Abstract
Context:
Recent prospective clinical trials have failed to confirm a unique benefit from normalization of glycemia on cardiovascular disease outcomes, despite evidence from basic vascular biology, epidemiological, and cohort studies.
Evidence Acquisition:
The literature was searched using the http://www.ncbi.nlm.nih.gov search engine including over 20 million citations on MEDLINE (1970 to present). Keyword searches included: atherosclerosis, cardiovascular, and glucose. Epidemiological, cohort, and interventional data on cardiovascular disease outcomes and glycemic control were reviewed along with analysis of recent reviews on this topic.
Evidence Synthesis:
High glucose activates a proatherogenic phenotype in all cell types in the vessel wall including endothelial cells, vascular smooth muscle cells, inflammatory cells, fibroblasts, and platelets, leading to a feedforward atherogenic response.
Epidemiological and Cohort Studies:
Epidemiological and cohort evidence indicates a clear and consistent correlation of glycemia with cardiovascular disease. A recent report of over 25,000 subjects with diabetes in the Swedish National Diabetes Registry verifies this relationship in contemporary practice.
Interventional Studies:
Prospective randomized interventions targeting a hemoglobin A1c of 6–6.5% for cardiovascular disease prevention failed to consistently decrease cardiovascular events or all-cause mortality.
Conclusions:
Basic vascular biology data plus epidemiological and cohort evidence would predict that glucose control should impact cardiovascular events. Prospective clinical trials demonstrate that current strategies that improve blood glucose do not achieve this goal but suggest that a period of optimal control may confer long-term cardiovascular disease benefit. Clinicians should target a hemoglobin A1c of 7% for the prevention of microvascular complications, individualized to avoid hypoglycemia.
The topic of this review is the role of glycemia in the cardiovascular complications of diabetes. We will present the irrefutable evidence that carbohydrate intolerance, with the concomitant abnormalities in glucose, protein, and free fatty acid metabolism, contributes to accelerated atherosclerosis in diabetes. We will discuss the mechanisms whereby hyperglycemia contributes to vascular disease. Next, we will provide a summary of the historical, epidemiological, and cohort studies suggesting a relationship between glycemia and cardiovascular events, also supporting a positive relationship between glucose and atherosclerosis. We will close with recent prospective clinical intervention studies indicating that the currently available strategies for aggressive glycemic control, which are metabolically imperfect, do not confer consistent benefit beyond “good” control in the context of optimized cholesterol and blood pressure management. This leaves us with a hemoglobin A1c of 7%, or as low as possible without hypoglycemia, as our target. As such, this issue may be moot because a target hemoglobin A1c of 7% is already our clinical objective to minimize the diabetes-specific microvascular complications including retinopathy, nephropathy, and neuropathy. The thesis of this review is that poor glucose control in diabetes accelerates atherosclerosis, but our current strategies for glycemic control fail to significantly impact cardiovascular disease (CVD) risk.
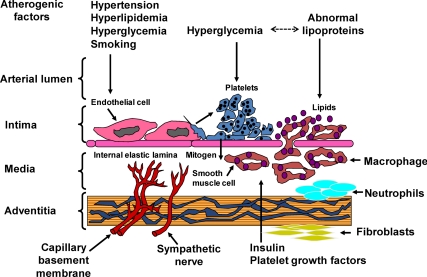
Overview of the Pathogenesis of Atherosclerosis and Mechanisms for the Impact of Glucose (Fig. 1)
Fig. 1.
Pathogenesis of atherosclerosis in the context of diabetes. [Adapted with permission from J. A. Colwell et al.: Am J Med 75:67–80, 1983 (4). © Elsevier.]
Atherosclerosis involves each of the cells in the vascular wall in a complex dysfunctional response to environmental stimuli (reviewed in Refs. 1 and 2). In healthy blood vessels, the endothelium regulates vasodilatation via nitric oxide production, repels platelets and immune cell adherence, blocks low-density lipoprotein (LDL) entrance into the subendothelial space, produces the antithrombotic protein tissue plasminogen activator (tPA), and maintains vascular smooth muscle cell quiescence, a contractile and nonproliferative phenotype (reviewed in Ref. 3). When the vasculature is challenged by proatherosclerotic stimuli such as hypertension, dyslipidemia, cigarette smoking, diabetes, or inflammation, the function of the endothelium changes completely by 180 degrees to a pathogenic phenotype. For example, efficient release of nitric oxide is impaired by reactive oxidant species, thereby losing endothelium-dependent relaxation. In addition, endothelial cells express adhesion molecules that attract and activate platelets and monocytes/macrophages. Endothelial cell damage secondary to proatherosclerotic stimuli induces loss of endothelial cell tight junctions permitting egress of LDL and modified LDL into the subendothelial space. Injured endothelium diminishes the production of tPA and produces plasminogen activator inhibitor-1 (PAI-1), a prothrombotic factor. Endothelial cells, activated platelets, and macrophages all produce cytokines and chemokines that attract additional inflammatory cells and stimulate proliferation and migration of vascular smooth muscle cells. Endothelial dysfunction sets into motion a feedforward cycle that leads to vascular inflammation, foam cell formation, thrombosis, and neointimal proliferation. To address the importance of glycemia as a contributor to the atherosclerosis, we will outline published work examining the impact of glucose on the processes outlined above.
Many studies have explored the specific impact of glucose on components of the vessel wall (Fig. 1) (4). In vivo exposure of healthy human subjects to acute glucose elevation leads to endothelial dysfunction as measured by endothelium-dependent vasomotion, oxidative stress, increased adhesion molecule expression, increased vascular permeability, and secretion of PAI-1 (5–8). In vitro, high glucose impairs insulin stimulation of endothelial nitric oxide synthase, increases PAI-1 expression, and decreases tPA expression in most studies (9, 10). Increased inflammation, adhesion molecule expression and growth factor production are also observed (11, 12). Hyperglycemia accelerates platelet turnover and activity (reviewed in Ref. 13). Analysis of the impact of high glucose on microvascular and macrovascular gap junction integrity reveals increased vascular permeability and decreases in connexin expression and function (14–16). Ion channels such as Ca(2+)-activated K(+) (big potassium) channels also become dysfunctional with exposure to streptozotocin-induced diabetes (17). This leads to abnormal calcium currents and sparks and consequent increased vascular tone (17). In addition, vascular smooth muscle cells exposed to hyperglycemia or advanced glycation end-products (AGE) express chemokines including macrophage chemotactic protein-1 and fractalkine, which increase vascular binding and activation of monocytes/macrophages (18). A number of recent studies have demonstrated that glucose elevation leads to epigenetic changes in gene promoters resulting in, for example, persistent inflammation despite only transient exposure to high glucose (19). In one report, H3 lysine-9 trimethylation regulatory region of proinflammatory genes such as macrophage chemotactic protein-1 and IL-6 led to persistent activation of a set of inflammatory genes (20). These new studies on epigenetic changes suggest that the legacy effect (which will be described in Prospective randomized studies correlating early glycemis control with late CVD prevention for clinical trials) may result from epigenetic programming induced by previous hyperglycemia or favorable glycemic control (overview in Ref. 21). In sum, the cellular components of the vasculature respond to high glucose with typical pathogenic changes observed in atherosclerosis.
A short overview of the mechanisms whereby high glucose affects vascular cell phenotype will be provided here (reviewed in Ref. 22). Glucose may enter endothelial cells, monocytes/macrophages, and vascular smooth muscle cells via GLUT-1 (glucose transporter 1), which increases flux through a number of pathological pathways. Glucose is in equilibrium with cellular and intravascular proteins. Hyperglycemia leads to binding of glucose moieties to amine groups on proteins and nucleic acids. AGE are a consequence of chronic hyperglycemia. Primary amino groups on proteins undergo reversible nonenzymatic glycosylation in the hyperglycemic environment. Subsequent reduction and Amadori rearrangements result in the irreversible formation of AGE. Elevated glucose leads to accumulation of diacylglycerol, which leads to the activation of protein kinase C (PKC). PKC impairs insulin action, which is antiinflammatory under physiological conditions in the vasculature, and some PKC isoforms induce endothelial dysfunction. Glycosylation with O-linked β-N-acetylglucosamine on serine and threonine residues, termed O-GlcNAcylation, affects the function of enzymes, transcription factors, and cytoskeletal proteins leading to abnormalities in cellular function (23). A high influx of glucose can be metabolized by aldose reductase in certain cells to sorbitol and fructose. These molecules can then cause osmotic and oxidative stress leading to abnormal cellular function. Perhaps most importantly, high substrate flux generates mitochondrial reactive oxygen species (reviewed in Ref. 24). The reactive oxygen species will activate poly (ADP-ribose) polymerase and block glucose oxidation, leading to additional flux through the pathways mentioned above and resulting in a vicious cycle of glucose toxicity (25). In endothelial cells, blocking AGE, PKC, poly (ADP-ribose) polymerase, or the accumulation of reactive oxygen species protects cells from glucose toxicity and induction of the pathological responses outlined above. These are the well-established mechanisms for glucose toxicity on macrovasculature and microvasculature.
Epidemiological Evidence for the Impact of Diabetes on Macrovascular Disease
Diabetes is currently treated as a CVD risk equivalent, based upon population studies including the East West Study and a study in Alaskan natives (26, 27). These and other cohort studies demonstrated an overall risk of approximately 2% per year for myocardial infarction in subjects with diabetes, which is similar to the Framingham risk equivalent for individuals with established coronary disease (28, 29). A CVD risk equivalent in clinical practice translates into recommendations for a target LDL less than 100 mg/dl, with a goal of less than 70 mg/dl considered reasonable (30, 31). Viewing diabetes as a CVD risk equivalent has been challenged because younger cohorts without concomitant metabolic syndrome are reported to have CVD risk that is equivalent to the general population (32). Even with this debate not fully resolved, it is clear that CVD is the leading cause of death in individuals with diabetes (www.cdc.gov/diabetes). Seventy-five percent of all hospitalizations for diabetes are for coronary artery disease. Death after acute myocardial infarction is 50% more common in people with diabetes (33). Plaque burden is more extensive and involves more vessels in people with diabetes, including youth (34, 35). In addition, after a myocardial infarction, congestive heart failure is more common and more progressive in people with diabetes, especially among women (36). Overall, the extent and consequences of CVD are greater in people with diabetes. However, the observation that people with diabetes have excess CVD does not mean that glucose causes CVD.
Epidemiological and Cohort Studies Suggesting Glucose as a Mediator of CVD
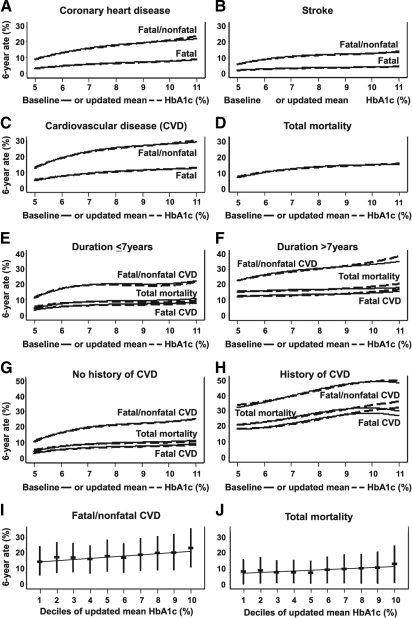
The diagnosis of diabetes is defined by the presence of hyperglycemia, so many epidemiological and cohort studies have examined the relationship between glucose control and cardiovascular risk. Balkau et al. (37) examined three cohorts of middle-aged men without diabetes. Nondiabetic men with 2-h glucose in the upper 20% after a glucose challenge, or the upper 2.5% of fasting blood glucose in the Whitehall Study (n = 10,025), the Paris Prospective Study (n = 6,629), and the Helsinki Policeman Study (n = 631) displayed an increase in all-cause mortality and CVD, with a relative risk of 1.8 (1.4–2.4) and 2.7 (1.7–4.4), respectively (reviewed in Ref. 37). A recent study by Selvin et al. (38) in nondiabetic subjects endorsed the correlation of hemoglobin A1c with CVD risk. Similar results in nondiabetic subjects were observed in the Rancho Bernardo Study, the Framingham Study, the Honolulu Heart Study, and the British Regional Heart Study (reviewed in Ref. 39). Of note, in the Framingham Study, women, but not men, had a higher incidence of cardiovascular events with elevated blood glucose (40). In the 1990s, a series of cohort studies including the Diabetes Intervention Study, the Wisconsin Epidemiological Study of Diabetic Retinopathy, the Framingham Study, the San Antonio Heart Study, and a number of studies from the Kuusito group in Finland demonstrated a 2- to 4-fold increase in CVD risk associated with either elevated hemoglobin A1c or fasting glucose (reviewed in Refs. 39 and 41). The Munich General Practitioner Project further endorses the relationship between glycemia and CVD (42). Most often cited is the United Kingdom Prospective Diabetes Study (UKPDS), in which there was an 11% increase in cardiovascular risk with every percentage point increase in hemoglobin A1c (43, 44). The Emerging Risk Factors Collaboration recently reviewed 102 prospective trials (data for 698,782 people) examining CVD risk in diabetes. They found that hyperglycemia, independent of other risk factors, confers approximately a 2-fold increased risk for all vascular diseases (38). In the Atherosclerosis Risk in Communities Study, Selvin et al. (45, 46) reported a consistent correlation between hemoglobin A1c and CVD, stroke, and peripheral vascular disease, regardless of diabetes status. Two recent reports from the Swedish National Diabetes Registry, one in type 1 diabetes and the other in type 2 diabetes, demonstrate significantly increased risk with increasing hemoglobin A1c (Fig. 2) (47, 48). The lowest risk was seen in individuals with a hemoglobin A1c of 6.5%, and substantially higher risks were seen with hemoglobin A1c of 8 to 9%.
Fig. 2.
Observational data from the Swedish National Registry. A significant correlation of glycemic control with cardiovascular events was demonstrated in 18,334 patients (age, 30–79 yr) followed for a mean of 5.6 yr (1997–2003). The endpoints of coronary heart disease (A), stroke (B), CVD (C), and total mortality (D) were assessed by quartile mean hemoglobin A1c (baseline or updated), and all demonstrated a significant correlation with hemoglobin A1c. E–J, A significant increase in events with increasing hemoglobin A1c was observed with or without previous CVD and regardless of duration of diabetes. HbA1c, Hemoglobin A1c. [Reprinted with permission from K. Eeg-Olofsson et al.: J Intern Med 268:471–482, 2010 (47). © Wiley.]
Epidemiological data from DECODE and the Honolulu Heart study suggest that postprandial glucose elevation is predictive of CVD risk (49, 50). In the Honolulu Heart study, glucose intolerance, as defined by elevated 1-h postglucose challenge blood glucose, significantly predicted CVD and all-cause mortality (49). Similarly, in the DECODE Study, glucose intolerance (impaired fasting glucose or impaired glucose tolerance) in response to a glucose tolerance test was predictive of CVD in the absence of overt diabetes (50). There have been few randomized studies examining the differential effect of postprandial vs. fasting hyperglycemia on CVD endpoints in diabetes. Surrogate endpoints have been used, such as carotid intima-medial thickness. Esposito et al. (51) compared the effects of repaglinide with glyburide in a 12-month study of subjects with type 2 diabetes. By the end of the study, the hemoglobin A1c decrease of 0.9% was similar between groups, but the repaglinide group achieved much lower area under the curve for glucose during a 2-h postmeal challenge compared with the glyburide group, whereas fasting glucose was lower in the glyburide group. Carotid intima-medial thickness decreased in the repaglinide group compared with the glyburide group. A study by Bibra et al. (52) compared the effects of three insulin regimens used for 24 months on intima-medial thickness and arterial stiffness in subjects with type 2 diabetes. Hemoglobin A1c and fasting glucose were similar during the study, but postmeal glucose correlated with intima-medial thickness and arterial stiffness. These studies are small and few in number and employ surrogate cardiovascular endpoints and a small number of subjects, but they suggest an effect of postprandial hyperglycemia in CVD risk.
These epidemiological and cohort studies compellingly endorse the hypothesis that hyperglycemia is associated with worse cardiovascular outcomes, yet these data do not directly address whether interventions to improve glycemia will decrease cardiovascular events.
Prospective Randomized Studies Correlating Early Glycemic Control with Late CVD Prevention
Two large prospective studies, the Diabetes Control and Complication Trial (DCCT) in type 1 diabetes and the UKPDS study in type 2 diabetes, were designed to examine the impact of glycemic control on microvascular events. In the DCCT, 1441 subjects with type 1 diabetes were randomized to receive either intensive or conventional glycemic therapy and were treated for a mean of 6.5 yr, reaching a hemoglobin A1c of 7.4 and 9.1% in the intensive and conventional therapy groups, respectively (53). By the end of the DCCT, subjects in the conventional treatment group had a higher prevalence of several CVD risk factors, including microalbuminuria and macroalbuminuria (54). There was also a trend toward decreased cardiovascular events in this very low risk cohort, but the study was underpowered to answer this question. The Epidemiology of Diabetes Interventions and Complications Trial (EDIC) followed individuals for an average of 11 yr after completing the DCCT, for a total of 17 yr of follow-up. After completion of the DCCT, subjects in the conventional therapy group were offered the opportunity to receive intensive treatment, and all participants returned to their primary care providers for further care. Subjects came in for annual visits, and hemoglobin A1c and an electrocardiogram were performed annually. Risk factors for CVD were measured every other year. The primary outcome was time to first definite cardiovascular event: nonfatal myocardial infarction or stroke, death from CVD, subclinical myocardial infarction, angina, or the need for revascularization with angioplasty or coronary artery bypass. The difference in mean hemoglobin A1c between treatment groups narrowed considerably during the EDIC follow-up study, almost converging, to a mean of 8.0% in the original intensive treatment group and 8.2% in the original conventional treatment group. By yr 11 of the EDIC study, the conventional treatment group continued to have a higher prevalence of microalbuminuria, macroalbuminuria, and serum creatinine greater than 2 mg/dl (53). A significantly greater number of cardiovascular events occurred in the conventional treatment group during DCCT/EDIC. Intensive treatment was associated with a 42% risk reduction in cumulative incidence of a first cardiovascular event and a 57% risk reduction for first occurrence of nonfatal myocardial infarction, stroke, or death from CVD (53). These remarkable findings demonstrated that an initial period of intensive glycemic control has a sustained beneficial effect to reduce risk for both micro- and macrovascular disease in type 1 diabetes.
The UKPDS randomized individuals with newly diagnosed type 2 diabetes to conventional therapy vs. intensive therapy for 10 yr. Intensive glycemic therapy was associated with reduced risk of microvascular complications but a nonsignificant reduction in relative risk of myocardial infarction. Subjects in the UKPDS were followed for an additional 10 yr after completion of the study (55). Of the 4209 originally randomized subjects, 3277 attended posttrial clinics every 3 yr, whereas annual questionnaires were used to follow subjects unable to attend clinics and to assess all subjects between yr 6 and 10. After the first year of posttrial monitoring, the difference in hemoglobin A1c between treatment groups was lost. However, the cumulative incidences for myocardial infarction and death from any cause were significantly lower in the intensive therapy groups (sulfonylurea-insulin and metformin) than on conventional therapy, as was the cumulative incidence for microvascular disease in the sulfonylurea-insulin group. Therefore, the sustained beneficial effect of glycemic control seen in DCCT/EDIC was also seen in the UKPDS follow-up study. The magnitude of cardiovascular risk reduction seen in the UKPDS follow-up study of type 2 diabetes seems to be smaller than that seen in EDIC, which involved individuals with type 1 diabetes.
Both the DCCT/EDIC and the UKPDS follow-up studies demonstrated that an interval of good glycemic control early on in diabetes has a sustained beneficial effect on long-term CVD risk. This legacy effect in the intensive therapy group comprised decreased incidence of both microvascular and macrovascular events, despite loss of optimal glycemic control and regression to a hemoglobin A1c similar to the standard control group (53, 55). These observations combined with the epidemiological and cohort analyses outlined earlier in this review to support the need to prospectively examine the impact of aggressive glycemic control on cardiovascular events as a primary outcome.
Large Prospective, Randomized Controlled Trials Examining the Impact of Glycemic Control on Cardiovascular Events and Mortality in Type 2 Diabetes (Table 1)
Table 1.
Baseline and end of study hemoglobin A1c, weight, glucose-lowering agents, and outcomes by treatment group in ACCORD, ADVANCE, and VADT (56, 58, 59)
| ACCORD | ADVANCE | VADT | |
|---|---|---|---|
| Duration of diabetes at baseline (yr) | 10 | 7.9 | 11.5 |
| Baseline hemoglobin A1c (%) | 8.1 | 7.5 | 9.4 |
| Known coronary artery disease at baseline (%) | 35 | 40 | |
| Known macrovascular disease at baseline (%) | 32 | ||
| On insulin at baseline (%) | 35 | 1.5 | 52 |
| On secretagogue at baseline (%) | 50 | 73 | |
| On thiazolidinedione at baseline (%) | 19 | 3.6 | |
| Median time of follow-up (yr) | 3.5 | 5 | 5.6 |
| Average achieved hemoglobin A1c (%)a | 7.5/6.4b | 7.3/6.5b | 8.4/6.9 |
| Primary outcome (number of subjects)a | 371/352 | 1116/1009b | 264/235 |
| Deaths from any cause (number of subjects)a | 203/257b | 533/498 | 95/102 |
| Weight change (kg)a | 0 | 4/8b | |
| On insulin at study end (%)a | 55/77 | 24/41 | 73/86c |
| On secretagogue at study end (%)a | 74/87 | 62/94 | 42/57c |
| On thiazolidinedione at study end (%)a | 58/92 | 11/17 | 58/68c |
| Weight gain > 10 kg at study end (%)a | 14/28b |
Data are expressed as standard/intensive.
P < 0.05 vs. standard therapy group.
Data at 4 yr (69).
The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study included 10,251 subjects with type 2 diabetes (56). The primary outcome was a composite endpoint of nonfatal myocardial infarction, nonfatal stroke, or death from cardiovascular causes. The median hemoglobin A1c at baseline was 8.1%, and median duration of diabetes at baseline was 10 yr. Subjects were assigned to treatment targeting a hemoglobin A1c below 6% (intensive therapy) or between 7 and 7.9% (standard therapy). Approximately 35% of the subjects had a known history of a cardiovascular event, 35% were receiving insulin therapy, 50% were on an insulin secretagogue, and 19% were receiving a thiazolidinedione. Within 4 months of randomization, the median hemoglobin A1c had decreased from 8.1 to 6.7% in the intensive therapy group and to 7.5% in the standard therapy group. At 1 yr, median hemoglobin A1c levels were stable at 6.4 and 7.5% in the intensive and standard therapy groups, respectively. By the time the study was stopped, 77.3 and 55.4% of subjects were receiving insulin therapy in the intensive and standard therapy groups, respectively, whereas 87 and 74% were receiving a secretagogue, and 92 and 58% were receiving a thiazolidinedione, respectively. The rate of death from any cause was higher in the intensive therapy group than in the standard therapy group after a mean of 3.5 of follow-up. This higher rate of death in the intensive therapy group was observed even after 5 yr of follow-up (57). The data and safety monitoring committee concluded that this increased rate of death in the intensive therapy group outweighed any potential benefits, so the committee recommended that the trial be discontinued for safety reasons. The trial was stopped prematurely, 17 months before the scheduled end of the study. Surprisingly, the primary outcome of the study was not different between groups (P = 0.16), with a slightly lower incidence of subjects achieving the primary outcome on intensive therapy (6.9%) vs. standard therapy (7.2%). The secondary outcomes of death from any cause and death from cardiovascular cause were higher in the intensive therapy group, potentially due at least in part to fatal or nonfatal congestive heart failure, but not to nonfatal myocardial infarction, which occurred significantly less frequently on intensive therapy. Further analysis of potential causes for increased all-cause mortality in the intensive therapy group suggested that there were fewer cardiovascular events in subjects without a prior history of CVD, or a baseline hemoglobin A1c of less than 8%. However, preliminary exploratory analyses of severe hypoglycemia after randomization and use of specific glucose-lowering drugs did not reveal a cause. Interestingly, there was marked weight gain during the study, with 28 and 14% of subjects on intensive therapy vs. standard therapy, respectively, gaining more than 10 kg at the time of study discontinuation compared with baseline.
Published in the same issue of the New England Journal of Medicine were results of the Action in Diabetes and Vascular Disease (ADVANCE) trial (58). This was a trial involving 11,140 subjects with type 2 diabetes with a mean baseline hemoglobin A1c of 7.5% and a mean duration of diabetes of 7.9 yr at baseline. Thirty-two percent of subjects had a history of major macrovascular disease, and 27% had a history of microalbuminuria at baseline. Subjects were randomized to undergo either intensive or standard glucose control. The primary endpoints were a composite of major macrovascular events (death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke) and major microvascular events (new or worsening nephropathy or retinopathy) assessed jointly and separately. Subjects were followed for a mean of 5 yr. The hemoglobin A1c reached a mean of 6.5% in the intensive control group and 7.3% in the standard control group. Only 1.5% of subjects were receiving insulin therapy, 73% were receiving a secretagogue (gliclazide, other sulfonylurea, or glinide), and 3.6% were receiving a thiazolidinedione at baseline. At the end of the study, 40.5 and 24.1% of subjects were receiving insulin in the intensive control and standard control groups, whereas 94 and 62% were receiving a secretagogue, and 17 and 11% were receiving a thiazolidinedione, respectively. Weight at baseline and at the end of the study was no different in either group. Event rates were lower than expected in both groups at 3 yr, so interim changes were made to increase the power of the study. Subjects in the intensive control group had a decreased incidence of a major macro- or microvascular event [hazard ratio, 0.90; 95% confidence interval (CI), 0.82–0.98; P = 0.01]. However, when assessed separately, this benefit seemed to be exclusively related to a reduction in the incidence of major microvascular events (hazard ratio, 0.86; 95% CI, 0.77–0.97; P = 0.01) and not major macrovascular events (hazard ratio, 0.94; 95% CI, 0.84–1.06; P = 0.32). In contrast to what was found in ACCORD, there was no difference in mortality between treatment groups (8.9% in the intensive control group vs. 9.6% in the standard control group; P = 0.28).
Finally, a smaller cohort involving 1791 military veterans was examined in the Veterans Affairs Diabetes Trial (VADT) (59). Subjects were randomized to intensive vs. standard glycemic control. The primary outcome was time from randomization to first occurrence of a major cardiovascular event, which was a composite of myocardial infarction, stroke, death from cardiovascular causes, congestive heart failure, surgery for vascular disease, inoperable coronary disease, and amputation for ischemic gangrene. Hemoglobin A1c at baseline was 9.4%, mean duration of diabetes was 11.5 yr, and 40% of subjects had a history of a cardiovascular event before starting the study. Subjects were followed for a mean of 5.6 yr. Median hemoglobin A1c was 8.4% in the standard therapy group and 6.9% in the intensive therapy group. By the end of the study, subjects in the intensive therapy group had gained 18 pounds, making the mean weight significantly greater than in the standard therapy group, which had gained an average of only 9 pounds. A total of 684 events were expected, with a 40% event rate in the standard therapy group. The observed event rate was lower, with 33.5% in the standard therapy group and 29.5% in the intensive therapy group, which represented a relative reduction of 11.9%. The effect of treatment did not seem to vary according to insulin use at baseline or prior history of a cardiovascular event. There was a slightly lower incidence of the primary outcome in the intensive therapy group, but this did not reach statistical significance (hazard ratio for intensive therapy group, 0.88; 95% CI, 0.74–1.05; P = 0.14). There was also no significant difference between groups in any individual component of the primary outcome, the rate of death from any cause, or microvascular complications. Selected data from ACCORD, ADVANCE, and VADT are presented in Table 1 for comparison.
Delving Deeper into Data from ACCORD, ADVANCE, and VADT
A number of analyses have subsequently been performed in the ACCORD cohort (60–63). Importantly, only a subset of the intensive treatment group was found to have a higher risk for mortality than the standard treatment group: those with an average hemoglobin A1c of more than 7%. Increased mortality was found to be associated with higher hemoglobin A1c at baseline, history of neuropathy, and higher hemoglobin A1c on treatment. The latter predicted mortality more strongly than hemoglobin A1c decrease in the first year or hemoglobin A1c during the last interval of follow-up. Interestingly, the relationship between hemoglobin A1c and risk for mortality varied by treatment group, with a linear increase in risk between a hemoglobin A1c of 6–9% in the intensive treatment group, and a “U-shaped” relationship in the standard treatment group. Individuals in the standard treatment group with the lowest risk for mortality had a hemoglobin A1c of 7–8%.
An analysis of severe hypoglycemia performed in the ADVANCE cohort (64) demonstrates a higher rate of severe hypoglycemia in the intensive intervention group compared with the standard intervention group, as expected. However, among individuals experiencing severe hypoglycemia, the annual mortality rate was lower in the intensive treatment group than in the standard treatment group (3.6 vs. 5.1%, respectively). Furthermore, there was no association among severe hypoglycemia, treatment group assignment, and risk of death (P = 0.30). Severe hypoglycemia was associated with higher risk for death, but the increased incidence of severe hypoglycemia in the intensive therapy group was not associated with greater risk of cardiovascular endpoints. Although individuals in the intensive therapy group had more severe hypoglycemia, there was lower mortality for those with severe hypoglycemia on intensive therapy. Having a history of hypoglycemia at baseline was associated with higher risk for mortality regardless of treatment assignment (60).
A secondary analysis of coronary artery calcium scores and cardiovascular events in a subset of individuals in the VADT shows that intensive glycemic control benefits individuals with minimal coronary atherosclerotic disease (65).
What Next?—Practice Implications and Gaps in Knowledge
Basic vascular biology data plus epidemiological and cohort evidence would predict that glucose control should impact cardiovascular events. It is clear that the current strategies that improve blood glucose do not achieve this goal (reviewed above and in Refs. 66 and 67). This leaves us with the intriguing question of why? Is it because concurrent treatment with statins, angiotensin converting enzyme inhibitors and angiotensin receptor blockers, aspirin, and other blood pressure-lowering agents already decrease the risk maximally? This is not likely because people with diabetes maintain an excess cardiovascular risk. If we consider recent data that even transient exposure to hyperglycemia can cause sustained epigenetic changes in target tissues as our explanation, then why does improved blood sugar control impact microvascular complications? It is possible that agents to normalize cholesterol and blood pressure are more successful because they achieve their target most of the 24-h day. In contrast, a hemoglobin A1c of 7% or even 6% can be achieved in the context of marked glycemic variability outside of the normal range (68). Until we have behavioral and pharmacological strategies that normalize carbohydrate metabolism, we cannot answer the scientific question of whether glucose lowering can decrease progression of atherosclerosis. Therapeutic interventions such as gastric bypass surgery, which often “cures” diabetes, may allow us to address this question in that unique cohort of obese subjects. Until such new evidence is available, reinforced by the recent cohort data from the Swedish population that individuals with lower hemoglobin A1c on contemporary medical regimens have improved cardiovascular outcomes, we should help our patients to achieve as close to normal of a hemoglobin A1c as possible without hypoglycemia. We know that this intervention decreases microvascular complications, which are responsible for considerable morbidity and mortality.
Acknowledgments
This work was supported by Veterans Administration Office of Research and Development in the form of a Merit Award (to J.E.B.R.), by a Career Development Award-2 (to C.C.L.W.), and by the National Institutes of Health (Grants DK64741, HL56481, and UL1 RR025780 to J.E.B.R. and Grant UL1 RR025780 to C.C.L.W.).
Disclosure Summary: J.E.B.R. has done investigator-initiated research with GlaxoSmithKline, Amylin/Lilly, and Bristol Myers Squibb and has served as a consultant for GlaxoSmithKline and Johnson & Johnson. C.C.L.W. has nothing to disclose.
Footnotes
- AGE
- Advanced glycation end-products
- CI
- confidence interval
- CVD
- cardiovascular disease
- LDL
- low-density lipoprotein
- PAI-1
- plasminogen activator inhibitor-1
- PKC
- protein kinase C
- tPA
- tissue plasminogen activator.
References
- 1. Reusch JE, Draznin BB. 2007. Atherosclerosis in diabetes and insulin resistance. Diabetes Obes Metab 9:455–463 [DOI] [PubMed] [Google Scholar]
- 2. Wang CC, Goalstone ML, Draznin B. 2004. Molecular mechanisms of insulin resistance that impact cardiovascular biology. Diabetes 53:2735–2740 [DOI] [PubMed] [Google Scholar]
- 3. Sitia S, Tomasoni L, Atzeni F, Ambrosio G, Cordiano C, Catapano A, Tramontana S, Perticone F, Naccarato P, Camici P, Picano E, Cortigiani L, Bevilacqua M, Milazzo L, Cusi D, Barlassina C, Sarzi-Puttini P, Turiel M. 2010. From endothelial dysfunction to atherosclerosis. Autoimmun Rev 9:830–834 [DOI] [PubMed] [Google Scholar]
- 4. Colwell JA, Winocour PD, Lopes-Virella M, Halushka PV. 1983. New concepts about the pathogenesis of atherosclerosis in diabetes mellitus. Am J Med 75:67–80 [DOI] [PubMed] [Google Scholar]
- 5. Ceriello A, Esposito K, Piconi L, Ihnat MA, Thorpe JE, Testa R, Boemi M, Giugliano D. 2008. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Diabetes 57:1349–1354 [DOI] [PubMed] [Google Scholar]
- 6. Otsuka A, Azuma K, Iesaki T, Sato F, Hirose T, Shimizu T, Tanaka Y, Daida H, Kawamori R, Watada H. 2005. Temporary hyperglycaemia provokes monocyte adhesion to endothelial cells in rat thoracic aorta. Diabetologia 48:2667–2674 [DOI] [PubMed] [Google Scholar]
- 7. Pandolfi A, Giaccari A, Cilli C, Alberta MM, Morviducci L, De Filippis EA, Buongiorno A, Pellegrini G, Capani F, Consoli A. 2001. Acute hyperglycemia and acute hyperinsulinemia decrease plasma fibrinolytic activity and increase plasminogen activator inhibitor type 1 in the rat. Acta Diabetol 38:71–76 [DOI] [PubMed] [Google Scholar]
- 8. Zuurbier CJ, Demirci C, Koeman A, Vink H, Ince C. 2005. Short-term hyperglycemia increases endothelial glycocalyx permeability and acutely decreases lineal density of capillaries with flowing red blood cells. J Appl Physiol 99:1471–1476 [DOI] [PubMed] [Google Scholar]
- 9. Velusamy T, Jain SK. 2011. Effects of high glucose and ketosis (acetoacetate, β-hydroxybutyrate) on PAI-1 secretion in human umbilical vascular endothelial cells. Clin Appl Thromb Hemost 17:288–292 [DOI] [PubMed] [Google Scholar]
- 10. Zhong W, Zou G, Gu J, Zhang J. 2010. L-Arginine attenuates high glucose-accelerated senescence in human umbilical vein endothelial cells. Diabetes Res Clin Pract 89:38–45 [DOI] [PubMed] [Google Scholar]
- 11. Piga R, Naito Y, Kokura S, Handa O, Yoshikawa T. 2009. Inhibitory effect of serotonin derivatives on high glucose-induced adhesion and migration of monocytes on human aortic endothelial cells. Brit J Nutr 102:264–272 [DOI] [PubMed] [Google Scholar]
- 12. Tang R, Li Q, Lv L, Dai H, Zheng M, Ma K, Liu B. 2010. Angiotensin II mediates the high-glucose-induced endothelial-to-mesenchymal transition in human aortic endothelial cells. Cardiovasc Diabetol 9:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colwell JA, Nesto RW. 2003. The platelet in diabetes: focus on prevention of ischemic events. Diabetes Care 26:2181–2188 [DOI] [PubMed] [Google Scholar]
- 14. Inoguchi T, Yu HY, Imamura M, Kakimoto M, Kuroki T, Maruyama T, Nawata H. 2001. Altered gap junction activity in cardiovascular tissues of diabetes. Med Electron Microsc 34:86–91 [DOI] [PubMed] [Google Scholar]
- 15. Li AF, Roy S. 2009. High glucose-induced downregulation of connexin 43 expression promotes apoptosis in microvascular endothelial cells. Invest Ophthalmol Vis Sci 50:1400–1407 [DOI] [PubMed] [Google Scholar]
- 16. Makino A, Platoshyn O, Suarez J, Yuan JX, Dillmann WH. 2008. Downregulation of connexin40 is associated with coronary endothelial cell dysfunction in streptozotocin-induced diabetic mice. Am J Physiol Cell Physiol 295:C221–C230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dong L, Zheng YM, Van Riper D, Rathore R, Liu QH, Singer HA, Wang YX. 2008. Functional and molecular evidence for impairment of calcium-activated potassium channels in type-1 diabetic cerebral artery smooth muscle cells. J Cereb Blood Flow Metab 28:377–386 [DOI] [PubMed] [Google Scholar]
- 18. Meng L, Park J, Cai Q, Lanting L, Reddy MA, Natarajan R. 2010. Diabetic conditions promote binding of monocytes to vascular smooth muscle cells and their subsequent differentiation. Am J Physiol Heart Circ Physiol 298:H736–H745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. El-Osta A, Brasacchio D, Yao D, Pocai A, Jones PL, Roeder RG, Cooper ME, Brownlee M. 2008. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med 205:2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Villeneuve LM, Reddy MA, Lanting LL, Wang M, Meng L, Natarajan R. 2008. Epigenetic histone H3 lysine 9 methylation in metabolic memory and inflammatory phenotype of vascular smooth muscle cells in diabetes. Proc Natl Acad Sci USA 105:9047–9052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Tonna S, El-Osta A, Cooper ME, Tikellis C. 2010. Metabolic memory and diabetic nephropathy: potential role for epigenetic mechanisms. Nat Rev Nephrol 6:332–341 [DOI] [PubMed] [Google Scholar]
- 22. Eckel RH, Wassef M, Chait A, Sobel B, Barrett E, King G, Lopes-Virella M, Reusch J, Ruderman N, Steiner G, Vlassara H. 2002. Prevention Conference VI: Diabetes and Cardiovascular Disease Writing Group II. Pathogenesis of atherosclerosis in diabetes. Circulation 105:e138–143 [DOI] [PubMed] [Google Scholar]
- 23. Lima VV, Giachini FR, Hardy DM, Webb RC, Tostes RC. 2011. O-GlcN acylation: a novel pathway contributing to the effects of endothelin in the vasculature. Am J Physiol Regul Integr Comp Physiol 300:R236–R250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brownlee M. 2005. The pathobiology of diabetic complications: a unifying mechanism. Diabetes 54:1615–1625 [DOI] [PubMed] [Google Scholar]
- 25. Du X, Matsumura T, Edelstein D, Rossetti L, Zsengellér Z, Szabó C, Brownlee M. 2003. Inhibition of GAPDH activity by poly(ADP-ribose) polymerase activates three major pathways of hyperglycemic damage in endothelial cells. J Clin Invest 112:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. 1998. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med 339:229–234 [DOI] [PubMed] [Google Scholar]
- 27. Schraer CD, Adler AI, Mayer AM, Halderson KR, Trimble BA. 1997. Diabetes complications and mortality among Alaska natives: 8 years of observation. Diabetes Care 20:314–321 [DOI] [PubMed] [Google Scholar]
- 28. Sheridan S, Pignone M, Mulrow C. 2003. Framingham-based tools to calculate the global risk of coronary heart disease: a systematic review of tools for clinicians. J Gen Intern Med 18:1039–1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tzoulaki I, Liberopoulos G, Ioannidis JP. 2009. Assessment of claims of improved prediction beyond the Framingham risk score. JAMA 302:2345–2352 [DOI] [PubMed] [Google Scholar]
- 30. Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. 2004. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 110:227–239 [DOI] [PubMed] [Google Scholar]
- 31. Feeman WE., Jr 2010. Diabetes mellitus is not a coronary heart disease equivalent. Am J Cardiol 106:754–755 [DOI] [PubMed] [Google Scholar]
- 32. Saely CH, Aczel S, Koch L, Schmid F, Marte T, Huber K, Drexel H. 2010. Diabetes as a coronary artery disease risk equivalent: before a change of paradigm? Eur J Cardiovasc Prev Rehabil 17:94–99 [DOI] [PubMed] [Google Scholar]
- 33. Sprafka JM, Burke GL, Folsom AR, McGovern PG, Hahn LP. 1991. Trends in prevalence of diabetes mellitus in patients with myocardial infarction and effect of diabetes on survival. The Minnesota Heart Survey. Diabetes Care 14:537–543 [DOI] [PubMed] [Google Scholar]
- 34. Ledru F, Ducimetière P, Battaglia S, Courbon D, Beverelli F, Guize L, Guermonprez JL, Diébold B. 2001. New diagnostic criteria for diabetes and coronary artery disease: insights from an angiographic study. J Am Coll Cardiol 37:1543–1550 [DOI] [PubMed] [Google Scholar]
- 35. McGill HC, Jr, McMahan CA, Malcom GT, Oalmann MC, Strong JP. 1995. Relation of glycohemoglobin and adiposity to atherosclerosis in youth. Pathobiological Determinants of Atherosclerosis in Youth (PDAY) Research Group. Arterioscler Thromb Vasc Biol 15:431–440 [DOI] [PubMed] [Google Scholar]
- 36. Jaffe AS, Spadaro JJ, Schechtman K, Roberts R, Geltman EM, Sobel BE. 1984. Increased congestive heart failure after myocardial infarction of modest extent in patients with diabetes mellitus. Am Heart J 108:31–37 [DOI] [PubMed] [Google Scholar]
- 37. Balkau B, Shipley M, Jarrett RJ, Pyörälä K, Pyörälä M, Forhan A, Eschwège E. 1998. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men: 20-year follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care 21:360–367 [DOI] [PubMed] [Google Scholar]
- 38. Emerging Risk Factors Collaboration, Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di Angelantonio E, Ingelsson E, Lawlor DA, Selvin E, Stampfer M, Stehouwer CD, Lewington S, Pennells L, Thompson A, Sattar N, White IR, Ray KK, Danesh J. 2010. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet 375:2215–2222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuusisto J, Laakso M. 1999. Prandial glucose regulation and cardiovascular disease in type 2 diabetes. Eur J Clin Invest 29(Suppl 2):7–11 [DOI] [PubMed] [Google Scholar]
- 40. Wilson PW, Cupples LA, Kannel WB. 1991. Is hyperglycemia associated with cardiovascular disease? The Framingham Study. Am Heart J 121:586–590 [DOI] [PubMed] [Google Scholar]
- 41. Wei M, Gaskill SP, Haffner SM, Stern MP. 1998. Effects of diabetes and level of glycemia on all-cause and cardiovascular mortality. The San Antonio Heart Study. Diabetes Care 21:1167–1172 [DOI] [PubMed] [Google Scholar]
- 42. Standl E, Balletshofer B, Dahl B, Weichenhain B, Stiegler H, Hörmann A, Holle R. 1996. Predictors of 10-year macrovascular and overall mortality in patients with NIDDM: the Munich General Practitioner Project. Diabetologia 39:1540–1545 [DOI] [PubMed] [Google Scholar]
- 43. 1998. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet 352:837–853 [PubMed] [Google Scholar]
- 44. Turner RC, Millns H, Neil HA, Stratton IM, Manley SE, Matthews DR, Holman RR. 1998. Risk factors for coronary artery disease in non-insulin dependent diabetes mellitus: United Kingdom Prospective Diabetes Study (UKPDS: 23). BMJ 316:823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, Coresh J, Brancati FL. 2010. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med 362:800–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Selvin E, Wattanakit K, Steffes MW, Coresh J, Sharrett AR. 2006. HbA1c and peripheral arterial disease in diabetes: the Atherosclerosis Risk in Communities study. Diabetes Care 29:877–882 [DOI] [PubMed] [Google Scholar]
- 47. Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjörnsdóttir S, Eliasson B. 2010. New aspects of HbA1c as a risk factor for cardiovascular diseases in type 2 diabetes: an observational study from the Swedish National Diabetes Register (NDR). J Intern Med 268:471–482 [DOI] [PubMed] [Google Scholar]
- 48. Eeg-Olofsson K, Cederholm J, Nilsson PM, Zethelius B, Svensson AM, Gudbjörnsdóttir S, Eliasson B. 2010. Glycemic control and cardiovascular disease in 7,454 patients with type 1 diabetes: an observational study from the Swedish National Diabetes Register (NDR). Diabetes Care 33:1640–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rodriguez BL, Lau N, Burchfiel CM, Abbott RD, Sharp DS, Yano K, Curb JD. 1999. Glucose intolerance and 23-year risk of coronary heart disease and total mortality: the Honolulu Heart Program. Diabetes Care 22:1262–1265 [DOI] [PubMed] [Google Scholar]
- 50. Balkau B, Hu G, Qiao Q, Tuomilehto J, Borch-Johnsen K, Pyörälä K. 2004. Prediction of the risk of cardiovascular mortality using a score that includes glucose as a risk factor. The DECODE Study. Diabetologia 47:2118–2128 [DOI] [PubMed] [Google Scholar]
- 51. Esposito K, Giugliano D, Nappo F, Marfella R. 2004. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation 110:214–219 [DOI] [PubMed] [Google Scholar]
- 52. Bibra H, Siegmund T, Ceriello A, Volozhyna M, Schumm-Draeger PM. 2009. Optimized postprandial glucose control is associated with improved cardiac/vascular function—comparison of three insulin regimens in well-controlled type 2 diabetes. Horm Metab Res 41:109–115 [DOI] [PubMed] [Google Scholar]
- 53. Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B. 2005. Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. 1993. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med 329:977–986 [DOI] [PubMed] [Google Scholar]
- 55. Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 2008. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med 359:1577–1589 [DOI] [PubMed] [Google Scholar]
- 56. Gerstein HC, Miller ME, Byington RP, Goff DC, Jr, Bigger JT, Buse JB, Cushman WC, Genuth S, Ismail-Beigi F, Grimm RH, Jr, Probstfield JL, Simons-Morton DG, Friedewald WT. 2008. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Gerstein HC, Miller ME, Genuth S, Ismail-Beigi F, Buse JB, Goff DC, Jr, Probstfield JL, Cushman WC, Ginsberg HN, Bigger JT, Grimm RH, Jr, Byington RP, Rosenberg YD, Friedewald WT. 2011. Long-term effects of intensive glucose lowering on cardiovascular outcomes. N Engl J Med 364:818–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Patel A, MacMahon S, Chalmers J, Neal B, Billot L, Woodward M, Marre M, Cooper M, Glasziou P, Grobbee D, Hamet P, Harrap S, Heller S, Liu L, Mancia G, Mogensen CE, Pan C, Poulter N, Rodgers A, Williams B, Bompoint S, de Galan BE, Joshi R, Travert F. 2008. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 59. Duckworth W, Abraira C, Moritz T, Reda D, Emanuele N, Reaven PD, Zieve FJ, Marks J, Davis SN, Hayward R, Warren SR, Goldman S, McCarren M, Vitek ME, Henderson WG, Huang GD. 2009. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 360:129–139 [DOI] [PubMed] [Google Scholar]
- 60. Bonds DE, Miller ME, Bergenstal RM, Buse JB, Byington RP, Cutler JA, Dudl RJ, Ismail-Beigi F, Kimel AR, Hoogwerf B, Horowitz KR, Savage PJ, Seaquist ER, Simmons DL, Sivitz WI, Speril-Hillen JM, Sweeney ME. 2010. The association between symptomatic, severe hypoglycaemia and mortality in type 2 diabetes: retrospective epidemiological analysis of the ACCORD study. BMJ 340:b4909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Calles-Escandón J, Lovato LC, Simons-Morton DG, Kendall DM, Pop-Busui R, Cohen RM, Bonds DE, Fonseca VA, Ismail-Beigi F, Banerji MA, Failor A, Hamilton B. 2010. Effect of intensive compared with standard glycemia treatment strategies on mortality by baseline subgroup characteristics: the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 33:721–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller ME, Bonds DE, Gerstein HC, Seaquist ER, Bergenstal RM, Calles-Escandon J, Childress RD, Craven TE, Cuddihy RM, Dailey G, Feinglos MN, Ismail-Beigi F, Largay JF, O'Connor PJ, Paul T, Savage PJ, Schubart UK, Sood A, Genuth S. 2010. The effects of baseline characteristics, glycaemia treatment approach, and glycated haemoglobin concentration on the risk of severe hypoglycaemia: post hoc epidemiological analysis of the ACCORD study. BMJ 340:b5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Riddle MC, Ambrosius WT, Brillon DJ, Buse JB, Byington RP, Cohen RM, Goff DC, Jr, Malozowski S, Margolis KL, Probstfield JL, Schnall A, Seaquist ER. 2010. Epidemiologic relationships between A1C and all-cause mortality during a median 3.4-year follow-up of glycemic treatment in the ACCORD trial. Diabetes Care 33:983–990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Zoungas S, Patel A, Chalmers J, de Galan BE, Li Q, Billot L, Woodward M, Ninomiya T, Neal B, MacMahon S, Grobbee DE, Kengne AP, Marre M, Heller S. 2010. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 65. Reaven PD, Moritz TE, Schwenke DC, Anderson RJ, Criqui M, Detrano R, Emanuele N, Kayshap M, Marks J, Mudaliar S, Harsha Rao R, Shah JH, Goldman S, Reda DJ, McCarren M, Abraira C, Duckworth W. 2009. Intensive glucose-lowering therapy reduces cardiovascular disease events in veterans affairs diabetes trial participants with lower calcified coronary atherosclerosis. Diabetes 58:2642–2648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Brown A, Reynolds LR, Bruemmer D. 2010. Intensive glycemic control and cardiovascular disease: an update. Nat Rev Cardiol 7:369–375 [DOI] [PubMed] [Google Scholar]
- 67. Skyler JS. 2010. Glycemia and cardiovascular diseases in type 2 diabetes. J Intern Med 268:468–470 [DOI] [PubMed] [Google Scholar]
- 68. Hirsch IB, Brownlee M. 2010. Beyond hemoglobin A1c–need for additional markers of risk for diabetic microvascular complications. JAMA 303:2291–2292 [DOI] [PubMed] [Google Scholar]
- 69. Abraira C, Duckworth WC, Moritz T; VADT Group 2009. Glycaemic separation and risk factor control in the Veterans Affairs Diabetes Trial: an interim report. Diabetes Obes Metab 11:150–156 [DOI] [PubMed] [Google Scholar]