limited knowledge of global recommendations and financing issues, as well as the implications of demand on vaccine supply and cost evolution. Many of the papers published on these topics have been based on studies that were observational and qualitative in nature, and only a few, for example, the Hib Initiative analysis by Shearer et al. [6], have tried a quantitative, multivariate analytical approach to assess the independent contribution of various factors. However, as was acknowledged in this last study, it is very difficult to conduct an analysis that comprehensively accounts for all important variables, many of which cannot be easily quantified.
3. Strategies to accelerate new vaccine introduction
The experience of the Hib Initiative, as well as other similar GAVI-funded projects for pneumococcal and rotavirus vaccines, has generated multiple lessons that have formed the basis of a proposed policy framework that organizes the process of vaccine introduction into a continuum from evidence to policy, implementation and access, in order to help accelerate new vaccine introduction in developing countries [16]. The strategic approach of the Hib Initiative focused on three major areas that provide a good illustration of the various steps that lead to the translation of evidence into policy, and then support the transition from policy to implementation: research, communications and advocacy, and coordination among various stakeholders (box 1) [15].
Box 1. A summary of lessons learned from the Hib Initiative.
— Develop a focused team that communicates regularly and has adequate oversight.
— Build a trusting relationship with countries through frequent communications on relevant needs.
— Support country-led advocacy and communication and link vaccination to disease burden.
— Anticipate research needs for future vaccines.
— Target research and surveillance towards data for decision-making, and programme sustainability.
— Facilitate country ownership of research activities and address programmatic research needs.
— Allow adequate time for research studies, account for vaccine introduction delays.
— Address programmatic research needs, e.g. impact of new vaccines on immunization programmes.
— Prepare early and carefully for implementation following a decision for vaccine introduction.
— Ensure that WHO regional offices have adequate resources and staff to coordinate vaccine activities.
— Large countries need additional support to address their vaccine decision needs.
— Develop clear and consistent messages about various evidence needs, including disease burden, role of surveillance, cost-effectiveness, financing and supply, programmatic issues and impact.
(a). Research
Research is important to generate the information needed to support an evidence-based decision, including the generation of data on disease burden, vaccine efficacy and cost-effectiveness. Research studies that evaluate the impact of vaccines are also important in order to justify the sustainability of the vaccine programme in the long term. In the case of Hib vaccines, there was already a significant body of information available by 2005. Therefore, the research strategy of the Hib Initiative focused mainly on addressing the gaps in Hib knowledge, and supporting research and surveillance activities that could provide evidence and capacity to sustain vaccine programmes beyond the period of GAVI support. These included studies on vaccine effectiveness, surveillance systems, modelling of disease burden and economics as well as studies to evaluate the programmatic impact of vaccine introduction. To address knowledge gaps, studies were specifically supported to assess the impact of Hib vaccine in Ethiopia, in order to have more data from the Horn of Africa, in Pakistan and Bangladesh to provide data on vaccine impact in South Asia, and in Vietnam to provide impact data from the Mekong Valley countries. In addition, studies were started in Mozambique to better understand the impact of Hib vaccination in populations with high HIV prevalence, and in The Gambia, an early introducing country of Hib vaccine, to assess whether a Hib booster dose, a common practice in developed countries, is needed in developing countries. To provide much needed local burden of disease estimates, the Hib Initiative, together with another GAVI project, the Pneumococcal Accelerated Development and Introduction Programme or Pneumo Accelerated Development and Introduction Programmes (ADIP) (http://www.pneumoADIP.org), supported the WHO Global Burden of Disease project to estimate the burden of Hib and pneumococcal disease in children younger than 5 years of age at the global, regional and country level, using mathematical modelling methods and the existing disease burden data [2]. To help build surveillance capacity and support monitoring and post-introduction evaluations of vaccine impact on disease, the Hib Initiative helped to establish, support or expand the routine surveillance networks in most WHO regions. Whenever possible, Hib Initiative activities were built on existing infrastructure: for example, the Pediatric Bacterial Meningitis network in the WHO African region, and the Bacterial Meningitis surveillance network in the WHO Eastern Mediterranean Region.
In addition to collection of data on disease burden, there was considerable interest in generating cost-effectiveness evidence to support and sustain vaccine decisions. The Hib Initiative supported several country-specific analyses and reviewed the existing, published studies [17]. In order to build capacity for such analysis at the country level, a web-based, interactive tool was developed to assist countries in estimating the cost-effectiveness of Hib vaccination and the impact of the vaccine on morbidity and mortality. In order to respond to country requests to measure the impact of Hib vaccine introduction not only on Hib disease but also on the immunization programme as a whole, the Hib Initiative assisted WHO in developing a post introduction evaluation (PIE) tool, that was based on a similar tool developed by WHO for the evaluation of hepatitis B vaccine introduction.
For new vaccines, such as pneumococcal and rotavirus vaccines, it was crucial to anticipate the need for data at the country and regional level and to plan the appropriate studies early enough, so that the information was available by the time that licensed vaccines became available. Both the PneumoADIP and the rotavirus vaccine programme (http://www.rotavirusvaccine.org) have done this, initiating studies early enough to provide data that can help countries make evidence-supported decisions on vaccine introduction. The key role of these studies is to provide the objective data on which a country can, at a national level, decide whether or not introduction of a new vaccine is a sound investment. A good example of anticipatory research comes from Mongolia. In 2002, UNICEF and WHO initiated a population-based active surveillance system for childhood bacterial meningitis in Mongolia. Results from two years of surveillance indicated significant rates of Hib, Streptococcus pneumoniae and Neisseria meningitidis meningitis [18]. Based on these findings, in 2005, Mongolia began phased introduction of pentavalent vaccine, which has a Hib component. Recognizing the potential opportunity to build upon the existing surveillance infrastructure, the Hib Initiative and the PneumoADIP provided continuing support for the surveillance system in 2007, enabling the government of Mongolia to measure the impact of Hib vaccine, building evidence to support a long-term investment in immunization against Hib disease, and to generate evidence needed for the introduction of pneumococcal vaccine.
To ensure that data from research studies result in policy change, it is important for countries to have ownership of these projects. A good example comes from Sri Lanka, which had a strong, ongoing bacterial disease surveillance system in various institutions, including academic and public hospitals, that was coordinated by the Epidemiology Unit at the Ministry of Health. Routine meetings were held to update various staff on the data generated by the surveillance system. Through the WHO local office, the Hib Initiative provided additional technical and financial support to this established surveillance programme. By late 2006, analysis of the data obtained revealed that the incidence of Hib disease in Sri Lanka was close to 20/100 000 among children less than 5 years old, indicating the need for national immunization. Sri Lanka, therefore, applied for GAVI support in 2007, and introduced the vaccine in 2008. The strong links between technical and policy staff within the Sri Lankan government facilitated timely, evidence-based public health decision-making. For all the research projects supported by the Hib Initiative and those listed above, the Hib Initiative required that Ministry of Health staff be involved in all aspects of the studies, from initial design to implementation, data analysis and interpretation, in order to ensure full country ownership and to decrease the gap between research and policy communities.
(b). Communications
Communication activities are crucial to ensure that all the pieces of evidence needed for an informed decision reach the relevant decision-makers and are therefore transformed into policy. Early in the project, the Hib Initiative determined what evidence was needed or perceived to be important for decision-making. Decision-making by national authorities appeared to be strongly influenced by the perceived level of disease burden. For example, in Asian or Eastern European countries where burden data were limited, Hib disease was seldom considered to be a problem and routine vaccination was not a priority. Therefore, the main objective of the Hib Initiative communications strategy was to ensure that decision-makers and other stakeholders had timely access to evidence that was relevant and understandable, to inform decisions about Hib vaccine introduction in their area. The Hib Initiative implemented extensive communications and advocacy efforts in order to increase awareness about the public health importance of Hib disease, vaccine efficacy, safety and cost-effectiveness. Regional fora were held in Asia, Africa, Europe and the Middle East, and attended by key stakeholders at country, regional and global levels, including ministries of health and finance officials, as well as representatives from community and professional organizations, partners and donors. In addition, direct meetings with authorities in these countries, particularly those with local or regional physicians and experts, were critical in communicating key messages and clarifying nuances of relevant studies. To generate political will at the country level to support the introduction of a new intervention, it is critical to ensure that the new intervention fits within the national health context. Thus, Hib vaccine was presented as an important tool for overall pneumonia prevention and a step towards achieving MDG4, rather than as an isolated intervention. A good example of this process comes from Cambodia where, as in many Asian countries, Hib disease was not seen as an urgent public health issue by decision-makers. Cambodia's immunization programme faced many programmatic challenges, and was not considering introducing a new vaccine. However, WHO local officers recognized the important role that Hib vaccine could play in preventing child mortality, linking it to child survival. With support from the Hib Initiative, they convened a symposium on pneumonia prevention that brought together various stakeholders from the health sector. Framing Hib vaccine in the context of child survival garnered increased support for this vaccine, and facilitated progress towards an application to the GAVI Alliance in September 2008.
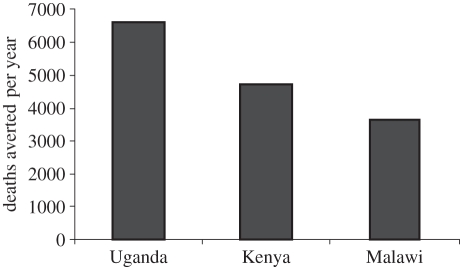
Communications materials were developed and customized to address regional and country needs. New publications reporting additional data on burden of disease or impact of the vaccine were highlighted and widely disseminated. The Hib Initiative customized its communication strategy based on regional perceptions, and created a sense of urgency by demonstrating the health and societal costs of delays in decisions. Figure 1 illustrates how data from surveillance studies can be used to visualize the deaths that could be averted by the vaccine [19–21]. In addition, the Hib Initiative communications strategy included risk communications and issues management, for example dealing with various problems that could affect vaccine introduction decisions or sustainability of programmes once the vaccine was introduced. The strategy focused on frequently publishing transparent and objective evidence-based updates on any new issues relevant to Hib vaccines. For example, in India, a strong anti-vaccine lobby was very resistant to the introduction of Hib vaccine. The Hib Initiative worked closely with respected Indian paediatricians and experts to write editorials in medical journals as well as newspapers highlighting the data available and putting it in the context of India's public health needs, rather than responding directly to the often poorly evidenced claims of the Indian anti-vaccine lobby (Hib India OpED: The Asian age, 2009: http://www.Hibaction.org/news/2009/20090128_HibIndiaOpEd.pdf). Another example comes from Sri Lanka, where shortly after the introduction of Hib vaccine, a few deaths were reported in babies who had recently received the vaccine. WHO launched an investigation of these deaths that later determined that they were not directly associated with Hib vaccine. The Hib Initiative followed closely the investigation and provided updates through its website and newsletter.
Figure 1.
The number of deaths averted per year following Hib vaccine introduction in three African countries (based on published data [19–21]).
(c). Programme coordination
Coordination among the various key partners or stakeholders is crucial to ensure the transition from policy to implementation. Vaccine introduction requires careful planning and collaboration among the various stakeholders involved, which include epidemiologists, vaccine scientists, economists, clinicians, behavioural scientists, advocates, policy analysts, communication specialists, politicians, health workers, communities, vaccine manufacturers, international agencies, donors and more. Pakistan provides a good illustration of such coordination as decisions to introduce a new vaccine in the country requires participation from Health, Finance, and Planning and Development ministries. In 2006, a team from the Hib Initiative visited Pakistan and met with key officials of all three ministries. While the Ministry of Health was aware of the health benefits of the vaccine, the Ministries of Finance, and Planning and Development were not initially aware of disease issues or the potential role of the vaccine in meeting MDGs. Once the staff of the Ministry of Planning and Development were sensitized to the health and societal costs of Hib disease and the benefits of prevention, they became advocates for vaccine adoption and together with the other ministries, played an important role in the final decision making. Despite the complexity of the decision-making process in Pakistan, the Hib Initiative team helped by bringing together the important officials involved in this process, and working closely with paediatricians and immunization officers in the country, delivering clear and consistent messages. The Hib Initiative coordinated all activities supporting programme implementation at the level of the WHO regional offices. To help countries plan adequately for vaccine introduction and to help them to prepare well-documented applications for review by GAVI, WHO staff worked closely with immunizations country staff to strengthen local planning and delivery capacity and ensure that logistical resources were adequate, including cold chain requirements, monitoring of adverse events and training of healthcare workers. Initial visits to various countries were conducted to assess and address obstacles for vaccine introduction and directly reach decision-makers. In addition to strong, evidence-based global policies recommending vaccine use, the other policies that are critical for vaccine introduction include credible policies to address the economic barriers to vaccine use, and a procurement strategy to facilitate a smooth process for vaccine introduction. The publication of GAVI co-financing guidelines that offered affordable co-payments until 2015 played an important role in accelerating decisions for vaccine introduction [15], by addressing country concerns about mid-term financial support. Analyses to forecast long-term demand and supply are very important to sustain vaccine supply and complement the existing short-term forecasts and tendering processes. The Hib Initiative, in order to ensure a healthy supply market and send clear messages about increasing demand, commissioned an analysis that explored potential global demand, including middle income countries, versus capacity, the number of products available and in the pipeline and the expected timing and production capacity of manufacturers. This analysis provided an important view of the vaccine landscape for many stakeholders and allowed modelling of price trends. While developing global strategies for vaccine introduction, it is important to recognize that large countries, owing to their diverse demographic, economic and healthcare characteristics, require special attention and customized strategies that carefully take into account their special needs. In the case of India, a country with a very large population and birth cohort as well as diverse geographic and socioeconomic characteristics, the Hib Initiative developed a separate strategy to assist the Ministry of Health to reach an evidence-based decision on the introduction of Hib vaccine. The strategy included stakeholders' analysis, a focused communications and advocacy strategy, and research activities such as conducting a cost-effectiveness analysis and an analysis of the burden of Hib disease and pneumonia in India.
4. Conclusion
The recent acceleration of Hib vaccine uptake suggests that focused strategies to accelerate the introduction of underutilized vaccines in developing countries are effective. Many of the lessons learnt from this recent experience have recently been reviewed [15] and are summarized in box 1. Significant advocacy and resources to understand and address countries' perceptions and needs, in addition to focused research, and extensive coordination efforts are often needed to accelerate introduction. However, it is important to customize the lessons learnt to suit the needs and public health context of various new vaccines. WHO, UNICEF and other partners continue to play a pivotal role in helping countries with new vaccine introduction [22]. Building on lessons learnt from the GAVI Hib Initiative and the ADIPs, various partners are now working together, under GAVI's Accelerated Vaccine Initiative (AVI) to accelerate the introduction of pneumococcal and rotavirus vaccines. SIVAC (http://www.sivacinitiative.org), a new project supported by the Bill and Melinda Gates Foundation, works closely with countries to support national independent immunization technical advisory groups to strengthen the process of evidence-based decision-making at the country level. Significant work still needs to be done to improve vaccine introduction in low–middle income countries, the pool of which is now increasing in size as more countries become no longer eligible for GAVI funding. As we continue to learn and work on improving the equity of access to new vaccines, the lessons learnt and strategies proposed above may provide useful guidance in accelerating the introduction of other life-saving vaccines into national programmes.
Acknowledgements
The author alone is responsible for the views expressed in this publication, which do not necessarily represent the views of the US Centers for Disease Control and Prevention.
References
- 1.Black R. E., et al. 2010. Global, regional and national causes of child mortality in 2008: a systematic analysis. Lancet 375, 1969–1987 10.1016/S0140-6736(10)60549-1 (doi:10.1016/S0140-6736(10)60549-1) [DOI] [PubMed] [Google Scholar]
- 2.Watt J. P. W. L., et al. 2009. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet 374, 903–911 10.1016/S0140-6736(09)61203-4 (doi:10.1016/S0140-6736(09)61203-4) [DOI] [PubMed] [Google Scholar]
- 3.Levine O. S., Cherian T., Shah R., Batson A. 2004. PneumoADIP: an example of translational research to accelerate pneumococcal vaccination in developing countries. J. Health Popul. Nutr. 22, 268–274 [PubMed] [Google Scholar]
- 4.Bloom D. E., Canning D., Weston M. 2005. The value of vaccination. World Econ. 6, 15 [Google Scholar]
- 5.Ojo L., et al. 2010. Global use of Haemophilus influenzae type b conjugate vaccine: an update of accelerated adoption in the developing world. Vaccine 28, 7117–7122 10.1016/j.vaccine.2010.07.074 (doi:10.1016/j.vaccine.2010.07.074) [DOI] [PubMed] [Google Scholar]
- 6.Shearer J., Stack M., Richmond M. 2010. Accelerating national decisions to adopt Haemophilus influenzae type b vaccine: a global multivariate analysis. PLoS Med. 16, e1000249. 10.1371/journal.pmed.1000249 (doi:10.1371/journal.pmed.1000249) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munira S., Fritzen S. 2007. What influences government adoption of vaccines in developing countries? A policy process analysis. Soc. Sci. Med. 65, 1751–1764 10.1016/j.socscimed.2007.05.054 (doi:10.1016/j.socscimed.2007.05.054) [DOI] [PubMed] [Google Scholar]
- 8.Wenger J. D., DiFabio J., Landaverde J. M., Levine O. S., Gaafar T. 1999. Introduction of Hib conjugate vaccines in the non-industrialized world: experience in four ‘newly adopting' countries. Vaccine 18, 736–742 10.1016/S0264-410X(99)00269-8 (doi:10.1016/S0264-410X(99)00269-8) [DOI] [PubMed] [Google Scholar]
- 9.Widdus R. 1999. Introduction of vaccines into the Third World. C. R. Acad. Sci. III 322, 999–1010 [DOI] [PubMed] [Google Scholar]
- 10.Lydon P., Levine R., Makinen M., Brenzel L., Mitchell V., Milstien J. B., Kamara L., Landry S. 2008. Introducing new vaccines in the poorest countries: what did we learn from the GAVI experience with financial sustainability? Vaccine 26, 6706–6716 10.1016/j.vaccine.2008.10.015 (doi:10.1016/j.vaccine.2008.10.015) [DOI] [PubMed] [Google Scholar]
- 11.Rossi I. A., Zuber P. L., Dumolard L., Walker D. G., Watt J. 2007. Introduction of Hib vaccine into national immunization programmes: a descriptive analysis of global trends. Vaccine 25, 7075–7080 10.1016/j.vaccine.2007.07.058 (doi:10.1016/j.vaccine.2007.07.058) [DOI] [PubMed] [Google Scholar]
- 12.World Health Organization 2004. WHO position statement on Hib conjugate vaccines. Wkly Epidemiol. Rec. 79, 17315168565 [Google Scholar]
- 13.World Health Organization 2006. WHO position paper on Hib conjugate vaccines. Wkly Epidemiol. Rec. 81, 445–452 [PubMed] [Google Scholar]
- 14.Danovaro-Holliday M. C., Garcia S., de Quadros C., Tambini G., Andrus J. K. 2008. Progress in vaccination against Haemophilus influenzae type b in the Americas. PLoS Med. 5, e87. 10.1371/journal.pmed.0050087 (doi:10.1371/journal.pmed.0050087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hajjeh R. A., et al. 2010. Supporting new vaccine introduction decisions: lessons learned from the Hib Initiative experience. Vaccine 28, 7123–7129 10.1016/j.vaccine.2010.07.028 (doi:10.1016/j.vaccine.2010.07.028) [DOI] [PubMed] [Google Scholar]
- 16.Levine O. S., et al. 2010. A policy framework for accelerating adoption of new vaccines. Hum. Vaccines 6, 1–4 10.4161/hv.6.12.13076 (doi:10.4161/hv.6.12.13076) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths U. K., Miners A. 2009. Economic evaluations of Haemophilus influenzae type b vaccine: systematic review of the literature. Exp. Rev. Pharmacoecon. Outcomes Res. 9, 333–946 10.1586/erp.09.38 (doi:10.1586/erp.09.38) [DOI] [PubMed] [Google Scholar]
- 18.Mendsaikhan J., et al. 2009. Childhood bacterial meningitis in Ulaanbaatar, Mongolia, 2002–2004. Clin. Infect. Dis. 48(Suppl. 2), S141–S146 10.1086/596493 (doi:10.1086/596493) [DOI] [PubMed] [Google Scholar]
- 19.Cowgill K. D., et al. 2006. Effectiveness of Haemophilus influenzae type b conjugate vaccine introduction into routine childhood immunization in Kenya. JAMA 296, 671–678 10.1001/jama.296.6.671 (doi:10.1001/jama.296.6.671) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daza P., et al. The impact of routine infant immunization with Haemophilus influenzae type b conjugate vaccine in Malawi, a country with high human immunodeficiency virus prevalence. Vaccine 24, 6232–6239 10.1016/j.vaccine.2006.05.076 (doi:10.1016/j.vaccine.2006.05.076) [DOI] [PubMed] [Google Scholar]
- 21.Lewis R. F., et al. 2008. Action for child survival: elimination of Haemophilus influenzae type b meningitis in Uganda. Bull. World Health Organ. 86, 292–301 10.2471/BLT.07.045336 (doi:10.2471/BLT.07.045336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization 2005 Vaccine introduction guidelines. See http://whqlibdoc.who.int/hq/2005/WHO_IVB_05.18.pdf