Abstract
Chronic, non-communicable diseases are the major cause of death and disability worldwide and have replaced infectious diseases as the major burden of society in large parts of the world. Despite the complexity of chronic diseases, relatively few predisposing risk factors have been identified by the World Health Organization. Those include smoking, alcohol abuse, obesity, high cholesterol and high blood pressure as the cause of many of these chronic conditions. Here, we discuss several examples of vaccines that target these risk factors with the aim of preventing the associated diseases and some of the challenges they face.
Keywords: therapeutic vaccines, chronic diseases, smoking cessation vaccine
1. Introduction
The successful introduction, use and continued development of vaccines against many of the most deadly infectious diseases during the past two centuries have brought numerous benefits to mankind. One prominent advantage is an increase in the average lifespan of the world's citizens. Indeed, the United Nations has reported that at the beginning of the twenty-first century, there were approximately 600 million individuals aged 60 years and over (http://www.un.org/esa/population/publications/worldageing19502050/). This number is expected to grow more than threefold by 2050. With many infectious diseases kept at bay, the type of diseases to which our ageing population is most prone has changed. The new major health threats of modern times are cardiovascular disease, cancer, chronic respiratory disease and diabetes, which account for more than half of the global burden of disease and death [1]. While the various aetiologies of these diseases are viewed as multi-factorial, perhaps surprisingly a relatively low number of risk factors are largely responsible for predisposition to these diseases. These factors include smoking (a major cause of cancer and respiratory disease), hypertension, (a key factor for cardiovascular disease) and obesity (closely related with development of type II diabetes). In this review, we discuss the concept that active vaccination can be used to target key molecules identified as risk-factors and thereby lessen the associated diseases. While numerous therapeutic vaccine approaches have been developed [2], in this review, we have chosen to describe our efforts to develop three virus-like particle (VLP)-based vaccines designed to induce antibodies against nicotine, angiotensin II or interleukin-1β (IL-1β) and thus provide a potential treatment of smoking addiction, hypertension and type II diabetes, respectively.
2. The technology
The disease-related molecules nicotine, angiotensin II and IL-1β represent a diverse and challenging set of antigens to target by active vaccination. To successfully neutralize haptens or endogenous small and large soluble polypeptides such as these, it is essential to induce sufficient amounts of high-affinity antibodies. For instance, our current experience indicates that for vaccination against nicotine to be successful, it is necessary to attain levels of nicotine-specific IgG of 10 µg ml [3]. This is orders of magnitude higher than what is usually required for protection against viral infection. To achieve this is not straightforward and requires that a combination of vaccine design elements are employed. The use of a vaccine platform based on VLPs represents an excellent means for optimizing antibody responses.
VLPs are supramolecular structures typically in the form of icosahedrons with diameters in the range 25–100 nm. They are composed of multiple copies of one or more recombinantly expressed viral structural proteins, which upon expression spontaneously assemble into particles [4,5]. Importantly they incorporate key immunological features of viruses responsible for the induction of fulminant B and T cell responses [6]. These include repetitive surfaces, particulate structure, induction of innate immunity through activation of pathogen associated molecular patterns and helper T cell epitopes [7,8]. Recombinantly expressed VLPs, while structurally similar to viruses, lack genetic information with replicative capacity and hence do not have the safety issues associated with whole virus vaccines.
We have used VLPs derived from the coat protein of the bacteriophage Qβ for which there is no pre-existing immunity in humans. The 14 kDa protein is highly expressed in Escherichia coli [9], which has permitted the development of an economical large-scale current good manufacturing practice production process. During VLP assembly, nucleic acids of the host, almost exclusively RNA, are packaged within the particle. Single-stranded RNA is the natural ligand for Toll-like receptors 7 and 8 (TLR7/8) [10]. Engagement of these receptors upregulates co-stimulatory molecules and cytokines, resulting in induction of effective immunity, which promotes antigen-specific IgG2a responses [11–13].
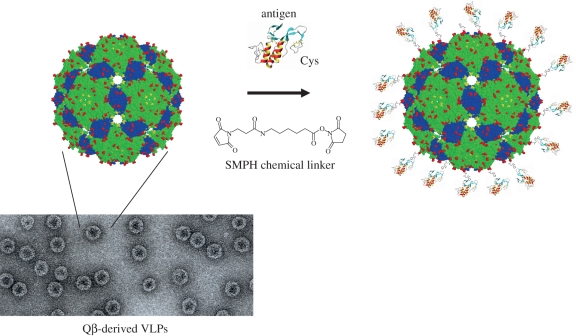
VLPs can also be used to generate antibody responses towards antigens not derived from the VLP's originating virus. This is achieved by linking a foreign antigen to the surface of a VLP. In this way, the intrinsic viral fingerprint of the VLP is passed onto the attached epitope, thereby rendering it as potent a B cell immunogen as the VLP. We have used chemical conjugation to attach antigens to the surface of Qβ-derived VLPs (figure 1). After coupling, the previously soluble antigen is rendered highly ordered and repetitive, triggering high antibody responses in animal models as well as humans [14–17].
Figure 1.
VLPs and the chemical coupling approach. The figure depicts a structural representation of a Qβ VLP obtained from the crystal structure of the phage Qβ. It also illustrates the modular approach to vaccine production whereby the protein antigen and VLP are separately produced and then covalently linked using a heterobifunctional chemical cross-linker such as succinimidyl-6-[(β-maleimidopropionamido) hexanoate] (SMPH). The resultant conjugate vaccine displays the target antigen in an ordered and highly repetitive fashion. Shown too is an electron-micrograph of 25–30 nm diameter icosahedral Qβ VLPs.
3. Smoking cessation vaccine
(a). Introduction
Smoking tobacco is the most frequent addictive habit worldwide. Despite extensive efforts to decrease and/or prevent smoking, in almost all parts of the world tobacco use remains the leading cause of preventable disease and death. It is estimated that there are 1.2 billion smokers worldwide, and 5 million deaths annually are related to tobacco use [18]. The major causes of death are lung cancer, coronary heart disease, chronic lung disease and stroke. Not only is smoking dangerous to individuals but it also causes excessive costs to the healthcare systems of our societies. By way of example, the total annual economic expenses associated with nicotine addiction are estimated to be over $150 billion in the USA [19]. Even in less-developed countries, chronic diseases caused by tobacco are currently overtaking the more traditional causes of deaths, and it is estimated that smoking kills similar numbers of people in the developing and developed worlds. An often underestimated aspect of smoking is that even in countries where infectious diseases are the main cause of early fatality, smoking increases the death toll by enhancing pulmonary diseases caused by pathogens. Indeed, smoking not only increases the incidence of clinical tuberculosis, but it has also been reported to cause half of the tuberculosis deaths in India [20].
Tobacco smoke contains a multitude of chemical substances, many of which are summarized under the term ‘tar’ and are deposited in upper airways and lungs. More than 40 of these substances are known carcinogens. Indeed, almost all of the cancers and lung diseases caused by tobacco smoking are due to toxins within the tar [19]. Addiction, however, is caused by a different substance, the alkaloid nicotine [21,22]. Nicotine is addictive since it stimulates the mesolimbic reward system, quickly leading to addiction [23]. Cigarette design has been optimized for rapid delivery of the drug to the brain, enhancing its addictive properties. Indeed, upon inhalation of cigarette smoke, nicotine is adsorbed in the lung and reaches the brain within seconds.
Current therapies for treating nicotine addiction principally target craving and withdrawal, and include nicotine replacement therapies (e.g. nicotine patches, gum and inhalers), Bupropion (an antidepressant inhibitor of norepinephrine and dopamine reuptake and nicotinic receptor antagonist) and Varenicline (a partial agonist of the nicotinic a4b2 acetylcholine receptor subtype).
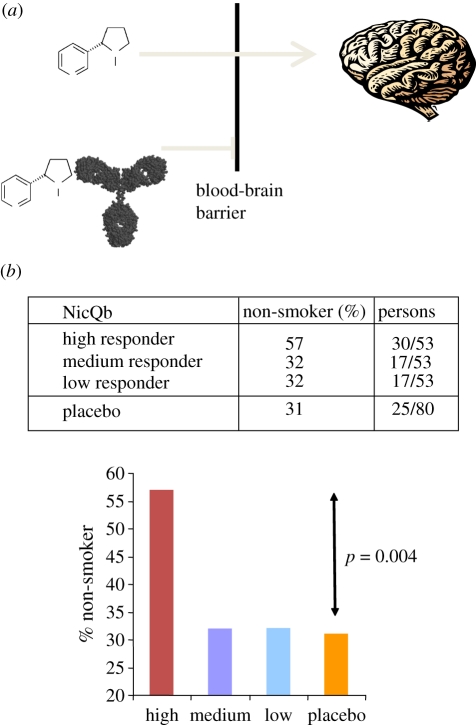
An alternative approach is to ‘remove’ the nicotine itself. This can be achieved by active vaccination, which induces anti-nicotine antibodies that act to prevent reinstatement of the positive reinforcement resulting from relapse. Proof of concept for this approach has been achieved in animals [3,24]. The mechanism for this is relatively simple. Anti-nicotine antibodies sequester nicotine in the blood, the nicotine–antibody complex is too large to cross the blood–brain barrier, and consequently, the flux of nicotine to the brain is altered (figure 2a). By altering the pharmacokinetics, the reward obtained from drug intake is decreased, the pleasure from reinitiating smoking is removed and the positive reinforcement cycle disrupted. Hence, immunized individuals may smoke a cigarette without their brain being exposed to the addictive nicotine. Such an approach may be particularly effective for relapse prevention, by allowing individuals who quit smoking to be weak for a moment, consume a cigarette and still remain non-smokers.
Figure 2.
Vaccination to prevent nicotine addiction. (a) Nicotine absorbed into the blood stream after smoking readily crosses the blood–brain barrier (BBB). Nicotine-specific antibodies induced by immunization with NiCQb bind nicotine in the blood. Because the antibody-sequestered nicotine is too large to cross the BBB, the flux of nicotine to the brain is inhibited. (b) Results from a phase II clinical study in smokers. 159 smokers receiving NicQb were classified into high, medium and low responders to the vaccine based on their anti-nicotine IgG titres. Thirty of the 53 high responders were continuously abstinent (non-smokers) compared with 31% in the placebo group. This result was highly statistically significant (p = 0.004) [28].
(b). Quantitative challenges
Cigarettes contain up to 500 µg of nicotine. Even though antibodies have been reported to block nicotine from entering the brain of rats at sub-stoichiometric concentrations [25], large amounts of antibodies are nevertheless required in order to significantly sequester such micromolar quantities of drug in the blood. Hence, the primary challenge for any smoking cessation vaccine will be to induce sufficient amounts of nicotine-binding antibodies. Indeed, current evidence from clinical studies suggests that the limiting factor for efficacy of vaccines targeting addictive substances such as nicotine and cocaine is the level of antibodies that can be induced [26,27].
(c). Efficacy in humans
An anti-nicotine vaccine (NicQb) comprising nicotine covalently coupled to the surface of Qβ VLPs via a succinate linkage has been produced. To study the immunogenicity of this vaccine, non-smoking human volunteers were immunized twice with 50 or 100 µg of NicQb in saline [3]. The higher dose was also formulated in alum. The vaccine was found to be safe and well tolerated and all volunteers responded with nicotine-specific IgG after the first immunization. The addition of alum increased the antibody titres by approximately twofold. The amount of anti-nicotine-specific IgG antibody induced was assessed by equilibrium dialysis and found to be in the region of 10 µg ml−1. To address the potential of NicQb to help people quit smoking a multi-centre double blind placebo-controlled phase II study was conducted in which 200 smokers were immunized with 100 µg of NicQb in alum and 100 were treated with a placebo [28]. To further increase the antibody responses obtained in the phase I study, individuals received five rather than two injections at monthly intervals. In addition, they received professional counselling to assist smoking cessation. One month into the study, coinciding with the second administration of vaccine, the subjects were instructed to quit smoking. Smoking cessation was defined as being continuously abstinent from smoking from month 2 till month 6 of the study.
As observed in the phase I study, all volunteers responded to vaccination by producing anti-nicotine IgG antibodies after the first injection and the titres could be further increased upon boosting. There was no evidence for smokers making inferior antibody responses compared with non-smokers. When smoking cessation rates were assessed by self-reported smoking status and by measuring CO in exhaled air, immunized individuals had a higher probability of quitting smoking than those on placebo (56% versus 40%, respectively) at month 2, when the difference was highest. However, the intention to treat analysis showed the difference was small and not statistically significant. Since antibody levels were expected to be possibly limiting, the population of immunized smokers was stratified into three equally sized groups, namely those that responded with low, medium and high antibody titres. When analysed this way, a robust significant effect was observed for the highest tertile; 57 per cent of the high antibody responders were continuously abstinent compared with 31 per cent of subjects who received placebo. By contrast, the medium and low antibody titre groups showed little difference compared with the placebo group (figure 2b). Hence, the amounts of antibodies induced by vaccination appear to have been limiting and need to be further increased in order to reach efficacious titres in the majority of smokers. This remains a challenging issue and a first attempt by Novartis to increase smoking cessation rates with NicQb by altering the vaccine regimen has failed to do so.
In addition to measuring antibody titres, an important parameter to take into account is the ‘quality’ of the induced antibodies. While antibodies with an affinity of 10−6 M may be readily measurable by enzyme-linked immunosorbent assay (ELISA) where antibodies bind bivalently and therefore with high avidity, such antibodies will certainly have little protective efficacy in vivo.
Nabi Biopharmaceuticals is developing NicVAX, a smoking cessation vaccine based on nicotine conjugated to recombinant Pseudomonas aeruginosa exoprotein A. As a consequence of promising phase II data [29], they are currently testing the efficacy of NicVAX in phase III studies.
In summary, the present data demonstrate that a vaccine against nicotine can be efficacious for smoking cessation in humans when high enough anti-nicotine antibody levels are achieved. The remaining challenge is to induce sufficient levels of antibody with sufficient quality in the majority of smokers.
4. Vaccination against hypertension
(a). Introduction
Hypertension is the most important risk factor for the development of cardiovascular events with potentially lethal outcome, such as heart-attacks and stroke. Pharmacological treatment of hypertension with both angiotensin converting enzyme inhibitors (ACEi) and angiotensin II type I receptor (AT1R) blockers (ARBs) has proved very successful in lowering high blood pressure and lessening disease sequelae. Nevertheless, only about 30–50% of hypertensive patients in the USA have adequate control of blood pressure [30]. In addition to insufficient diagnosis and drug prescription, low patient compliance is a major reason for the high number of patients with uncontrolled hypertension [31]. Therefore, improving patient compliance is a goal of newly developed anti-hypertensive therapies. Important reasons for the poor adherence of patients to their treatment include the presence of side effects and general reluctance to take long-term drug medication in the absence of symptoms [32]. A second issue for the treatment of hypertension with classical drugs is the so-called morning pressure surge, a steep increase in blood pressure that occurs early in the morning prior to waking. At this time, drug levels are usually at their lowest, since most patients take their hypertension medication with breakfast.
Active vaccination with the aim of inducing long-lived antibody responses that target key regulators of blood pressure such as angiotensin II represents an alternative approach to treating hypertension and minimizing the more severe outcomes of the condition. Immunotherapy also has the potential to address the issues of compliance and morning pressure surge. An immunization regimen consisting of a few injections per year would considerably simplify treatment and thereby potentially promote higher adherence to treatment. Furthermore, vaccination may enhance control of the morning pressure surge since antibody levels remain constant over any 24 h period and levels early in the morning will not be different from those during the rest of the day. A further advantage of the approach is that angiotensin II has been chronically blocked by pharmaceutical intervention in millions of hypertensive patients. It is therefore unlikely that vaccine-induced blockage of the molecule will have unexpected side-effects, compromising the safety of the vaccine.
(b). Efficacy in humans
Antibodies have the potential to block the renin/angiotensin axis at various levels. Renin, which cleaves angiotensinogen into angiotensin I is a possible target. However, since renin is an abundant membrane-bound protein in the kidney, it may be prone to cause glomerulonephritis owing to immune complex formation. Indeed, preclinical experiments have revealed lethal kidney disease in immunized animals [33]. In contrast, angiotensin I and II are small soluble peptides of 10 and 8 amino acids, respectively, and are therefore too small to allow immune complexes to form. Both these peptides have been targeted with vaccines.
The biotechnology company Protherics tested an anti-angiotensin vaccine comprising angiotensin I peptides coupled to either tetanus toxoid or to keyhole limpet haemocyanin. Preclinical studies in rats showed the vaccines attenuated the anticipated blood pressure increase following intravenous administration of angiotensin I. However, in a subsequent placebo-controlled clinical study in hypertensive subjects, the vaccine PMD3117 did not influence clinical blood pressure readings or ambulatory blood pressure measurements. It was concluded that the antibody levels generated with PMD3117 were too small to provide sufficient blockage of angiotensin I [34].
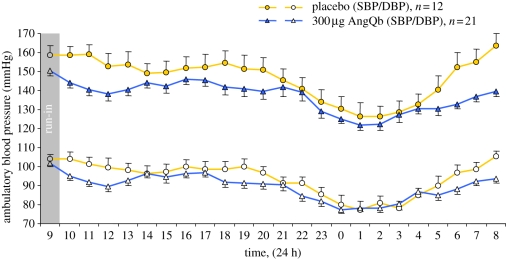
As an alternative approach, we chose to immunize against angiotensin II by coupling the eight amino acid peptide to Qβ VLPs (AngQb). Preclinical experiments demonstrated high levels of angiotensin II-specific IgG antibodies, which were essentially able to control blood pressure in spontaneously hypertensive rats [35]. A phase I study in human volunteers indicated that the AngQb was safe and well tolerated and also highly immunogenic in humans. AngQb induced specific IgG responses after a single immunization. A multi-centre, double-blind placebo-controlled phase II study in 72 mild to moderate hypertensive patients confirmed the high immunogenicity of the vaccine [36]. Importantly, after three injections of 300 µg of the vaccine, daytime systolic and diastolic blood pressure were reduced by 9 and 4 mmHg, respectively, compared with placebo. Most prominent was the reduction of blood pressure early in the morning and morning pressure surge was clearly reduced in immunized individuals (figure 3). Measuring the binding properties of the antibodies revealed a strong affinity maturation of the IgG response subsequent to the third injection at week 14 of the study. An approximately 10-fold increase in affinity from 20 nM at week 6 to 1–2 nM at week 14 was observed. This increased affinity of the antibodies resulted in sequestration of angiotensin II in the blood by specific antibodies, leading to a more than 10-fold increase in total angiotensin II levels (antibody bound angiotensin II and free angiotensin II). Specifically, while concentrations of total angiotensin II were in the region of 5 pM at week 6, the concentration at week 14 was around 90 pM. Despite the overall increase in the levels of total angiotensin II, the amount of free angiotensin II was actually reduced in the immunized volunteers.
Figure 3.
Vaccination to treat hypertension. Profiles of mean hourly ambulatory blood pressure measurements from 21 individuals immunized with 300 µg of the anti-hypertension vaccine AngQb compared with the corresponding placebo group (n = 12) at week 14 of the phase II study. The difference from placebo in the change from baseline in mean ambulatory blood pressure at week 14 was −9.0/–4.0 mmHg (p = 0.015 for systolic blood pressure, p = 0.064 for diastolic blood pressure). The blood pressure surge between 5.00 and 8.00 h was reduced compared with placebo after baseline correction (p = 0.012 and p = 0.036, respectively) with a change at 8.00 h of −25 mmHg in systolic blood pressure (p < 0.0001) and −13 mmHg in diastolic blood pressure (p = 0.0035).
In a second study, we tried to further improve these results by inducing higher antibody titres. We attempted to do this by increasing the number and the frequency of injections. While this new regimen increased antibody titres, the reduction of blood pressure was surprisingly smaller compared with the previous study. Interestingly, the reduced efficacy correlated with reduced affinities (two to three times lower) of the antibodies induced by the altered vaccination regimen, suggesting that affinities might have been limiting for efficacy.
In summary, it has been shown that immunization against angiotensin II can result in significantly reduced blood pressure in hypertensive patients. However, as observed for nicotine, it may be important to consider the quality of antibodies in addition to their quantity. A factor affecting antibody quality may be the vaccination regimen used, a parameter that has perhaps been previously underestimated in the development of therapeutic vaccines.
5. Vaccination against type II diabetes
(a). Introduction
The number of type II diabetic patients has dramatically increased over the past few decades and the disease has now reached epidemic proportions. Changes in dietary habits are the leading cause of this increase in prevalence. It has been known for some time that pro-inflammatory cytokines are produced in fat tissues and may in part be responsible for insulin resistance and islet beta cell dysfunction, the two major characteristics of type II diabetes [37]. The cytokine IL-1β may cause islet cell apoptosis and has been described as the primary agonist in the loss of beta-cell mass in type II diabetes [38,39]. Of particular relevance is the observation that blocking IL-1 may improve symptoms of type II diabetes in patients [40]. A potential role for IL-1β in the disease has further been supported by the finding that amyloid polypeptide produced by islet cells activates the NLRP3 inflammasome, causing local production of the cytokine [41].
(b). The vaccine
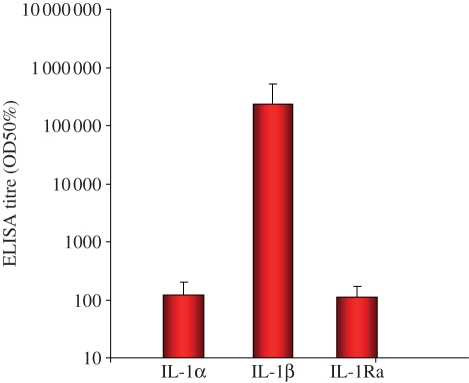
In order to be able to block IL-1β and provide a long-term treatment of type II diabetes, we have initiated the development of a vaccine against IL-1β. Our experience with peptide-based vaccines has taught us that while preclinical efficacy can be readily demonstrated in animal models, it is rather more challenging to achieve clinical efficacy with such vaccines. Hence, we decided to generate a vaccine comprising full-length IL-1β protein coupled to Qβ VLP (IL1bQb). Immunization of mice with such a vaccine induced high neutralizing antibody titres with specificity for IL-1β but not the related family members with most sequence homology, IL-1α and IL-1Ra (figure 4). Moreover, IL1bQb protected animals against collagen-induced rheumatoid arthritis [42].
Figure 4.
Vaccination to prevent type II diabetes. Immunogenicity and specificity of anti-IL-1β IgG antibodies induced by immunization with IL1bQb. Mice (n = 5) were immunized on days 0, 21 and 42 with 300 µg of IL1bQb in alum. Sera were collected on day 56 and analysed by ELISA to measure anti-IL1β response and test cross-reactivity with IL-1α and IL-1 receptor antagonist (IL-1Ra).
IL-1β is a highly bioactive molecule, active at doses of less than 10 ng kg−1 in humans. A typical dose of IL1bQb delivers microgram quantities of IL-1β. Hence, the possibility of inducing vaccine-related acute toxicity exists. In order to reduce this risk, we screened more than 20 different IL-1β mutants for their ability to bind IL-1 receptor, trigger IL-6 release and induce neutralizing antibodies. Many of the mutant IL-1β molecules we screened demonstrated loss of activity concomitant with decreased receptor binding suggesting altered tertiary structure; an undesirable characteristic for a vaccine antigen. However, a mutant IL-1β was identified with the required traits of low bioactivity, authentic structure and antigenicity [43]. Moreover, we found that by coupling IL-1β with Qβ VLP, the bioactivity of the molecule was further reduced by a factor of approximately 100 in vitro. Importantly, antibody responses against this mutant form of IL-1β protected mice in models of rheumatoid arthritis and ameliorated the diabetic phenotype in a murine diet-induced obesity model [44].
Toxicity studies with the detoxified IL1bQb performed in primates and rodents showed that human, monkey and murine versions of the vaccine were well tolerated as was anticipated from our antigen screening studies. In addition, the vaccine was highly immunogenic in primates and rodents and induced high titres of neutralizing antibodies (P. Maurer 2010, unpublished data). From a safety perspective, the effects of active vaccination with IL1bQb on the normal physiological processes regulated by IL-1β can be judged by analysing the extensive therapeutic experience obtained with IL-1α/β-neutralizing biologicals and anti-IL-1 monoclonal antibodies. IL-1α and -β are involved in the host response to infections, particularly those involving intracellular pathogens. Indeed IL-1R1- and IL-1α/β-knockout mice have increased susceptibility to infection with Mycobacterium tuberculosis and Listeria monocytogenes. However, IL1bQb may actually have safety advantages over biologicals, which block both IL-1α and -1β such as Kineret which acts as a competitive inhibitor for the binding of both IL-1α and IL-1β to IL-1 receptors. IL1bQb induces antibodies that are highly specific for IL-1β and do not neutralize IL-1α [42]. We have also demonstrated that blocking of IL-1α but not of IL-1β through active vaccination resulted in increased susceptibility to chronic infection with M. tuberculosis suggesting that IL-1α is the major mediator of the IL-1RI-dependent and protective innate immune responses to mycobacteria in mice [45]. An analysis of multiple clinical studies performed with the IL-1 receptor antagonist Kineret shows a slight increase in the incidence of serious infections (1.8% in Kineret-treated versus 0.7% in placebo group). Extensive post-marketing surveys and available clinical data indicate that neutralization of IL-1 activity is generally safe and without systemic toxicities.
The vaccine IL1bQb is currently undergoing phase I testing in patients with mild type II diabetes to determine safety/tolerability, regimen and immunogenicity. After the completion of the study, we plan to expand clinical testing into a phase II study to test for efficacy in type II diabetes patients using an optimized dosing regimen.
6. Conclusion
It has recently become evident that vaccination targeting the molecular risk factors associated with chronic non-communicable diseases is a possible means to control some major global diseases. Successes with vaccines for smoking cessation and hypertension have highlighted the significant potential of such drugs. Advances in our understanding of immunology, vaccinology and molecular and cellular biology have provided an opportunity for the concept of vaccination to be ‘recycled’ and newly applied. Vaccines still remain the most relevant means of preventing infectious disease but their use may be expanded to an additional and similarly important field: the prevention of chronic non-infectious diseases. The use of rational vaccine design to target and minimize the risk factors associated with chronic disease may yet again help mankind control the threat of the ‘new pestilences’ of the modern era.
However, for the promise to be fulfilled, the challenges that confront the development of therapeutic vaccines need to be met. As clinical testing of therapeutic B cell vaccines advances (more than 20 independent approaches have been trialled in the past decade), it has become possible to identify some of these. Salient among them is the need to induce antibodies in sufficient amounts and of appropriate affinities. Determining the quantities of antibodies and the range of affinities that need be reached in order to achieve clinical efficacy is a complex pharmacometric matter, which is influenced by many target-specific factors such as concentration, size and quaternary structure. Nevertheless, both antibody affinity and quantity may be controlled by the use of properly adjuvanted vaccines displaying favourably spaced antigens with the correct three-dimensional configuration applied with an optimal regimen.
References
- 1.World Health Organization 2003. WHO World Health Report 2002—reducing risks, promoting healthy life, pp. 49–97. See http://www.who.int/whr/2002/en/index.html. [DOI] [PubMed]
- 2.Rohn T. A., Bachmann M. F. 2010. Vaccines against non-communicable diseases. Curr. Opin. Immunol. 22, 391–396 10.1016/j.coi.2010.02.009 (doi:10.1016/j.coi.2010.02.009) [DOI] [PubMed] [Google Scholar]
- 3.Maurer P., Jennings G. T., Willers J., Rohner F., Lindman Y., Roubicek K., Renner W. A., Muller P., Bachmann M. F. 2005. A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur. J. Immunol. 35, 2031–2040 10.1002/eji.200526285 (doi:10.1002/eji.200526285) [DOI] [PubMed] [Google Scholar]
- 4.Johnson J. E., Chiu W. 2000. Structures of virus and virus-like particles. Curr. Opin. Struct. Biol. 10, 229–235 10.1016/S0959-440X(00)00073-7 (doi:10.1016/S0959-440X(00)00073-7) [DOI] [PubMed] [Google Scholar]
- 5.Pumpens P., Grens E. 1999. Hepatitis B core particles as a universal display model: a structure-function basis for development. FEBS Lett. 442, 1–6 10.1016/S0014-5793(98)01599-3 (doi:10.1016/S0014-5793(98)01599-3) [DOI] [PubMed] [Google Scholar]
- 6.Jennings G. T., Bachmann M. F. 2007. Designing recombinant vaccines with viral properties: a rational approach to more effective vaccines. Curr. Mol. Med. 7, 143–155 10.2174/156652407780059140 (doi:10.2174/156652407780059140) [DOI] [PubMed] [Google Scholar]
- 7.Bachmann M. F., Rohrer U. H., Kundig T. M., Burki K., Hengartner H., Zinkernagel R. M. 1993. The influence of antigen organization on B cell responsiveness. Science 262, 1448–1451 10.1126/science.8248784 (doi:10.1126/science.8248784) [DOI] [PubMed] [Google Scholar]
- 8.Bachmann M. F., Jennings G. T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat. Rev. Immunol. 10, 787–796 10.1038/nri2868 (doi:10.1038/nri2868) [DOI] [PubMed] [Google Scholar]
- 9.Kozlovska T. M., Cielens I., Dreilinna D., Dislers A., Baumanis V., Ose V., Pumpens P. 1993. Recombinant RNA phage Q beta capsid particles synthesized and self-assembled in Escherichia coli. Gene 137, 133–137 10.1016/0378-1119(93)90261-Z (doi:10.1016/0378-1119(93)90261-Z) [DOI] [PubMed] [Google Scholar]
- 10.Heil F., Hemmi H., Hochrein H., Ampenberger F., Kirschning C., Akira S., Lipford G., Wagner H., Bauer S. 2004. Species-specific recognition of single-stranded RNA via Toll-like receptor 7 and 8. Science 303, 1526–1529 10.1126/science.1093620 (doi:10.1126/science.1093620) [DOI] [PubMed] [Google Scholar]
- 11.Jegerlehner A., Maurer P., Bessa J., Hinton H. J., Kopf M., Bachmann M. F. 2007. TLR9 signaling in B cells determines class switch recombination to IgG2a. J. Immunol. 178, 2415–2420 [DOI] [PubMed] [Google Scholar]
- 12.Bessa J., Jegerlehner A., Hinton H. J., Pumpens P., Saudan P., Schneider P., Bachmann M. F. 2009. Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J. Immunol. 183, 3788–3799 10.4049/jimmunol.0804004 (doi:10.4049/jimmunol.0804004) [DOI] [PubMed] [Google Scholar]
- 13.Hou B., et al. 2011. Selective utilization of Toll-like receptor and MyD88 signaling in B cells for enhancement of the antiviral germinal center response. Immunity 34, 375–384 10.1016/j.immuni.2011.01.011 (doi:10.1016/j.immuni.2011.01.011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spohn G., Schwarz K., Maurer P., Illges H., Rajasekaran N., Choi Y., Jennings G. T., Bachmann M. F. 2005. Protection against osteoporosis by active immunization with TRANCE/RANKL displayed on virus-like particles. J. Immunol. 175, 6211–6218 [DOI] [PubMed] [Google Scholar]
- 15.Spohn G., et al. 2007. A virus-like particle-based vaccine selectively targeting soluble TNF-alpha protects from arthritis without inducing reactivation of latent tuberculosis. J. Immunol. 178, 7450–7457 [DOI] [PubMed] [Google Scholar]
- 16.Rohn T. A., Jennings G. T., Hernandez M., Grest P., Beck M., Zou Y., Kopf M., Bachmann M. F. 2006. Vaccination against IL-17 suppresses autoimmune arthritis and encephalomyelitis. Eur. J. Immunol. 36, 2857–2867 10.1002/eji.200636658 (doi:10.1002/eji.200636658) [DOI] [PubMed] [Google Scholar]
- 17.Kundig T. M., et al. 2006. Der p 1 peptide on virus-like particles is safe and highly immunogenic in healthy adults. J. Allergy Clin. Immunol. 117, 1470–1476 10.1016/j.jaci.2006.01.040 (doi:10.1016/j.jaci.2006.01.040) [DOI] [PubMed] [Google Scholar]
- 18.World Health Organization 2004. Tobaco free initiative: building blocks for tobacco control: a handbook. See http://www.who.int/tobacco/resources/publications/tobaccocontrol_handbook/en/.
- 19.US Department of Health and Human Services 2004. The health consequences of smoking: a report of the Surgeon General. pp. 1–33. See http://www.cdc.gov/tobacco/data_statistics/sgr/2004/pdfs/chapter1.pdf. [Google Scholar]
- 20.Gajalakshmi V., Peto R., Kanaka T. S., Jha P. 2003. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43000 adult male deaths and 35000 controls. Lancet 362, 507–515 10.1016/S0140-6736(03)14109-8 (doi:10.1016/S0140-6736(03)14109-8) [DOI] [PubMed] [Google Scholar]
- 21.Harvey D. M., Yasar S., Heishman S. J., Panlilio L. V., Henningfield J. E., Goldberg S. R. 2004. Nicotine serves as an effective reinforcer of intravenous drug-taking behavior in human cigarette smokers. Psychopharmacology 175, 134–142 10.1007/s00213-004-1818-6 (doi:10.1007/s00213-004-1818-6) [DOI] [PubMed] [Google Scholar]
- 22.Le Foll B., Goldberg S. R. 2006. Nicotine as a typical drug of abuse in experimental animals and humans. Psychopharmacology 184, 367–381 10.1007/s00213-005-0155-8 (doi:10.1007/s00213-005-0155-8) [DOI] [PubMed] [Google Scholar]
- 23.Le Foll B., Goldberg S. R. 2005. Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol. Sci. 26, 287–293 10.1016/j.tips.2005.04.005 (doi:10.1016/j.tips.2005.04.005) [DOI] [PubMed] [Google Scholar]
- 24.Pentel P. R., et al. 2000. A nicotine conjugate vaccine reduces nicotine distribution to brain and attenuates its behavioral and cardiovascular effects in rats. Pharmacol. Biochem. Behav. 65, 191–198 10.1016/S0091-3057(99)00206-3 (doi:10.1016/S0091-3057(99)00206-3) [DOI] [PubMed] [Google Scholar]
- 25.Hieda Y., Keyler D. E., Ennifar S., Fattom A., Pentel P. R. 2000. Vaccination against nicotine during continued nicotine administration in rats: immunogenicity of the vaccine and effects on nicotine distribution to brain. Int. J. Immunopharmacol. 22, 809–819 10.1016/S0192-0561(00)00042-4 (doi:10.1016/S0192-0561(00)00042-4) [DOI] [PubMed] [Google Scholar]
- 26.Cerny E. H., Cerny T. 2009. Vaccines against nicotine. Hum. Vaccine 5, 200–205 10.4161/hv.5.4.7310 (doi:10.4161/hv.5.4.7310) [DOI] [PubMed] [Google Scholar]
- 27.Orson F. M., Kinsey B. M., Singh R. A., Wu Y., Kosten T. R. 2009. Vaccines for cocaine abuse. Hum. Vaccine 5, 194–199 10.4161/hv.5.4.7457 (doi:10.4161/hv.5.4.7457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cornuz J., et al. 2008. A vaccine against nicotine for smoking cessation: a randomized controlled trial. PLoS ONE 3, e2547. 10.1371/journal.pone.0002547 (doi:10.1371/journal.pone.0002547) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hatsukami D. K., et al. Immunogenicity and smoking-cessation outcomes for a novel nicotine immunotherapeutic. Clin. Pharmacol. Ther. 89, 392–399 10.1038/clpt.2010.317 (doi:10.1038/clpt.2010.317) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott W. J. 2003. The economic impact of hypertension. J. Clin. Hypertens. 5, 3–13 10.1111/j.1524-6175.2003.02463.x (doi:10.1111/j.1524-6175.2003.02463.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burnier M. 2000. Long-term compliance with antihypertensive therapy: another facet of chronotherapeutics in hypertension. Blood Press Monit. 5, S31–S34 10.1097/00126097-200005001-00006 (doi:10.1097/00126097-200005001-00006) [DOI] [PubMed] [Google Scholar]
- 32.Osterberg L., Blaschke T. 2005. Adherence to medication. N. Engl J. Med. 353, 487–497 10.1056/NEJMra050100 (doi:10.1056/NEJMra050100) [DOI] [PubMed] [Google Scholar]
- 33.Michel J. B., et al. 1989. Immunological approach to blockade of the renin-substrate reaction. J. Hypertens. (Suppl. 7), S63–S70 [DOI] [PubMed] [Google Scholar]
- 34.Brown M. J., Coltart J., Gunewardena K., Ritter J. M., Auton T. R., Glover J. F. 2004. Randomized double-blind placebo-controlled study of an angiotensin immunotherapeutic vaccine (PMD3117) in hypertensive subjects. Clin. Sci.(Lond) 107, 167–173 10.1042/CS20030381 (doi:10.1042/CS20030381) [DOI] [PubMed] [Google Scholar]
- 35.Ambuhl P. M., et al. 2007. A vaccine for hypertension based on virus-like particles: preclinical efficacy and phase I safety and immunogenicity. J. Hypertens. 25, 63–72 10.1097/HJH.0b013e32800ff5d6 (doi:10.1097/HJH.0b013e32800ff5d6) [DOI] [PubMed] [Google Scholar]
- 36.Tissot A. C., et al. 2008. Effect of immunisation against angiotensin II with CYT006-AngQb on ambulatory blood pressure: a double-blind, randomised, placebo-controlled phase IIa study. Lancet 371, 821–827 10.1016/S0140-6736(08)60381-5 (doi:10.1016/S0140-6736(08)60381-5) [DOI] [PubMed] [Google Scholar]
- 37.Kahn S. E., Hull R. L., Utzschneider K. M. 2006. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature 444, 840–846 10.1038/nature05482 (doi:10.1038/nature05482) [DOI] [PubMed] [Google Scholar]
- 38.Bendtzen K., Mandrup-Poulsen T., Nerup J., Nielsen J. H., Dinarello C. A., Svenson M. 1986. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science 232, 1545–1547 10.1126/science.3086977 (doi:10.1126/science.3086977) [DOI] [PubMed] [Google Scholar]
- 39.Dinarello C. A., Donath M. Y., Mandrup-Poulsen T. 2010. Role of IL-1beta in type 2 diabetes. Curr. Opin. Endocrinol. Diabetes Obes. 17, 314–321 [DOI] [PubMed] [Google Scholar]
- 40.Larsen C. M., Faulenbach M., Vaag A., Volund A., Ehses J. A., Seifert B., Mandrup-Poulsen T., Donath M. Y. 2007. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N. Engl. J. Med. 356, 1517–1526 10.1056/NEJMoa065213 (doi:10.1056/NEJMoa065213) [DOI] [PubMed] [Google Scholar]
- 41.Masters S. L., et al. 2010. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat. Immunol. 11, 897–904 10.1038/ni.1935 (doi:10.1038/ni.1935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spohn G., Keller I., Beck M., Grest P., Jennings G. T., Bachmann M. F. 2008. Active immunization with IL-1 displayed on virus-like particles protects from autoimmune arthritis. Eur. J. Immunol. 38, 877–887 10.1002/eji.200737989 (doi:10.1002/eji.200737989) [DOI] [PubMed] [Google Scholar]
- 43.Jennings G. T., Bachmann M. F. 2009. Immunodrugs: therapeutic VLP-based vaccines for chronic diseases. Annu. Rev. Pharmacol. Toxicol. 49, 303–326 10.1146/annurev-pharmtox-061008-103129 (doi:10.1146/annurev-pharmtox-061008-103129) [DOI] [PubMed] [Google Scholar]
- 44.Spohn G., et al. In preparation Preclinical development of a therapeutic vaccine against type II diabetes. [Google Scholar]
- 45.Guler R., Parihar S. P., Spohn G., Johansen P., Brombacher F., Bachmann M. F. Blocking IL-1alpha but not IL-1beta increases susceptibility to chronic Mycobacterium tuberculosis infection in mice. Vaccine 29, 1339–1346 10.1016/j.vaccine.2010.10.045 (doi:10.1016/j.vaccine.2010.10.045) [DOI] [PubMed] [Google Scholar]