Abstract
Context:
Preeclampsia is associated with elevated levels of proinflammatory cytokines, excess decidual macrophages, and dendritic cells. IL-1β- or TNF-α-stimulated leukocyte-free first trimester decidual cells produced abundant macrophage- and dendritic cell-recruiting chemokines identified in preeclamptic decidua.
Objective:
The relative potency of IL-1β- or TNF-α-induced first trimester decidual cell-secreted chemokines in chemoattracting macrophages or dendritic cells and the signaling pathways involved in the expression of these chemokines were evaluated.
Interventions and Main Outcome Measures:
First trimester decidual cells were treated with estradiol + medroxyprogesterone acetate ± IL-1β or TNF-α. The chemotaxis assay was performed by incubating conditioned medium from first trimester decidual cells with neutralizing antibody for six chemokines. The activation of each signaling pathway was examined by Western blotting, flow cytometry, confocal microscopy, and ELISA with or without kinase and nuclear factor κB (NFκB) inhibitors.
Results:
Neutralization of CCL2 and CCL5 significantly reduced chemotaxis of monocyte and dendritic cells up to 50 and 36%, respectively. NFκB and MAPK (MAPK kinase, JUN NH2-terminal kinase, p38 kinase) pathways were activated by IL-1β or TNF-α in first trimester decidual cells. In IL-1β- or TNF-α-stimulated first trimester decidual cells, NFκB inhibitor suppressed production of all six chemokines; JUN NH2-terminal kinase inhibitor inhibited secretion of CCL2, CCL4, and CCL5; and MAPK kinase and p38 inhibitor decreased production of CXCL8.
Conclusions:
Up-regulation of CCL2 and CCL5 by first trimester decidual cells in response to proinflammatory stimuli may account for the accumulation of macrophages and dendritic cells in preeclamptic decidua. These chemokines and underlying IL-1β- or TNF-α-induced signaling molecules are potential diagnostic and therapeutic targets for preeclampsia.
A successful pregnancy requires subtle adjustments of the maternal immunity that enables it to tolerate the fetal semi-allograft, yet defend against potential pregnancy-terminating pathogens present in the urogenital tract. Macrophages (Mφ) and dendritic cells (DC) mediate this somewhat contradictory yet complementary action by acting as major antigen-presenting cells that protect against pathogen invasion and provide a link between the innate and adaptive immune systems.
In early pregnancy, blastocyst-derived extravillous trophoblasts (EVT) invade underlying decidua and remodel spiral arteries into high conductance vessels that enhance uteroplacental blood flow to the developing fetal-placental unit (1). These events are accompanied by decidual infiltration of uterine natural killer cells (NK) (2), Mφ (3, 4), and DC (5, 6). Most studies have focused on the role of uterine NK in promoting angiogenic remodeling of decidual vasculature (7). However, recognition is increasing that Mφ and DC play roles in promoting placental development, angiogenesis, and decidual remodeling by secreting vascular endothelial growth factor, matrix metalloproteinases, fibroblast growth factor, fibronectin, collagen, TGF-β, IL-8, and sFlt-1 (8, 9). Studies from our laboratory (5) and others (10, 11) indicate that preeclampsia is accompanied by a decidual excess of Mφ and, as we reported, of DC (12). A significant subset of cases of preeclampsia are associated with underlying maternal infections accompanied by elevated levels of the proinflammatory cytokines, IL-1β and TNF-α (13). Specifically, Mφ-derived TNF-α has been shown to impair trophoblast invasiveness by inducing trophoblast apoptosis and/or by enhancing expression of plasminogen activator inhibitor-1, which inhibits urokinase-mediated extracellular matrix degradation at the leading edge of trophoblast invasion (14–16). In vitro, our laboratory confirmed that Mφ can impair trophoblast invasion (5).
Chemokines are 8- to 14-kDa cytokines required for the recruitment of immune cells to the site of infection or inflammation. As a mediator for cell recruitment, chemokines are known to be important in inflammatory disease, including rheumatoid arthritis, osteoarthritis, inflammatory bowel disease, multiple sclerosis, and transplant rejection (17, 18). Our previous studies revealed that resident decidual cells, the predominant cell type encountered by invading EVT, produced tremendous amounts of such monocyte (MO)/Mφ- and DC-recruiting chemokines as CCL2, CCL20, CCL4, CCL5, CCL7, and CXCL8 in response to IL-1β and TNF-α stimulation (19). Moreover, elevated levels of these chemokines were also observed in decidual cells of preeclamptic vs. gestational age-matched control patients by immunostaining and parallel ELISA measurements of decidual extracts, thereby emphasizing the involvement of Mφ, DC, and their recruiting chemokines in the pathology of preeclampsia (19). These preeclampsia-related chemokines not only exhibit redundant activity, but are also promiscuous because several of these are chemoattractants for Mφ as well as immature DC (iDC) (20, 21). Moreover, nuclear factor κB (NFκB), MAPK, and phosphatidylinositol 3-kinases (PI3K) signaling pathways have all been shown to mediate IL-1β- and TNF-α-induced synthesis of these chemokines in other cell types (22, 23). However, this role has not been assessed in primary human decidual cells. Therefore, the current study sought first to identify the specific chemokine(s) that bear the primary responsibility for mediating Mφ and DC excess that characterizes the preeclamptic decidua; and second, to identify the specific signaling pathway(s) that mediate the synthesis of the identified chemokines. We provide evidence that IL-1β and TNF-α signal mainly through NFκB and MAPK pathways to induce MO/Mφ- and DC-recruiting chemokine production in first trimester decidual cells. Among these chemokines, CCL2 is the main chemoattractant for MO, and CCL5 is the main chemoattractant for iDC.
Materials and Methods
Primary cell culture of first trimester decidual cell
First trimester decidual cells were isolated and cultured as previously described (19). Briefly, decidual specimens from elective termination of 6- to 12-wk gestation were obtained under Institutional Review Board approval at Bellevue Hospital (New York, NY). Tissue was minced and digested, followed by purification on 60/50/40% Percoll gradient. The decidual cells were cultured in T75 flasks until they were 95–100% confluent. The cells were then propagated until free of immune cell contamination. The purity of cells was determined by flow cytometric analysis of CD14 and CD45 expression. The leukocyte-free confluent cells were primed with estradiol (10−8 m) + medroxyprogesterone acetate (10−7 m) for 7 d. Decidualization-related changes including enhanced expression of prolactin and plasminogen activator inhibitor-1, as well as inhibited expression of interstitial collagenase and stromelysin-1, were examined (data not shown). These cells were stimulated in serum free medium ± 10 ng/ml IL-1β or TNF-α (R&D Systems, Minneapolis, MN) for 24 h. The conditioned medium (CM) was stored at −80 C for further usage. Distinct batches of the primary first trimester decidual cells from different patients were used in different replicates of chemotaxis assay, ELISA, Western blot, and immunostaining.
Immune cell culture
Peripheral blood mononuclear cells from healthy reproductive-age female donors were collected from the interphase after Ficoll–Hypaque (GE Healthcare, Piscataway, NJ) centrifugation. MO were purified with anti-CD14 paramagnetic beads from Miltenyi Biotec (Auburn, CA) according to the manufacturer's instructions. The purity of MO was determined by flow cytometric analysis, confirming that at least 95% purity was achieved. iDC were differentiated in vitro by culturing MO with 50 ng/ml granulocyte-Mφ colony-stimulating factor and 20 ng/ml IL-4 (R&D Systems) in AIM-V medium (Invitrogen, Carlsbad, CA) for 5 d. Fresh medium containing equal amounts of granulocyte-Mφ colony-stimulating factor and IL-4 was added on d 3 (20% vol). After 5 d of culture, the loosely attached cells were harvested, and the viability was examined by trypan blue exclusion (>95%). The purity of iDC determined by flow cytometric analysis of CD11C and CCR6 expression was greater than 90%. The maturity of DC was confirmed by the assessment of such maturation markers as CD80, CD83, CD86, and HLA-DR. In parallel cultures, cells activated with 100 ng/ml lipopolysaccharide (LPS) for 24 h served as a positive control. The THP-1 cells, a human monocytic leukemic cell line (24), were grown in RPMI 1640 medium supplemented with 10% fetal calf serum. The Mφ and DC used in each replicate were also developed from MO isolated from different donors.
Chemotaxis assay
The chemotactic effects of CM were assayed using THP-1 cells, MO, or iDC as previously described. Briefly, 5 × 105 THP-1 cells or MO in 400 μl Opti-MEM (Invitrogen) or 1 × 105 iDC in 300 μl Opti-MEM were placed in cell culture inserts with 8.0 μm polyethylene terephthalate membranes (BD Biosciences, San Jose, CA). The inserts were placed in 24-well plates containing 500 μl CM treated with or without neutralizing antibodies. CM from IL-1β- or TNF-α-treated first trimester decidual cell cultures were incubated with anti-CCL2 (monocyte chemotactic protein-1; 10 μg/ml), -CCL20 (macrophage inflammatory protein-3α; 20 μg/ml), -CCL4 (macrophage inflammatory protein-1β; 5 μg/ml), -CCL5 (regulated upon activation, normal T-cell expressed, and secreted; 10 μg/ml), -CCL7 (monocyte chemotactic protein-3; 10 μg/ml), or -CXCL8 (IL-8; 128 μg/ml) neutralizing antibody, or a combination of all of the antibodies (antibodies cocktail) (R&D Systems) for 1 h at room temperature. The antibody concentrations used were optimized based on chemokine levels in the CM and the neutralization curve of each neutralizing antibody from the manufacturer. A dose-dependent study for chemotaxis assay was performed with THP-1 cells to determine the appropriate concentration of each antibody. An excess amount of neutralizing antibody was added to all of the chemotaxis studies. The corresponding IgG isotype was used as a negative control. Anti-CXCL8 and -CCL20 were only used for THP-1/MO or DC, respectively. After 5 or 3 h of incubation at 37 C in 5% CO2, the cells remaining in the inserts were aspirated, and the migrated cells were harvested by washing the bottom of the inserts with CM in the well. The migrated cells in the CM were then counted for 1 min with FACSCalibur using CellQuest software (BD Biosciences, Franklin Lakes, NJ). The percentage inhibition by neutralizing antibody was calculated as follows: (cell number chemoattracted by CM − cell number chemoattracted by neutralizing antibody treated CM/cell number chemoattracted by CM) × 100.
Signaling pathways of chemokine production
To study the signaling pathway of IL-1β- and TNF-α-induced chemokine production, first trimester decidual cells were preincubated with MAPK kinase (MEK) inhibitor (PD980589, 20 μm), JUN NH2-terminal kinase (JNK) inhibitor (SP600125, 20 μm), p38 kinase inhibitor (SB203580, 1 μm) (Sigma-Aldrich Co., St. Louis, MO), NFκB inhibitor (BAY11-7082, 5 μm), or PI3K inhibitor (Wortmannin, 5 μm) (Invitrogen), for 1 h before stimulation with IL-1β or TNF-α for 5, 10, or 30 min. CM was collected for the measurement of chemokines, including CCL2, CCL20, CCL4, CCL5, CCL7, and CXCL8 using Multiplex-ELISA (Aushon Biosystems, Billerica, MA). Total protein was extracted from whole cell lysate for Western blot analysis.
Western blot
Thirty micrograms of total protein were denatured and separated by 12% SDS-PAGE and transferred to 0.45-μm nitrocellulose membranes. Membranes were blocked with Odyssey blocking buffer (LI-COR, Lincoln, NE) and incubated with mouse antihuman Hsp90, rabbit anti-phospho-MEK1/2 (Ser217/221), anti-MEK1/2, anti-phospho-SAPK/JNK, anti-SAPK/JNK, anti-phospho-NFκB p65 subunit (Ser536) (93H1), anti-NFκB p65 subunit, anti-phospho-p38 MAPK (Thr180/Tyr182), anti-p38 MAPK, anti-phospho-PI3K p85(Tyr458)/p55 (Tyr199), or anti-PI3K p85 (Cell Signaling, Beverly, MA) and followed by incubating with IRDye680-conjugated donkey antimouse IgG or Alexa Fluor 800-conjugated donkey antirabbit IgG (Rockland, Gilbertsville, PA). Signals were scanned using the Odyssey infrared imaging system (LI-COR).
Measurement of phosphorylated protein kinase
First trimester decidual cells were stimulated with or without 10 ng/ml IL-1β or TNF-α for 10 min. The activated cells were harvested, fixed, permeabilized, and incubated with rabbit anti-phospho-MEK1/2 (Ser217/221), anti-phospho-SAPK/JNK, anti-phospho-NFκB p65 (Ser536) (93H1), anti-phospho-p38 MAPK (Thr180/Tyr182), followed by incubating with Alexa Fluor 488-conjugated goat-antirabbit antibody (Cell Signaling) according to the manufacturer's instruction. Finally, the cells were analyzed by FACSCalibur using CellQuest Pro software (BD Biosciences). A total of 104 events were recorded for each sample.
Immunofluorescence microscopy
For the phosphorylated protein staining, first trimester decidual cells were cultured on eight-chamber slides. After stimulation with IL-1β- or TNF-α, cells were fixed, permeabilized, and incubated with rabbit anti-phospho-MEK1/2 (Ser217/221), anti-phospho-SAPK/JNK, anti-phospho-NFκB p65 (Ser536) (93H1), or anti-phospho-p38 MAPK (Thr180/Tyr182) overnight at 4 C. The slides were then incubated with Alexa Fluor 488 rabbit antigoat IgG. The cytoskeletal F-actin was labeled with Alexa Fluor 633 phalloidin (Invitrogen). Nuclei were stained with 4′, 6-diamidino-2-phenylindole (DAPI) using VECTASHIELD Hard + Set mounting medium (Vector Laboratories, Burlingame, CA). The staining was analyzed using Zeiss LSM 510NLO confocal fluorescence microscope (×63 objective) (Zeiss, Thornwood, NY).
Statistical analysis
The results were presented as mean ± sem. A two-sample assuming unequal variances Student t test was applied to test the statistical significance while P < 0.05.
Results
CCL2 is the most dominant IL-1β- or TNF-α-stimulated first trimester decidual cell-secreted chemoattractant for THP-1 cells and MO
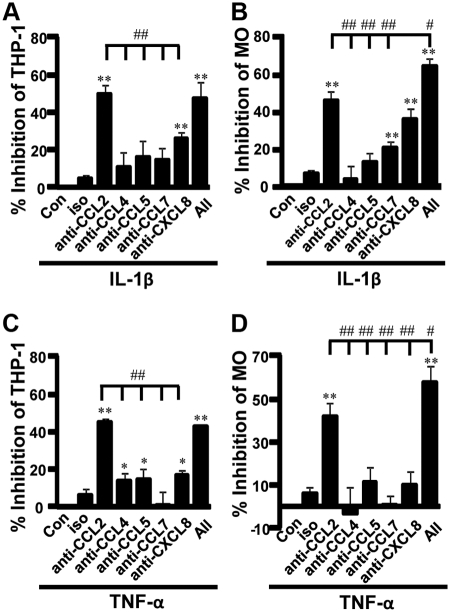
Chemotaxis of THP-1 cells induced by CM from IL-1β-stimulated decidual cells was significantly inhibited by anti-CCL2, anti-CXCL8, and a cocktail of these neutralizing antibodies by 49, 26, and 48%, respectively (Fig. 1A). The inhibition of THP-1 chemotaxis by anti-CCL2 was significantly higher than that by anti-CCL4, -CCL5, -CCL7, and -CXCL8 (Fig. 1A). Peripheral MO were also used to further confirm the chemotactic effects. The chemotaxis in MO induced by CM from IL-1β-stimulated decidual cells was inhibited by anti-CCL2, -CCL7, -CXCL8, and antibody cocktail by 44, 18, 34, and 63%, respectively (Fig. 1B). The inhibition of MO chemotaxis by anti-CCL2 was again significantly greater than that by anti-CCL4, -CCL5, and -CCL7; however, it was lower than the inhibition by the combined antibody cocktail (Fig. 1B).
Fig. 1.
CCL2 is the most potent chemoattractant for THP-1 cells and MO secreted by IL-1β- or TNF-α-stimulated first trimester decidual cells. The relative chemotaxis in THP-1 cells (A and C) or MO (B and D) by chemokines produced by IL-1β- or TNF-α-stimulated first trimester decidual cells, was evaluated by incubation with or without (Con) excess each individual neutralizing antibody to CCL2, CCL4, CCL5, CCL7, CXCL8, or antibody cocktail (All). Cells (5 × 105) placed in upper chambers of 24-well plates were allowed to migrate into antibody-treated CM for 3 h. Migrated cells were then collected and counted with FACSCalibur using CellQuest software. The results were shown as the percentage inhibition by neutralizing antibody. *, P < 0.05; **, P < 0.01, each group vs. Con. #, P < 0.05; ##, P < 0.01, vs. anti-CCL2. Data are shown as mean ± sem (n = 8).
In CM from TNF-α-stimulated first trimester decidual cells, anti-CCL2, anti-CCL4, anti-CCL5, anti-CXCL8, and antibody cocktail significantly inhibited the chemoattraction in THP-1 cells from a low of 13% with anti-CCL4 to a high of 45% with anti-CCL2 (Fig. 1C). The inhibition by anti-CCL2 was significantly greater than that by the other chemokine-neutralizing antibodies, anti-CCL4, -CCL5, -CCL7, and -CXCL8 (Fig. 1C). However, the chemotaxis in peripheral MO was only significantly inhibited by anti-CCL2 (42%) and antibody cocktail (58%) (Fig. 1D). Moreover, the inhibition of MO chemotaxis by anti-CCL2 was also significantly higher than that by anti-CCL4, -CCL5, -CCL7, and -CXCL8, whereas antibody cocktail exhibited the most potent inhibition (Fig. 1D).
CCL5 is the most dominant IL-1β- or TNF-α-stimulated first trimester decidual cell-secreted chemoattractant for iDC
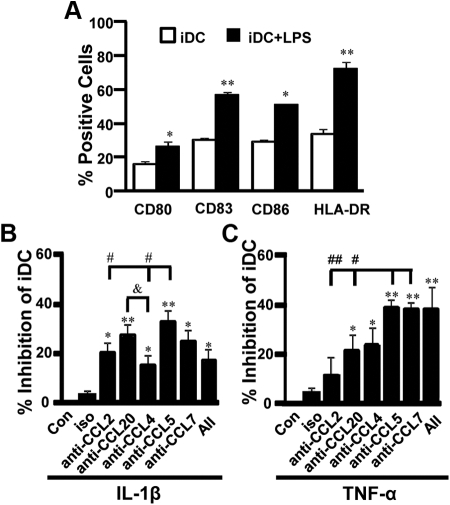
The DC developed in AIM-V serum free medium expressed significantly lower levels of all the maturation markers compared with cells treated with LPS. These observations indicate that the cells used in the DC chemotaxis assay in the current study were iDC (Fig. 2A). The chemotaxis in MO-derived DC induced by CM from IL-1β-stimulated first trimester decidual cells was significantly inhibited by anti-CCL2, -CCL20, -CCL4 -CCL5, -CCL7, and their cocktail by 18, 25, 13, 31, 23, and 16%, respectively (Fig. 2B). The inhibition of chemotaxis by anti-CCL5 was significantly greater than that by anti-CCL2 and -CCL4, but not anti-CCL20 or -CCL7 (Fig. 2B).
Fig. 2.
CCL5 is the most potent chemoattractant for iDC secreted by IL-1β- or TNF-α-stimulated first trimester decidual cells. A, The expression of DC maturation markers in DC treated with or without LPS. The relative chemotaxis in iDC by chemokines produced by IL-1β- (B) or TNF-α (C)-stimulated first trimester decidual cells was evaluated by placing iDC (1 × 105) in the upper chambers of 24-well plates and migrating iDC into CM treated with or without (Con) antibody for 5 h. The results were shown as the percentage inhibition by neutralizing antibody. *, P < 0.05; **, P < 0.01, each group vs. Con. #, P < 0.05; ##, P < 0.01, vs. anti-CCL5 or anti-CCL7. &, P < 0.05 vs. anti-CCL20. Data are shown as mean ± sem (n = 8).
In CM from TNF-α-stimulated cells, the chemotaxis in DC was significantly suppressed by anti-CCL20, -CCL4, -CCL5, -CCL7, and their cocktail from 19 to 36% (Fig. 2C). No significant inhibition was achieved, however, when cells were exposed to anti-CCL2 (Fig. 2C). The inhibition of chemotaxis by anti-CCL5 or anti-CCL7 was significantly greater than that by anti-CCL2 and -CCL20 (Fig. 2C).
Signaling pathways in first trimester decidual cells in response to IL-1β or TNF-α
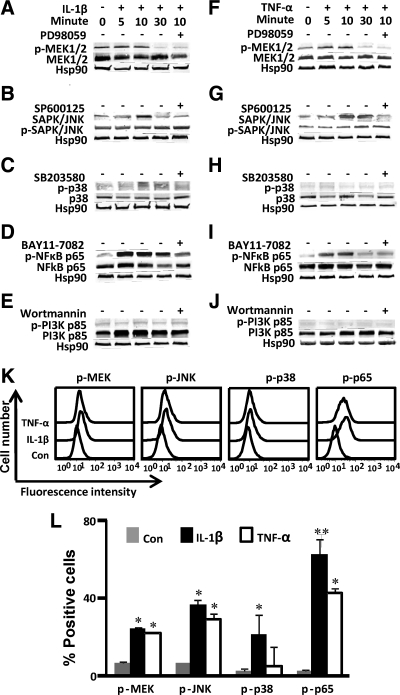
In IL-1β-stimulated cells, the phosphorylation of MEK (Fig. 3A), JNK (Fig. 3B), p38 kinase (Fig. 3C), and NFκB p65 subunit (Fig. 3D), but not PI3K (Fig. 3E), was elevated without any significant changes in the total protein levels. Maximal phosphorylation of MEK, JNK, p38 kinase, and NFκB p65 subunit was observed at 10 min after stimulation. Phosphorylation of MEK (Fig. 3F), JNK (Fig. 3G), and NFκB p65 subunit (Fig. 3I), but not p38 kinase (Fig. 3H) or PI3K (Fig. 3J), was induced without any significant changes in the total protein levels after the treatment of TNF-α. Maximal phosphorylation of these molecules was observed at 10 min after stimulation. To confirm such regulation, first trimester decidual cells were preincubated with MAPK inhibitors and NFκB inhibitor before IL-1β or TNF-α stimulation. MEK inhibitor (PD980589) (Fig. 3, A and F), JNK inhibitor (SP600125) (Fig. 3, B and G), p38 kinase inhibitor (SB203580) (Fig. 3C) and NFκB inhibitor (BAY11-7082) (Fig. 3, D and I) effectively inhibited the phosphorylation of each corresponding molecule.
Fig. 3.
Phosphorylation of MAPK and NFκB p65 in IL-1β- or TNF-α- stimulated first trimester decidual cells. First trimester decidual cells were stimulated with IL-1β (10 ng/ml) or TNF-α (10 ng/ml) for 5, 10, or 30 min. The phosphorylated and total protein of MEK kinase (A and B), JNK kinase (C and D), p38 kinase (E and F), NFκB p65 subunit (G and H), and PI3K kinase (I and J) were identified using Western blotting. K, The intracellular staining of phosphorylated MAPK and NFκB p65 in unstimulated (Con), IL-1β- or TNF-α-stimulated first trimester decidual cells (10 min) was analyzed by FACSCalibur. L, Summary of flow cytometric analysis for panel K. Data are shown as mean ± sem (n = 3). *, P < 0.05; **, P < 0.01, IL-1β or TNF-α vs. Con.
Flow cytometric analysis was employed to semiquantify the phosphorylation of the MAPKs and NFκB p65 subunit. In IL-1β-stimulated cells, the percentage of phosphorylated MEK, JNK, p38 kinase, and NFκB p65 subunit-positive cells was 24.4, 36.4, 21.5, and 62.8%, respectively (Fig. 3, K and L). In TNF-α-stimulated cells, the percentage of phosphorylated MEK, JNK, p38 kinase, and NFκB p65 subunit-positive cells was 22.0, 29.2, 6.54, and 42.7%, respectively (Fig. 3, K and L), whereas in unstimulated decidual cells, the percentage of phosphorylated MEK, JNK, p38 kinase, and NFκB p65 subunit-positive cells was 6.44, 6.47, 2.46, 2.8%, respectively (Fig. 3, K and L). Consistent with Western blotting analysis, the phosphorylation of MEK, JNK kinase, and NFκB p65 subunit was significantly up-regulated in IL-1β- or TNF-α-stimulated first trimester decidual cells; however, the phosphorylation of p38 kinase was relatively low compared with phosphorylated MEK, JNK kinases, and NFκB p65 subunit.
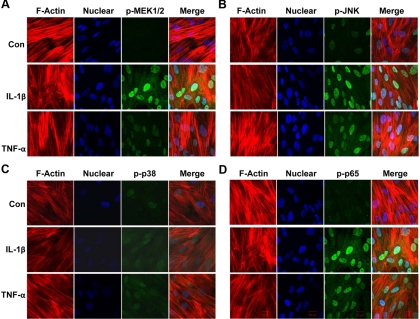
Nuclear translocation of phosphorylated MEK, JNK, p38 kinase, and NFκB p65 subunit was used to confirm the activation of these molecules using confocal microscopy. The cytoskeletal F-actin and nuclei were stained by Alexa Fluor 633 phalloidin (red) and DAPI (blue), respectively. After the treatment of IL-1β or TNF-α, intense staining of phosphorylated MEK (Fig. 4A), JNK (Fig. 4B), and NFκB p65 subunit (Fig. 4D) (green) was found in the nuclei. The nuclear phosphorylated p38 kinase (Fig. 4C) was increased in IL-1β- or TNF-α-stimulated decidual cells compared with control cells, although its intensity was lower than that of phosphorylated MEK, JNK, and NFκB p65 subunit. These results were consistent with the results from Western blotting and flow cytometric analysis.
Fig. 4.
The nuclear expression of phosphorylated MEK, JNK, p38, and NFκB p65 by IL-1β- or TNF-α-treated first trimester decidual cells. First trimester decidual cells were cultured in eight-chamber slides and stimulated with IL1-β or TNF-α for 10 min. The cells were then fixed, permeabilized, and incubated with anti-phospho-MEK1/2 (A), anti-phospho-SAPK/JNK (B), anti-phospho-p38 (C), and anti-phospho-NFκB p65 (D) followed by staining with Alexa Fluor 488 conjugated secondary antibody (green). The cell cytoskeletal F-actin was labeled with Alexa Fluor 633 phalloidin (red). Nuclei were stained with DAPI (blue). The slides were examined by a Zeiss LSM 510NLO confocal fluorescence microscope (×63 objective).
Production of chemokines in IL-1β- or TNF-α-stimulated first trimester decidual cells mainly signals through MAPK and NFκB pathways
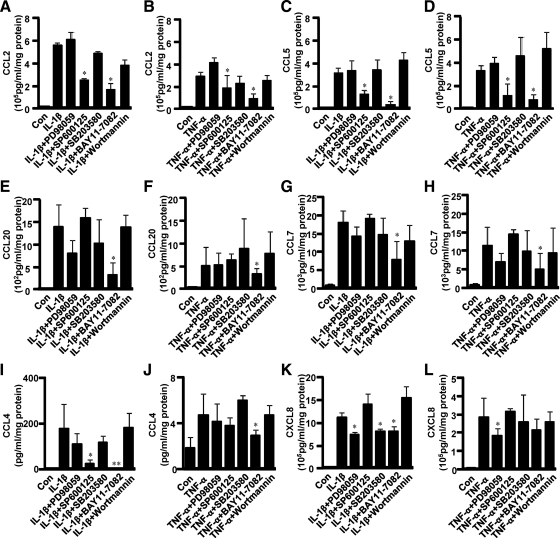
CCL2 (Fig. 5, A and B) and CCL5 production (Fig. 5, C and D) induced by IL-1β or TNF-α was significantly suppressed by the inhibitors of JNK (SP610025) and NFκB (BAY11-7082) from 2- to 14-fold. CCL20 (Fig. 5, E and F) and CCL7 production (Fig. 5, G and H) induced by IL-1β or TNF-α was down-regulated by NFκB inhibitor from 2- to 4-fold. CCL4 production (Fig. 5I) induced by IL-1β was suppressed by JNK inhibitor and NFκB inhibitor by 7- and 95-fold, respectively. TNF-α-induced CCL4 (Fig. 5J) was inhibited by NFκB inhibitor by 1.6-fold. IL-1β-induced CXCL8 production (Fig. 5K) was suppressed by MEK inhibitor (PD98059), p38 inhibitor (SP203580), and NFκB inhibitor, each by 1.5-fold. TNF-α-induced CXCL8 production (Fig. 5L) was reduced by MEK inhibitor by 1.6-fold. By contrast, the PI3K inhibitor (Wortmannin) exhibited no effect on either IL-1β- or TNF-α-induced chemokine expression.
Fig. 5.
Effect of MAPK, NFκB, and PI3K inhibitors on IL-1β- or TNF-α-induced chemokine production in first trimester decidual cells. First trimester decidual cells were preincubated with MEK inhibitor (PD98059), JNK inhibitor (SP600125), p38 inhibitor (SB203580), NFκB inhibitor (BAY11-7082), or PI3K inhibitor (Wortmannin) for 1 h, followed by stimulation with IL-1β (10 ng/ml) or TNF-α (10 ng/ml) for 24 h. Each chemokine, including CCL2 (A and B), CCL5 (C and D), CCL20 (E and F), CCL7 (G and H), CCL4 (I and J), and CXCL8 (K and L), secreted in CM was examined by Multi-ELISA array assay. The data are shown as mean ± sem (n = 3). *, P < 0.05; **, P < 0.01, inhibitor-treated groups vs. IL-1β or TNF-α.
Discussion
Despite intense investigation into the causes of preeclampsia, delivery of the placenta remains the only reliable treatment to relieve its symptoms (13). The root causes of this dilemma lie in the dichotomy between recognition of its symptoms, usually in third trimester, and the initiation of its pathogenesis, which frequently reflects immune maladaptation at the implantation site in the first trimester (25). As mentioned, our previous findings suggested the potential roles of Mφ, DC, and their recruiting chemokines in the pathogenesis of preeclampsia.
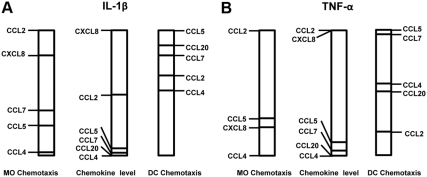
The well-established redundant and promiscuous actions of chemokines in recruiting immune cell types have been an obstacle in identifying individual dominance in situations where several chemokines are up-regulated by proinflammatory stimuli. Seeking to overcome this impediment, the current study evaluated the CM of cultured first trimester decidual cells incubated with IL-1β or TNF-α for: 1) measurement of chemoattractant activities after incubation of CM with antibody to each chemokine or a cocktail of antibodies to simultaneously neutralize all six chemokines; and 2) the measurement of the six chemokines by specific ELISA. Scrutiny of the relative results displayed in Fig. 6, A and B, reveals striking differences between: 1) the levels of a specific chemokine in CM compared with its chemoattractant activity for MO and iDC; 2) the profile of chemokines mediating MO- vs. iDC-recruiting activity; and 3) IL-1β- vs. TNF-α-induced chemokine levels and the different resulting chemoattractant activities.
Fig. 6.
Comparison of chemokine expression vs. the resulting effects on MO and iDC chemotaxis in IL-1β- or TNF-α-stimulated first trimester decidual cells. A, Chemotaxis in MO and iDC induced by CM from IL-1β-stimulated first trimester decidual cells vs. the levels of their recruiting chemokines in the CM. B, Chemotaxis in MO and iDC induced by CM from TNF-α-stimulated first trimester decidual cells vs. the levels of their recruiting chemokines in the CM.
As indicated in Fig. 5, CM from IL-1β-treated decidual cells contained about 2-fold of CXCL8 than CCL2 with much lower levels of CCL5, CCL7, CCL20, and CCL4. Although CXCL8 and CCL2 were both the dominant MO chemoattractants in the decidual cell CM, CCL2 exhibited greater MO chemoattractant activity than CXCL8. CXCL8 has also been shown to recruit NK cells and neutrophils (26, 27). Consistent with their differential roles in mediating immune responses, the profile of IL-1β-enhanced chemokine effects on iDC-recruiting activity was strikingly different than that for MO chemoattraction. The most marked differences were evident in the dominating effects on iDC chemoattraction elicited by CCL5, which displayed only moderate MO chemoattraction, and the minor effect on iDC chemoattraction elicited by CCL2, which was prominent in attracting MO. Incubation of first trimester decidual cells with TNF-α shared many features with IL-1β in terms of the profile of induced chemokine expression and the resulting effects on MO and iDC chemotaxis. However, some additional comparative differences were also evident. These included: 1) lower expression of CCL2, CCL20, CCL4, CCL7, and CXCL8, but higher expression of CCL5 in response to TNF-α than to IL-1β; 2) for MO chemotaxis, a much lower CXCL8-mediated response elicited by TNF-α than by IL-1β; 3) for iDC chemotaxis, a greater relative effect of TNF-α on CCL7 mediation and a reciprocally lower effect on CCL20 mediation. In addition to chemoattractant activities, several chemokines have distinct roles in the activation of Mφ and DC (28). Therefore, the implication of chemokines secreted by first trimester decidual cells on the activation of Mφ and DC in the pathology of preeclampsia needs to be further determined. In cultures of several cell types, IL-1β and TNF-α affect the expression of numerous chemokines mediated by NFκB, MAPK, and PI3K signaling pathways (22, 23, 29). Our observations reveal that IL-1β and TNF-α signal through NFκB and MAPK (including JNK, MEK, and p38 kinase), but not PI3K, pathways to regulate downstream gene expression in decidual cells. We also show for the first time that NFκB is the dominant pathway responsible for the production of six MO/Mφ- and DC-recruiting chemokines tested here in IL-1β- or TNF-α-stimulated first trimester decidual cells. In activating these signaling pathways, IL-1β proved to be more effective than TNF-α, which may reflect the relatively lower chemokine levels induced by TNF-α (Fig. 6). Compared with NFκB and MAPK signaling pathways, little attention has focused on PI3K as a downstream effector of IL-1β. Although NFκB is an important downstream target of PI3K pathway signaled via AKT in some cell types (30), treatment of decidual cells with PI3K inhibitor did not attenuate IL-1β- or TNF-α-induced chemokine production, indicating that NFκB activation is independent of PI3K in first trimester decidual cells. Compared with studies in EVT stimulated by TNF-α (31), the current study shows that NFκB is uniquely involved in the signaling pathways induced by IL-1β and TNF-α in first trimester decidual cells.
At the fetal-maternal interface, decidual cells, immune cells, as well as villous and extravillous trophoblasts have been shown to express IL-1β, TNF-α, interferon-γ, and their receptors. Among these, IL-1β was found to promote EVT invasion by enhancing matrix metalloproteinase activity and increasing leptin secretion by decidual cells (32). By contrast, TNF-α added alone or in combination with interferon-γ, was shown to inhibit EVT invasion (33, 34). These reports suggest that the balance in the local cytokine milieu may be critical in determining the effectiveness of EVT invasion. In addition, several cell types in both the fetal and maternal compartments were found to express an array of chemokines (31, 35–37). Among these, CCL4, CCL5, CXCL12, CXCL10, and CXCL11 are potent NK cell chemoattractants. CCL2, CCL5, and CCL7 are potent MO chemoattractants, and CCL4, CCL5, CCL7, CCL20, and CCL21 have been shown to chemoattract DC. Alternatively, CX3CL1, CCL14, and CCL4 have been shown to promote EVT migration (36). Moreover, peripheral endothelial progenitor cells that are ultimately involved in angiogenesis and vasculogenesis display chemokine receptors CCR2 and CCR5 (38), indicating that chemokines secreted by first trimester decidual cells in our study such as CCL2, CCL5, and CCL7 may chemoattract endothelial progenitor cells to the implantation sites. In the decidua, EVT, NK cells, as well as Mφ express a repertoire of chemokine receptors known to bind to all of the chemokines described above. Taken together, the results described above indicate that balanced expression of an array of cytokines and chemokines present at the fetal-maternal interface play important roles in recruiting immune cells, especially NK cells and MO/Mφ, and in directing EVT invasion that are crucial in promoting normal periimplantational events. Conversely, abnormally high placental and serum level of IL-1β, TNF-α, and specific chemokines, such as CCL2, CXCL8, and CXCL10, are associated with such pregnancy complications as preterm birth, intrauterine growth restriction and preeclampsia (24, 39). Consistently, the results from the current study and our recent publications indicate that incubation of leukocyte-free first trimester decidual cells, the major cell type at the fetal-maternal interface, with IL-1β or TNF-α markedly enhances expression of several chemokines responsible for elevated numbers of leukocytes, especially Mφ and DC, at the implantation site, thereby constituting a major influence in determining the balance between normal and abnormal periimplantational events.
Normal pregnancy represents a mild inflammatory state, and exaggerated inflammation is characteristic of several adverse pregnancy outcomes. Our results suggest that after activation with proinflammatory cytokines, decidual cells produce potent MO/Mφ- and iDC-recruiting chemokines, particularly CCL2 and CCL5. Upon activation, Mφ and DC secrete an array of proinflammatory cytokines, which in turn chemoattract and activate additional Mφ/MO and DC to promote a local proinflammatory feedforward cycle. Consistent with the observation of up-regulation of proinflammatory cytokines and chemokines in preeclamptic women (39) and an animal model of preeclampsia (40), the current study sheds further light on the mechanistic roles of CCL2 and CCL5 during the pathogenesis of preeclampsia. Moreover, these findings suggest the possibility that abnormal levels of CCL2 and CCL5 in maternal circulation may serve as potential early markers for clinical diagnosis (39).
In conclusion, our results reveal key differential responses to IL-1β and TNF-α between levels of specific chemokines in leukocyte-free first trimester decidual cells and their chemotactic effects for Mφ/MO and iDC that could impact on the pathogenesis of preeclampsia. Our data also demonstrate that decidual cell-secreted CCL2 and CCL5 may play an essential role in the recruitment of MO and DC, respectively, to preeclamptic decidua in vivo. Moreover, these chemokines, especially CCL2 and CCL5, share similarities in NFκB and JNK/MAPK signaling pathways in first trimester decidual cells. These findings suggest that therapies aimed to neutralize the chemokines and/or the underlining signaling pathways may be effective in treating preeclampsia.
Acknowledgments
We are very grateful to Dr. Fred Schatz and Dr. Salley Pels for their critical suggestions and editing of the manuscript.
This work is supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health Grant 5R01HD056123 (to S.J.H.).
Disclosure Summary: None of the authors disclose any conflict of interests.
Footnotes
- CM
- Conditioned medium
- DAPI
- 4′, 6-diamidino-2-phenylindole
- DC
- dendritic cell
- EVT
- extravillous trophoblast
- iDC
- immature DC
- JNK
- JUN NH2-terminal kinase
- LPS
- lipopolysaccharide
- MEK
- MAPK kinase
- MO
- monocyte
- Mφ
- macrophage
- NFκB
- nuclear factor κB
- NK
- natural killer cell
- PI3K
- phosphatidylinositol 3-kinase.
References
- 1. Reister F, Heyl W, Kaufmann P, Rath W. 1999. [Trophoblast invasion in pre-eclampsia]. Zentralbl Gynakol 121:587–590 [PubMed] [Google Scholar]
- 2. Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, Gazit R, Yutkin V, Benharroch D, Porgador A, Keshet E, Yagel S, Mandelboim O. 2006. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med 12:1065–1074 [DOI] [PubMed] [Google Scholar]
- 3. Baines MG, Duclos AJ, Antecka E, Haddad EK. 1997. Decidual infiltration and activation of macrophages leads to early embryo loss. Am J Reprod Immunol 37:471–477 [DOI] [PubMed] [Google Scholar]
- 4. Reister F, Frank HG, Heyl W, Kosanke G, Huppertz B, Schröder W, Kaufmann P, Rath W. 1999. The distribution of macrophages in spiral arteries of the placental bed in pre-eclampsia differs from that in healthy patients. Placenta 20:229–233 [DOI] [PubMed] [Google Scholar]
- 5. Huang SJ, Chen CP, Schatz F, Rahman M, Abrahams VM, Lockwood CJ. 2008. Pre-eclampsia is associated with dendritic cell recruitment into the uterine decidua. J Pathol 214:328–336 [DOI] [PubMed] [Google Scholar]
- 6. Plaks V, Birnberg T, Berkutzki T, Sela S, BenYashar A, Kalchenko V, Mor G, Keshet E, Dekel N, Neeman M, Jung S. 2008. Uterine DCs are crucial for decidua formation during embryo implantation in mice. J Clin Invest 118:3954–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Li XF, Charnock-Jones DS, Zhang E, Hiby S, Malik S, Day K, Licence D, Bowen JM, Gardner L, King A, Loke YW, Smith SK. 2001. Angiogenic growth factor messenger ribonucleic acids in uterine natural killer cells. J Clin Endocrinol Metab 86:1823–1834 [DOI] [PubMed] [Google Scholar]
- 8. Kammerer U, Kruse A, Barrientos G, Arck PC, Blois SM. 2008. Role of dendritic cells in the regulation of maternal immune responses to the fetus during mammalian gestation. Immunol Invest 37:499–533 [DOI] [PubMed] [Google Scholar]
- 9. Renaud SJ, Graham CH. 2008. The role of macrophages in utero-placental interactions during normal and pathological pregnancy. Immunol Invest 37:535–564 [DOI] [PubMed] [Google Scholar]
- 10. Kim JS, Romero R, Cushenberry E, Kim YM, Erez O, Nien JK, Yoon BH, Espinoza J, Kim CJ. 2007. Distribution of CD14+ and CD68+ macrophages in the placental bed and basal plate of women with preeclampsia and preterm labor. Placenta 28:571–576 [DOI] [PubMed] [Google Scholar]
- 11. Rieger L, Honig A, Sütterlin M, Kapp M, Dietl J, Ruck P, Kämmerer U. 2004. Antigen-presenting cells in human endometrium during the menstrual cycle compared to early pregnancy. J Soc Gynecol Investig 11:488–493 [DOI] [PubMed] [Google Scholar]
- 12. Huang SJ, Zenclussen AC, Chen CP, Basar M, Yang H, Arcuri F, Li M, Kocamaz E, Buchwalder L, Rahman M, Kayisli U, Schatz F, Toti P, Lockwood CJ. 2010. The implication of aberrant GM-CSF expression in decidual cells in the pathogenesis of preeclampsia. Am J Pathol 177:2472–2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sibai B, Dekker G, Kupferminc M. 2005. Pre-eclampsia. Lancet 365:785–799 [DOI] [PubMed] [Google Scholar]
- 14. Bauer S, Pollheimer J, Hartmann J, Husslein P, Aplin JD, Knöfler M. 2004. Tumor necrosis factor-α inhibits trophoblast migration through elevation of plasminogen activator inhibitor-1 in first-trimester villous explant cultures. J Clin Endocrinol Metab 89:812–822 [DOI] [PubMed] [Google Scholar]
- 15. Reister F, Frank HG, Kingdom JC, Heyl W, Kaufmann P, Rath W, Huppertz B. 2001. Macrophage-induced apoptosis limits endovascular trophoblast invasion in the uterine wall of preeclamptic women. Lab Invest 81:1143–1152 [DOI] [PubMed] [Google Scholar]
- 16. Renaud SJ, Postovit LM, Macdonald-Goodfellow SK, McDonald GT, Caldwell JD, Graham CH. 2005. Activated macrophages inhibit human cytotrophoblast invasiveness in vitro. Biol Reprod 73:237–243 [DOI] [PubMed] [Google Scholar]
- 17. Baggiolini M, Dewald B, Moser B. 1997. Human chemokines: an update. Annu Rev Immunol 15:675–705 [DOI] [PubMed] [Google Scholar]
- 18. Gerard C, Rollins BJ. 2001. Chemokines and disease. Nat Immunol 2:108–115 [DOI] [PubMed] [Google Scholar]
- 19. Huang SJ, Schatz F, Masch R, Rahman M, Buchwalder L, Niven-Fairchild T, Tang C, Abrahams VM, Krikun G, Lockwood CJ. 2006. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Reprod Immunol 72:60–73 [DOI] [PubMed] [Google Scholar]
- 20. de la Rosa G, Longo N, Rodríguez-Fernández JL, Puig-Kroger A, Pineda A, Corbí AL, Sánchez-Mateos P. 2003. Migration of human blood dendritic cells across endothelial cell monolayers: adhesion molecules and chemokines involved in subset-specific transmigration. J Leukoc Biol 73:639–649 [DOI] [PubMed] [Google Scholar]
- 21. Thorley AJ, Goldstraw P, Young A, Tetley TD. 2005. Primary human alveolar type II epithelial cell CCL20 (macrophage inflammatory protein-3α)-induced dendritic cell migration. Am J Respir Cell Mol Biol 32:262–267 [DOI] [PubMed] [Google Scholar]
- 22. Cahill CM, Rogers JT. 2008. Interleukin (IL) 1β induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IκB kinase α pathway targeting activator protein-1. J Biol Chem 283:25900–25912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim MO, Suh HS, Brosnan CF, Lee SC. 2004. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-β. J Neurochem 90:297–308 [DOI] [PubMed] [Google Scholar]
- 24. Tosun M, Celik H, Avci B, Yavuz E, Alper T, Malatyalioðlu E. 2010. Maternal and umbilical serum levels of interleukin-6, interleukin-8, and tumor necrosis factor-α in normal pregnancies and in pregnancies complicated by preeclampsia. J Matern Fetal Neonatal Med 23:880–886 [DOI] [PubMed] [Google Scholar]
- 25. Redman CW, Sargent IL. 2005. Latest advances in understanding preeclampsia. Science 308:1592–1594 [DOI] [PubMed] [Google Scholar]
- 26. De Oliveira LG, Lash GE, Murray-Dunning C, Bulmer JN, Innes BA, Searle RF, Sass N, Robson SC. 2010. Role of interleukin 8 in uterine natural killer cell regulation of extravillous trophoblast cell invasion. Placenta 31:595–601 [DOI] [PubMed] [Google Scholar]
- 27. Gupta AK, Hasler P, Holzgreve W, Hahn S. 2007. Neutrophil NETs: a novel contributor to preeclampsia-associated placental hypoxia? Semin Immunopathol 29:163–167 [DOI] [PubMed] [Google Scholar]
- 28. Jimenez F, Quinones MP, Martinez HG, Estrada CA, Clark K, Garavito E, Ibarra J, Melby PC, Ahuja SS. 2010. CCR2 plays a critical role in dendritic cell maturation: possible role of CCL2 and NF-κB. J Immunol 184:5571–5581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Inoue Y, Tsushima H, Ando K, Sawayama Y, Sakai M, Yamasaki R, Matsuo E, Tsutsumi C, Imaizumi Y, Iwanaga M, Imanishi D, Taguchi J, Miyazaki Y, Tomonaga M. 2006. Chemokine expression in human erythroid leukemia cell line AS-E2: macrophage inflammatory protein-3α/CCL20 is induced by inflammatory cytokines. Exp Hematol 34:19–26 [DOI] [PubMed] [Google Scholar]
- 30. Kane LP, Shapiro VS, Stokoe D, Weiss A. 1999. Induction of NF-κB by the Akt/PKB kinase. Curr Biol 9:601–604 [DOI] [PubMed] [Google Scholar]
- 31. Renaud SJ, Sullivan R, Graham CH. 2009. Tumour necrosis factor α stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta 30:313–319 [DOI] [PubMed] [Google Scholar]
- 32. Fontana VA, Sanchez M, Cebral E, Calvo JC. 2010. Interleukin-1 β regulates metalloproteinase activity and leptin secretion in a cytotrophoblast model. Biocell 34:37–43 [PubMed] [Google Scholar]
- 33. Jokhi PP, King A, Loke YW. 1997. Cytokine production and cytokine receptor expression by cells of the human first trimester placental-uterine interface. Cytokine 9:126–137 [DOI] [PubMed] [Google Scholar]
- 34. Otun HA, Lash GE, Innes BA, Bulmer JN, Naruse K, Hannon T, Searle RF, Robson SC. 2011. Effect of tumour necrosis factor-α in combination with interferon-γ on first trimester extravillous trophoblast invasion. J Reprod Immunol 88:1–11 [DOI] [PubMed] [Google Scholar]
- 35. Hanna J, Wald O, Goldman-Wohl D, Prus D, Markel G, Gazit R, Katz G, Haimov-Kochman R, Fujii N, Yagel S, Peled A, Mandelboim O. 2003. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16- human natural killer cells. Blood 102:1569–1577 [DOI] [PubMed] [Google Scholar]
- 36. Hannan NJ, Jones RL, White CA, Salamonsen LA. 2006. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod 74:896–904 [DOI] [PubMed] [Google Scholar]
- 37. Red-Horse K, Drake PM, Fisher SJ. 2004. Human pregnancy: the role of chemokine networks at the fetal-maternal interface. Expert Rev Mol Med 6:1–14 [DOI] [PubMed] [Google Scholar]
- 38. Spring H, Schüler T, Arnold B, Hämmerling GJ, Ganss R. 2005. Chemokines direct endothelial progenitors into tumor neovessels. Proc Natl Acad Sci USA 102:18111–18116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Szarka A, Rigó J, Jr, Lázár L, Beko G, Molvarec A. 2010. Circulating cytokines, chemokines and adhesion molecules in normal pregnancy and preeclampsia determined by multiplex suspension array. BMC Immunol 11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tinsley JH, Chiasson VL, South S, Mahajan A, Mitchell BM. 2009. Immunosuppression improves blood pressure and endothelial function in a rat model of pregnancy-induced hypertension. Am J Hypertens 22:1107–1114 [DOI] [PubMed] [Google Scholar]